Abstract
Objective
To test for an association between surgical delay and overall survival (OS) for patients with T2 renal masses. Many health care systems are balancing resources to manage the current COVID-19 pandemic, which may result in surgical delay for patients with large renal masses.
Methods
Using Cox proportional hazard models, we analyzed data from the National Cancer Database for patients undergoing extirpative surgery for clinical T2N0M0 renal masses between 2004 and 2015. Study outcomes were to assess for an association between surgical delay with OS and pathologic stage.
Results
We identified 11,848 patients who underwent extirpative surgery for clinical T2 renal masses. Compared with patients undergoing surgery within 2 months of diagnosis, we found worse OS for patients with a surgical delay of 3-4 months (hazard ratio [HR] 1.12, 95% confidence interval [CI] 1.00-1.25) or 5-6 months (HR 1.51, 95% CI 1.19-1.91). Considering only healthy patients with Charlson Comorbidity Index = 0, worse OS was associated with surgical delay of 5-6 months (HR 1.68, 95% CI 1.21-2.34, P= .002) but not 3-4 months (HR 1.08, 95% CI 0.93-1.26, P = 309). Pathologic stage (pT or pN) was not associated with surgical delay.
Conclusion
Prolonged surgical delay (5-6 months) for patients with T2 renal tumors appears to have a negative impact on OS while shorter surgical delay (3-4 months) was not associated with worse OS in healthy patients. The data presented in this study may help patients and providers to weigh the risk of surgical delay versus the risk of iatrogenic SARS-CoV-2 exposure during resurgent waves of the COVID-19 pandemic.
The current COVID-19 pandemic has disrupted systems of care. In these turbulent times, triage of clinical care delivery and appropriate calibration of care intensity have taken center stage. Oncology providers, in particular, are challenged to balance the risks of cancer progression versus the risks of COVID-19 morbidity and mortality, while potentially being constrained by limited healthcare resources.1 In patients with localized kidney cancer, for whom surgery presents an opportunity for oncologic cure, nuanced clinical decision-making is particularly critical.
Active surveillance for small renal masses (<4 cm in size) is a well-established and safe management strategy.2, 3, 4, 5, 6 Although larger renal tumors are known to harbor higher oncologic risks, it remains unclear to what extenta short to intermediate term delay in time to definitive management may impact survival. Prior studies on larger renal masses are limited by relatively small numbers from single institutions7, 8, 9 or describe preselected patients who were poor surgical candidates.10, 11, 12, 13, 14, 15 There is a paucity of literature evaluating the impact of surgical delay on survival in the setting of large renal masses in contemporary patient cohorts.
In the setting of ongoing resource limitations and the risk of iatrogenic exposure of patients and providers to SARS-CoV-2, the decision to proceed with surgery for large renal masses pivots on the ability to assess the risk of surgical delay. As such, additional data are needed to guide these decisions. To this end, this study was designed to evaluate overall survival (OS) in patients with clinical stage T2 renal mass (≥7 cm; cT2) undergoing immediate versus delayed nephrectomy. We hypothesized that patients with a cT2 renal mass who experienced greater surgical delay would have worse OS when compared with patients who underwent immediate nephrectomy.
METHODS
Study Design
We conducted a retrospective review of patients undergoing extirpative surgery for clinical T2 renal masses in the National Cancer Database (NCDB) from 2004 to 2015. The NCDB, a hospital-based registry, harnesses clinical data from more than 1500 Commission on Cancer (CoC) accredited institutions in the United States and Puerto Rico. It is estimated that approximately 70% of all new cancer diagnoses in the United States are captured in the NCDB.16, 17, 18, 19 The NCDB is a joint project of the CoC of the American College of Surgeons and the American Cancer Society. The data analyzed in the study were obtained from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. This study was deemed exempt from IRB review by the Wayne State University Institutional Review Board.
Study Population
Patients from the NCDB were included in this study if they had a clinical stage T2N0M0 renal mass and underwent radical or partial nephrectomy within 6 months of diagnosis during the period 2004-2015 (n = 41,730). Patients were excluded if they had nonrenal cell carcinoma histology (n = 17,029), uninterpretable date of diagnosis (n = 8,924), any neoadjuvant radiotherapy or systemic therapy (n = 3,716), or a mass larger than 20 cm (n = 213). The final analytic cohort included 11,848 patients.
Study Outcomes
The primary objective of this study was to test for an association between surgical delay and overall survival (OS). Surgical delay was calculated as the interval between the date of diagnosis (defined by the North American Association of Cancer Registries as the date of initial diagnosis by a recognized medical practitioner either clinically or microscopically) and the date on which the surgical procedure was performed. Patients were divided into categories based on time from diagnosis to surgery: ≤2 months, 3-4 months, and 5-6 months after diagnosis. To avoid immortal persontime bias, follow-up was calculated as the interval from surgery to death or last clinical contact.20 A sensitivity analysis was performed with OS calculated as the interval from diagnosis to death or last clinical contact. Due to concern that comorbidity may be related to OS and surgical delay, a subgroup analysis was performed of patients with a comorbidity score of 0. Secondary outcomes of interest were to test for an association between surgical delay and upgrading to pathologic T3/4 disease or node positive (pN+) in patients who underwent a lymphadenectomy.
Statistical Analysis
The Kaplan-Meier method was used to estimate survival curves stratified by surgical delay. Multivariable Cox proportional hazard models with robust standard errors clustered by hospital were fit to assess for an association between surgical delay and overall survival. The main independent variable was surgical delay, treated as both a continuous variable and as an unordered categorical variable comparing categories of surgical delay against surgery within 2 months of diagnosis. The multivariable models were adjusted for factors available within the NCDB known or believed to be associated with surgical delay or overall survival: age, sex, year of diagnosis, race (white vs non-white), size of the renal mass in cm, urban/rurality index (metropolitan vs urban vs rural as defined by the USDA Economic Research Service), travel distance from the hospital (defined by the NCDB as distance from the center of the patient's zip code of residence and to the treating hospital's address), education status (as defined by the proportion of people age 25 and older without a high school degree in the patient's zip code of residence), income (as defined as median income of the patient's zip code of residence), Charlson-Deyo comorbidity score (calculated by the NCDB), hospital type (community cancer program vs comprehensive community cancer program vs academic/research program versus integrated network cancer program), and transfer status (as defined by whether the patient was treated at the diagnosing institution vs a different institution). The multivariable Cox proportional hazards model was used to estimate the adjusted 3-, 5-, and 7-year OS probability. Multivariable logistic regressions models were fit for the secondary outcomes of pathological upgrading and pN+ disease. Models for the secondary outcomes were adjusted for the same covariates as the primary outcome and clustered by hospital. All statistical tests were two-sided with significance set at 0.05, and statistical analysis was performed with Stata version 15.1 (StataCorp, College Station, TX).
RESULTS
Demographics
Median follow-up of the 11,848 patients in our study was 41.7 months (interquartile range (IQR): 19.8-71.3). Median age of the cohort was 61 years (IQR: 52-69). Median renal mass size was 8 cm (IQR: 7-10 cm). The median time from diagnosis to surgery was 4 weeks (IQR: 2-7 weeks), with 10,146 (85.6%) patients undergoing surgery within 2 months, 1453 (12.3%) patients within 3-4 months, 249 (2.1%) patients within 5-6 months of diagnosis. Clinical, demographic, and oncological parameters for different categories of surgical delay are shown in Table 1 . We noted that patients with longer surgical delay tended to be older and less healthy.
Table 1.
Clinical, demographic, and oncological parameters for patients treated within 1-2 months, 3-4 months, and 5-6 months of diagnosis. Categorical measures compared with the Chi-squared test and continuous measure compared with the Kruskal-Wallis test
| ≤2 Months |
3-4 Months |
5-6 Months |
|||||
|---|---|---|---|---|---|---|---|
| N/Median | %/IQR | N/Median | %/IQR | N/Median | %/IQR | P value | |
| Age | 60 | 52-69 | 64 | 55-73 | 64 | 55-72 | <.001 |
| Sex | .876 | ||||||
| Male | 6362 | 62.7 | 914 | 62.9 | 160 | 64.3 | |
| Female | 3784 | 37.3 | 538 | 37.1 | 89 | 35.7 | |
| Tumor Size (cm) | 8 | 7-10 | 8 | 7-9 | 8 | 7-9 | <.001 |
| Race | <.001 | ||||||
| White | 9001 | 88.7 | 1211 | 83.3 | 190 | 76.3 | |
| non-white | 1145 | 11.3 | 242 | 16.7 | 59 | 23.7 | |
| Distance (miles) | 11.7 | 5.0-28.9 | 12.3 | 5.1-34.3 | 13.4 | 5.6-40.4 | .0336 |
| Insurance Type | <.001 | ||||||
| Private | 5352 | 52.8 | 500 | 34.4 | 75 | 30.1 | |
| Government | 4229 | 41.7 | 871 | 59.9 | 161 | 64.7 | |
| Unknown/Not insured | 565 | 5.6 | 82 | 5.6 | 12 | 5.2 | |
| Education | <.001 | ||||||
| ≥17.6 | 1904 | 19.0 | 359 | 25.1 | 77 | 31.3 | |
| 10.9-17.5 | 2608 | 26.0 | 416 | 29.1 | 72 | 29.3 | |
| 6.3-10.8 | 2979 | 29.7 | 399 | 27.9 | 63 | 25.6 | |
| <6.3 | 2545 | 25.4 | 254 | 17.8 | 34 | 13.8 | |
| Income ($/year) | <.001 | ||||||
| <40,227 | 1776 | 17.7 | 322 | 22.6 | 67 | 27.2 | |
| 40,227-50,353 | 2284 | 22.8 | 353 | 24.8 | 70 | 28.5 | |
| 50,354-63,332 | 2509 | 25.0 | 354 | 24.8 | 50 | 20.3 | |
| ≥63,333 | 3449 | 34.4 | 397 | 27.8 | 59 | 24.0 | |
| Comorbidity Index | <.001 | ||||||
| 0 | 7232 | 71.3 | 886 | 61.0 | 138 | 55.4 | |
| 1 | 2213 | 21.8 | 374 | 25.7 | 68 | 27.2 | |
| ≥2 | 701 | 6.9 | 193 | 13.3 | 43 | 17.3 | |
| Facility Type | <.001 | ||||||
| Community Cancer Program | 785 | 8.1 | 82 | 5.8 | 14 | 5.8 | |
| Comprehensive Community Cancer Program | 4115 | 42.5 | 486 | 34.4 | 74 | 30.6 | |
| Academic/Research Program | 3430 | 35.4 | 645 | 45.6 | 120 | 49.6 | |
| Integrated Network Cancer Program | 1352 | 14.0 | 202 | 14.3 | 34 | 14.1 | |
| Treated at Diagnosing Facility | <.001 | ||||||
| Yes – No transfer in care | 6950 | 68 | 827 | 56.9 | 123 | 49.4 | |
| No – Care was transferred | 3196 | 32 | 626 | 43.1 | 126 | 50.6 | |
*Cells for year of diagnosis and urban/rurality index suppressed per the NCDB data use agreement.
Primary Outcomes
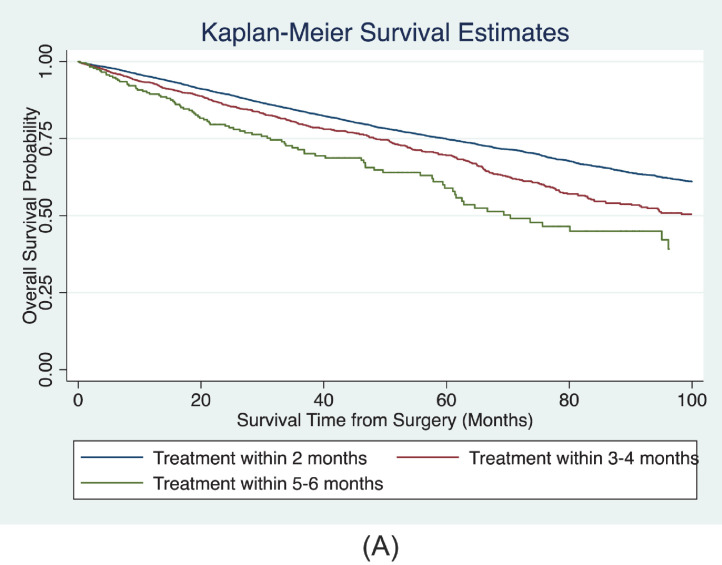
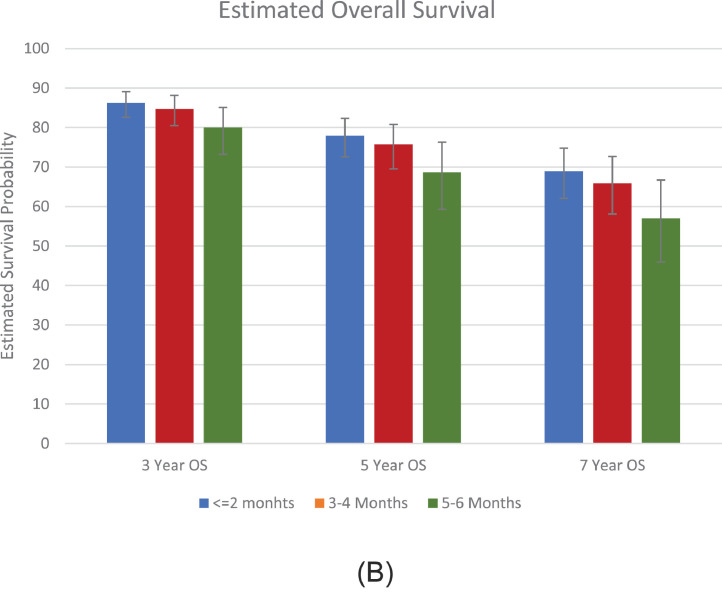
The Kaplan-Meier curves of unadjusted OS for patients with different categories of surgical delay are shown in in Figure 1A . There were 2,806 deaths during follow-up. In the multivariable Cox proportional hazards models, we detected a significant association between surgical delay and OS (Table 2A ). Adjusted ORs for the covariates included in the model are shown in the Supplemental Table. Compared with patients treated within 2 months of diagnosis, patients treated within 3-4 months (hazard ratio [HR] 1.12, 95% confidence interval [CI] 1.00-1.25, P= .042) and 5-6 months (HR 1.51, 95% CI 1.19-1.91, P= .001) had worse overall survival. Surgical delay modeled as a continuous variable demonstrated a 10% increased hazard of all-cause mortality for each month interval between diagnosis and surgery (HR 1.10, 95% CI 1.06-1.14, P <.001). The estimated 3-, 5-, and 7-year OS probability for categories of surgical delay are shown in Figure 1B . The predicted 5-year OS for patients treated within 2 months of surgery was 78% (95% CI: 73%-82%) compared with 76% (95% CI 70%-81%), and 69% (95% CI: 59%-76%) for patients treated within 3-4 months and 5-6 months after diagnosis.
Figure 1A.
Unadjusted Kaplan-Meier OS survival probability by category of surgical delay. (Color version available online.)
Table 2A.
Multivariable Cox proportional hazards models of association of surgical delay and overall survival in the entire cohort and a subgroup of patients with CCI = 0. Surgical delay is treated as an unordered categorical variable in model 1 and a continuous variable in model 2. Overall survival was calculated as the interval from surgery to either death or last clinical contact
| Time from Diagnosis to Surgery: Entire Cohort | HR | 95% CI | P | Time from Diagnosis to Surgery: CCI = 0 | HR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Model 1: | Model 1: | ||||||
| ≤2 months | ref | ref | ref | ≤2 Months | ref | ref | ref |
| 3-4 months | 1.12 | 1.00-1.25 | .042 | 3-4 Months | 1.08 | 0.93-1.26 | .309 |
| 5-6 months | 1.51 | 1.19-1.91 | .001 | 5-6 Months | 1.68 | 1.21-2.34 | .002 |
| Model 2: | Model 2: | ||||||
| Continuous per month | 1.10 | 1.06-1.14 | <.001 | Continuous per month | 1.11 | 1.06-1.17 | <.001 |
Adjusted for tumor size, age, sex, year of surgery, race, urban/rurality index, distance from the hospital, insurance type, education status, income, comorbidity index, hospital type, and transfer status.
Figure 1B.
Estimated 3-, 5-, and 7-year OS probability adjusted for factors in the multivariable Cox model. Error bars display 95% CI. (Color version available online.)
Surgical delay may be related to the presence of comorbidities. To address this potential bias, we repeated the analysis including only patients with a Charlson Comorbidity Index (CCI) of 0 (n=8,256). There were 1,806 deaths in this subgroup. We identified 7,232 patients (87.6%) treated within 2 months, 866 patients (10.7%) treated within 3-4 months, and 138 patients (1.7%) treated within 5-6 months of diagnosis. Multivariable Cox proportional hazard models restricted to patients with a CCI of 0 continued to show an association of surgical delay with OS (Table 2A). Patients treated within 5-6 months of diagnosis had worse OS compared with patients treated within 2 months of diagnosis (HR 1.68, 95% CI 1.21-2.34, P= .002). However, healthy patients treated 3-4 months after diagnosis had similar OS compared with patients treated within 2 months of diagnosis (HR 1.08, 95% CI 0.93-1.26, P= .309). Surgical delay modeled as a continuous variable demonstrated a 11% increased hazard of all-cause mortality for each month interval between diagnosis and surgery in healthy patients (HR 1.11, 95% CI 1.06-1.17, P<.001).
Using the entire cohort and the subgroup of patients with a comorbidity index of 0, we performed a sensitivity analysis with OS measured as the interval from diagnosis to death or last clinical contact (Table 2B ). These results demonstrated similar results as we continued to appreciate patients with surgical delay of 5-6 months had worse OS compared with patients treated within 2 months of diagnosis in the entire cohort (HR 1.38, 95% CI 1.10-1.73, P= .006) and in the cohort restricted to patients with a CCI of 0 (HR 1.52, 95% CI 1.11-2.08, P= .009).
Table 2B.
Sensitivity analysis for the association of surgical delay and overall survival in multivariable Cox proportional hazard models for the entire cohort and a subgroup of patients with CCI = 0. Surgical delay is treated as an unordered categorical variable in model 1 and a continuous variable in model 2. Overall survival was calculated as the interval from diagnosis to either death or last clinical contact
| Time from Diagnosis to Surgery: Entire Cohort | HR | 95% CI | P | Time from Diagnosis to Surgery: CCI = 0 | HR | 95% CI | P |
|---|---|---|---|---|---|---|---|
| Model 1: | Model 1: | ||||||
| ≤2 Months | ref | ref | ref | ≤2 Months | Ref | ref | ref |
| 3-4 Months | 1.06 | 0.96-1.18 | .255 | 3-4 Months | 1.05 | 0.90-1.21 | .549 |
| 5-6 Months | 1.38 | 1.10-1.73 | .006 | 5-6 Months | 1.52 | 1.11-2.08 | .009 |
| Model 2: | Model 2: | ||||||
| Continuous per month | 1.07 | 1.03-1.11 | <.001 | Continuous per month | 1.09 | 1.04-1.14 | .001 |
Adjusted for tumor size, age, sex, year of surgery, race, urban/rurality index, distance from the hospital, insurance type, education status, income, comorbidity index, hospital type, and transfer status.
Secondary Outcomes
We did not observe a significant association between surgical delay and upgrading or pN+ disease. Similar odds of upgrading and pN+ disease were seen in patients treated 3-4 and 5-6 months after diagnosis compared with patients treated within 2 months of diagnosis (Tables 3A and B ).
Table 3A.
Multivariable logistic regression model for the association of surgical delay and pathologic upgrading
| Surgical Delay | OR | 95% CI | P |
|---|---|---|---|
| ≤2 months | ref | ref | Ref |
| 3-4 months | 0.9 | 0.77-1.04 | .138 |
| 5-6 months | 1.07 | 0.77-1.48 | .673 |
Adjusted for tumor size, age, sex, year of surgery, race, urban/rurality index, distance from the hospital, insurance type, education status, income, comorbidity index, hospital type, and transfer status.
Table 3B.
Multivariable logistic regression model for the association of surgical delay and pN+
| Surgical Delay | OR | 95% CI | P |
|---|---|---|---|
| ≤2 months | ref | ref | Ref |
| 3-4 months | 0.82 | 0.47-1.43 | .475 |
| 5-6 months | 1.41 | 0.52-3.83 | .504 |
Adjusted for tumor size, age, sex, year of surgery, race, urban/rurality index, distance from the hospital, insurance type, education status, income, comorbidity index, hospital type, and transfer status.
COMMENT
As resurgent waves of the COVID-19 pandemic continue to strain hospital systems’ resources and personnel, nonemergent surgeries may be delayed.21 The goal of this study was to assess the association of surgical delay with OS in patients with large renal masses. To this end, we report that patients with cT2 renal masses who experienced prolonged delays in surgical treatment exhibited decreased OS when compared to patients who underwent surgery soon after diagnosis. A similar association was seen when we restricted the cohort to patients with a comorbidity index of 0, although shorter surgical delay (3-4 months) was not associated with worse OS in healthy patients. We did not observe a significant association of surgical delay with the secondary outcomes of pathologic upgrading and pN+ disease.
The implications of treatment delay for patients with cancer is understandably on many patients’ and clinicians’ minds during these uncertain times.22, 23, 24. The literature is limited in addressing the association surgical delay with oncologic outcomes in patients with large renal masses. Published reports largely consist of retrospective single institution studies.7, 8, 9 , 25 or focus on poor surgical candidates undergoing observation of large renal masses.10, 11, 12, 13, 14 Similar to our results, Mano et al retrospectively reviewed 1,278 patients at a single center and found that increasing surgical delay for renal masses >4 cm (median tumor size, 6.2 cm) was significantly associated with decreased OS after adjusting for patient and tumor characteristics.8 Interestingly, these investigators did not find surgical delay to be associated with tumor upstaging, recurrence, or cancer-specific survival. In contrast, a study conducted by Kim et al retrospectively reviewed 319 patients with cT2 or greater renal cancer who underwent radical nephrectomy at a single institution and found no difference in survival or oncologic outcomes when comparing patients who underwent surgery within 1 month to those who had surgery within 1-3 months after diagnosis, suggesting that the harm of a short delay, if it exists, may be difficult to detect in a small cohort.7
Furthermore, recent reports by Khorana et al and Turaga et al utilized the NCDB to evaluate the broader association of treatment delays and OS in multiple cancer types, including renal cancer.26 , 27 These studies included patients with renal masses of all sizes and demonstrated an association between prolonged treatment delay and decreased overall survival. In both studies, the median time to surgery was less than one week, suggesting possible inaccuracies in the recording of surgical delay. Indeed, a strength of our study was the inclusion only of patients in which surgical delay could be accurately characterized.
Our work continues to build upon the limited data regarding surgical delay in patients with cT2 renal masses by utilizing a large, contemporary, nationally representative cohort to suggest a small, but significant, absolute difference in OS in patients with prolonged surgical delay. We did not observe a significant association of the secondary outcomes of pathologic upgrading and pN+ disease with treatment delay. Furthermore, when restricting the analysis to healthy patients with CCI of 0, a short delay was not associated with worse OS. These data suggest that a short treatment delay due to the COVID pandemic may not affect survival nor worsen oncological outcomes of upgrading or node positivity. During the ongoing COVID-19 pandemic, the harms of surgical delay must be carefully weighed against multiple other considerations, including the risk of iatrogenic exposure to the virus, the compromise of social distancing during care delivery, and allocation of limited health resources.1 This calculus must be individualized, informed by accurate estimates of the risks involved, and include active patient participation when deciding on the optimal timing of surgery. Furthermore, as the current pandemic resolves, these data may provide reassurance to patients who require a short delay to allow for medical maximization and improved control of chronic medical conditions prior to proceeding with surgery.
This study has several limitations. First, although we attempted to adjust for confounding variables in our multivariable models, no statistical adjustment of data obtained from preselected cohorts can fully account for both measured and unmeasured confounders. Granular details regarding specific comorbidities or oncologic characteristics, such as imaging findings or the health of the contralateral kidney, are not available in the NCDB. Second, the cause for delay in surgeries is unknown and the generalizability of these results to patients who have deferred surgery during the COVID-19 pandemic remains unclear. It remains possible that generally sicker and unhealthier patients may take longer to proceed to surgery due to more intense surgical clearance and medical optimization processes. Last, this study was limited by the variables collected by the NCDB. Oncologic outcomes such as disease recurrence, progression, and cancer specific mortality were not available. Despite these limitations, this study uses current data from a national database with a large cohort providing a level of power that is otherwise not present in the current literature.
CONCLUSION
As the COVID-19 pandemic continues to strain healthcare systems, delays in nonemergent surgery are inevitable. As gleaned from this large NCDB cohort, patients with cT2 renal masses may not have worse OS or oncological outcomes as a result of short surgical delay. Prolonged surgical delay was associated with worse OS and should be minimized when possible. These data can help inform patient counseling when deciding on the timing of nephrectomy by weighing the risk of surgical delay against the risk of iatrogenic SARS-CoV-2 exposure.
Footnotes
Conflicts of Interest:All the authors have no conflict of interest to disclose.
Financial Disclosure:The authors declare that they have no relevant financial interests.
Kevin Ginsburg attests to the accuracy of the references and all statements made in the following documents.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urology.2020.09.010.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med. 2020;172(11):756–758. doi: 10.7326/M20-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell S, Uzzo RG, Allaf ME. Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520–529. doi: 10.1016/j.juro.2017.04.100. [DOI] [PubMed] [Google Scholar]
- 3.Alam R, Patel HD, Osumah T. Comparative effectiveness of management options for patients with small renal masses: a prospective cohort study. BJU Int. 2019;123:42–50. doi: 10.1111/bju.14490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntosh AG, Ristau BT, Ruth K. Active surveillance for localized renal masses: tumor growth, delayed intervention rates, and >5-yr clinical outcomes. Eur Urol. 2018;74:157–164. doi: 10.1016/j.eururo.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Crispen PL, Viterbo R, Fox EB, Greenberg RE, Chen DY, Uzzo RG. Delayed intervention of sporadic renal masses undergoing active surveillance. Cancer. 2008;112:1051–1057. doi: 10.1002/cncr.23268. [DOI] [PubMed] [Google Scholar]
- 6.Rais-Bahrami S, Guzzo TJ, Jarrett TW, Kavoussi LR, Allaf ME. Incidentally discovered renal masses: oncological and perioperative outcomes in patients with delayed surgical intervention. BJU Int. 2009;103:1355–1358. doi: 10.1111/j.1464-410X.2008.08242.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, You D, Jeong IG. The impact of delaying radical nephrectomy for stage II or higher renal cell carcinoma. J Cancer Res Clin Oncol. 2012;138:1561–1567. doi: 10.1007/s00432-012-1230-2. [DOI] [PubMed] [Google Scholar]
- 8.Mano R, Vertosick EA, Hakimi AA. The effect of delaying nephrectomy on oncologic outcomes in patients with renal tumors greater than 4 cm. Urol Oncol. 2016;34 doi: 10.1016/j.urolonc.2015.12.001. 239.e231-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stec AA, Coons BJ, Chang SS. Waiting time from initial urological consultation to nephrectomy for renal cell carcinoma–does it affect survival? J Urol. 2008;179:2152–2157. doi: 10.1016/j.juro.2008.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beisland C, Hjelle KM, Reisaeter LA, Bostad L. Observation should be considered as an alternative in management of renal masses in older and comorbid patients. Eur Urol. 2009;55:1419–1427. doi: 10.1016/j.eururo.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Lamb GW, Bromwich EJ, Vasey P, Aitchison M. Management of renal masses in patients medically unsuitable for nephrectomy–natural history, complications, and outcome. Urology. 2004;64:909–913. doi: 10.1016/j.urology.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 12.Marzouk K, Tin A, Liu N. The natural history of large renal masses followed on observation. Urol Oncol. 2018;36 doi: 10.1016/j.urolonc.2018.05.002. 362.e317–362.e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrazin R, Smaldone MC, Kutikov A. Growth kinetics and short-term outcomes of cT1b and cT2 renal masses under active surveillance. J Urol. 2014;192:659–664. doi: 10.1016/j.juro.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mues AC, Haramis G, Badani K. Active surveillance for larger (cT1bN0M0 and cT2N0M0) renal cortical neoplasms. Urology. 2010;76:620–623. doi: 10.1016/j.urology.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Touma NJ, Hosier GW, Di Lena MA. Growth rates and outcomes of observed large renal masses. Can Urol Assoc J. 2018:276–281. doi: 10.5489/cuaj.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohanty S, Bilimoria KY. Comparing national cancer registries: the National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol. 2014;109:629–630. doi: 10.1002/jso.23568. [DOI] [PubMed] [Google Scholar]
- 17.Merkow RP, Rademaker AW, Bilimoria KY. Practical guide to surgical data sets: National Cancer Database (NCDB) JAMA Surg. 2018;153:850–851. doi: 10.1001/jamasurg.2018.0492. [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boffa DJ, Rosen JE, Mallin K. Using the national cancer database for outcomes research: a review. JAMA Oncol. 2017;3:1722–1728. doi: 10.1001/jamaoncol.2016.6905. [DOI] [PubMed] [Google Scholar]
- 20.Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 21.ACo S. COVID-19: guidance for triage of non-emergent surgical procedures. 2020.
- 22.Bell SA, Banerjee M, Griggs JJ, Iwashyna TJ, Davis MA. The effect of exposure to disaster on cancer survival. J Gen Intern Med. 2020;35:380–382. doi: 10.1007/s11606-019-05465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Man RX, Lack DA, Wyatt CE, Murray V. The effect of natural disasters on cancer care: a systematic review. Lancet Oncol. 2018;19:e482–e499. doi: 10.1016/S1470-2045(18)30412-1. [DOI] [PubMed] [Google Scholar]
- 24.Ginsburg KB, Curtis G, Timar R, George AK, Cher ML. Delayed radical prostatectomy is not associated with adverse oncological outcomes: implications for men experiencing surgical delay due to the COVID-19 pandemic. J Urol. 2020;204(4):720–725. doi: 10.1097/JU.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 25.Martinez CH, Martin P, Chalasani V. How long can patients with renal cell carcinoma wait for surgery without compromising pathological outcomes? Can Urol Assoc J. 2011;5:E148–E151. doi: 10.5489/cuaj.10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turaga KK, Girotra S. Are we harming cancer patients by delaying their cancer surgery during the COVID-19 pandemic? Ann Surg. 2020 doi: 10.1097/SLA.0000000000003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khorana AA, Tullio K, Elson P. Time to initial cancer treatment in the United States and association with survival over time: an observational study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.