Abstract
Endoplasmic reticulum (ER) stress, defined as prolonged disturbances in protein folding and accumulation of unfolded proteins in the ER. Perturbation of the ER, such as distribution of oxidative stress, iron imbalance, Ca2+ leakage, protein overload, and hypoxia, can cause ER stress. The cell reacts to ER stress by activating protective pathways, called the unfolded protein response (UPR), which is comprised of cellular mechanisms aimed for maintaining cellular homeostasis or, in case of excessively severe stress, at the initiation of cellular apoptosis. The three UPR signaling pathways from the ER stress sensors are initiated by activating transcription factor 6, inositol requiring enzyme 1, and protein kinase RNA-activated-like ER kinase. A number of physiological and pathological conditions, environmental toxicants and variety of pharmacological agents showed disruption of proper ER functions and thereby cause ER stress in male reproductive organ in rat model. The present review summarizes the existing data concerning the molecular and biological mechanism of ER stress in male reproduction and male infertility. ER stress initiated cell death pathway has been related to several diseases, including hypoxia, heath disease, diabetes, and Parkinson's disease. Although there is not enough evidence to prove the relationship between ER stress and male infertility in human, most studies in this review found that ER stress was correlated with male reproduction and infertility in animal models. The ER stress could be novel signaling pathway of regulating male reproductive cellular apoptosis. Infertility might be a result of disturbing the ER stress response during the process of male reproduction.
Keywords: Apoptosis, Endoplasmic reticulum stress, Infertility, Oxidative stress, Reproduction
INTRODUCTION
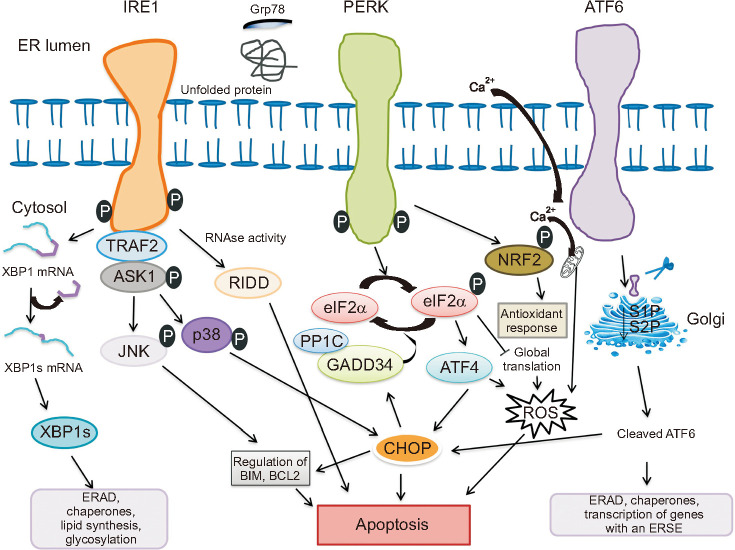
The endoplasmic reticulum (ER) is an important organelle for eukaryotic cells survival and development [1,2]. ER is responsible for biosynthesis, folding, assembly and modification of most secreted and a transmembrane protein, calcium homeostasis, lipid, and steroid synthesis in cells [3]. Approximately one-third of cellular proteins production and folding occur in ER [4]. Increase protein synthesis beyond the capacity for folding nascent polypeptides in the cells or excess accumulation of unfolded/misfolded proteins within the ER lumen will disrupt ER homeostasis and trigger the unfolded protein response (UPR) and eventually leads to ER stress [5]. The UPR coordinated primarily by three ER membrane-associated proteins: inositol requiring enzyme 1 (IRE1), activating transcription factor 6 (ATF6), and protein kinase RNA-like ER kinase (PERK) (Fig. 1) [3,6]. Under normal state, ER chaperone protein binding immunoglobulin protein or glucose-regulated protein 78 kDa (BiP/Grp78) interacts with three sensors (IRE1, PERK, and ATF6), promotes cell survival by reducing unfolded/misfolded protein levels and hold these three pathways inactive when misfolded protein are absent [7]. Under ER stress, BiP/Grp78 is titrated away by misfolded proteins, leaving the three sensors (IRE1, PERK, and ATF6) activate by inducing phosphorylation and homodimerization of IRE1 and PERK as well as relocalization of ATF6 to the Golgi [8,9]. Induction of ER stress activates a constitutive protein degradation pathway termed as endoplasmic reticulum associated degradation (ERAD) [10]. Accumulation of unfolded or misfolded protein reduces the entry of protein into the ER by attenuating protein translation, and degradation of misfolded protein by retrograde transport from ER into cytosol through the ubiquitin-proteasome system: a process named ERAD I [11]. When retrotranslocon/ERAD I is impaired mutant dysferlin aggregates on the ER and degraded by the autophagy/lysosome system also known as ERAD II pathway [12]. Prolonged ER stress result apoptosis through activation of C/EBP homologous protein (CHOP), c-Jun N-terminal kinase (JNK) and caspase-12 pathway in human and cleaved caspase-12 in mice [13].
Fig. 1. Endoplasmic reticulum (ER) stress induces the unfolded protein response signaling by three types of ER stress sensors: inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like ER kinase (PERK) and activating transcription factor 6 (ATF6). Misfolded proteins sequestrate glucose-regulated protein 78 kDa (Grp78), thus allowing activation of three ER membrane-associated proteins. Activated IRE1 cleaves X box-binding protein 1 (XBP1) mRNA to a spliced form (XBP1s) that is translated to gene sets involved in ER-associated protein degradation (ERAD), ER chaperones, lipid biosynthesis and glycosylation. Along with selective XBP1 mRNA splicing, other mRNA are degraded through regulated IRE1-dependent decay (RIDD). In addition, IRE1 activates c-Jun N-treminal kinase (JNK) and p38 by IRE1-tumor necrosis factor receptor associated factor 2 (TRAF2)-apoptotic signaling kinase-1 (ASK1) complex and induce apoptosis by inhibiting anti-apoptotic proteins. Activation of PERK phosphorylates eukaryotic translation initiator factor 2α (eIF2α), selective induction of ATF4 and its downstream protein C/EBP homologous protein (CHOP), resulting in apoptosis. This pathway is negatively regulated by a phosphate complex protein phosphate 1c (PP1c) and stress-induced regulatory subunit DNA damage-inducible protein 34 kDA (GADD34). In addition PERK also activates nuclear factor erythroid 2-related factor 2 (NRF2), which induce antioxidant responses. ATF6 is translocated into golgi where it is cleaved by the site 1 and site 2 protease to release the transcription factor that regulates chaperones expression, transcription of genes with an ER stress response elements (ERSE), ERAD and CHOP.
IRE1, a type I transmembrane protein, is the highly conserved ER stress sensor responsible for protein kinase and endoribonuclease activities [14]. Upon ER stress, IRE1 RNase is activated through conformational change, oligomerization of IRE1 in ER membrane and autophosphorylation of IRE1 cytosolic domain [15]. The cytosolic RNase domain processes an intron from the X box-binding protein 1 (XBP1) mRNA to generate a potent transcription activator XBP1 protein [16]. XBP1 protein binds to the promoters of its target genes involved in UPR and proteins contributing to ERAD, restore homeostasis and thereby cytoprotection [14]. When the attempt to rebalance ER homeostasis fails, regulated IRE1-dependent decay of mRNA (RIDD) process promotes the degradation of mRNAs encoding mostly ER-targeted proteins involved in protein folding and can either preserve ER homeostasis or enhance apoptosis [17,18]. RIDD activation without XBP1 splicing predominantly leads to apoptosis [14]. In addition to its cytoprotective function, IRE1 interact with tumor necrosis factor receptor associated factor 2 (TRAF2) and the IRE1-TRAF2 complex stimulates activation of the apoptotic signaling kinase-1 (ASK1), also known as mitogen-activated protein kinase kinase [19]. IRE1-TRAF2-ASK1 complex activate downstream of stress kinases JNK and p38 MAPK signaling pathway, which further activate CHOP to contribute to reactive oxygen species (ROS) generation and enhance apoptosis [20,21].
ATF6 is a type II transmembrane glycoprotein, another ER stress transducers encode basic Leucine zipper (bZIP) transcription factors, in the ER membrane that upon ER stress translocate from the ER to the Golgi membrane. Following translocation to Golgi apparatus, ATF6 is cleaved sequentially by serine protease site-1 (S1P) in the luminal domain and metalloprotease site-2 protease (S2P) in N-terminal portion, releasing a cytosolic fragment (ATF6f) by binding to ATF/cAMP response element (CRE) and ER stress-response element (ERSE-1) that leads to induction of chaperones, such as BiP/Grp78, glucose-regulated protein 94 (Grp94), transcription factors CHOP and XBP1 [22,23].
PERK is essential type I transmembrane kinase contains a large ER luminal “stress sensing” domain responsible for attenuation of mRNA translation under ER stress and help to reduce the flux of protein entering the ER to alleviate ER stress [24]. Activated PERK phosphorylates eukaryotic translation initiation factor 2 (eIF2α) at serine-51 which inhibits the reactivation of eIF2 into its guanosine triphosphate-bound form (initial phase of polypeptide synthesis) and enhance translation of activating transcriptional factor 4 (ATF4) mRNA [25]. Under prolonged ER stress conditions, ATF4 induce transcription of CHOP and DNA damage-inducible protein 34 kDA (GADD34) [26]. CHOP induces apoptosis via direct inhibition of B cell lymphoma 2 (BCL-2) transcription and induction of BCL-2-interacting mediator of cell death (BIM) expression [27]. GADD34 is regulatory subunit of protein phosphatase 1c (PP1c) that counteracts PERK by dephosphorylating eIF2α and restore normal protein synthesis [28]. Besides eIF2α, Upon ER stress activated PERK also phosphorylates bZIP Cap (n) Collar transcription factor (Nrf2), a transcription factor involved in redox metabolism [29]. PERK-peIF2α-ATF4 complex pathway also facilitates activation of ATF6 and its target genes [29,30].
Previous studies have reported that ER stress plays key roles in immune response, disease such as diabetes, Alzheimer's, Parkinson's disease and, various type of cancer [31,32,33]. A variety of physiological states, environmental stimuli and pharmaceutical agents are responsible for disruption of the ER homeostasis, causing ER stress in male reproductive system [34,35,36,37]. It is well accepted that oxidative stress is common mode of action in testicular dysfunction and male infertility [38,39,40,41]. Intriguingly, recent evidence demonstrated that oxidative stress, including reactive oxygen species (ROS) production, can induce ER stress [32,42]. Production of ROS has been linked with oxidative stress and ER stress [42]. Oxidative stress elicits a reduction-oxidation (redox) imbalance [33]. Alteration of redox homeostasis in ER disturbs ER protein folding and causes ER stress, and accumulation of ROS in the ER and mitochondria [35]. Furthermore, oxidative protein folding in the ER of eukaryotic cells generates ROS as a byproduct and cause oxidative stress [32]. Cellular antioxidant mechanism is an indispensable to scavenge and reduce ROS production, to counteract crosstalk between oxidative stress and ER stress [32]. The aim of this review was to summarize the research studies associated with male reproduction and infertility, and insight the role of noble ER stress signaling pathway that regulates apoptosis in male reproductive tissue.
INVOLVEMENT OF ENDOPLASMIC RETICULUM STRESS IN TESTIS AND GERM CELLS
Testes are male reproductive gland in human and animals where the germ cells develop to sperm cell by the process of mitosis and meiosis, and it is also the predominant site of androgen biosynthesis. Testicular temperature below body temperature is favorable for normal spermatogenesis. Pathological conditions like varicocele, cryptorchidism, and febrile episodes increase the testis temperature and compromised spermatogenesis, which may lead to male infertility [43]. ER stress chaperone Grp78 predominantly presents in pachytene spermatocytes, suggest ER stress signaling pathway have important role in the process of spermatogenesis [44]. A study in Drosophila male reports ER stress gene are highly express in male accessory gland and induced strong ER stress decrease fertility [7]. A study by Kim et al [13] reported that repetitive cycle of testicular hyperthermia leads to ER stress associated apoptosis of spermatocytes in mouse testis by activation of IRE1-JNK and PERK pathway. In support of these observation, recent study from our lab report varicocele-induced infertility in rat model by ROS-dependent ER stress in testis [35]. In a study on testicular injury after torsion/detorsion in rat model reported the germ cell apoptosis in testis by upregulate eIF2α and CHOP, which insight the role of ER stress in testicular ischemia/reperfusion injury [45]. Aging in men is associated with decline in the testosterone level by Leydig cell steroidogenic function degeneration [46]. Degenerative changes in germ cells in aging rat showed oxidative stress and ER stress signaling pathway play important role [47,48]. Two independent study provide evidence that hypoxia downregulate androgen biosynthesizing genes such as steroidogenic acute regulatory protein (StAR) and 3-β-hydroxysteroid dehydrogenase (3β-HSD) in testis of rat by increasing calcium influx, oxidative stress and upregulation of ER stress signaling molecule Grp78, PERK and CHOP [49,50].
In a streptozotocin-induced diabetic rat model showed crosstalk between mitochondrial and ER-dependendent testicular cell death by JNK pathway that upregulated pro-apoptotic protein cleaved caspase-3, Bcl-2 associated X protein (Bax), Bcl-2-associated death promoter (Bad) and BH3 interacting-domain agonist (Bid) [51]. Another study in streptozotocin-induced diabetic animal model showed activation of testicular oxidative stress; mitochondrial cell death and associated p38 MAPK and p53 signaling; and ER stress and associated cell death [36,52]. Similarly, in vitro study, advanced glycation end products, a heterogeneous compound in the progression of diabetes, inhibit the generation of testosterone by upregulation the ER stress related protein Grp78 and CHOP in Leydig cells [53]. Human chorionic gonadotropin (hCG) secreted by placenta, is heterodimeric protein hormone to luteinizing hormone and exogenous hCG has been used clinically to increase plasma testosterone in disease, such as hypogonadotropic hypogonadism, cryptorchidism. Park et al [54] reported that excess hCG stimulation is associated with ER stress-induced apoptosis in mouse Leydig tumor (mLTC-1) cells and rat testis. Moreover, activation of ATF6 pathway downregulate androgen biosynthesizing gene 3β-HSD.
There are many environment pollutants in the group of endocrine-disrupting chemicals (EDCs) associated with the impairment of male reproductive function [55]. Bisphenol A (BPA), one of the EDC leads to apoptosis in mouse testicular Sertoli cells by JNK/p38 MAPK and PERK pathway [56,57]. Another in vivo study showed that ROS mediated PERK/EIF2α/CHOP pathway plays important role in BPA-induced apoptosis in germ cell [58]. Furthermore, low-dose and combined exposure to BPA and diethylstilbestrol in rat study reported the upregulation of ER stress molecular sensor IRE1 and CHOP, suggest that ER stress mediated apoptosis in germ cell [59]. Reproductive toxicant microcystin-LR showed germ cell apoptosis in testis of zebrafish by upregulation of ER stress sensors Grp78, and eIF2s1, a downstream target of the PERK pathway and MAPK8, a zebrafish homolog of JNK [2]. 4-nonylphenol (NP)-induced apoptosis in the rat testicular Sertoli cells by increased intracellular Ca2+ and upregulated the expression of ER stress signaling target genes Grp78, protein disulfide isomerase (ERp57) and GADD153 [60]. Furthermore, NP-induces autophagy antagonizing apoptotic cell death in Sertoli cells by ROS-mediated JNK-dependent autophagy as survival mechanisms against apoptosis [61]. Cadmium is major occupational and environmental toxicant that induces gem cell apoptosis in the testis of mice by upregulation of ROS, ER stress signaling and mitochondrial pathway [62,63,64]. Nickel is other occupational toxicants involved in apoptosis of rat Leydig cells by ROS-mediated ER stress and mitochondrial apoptosis pathway [65]. Similarly, Lead (Pb) induced testicular toxicity in chicken by oxidative stress, ER stress and apoptosis via CHOP/caspase-3 signaling pathway [66]. Fine particulate matter 2.5 (PM2.5) is a part of atmospheric dust that has reproductive toxicity effect and promotes testicular germ cell apoptosis through upregulation of ER stress molecular sensor Grp78, XBP1 and CHOP, and caspase-12 [3]. Fluoride is an essential element and accumulated in different organ of human body. It is also well known natural pollutant responsible for reproductive toxicant. Zhang et al [37] reported that sodium fluorite (NaF) induced germ cell apoptosis in testis by increasing oxidative stress and ER stress protein Grp78, IRE1 and CHOP. Moreover, upregulated pro-inflammatory cytokines, tumor necrosis factor-α, interleukin-1β, inducible nitric oxide synthase and cyclooxygenase-2, in a nuclear factor-κB-dependent manner suggest that oxidative stress, ER stress and inflammation are the molecular mechanism to fluorite-induced testicular toxicity. In support of this observation, in vitro study reported NaF-induced apoptosis of Sertoli cells by ROS-mediated ER stress pathway [67]. Dibutyl phthalate (DBP) is EDCs that induced testicular toxicity in Sertoli cells by hyperphosphorylation of vimentin and upregulation of ER stress [68]. Another study by Zhang et al [69] reported that DBP induced ER stress mediated apoptosis by CHOP activation and also triggered autophagy as a protective effect against germ cell death, suggest the role of ER stress in both cell survival and cell death mechanism. A study in low dose radiation (LDR) reported ROS mediated ER stress and apoptosis in testicular cells in mice, and LDR-induced ER stress mechanism were regulated by IRE1, PERK and ATF6 pathways [70]. Zearalenone (ZEN) is widely distributed mycotoxin, produced by Fusarium species and can exert estrogen-like activity. Zheng et al [71] reported ZEN-induced germ cell death by ROS mediated ER stress in mouse Sertoli cell. Furthermore, ER stress regulates ATP/AMPK pathway, suggest crosstalk between ROS-mediated ER stress and ATP/AMPK pathway and plays key role in ZEN induced cell cycle arrest and cell apoptosis. Another study showed ZEN-induced apoptosis in mouse Leydig cell by the activation of ER stress markers Grp78, CHOP and caspase-12 [72].
Drug-induced toxicity leads to male infertility and some side effects can be severe as infertility may persist in some cases. In studies, cisplatin induced testicular toxicity in rat by ROS-mediated ER stress [34,73]. Another study reported cisplatin-induced testicular damage by oxidative stress, ER stress and MAPK pathway [74]. Similarly, in vitro study in mouse Leydig MTTC-1 cell reported ER stress mediated apoptosis by upregulation of ER stress proteins PERK, EIF2α and ATF4; ER stress apoptosis-relative proteins caspase-3, caspase-7 and caspase-12, and autophagy protein microtubule-associated protein 1A/1B-light chain 3 (LC3II) and autophagy related 5 (Atg5) suggest ER stress mediated apoptosis and autophagy are the mechanism involved in spermatogenic dysfunction [75]. Recent study from our lab reported adriamycin-induced testicular dysfunction by the crosstalk between ER stress and mitochondrial mediated signaling pathway [76]. Another commonly used chemotherapy drug busulfan-induced apoptosis in both testicular germ cells in mouse and C18-4 cell line by upregulation of IRE1 and PERK pathway [77]. Finasteride is a 5α-reductase inhibitor used to treat benign prostate hyperplasia and male pattern baldness. Soni et al [78] reported the testicular germ cell apoptosis by finasteride treatment in rat and the main mechanism is associated with ROS mediated ER stress. All this study suggests that oxidative stress and ER stress could be the important molecular mechanism involved in drug liability and toxicity.
INVOLVEMENT OF ENDOPLASMIC RETICULUM STRESS IN EPIDIDYMIS
Epididymis is a tube connects to testicle to vas deference where the sperm undergo further maturation and is primary storage site for mature sperm. Zhu at al [79] reported that cigarette smoking alters the epididymis protein profile responsible for energy metabolism, reproduction and structural molecule activity, thereby impairing epididymis function and sperm maturation. Oxidative stress and ER stress pathway is associated mechanism involved in epididymis protein profile alteration in mice epididymis. Another study showed PM2.5-induced apoptosis in epididymis by upregulation of ER stress molecular sensor Grp78, XBP1 and CHOP, and caspase-12 [3]. In summary, ER stress may impair the function of epididymis. These data provide novel insight into the mechanism of sperm maturation.
INVOLVEMWNT OF ENDOPLASMIC RETICULUM STRESS IN SPERM
A matured sperm doesn't contain ER. However, resident protein of the ER such as ER protein 29 (ERp29) and calreticulin are transported to the sperm and plays important role in acrosome reaction and sperm fertilization in rat [80,81]. Results from the study shown that ER-stress mediated germ cell apoptosis as well as potentially decreased in the cation channels of sperm (CatSper) in epididymal sperm by cisplatin in rat [34]. In their opinion, ER-stress in testicular germ cell may alter the CatSper channels of sperm that play important role in sperm maturation and hyperactivation in sperm. Nagamori et al [82,83] reported that testis-specific bZip type transcription factor (Tisp40) gene is predominantly express during spermatogenesis in rat testis and disruption of Tisp40 leads to ER stress mediated apoptosis of meiotic/post meiotic germ cells. This study suggests Tisp40 regulates the maturation of sperm head nuclei by unique UPR system survival mechanisms against ER-stress induced apoptosis. In summary, these findings suggest that ER stress signaling pathway may play important role in sperm maturation and fertilization.
CONCLUSIONS
Current literature provide strong evidence that ER-stress regulates maintenance of cellular homeostasis and apoptosis in male reproductive organs and linked with several mechanisms such as direct activation of proteases, kinases, transcription factors, Bcl2-family protein and their modulators. Varieties of environmental, physiological pathological and drug treatment in animal models induced infertility by ER stress signaling pathway insight the precise knowledge of how these pathways cause cellular homeostasis or leads to apoptosis (Table 1). Evidence for the role of ER stress induced cell death in male reproduction could make this process an attractive target for therapy. Based on this basic research evidence, further clinical studies are needed for greater understanding of the mechanisms of ER-stress signaling pathway in male infertility. Such information is expected to help the pathophysiological understanding and effectively treat or prevent ER-stress associated male infertility.
Table 1. Relevance of ER stress in reproductive infertility in male.
| Agents/State | ER stress molecule | Role of ER stress | Finding | Study (ref.) |
|---|---|---|---|---|
| Diabetes mellitus | Grp78, p-JNK, CHOP | Upregulate oxidative stress, mitochondrial cell death pathway and ER stress mediated testicular germ cell apoptosis. | Testicular dysfunction by oxidative stress and ER stress mediated germ cell apoptosis. | 51,36,52 |
| AGEs treated rat Leydig cells | Grp78, CHOP | Upregulation of ROS-mediated ER stress decreases the levels of StAR, 3-β-HSD and cholesterol side-chain cleavage enzyme (P450scc) in Leydig cells. | Inhibit testosterone biosynthesis by upregulation of ER stress. | 53 |
| Hypoxia | BiP, PERK, CHOP | Hypoxia upregulate BiP, PERK and CHOP level and downregulate StAR and 3-β-HSD in rat testis. | Hypoxia induced ER stress in testis and compromised testosterone biosynthesis. | 49,50 |
| Testicular hyperthermia | Grp78, PERK/p-eIF2α, ATF6, p-IRE1α, p-JNK, CHOP | Testicular hyperthermia leads to UPR pathway for cell survival and adaptation. However repeated cycle of hyperthermia promotes ER stress-mediated testicular cell apoptosis in rat. | Increase apoptosis of germ cell in testis may lead to infertility. | 13 |
| Varicocele | Grp78, p-IRE1α, p-JNK | ROS-mediated upregulation of ER stress sensor molecule Grp78, p-IRE1α and p-JNK in rat testis triggers apoptosis. | Varicocele-induced infertility by ROS-mediated ER stress signaling pathway. | 35 |
| T/D induced testicular injury | p-eIF2α, CHOP | T/D induced testicular injury upregulated p-eIF2α and CHOP in rat. | ER stress-mediated apoptotic pathway lead to infertility in testicular injury after testicular torsion. | 45 |
| Ageing | Grp78, p-PERK/p-eIF2α, ATF6, p-IRE1α, p-JNK, CHOP | Ageing rat model accompanied by upregulated oxidative stress and ER stress protein level in germ cell. | Accelerating aging-related testicular dysfunction by upregulating ER stress-mediated testicular germ cell apoptosis. | 48,47 |
| hCG | Grp78, p-JNK, p-IRE1α, ATF6, p-eIF2α, ATF4,CHOP | hCG induced ER stress-mediated apoptosis in mice testis and mouse Leydig tumor (mLTC-1) cells and downregulate the expression of steroidogenic enzymes. | ER stress mediated apoptosis in Leydig cells cause infertility by downregulation of testosterone biosynthesis. | 54 |
| BPA | p-IRE1, p-JNK, PERK, eIF2α, CHOP, | BPA upregulated ER stress sensor protein in both in vivo and in vitro of rat germ cell. | ER stress mediated germ cell apoptosis leads to infertility. | 56,57,58,59 |
| MCLR | Grp78, eIF2s1 | Upregulate the ER stress protein in zebrafish testes. | MCLR induced infertility by activation of ER stress mediated testicular germ cell apoptosis in zebrafish. | 2 |
| NP | Grp78, GADD153, p-JNK, protein disulfide isomerase (ERp57) | Upregulated the expression of ER stress signaling target genes Grp78, protein disulfide isomerase (ERp57) and GADD153 in rat sertoli cells. Moreover, survival mechanisms against apoptosis by ROS-mediated JNK-dependent autophagy. | NP induced ER stress mediated apoptosis in testicular sertoli cell and decrease male fertility. | 60,61 |
| Cadmium | Grp78, XBP1, p-eIF2α, p-JNK, CHOP, p-IRE1α | Upregulation of ER stress signaling protein and mitochondrial pathway in germ cell of rat testes. | Testicular dysfunction by ER stress mediated germ cell apoptosis. | 62,63,64 |
| Nickel | Grp78, GADD153 | ROS mediated upregulation of mitochondria and ER stress signaling pathway in rat Leydig cells. | NiSO4-induced apoptosis in rat Leydig cells by ROS-dependent mitochondria and ER stress signaling pathway. | 65 |
| Lead (Pb) | Grp78, PERK, p-eIF2α, ATF4, CHOP | Upregulate the expression of oxidative stress and ER sensor proteins Grp78, PERK, eIF2α, ATF4 and CHOP in the chicken testis. | Pb induced apoptosis in chicken testis by oxidative stress and ER stress pathway. | 66 |
| Particulate matter 2.5 | Grp78, XBP1, CHOP | Upregulation of Grp78, XBP1 and CHOP in rat testis and epididymis. | Testicular toxicity by ER stress mediated apoptosis in rat testis and epididymis. | 3 |
| Fluoride | Grp78, IRE1, PERK, p-eIF2α, CHOP | Upregulation of ROS mediated ER stress and inflammation in rat germ cell. | Reproductive dysfunction by ER stress mediated germ cell apoptosis. | 37,67 |
| Dibutyl phthalate | Grp78, ATF6, p-eIF2α, CHOP | Upregulation of CHOP in rat germ cells. ER stress triggered autophagy as a protective effect against germ cell death. | Increase ER stress mediated germ cell apoptosis in rat sertoli cells and mouse spermatocyte-derived GC-2 cells. | 68,69 |
| LRD | Grp78, p-PERK, p-eIF2α, ATF6, p-IRE1α, XBP1, p-JNK, CHOP | Upregulation of CHOP by PERK, ATF6 and IRE1 pathway in mice testis. | LRD induced germ cell apoptosis by ER stress signaling pathway. | 70 |
| ZEN | Grp78, CHOP, ATF6, PERK | Upregulation of ER sensor protein in mouse Sertoli cells and Leydig cells. | ZEN-induced apoptosis in Sertoli cells and Leydig cells by ER stress signaling pathway. | 71,72 |
| Cisplatin | Grp78, p-IRE1, p-JNK, ATF6, PERK, p-eIF2α, CHOP | Increased oxidative stress and ER stress protein level in rat germ cell in vivo and in vitro. | Testicular dysfunction by oxidative stress and ER stress mediated apoptosis. | 34,73,74,75 |
| Adriamycin | Grp78, p-IRE1, p-JNK | Increased oxidative stress, ER stress and mitochondrial signaling protein in rat testis. | Testicular dysfunction by oxidative stress, ER stress and mitochondrial mediated apoptosis signaling pathway. | 76 |
| Busulfan | Grp78, ATF6, p-IRE1, XBP1, CHOP | Increased ER stress sensor protein in both mouse testis (in vivo) and C18-4 cell line (in vitro). | Spermatogonial stem cell apoptosis by ER stress signaling pathway. | 77 |
| Finasteride | Grp78, p-IRE1α, p-JNK | Upregulation of Grp78, p-IRE1α and p-JNK in rat testis. | ER stress mediated apoptosis may lead to infertility in rat. | 78 |
ER: endoplasmic reticulum, AGE: glycation end products, T/D: torsion/detorsion, hCG: human chorionic gonadotropin, BPA: bisphenol A, MCLR: microcystin-LR, NP: 4-nonylphenol, Pb: Lead, LRD: low dose radiation, ZEN: Zearalenone, Grp78: glucose-regulated protein 78 kDa, p-JNK: phosphorylated c-Jun N-terminal kinase, CHOP: C/EBP homologous protein, BiP: binding immunoglobulin protein, PERK: protein kinase RNA-like ER kinase, p-eIF2α: phosphorylated eukaryotic translation initiation factor 2, ATF6: activating transcription factor 6, p-IRE1: phosphorylated inositol requiring enzyme 1, p-PERK: phosphorylated protein kinase RNA-like ER kinase, eIF2: eukaryotic translation initiation factor 2, GADD153: DNA damage-inducible protein 153 kDA, XBP1: X box-binding protein 1, IRE1: inositol requiring enzyme 1, ROS: reactive oxygen species, StAR: steroidogenic acute regulatory protein, 3β-HSD: 3-β-hydroxysteroid dehydrogenase, UPR: unfolded protein response.
ACKNOWLEDGEMENTS
This study was supported by grants from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI14C0018).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Research conception & design: KKK, JKP.
- Data acquisition: KKK, YSS, BRC, HKK, JKP.
- Data analysis and interpretation: KKK, JKP.
- Drafting of the manuscript: KKK, JKP.
- Critical revision of the manuscript: KKK, JKP.
- Approval of final manuscript: KKK, YSS, BRC, HKK, JKP.
References
- 1.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao S, Liu Y, Wang F, Xu D, Xie P. N-acetylcysteine protects against microcystin-LR-induced endoplasmic reticulum stress and germ cell apoptosis in zebrafish testes. Chemosphere. 2018;204:463–473. doi: 10.1016/j.chemosphere.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Liu X, Jin X, Su R, Li Z. The reproductive toxicology of male SD rats after PM2.5 exposure mediated by the stimulation of endoplasmic reticulum stress. Chemosphere. 2017;189:547–555. doi: 10.1016/j.chemosphere.2017.09.082. [DOI] [PubMed] [Google Scholar]
- 4.Almanza A, Carlesso A, Chintha C, Creedican S, Doultsinos D, Leuzzi B, et al. Endoplasmic reticulum stress signalling - from basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzel E, Arlier S, Guzeloglu-Kayisli O, Tabak MS, Ekiz T, Semerci N, et al. Endoplasmic reticulum stress and homeostasis in reproductive physiology and pathology. Int J Mol Sci. 2017;18:E792. doi: 10.3390/ijms18040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engin F, Hotamisligil GS. Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab. 2010;12(Suppl 2):108–115. doi: 10.1111/j.1463-1326.2010.01282.x. [DOI] [PubMed] [Google Scholar]
- 7.Chow CY, Avila FW, Clark AG, Wolfner MF. Induction of excessive endoplasmic reticulum stress in the Drosophila male accessory gland results in infertility. PLoS One. 2015;10:e0119386. doi: 10.1371/journal.pone.0119386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pincus D, Chevalier MW, Aragón T, van Anken E, Vidal SE, El-Samad H, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong J, Wang XZ, Wang T, Chen JJ, Xie XY, Hu H, et al. Molecular signal networks and regulating mechanisms of the unfolded protein response. J Zhejiang Univ Sci B. 2017;18:1–14. doi: 10.1631/jzus.B1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christianson JC, Ye Y. Cleaning up in the endoplasmic reticulum: ubiquitin in charge. Nat Struct Mol Biol. 2014;21:325–335. doi: 10.1038/nsmb.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, et al. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II) Hum Mol Genet. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Park SJ, Kim TS, Park HJ, Park J, Kim BK, et al. Testicular hyperthermia induces Unfolded Protein Response signaling activation in spermatocyte. Biochem Biophys Res Commun. 2013;434:861–866. doi: 10.1016/j.bbrc.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Wu J, He GT, Zhang WJ, Xu J, Huang QB. IRE1α signaling pathways involved in mammalian cell fate determination. Cell Physiol Biochem. 2016;38:847–858. doi: 10.1159/000443039. [DOI] [PubMed] [Google Scholar]
- 15.Maurel M, Chevet E, Tavernier J, Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci. 2014;39:245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Abdullah A, Ravanan P. The unknown face of IRE1α - beyond ER stress. Eur J Cell Biol. 2018;97:359–368. doi: 10.1016/j.ejcb.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Coelho DS, Domingos PM. Physiological roles of regulated Ire1 dependent decay. Front Genet. 2014;5:76. doi: 10.3389/fgene.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore K, Hollien J. Ire1-mediated decay in mammalian cells relies on mRNA sequence, structure, and translational status. Mol Biol Cell. 2015;26:2873–2884. doi: 10.1091/mbc.E15-02-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem. 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- 21.Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal. 2002;4:415–425. doi: 10.1089/15230860260196218. [DOI] [PubMed] [Google Scholar]
- 22.Hillary RF, FitzGerald U. A lifetime of stress: ATF6 in development and homeostasis. J Biomed Sci. 2018;25:48. doi: 10.1186/s12929-018-0453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraskiewicz H, FitzGerald U. InterfERing with endoplasmic reticulum stress. Trends Pharmacol Sci. 2012;33:53–63. doi: 10.1016/j.tips.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 25.Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69:169–181. doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 27.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 29.Sovolyova N, Healy S, Samali A, Logue SE. Stressed to death - mechanisms of ER stress-induced cell death. Biol Chem. 2014;395:1–13. doi: 10.1515/hsz-2013-0174. [DOI] [PubMed] [Google Scholar]
- 30.Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. 2011;22:4390–4405. doi: 10.1091/mbc.E11-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soni KK, Kim HK, Choi BR, Karna KK, You JH, Cha JS, et al. Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: reactive oxygen species and endoplasmic reticulum stress. Drug Des Devel Ther. 2016;10:3959–3968. doi: 10.2147/DDDT.S120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soni KK, Zhang LT, Choi BR, Karna KK, You JH, Shin YS, et al. Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1α (p-IRE1α) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways. Pharm Biol. 2018;56:94–103. doi: 10.1080/13880209.2017.1421672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang X, Zhang C, Xin Y, Huang Z, Tan Y, Huang Y, et al. Protective effect of FGF21 on type 1 diabetes-induced testicular apoptotic cell death probably via both mitochondrial- and endoplasmic reticulum stress-dependent pathways in the mouse model. Toxicol Lett. 2013;219:65–76. doi: 10.1016/j.toxlet.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Jiang C, Liu H, Guan Z, Zeng Q, Zhang C, et al. Fluoride-elicited developmental testicular toxicity in rats: roles of endoplasmic reticulum stress and inflammatory response. Toxicol Appl Pharmacol. 2013;271:206–215. doi: 10.1016/j.taap.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 38.Darbandi M, Darbandi S, Agarwal A, Sengupta P, Durairajanayagam D, Henkel R, et al. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol. 2018;16:87. doi: 10.1186/s12958-018-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50:e13126. doi: 10.1111/and.13126. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park YS, Lee SH, Choi HW, Lee HS, Lee JS, Seo JT. Abnormal human sperm parameters contribute to sperm DNA fragmentation in men with varicocele. World J Mens Health. 2018;36:239–247. doi: 10.5534/wjmh.180014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword. Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 43.Durairajanayagam D, Agarwal A, Ong C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod Biomed Online. 2015;30:14–27. doi: 10.1016/j.rbmo.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 44.Huo R, Zhu YF, Ma X, Lin M, Zhou ZM, Sha JH. Differential expression of glucose-regulated protein 78 during spermatogenesis. Cell Tissue Res. 2004;316:359–367. doi: 10.1007/s00441-004-0885-7. [DOI] [PubMed] [Google Scholar]
- 45.Huang KH, Weng TI, Huang HY, Huang KD, Lin WC, Chen SC, et al. Honokiol attenuates torsion/detorsion-induced testicular injury in rat testis by way of suppressing endoplasmic reticulum stress-related apoptosis. Urology. 2012;79:967.e5–967.e11. doi: 10.1016/j.urology.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 46.Beattie MC, Adekola L, Papadopoulos V, Chen H, Zirkin BR. Leydig cell aging and hypogonadism. Exp Gerontol. 2015;68:87–91. doi: 10.1016/j.exger.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang D, Wei W, Xie F, Zhu X, Zheng L, Lv Z. Steroidogenesis decline accompanied with reduced antioxidation and endoplasmic reticulum stress in mice testes during ageing. Andrologia. 2018;50 doi: 10.1111/and.12816. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Ma N, Liu Z, Wang T, Yuan C, He Y, et al. Protective effect of Wuzi Yanzong recipe on testicular dysfunction through inhibition of germ cell apoptosis in ageing rats via endoplasmic reticulum stress. Andrologia. 2019;51:e13181. doi: 10.1111/and.13181. [DOI] [PubMed] [Google Scholar]
- 49.Liu GL, Yu F, Dai DZ, Zhang GL, Zhang C, Dai Y. Endoplasmic reticulum stress mediating downregulated StAR and 3-beta-HSD and low plasma testosterone caused by hypoxia is attenuated by CPU86017-RS and nifedipine. J Biomed Sci. 2012;19:4. doi: 10.1186/1423-0127-19-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang GL, Dai DZ, Zhang C, Dai Y. Apocynin and raisanberine alleviate intermittent hypoxia induced abnormal StAR and 3β-HSD and low testosterone by suppressing endoplasmic reticulum stress and activated p66Shc in rat testes. Reprod Toxicol. 2013;36:60–70. doi: 10.1016/j.reprotox.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Rashid K, Sil PC. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim Biophys Acta. 2015;1852:70–82. doi: 10.1016/j.bbadis.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Tan Y, Dai J, Li B, Guo L, Cui J, et al. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett. 2011;200:100–106. doi: 10.1016/j.toxlet.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Zhao YT, Qi YW, Hu CY, Chen SH, Liu Y. Advanced glycation end products inhibit testosterone secretion by rat Leydig cells by inducing oxidative stress and endoplasmic reticulum stress. Int J Mol Med. 2016;38:659–665. doi: 10.3892/ijmm.2016.2645. [DOI] [PubMed] [Google Scholar]
- 54.Park SJ, Kim TS, Park CK, Lee SH, Kim JM, Lee KS, et al. hCG-induced endoplasmic reticulum stress triggers apoptosis and reduces steroidogenic enzyme expression through activating transcription factor 6 in Leydig cells of the testis. J Mol Endocrinol. 2013;50:151–166. doi: 10.1530/JME-12-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman MS, Kwon WS, Lee JS, Yoon SJ, Ryu BY, Pang MG. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci Rep. 2015;5:9169. doi: 10.1038/srep09169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabuchi Y, Takasaki I, Kondo T. Identification of genetic networks involved in the cell injury accompanying endoplasmic reticulum stress induced by bisphenol A in testicular Sertoli cells. Biochem Biophys Res Commun. 2006;345:1044–1050. doi: 10.1016/j.bbrc.2006.04.177. [DOI] [PubMed] [Google Scholar]
- 57.Qi S, Fu W, Wang C, Liu C, Quan C, Kourouma A, et al. BPA-induced apoptosis of rat Sertoli cells through Fas/FasL and JNKs/p38 MAPK pathways. Reprod Toxicol. 2014;50:108–116. doi: 10.1016/j.reprotox.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Yin L, Dai Y, Cui Z, Jiang X, Liu W, Han F, et al. The regulation of cellular apoptosis by the ROS-triggered PERK/EIF2α/chop pathway plays a vital role in bisphenol A-induced male reproductive toxicity. Toxicol Appl Pharmacol. 2017;314:98–108. doi: 10.1016/j.taap.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Jiang X, Chen HQ, Cui ZH, Yin L, Zhang WL, Liu WB, et al. Low-dose and combined effects of oral exposure to bisphenol A and diethylstilbestrol on the male reproductive system in adult Sprague-Dawley rats. Environ Toxicol Pharmacol. 2016;43:94–102. doi: 10.1016/j.etap.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Gong Y, Wu J, Huang Y, Shen S, Han X. Nonylphenol induces apoptosis in rat testicular Sertoli cells via endoplasmic reticulum stress. Toxicol Lett. 2009;186:84–95. doi: 10.1016/j.toxlet.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Duan P, Hu C, Quan C, Yu T, Zhou W, Yuan M, et al. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology. 2016;341-343:28–40. doi: 10.1016/j.tox.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 62.Ji YL, Wang H, Zhang C, Zhang Y, Zhao M, Chen YH, et al. N-acetylcysteine protects against cadmium-induced germ cell apoptosis by inhibiting endoplasmic reticulum stress in testes. Asian J Androl. 2013;15:290–296. doi: 10.1038/aja.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ji YL, Wang H, Zhao XF, Wang Q, Zhang C, Zhang Y, et al. Crosstalk between endoplasmic reticulum stress and mitochondrial pathway mediates cadmium-induced germ cell apoptosis in testes. Toxicol Sci. 2011;124:446–459. doi: 10.1093/toxsci/kfr232. [DOI] [PubMed] [Google Scholar]
- 64.Ji YL, Wang Z, Wang H, Zhang C, Zhang Y, Zhao M, et al. Ascorbic acid protects against cadmium-induced endoplasmic reticulum stress and germ cell apoptosis in testes. Reprod Toxicol. 2012;34:357–363. doi: 10.1016/j.reprotox.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 65.Zou L, Su L, Sun Y, Han A, Chang X, Zhu A, et al. Nickel sulfate induced apoptosis via activating ROS-dependent mitochondria and endoplasmic reticulum stress pathways in rat Leydig cells. Environ Toxicol. 2017;32:1918–1926. doi: 10.1002/tox.22414. [DOI] [PubMed] [Google Scholar]
- 66.Huang H, An Y, Jiao W, Wang J, Li S, Teng X. CHOP/caspase-3 signal pathway involves in mitigative effect of selenium on lead-induced apoptosis via endoplasmic reticulum pathway in chicken testes. Environ Sci Pollut Res Int. 2018;25:18838–18845. doi: 10.1007/s11356-018-1950-1. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, Lin X, Huang H, Feng D, Ba Y, Cheng X, et al. Sodium fluoride induces apoptosis through reactive oxygen species-mediated endoplasmic reticulum stress pathway in Sertoli cells. J Environ Sci (China) 2015;30:81–89. doi: 10.1016/j.jes.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Wang X, Liu T, Mo M, Ao L, Liu J, et al. ZnSO4 rescued vimentin from collapse in DBP-exposed Sertoli cells by attenuating ER stress and apoptosis. Toxicol In Vitro. 2018;48:195–204. doi: 10.1016/j.tiv.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 69.Zhang G, Liu K, Ling X, Wang Z, Zou P, Wang X, et al. DBPinduced endoplasmic reticulum stress in male germ cells causes autophagy, which has a cytoprotective role against apoptosis in vitro and in vivo. Toxicol Lett. 2016;245:86–98. doi: 10.1016/j.toxlet.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Wang ZC, Wang JF, Li YB, Guo CX, Liu Y, Fang F, et al. Involvement of endoplasmic reticulum stress in apoptosis of testicular cells induced by low-dose radiation. J Huazhong Univ Sci Technolog Med Sci. 2013;33:551–558. doi: 10.1007/s11596-013-1157-0. [DOI] [PubMed] [Google Scholar]
- 71.Zheng WL, Wang BJ, Wang L, Shan YP, Zou H, Song RL, et al. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins (Basel) 2018;10:E24. doi: 10.3390/toxins10010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin P, Chen F, Sun J, Zhou J, Wang X, Wang N, et al. Mycotoxin zearalenone induces apoptosis in mouse Leydig cells via an endoplasmic reticulum stress-dependent signalling pathway. Reprod Toxicol. 2015;52:71–77. doi: 10.1016/j.reprotox.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 73.Soni KK, Zhang LT, You JH, Lee SW, Kim CY, Cui WS, et al. The effects of MOTILIPERM on cisplatin induced testicular toxicity in Sprague-Dawley rats. Cancer Cell Int. 2015;15:121. doi: 10.1186/s12935-015-0274-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shati AA. Resveratrol improves sperm parameter and testicular apoptosis in cisplatin-treated rats: effects on ERK1/2, JNK, and Akt pathways. Syst Biol Reprod Med. 2019;65:236–249. doi: 10.1080/19396368.2018.1541114. [DOI] [PubMed] [Google Scholar]
- 75.Yang F, Wei Y, Liao B, Wei G, Qin H, Pang X, et al. Lycium barbarum polysaccharide prevents cisplatin-induced MLTC-1 cell apoptosis and autophagy via regulating endoplasmic reticulum stress pathway. Drug Des Devel Ther. 2018;12:3211–3219. doi: 10.2147/DDDT.S176316. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Karna KK, Choi BR, You JH, Shin YS, Soni KK, Cui WS, et al. Cross-talk between ER stress and mitochondrial pathway mediated adriamycin-induced testicular toxicity and DA-9401 modulate adriamycin-induced apoptosis in Sprague-Dawley rats. Cancer Cell Int. 2019;19:85. doi: 10.1186/s12935-019-0805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cui Y, Ren L, Li B, Fang J, Zhai Y, He X, et al. Melatonin relieves busulfan-induced spermatogonial stem cell apoptosis of mouse testis by inhibiting endoplasmic reticulum stress. Cell Physiol Biochem. 2017;44:2407–2421. doi: 10.1159/000486165. [DOI] [PubMed] [Google Scholar]
- 78.Soni KK, Shin YS, Choi BR, Karna KK, Kim HK, Lee SW, et al. Protective effect of DA-9401 in finasteride-induced apoptosis in rat testis: inositol requiring kinase 1 and c-Jun N-terminal kinase pathway. Drug Des Devel Ther. 2017;11:2969–2979. doi: 10.2147/DDDT.S140543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu Z, Xu W, Dai J, Chen X, Zhao X, Fang P, et al. The alteration of protein profile induced by cigarette smoking via oxidative stress in mice epididymis. Int J Biochem Cell Biol. 2013;45:571–582. doi: 10.1016/j.biocel.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Ying X, Liu Y, Guo Q, Qu F, Guo W, Zhu Y, et al. Endoplasmic reticulum protein 29 (ERp29), a protein related to sperm maturation is involved in sperm-oocyte fusion in mouse. Reprod Biol Endocrinol. 2010;8:10. doi: 10.1186/1477-7827-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakamura M, Moriya M, Baba T, Michikawa Y, Yamanobe T, Arai K, et al. An endoplasmic reticulum protein, calreticulin, is transported into the acrosome of rat sperm. Exp Cell Res. 1993;205:101–110. doi: 10.1006/excr.1993.1063. [DOI] [PubMed] [Google Scholar]
- 82.Nagamori I, Yabuta N, Fujii T, Tanaka H, Yomogida K, Nishimune Y, et al. Tisp40, a spermatid specific bZip transcription factor, functions by binding to the unfolded protein response element via the Rip pathway. Genes Cells. 2005;10:575–594. doi: 10.1111/j.1365-2443.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 83.Nagamori I, Yomogida K, Ikawa M, Okabe M, Yabuta N, Nojima H. The testes-specific bZip type transcription factor Tisp40 plays a role in ER stress responses and chromatin packaging during spermiogenesis. Genes Cells. 2006;11:1161–1171. doi: 10.1111/j.1365-2443.2006.01013.x. [DOI] [PubMed] [Google Scholar]