Abstract
Background
Coronavirus disease 2019 (COVID-19) has been associated to microvascular alterations. We screened the fundus of patients with COVID-19 to detect alterations of the retina and its vasculature and to assess possible correlations with clinical parameters.
Methods
Cross-sectional study. The presence of retinal alterations in patients with COVID-19 and subjects unexposed to the virus was assessed using fundus photographs and their prevalence was compared. Mean arteries diameter (MAD) and mean veins diameter (MVD) were compared between patients and unexposed subjects with multiple linear regression including age, sex, ethnicity, body mass index, smoking/alcohol consumption, hypertension, hyperlipidaemia, diabetes as covariates. The influence of clinical/lab parameters on retinal findings was tested in COVID-19 patients.
Findings
54 patients and 133 unexposed subjects were enrolled. Retinal findings in COVID-19 included: haemorrhages (9·25%), cotton wools spots (7·4%), dilated veins (27·7%), tortuous vessels (12·9%). Both MAD and MVD were higher in COVID-19 patients compared to unexposed subjects (98·3 ± 15·3 µm vs 91·9 ± 11·7 µm, p = 0.006 and 138·5 ± 21·5 µm vs 123·2 ± 13·0 µm, p<0.0001, respectively). In multiple regression accounting for covariates MVD was positively associated with COVID-19 both in severe (coefficient 30·3, CI95% 18·1–42·4) and non-severe (coefficient 10·3, CI95% 1·6–19·0) cases compared to unexposed subjects. In COVID-19 patients MVD was negatively correlated with the time from symptoms onset (coefficient −1·0, CI 95% −1·89 to −0·20) and positively correlated with disease severity (coefficient 22·0, CI 95% 5·2–38·9).
Interpretation
COVID-19 can affect the retina. Retinal veins diameter seems directly correlated with the disease severity. Its assessment could have possible applications in the management of COVID-19.
Funding
None.
Keywords: COVID-19, Retina, Vessels, Veins, Vasculature, SARS-CoV-2, Eye, Uveitis, Coronavirus, Arteries, Endothelium
Research in context.
Evidence before this study
We searched PubMed and Embase on June 1, 2020, for studies reporting retinal examination during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. We used the search terms (“COVID-19”OR “SARS-CoV-2”OR “coronavirus”) AND (“vascular” OR “vessel” OR “vein” OR “artery” OR “retina” OR “retinal findings”) without language or date restrictions. There is increasing evidence that SARS-CoV-2 can affect the endothelium and induce important changes to the vessels in many body districts without any report focusing on the retinal vessels. We identified one manuscript reporting twelve COVID-19 patients describing only retinal alterations and one report providing pathological evidence of the presence of SARS-CoV-2 in the retina.
Added value of this study
In the present study we screened the fundus of fifty-four COVID-19 patients with SARS-CoV-2 infection to identify alterations to the retina and the retinal vasculature and we compared them to one hundred and thirty-three unexposed subjects. For the first time we observed that both retinal arteries and veins were dilated in COVID-19 patients with the veins being significantly larger both in severe and non-severe cases compared to unexposed subjects. In addition, retinal veins diameter was significantly and positively correlated to disease severity [coefficient 22·0 (95% Confidence Interval 5·2–38·9)] and significantly and negatively influenced by the time between symptoms onset and the day of the fundus examination [coefficient −1·0 (95% Confidence Interval −1·89 to −0·20)].
Implications of all the available evidence
Retinal examination offers a unique opportunity to analyze vessels in vivo. Fundus examination is quick, not expensive and relatively non-invasive. If our data will be confirmed, retinal veins diameter could represent a useful parameter to monitor the inflammatory response and/or the endothelial damage in COVID-19. Retinal changes should be further investigated in prospective studies to understand their possible applications in the diagnosis and management of COVID-19.
Alt-text: Unlabelled box
1. Introduction
In December 2019 China experienced an outbreak of a new Coronavirus, Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2). The disease that quickly reached the size of a pandemic in early 2020 [1] was named coronavirus disease 2019 (COVID-19) by the World Health Organization. The majority of SARS-CoV-2 infections display mild to moderate symptoms. Nevertheless, approximately one out of five patients require hospital admission of whom approximately 16% require intensive care assistance [2].
The reason why the clinical course may range from mild to severe is still poorly understood, but it could be speculated that the host immune system could play a role in defining the clinical course of SARS-CoV-2 infection. In particular, some authors have suggested the cytokine storm as the underling mechanism leading to the acute respiratory distress syndrome (ARDS) development [3]. Moreover, different studies have reported a direct correlation between levels of inflammatory cytokines, such as Interleukin-6 (IL-6), and the disease severity [4].
Coagulation disorders are also common in patients with SARS-CoV-2 infection, especially in those with severe disease. Blood hypercoagulability, elevated D-Dimer levels, prothrombin time and partial thromboplastin time prolongation, fibrin degradation products increase are consistently reported [5]. In addition, several studies found diffuse endothelial damage in COVID-19 patients and thromboembolic events affecting different body districts other than the lungs have been reported [6,7].
The examination of the ocular fundus allows a thorough evaluation of the retina and its vasculature. The arteries and veins of the retina represent a window on the vascular system as they are exposed to the same pathological processes and show changes in many systemic diseases [8]. The acquisition of retinal color photographs is fast, non-invasive and is commonly used to screen large populations of patients with systemic diseases including hypertension and diabetes both to identify new cases and follow those with an established diagnosis [8,9].
Alterations of the retina are also commonly seen in patients with viral diseases. These pathologic phenomena can occur due to a direct cytopathic effect of the microorganism on retinal neurons as in case of cytomegalovirus [10], or secondary to a damage to the microvasculature when the virus targets the vessels endothelium like in HIV retinopathy [11]. Interestingly, SARS-CoV-2 is able to infect endothelial cells [12] and has been detected in the retina [13]. The aim of this study was to screen the fundus of patients with SARS-CoV-2 infection in order to detect possible alterations of the retina or the retinal vasculature related to COVID-19 and to assess the possible correlations with the clinical parameters.
2. Methods
This was a cross sectional, monocentric study, conducted at the Luigi Sacco Hospital, Department of Biomedical and Clinical Science “L. Sacco”, University of Milan, Milan, Italy. The study title was "ScrEening the Retina in Patients wIth COVID-19″ (SERPICO-19). The study was approved by the local ethic committee (Protocol Number 2020/ST/088) and written informed consent was obtained from all participants. The study adhered to the tenants of the declaration of Helsinki.
2.1. Population
Consecutive patients admitted in May 2020 to the infectious diseases Department of the Luigi Sacco Hospital diagnosed with SARS-CoV-2 infection were asked to participate the study. To be included in the study patients had to: (1) have a positive nasopharyngeal swab (2) be able to understand the informed consent and agree to be part of the study, (3) be able to sit in front of the retinal fundus camera for the time needed to acquire the retinal images (~2 min) and (4) have symptoms onset within 30 days before the fundus screening.
Patients with a previous intensive care unit stay and those with known retinal disorders (i.e. diabetic retinopathy) were excluded.
A group of subjects among hospital and university staff, unexposed to the virus, was enrolled. All people employed in the ophthalmology and the infectious diseases department were informed about the current study and were invited to participate. All those who were asymptomatic during the previous month and tested negative for SARS-CoV-2 specific antibodies and agreed to participate were enrolled. Subjects with known retinal disorders (i.e. diabetic retinopathy) were excluded.
2.2. Procedures
Patients included in the study were asked to fill a questionnaire describing their ocular symptoms since the beginning of the COVID-19 systemic symptoms. All enrolled subjects underwent pupils dilation of both eyes using mydriatic drops (Tropicamide 1%) 15 min prior to the acquisition of retinal images. Two sets of fundus photos, one for each eye, were acquired in all subjects with the Digital Retinography System (DRS) fundus camera (CenterVue, Padua, Italy). Each set was constituted by four photos, with a 45°×40° field of view each and a resolution of 48 pixels/degree, two centered on the macular area and two on the optic nerve head. Each pair of photos included a monochromatic “Red-Free” image and a color image. Red-Free images are commonly used in addition to standard color photos to detect haemorrhages and cotton wools within the retina as the wavelength used to acquire them offers the best contrast to highlight this alterations.
2.3. Images analysis
All images were assessed by two retinal specialists (FZ and SP) to evaluate their quality. If the fundus details were not visible due to acquisition artifacts or media opacities in more than 2/4 photos the eye was not included in the analysis, if both eyes were graded “unreadable” the subject was excluded. For all included eyes the same retinal specialists assessed the images for the presence of any abnormal findings affecting the retina and/or the retinal vessels. In case of disagreement, a third senior retinal specialist (AI) was asked to evaluate the image.
The fundus color photo centered onto the optic nerve of one eye randomly selected from each subject (from both groups) was then analyzed to assess the mean retinal vessels diameter using a semi-automatic approach. Retinal vessels are not significantly different between the eyes of the same subject so the use of a single eye is widely accepted for this kind of analysis [14]. In brief, retinal images were acquired and processed using the Automated Retinal Image analyzer (ARIA, V1-09-12-11), an opensource software developed on the MATLAB platform (MATLAB R2020a – update 1 (9.8.0.1359463)) [15].
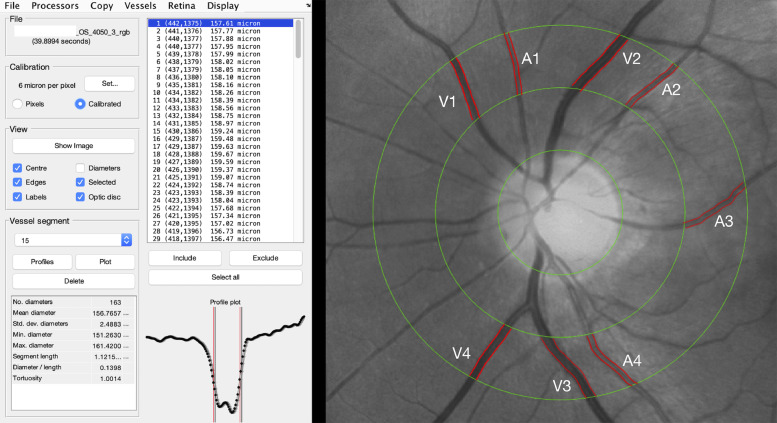
The software automatically recognizes the retinal vessels and allows the operator to select those of interest and to analyze their mean diameter at a certain distance from their origin. In particular, we calculated the diameter of arteries and veins between 0·5 and 1 disk diameters from the optic disk margin (Fig. 1). This is in line with what reported in the major studies on retinal vessels diameter [16,17].
Fig. 1.
Retinal vessels analysis. The ARIA (automatic retinal image analyzer) software allowed to perform a semi-automatic assessment of retinal vessels diameter with an area of interest around the optic nerve area. For the purpose of the study, the main vessels within 0·5 and 1 disk diameter around the optic nerve head were automatically segmented by the software. The operator chose the four main veins and arteries and averaged their diameters automatically calculated by the software to obtain the mean arteries diameter (MAV) and the mean veins diameter (MVD), respectively.
For the purpose of the study we measured the diameter of the four main veins and the four main arteries comprised within the studied area and we obtained the mean artery diameter (MAD) and the mean veins diameter (MVD) by averaging the four arterial and venous values respectively.
Electronic charts of all patients were reviewed to collect demographic clinical and laboratory relevant parameters. The list included: age, sex, ethnicity, body mass index (BMI), smoking, alcohol consumption, presence of comorbidities (systemic hypertension, diabetes, dyslipidaemia, history of coronary disease/stroke, tuberculosis and HIV infection), COVID-19 related symptoms (cough, fever), time between the symptoms onset and the day of the fundus photography, systolic and diastolic blood pressure on the day of the fundus, oxygen support if needed (low flow oxygen, Venturi mask, continuous positive airway pressure), treatment received (anticoagulant, antiplatelet, hydroxychloroquine, remdesivir, lopinavir/ritonavir, tocilizumab, steroids). Treatments and comorbidities were considered as binary variables (YES/NO) without accounting for dosage or duration.
Laboratory parameters included haematocrit, white blood cells, neutrophils, lymphocytes and platelets count, prothrombin time (PT), partial thromboplastin time (PTT), Fibrinogen, D-Dimer, C reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), creatinine, creatine kinase, and albumin tested within three days prior to fundus photograph collection.
Patients were classified as having severe COVID-19 if they had any of the following: hypoxia (oxygen saturation ≤ 93% on room air or PaO2/FiO2 <300 mmHg), tachypnoea (respiratory rate >30 breaths per minute) or respiratory distress, more than 50% involvement of the lung parenchyma on chest imaging. The remaining patients were classified as non-severe[18].
Information regarding the age, sex, ethnicity, body mass index (BMI), smoking, alcohol consumption, presence of comorbidities (systemic hypertension, diabetes, dyslipidaemia, history of coronary disease/stroke, tuberculosis and HIV infection) were also collected in unexposed subjects. Comorbidities were considered as binary variables (YES/NO) without further characterization.
The primary outcome of the study was to assess the presence of alterations of the retina or the retinal vasculature in COVID-19 patients compared to unexposed subjects. The secondary outcomes were the association of demographic, clinical and laboratory parameters with retinal findings in the COVID-19 group.
2.4. Statistical analysis
Descriptive statistics for continuous variables included the mean, standard deviation (SD), and ranges where appropriate. The prevalence of demographic qualitative variables was reported in percentage. The prevalence of retinal findings in COVID-19 patients and unexposed subjects was compared using Fisher's exact test for binary variables and Student's t-test for continuous variables. The effect of severe and non-severe COVID-19 on MAD and MVD, was tested using multiple linear regression analyses considering unexposed subjects as reference and including factors known to have a possible effect on retinal vessels (age, sex, ethnicity, BMI, systemic hypertension, diabetes, smoking, alcohol consumption, dyslipidaemia, history of coronary disease/stroke) as covariates. Pairwise comparisons using the Bonferroni correction within the same model allowed to investigate the contrast between non-severe COVID-19 cases, severe COVID-19 cases and unexposed subjects accounting for the abovementioned covariates. Linear regression analysis was used to assess the effect clinical and laboratory features on retinal findings, MAD and MVD in COVID-19 patients. The effect of each variable was tested using simple linear regression. Those with a significant effect were then tested in a multiple linear regression including all the above mentioned covariates. Normal distribution of the data was tested before the analysis and in case of co-linearity only the variable with the stronger effect was kept in the model. The statistical analyses were run on R Studio (Version 1.1.383, R Project, www.r-project.org). P values <0·05 were considered statistically significant.
2.5. Role of funding source
None.
3. Results
Eighty-five patients were asked to participate the study and all of them agreed. Of these two were excluded as the quality of the images was too bad. Of the 83 remaining, 19 were excluded because they had been treated in intensive care unit before the fundus pictures collection and 10 because the time between the COVID-19 symptoms onset and the day when the fundus photos were performed was longer than 30 days. As a result, the complete analysis was conducted on the remaining 54 patients. A total of 133 unexposed subjects were also enrolled. Detailed demographics and clinical features of the subjects included in the study are reported in Table 1. Ocular symptoms were referred by 27·7% of patients, details are reported in Table 2. Detailed description of the laboratory parameters in COVID-19 patients are reported in Table 3.
Table 1.
Demographics and clinical features of subjects enrolled in the study.
| COVID-19 patients | Unexposed subjects | P value | |
|---|---|---|---|
| n = 54 | n = 133 | ||
| Age, mean years (SD, range) | 49·9 (15·6, 23–82) | 44·2 (12·8, 25–69) | 0·01a |
| Gender, n (%) males | 38 (70·3) | 67 (50·4) | 0·01b |
| Ethnicity, n (%) | <0·0001b | ||
| Caucasian | 27 (50·0) | 127 (95·5) | |
| Latin-American | 11 (20·3) | 1 (0·75) | |
| Indian | 12 (22·2) | 1 (0·75) | |
| Asian | 1 (1·8) | 1 (0·75) | |
| African | 3 (5·5) | 3 (2·25) | |
| Body mass index (Kg/m2), mean (SD, range) | 25·8 (4·0, 20·2–38·5) | 24·5 (4·3, 17–41) | 0·03a |
| Systolic arterial pressure (mmHg), mean (SD, range) | 116·5 (13·2, 90–150) | NA | NA |
| Diastolic arterial pressure (mmHg), mean (SD, range) | 70·9 (8·6, 50–90) | NA | NA |
| Comorbidities, n (%) | |||
| Systemic hypertension | 16 (29·6) | 24 (18·0) | 0·10b |
| Diabetes | 8 (14·8) | 9 (6·7) | 0·09b |
| HIV | 3 (5·5) | 1 (0·75) | 0·07b |
| TB | 2 (3·7) | 0 (0·0) | 0·08b |
| Alcohol consumption | 12 (22·2) | 38 (28·5) | 0·46b |
| Smoking | 13 (24·1) | 39 (29·3) | 0·58b |
| Dyslipidemia | 7 (12·9) | 16 (12·0) | 0·81b |
| Coronary disease/stroke | 5 (9·2) | 6 (4·5) | 0·30b |
| Symptoms-fundus days, mean (SD, range) | 13·6 (7·0, 4–29) | NA | NA |
| COVID-19 clinical features, n (%) | NA | NA | |
| Fever | 50 (92·5) | ||
| Cough | 41 (75·92) | ||
| Pneumonia | 45 (83·3) | ||
| Deep venous thrombosis | 1 (1·8) | ||
| COVID-19 severity, n (%) severe | 14 (25·9) | NA | NA |
| Oxygen supply, n (%) | NA | NA | |
| Venturi | 20 (37·0) | ||
| CPAP | 10 (18·5) | ||
| Therapy, n (%) | NA | NA | |
| Anticoagulant prophylaxis | 45 (83·3) | ||
| Anticoagulant therapy | 5 (9·3) | ||
| Antiplatelet | 9 (16·7) | ||
| Hydroxychloroquine | 34 (62·9) | ||
| LPV/r | 5 (9·3) | ||
| Remdesivir | 10 (18·5) | ||
| Tocilizumab | 1 (1·8) | ||
| Steroids | 5 (9·3) |
COVID-19, Coronavirus Disease 19; n, number; SD, Standard Deviation; NA, not available; HIV, Human Immunodeficiency Virus; TB, Tuberculosis; CPAP, Continuous-Positive Airway Pressure; LPV/r, Lopinavir/ritonavir.
t-test.
Fisher's exact test.
Table 2.
Ocular symptoms reported by SARS-CoV-2 patients included in the study.
| Number of patients (%) | |
|---|---|
| Any symptoms | 15 (27·7) |
| Far vision difficulties | 0 (0) |
| Near vision difficulties | 1 (1·8) |
| Redness | 2 (3·6) |
| Burning sensation | 12 (22·2) |
| Photophobia | 1 (1·8) |
| Floaters | 0 (0) |
| Phosphenes | 0 (0) |
Table 3.
Laboratory parametersa in COVID-19 patients enrolled in the study.
| Parameter (units) | Mean (SD, range) |
|---|---|
| HTC (%) | 38·1 (5·7, 26–48) |
| WBC (×106/L) | 6864·3 (2623·3, 2920–16,120) |
| Neutrophils (×106/L) | 4239·8 (2379·8, 1500–14,160) |
| Lymphocytes (×106/L) | 1806·9 (748·6, 580–4500) |
| Platelets (×109/L) | 316 (126·9, 147–663) |
| PT (ratio) | 1·19 (0·13, 0·97–1·61) |
| PTT (ratio) | 1·16 (0·14, 0·9–1·69) |
| Fibrinogen (mg/dL) | 550·2 (109·0, 320–701) |
| D-Dimer (μg/L) | 956·7 (1200·5, 200–7609) |
| CRP (mg/L) | 26·2 (36·7, 1–188) |
| Ferritin (μg/L) | 662 (668·5, 6–2478) |
| LDH (U/L) | 270·9 (78·4, 175–537) |
| Creatinine (mg/dL) | 1·18 (1·32, 0·44–8·22) |
| CK (U/L) | 104·7 (118·9, 12–553) |
| Albumin (g/L) | 32·9 (5·7, 21–43) |
COVID-19, Coronavirus disease 2019; SD, Standard Deviation; HTC, Hematocrit; WBC, White Blood Cells; PT, Prothrombin Time; PTT, Partial Thromboplastin Time; CRP, C-Reactive Protein; LDH, Lactic acid Dehydrogenase; CK, Creatine Kinase.
The closest available within 3 days prior to the fundus examination.
3.1. Retinal findings in COVID-19 patients
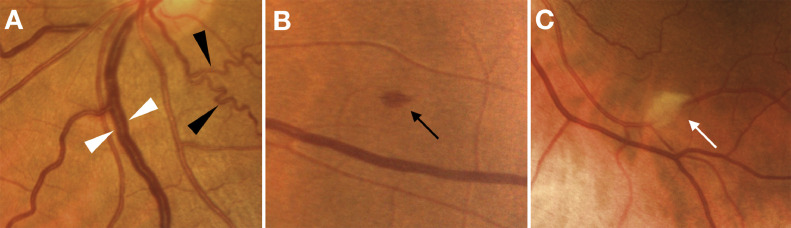
Of the 54 patients included in the analysis 50 (92·5%) had good quality images in both eyes, while four (7·5%) were evaluated in the only eye available. Retinal haemorrhages were found in at least one eye of five patients (9·25%), cotton wools spots were found in four patients (7·4%), drusen were observed in six patients (11·1%). At funduscopic examination dilated veins were observed in 15 patients (27·7%) and tortuous vessels in seven patients (12·9%). These alterations were present in only a minority of unexposed subjects. Comparisons are reported in Table 4. Photographical examples of retinal abnormalities are shown in Fig. 2.
Table 4.
Retinal findings in subjects included in the study.
| COVID-19 patients (n = 54) | Unexposed subjects (n = 133) | p-valuec | |
|---|---|---|---|
| Retinal hemorrhages patients (percentage) |
5 (9·25) | 2 (1·5) | 0·01 |
| Cotton wool spots patients (percentage) |
4 (7·4) | 0 | 0·006 |
| Drusen patients (percentage) |
6 (11·1) | 10 (7·5) | 0·4 |
| Dilated veinsa patients (percentage) |
15 (27·7) | 4 (3·0) | 0·0001 |
| Tortuous vesselsa patients (percentage) |
7 (12·9) | 9 (6·7) | 0·24 |
| Mean vein diameterb µm (SD, range) | 138·5 (21·5, 98·5–203) | 123·2 (13·0, 91·1–156·7) | <0·0001 |
| Mean artery diameterb µm (SD, range) | 98·3 (15·3, 71·4–131·8) | 91·9 (11·7, 63·0–119·6) | 0·006 |
COVID-19, Coronavirus disease 2019; SD, Standard Deviation.
According to qualitative evaluation performed by the ophthalmologists.
Measured using the semi-automatic computer assisted method.
Fisher exact test for qualitative variables, t-test for continuous variables.
Fig. 2.
Retinal findings in Coronavirus Diseases 2019 patients. Dilated veins (white arrowheads) and tortuous vessels (black arrowheads) (A), a retinal haemorrhage (B) and a cotton wool spot (C) as seen on color fundus photos.
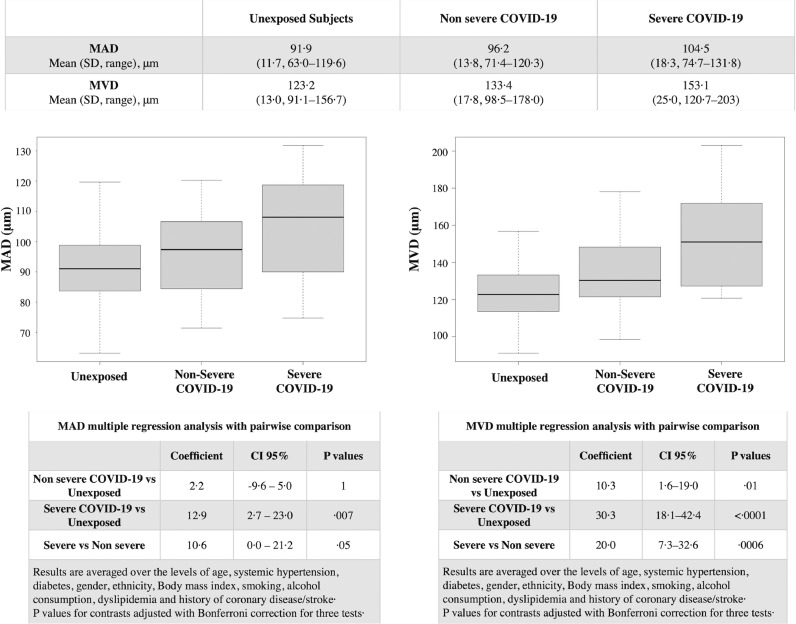
Mean arteries diameter was 98·3 ± 15·3 µm in COVID-19 patients and 91·9 ± 11·7 µm in unexposed subjects, with this difference being statistically significant (p = 0.006). Similarly, MVD was higher in COVID-19 patients (138·5 ± 21·5 µm) compared to unexposed subjects (123·2 ± 13·0 µm) and the difference was statistically significant (p<0.0001). (Table 4)
Multiple linear regression analysis accounting for the effect of covariates and using unexposed subjects as reference showed that MAD was positively associated with COVID-19 with the association being statistically significant only for severe cases (coefficient 12·9, Confidence interval (CI) 95% 2·7–23·0, p = 0·007) (Table 5). MVD showed a significant and positive association with COVID-19 infection for both severe (coefficient 30·3, CI 95% 18·1–42·4, p<0.001) and non-severe (coefficient 10·3, CI 95% 1·6–19·0, p = 0·01) cases compared to unexposed subjects (Table 5). The pairwise comparison showed a significant difference in MVD also between severe and non-severe cases with severe patients having a higher MVD (Fig. 3).
Table 5.
Multiple linear regression showing the effect of COVID-19, demographics and clinical factors on retinal arteries and veins mean diameters.
| Mean arteries diameter | Mean veins diameter | |||
|---|---|---|---|---|
| Coefficient (CI 95%) | pb | Coefficient (CI 95%) | pb | |
| Referencea | 87·4 (74·2–100·6) | 117·3 (101·6–133·1) | ||
| COVID-19 (Mild) | 2·2 (−3·7–8·2) | 0·45 | 10·2 (3·1–17·4) | 0·004 |
| COVID-19 (Severe) | 12·8 (4·5 – 21·1) | 0·002 | 30·2 (20·3–40·1) | <0·0001 |
| Age (×1 year) | −0·0 (−0·1–0·1) | 0·97 | −0·0 (−0·2–0·1) | 0·92 |
| Gender (Male) | 0·4 (−3·8–4·7) | 0·82 | −2·6 (−7·7–2·4) | 0·31 |
| Ethnicity: | ||||
| Asian | 1·7 (−25·5–29·0) | 0·89 | −11·6 (−44·1–20·7) | 0·47 |
| Latin | 4·9 (−3·9–13·9) | 0·27 | −2·7 (−13·3–7·9) | 0·61 |
| Indian | 1·9 (−6·9–10·8) | 0·66 | 4·7 (−5·8–15·3) | 0·37 |
| African | −1·6 (−12·7–9·5) | 0·77 | −9·6 (−22·9–3·6) | 0·15 |
| BMI (×1 point increase) | 0·1 (−0·3–0·6) | 0·51 | 0·2 (−0·3–0·8) | 0·48 |
| Smoking (Yes) | 2·1 (−2·5–6·7) | 0·36 | 4·2 (−1·2–9·8) | 0·12 |
| Alcohol consumption (Yes) | 0·5 (−4·2–5·3) | 0·81 | 3·1 (−2·5–8·8) | 0·28 |
| Systemic hypertension (Yes) | −4·8 (−10·3–0·7) | 0·08 | 0·4 (−6·1–7·0) | 0·89 |
| Diabetes (Yes) | 1·0 (−8·3–10·4) | 0·82 | −0·4 (−11·6–10·7) | 0·93 |
| Dyslipidemia (Yes) | −1·6 (−9·2–5·9) | 0·67 | −0·9 (−9·9–8·0) | 0·83 |
| History of coronary disease/stroke (Yes) | 7·0 (−3·3–17·4) | 0·18 | 8·3 (−4·0–20·7) | 0·18 |
CI= Confidence interval; COVID-19 = Coronavirus Diseases 2019; BMI= Body Mass Index; p = p value.
Reference = Subject unexposed to COVID-19, Age=0, Gender=female, Ethnicity=Caucasian, BMI=0, Smoking=NO, Alcohol consumption=NO, Systemic Hypertension=NO, Diabetes=NO, Dyslipidemia=NO, History of coronary disease/stroke=NO.
p value <0.05 was considered to be statistically significant.
Fig. 3.
Mean arteries diameter (MAD) and mean veins diameter (MVD) in non-severe and severe cases of Coronavirus Disease 2019 (COVID-19) infection compared to unexposed subjects. Both MAD and MVD was higher in COVID-19 patients compared to unexposed subjects. The positive effect of COVID-19 on MAV was significant in severe patients compared to unexposed subjects in the multiple linear regression analysis, accounting for all covariates. The positive effect of COVID-19 was significant in both non severe and severe cases compared to unexposed subjects in the multiple linear regression analysis. Pairwise comparison accounting for covariates also showed a positive and significant effect on MVD of severe vs non severe cases.
3.2. Correlations between retinal findings, demographic and clinical features in COVID-19 patients
The presence of retinal haemorrhages and cotton wools was not significantly associated with demographics or systemic diseases including hypertension, diabetes, tuberculosis and HIV infection. Neither dilated veins nor tortuous vessels, as detected by the clinicians on fundus photographs, significantly correlated with any demographic or clinical/lab parameter. Despite this, the MVD was significantly higher in eyes having dilated veins compared to those graded normal at funduscopic examination (p = 0·004).
Demographics, clinical and laboratory parameters that had a significant influence on the MAD in simple linear regression analysis in COVID-19 patients are reported in Table 6. The effects of the remaining variables listed in Tables 1 and 3 are not reported as they were not significant.
Table 6.
Simple and multiple linear regression analysis of factors influencing MAD in COVID-19 patients.
| Simple linear regression |
||
|---|---|---|
| Clinical/Laboratory parameters | Coefficient (CI 95%) | P value |
| PT (× 1 point of PT) | 34·1 (2·9–65·3) | 0·03 |
| Creatinine (× 1 mg/dL more) | −4·1 (−7·2 to −1·0) | 0·01 |
| Multiple linear regression for MAD in COVID-19 patients |
||
|---|---|---|
| Coefficient (CI 95%) | P value | |
| Referencea | 79·7 (19·9–139·5) | n/a |
| PT (× 1 point of PT) | 26·6 (−20·0–60·3) | 0·31 |
| Creatinine (× 1 mg/dL more) | −4·6 (−8·9–0.2) | 0·04 |
MAD, Mean Arteries Diameter; COVID-19, Coronavirus disease 2019; CI, Confidence Interval; PT, Prothrombin Time.
All clinical and laboratory parameters not reported in the current table did not have a significant effect on MVD when tested using a simple linear regression.
All covariates listed in the REFERENCE were included in the multivariate analysis but are not reported in the table as their influence was not statistically significant.
Reference = COVID-19 patient, Creatinine=0, PT=0, Age=0, Gender=female, Ethnicity=Caucasian, BMI=0, Smoking=NO, Alcohol consumption=NO, Systemic Hypertension=NO, Diabetes=NO, Dyslipidaemia=NO, History of coronary disease/stroke=NO.
A complete list of the demographics, clinical and laboratory parameters that had a significant influence on the MVD in simple and multiple linear regression analysis in COVID-19 patients are reported in Table 7. The effects of the remaining variables listed in Tables 1 and 3 are not reported as they were not significant. In the multiple regression analysis the MVD was significantly and negatively correlated with the time between the symptoms onset and the day of the fundus photos (coefficient −1·0, CI 95% −1·89 to −0·20, p = 0·01) and was positively and significantly influenced by the disease severity (coefficient 22·0, CI 95% 5·2–38·9, p = 0·01) (Table 7).
Table 7.
Simple and multiple linear regression analysis of factors influencing MVD in COVID-19 patients.
| Simple linear regression |
||
|---|---|---|
| Clinical/Laboratory parameters | Coefficient (CI 95%) | P value |
| Symptoms to fundus days (×1 day) | −0·7 (−1·6–0) | 0·05 |
| COVID-19 Severity (severe) | 19·7 (7·3–32·1) | 0·002 |
| Ethnicity (African) | −29·0 (−54·5 to −3·5) | 0·02 |
| PT (× 1 point of PT) | 63·97 (21·13–106·8) | 0·004 |
| Multiple linear regression for MVD in COVID-19 patients |
||
|---|---|---|
| Coefficient (CI 95%) | P value | |
| Referencea | 135·9 (69·0–202·8) | n/a |
| Symptoms to fundus days (×1 day) | −1·0 (−1·89 to −0·20) | 0·01 |
| COVID-19 Severity (severe) | 22·0 (5·2–38·9) | 0·01 |
| Ethnicity (African) | −18·2 (−44·1–6·0) | 0·16 |
| PT (× 1 point of PT) | 8·2 (−45·3–6·1) | 0·75 |
MVD, Mean Vein Diameter; COVID-19, Coronavirus disease 2019; CI, Confidence Interval; PT, Prothrombin Time.
All clinical and laboratory parameters not reported in the current table did not have a significant effect on MVD when tested using a simple linear regression.
All covariates listed in the REFERENCE were included in the multivariate analysis but are not reported in the table as their influence was not statistically significant.
Reference = COVID-19 patient with MILD disease, Symptoms to fundus days=0, PT=0, Age=0, Gender=female, Ethnicity=Caucasian, BMI=0, Smoking=NO, Alcohol consumption=NO, Systemic Hypertension=NO, Diabetes=NO, Dyslipidaemia=NO, History of coronary disease/stroke=NO.
Treatments including different types of oxygen supply as well as systemic drugs (anticoagulant, antiaggregant, hydroxychloroquine, remdesivir, lopinavir/ritonavir, tocilizumab, steroids) did not have a significant effect on MAD and MVD.
There was no correlation between ocular symptoms collected in the questionnaire and any of the fundus changes.
4. Discussion
Retinal examination offers a unique opportunity to analyze vessels in vivo [19]. Retinal arteries and veins diameters have been extensively studied and their variations are significantly related to several systemic factors [17,20]. In this study we analyzed the retina of patients with COVID-19 within 30 days from the onset of systemic symptoms. We found that both retinal arteries and veins were larger compared to unexposed subjects. In addition, veins diameter was larger in more severe cases and showed an inverse correlation with time to symptoms onset.
Retinal veins passively enlarge due to an impaired drainage [21], while arteries actively dilate in response to O2 decrease [22] or CO2 increase [23]. In addition, both type of vessels can dilate under the effect of inflammatory mediators [24]. In our population the diameter of retinal veins was significantly larger in patients with COVID-19 compared to unexposed subjects and their size positively correlated with the disease severity. Subsegmental vascular enlargement has been reported even on chest computerized tomography in patients with COVID-19 [25,26]. This dilatation of pulmonary vessels might be attributed to an increased blood supply due to the inflammatory response [25,26], but also to a direct endothelial damage. [7] Our study does not allow to identify the pathophysiology of vessels enlargement but a mechanism similar to that reported for pulmonary vessels could be responsible for the changes we observed the retina. The larger impact on veins diameter could depend on the higher lassitude of the veins walls compared to those of arteries or to a different expression of inflammatory response receptors [19,24].
Veins diameter significantly and negatively correlated with the time interval between the systemic symptoms onset and the day when the fundus photo was performed. This suggests that the veins dilation could reach its highest peak when the immune response starts to kick in and the inflammatory mediators levels increase in the blood stream. The progressive control of the disease and inflammation following hospitalization and treatments could explain the parallel decrease in the vessels dilation [27].
We found no significant effect of oxygen supply or systemic drugs on MAD and MVD in COVID-19 patients. However these results have to be interpreted with extreme caution. In fact, the study was not designed to assess the effect of drugs and the absence of significance could simply depend on the very limited sample size. In addition all treatments were considered as binary variables with no regard to the dosage or the duration, further limiting the significance of this analysis.
It has to be underlined that retinal vessels dilation was not always detectable by clinical funduscopic examination. By contrast, the computer based analysis that we used allowed us to detect minimal changes, invisible to the naked eye. In addition, semi-automatic analysis of fundus photographs is quick and offers an objective evaluation to monitor changes overtime [28].
Along with vascular changes in COVID-19 patients we also found retinal haemorrhages and cotton wools, both common signs of retinal microangiopathy [29]. Similar alterations have been recently reported in a small number of COVID-19 patients [30]. The authors also described lesions in the inner retinal layers using optical coherence tomography (OCT) an imaging technique more sophisticated than funduscopic examination. However, concerns have been expressed by a large number of experts about the possible misinterpretation of these findings ascribed to COVID-19 [31]. It is hard to tell whether retinal haemorrhages and cotton wools are secondary to COVID-19 or just incidental findings, given the high prevalence of hypertension and diabetes in our cohort. We did not find a clear correlation between the presence of these retinal signs and comorbidities, but it is possible that the virus itself, the inflammatory response or the treatment had a stronger effect in patients with an already fragile microvasculature due to their underlying conditions.
Ocular symptoms were not associated with retinal alterations in our study. This is reasonable considering that most of the referred symptoms were not affecting the vision and were likely caused by ocular surface alterations, frequently reported in COVID-19 patients [32,33]. On the other hand the lack of association is a relevant finding. In fact it highlights the importance of evaluating the fundus in all COVID-19 patients, regardless of their complains and stresses the importance of a routine funduscopic examination.
We wish to acknowledge the limitations of our study. First of all, we had a small sample size. This limited the power of our statistical analysis and did not allow us to perform more analysis on the impact of clinical parameters on MAD and MVD including drugs. The group of unexposed subjects was not characterized as accurately as the COVID-19 patients due to the emergency conditions under which the study was conducted. In addition, a group of subjects with systemic inflammation other than COVID-19 should be investigated in further studies in order to understand whether our findings were specific for COVID-19 or just a sign of systemic inflammation. We found that the MVD was inversely correlated with the time between the symptoms onset and the day of the fundus photo, but this was a cross sectional study so each patient was only imaged a single time. A second examination of the fundus would allow to compare veins of the same subject at different times and hence establish if their diameter actually decreases overtime. In fact a longitudinal study collecting follow-up images to assess the evolution of retinal and vascular changes in these patients is presently ongoing at our center. Finally collecting fundus images at the very beginning of the disease and in asymptomatic cases could complete the picture and give more clues about the role of the inflammatory response in the development of retinal findings.
In summary we found that COVID-19 can induce important changes at the level of the retina, most of them affecting the retinal vasculature and particularly veins. The entity of such changes was directly correlated with the disease severity and seemed to affect patients early in the disease course. We were not able to establish whether retinal changes were caused by the virus itself or by the immune response of the host. Nonetheless, if our data will be confirmed, retinal veins diameter could represent a useful parameter to monitor the inflammatory response and/or the endothelial damage in COVID-19. Considering the non-invasive nature of fundus examination, retinal changes should be further investigated in prospective studies to understand their possible applications in the diagnosis and management of COVID-19.
4.1. Data sharing statement
Anonymized dataset and statistical analysis script will be available and provided upon request. The sharing of clinical images (fundus photographs) has not been discussed in the original approval of the study by the local ethic committee so it was not proposed to the patients in the informed consent. This data could be available upon request only if the sharing will be approved by the local committee and by the single patient.
5. Funding
None.
Declaration of Interests
Dr. Invernizzi reports personal fees from Novartis, personal fees from Bayer, outside the submitted work.
Dr. Giacomelli reports personal fees from Mylan, non-financial support from Gilead, outside the submitted work.
Dr. Rizzardini reports personal fees and non-financial support from Gilead, personal fees and non-financial support from ViiV, personal fees and non-financial support from MSD, personal fees and non-financial support from AbbVie, outside the submitted work.
Dr. Galli reports personal fees and non-financial support from Gilead, personal fees and non-financial support from BMS, personal fees and non-financial support from ViiV, personal fees and non-financial support from MSD, personal fees and non-financial support from AbbVie, personal fees and non-financial support from Janssen, personal fees and non-financial support from Roche, outside the submitted work.
Dr. Antinori reports personal fees and non-financial support from Pfizer, personal fees and non-financial support from Merck Sharp & Dome, outside the submitted work.
Dr. Staurenghi reports grants and personal fees from Heidelberg Engineering, grants from Optos, other from Ocular Instruments, grants from Optovue, grants from Quantel Medical, grants and personal fees from Centervue, grants from Carl Zeiss Meditec, grants and personal fees from Nidek, personal fees from Apellis, personal fees from Allergan, personal fees from Bayer, personal fees from Boheringer, grants from Topcon, personal fees from Genentech, personal fees from Novartis, personal fees from Roche, personal fees from Chengdu Kanghong Biotechnology Co, personal fees from Kyoto Drug Discovery & Development Co, outside the submitted work.
All other authors have nothing to disclose.
References
- 1.Jee Y. WHO International Health Regulations Emergency Committee for the COVID-19 outbreak. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terpos E., Ntanasis-Stathopoulos I., Elalamy I. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intens Care Med. 2020:1–10. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):12–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong T.Y., Klein R., Sharrett A.R. The prevalence and risk factors of retinal microvascular abnormalities in older persons: the Cardiovascular Health Study. Ophthalmology. 2003;110(4):658–666. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 9.Bresnick G.H., Mukamel D.B., Dickinson J.C., Cole D.R. A screening approach to the surveillance of patients with diabetes for the presence of vision-threatening retinopathy. Ophthalmology. 2000;107(1):19–24. doi: 10.1016/s0161-6420(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 10.Port A.D., Orlin A., Kiss S., Patel S., D'Amico D.J., Gupta M.P. Cytomegalovirus retinitis: a review. J Ocul Pharmacol Ther. 2017;33(4):224–234. doi: 10.1089/jop.2016.0140. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A., Invernizzi A., Acquistapace A. Analysis of retinochoroidal vasculature in human immunodeficiency virus infection using spectral-domain OCT angiography. Ophthalmol Retina. 2017;1(6):545–554. doi: 10.1016/j.oret.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casagrande M., Fitzek A., Püschel K. Detection of SARS-CoV-2 in human retinal biopsies of deceased COVID-19 patients. Ocul Immunol Inflamm. 2020:1–5. doi: 10.1080/09273948.2020.1770301. [DOI] [PubMed] [Google Scholar]
- 14.Wong T.Y., Knudtson M.D., Klein R., Klein B.E., Meuer S.M., Hubbard L.D. Computer-assisted measurement of retinal vessel diameters in the Beaver Dam Eye Study: methodology, correlation between eyes, and effect of refractive errors. Ophthalmology. 2004;111(6):1183–1190. doi: 10.1016/j.ophtha.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 15.Bankhead P., Scholfield C.N., McGeown J.G., Curtis T.M. Fast retinal vessel detection and measurement using wavelets and edge location refinement. PLoS One. 2012;7(3):e32435. doi: 10.1371/journal.pone.0032435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knudtson M.D., Lee K.E., Hubbard L.D., Wong T.Y., Klein R., Klein B.E. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27(3):143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 17.Leung H., Wang J.J., Rochtchina E. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003;44(7):2900–2904. doi: 10.1167/iovs.02-1114. [DOI] [PubMed] [Google Scholar]
- 18.COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed [15 May 2020]. [PubMed]
- 19.Ikram M.K., Ong Y.T., Cheung C.Y., Wong T.Y. Retinal vascular caliber measurements: clinical significance, current knowledge and future perspectives. Ophthalmologica. 2013;229(3):125–136. doi: 10.1159/000342158. [DOI] [PubMed] [Google Scholar]
- 20.Köchli S., Endes K., Infanger D., Zahner L., Hanssen H. Obesity, blood pressure, and retinal vessels: a meta-analysis. Pediatrics. 2018;141(6) doi: 10.1542/peds.2017-4090. [DOI] [PubMed] [Google Scholar]
- 21.Bek T. Diameter changes of retinal vessels in diabetic retinopathy. Curr Diab Rep. 2017;17(10):82. doi: 10.1007/s11892-017-0909-9. [DOI] [PubMed] [Google Scholar]
- 22.Cheng R.W., Yusof F., Tsui E. Relationship between retinal blood flow and arterial oxygen. J Physiol. 2016;594(3):625–640. doi: 10.1113/JP271182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorner G.T., Garhoefer G., Zawinka C., Kiss B., Schmetterer L. Response of retinal blood flow to CO2-breathing in humans. Eur J Ophthalmol. 2002;12(6):459–466. doi: 10.1177/112067210201200603. [DOI] [PubMed] [Google Scholar]
- 24.de Jong F.J., Ikram M.K., Witteman J.C., Hofman A., de Jong P.T., Breteler M.M. Retinal vessel diameters and the role of inflammation in cerebrovascular disease. Ann Neurol. 2007;61(5):491–495. doi: 10.1002/ana.21129. [DOI] [PubMed] [Google Scholar]
- 25.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214(6):1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 26.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020:1–9. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Z., Cai T., Fan L. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–339. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akbar S., Sharif M., Akram M.U., Saba T., Mahmood T., Kolivand M. Automated techniques for blood vessels segmentation through fundus retinal images: a review. Microsc Res Tech. 2019;82(2):153–170. doi: 10.1002/jemt.23172. [DOI] [PubMed] [Google Scholar]
- 29.Wong T.Y., McIntosh R. Hypertensive retinopathy signs as risk indicators of cardiovascular morbidity and mortality. Br Med Bull. 2005;73–74:57–70. doi: 10.1093/bmb/ldh050. [DOI] [PubMed] [Google Scholar]
- 30.Marinho P.M., Marcos A.A.A., Romano A.C., Nascimento H., Belfort R., Jr. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vavvas D.G., Sarraf D., Sadda S.R. Concerns about the interpretation of OCT and fundus findings in COVID-19 patients in recent Lancet publication. Eye. 2020:1–2. doi: 10.1038/s41433-020-1084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daruich A., Martin D., Bremond-Gignac D. Ocular manifestation as first sign of Coronavirus Disease 2019 (COVID-19): interest of telemedicine during the pandemic context. J Fr Ophtalmol. 2020;43(5):389–391. doi: 10.1016/j.jfo.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu P., Duan F., Luo C. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]