Abstract
Background:
Catheter ablation is an increasingly utilized treatment for symptomatic atrial fibrillation (AF). However, there are limited prospective, nationwide data on patient selection and procedural characteristics. This study describes patient characteristics, techniques, treatment patterns, and safety outcomes of patients undergoing AF ablation.
Methods:
A total of 3,139 patients undergoing AF ablation between 2016-2018 in the Get With The Guidelines-Atrial Fibrillation registry from 24 US centers were included. Patient demographics, medical history, procedural details and complications were abstracted. Differences between paroxysmal and persistent AF patients were compared using Pearson’s Chi-square and Wilcoxon rank-sum tests.
Results:
Patients undergoing AF ablation were predominantly male (63.9%) and Caucasian (93.2%) with a median age of 65. Hypertension was the most common comorbidity (67.6%) and persistent AF patients had more comorbidities than paroxysmal AF patients. Drug refractory, paroxysmal AF was the most common ablation indication (Class I, 53.6%) followed by drug refractory, persistent AF (Class I, 41.8%). Radiofrequency (RF) ablation with contact force (CF) sensing was the most common ablation modality (70.5%); 23.7% of patients underwent cryoballoon ablation. Pulmonary vein isolation (PVI) was performed in 94.6% of de novo ablations; the most common adjunctive lesions included left atrial roof or posterior/inferior lines, and cavotricuspid isthmus ablation. Complications were uncommon (5.1%), and were life-threatening in 0.7% of cases.
Conclusions:
More than 98% of AF ablations among participating sites are performed for Class I or Class IIA indications. CF-guided RF ablation is the dominant technique and PVI the principal lesion set. In-hospital complications are uncommon and rarely life-threatening.
Introduction
Catheter ablation for atrial fibrillation (AF) has grown significantly as a mainstream therapy with a more than 5-fold increase in utilization between 1999 and 2013.1 During this time period, professional society guidelines have endorsed catheter ablation as a rhythm control strategy across a range of clinical scenarios.2,3 Single-center analyses, European registry studies, and analyses of US administrative claims data have demonstrated an improvement in the safety profile of AF ablation over time.4-11 However, few data are available describing national practice patterns of procedural techniques and ablation lesion patterns. Additionally, given the major advances and procedural approaches to AF in recent years,12 analysis of a more contemporary cohort may provide a more accurate assessment of procedural patterns and the safety profile of catheter ablation.
The Get With The Guidelines-Atrial Fibrillation (GWTG-AFIB) registry quality improvement program introduced an ablation module in 2016 that allowed participating sites to report patient characteristics, procedural methods and complication rates of patients undergoing catheter ablation for AF. In this study, we aimed to (1) evaluate the patient characteristics and indications among patients undergoing AF ablation, (2) describe procedural characteristics/techniques and compare them by AF subtype (paroxysmal vs persistent), and (3) assess the frequency of complications.
Methods
Data Collection and Components:
The data used for the analyses presented here were collected as part of the GWTG-AFIB registry. The details of this registry have been described previously.13 Briefly, GWTG-AFIB is a national, voluntary quality improvement initiative that began in 2013 in partnership with the AHA and HRS. The goal for the registry is to improve the adoption of guideline-directed therapies and outcomes in patients with AF. The main GWTG-AFIB registry collects data on medical history, hospital care and outcomes of AF patients via an online, interactive case report form. In 2016, a sub-registry module was introduced that allows sites to enroll patients undergoing catheter ablation. Beyond the data collected in the main GWTG-AFIB registry, the ablation module collects data on the indications for ablation, rhythm control history, procedural characteristics, and safety outcomes. The present analysis focuses on patient selection, techniques, and safety outcomes. Participating institutions complied with local regulatory and privacy guidelines, including institutional review board approval where required. Since the GWTG-AFIB registry is a quality improvement program, some institutions consider the program exempt from IRB review. IQVIA served as the registry coordinating center and the Duke Clinical Research Institute served as the analytic core; IRB approval was established for all analytic activities at the analytic core. Data was abstracted by trained personnel using standardized definitions for all data elements.13
Indications for Catheter Ablation:
The relative frequency of indications for catheter ablation for AF were reported based on the 2014 AHA/ACC/HRS guidelines, given the study period of 2016-2018 preceded the release of the 2019 Focused Update of these guidelines.2,14 Namely, catheter ablation was considered a Class I indication for symptomatic paroxysmal AF refractory to at least one antiarrhythmic drug (AAD), Class IIA for symptomatic persistent AF refractory to at least one AAD, Class IIA as first-line therapy for paroxysmal AF and Class IIB for first-line therapy for persistent AF. The 2019 Focused Update added a Class IIB indication for catheter ablation for treatment of patients with symptomatic AF and heart failure with reduced ejection fraction. This change would does not affect the class of recommendations of any of the patients included in the present analysis; however, patients meeting this indication may not be well represented in the study population.
Procedural Characteristics, Safety Endpoints, and Quality Measures:
The characteristics of catheter ablation procedures were reported, including pre-procedural management/imaging, sedation strategy, intra-procedural imaging, ablation technique/lesion sets, provocative testing and procedure times. These variables were compared between AF subtypes (paroxysmal vs persistent). Paroxysmal AF was defined as AF self-terminating within seven days of recognized onset; whereas, persistent AF was defined as AF self-terminating beyond seven days or was terminated electrically or pharmacologically. Frequency of intra-procedural and post-procedural complications were reported for all patients as well as by AF subtype. As an exploratory analysis, the baseline characteristics and procedural management of patients with and without post-procedural complications were compared.
Statistical Analysis
Baseline patient characteristics were described overall and by AF subtype using percentages for categorical variables and medians with interquartile range (IQR, 25th - 75th percentiles) for continuous variables. Differences in these characteristics were compared using Pearson’s Chi-square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Comparisons were made using non-missing data only. All tests were 2-sided and statistical significance was declared when p<0.05. All analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC).
Results
Study Population and Baseline Characteristics
Between January 4, 2013 and November 22, 2018 there were 65,565 patients who were registered from 141 sites participating in GWTG-AFIB. We excluded patients who were missing gender information (n=131), received comfort care only on hospital day 0 or 1 (n=822), from hospitals without the ablation module (n=61,102), were missing all data on ablation technique (n=187), received cavotricuspid isthmus (CTI) ablation only (n=115), had long-standing persistent AF (n=32), had other atrial arrhythmias such as left atrial (LA) flutter or LA tachycardia (n=35), or were missing data on their rhythm (n=2). The final cohort contained 3,139 patients from 24 hospitals with a median of 84 patients per site (IQR 10-236). Of participating sites, 85.1% were academic teaching hospitals with more than half (54.2%) having 500 or more beds (site characteristics in Supplemental Table).
Baseline characteristics are presented in Table 1. The median age was 65 years (58-71), 36.1% of patients were female, 93.2% were Caucasian and 2.6% of patients were African American. Most patients had private insurance (64.6%), although a substantial minority (29.9%) had Medicare with a higher prevalence among patients with persistent AF compared with paroxysmal AF (33.7% vs 27.1%, p=0.001). The most common comorbidities were hypertension (67.6%), obstructive sleep apnea (OSA, 31.9%), stage 3 chronic kidney disease (CKD, 24.3%), coronary artery disease (18.9%), diabetes mellitus (18.8%), and heart failure (18.2%), with 8.2% of included patients having a left ventricular ejection fraction <40%. Each of these conditions were more prevalent among patients with persistent AF (p<0.001 for all). Median CHA2DS2VASc score15 was 2 (1-4) and median HAS-BLED score16 was 2 (1-2); both were higher among persistent AF patients (p<0.001).The median LA diameter was 4.2cm (3.7-4.8) for all patients, with persistent AF patients having larger diameters than paroxysmal AF patients (4.4 [4.0-5.0]) vs 4.0 [3.5-4.5], p<0.0001).
Table 1: Baseline Characteristics:
Demographics and medical history for all included patients and by AF subtype. Data presented as median (IQR) or N (%) as appropriate.
| Overall | Paroxysmal AF | Persistent AF | P-value | |
|---|---|---|---|---|
| N | 3139 | 1778 (56.6%) | 1361 (43.4%) | |
| Age, years | 65 (58-71) | 64 (57-70) | 66 (60-72) | <0.0001 |
| Female | 1132 (36.1%) | 711 (40%) | 421 (30.9%) | <0.0001 |
| Race/ethnicity | 0.6949 | |||
| White | 2924 (93.2%) | 1653 (93.0%) | 1271 (93.4%) | |
| Black/African American | 80 (2.6%) | 43 (2.4%) | 37 (2.7%) | |
| Hispanic | 28 (0.9%) | 19 (1.1%) | 9 (0.7%) | |
| Other | 107 (3.4%) | 63 (3.5%) | 44 (3.2%) | |
| Insurance | 0.0010 | |||
| Private | 2021 (64.6%) | 1200 (67.7%) | 821 (60.4%) | |
| Medicare | 483 (15.4%) | 251 (14.2%) | 232 (17.1%) | |
| Private + Medicare | 453 (14.5%) | 228 (12.9%) | 225 (16.6%) | |
| Medicaid | 152 (4.8%) | 81 (4.6%) | 71 (5.2%) | |
| None | 22 (0.7%) | 12 (0.7%) | 10 (0.74%) | |
| Hypertension | 2122 (67.6%) | 1102 (62.0%) | 1020 (74.9%) | <0.0001 |
| OSA | 1000 (31.9%) | 505 (28.4%) | 495 (36.4%) | <0.0001 |
| Coronary artery disease | 593 (18.9%) | 295 (16.6%) | 298 (21.9%) | 0.0002 |
| Diabetes Mellitus | 589 (18.8%) | 284 (16%) | 305 (22.4%) | <0.0001 |
| Heart Failure | 571 (18.2%) | 201 (11.3%) | 370 (27.2%) | <0.0001 |
| LVEF <40% | 243 (8.2%) | 80 (4.8%) | 163 (12.5%) | <0.0001 |
| Thyroid disease | 505 (16.1%) | 284 (16.0%) | 221 (16.2%) | 0.8413 |
| On dialysis | 6 (0.2%) | 3 (0.2%) | 3 (0.2%) | 0.7425 |
| Stage 3 CKD | 623 (24.3%) | 308 (22.0%) | 315 (27.2%) | 0.0023 |
| Cancer | 365 (11.6%) | 201 (11.3%) | 164 (12.1%) | 0.5187 |
| COPD | 256 (8.2%) | 129 (7.3%) | 127 (9.3%) | 0.0352 |
| CVA/TIA | 245 (7.8%) | 129 (7.3%) | 116 (8.5%) | 0.1895 |
| Peripheral vascular disease | 95 (3.0%) | 37 (2.1%) | 58 (4.3%) | 0.0004 |
| Family history of AF | 82 (2.6%) | 39 (2.2%) | 43 (3.2%) | 0.0927 |
| Left atrial diameter, cm | 4.2 (3.7-4.8) | 4.0 (3.5-4.5) | 4.4 (4.0-5.0) | <0.0001 |
| CHA2DS2VASc score | 2 (1-4) | 2 (1-3) | 3 (2-4) | <0.0001 |
| ORBIT bleeding score | 1 (0-2) | 1 (0-2) | 1 (0-2) | 0.0003 |
| HAS-BLED Score | 2 (1-2) | 1 (1-2) | 2 (1-2) | <0.0001 |
| Prior major bleeding | 94 (3.0%) | 44 (2.5%) | 50 (3.7%) | 0.0151 |
| Prior ablations for AF | 0.0002 | |||
| 0 | 2447 (78.1%) | 1432 (80.6%) | 1015 (74.8%) | |
| 1 | 563 (18.0%) | 293 (16.5%) | 270 (19.9%) | |
| 2 | 95 (3.0%) | 42 (2.4%) | 53 (3.9%) | |
| ≥3 | 29 (0.9%) | 10 (0.6%) | 19 (1.4%) |
Abbreviations: AF = atrial fibrillation; OSA = obstructive sleep apnea; LVEF = left ventricular ejection fraction; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CVA/TIA = cerebrovascular accident/transient ischemic attack
Catheter Ablation Frequency by Indication
Most patients (78.1%) underwent first-time (de novo) ablation. A total of 18.0% had had one prior ablation, 3.0% had had two prior ablations and less than 1% had had ≥3 prior ablations (Table 1). Patients with persistent AF were more likely to have undergone prior ablation (p=0.0002). The most common indication for catheter ablation was paroxysmal AF refractory to at least one AAD (53.6%) followed by drug refractory persistent AF (41.8%) (Figure 1). Less than 1 in 20 procedures were performed as first line therapy for paroxysmal AF (3.0%) or persistent AF (1.6%).
Figure 1. Indications for Catheter Ablation:
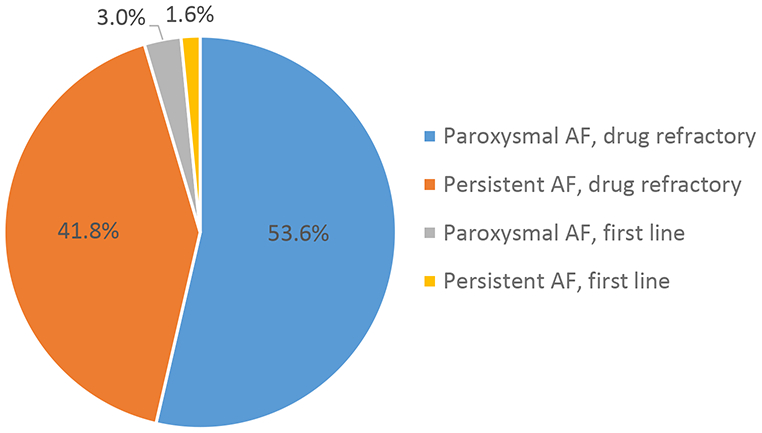
Percentage of patients referred for catheter ablation for paroxysmal atrial fibrillation (AF) refractory to at least one antiarrhythmic drug (AAD), persistent AF refractory to at least one AAD, first line therapy for paroxysmal AF, and first line therapy for persistent AF.
Procedural Techniques and Characteristics
Patients were treated with AADs at the time of admission for their ablation hospitalization 59.6% of the time (Figure 2). The most commonly used AAD was flecainide (14.6%). Patients with paroxysmal AF were more likely to receive AADs overall (63.4% vs 54.6%) and more likely to receive flecainide, sotalol, dronedarone or propafenone (p<0.0001 for all). Persistent AF patients were more likely to receive amiodarone or dofetilide. Peri-procedural management strategies are described in Table 2. The most common peri-procedural anticoagulation strategy was uninterrupted anticoagulation (defined as no interruption to the patient’s anticoagulant medication regimen prior to the ablation procedure, 73.6%); bridging (temporary interruption of a patient’s chronic anticoagulant medication with use of a short-acting parenteral anticoagulant) was uncommon (4.3%). Pre-procedure transesophageal echocardiography (TEE) was performed in 49.0% of patients, more commonly among persistent AF patients (p<0.0001). Pre-procedure computed tomography (CT) was used in most cases (60.3%), while magnetic resonance imaging (MRI) was uncommon (2.9%).
Figure 2. Antiarrhythmic Use:
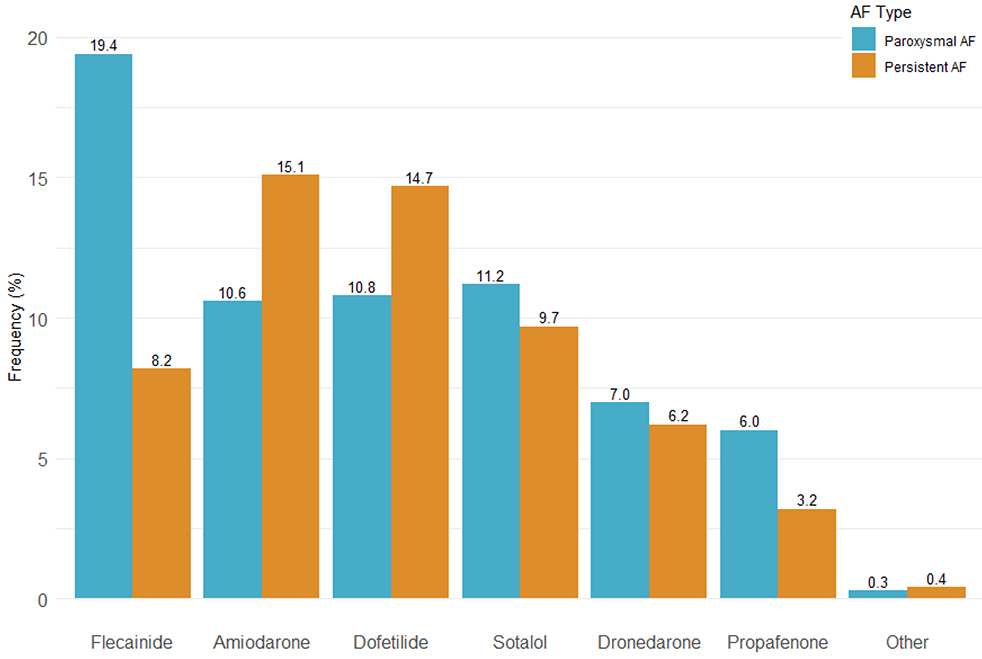
Frequency of antiarrhythmic use prior to catheter ablation subgrouped by atrial fibrillation (AF) subtype. P<0.0001 for all comparisons between paroxysmal and persistent AF.
Table 2: Procedural Characteristics:
Use of peri-procedural anticoagulation (AC), imaging, catheter types and provocation testing as well as procedural times for overall population and by AF subtype. Data presented as N (%) or median (IQR) as appropriate.
| Overall | Paroxysmal AF | Persistent AF | p-value | |
|---|---|---|---|---|
| N | 3139 | 1778 (56.6%) | 1361 (43.4%) | |
| Peri-procedure AC strategy | 0.0134 | |||
| Uninterrupted | 2280 (73.6%) | 1299 (74.4%) | 981 (72.4%) | |
| Interrupted | 688 (22.2%) | 389 (22.3%) | 299 (22.1%) | |
| Bridging | 132 (4.3%) | 58 (3.3%) | 74 (5.5%) | |
| Pre-procedure Imaging | ||||
| Preoperative TEE | 1535 (49.0%) | 762 (43.0%) | 773 (56.9%) | <0.0001 |
| CT | 1889 (60.3%) | 1106 (62.3%) | 783 (57.7%) | 0.0079 |
| MRI | 91 (2.9%) | 66 (3.7%) | 25 (1.8%) | 0.0019 |
| Intra-procedure Imaging | ||||
| Intra-cardiac echocardiography | 2867 (91.5%) | 1628 (91.8%) | 1239 (91.2%) | 0.5954 |
| 3D electroanatomic mapping | 2843 (90.8%) | 1602 (90.3%) | 1241 (91.4%) | 0.3008 |
| Intra-operative TEE | 436 (13.9%) | 248 (14.0%) | 188 (13.8%) | 0.9134 |
| Rotational angiography | 176 (5.6%) | 103 (5.8%) | 73 (5.4%) | 0.6042 |
| Energy and catheter type | ||||
| Irrigated RF with contact force | 2206 (70.5%) | 1222 (69.0%) | 984 (72.5%) | 0.0335 |
| Irrigated RF without contact force | 271 (8.7%) | 135 (7.6%) | 136 (10.0%) | 0.0182 |
| Cryoballoon | 743 (23.7%) | 468 (26.4%) | 275 (20.2%) | <0.0001 |
| Other | 53 (1.7%) | 29 (1.6%) | 24 (1.8%) | 0.7788 |
| Provocation testing* | ||||
| Burst pacing | 1652 (68.5%) | 942 (67.9%) | 710 (69.4%) | 0.4222 |
| Isoproternol | 1148 (47.6%) | 626 (45.1%) | 552 (51.0%) | 0.0040 |
| Adenosine | 602 (25.0%) | 394 (28.4%) | 208 (20.3%) | <0.0001 |
| Other | 224 (9.3%) | 149 (10.7%) | 75 (7.3%) | 0.0044 |
| Procedure time (minutes) | ||||
| Ablation time | 37 (17-59) | 35 (17.5-53) | 41 (17-67) | <0.0001 |
| Fluoroscopy time | 16 (8-28) | 15 (7-26) | 18 (10-30) | <0.0001 |
| Total procedure time | 180 (140-230) | 174 (137-218) | 190 (146-240) | <0.0001 |
Abbreviations: AF= atrial fibrillation; AC = anticoagulation; CT = computed tomography; MRI = magnetic resonance imaging; TEE = transesophageal echocardiography; RF = radiofrequency.
All patients in this cohort received provocation testing of some type.
The most common sedation strategy was general anesthesia with endotracheal intubation (87.7%), although 5.9% of patients had conscious sedation and 5.8% of patients had general anesthesia with use of a laryngeal mask airway. Intracardiac echocardiography and 3D electroanatomic mapping were used in more than 90% of cases; whereas, intraoperative TEE and rotational angiography use were uncommon.
The most common energy modality utilized was irrigated radiofrequency (RF) ablation with contact force (CF) sensing (70.5%) followed by cryoballoon (23.7%). Patients with paroxysmal AF were more likely to undergo cryoballoon ablation than persistent AF patients. Provocative testing was common and done most frequently with burst pacing (68.5%). Isoproteronol and adenosine were also commonly used, with isoproterenol more frequently used in persistent AF patients and adenosine more commonly used in paroxysmal AF patients. Median procedure times were 180 minutes (IQR 140-230), with 37 minutes of ablation (IQR 17-59) and 16 minutes of fluoroscopy (IQR 8-28). Persistent AF patients had longer ablation, fluoroscopy and total procedure times (p<0.0001 for all).
Figure 3 illustrates the lesion sets employed in patients undergoing ablation for the first time (N=2,418, 77% of patients). Overall, pulmonary vein isolation (PVI) was performed in 94.6% of cases. The most common adjunctive lesion set was right sided CTI ablation for atrial flutter (32.9%), LA roof line (30.8%), and LA posterior/inferior line (24.0%). Persistent AF patients were more likely to receive a LA roof line (42.1% vs 21.7%, p<0.0001), LA posterior/inferior line (36.0% vs 14.3%, p<0.0001), posterior wall (PW) isolation (24.0% vs 8.4%, p<0.0001), lateral mitral isthmus line (5.4% vs 2.7%, p=0.0020), or septal mitral isthmus line (5.4% vs 3.1%, p=0.0062). Complex fractionated electrogram ablation (CFAE) and focal impulse and rotor modulation (FIRM) were performed in 9.2% and 10.7% of patients respectively, with higher rates in persistent AF (11.4% vs 7.4% p = 0.0015 for CFAE, 14.0% vs 8.1% p<0.0001 for FIRM). Among patients with right sided CTI ablation for atrial flutter, 8.4% of patients had a documented baseline rhythm of atrial flutter (typical or atypical).
Figure 3. Ablation Techniques for First Ablations:
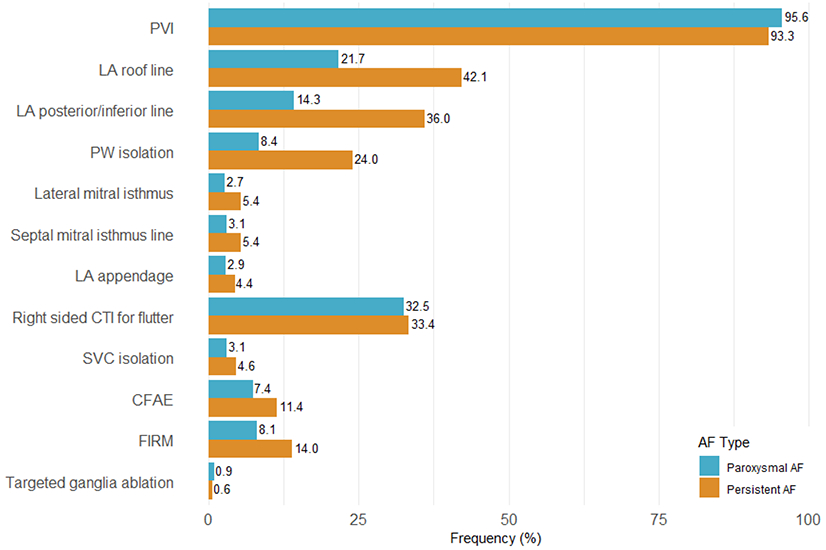
Frequency of different ablation lesion sets for patients undergoing first ablations (N=2418) subgrouped by atrial fibrillation (AF) subtype. Abbreviations: PVI = pulmonary vein isolation; LA = left atrial; PW = posterior wall; CTI = cavotricuspid isthmus; SVC = superior vena cava; CFAE = complex fractionated atrial electrogram; FIRM = focal impulse and rotor modulation
Figure 4 illustrates the lesion sets employed stratified by first time vs repeat ablation (N=721). Patients undergoing repeat ablation were less likely to receive PVI (94.6% vs 81.4%, p<0.0001) and were more likely to receive adjunctive lesions including LA posterior/inferior line (33.4% vs 24.0%, p<0.0001), lateral mitral isthmus line (13.0% vs 3.9%, p<0.0001), septal mitral isthmus line (11.1% vs 4.1%), LA appendage isolation (6.9% vs 3.6%, p=0.0002), SVC isolation (8.6% vs 3.8%, p<0.0001), and CFAE (13.6% vs 9.2%, p=0001).
Figure 4. Ablation Techniques for First Time vs Repeat Ablations:
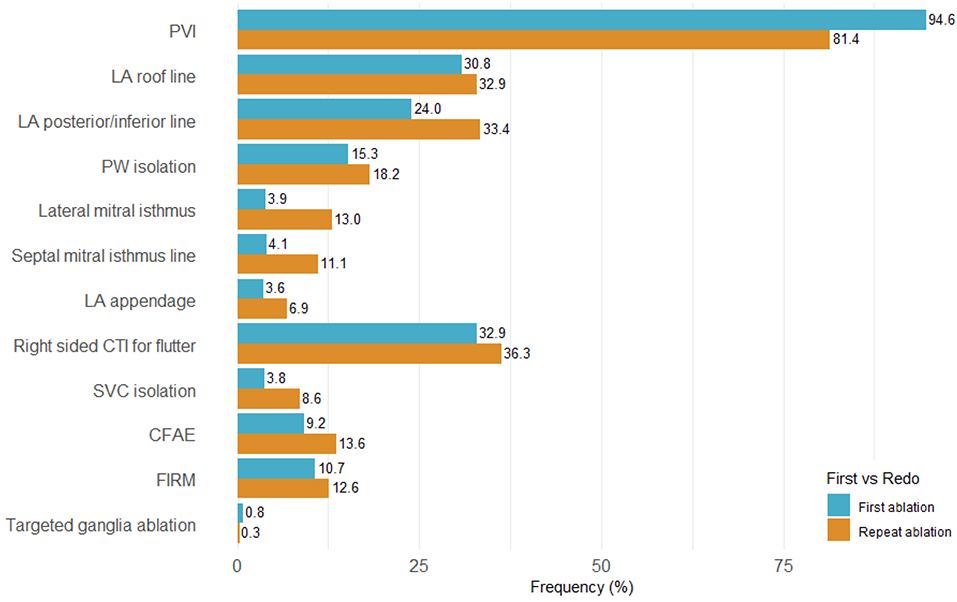
Frequency of different ablation lesion sets for patients undergoing first time ablation (N=2418) vs repeat ablations (N=721). Abbreviations: PVI = pulmonary vein isolation; LA = left atrial; PW = posterior wall; CTI = cavotricuspid isthmus; SVC = superior vena cava; CFAE = complex fractionated atrial electrogram; FIRM = focal impulse and rotor modulation
Safety Outcomes
In-hospital complications were noted in 160 patients (5.1% of the total population, Table 3). Complications were more common in patients with persistent AF compared with paroxysmal AF (91 cases [6.7%] vs 69 cases [3.9%], p=0.0004). The most common complications reported were volume overload (39 cases, 1.2%), hematoma (33 cases, 1.1%), hemopericardium (14 cases, 0.4%), need for pericardiocentesis (11 cases, 0.4%), and urinary tract infection (11 cases, 0.4%). Life-threatening complications were uncommon, with nine cases of tamponade, seven cases of hemorrhage requiring transfusion, four strokes or transient ischemic attacks (TIAs), and one death. There were no reports of atrioesophageal fistula.
Table 3: Complications:
Number of intra-procedural or immediate post-procedure complications for all patients and by AF subtype.
| Overall | Paroxysmal AF | Persistent AF | p-value | |
|---|---|---|---|---|
| N | 3139 | 1778 (56.6%) | 1361 (43.4%) | N |
| Total cases with complications | 160 (5.1%) | 69 (3.9%) | 91 (6.7%) | 0.0004 |
| Volume overload | 39 (1.2%) | 14 (0.8%) | 25 (1.8%) | 0.0086 |
| Hematoma | 33 (1.1%) | 14 (0.8%) | 19 (1.4%) | 0.0979 |
| Hemopericardium | 14 (0.4%) | 10 (0.6%) | 4 (0.3%) | 0.2628 |
| Pericardiocentesis | 11 (0.4%) | 7 (0.4%) | 4 (0.3%) | 0.6762 |
| Urinary tract infection | 11 (0.4%) | 8 (0.4%) | 3 (0.2%) | 0.2805 |
| Anesthesia complication | 9 (0.3%) | 4 (0.2%) | 5 (0.4%) | 0.4602 |
| Tamponade | 9 (0.3%) | 8 (0.4%) | 1 (0.08%) | 0.0559 |
| Pseudoaneurysm | 8 (0.2%) | 3 (0.2%) | 5 (0.4%) | 0.2744 |
| Hemorrhage requiring transfusion | 7 (0.2%) | 3 (0.2%) | 4 (0.3%) | 0.4618 |
| Phrenic nerve injury | 6 (0.2%) | 1 (0.1%) | 5 (0.4%) | 0.0481 |
| Retroperitoneal bleed | 3 (0.1%) | 1 (0.1%) | 2 (0.2%) | 0.4154 |
| Arteriovenous fistula | 3 (0.1%) | 0 (0.0%) | 3 (0.2%) | 0.0477 |
| Aspiration | 3 (0.1%) | 2 (0.1%) | 1 (0.07%) | 0.7256 |
| Stroke | 3 (0.1%) | 2 (0.1%) | 1 (0.07%) | 0.7256 |
| Transient ischemic attack | 1 (0.03%) | 1 (0.1%) | 0 (0.0%) | 0.3815 |
| Perforation or tamponade requiring surgery | 1 (0.03%) | 1 (0.1%) | 0 (0.0%) | 0.3815 |
| Death | 1 (0.03%) | 0 (0.0%) | 1 (0.07%) | 0.2532 |
| Other | 45 (1.4%) | 18 (1.0%) | 27 (2.0%) | 0.0234 |
The demographics and medical history of patients who experienced a procedural complication and those who did not are compared in Table 4. Patients who had procedural complications were more likely to be older, female, and have hypertension, heart failure stage 3 CKD or COPD. They were also more likely to have larger left atria and have higher thromboembolic and/or bleeding risk scores. Procedural management patterns by complication presence are described in Table 5. Patients who had complications were less likely to have interrupted anticoagulation. They were more likely to have a preoperative TEE, intraoperative intra-cardiac echocardiography (ICE) and less likely to have had rotational angiography. Use of irrigated RF with CF was more frequent among those with complications, whereas cryoballoon use was less frequent. Frequency of specific complication types was similar across energy and catheter types with the exception of volume overload/pulmonary edema which was seen exclusively among patients with RF and contact force (36/2206 patients [1.6%]) and RF without contact force (3/271 patients, [1.1%]) and was not seen in the 626 patients receiving cryoballoon ablation (N=626) or in whom “other” was selected as their energy and catheter type (N=27). Patients with complications more frequently had provocative testing with isoproterenol as well as longer fluoroscopy and total procedure times. Patients with complications had higher rates of ablation on the LA roof, LA posterior/inferior wall, LA appendage, right sided CTI and more targeted ganglion ablation. Complication rates were similar across regions. While 94.4% of patients with complications were treated at hospitals identified as academic or teaching hospitals; it should be noted that, 85.1% of patients were treated at this hospital type (Supplemental Table). Patients with complications were treated at sites with higher case volumes than those without complications (245 vs 244 cases over the study period, p=0.01).
Table 4: Baseline characteristics by complication occurrence:
Demographics and medical history for all included patients and by complication occurrence. Data presented as median (IQR) or N (%) as appropriate
| Overall | No complication | Complication | P-value | |
|---|---|---|---|---|
| N | 3138* | 2978 (94.9%) | 160 (5.1%) | |
| Age, years | 65 (58-71) | 65 (58-71) | 68 (62-73) | <0.0001 |
| Female | 1131 (36.0%) | 1053 (35.4%) | 78 (48.8%) | 0.0006 |
| Hypertension | 2121 (67.6%) | 1999 (67.1%) | 122 (76.2%) | 0.0163 |
| OSA | 999 (31.8%) | 949 (31.9%) | 50 (31.3%) | 0.8704 |
| Coronary artery disease | 593 (18.9%) | 555 (18.6%) | 38 (23.8%) | 0.1076 |
| Diabetes Mellitus | 589 (18.8%) | 563 (18.9%) | 26 (16.2%) | 0.4021 |
| Heart Failure | 570 (18.2%) | 525 (17.6%) | 45 (28.1%) | 0.0008 |
| LVEF <40% | 243 (8.2%) | 229 (8.1%) | 14 (9.2%) | 0.6488 |
| Thyroid disease | 505 (16.1%) | 473 (15.8%) | 32 (20.0%) | 0.1675 |
| On dialysis | 6 (0.2%) | 6 (0.2%) | 0 (0.0%) | 0.5699 |
| Stage 3 CKD | 622 (24.3%) | 576 (23.9%) | 46 (31.3%) | 0.0414 |
| Cancer | 364 (11.6%) | 343 (11.5%) | 21 (13.1%) | 0.5363 |
| COPD | 256 (8.2%) | 233 (7.8%) | 23 (14.4%) | 0.0032 |
| CVA/TIA | 245 (7.8%) | 231 (7.8%) | 14 (8.8%) | 0.6483 |
| Peripheral vascular disease | 95 (3.0%) | 89 (3.0%) | 6 (3.8%) | 0.5840 |
| Family history of AF | 82 (2.6%) | 77 (2.6%) | 5 (3.1%) | 0.6770 |
| Left atrial diameter, cm | 4.2 (3.7-4.8) | 4.2 (3.7-4.8) | 4.4 (3.9-5.0) | 0.0490 |
| CHA2DS2VASc score | 2 (1-4) | 2 (1-4) | 3 (2-4) | <0.0001 |
| ORBIT bleeding score | 1 (0-2) | 1 (0-2) | 1 (0-3) | <0.0001 |
| HAS-BLED Score | 2 (1-2) | 1 (1-2) | 2 (1.5-3) | <0.0001 |
| Prior major bleeding | 94 (3.0%) | 86 (2.9%) | 8 (5.0%) | 0.1241 |
One patient had missing information regarding complications so these analysis are out of 3,138 patients. Abbreviations: OSA = obstructive sleep apnea; LVEF = left ventricular ejection fraction; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; CVA/TIA = cerebrovascular accident/transient ischemic attack; AF = atrial fibrillation
Table 5: Procedural characteristics by complication occurrence:
Procedural characteristics for patients by complication occurrence. Data presented as median (IQR) or N (%) as appropriate
| Overall | No complication | Complication | P-value | |
|---|---|---|---|---|
| N | 3138* | 2978 (94.9%) | 160 (5.1%) | |
| Peri-procedure AC strategy | <.0001 | |||
| Uninterrupted | 2279 (73.5%) | 2150 (73.1%) | 129 (81.1%) | |
| Interrupted | 688 (22.2%) | 673 (22.9%) | 15 (9.4%) | |
| Bridging | 132 (4.3%) | 117 (4.0%) | 15 (9.4%) | |
| Pre-procedure Imaging | ||||
| Preoperative TEE | 1534 (49.0%) | 1438 (48.4%) | 96 (60.0%) | 0.0043 |
| CT | 1888 (60.3%) | 1785 (60.1%) | 103 (64.4%) | 0.2796 |
| MRI | 91 (2.9%) | 89 (3.0%) | 2 (1.2%) | 0.2005 |
| Intra-procedure Imaging | ||||
| Intra-cardiac echocardiography | 2866 (91.5%) | 2712 (91.3%) | 154 (96.2%) | 0.0279 |
| 3D electroanatomic mapping | 2842 (90.8%) | 2690 (90.5%) | 152 (95.0%) | 0.0578 |
| Intra-operative TEE | 435 (13.9%) | 407 (13.7%) | 28 (17.5%) | 0.1758 |
| Rotational angiography | 176 (5.6%) | 173 (5.8%) | 3 (1.9%) | 0.0347 |
| Energy and catheter type | ||||
| Irrigated RF with contact force | 2205 (70.5%) | 2076 (69.9%) | 129 (80.6%) | 0.0038 |
| Irrigated RF without contact force | 271 (8.7%) | 257 (8.7%) | 14 (8.8%) | 0.9672 |
| Cryoballoon | 743 (23.7%) | 725 (24.4%) | 18 (11.2%) | 0.0001 |
| Other | 53 (1.7%) | 51 (1.7%) | 2 (1.2%) | 0.6552 |
| Provocation testing | ||||
| Burst pacing | 1652 (68.5%) | 1563 (68.5%) | 89 (69.0%) | 0.9110 |
| Isoproternol | 1147 (47.6%) | 1063 (46.6%) | 84 (65.1%) | <0.0001 |
| Adenosine | 602 (25.0%) | 573 (25.1%) | 29 (22.5%) | 0.5005 |
| Other | 224 (9.3%) | 221 (9.7%) | 3 (2.3%) | 0.0051 |
| Procedure time (minutes) | ||||
| Ablation time | 37 (17-59) | 38 (18-59) | 36 (0-88) | 0.9531 |
| Fluoroscopy time | 16 (8-28) | 16 (8-28) | 20 (11-32) | 0.0081 |
| Total procedure time | 180 (140-230) | 179 (140-226) | 215 (164-273) | <0.0001 |
| Lesion sets | ||||
| PVI | 2875 (91.6%) | 2736 (91.9%) | 139 (86.9%) | 0.0262 |
| LA roof line | 872 (31.3%) | 8510 (30.8%) | 62 (40.8%) | 0.0097 |
| LA posterior/inferior line | 731 (24.3%) | 679 (25.8%) | 52 (34.2%) | 0.0219 |
| Lateral mitral isthmus | 170 (6.1%) | 156 (5.9%) | 14 (9.2%) | 0.1003 |
| Septal mitral isthmus | 162 (5.8%) | 150 (5.7%) | 12 (7.9%) | 0.2610 |
| LA appendage | 122 (4.4%) | 110 (4.2%) | 12 (7.9%) | 0.0296 |
| R sided CTI for flutter | 938 (33.7%) | 875 (33.2%) | 63 (41.4%) | 0.0375 |
| SVC isolation | 137 (5.0%) | 126 (4.8%) | 11 (7.2%) | 0.1747 |
| CFAE | 285 (10.2%) | 269 (10.2%) | 16 (10.5%) | 0.9037 |
| FIRM | 311 (11.2%) | 296 (11.2%) | 15 (9.9%) | 0.6001 |
| Targeted ganglion ablation | 19 (0.7%) | 15 (0.6%) | 4 (2.6%) | 0.0027 |
| Hospital characteristics | ||||
| Region | 0.0893 | |||
| West | 400 (12.8%) | 379 (12.7%) | 21 (13.1%) | |
| South | 1273 (40.6%) | 1194 (40.1%) | 79 (49.4%) | |
| Midwest | 359 (11.4%) | 346 (11.6%) | 13 (8.1%) | |
| Northeast | 1106 (35.2%) | 1059 (35.6%) | 47 (29.4%) | |
| Academic/Teaching Hospital | 2648 (85.1%) | 2497 (84.6%) | 151 (94.4%) | 0.0007 |
| Ablation Case Volume | 244 (192-404) | 244 (192-404) | 245 (229-487) | 0.0109 |
One patient had missing information regarding complications so these analysis are out of 3,138 patients. Abbreviations: AF= atrial fibrillation; AC = anticoagulation; CT = computed tomography; MRI = magnetic resonance imaging; TEE = transesophageal echocardiography; RF = radiofrequency
Discussion
In this analysis of greater than 3,000 patients undergoing catheter ablation across 24 US centers, catheter ablation for AF was highly consistent with guideline recommendations. The vast majority (>95%) of cases were performed for Class I or Class IIA indications, most patients received antiarrhythmic drug therapy prior to ablation, and anticoagulation was typically not interrupted for the procedure. Open-irrigated RF ablation with CF sensing was the most common ablation modality used, although cryoballoon ablation accounted for nearly a quarter of all cases. Beyond PVI, the most common adjunctive ablation included ablation of the CTI, the LA roof/posterior wall, and rotor ablation. In-hospital complications were uncommon and mortality was rare. These findings confirm and extend prior observations that catheter ablation at experienced centers is safe.
The demographics of the current study of patients undergoing AF ablation are similar to those reported in the World Wide Survey (WWS), National Inpatient Sample (NIS), and broader analyses of AF patients in the GWTG-AFIB registry.11,17,18 Compared with the GWTG-AFIB patients with elevated CHA2DS2-VASc scores, this catheter ablation population had a lower prevalence of women, as well as African American, and Hispanic patients. Sex and racial disparities have been observed in the GWTG-Heart Failure registry as well as other studies demonstrating that minorities are less likely to receive invasive cardiovascular therapies.19,20 These disparities may have contributed to their under-representation in the present study.
The majority of patients were referred for ablation for a Class I indication (drug refractory, paroxysmal AF), with a large proportion referred for Class IIA indications (drug refractory, persistent AF). Less than 1 in 20 patients were referred for first line therapy for paroxysmal or persistent AF. The fact that more than 95% of ablations were performed for Class IA or IIA indications suggests that AF ablation practice is very consistent with guideline recommendations.
The 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter ablation for AF recommends performance of the ablation procedure without interruption of warfarin (Class I), dabigatran (Class I), rivaroxaban (Class I), or another direct acting oral anticoagulant (Class IIA).21 Alternatively, the consensus recommendations counsel that holding 1-2 doses with resumption after the procedure is reasonable (Class IIA).21 These data from GWTG-AFIB suggest that anticoagulation in clinical practice is highly consistent with these recommendations. The COMPARE trial randomized patients to continuous warfarin vs a low molecular weight heparin bridge and showed that thromboembolic events were higher with the bridging strategy.22 Thus, it is not surprising that only 4.3% of patients in our study had bridging of anticoagulation in the peri-procedural period.
Open-irrigated radiofrequency ablation was the dominant energy source in the registry with a substantial minority of patients receiving cryoballoon-based ablation. The FIRE AND ICE trial demonstrating non-inferiority of cryoballoon ablation to RF ablation was published in 2016 (near the beginning of this study period).23 While most cryoballoon ablations were done in patients with paroxysmal AF, 37% of the patients receiving cryoballoon ablation had persistent AF despite the absence of randomized data in these patients. While observational data has been promising,24 ongoing randomized trials of cryoballoon ablation for the treatment of persistent AF, such as IRON-ICE trial will provide valuable data to guide future practice (ClinicalTrials.gov NCT03365700).
The 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement affirms that PVI should be the cornerstone of AF ablation. The most frequent lesion sets employed in adjunctive ablation were CTI ablation, LA roof/posterior wall ablation, and catheter ablation of focal sources. Randomized comparisons of adjunctive substrate ablation have been neutral. In the STAR AF-II trial, neither linear ablation nor CFAE improved the rate of recurrence of AF for persistent AF.25,26 Similarly, FIRM ablation has been shown to have no additional benefit over PVI in persistent AF in the recently presented REAFFIRM trial (ClinicalTrials.gov NCT02274857); however, the results of this trial were not available during the study period. There has been enthusiasm regarding ablation of the posterior wall, particularly given its shared embryologic origin with the pulmonary veins.27 Some randomized data suggests that addition of posterior wall isolation to PVI improves maintenance of sinus rhythm.28 This study demonstrates that, while PVI continues to be the cornerstone of AF ablation, adjunctive ablation is commonly performed. The uncertainty over the benefit of adjunctive ablation is supported by the significant heterogeneity in its use in clinical practice, including in GWTG-AFIB.
In-hospital complications are uncommon following AF ablation, but they remain an important concern for electrophysiologists, referring physicians, and patients. These data confirm that AF ablation is a safe procedure with a low in-hospital complication rate in specialized centers. This is particularly true with respect to potentially life-threatening complications. A total of 21 life-threatening complications were reported for the 3,139 patients (nine cases of tamponade, seven hemorrhages requiring transfusion, three strokes, one TIA, and one death) representing less than 1% of all patients. These overall complication rates and in-hospital mortality are in line with those previously reported from prior studies, including the WWS, as well as the NIS and provides insight into the real world experience of modern AF ablation.5,17 Rates of postoperative hemorrhage, pericardial complications, and neurological complications were lower in the GWTG-AFIB cohort compared to the NIS (2.86% vs 0.2%, 1.99% vs 0.1%, and 1.05% vs 0.1% respectively). Higher ablation volume has been shown to be associated with a lower odds of mortality and complications.11 Thus, it is possible that the lower observed rates of these complications is a reflection of the experience of the enrolling centers which were predominantly (85.1%) academic teaching hospitals.
While the low number of overall in-hospital complications limits the ability to identify predictors or drivers of complications, we did describe the characteristics and procedural management of patients who had or did not have procedural complications. Not surprisingly, patients with higher baseline risk profiles had higher rates of complications as has been demonstrated in analysis of the NIS.11 The higher use of preoperative TEE among patients with complications may be reflective of clinicians using this pre-procedure imaging more frequently in high risk patients. The higher rates of ICE use among patients with complications may follow a similar trend; alternatively, the larger bore venous access that ICE requires may carry additional risk or ICE may be introduced as a reaction to a complication (e.g. to assess for effusion). Use of contact force-guided RF was more common in patients with complications; whereas cryoballoon ablation was less common. Additionally, patients with complications had more non-PVI ablation. This is consistent with other studies demonstrating higher complication rates with RF ablation compared to cryoballoon and more complications with additional ablation beyond PVI. RF ablation is often performed using continuously irrigated catheters which may increase the risk of pulmonary edema, one of the more frequently reported complications. At present, there is no class I indication for non-PVI ablation in the LA in the 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement.21,29 However, clinicians and investigators continue to pursue ablation beyond PVI in an attempt to improve AF-free survival. Ongoing randomized trials will help clarify the role of non-PVI ablation moving forward. Given the association between ablation of non-PV triggers and improved outcomes,30 provocation testing with isoproterenol is often used to identify non-pulmonary vein triggers of AF, and thus may be closely related to non-PVI ablation, and also lacks a Class I indication.21,31 Patients with complications were seen at sites that had higher median ablation case volumes. While this difference was statistically significant, the relatively similar absolute values of case volumes suggest that this is likely a reflection of high volume centers amassing more complications as a result of doing more procedures. This analysis compared patients with any complication to those with no complications and therefore equally weighted minor complications (e.g. urinary tract infections) and serious events (e.g. tamponade, death). Thus, the overall results are meant to be descriptive, and hypothesis generating; as with any observational analysis, no causal relationship should be inferred.
The study has several limitations. Data were collected over a three year span (2016-2018) during which time several large randomized controlled trials were published and thus may not fully reflect current practice patterns.32,33 The 3,139 patients in this study came from only 24 sites with a median of 84 patients per site and thus may not be representative of patterns in smaller, low-volume practices. Additionally, sites participating in the GWTG-AFIB registry may be more likely to deliver guideline-indicated therapy, which may have introduced selection bias. Sites enrolled consecutive patients to the registry to get as complete an assessment of care patterns as possible without selection bias within sites. Data entry and medical chart review was performed by providers without external audit which raises the potential for inaccurate reporting; however, providers receive training on data entry and a recent analysis of the similarly designed GWTG-Stroke initiative demonstrated an overall composite accuracy of data entry of 96.1% suggesting that the data abstraction in GWTG registries is reliable.34 The present analysis evaluated in-hospital complications and does not capture complications that may present after hospital discharge such as atrioesophageal fistulae. Future analyses are planned to evaluate medium to long term outcomes including complications, mortality, readmission and resource utilization.
Conclusions
Catheter ablation of AF in the United States among 24 sites participating in the GWTG-AFIB quality improvement registry is performed for Class I or Class IIA indications in more than 98% of cases, consistent with current guideline recommendations. CF-guided RF is the most common ablation modality and PVI remains the dominant ablation lesion set. Major in-hospital complications including tamponade, stroke and death are infrequent.
Supplementary Material
Acknowledgments
Disclosures:
ZL is supported in part by an NIH T32 training grant (#5T32HL069749) and receives grant support from Boston Scientific. DNH reports receiving grants from Janssen Scientific. RAM reports no relevant conflicts of interest. ABC serves as a consultant to Janssen Pharmaceuticals, Abbott, Novartis, Sanofi Aventis, Milestone Pharmaceuticals and has received honoraria for speaking from Medtronic, Inc., Abbott, Novartis, and Biotronik. JDD reports consulting for Abbott, Boston Scientific, Medtronic, and Phillips. ND reports consulting for Amgen, Boehringer Ingelheim, Cytokinetics, Relypsa, and Novartis. KAE receives grants and honoraria and was a clinical investigator and consultant for Boston Scientific, Medtronic, St. Jude Medical, and Biotronik. GKF has received EP Fellowship Training Program stipends from Medtronic, Abbott/St. Jude Medical, Biotronik, Boston Scientific, and Biosense Webster; holds stock options in Medwaves, Acutus, Perminova, and toSense; has served on Scientific Advisory Boards for Medwaves and Acutus; is cofounder of Perminova; and has received research support from Circa Scientific. GCF reports consulting for Abbott, Bayer, Janssen, Medtronic, and Novartis. DSF reports no relevant conflicts of interest. JLH reports no relevant conflicts of interest. BPK discloses that he receives honoraria for speaking or consulting from, and his hospital receives fellowship support from, Abbott, Biosense Webster, Biotronik, Boston Scientific, and Medtronic, and he also receives honoraria for speaking or consulting from Baylis Medical and Sanofi. JAJ reports no relevant conflicts of interest. AMR receives study report for clinical research (to hospital) from Bardy Dx, Boehringer Ingelheim, and Boston Scientific, serves on a research steering committee (without honoraria) for Boston Scientific and Apple Inc, and received royalties from Up-to-Date. MSS reports no relevant conflicts of interest. Outside of the submitted work, MPT reports grants from Apple Inc, Janssen Inc, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, American Heart Association, SentreHeart, personal fees from Medtronic Inc, Abbott, Precision Health Economics, iBeat Inc, iRhythm, Novartis, Biotronik, Sanofi-Aventis, Pfizer, consulting fees and equity from AliveCor , grants and personal fees from Cardiva Medical, and Medtronic. WRL reports no disclosures. JPP receives grants for clinical research from Abbott, American Heart Association, Boston Scientific, Gilead, Janssen Pharmaceuticals, and the NHLBI and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, Johnson & Johnson, LivaNova, Medtronic, Milestone, Oliver Wyman Health, Sanofi, Philips, and Up-to-Date.
References:
- 1.Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National Trends in Atrial Fibrillation Hospitalization, Readmission, and Mortality for Medicare Beneficiaries, 1999–2013. Circulation. 2017;135(13):1227–1239. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. 2014;64(21):e1–e76. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European heart journal. 2016;37(38):2893–2962. [DOI] [PubMed] [Google Scholar]
- 4.Muthalaly RG, John RM, Schaeffer B, et al. Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. Journal of cardiovascular electrophysiology. 2018;29(6):854–860. [DOI] [PubMed] [Google Scholar]
- 5.Abdur Rehman K, Wazni OM, Barakat AF, et al. Life-Threatening Complications of Atrial Fibrillation Ablation: 16-Year Experience in a Large Prospective Tertiary Care Cohort. JACC Clinical electrophysiology. 2019;5(3):284–291. [DOI] [PubMed] [Google Scholar]
- 6.Arbelo E, Brugada J, Blomstrom-Lundqvist C, et al. Contemporary management of patients undergoing atrial fibrillation ablation: in-hospital and 1-year follow-up findings from the ESC-EHRA atrial fibrillation ablation long-term registry. European heart journal. 2017;38(17):1303–1316. [DOI] [PubMed] [Google Scholar]
- 7.Holmqvist F, Kesek M, Englund A, et al. A decade of catheter ablation of cardiac arrhythmias in Sweden: ablation practices and outcomes. European heart journal. 2019;40(10):820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccini JP, Sinner MF, Greiner MA, et al. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126(18):2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noseworthy PA, Yao X, Deshmukh AJ, et al. Patterns of Anticoagulation Use and Cardioembolic Risk After Catheter Ablation for Atrial Fibrillation. Journal of the American Heart Association. 2015;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ullal AJ, Kaiser DW, Fan J, et al. Safety and Clinical Outcomes of Catheter Ablation of Atrial Fibrillation in Patients With Chronic Kidney Disease. Journal of cardiovascular electrophysiology. 2017;28(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripathi B, Arora S, Kumar V, et al. Temporal trends of in-hospital complications associated with catheter ablation of atrial fibrillation in the United States: An update from Nationwide Inpatient Sample database (2011–2014). Journal of cardiovascular electrophysiology. 2018;29(5):715–724. [DOI] [PubMed] [Google Scholar]
- 12.Kece F, Zeppenfeld K, Trines SA. The Impact of Advances in Atrial Fibrillation Ablation Devices on the Incidence and Prevention of Complications. Arrhythmia & electrophysiology review. 2018;7(3):169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis WR, Piccini JP, Turakhia MP, et al. Get With The Guidelines AFIB: novel quality improvement registry for hospitalized patients with atrial fibrillation. Circulation Cardiovascular quality and outcomes. 2014;7(5):770–777. [DOI] [PubMed] [Google Scholar]
- 14.January CT, Wann LS, Calkins H, et al. 2019. AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. 2019:25873. [DOI] [PubMed] [Google Scholar]
- 15.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. [DOI] [PubMed] [Google Scholar]
- 16.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. [DOI] [PubMed] [Google Scholar]
- 17.Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation Arrhythmia and electrophysiology. 2010;3(1):32–38. [DOI] [PubMed] [Google Scholar]
- 18.Piccini JP, Xu H, Cox M, et al. Adherence to Guideline-Directed Stroke Prevention Therapy for Atrial Fibrillation Is Achievable. Circulation. 2019;139(12):1497–1506. [DOI] [PubMed] [Google Scholar]
- 19.Al-Khatib SM, Hellkamp AS, Hernandez AF, et al. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation. 2012;125(9):1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewey J, Choudhry NK. The current state of ethnic and racial disparities in cardiovascular care: lessons from the past and opportunities for the future. Current cardiology reports. 2014;16(10):530. [DOI] [PubMed] [Google Scholar]
- 21.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Biase L, Burkhardt JD, Santangeli P, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation. 2014;129(25):2638–2644. [DOI] [PubMed] [Google Scholar]
- 23.Kuck K-H, Brugada J, Fürnkranz A, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. New England Journal of Medicine. 2016;374(23):2235–2245. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann E, Straube F, Wegscheider K, et al. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma A, Mantovan R, Macle L, et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): a randomized, multicentre, international trial. European heart journal. 2010;31(11):1344–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verma A, Jiang C-y, Betts TR, et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. New England Journal of Medicine. 2015;372(19):1812–1822. [DOI] [PubMed] [Google Scholar]
- 27.Douglas YL, Jongbloed MR, Gittenberger-de Groot AC, et al. Histology of vascular myocardial wall of left atrial body after pulmonary venous incorporation. Am J Cardiol. 2006;97(5):662–670. [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Shin SY, Na JO, et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation?: A prospective randomized clinical trial. Int J Cardiol. 2015;181:277–283. [DOI] [PubMed] [Google Scholar]
- 29.Chun KRJ, Perrotta L, Bordignon S, et al. Complications in Catheter Ablation of Atrial Fibrillation in 3,000 Consecutive Procedures. Balloon Versus Radiofrequency Current Ablation. 2017;3(2):154–161. [DOI] [PubMed] [Google Scholar]
- 30.Mohanty S, Mohanty P, Di Biase L, et al. Long-term follow-up of patients with paroxysmal atrial fibrillation and severe left atrial scarring: comparison between pulmonary vein antrum isolation only or pulmonary vein isolation combined with either scar homogenization or trigger ablation. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2017;19(11):1790–1797. [DOI] [PubMed] [Google Scholar]
- 31.Santangeli P, Marchlinski FE. Techniques for the provocation, localization, and ablation of non-pulmonary vein triggers for atrial fibrillation. Heart rhythm. 2017;14(7):1087–1096. [DOI] [PubMed] [Google Scholar]
- 32.Marrouche NF, Brachmann J, Andresen D, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. New England Journal of Medicine. 2018;378(5):417–427. [DOI] [PubMed] [Google Scholar]
- 33.Packer DL, Mark DB, Robb RA, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical TrialEffect of Catheter Ablation vs Antiarrhythmic Drugs on Mortality, Stroke, Bleeding, and Cardiac Arrest in AFEffect of Catheter Ablation vs Antiarrhythmic Drugs on Mortality, Stroke, Bleeding, and Cardiac Arrest in AF. JAMA. 2019;321(13):1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xian Y, Fonarow GC, Reeves MJ, et al. Data quality in the American Heart Association Get With The Guidelines-Stroke (GWTG-Stroke): results from a national data validation audit. American heart journal. 2012;163(3):392–398, 398.e391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


