Abstract
Purpose
To examine the therapeutic effect of Vactosertib, a small molecule inhibitor of transforming growth factor-β (TGF-β) type I receptor (activin receptor-like kinase-5, ALK5), in an experimental model of Peyronie's disease (PD) and determining anti-fibrotic mechanisms of Vactosertib in primary fibroblasts derived from human PD plaques.
Materials and Methods
Male rats were randomly divided into three groups (n=6 per group); control rats without treatment; PD rats receiving vehicle; and PD rats receiving Vactosertib (10 mg/kg). PD-like plaques were induced by administering 100 µL of each of human fibrin and thrombin solutions into the tunica albuginea on days 0 and 5. Vactosertib was given orally five times a week for 2 weeks. On day 30, we performed electrical stimulation of the cavernous nerve to measure erectile function, and the penis was obtained for histological examination. Fibroblasts isolated from human PD plaques were used to determine the anti-fibrotic effects of Vactosertib in vitro.
Results
Vactosertib induced significant regression of fibrotic plaques in PD rats in vivo through reduced infiltration of inflammatory cells and reduced expression of phospho-Smad2, which recovered erectile function. Vactosertib also abrogated TGF-β1-induced enhancement of extracellular matrix protein production and hydroxyproline content in PD fibroblasts in vitro by hindering the TGF-β1-induced Smad2/3 phosphorylation and nuclear translocation, and fibroblast-to-myofibroblast transdifferentiation.
Conclusions
In view of the critical role of TGF-β and the Smad pathway in the pathogenesis of PD, inhibition of this pathway with an ALK5 inhibitor may represent a novel, targeted therapy for PD.
Keywords: Activin receptors, Fibrosis, Peyronie's disease, Transforming growth factor beta
INTRODUCTION
Peyronie's disease (PD) is characterized by excessive fibrosis and plaque formation in the tunica albuginea (TA), resulting penile deformities and pain on erectile status [1,2]. Inflammatory process and dysregulated wound healing following repeated trauma to the penis resulting from sexual intercourse are known to be responsible for fibrotic processes [3,4]. Despite promising clinical results with intralesional injection of collagenase clostridium histolyticum [5], surgical intervention is still the mainstay of treatment for PD [6,7]. Therefore, novel medical treatments are needed, and they may emerge from our increased knowledge of this disorder at the cellular and molecular levels.
Transforming growth factor-β1 (TGF-β1), a member of TGF-β superfamily, is known as a potent pro-fibrotic factor and is upregulated in human PD plaques [8,9,10]. Previous studies have shown that the expression and activity of the Smad transcriptional factors are increased in primary cultured fibroblast [9] and in fibrotic plaque tissues from PD patients [10]. TGF-β1 has also been regarded as a key component related to tissue fibrosis in a variety of organs [11]. The profibrotic effects of TGF-β1 include increased synthesis of extracellular matrix proteins and the induction of myofibrotic differentiation [11]. The desire to block TGF-β1 signaling has led to the development of several small molecule inhibitors that block the binding of TGF-β1 to its receptors. We recently observed that antagonizing TGF-β signaling through the use of a small molecule inhibitor of activin receptor-like kinase 5 (ALK5) (IN-1130), a TGF-β type I receptor, induced the regression of fibrotic plaques in PD rats in vivo [12]. However, the oral bioavailability of IN-1130 and its kinase selectivity against p38α, one of the most homologous kinase domains to that of ALK5, is relatively low in mice and rats [13], which greatly limits study for its clinical application.
Recently, our colleague developed a novel ALK5 inhibitor, N ((4-([1,2,4]triazolo[1,5 a]pyridin-6-yl)-5-(6-methylpyridin-2-yl) 1H imidazol-2-yl)methyl)-2-fluoroaniline (Vactosertib; Medpacto Inc., Seoul, Korea), as an anti-fibrotic or cancer immunotherapeutic agent, which is a highly potent, selective, and orally bioavailable ALK5 inhibitor [13]. The safety and efficacy of Vactosertib has been documented in animal models of hepatic, renal, and pulmonary fibrosis [14,15,16]. Because of the favorable pharmacologic, pharmacokinetic, and toxicologic profiles of Vactosertib, a first-in-human dose escalation study of Vactosertib in subjects with advanced-stage solid tumors is in progress in the United States (NCT02160106, National Institutes of Health [NIH]).
In the present study, the anti-fibrotic effects of Vactosertib were determined in a rat model of PD induced by multiple intratunical administration of fibrin and in primary cultured fibroblasts derived from human PD plaques.
MATERIALS AND METHODS
1. Animals and study design
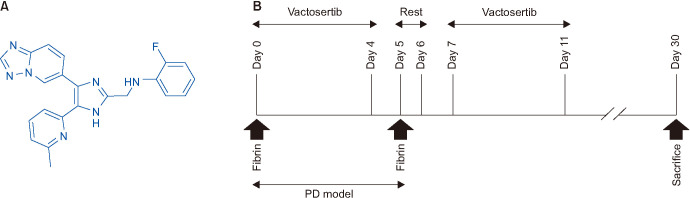
Four-month-old male Sprague-Dawley (SD) rats were used in this study. To establish an optimal condition to generate PD-like plaques, rats received repeated injections of fibrin (Greenplast; Green Cross Co., Yongin, Korea) into the TA at 100 µL of each of human fibrin and thrombin solutions on days 0 and 5 as we previously described [17]. Then, we determined the efficacy of Vactosertib (molecular weight, 399.42) in a rat model of PD. The chemical structure of Vactosertib and the experimental design are illustrated in Fig. 1.
Fig. 1. The chemical structure of Vactosertib and study design. (A) Structures of Vactosertib. (B) Study protocol. Rats received injections consist of 100 µL of human fibrin and 100 µL thrombin solutions on days 0 and 5, respectively into the tunica albuginea to induced Peyronie's disease (PD)-like plaques. Vactosertib (10 mg/kg) or vehicle (simulated gastric fluid) was given orally five times a week for 2 weeks. On day 30, erectile function was assessed, and erectile tissue was harvested for histological examination.
Age-matched SD rats were randomly divided into the following three groups (n=6 per group): control without treatment; PD rats receiving vehicle; and PD rats receiving Vactosertib (10 mg/kg). Vactosertib was dissolved in an artificial gastric fluid formulation consisting of 7 mL of 37% hydrochloric acid, 2.0 g of NaCl, and 3.2 g of pepsin in 1,000 mL of water. The artificial gastric fluid was used as a vehicle. Vactosertib or vehicle was given orally five times a week for 2 weeks. Evaluation of erectile function was done at day 30 by cavernous nerve electrical stimulation. The penis was then harvested for histologic examination.
2. Measurement of erectile function
Rats were anesthetized with ketamine (100 mg/kg) and xylazine (5 mg/kg) intramuscularly. Erectile function was measured as we previously described [18]. The platinum wire electrodes were placed around the cavernous nerve under the dissecting microscope (Zeiss, Göttingen, Germany). Electrical stimulation was performed with 3 V current, 12 Hz pulse frequency, and 1 ms pulse width for 1 minute duration. During tumescence, the maximal intracavernous pressure (ICP) and total ICP were recorded. The total ICP of tumescence, expressed by area under the curve, was measured from the beginning of cavernous nerve stimulation to 20 seconds after stimulus termination. Noninvasive computerized tail-cuff system was used for measuring systemic blood pressure (BP-2000; Visitech Systems, Apex, NC, USA). The ratios of maximum ICP to mean systolic blood pressure (MSBP) and total ICP to MSBP were calculated to normalize the variations in systemic blood pressure.
3. Histological examinations
A midportion of penile segment was collected and fixed in 10% formalin phosphate-buffered solution (PBS) immediately before paraffin embedding. The sections were stained with H&E. The collagen deposition and disorganization in the fibrotic plaque were determined with Masson trichrome staining. Quantitative image analysis of plaque area was performed using image processing program (NIH ImageJ 1.34; http://rsbweb.nih.gov/ij/), and we analyzed two sections from each animal. The plaque area of the vehicle-treated PD group was arbitrarily set to 100%, and the area of each group was expressed as a percentage of the vehicle-treated PD group.
For immunohistochemistry, tissue sections were incubated with an antibody against vimentin (a fibroblast marker; Sigma-Aldrich, St. Louis, MO, USA; 1:100) or phospho-Smad2 (p-Smad2; Cell Signaling, Beverly, MA, USA; 1:100), followed by processing with a Histostain®-Plus Bulk Kit (Zymed Laboratories, San Francisco, CA, USA).
4. Primary fibroblast culture
The plaque tissue samples were collected from patients with PD who underwent surgical correction after obtaining informed consent from all the subjects. All of procedures were performed in accordance with relevant approved guidelines by the Inha University Institutional Review Board (IRB No. 2007-730). Fibroblasts were isolated from human PD plaques as previously described [10].
5. Western blot analysis
The fibroblasts were cultured in serum free media and pretreated for 24 hours with Vactosertib (400 ng/mL). After 24 hours, the fibroblasts then treated for 48 hours with 10 ng/mL TGF-β1 (R&D Systems Inc., Minneapolis, MN, USA) to detect the protein expression of collagen subtypes, plasminogen activator inhibitor-1 (PAI-1), fibronectin, and smooth muscle α-actin or treated with TGF-β1 for 1 hour to observe the protein expression of p-Smad2, phospho-Smad3 (p-Smad3), total Smad2/3, phospho-p38 (p-p38), and total p38. Equivalent amount of protein from whole-cell lysate (50 µg/lane) were electrophoresed on 8% sodium dodecyl sulphate-polyacrylamide gels before transferred to nitrocellulose membranes. The membranes were probed with antibodies against PAI-1 (Abcam, Cambridge, UK; 1:300), fibronectin (Abcam; 1:300), collagen I (Abcam; 1:300), collagen III (Abcam; 1:300), collagen IV (Abcam; 1:300), smooth muscle α-actin (Sigma-Aldrich; 1:300), p-Smad2 (Ser465/Ser467, Cell Signaling; 1:200), p-Smad3 (Ser423/Ser425, Cell Signaling; 1:200), Smad2/3 (Cell Signaling; 1:200), p-p38 (Thr180/Tyr182, Cell Signaling; 1:200), p38(Cell Signaling; 1:200), or β-actin (Abcam). The results were quantified by densitometry.
6. Fluorescent immunocytochemistry
The fibroblasts were cultured on sterile coverslips (Marienfeld Laboratory, Lauda-Königshofen, Germany) and grown until nearly confluent. The cells were rinsed with PBS for three times and then fixed in 4% paraformaldehyde and 100% methanol for 10 minutes at 4℃. Cells in single chambered slide were incubated with antibodies against F-actin (a filamentous cytoskeleton; Life technologies, Eugene, OR, USA; 1:300), PAI-1 (Abcam; 1:300), fibronectin (Abcam; 1:300), collagen I (Abcam; 1:300), collagen IV (Abcam; 1:300), smooth muscle α-actin (Sigma-Aldrich; 1:300), or Smad2/3 (Cell Signaling; 1:200) overnight at 4℃ in a moist chamber. After several washes with PBS, the slides were incubated with fluorescein isothiocyanate-conjugated (Zymed Laboratories, South San Francisco, CA, USA; 1:300) or tetramethyl rhodamine isothiocyanate-conjugated (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA; 1:300) secondary antibodies for 2 hours at room temperature. Slides were cover slipped with mounting medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA, USA) to label nuclei. Fluorescence signals were visualized and images were taken using FV1000 confocal microscope (Olympus, Tokyo, Japan) under identical exposure settings.
7. Hydroxyproline assay
Collagen protein levels were estimated by hydroxyproline determinations as previously described [19]. Briefly, aliquots of standard hydroxyproline or fibroblast samples were hydrolyzed in an alkali solution and mixed with a buffered chloramine-T reagent for 25 minutes at room temperature. Ehrlich's reagent was added to produce the chromophore. Using a spectrophotometer, the reddish-purple complex of absorbance was measured at 550 nm. Absorbance measurement was recorded and plotted against the concentration of standard hydroxyproline. The concentration of hydroxyproline in fibroblast lysates was calculated based on the standard curve created for each microplate.
8. Statistical analysis
The results of each measurement are represented by the means±standard errors. Comparisons of parametric data between groups were performed by one-way analysis of variance followed by Newman-Keuls post hoc tests for the multiple comparisons and Kruskal-Wallis tests were used for nonparametric data. Statistical analysis was done with Sigmastat v3.5 software (Systat Software Inc., Richmond, CA, USA). The data were tested for its normality and homogeneity variance. The p-values less than 5% were considered as statistically significant.
9. Ethics statement
All experiments were conducted in accordance with laboratory animal and institutional animal care guidelines. Protocols used in this study were approved by the Institutional Animal Care and Use Subcommittee (IACUC) of Inha University (IACUC No. 170126-477). All tissue donors provided informed consent, and the experiments were approved by the Ethics Committee and the Institutional Review Board of Inha University (No. 2007-730).
RESULTS
1. Vactosertib promotes the regression of fibrotic plaques in Peyronie's disease rats through reduced inflammatory cell infiltration
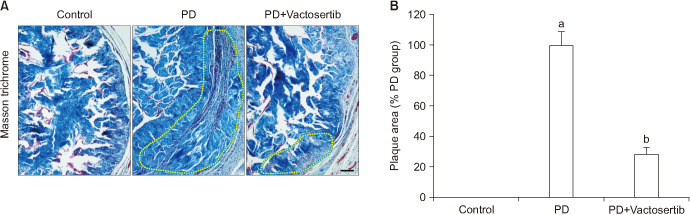
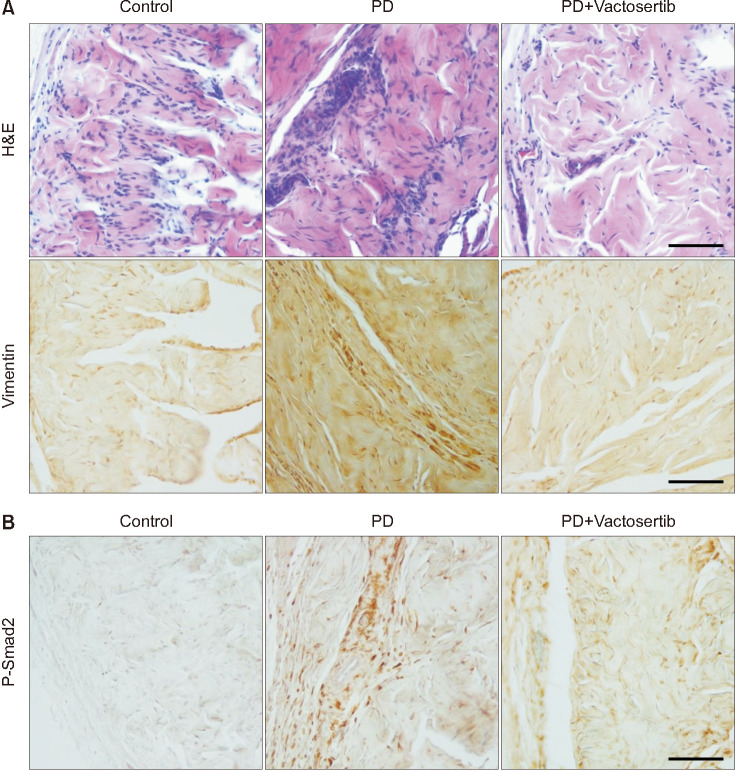
Repeated intratunical injections of fibrin induced fibrotic scarring in the TA. PD rats treated with Vactosertib showed a remarkable regression of fibrotic plaques and improved the disorganization of collagen distribution (Fig. 2). H&E and immunohistochemical staining for vimentin revealed infiltration of lymphocytes and fibroblasts in fibrotic plaques of the vehicle-treated PD rats. Oral administration of Vactosertib significantly decreased inflammatory cell infiltration (Fig. 3A).
Fig. 2. Vactosertib promotes the regression of fibrotic plaques. (A) Representative figures of Masson trichrome staining in age-matched controls and Peyronie's disease (PD) rats that received Vactosertib (10 mg/kg) or vehicle. Dotted line represents the area of fibrotic plaques. Scale bar indicates 100 µm. (B) Quantification of the plaque areas. An image analyzer was used to quantitate the plaque areas. The area of the vehicle-treated PD group was arbitrarily set to 100%. Each bar depicts the mean value (±standard errors) of n=6 animals per group. ap<0.01, compared with the control group; bp<0.01, compared with the PD+Vactosertib group.
Fig. 3. Vactosertib reduces inflammatory cell infiltration and suppresses the expression of phospho-Smad2 (p-Smad2). (A) Representative images of H&E staining and immunohistochemical staining for vimentin in age-matched controls and Peyronie's disease (PD) rats that received Vactosertib (10 mg/kg) or vehicle. (B) Representative immunohistochemical staining for p-Smad2 in each group. Scale bar indicates 100 µm (H&E staining and immunostaining for vimentin and p-Smad2). The results were similar among four independent experiments.
2. Vactosertib suppresses the transnuclear expression and phosphorylation of Smad2/3 in Peyronie's disease rats in vivo and in human Peyronie's disease fibroblasts in vitro
To examine the effect of Vactosertib on the TGF-β signaling pathway, we performed immunohistochemical staining for the phosphorylated form of Smad2. P-Smad2 immunoreactivity was weak in control rats. Strong expression of p-Smad2 was noted in fibrotic plaques in the vehicle-treated PD rats, whereas the expression was reduced significantly in PD rats treated with Vactosertib (Fig. 3B).
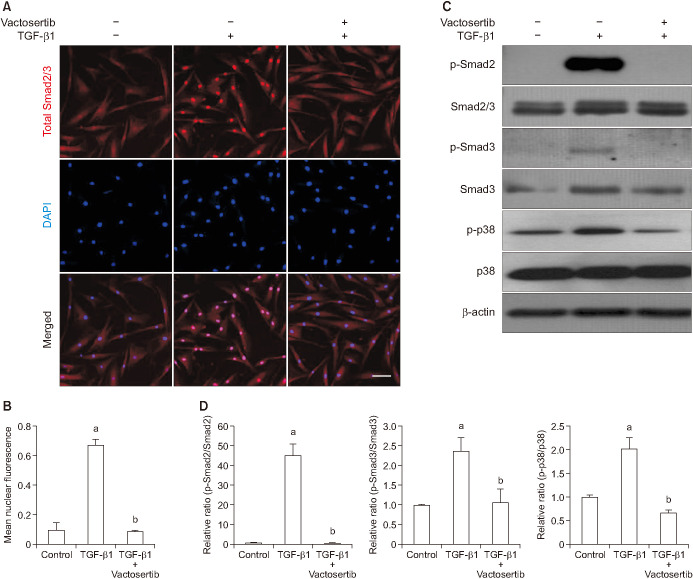
We also performed immunofluorescent staining of fibroblasts with an antibody against total Smad2 (which also recognizes Smad3) to determine whether ALK5 mediates TGF-β1-induced nuclear translocation of Smad2/3. Vactosertib significantly reduced TGF-β1-induced nuclear accumulation of Smad proteins (Fig. 4A, 4B). Western blot analysis revealed a high level of p-Smad2 and p-Smad3 in response to a 1-hour treatment with TGF-β1. Smad2 and Smad3 phosphorylation was profoundly inhibited by preincubation with Vactosertib (Fig. 4C, 4D).
Fig. 4. Vactosertib suppresses Smad2 and Smad3 phosphorylation and nuclear translocation. Fibroblasts were pretreated for 24 hours with Vactosertib (400 ng/mL) then exposed with transforming growth factor-β (TGF-β1) (10 ng/mL) for 1 hour. (A) Representative fluorescent immunocytochemistry of primary human fibroblasts cells stained with antibodies against total Smad2/3. Nuclei was dyed using DNA dye, DAPI (4,6-diamidino-2-phenylindole). Scale bar indicates 100 µm. (B) Nuclear fluorescence signal was quantified for all cells. Each bar depicts the mean value (±standard errors) of four experiments per group. ap<0.05, compared with the control group; bp<0.05, compared with the TGF-β1 group. (C) Representative Western blot analysis for phosphorylated Smad2 (p-Smad2), phosphorylated Smad3 (p-Smad3), total Smad2/3, phospho-p38 (p-p38), and p38. (D) Data are expressed as the ratio of phosphorylated protein/total protein. The data measured relative to the control group ratio is arbitrarily presented as 1. Each bar depicts the mean value (±standard errors) of four experiments per group. ap<0.05, compared with the control group; bp<0.05, compared with the TGF-β1 group.
p38 kinase is a family of mitogen-activated protein (MAP) kinases that phosphorylate Smad2 [20]. Vactosertib significantly reduced TGF-β1-induced phosphorylation of p38 (Fig. 4C, 4D).
3. Vactosertib inhibits myofibroblastic differentiation induced by transforming growth factor-β1 in human Peyronie's disease fibroblasts
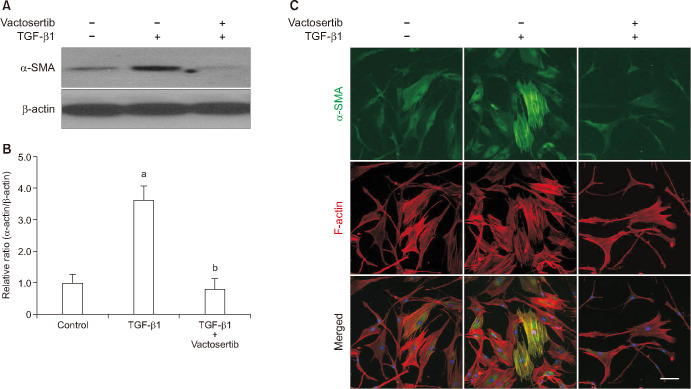
The expression of smooth muscle α-actin, a marker for myofibroblasts, at the protein level was determined by Western blot and immunocytochemistry. Treatment of PD fibroblasts with TGF-β1 resulted in an increase in α-actin fiber formation, which was attenuated by treatment with Vactosertib (Fig. 5).
Fig. 5. Vactosertib inhibits transforming growth factor-β (TGF-β1)-induced myofibroblastic differentiation in fibroblasts. Fibroblasts were pretreated for 24 hours with Vactosertib (400 ng/mL) then exposed with TGF-β1 (10 ng/mL) for 48 hours. (A) Representative Western blot for alpha smooth muscle actin (α-SMA) expression in fibroblasts. (B) Data are presented as relative density of α-SMA protein normalized to β-actin. Each bar depicts the mean value (±standard errors) of four experiments per group. The data measured relative to the control group ratio is arbitrarily presented as 1. ap<0.05, compared with the control group; bp<0.05, compared with the TGF-β1 group. (C) Representative fluorescent immunocytochemistry of fibroblasts with antibodies against α-SMA and F-actin. Scale bar indicates 100 µm. The results were similar among four independent experiments.
4. Vactosertib decreases the production of hydroxyproline and extracellular matrix proteins induced by transforming growth factor-β1 in human Peyronie's disease fibroblasts
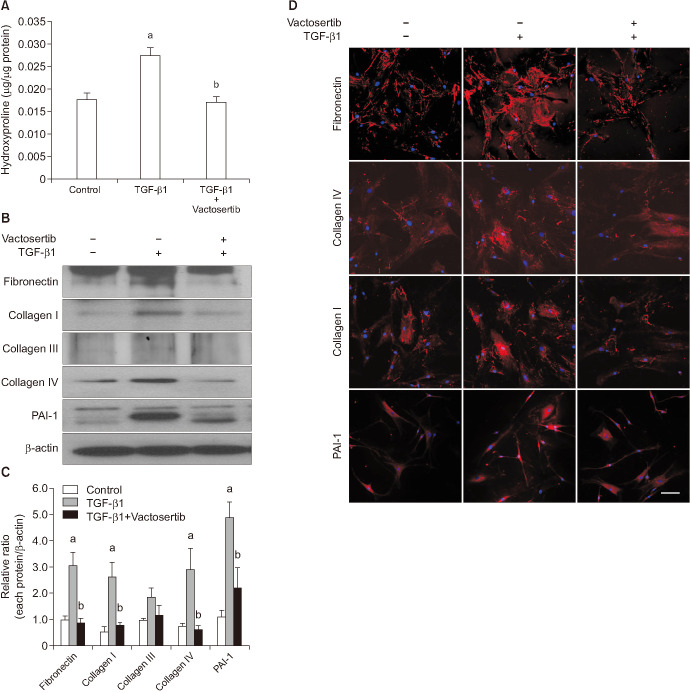
The amount of hydroxyproline was measured to determine total collagen content. The hydroxyproline content in TGF-β1-treated fibroblasts was significantly increased compared with that in untreated fibroblasts. Collagen production induced by TGF-β1 was significantly reduced by Vactosertib treatment (Fig. 6A).
Fig. 6. Vactosertib decreases transforming growth factor-β (TGF-β1)-induced production of hydroxyproline and extracellular matrix proteins. Fibroblasts were pretreated for 24 hours with Vactosertib (400 ng/mL) then exposed with TGF-β1 (10 ng/mL) for 48 hours. (A) Hydroxyproline assay. Each bar depicts the mean value (±standard errors) of four experiments per group. ap<0.05, compared with the control group; bp<0.05, compared with the TGF-β1 group. (B) Representative Western blot for plasminogen activator inhibitor-1 (PAI-1), collagen I, collagen III, collagen IV, and fibronectin expressions in fibroblasts. (C) Data are presented as the relative density of each protein normalized to β-actin. Each bar depicts the mean value (±standard errors) of four experiments per group. ap<0.05, compared with the control group; bp<0.05, compared with the TGF-β1 group. (D) Representative fluorescent immunocytochemistry of fibroblasts with antibodies against PAI-1, fibronectin, collagen I, and collagen IV. Nuclei were stained with DAPI (4,6-diamidino-2-phenylindole). Scale bar indicates 100 µm. The results were similar among four independent experiments.
Both Western blot analysis and fluorescent immunocytochemistry showed that Vactosertib significantly inhibited TGF-β1-induced production of extracellular matrix proteins, such as PAI-1, fibronectin, collagen I, and collagen IV in PD fibroblasts (Fig. 6B–6D). However, no significant difference was noted in collagen III expression among three experimental groups (Fig. 6B, 6C).
5. Vactosertib restores erectile function in Peyronie's disease rats
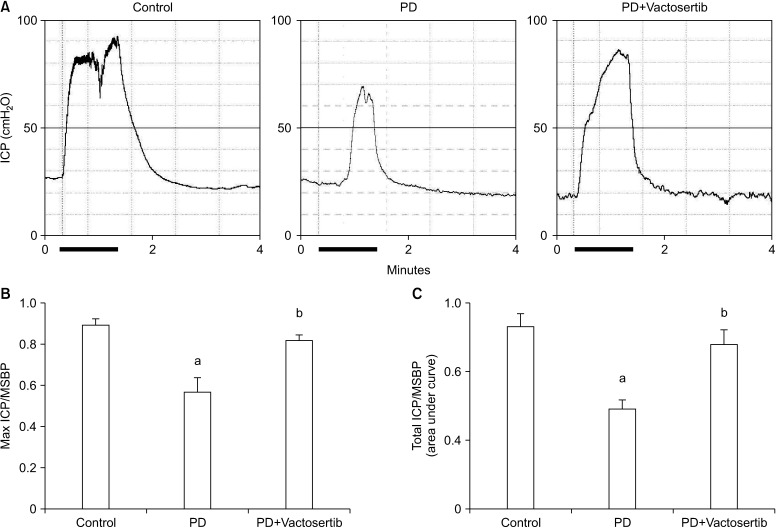
Representative ICP traces during stimulation of the cavernous nerve with 3 V current, 12 Hz pulse frequency, and 1 ms pulse width in age-matched control and PD rats treated with vehicle or Vactosertib are shown in Fig. 7A. The ratios of maximal ICP and total ICP to MSBP were significantly lower in the vehicle-treated PD rats than in age-matched controls. Oral administration of Vactosertib completely restored erectile function in PD rats (Fig. 7B, 7C).
Fig. 7. Vactosertib restores erectile function in Peyronie's disease (PD) rats. (A) Representative intracavernous pressure (ICP) responses in age-matched controls and PD rats that received Vactosertib (10 mg/kg) or vehicle. Stimulus interval is indicated by the solid bar (1 minute). (B, C) Ratios of the mean maximal ICP/mean systolic blood pressure (MSBP) and total ICP (area under the curve) to MSBP. Each bar depicts the mean value (±standard errors) of n=6 animals per group. ap<0.05, compared with the control group; bp<0.05, compared with the PD+Vactosertib group.
DISCUSSION
We showed here that oral administration of Vactosertib decreased infiltration of inflammatory cells, suppressed the expression of p-Smad2, and ameliorated tunica fibrosis in PD rats in vivo. Furthermore, Vactosertib also diminished TGF-β1-induced extracellular matrix production and hydroxyproline content in human PD fibroblasts in vitro by blocking TGF-β1-induced nuclear translocation of Smad2 and Smad3, and by inhibiting TGF-β1-induced differentiation of fibroblasts into myofibroblasts. Finally, sVactosertib induced a significant recovery of erectile function in PD rats.
In the present study, Vactosertib significantly reduced infiltration of inflammatory cells in PD rats. Local delivery of fibrin glue into the TA increases the expression of TGF-β1 in the fibrotic area [21]. TGF-β is mitogenic for inflammatory cells and promotes the accumulation of extracellular matrix proteins [22]. Moreover, TGF-β is produced by multiple cell types, including fibroblasts, myofibroblasts, and lymphocytes [23,24]. Vactosertib also attenuated TGF-β1-induced Smad2/3 phosphorylation and nuclear translocation in human PD fibroblasts in vitro, and decreased p-Smad2 expression in PD rats in vivo, which results in a decrease in the production of extracellular matrix proteins. Therefore, reduced infiltration of inflammatory cells and blockage of the TGF-β signaling pathway may play a major role in the Vactosertib-mediated regression of fibrosis.
A previous study in renal epithelial cells reported that TGF-β1 can regulate extracellular matrix genes by several mechanisms, including the Smad pathway, the p38 MAP kinase pathway, and a combination of the two, as well as other kinase pathways [25]. In the present study, Vactosertib also abrogated TGF-β1-induced phosphorylation of p38. From this finding, we believe that antifibrotic therapies aimed at inhibiting both the Smad and p38 MAP kinase pathways would be more effective than blocking a single pathway.
TGF-β activates Smad2 and Smad3 upon ligand binding, which in turn induces myofibroblastic differentiation and promotes the synthesis of extracellular matrix proteins [26]. In the present study, treating PD fibroblasts with TGF-β1 induced myofibroblastic differentiation, as evidenced by both Western blot analysis and fluorescent immunocytochemistry for smooth muscle α-actin. Vactosertib suppressed the TGF-β1-induced fibroblast-to-myofibroblast transition. Because myofibroblasts are the main cell type that responsible for the production of extracellular matrix proteins, blocking myofibroblastic differentiation is another important mechanism responsible for the Vactosertib-mediated amelioration of fibrotic processes.
Previous study reported that erectile function was impaired in elastin haploinsufficient mice [27], indicating importance of elastin component in normal penile erection. We previously reported that a small molecule inhibitor of ALK5 (IN-1130) restored elastin fibers in PD rats in vivo [12]. However, we did not measure the effects of Vactosertib on elastin fibers and this is a limitation of our study.
Small molecule inhibitors of ALK5, such as SB-505124, SB-525334, GW6604, SD-208, and LY-2157299, were devised to directly block the catalytic activity of ALK5 [13]. Among them, LY-2157299 has progressed to phase II clinical trials for various cancers [28]. A preclinical study in 4-week-old rats with Vactosertib at doses up to 120 mg/kg revealed little toxicity. The maximum tolerated dosage of Vactosertib in rats appeared to be 50 mg/kg/d for males and 20 mg/kg/d for females. Notably, a daily dose of 2.5 mg/kg of Vactosertib was more efficacious than a daily dose of 150 mg/kg of LY-2157299 against melanoma [29]. Additionally, Vactosertib prolonged the lifespan of the hepatic fibrosis model induced by carbon tetrachloride or bile duct ligation as well as the bleomycin-induced pulmonary fibrosis model [13,16]. Currently, phase I clinical trials for patients with advanced solid tumors (NCT02160106, NIH) and phase 1/2 clinical trials in myelodysplastic syndrome (NCT03074006, NIH) have been initiated to investigate the safety, tolerability, and efficacy of Vactosertib. However, no anti-TGF-β therapy is currently available for fibrotic diseases. Therefore, further clinical trials are necessary to validate the therapeutic potential of Vactosertib in patients with PD. Moreover, additional studies are needed to address whether Vactosertib induces the regression of fibrotic plaques when administered at a chronic or stable phase of disease.
CONCLUSIONS
Vactosertib promoted the regression of tunica fibrosis by blocking the TGF-β pathway and inhibiting infiltration of inflammatory cell in PD rats in vivo. Vactosertib also abrogated TGF-β1-induced extracellular matrix production and differentiation of fibroblasts into myofibroblasts by blocking the TGF-β1-induced activation of the Smad2/3 pathway in human PD fibroblasts in vitro. Inhibition of the TGF-β pathway via a novel ALK5 inhibitor, especially as an orally bioavailable formulation, may represent a viable therapeutic strategy for the treatment of PD.
ACKNOWLEDGEMENTS
This work was supported by a National Research Foundation of Korea (NRF) grant (Jun-Kyu Suh, 2016R1A2B4013130) and by a Medical Research Center grant (Ji-Kan Ryu, 2014R1A5A2009392) funded by the Korean government (Ministry of Science, ICT and Future Planning).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: KMS, DYC, JKR, JKS.
- Data curation: KMS, MJC, KG, NNM, AL.
- Formal analysis: DYC, MHK, JO, GNY.
- Methodology: DKK.
- Software: MJC, MHK.
- Funding acquisition: JKS, JKR.
- Writing—original draft: KMS, DYC, JKR.
- Writing—review & editing: JKS.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/BHEFNR.
References
- 1.Gholami SS, Gonzalez-Cadavid NF, Lin CS, Rajfer J, Lue TF. Peyronie's disease: a review. J Urol. 2003;169:1234–1241. doi: 10.1097/01.ju.0000053800.62741.fe. [DOI] [PubMed] [Google Scholar]
- 2.Hellstrom WJ, Bivalacqua TJ. Peyronie's disease: etiology, medical, and surgical therapy. J Androl. 2000;21:347–354. [PubMed] [Google Scholar]
- 3.Devine CJ, Jr, Somers KD, Jordan SG, Schlossberg SM. Proposal: trauma as the cause of the Peyronie's lesion. J Urol. 1997;157:285–290. doi: 10.1016/s0022-5347(01)65361-8. [DOI] [PubMed] [Google Scholar]
- 4.Jarow JP, Lowe FC. Penile trauma: an etiologic factor in Peyronie's disease and erectile dysfunction. J Urol. 1997;158:1388–1390. doi: 10.1016/s0022-5347(01)64222-8. [DOI] [PubMed] [Google Scholar]
- 5.Sun AJ, Li S, Eisenberg ML. The impact of clostridium histolyticum collagenase on the prevalence and management of Peyronie's disease in the United States. World J Mens Health. 2019;37:234–239. doi: 10.5534/wjmh.180073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung E. Penile reconstructive surgery in Peyronie disease: challenges in restoring normal penis size, shape, and function. World J Mens Health. 2018 doi: 10.5534/wjmh.170056. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joice GA, Burnett AL. Nonsurgical interventions for Peyronie's disease: update as of 2016. World J Mens Health. 2016;34:65–72. doi: 10.5534/wjmh.2016.34.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sakka AI, Hassoba HM, Pillarisetty RJ, Dahiya R, Lue TF. Peyronie's disease is associated with an increase in transforming growth factor-beta protein expression. J Urol. 1997;158:1391–1394. [PubMed] [Google Scholar]
- 9.Haag SM, Hauck EW, Szardening-Kirchner C, Diemer T, Cha ES, Weidner W, et al. Alterations in the transforming growth factor (TGF)-beta pathway as a potential factor in the pathogenesis of Peyronie's disease. Eur Urol. 2007;51:255–261. doi: 10.1016/j.eururo.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Piao S, Choi MJ, Tumurbaatar M, Kim WJ, Jin HR, Shin SH, et al. Transforming growth factor (TGF)-β type I receptor kinase (ALK5) inhibitor alleviates profibrotic TGF-β1 responses in fibroblasts derived from Peyronie's plaque. J Sex Med. 2010;7:3385–3395. doi: 10.1111/j.1743-6109.2010.01753.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Wang DR, Cao YL. TGF-beta: a fibrotic factor in wound scarring and a potential target for anti-scarring gene therapy. Curr Gene Ther. 2004;4:123–136. doi: 10.2174/1566523044578004. [DOI] [PubMed] [Google Scholar]
- 12.Ryu JK, Piao S, Shin HY, Choi MJ, Zhang LW, Jin HR, et al. IN-1130, a novel transforming growth factor-beta type I receptor kinase (activin receptor-like kinase 5) inhibitor, promotes regression of fibrotic plaque and corrects penile curvature in a rat model of Peyronie's disease. J Sex Med. 2009;6:1284–1296. doi: 10.1111/j.1743-6109.2009.01216.x. [DOI] [PubMed] [Google Scholar]
- 13.Jin CH, Krishnaiah M, Sreenu D, Subrahmanyam VB, Rao KS, Lee HJ, et al. Discovery of N-((4-([1,2,4]Triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazol-2-yl)methyl)-2-fluoroaniline (EW-7197): a highly potent, selective, and orally bioavailable inhibitor of TGF-β type I receptor kinase as cancer immunotherapeutic/antifibrotic agent. J Med Chem. 2014;57:4213–4238. doi: 10.1021/jm500115w. [DOI] [PubMed] [Google Scholar]
- 14.Jun EJ, Park JH, Tsauo J, Yang SG, Kim DK, Kim KY, et al. EW-7197, an activin-like kinase 5 inhibitor, suppresses granulation tissue after stent placement in rat esophagus. Gastrointest Endosc. 2017;86:219–228. doi: 10.1016/j.gie.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Park SA, Kim CH, Park SY, Kim JS, Kim DK, et al. TGF-β type I receptor kinase inhibitor EW-7197 suppresses cholestatic liver fibrosis by inhibiting HIF1α-induced epithelial mesenchymal transition. Cell Physiol Biochem. 2016;38:571–588. doi: 10.1159/000438651. [DOI] [PubMed] [Google Scholar]
- 16.Park SA, Kim MJ, Park SY, Kim JS, Lee SJ, Woo HA, et al. EW-7197 inhibits hepatic, renal, and pulmonary fibrosis by blocking TGF-β/Smad and ROS signaling. Cell Mol Life Sci. 2015;72:2023–2039. doi: 10.1007/s00018-014-1798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon KD, Choi MJ, Park JM, Song KM, Kwon MH, Batbold D, et al. Silencing histone deacetylase 2 using small hairpin RNA induces regression of fibrotic plaque in a rat model of Peyronie's disease. BJU Int. 2014;114:926–936. doi: 10.1111/bju.12812. [DOI] [PubMed] [Google Scholar]
- 18.Piao S, Ryu JK, Shin HY, Zhang L, Song SU, Han JY, et al. Repeated intratunical injection of adenovirus expressing transforming growth factor-beta1 in a rat induces penile curvature with tunical fibrotic plaque: a useful model for the study of Peyronie's disease. Int J Androl. 2008;31:346–353. doi: 10.1111/j.1365-2605.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 19.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 20.Burch ML, Yang SN, Ballinger ML, Getachew R, Osman N, Little PJ. TGF-beta stimulates biglycan synthesis via p38 and ERK phosphorylation of the linker region of Smad2. Cell Mol Life Sci. 2010;67:2077–2090. doi: 10.1007/s00018-010-0315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davila HH, Ferrini MG, Rajfer J, Gonzalez-Cadavid NF. Fibrin as an inducer of fibrosis in the tunica albuginea of the rat: a new animal model of Peyronie's disease. BJU Int. 2003;91:830–838. doi: 10.1046/j.1464-410x.2003.04224.x. [DOI] [PubMed] [Google Scholar]
- 22.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF-beta activation. J Cell Sci. 2003;116(Pt 2):217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 24.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 25.Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 26.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hidalgo-Tamola J, Luttrell I, Jiang X, Li D, Mecham RP, Chitaley K. Characterization of erectile function in elastin haploinsufficicent mice. J Sex Med. 2011;8:3075–3085. doi: 10.1111/j.1743-6109.2011.02454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon JH, Jung SM, Park SH, Kato M, Yamashita T, Lee IK, et al. Activin receptor-like kinase5 inhibition suppresses mouse melanoma by ubiquitin degradation of Smad4, thereby derepressing eomesodermin in cytotoxic T lymphocytes. EMBO Mol Med. 2013;5:1720–1739. doi: 10.1002/emmm.201302524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/BHEFNR.