Abstract
Purpose
Although five-alpha reductase inhibitor (5-ARI) is one of standard treatment for benign prostatic hyperplasia (BPH) or alopecia, potential complications after 5-ARI have been issues recently. This study aimed to investigate the risk of depression after taking 5-ARI and to quantify the risk using meta-analysis.
Materials and Methods
A total of 209,940 patients including 207,798 in 5-ARI treatment groups and 110,118 in control groups from five studies were included for final analysis. Inclusion criteria for finial analysis incudes clinical outcomes regarding depression risk in BPH or alopecia patients. Overall hazard ratio (HR) and odds ratio (OR) for depression were analyzed. Moderator analysis and sensitivity analysis were performed to determine whether HR or OR could be affected by any variables, including number of patients, age, study type, and control type.
Results
The pooled overall HRs for the 5-ARI medication was 1.23 (95% confidence interval [CI], 0.99–1.54) in a random effects model. The pooled overall ORs for the 5-ARI medication was 1.19 (95% CI, 0.95–1.49) in random effects model. The sub-group analysis showed that non-cohort studies had higher values of HR and OR than cohort studies. Moderator analysis using meta-regression showed that there were no variables that affect the significant difference in HR and OR outcomes. However, in sensitivity analysis, HR was significantly increased by age (p=0.040).
Conclusions
Overall risk of depression after 5-ARI was significantly not high, however its clinical importance needs validation by further studies. These quantitative results could provide useful information for both clinicians and patients.
Keywords: Alopecia, Depression, Prostatic hyperplasia, Suicide, 5-alpha reductase inhibitors
INTRODUCTION
Five-alpha reductase inhibitor (5-ARI) is one of the standard treatments for benign prostatic hyperplasia (BPH)/lower urinary tract symptoms (LUTS), together with alpha blockers for BPH [1,2]. Considering the recent and increasing shift toward medication instead of surgical therapy [3], the clinical efficacy of 5-ARI is in reducing both complications related to BPH/LUTS and the need to perform surgery [4,5]. Five-ARI is also standard treatment in androgenic alopecia, especially for male pattern hair loss (MPHL) because androgen is the most common cause of hair loss [6].
Five-ARI blocks the conversion of testosterone to dihydrotestosterone, which accounts for the efficacy of its use in the treatment of BPH/LUTS and MPHL, by reducing prostate volume or promoting regeneration of hair follicles [6,7]. To date, there are two types of 5-ARIs, including finasteride and dutasteride. While finasteride inhibits only type 2 5-alpha reductase (5-AR), dutasteride inhibits both type 1 and 2 5-AR, but both medications have equal efficacy [8].
There have been controversy about long term safety of 5-ARI due to limited evidence has been introduced to date. Due to the potent androgenic activity of dihydrotestosterone, adverse events related to 5-ARI have been reported for 20 years, especially sexual adverse events [9,10,11]. Moreover, recently, depressive symptoms and suicidal thoughts were more prevalent among the formal users of finasteride who experienced persistent sexual adverse events than the controls [9].
Although there is a controversy, a considerable number of men have reported intolerable adverse effects after initiating finasteride therapy, and they continue to experience these effects after stopping the medication [9,10]. These peripheral or secondary effects have undesirable consequences that are collectively becoming known as post-finasteride syndrome [11]. Currently, US Food and Drug Administration and Health Canada have warned about this crucial issue of adverse events using finasteride during their use for BPH/LUTS and alopecia [12,13].
However, there is only limited academic evidence of adverse events related to taking 5-ARI, including depression and suicidal thoughts or suicide attempts, and those evaluating the evidence are drawing conflicting conclusions [14,15,16]. Although two prior clinical studies (which are secondary analyses of a previous clinical trial and cohort study) showed the association between depression risk and 5-ARI medication [14,15], recent large case control studies have shown limited association between the use of 5-ARI medication and depression or the risk of suicide attempts [16].
Considering the social burden of depression and suicide attempts, together with that of BPH/LUTS or alopecia [17], more evidence is needed in order to develop better information for both clinicians and patients, which could have benefits regarding shared decision making about 5-ARI use. Our goal of this study is to suggest quantitative information about the risk of depression and suicide attempts after 5-ARI use through the use of systematic review and meta-analysis from current observational studies.
MATERIALS AND METHODS
1. Ethics statement
This systematic review and meta-analysis were performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [18]. This is a literature-based study, ethical approval is unnecessary.
2. Data sources and literature searches
The electronic databases screened were MEDLINE (1966 through January 2017) and Cochrane Library (1993 through January 2017). Medical subject headings terms were used. The subject headings and text keywords included interventions (5-alpha Reductase Inhibitors; azasteroids, finasteride, and/or dutasteride) and outcomes (depression disorder, suicide and/or suicide thinking). The searches were limited to human studies and performed for all languages & all study types. The same reference searching was adopted for the EMBASE using Emtree (Embase subject headings).
3. Study selection
Study inclusion criteria were as follows: 1) interventions included administration of 5-ARI, 2) participants were diagnosed with depression disorder, suicide and/or suicidal thinking. Two of us (JHK and SRS) independently screened the titles and abstracts of all articles using predefined inclusion criteria. The full-text articles were examined independently by two of authors (JHK and BIC) to determine whether or not they met the inclusion criteria. Then, two of authors (JHK and BIC) independently extracted data using a data extraction form. Final inclusion articles were determined by the all investigators' evaluation discussions, using a point estimation system for each article. References and data for each included study were carefully crosschecked to ensure that no overlapping data were present and to maintain the integrity of the meta-analysis.
4. Types of interventions and outcomes
The experimental group received 5-ARIs and the control group received non-exposure of 5-ARI, placebo, or adrenergic alpha-antagonist. Outcomes measured hazard ratio (HR) and odds ratio (OR).
5. Types of moderators
In order to identify the moderator effect, continuous variables (age and number of patients) and binary variables (study type: cohort/non-cohort; control type: adrenergic alpha-antagonist/non-expose of 5-ARI) were analyzed.
6. Meta-analysis assessment of outcome findings and statistical analysis
Included studies are those of large populations and long periods - at least one year. These kinds of time-to-event outcomes are most appropriately analyzed using HR. For the absence of individual patient data or HR, Tierney et al [19] well described the practical methods for incorporating summary time-to-event data into meta-analysis. The HRs of three studies [9,14,20] were calculated using Tierney's practical guide [19].
For overall effect size of OR, two studies [9,15] did not report ORs. We calculated ORs using crude depression events. In order to gain deep understanding, we displayed two types of statistical models as the fixed-effects model and the random-effects model. Meta-regression analysis was conducted for each moderator. To examine potential moderators (e.g., number of patients, age, study type, and control type), we used a restricted maximum likelihood estimator of the variance of the true effects.
Sensitivity analysis was conducted for robust interpretation. Due to the fact that one study [9] has relatively small sample sizes and different indication for medication, we excluded that study [9] in sensitivity analysis.
A two-sided p-value ≤0.05 or not contained of null value (HR=1 or OR=1) within the 95% was considered to be significant. The abovementioned analyses were conducted with STATA ver. 14.2 software (Stata Corp. LP, College Station, TX, USA).
7. Quality assessment
Newcastle–Ottawa quality scale was used to evaluate for the quality assessment for the case-control and cohort study. We assessed the following three parameters: (1) appropriate selection, (2) comparability of research design or statistical analysis, (3) outcome/exposure ascertainment and research procedure. We graded each parameter as a star; a study can be awarded a maximum of one star for each item in selection and outcome/exposure, but a maximum of two stars can be awarded in comparability. The quality power of the evidence related to the estimation of benefits and disadvantages were displayed according to specific conditions.
8. Assessment of heterogeneity
Statistical heterogeneity was evaluated by the Cochran's Q test and the I2 statistic. Either a Cochran's Q statistic p<0.1 or an I2 statistic >50% indicated the existence of significant heterogeneity between studies. However, a non-significant χ2 test result (p≥0.1) or I2 statistic (≤50%) indicated a lack of evidence for heterogeneity, but it did not necessarily imply homogeneity because there may have been insufficient statistical power to detect heterogeneity. So, we used two types of statistical models (fixed-effects and random-effects model).
9. Assessment of potential publication bias
We performed comprehensive meta-analysis to examine any potential publication bias in the studies, and the results were shown by funnel plot. In the absence of publication bias, the studies will be distributed symmetrically by the combined effect size. The Begg and Mazumdar rank correlation test and the Egger linear regression test were performed for publication biases.
RESULTS
1. Study selection
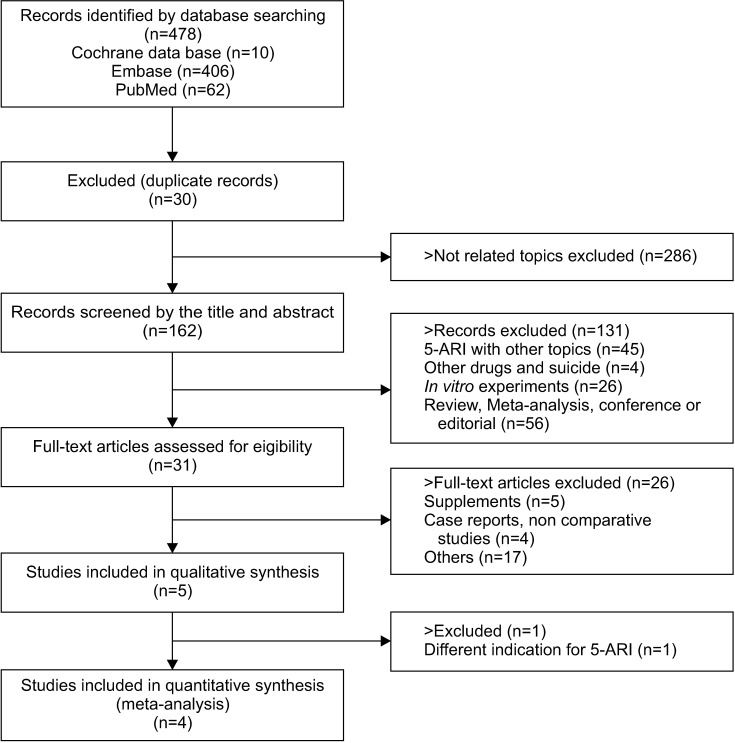
The initial search identified a total of 478 articles from electronic databases (PubMed, 62; Cochrane, 10; Embase, 406). Thirty studies were excluded because they contained overlapping data or appeared in more than one database. Upon more detailed review, an additional 417 papers were excluded for the following reasons: unrelated topic (n=286), 5-ARI with other topics (n=45), other drugs and suicide (n=4), in vitro experiment (n=26), review paper or editorials (n=56). After screening the titles and abstracts, 31 studies were determined to be eligible for intensive screening. Of these, 26 studies were further eliminated as they were duplicated supplements and had insufficient outcome data. Finally, five studies met our selection criteria and were included in the meta-analysis (Fig. 1).
Fig. 1. Flow chart for study inclusion.
A systematic review of the five studies was conducted to assess detailed experimental differences and subject descriptions for qualitative analysis (Table 1). However, for quantitative analysis only four studies were finally included. Three were cohort studies with large population comparative studies that included a total of 207,798 subjects (99,627 treatment and 108,171 control subjects), and one observational cross-section study that included a total of 2,052 subjects (134 treatment and 1,918 control subjects).
Table 1. Characteristics of included trials.
| Study | Country | Study design | Subject for analysis treatment (n) | Subject for analysis control (n) | Medication duration | Subject description | Indication for medication | Assessment for depression |
|---|---|---|---|---|---|---|---|---|
| Irwig (2012) [9] | USA | Case-control, retrospective study | Finasteride (61) | Control (29) | <1 year (51%), ≥1 year (49%) | Mean age 31.7 years (finasteride) and 26.2 years (control). Finasteride group was taking the medication for at least 3 months and control group was never used finasteride. | Male pattern hair loss | Beck Depression Inventory-II |
| Pietrzyk et al (2015) [14] | Poland | Observation, cross section study | 5-ARI only (134) | AB only (1,918) | <1 year (21.4%), ≥1 year (78.6%) | Mean age 65.8 years. 4,035 men diagnosed with BPH, control group was never used 5-ARI. | BPH | Beck Depression Inventory |
| Unger et al (2016) [15] | USA | Cohort study (prospective study) | Finasteride (6,941) | Placebo (6,994) | At least 7 years | Mean age 63.5 years (finasteride) and 63.6 years (placebo). A total of 18,880 patients from PCPT were randomly assigned to finasteride and placebo for 7 years. And then PCPT was linked to Medicare claims 13,395 patients. | To identify the relationship between finasteride and prostate cancer | ICD-9 diagnostic codes |
| Welk et al (2017) [16] | Canada | Cohort study (retrospective, propensity score matching study) | 5-ARI (89,844) | Control (89,844) | 1.57–1.60 years | Mean age 75 years. It used 7 types of administrative data sources at Ontario province in Canada. And then it matched with a 5-ARI prescription to those without any 5-ARI prescriptions. Control goup is unexposed of 5-ARI. | To identify the relationship between 5-ARI and depression | ICD-9 & 10 diagnostic codes |
| Hagberg et al (2017) [20] | UK | Cohort study (matching for nested case-control study) | 5-ARI only (2,842) | AB only (11,333) | 7.6 (5-ARI only) & 7.7 (AB only) person-years/per 1,000 person-years | A 78.4% of total patients are more than 60 years old who identified 77,732 men with benign prostatic hyperplasia. It was conducted using the UK's Clinical Practice Research Datalink. | BPH | Depression diagnostic code and who received a prescription for an antidepressant |
5-ARI: alpha reductase inhibitor, AB: adrenergic alpha-antagonist, BPH: benign prostatic hyperplasia, PCPT: Prostate Cancer Prevention Trial, ICD: International Classification of Diseases.
2. Quality assessment
Authors critically appraised the selected studies using criteria given by Newcastle–Ottawa quality scale, and then final quality evaluation was discussed among all investigators (Table 2). All three cohort studies were ranked good quality, and one retrospective case-control study [9] was ranked poor quality due to its relatively small sample size and the fact that its bias control was not adjusted for research analysis.
Table 2. Newcastle-Ottawa quality assessment form for case-control study and cohort study.
| Variable | Selection | Comparability | Outcome/Exposure | Total score | Quality power | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| For case-control study | Adequate definition of case | Representativeness of case | Selection of control | Definition of control | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non response rate | ||
| Irwig (2012) [9] | ★ | ★ | ★ | ★ | ★ | ★★★★★ (5) | Poor | |||
| Pietrzyk et al (2015) [14] | ★ | ★ | ★ | ★★ | ★ | ★ | ★★★★★★★ (7) | Good | ||
| For cohort study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of interest not present at start of study | Comparability of cohorts on the basis of the design or analysis | Ascertainment of outcome | Adequacy of duration of follow-up | Adequacy of completeness of follow-up | ||
| Unger et al (2016) [15] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★★★★★★★★ (8) | Good | |
| Welk et al (2017) [16] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★★★★★★★★ (8) | Good | |
| Hagberg et al (2017) [20] | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★★★★★★★★ (8) | Good | |
A study can be awarded a maximum of one star for each numbered item except for Comparability. A maximum of two stars can be awarded for Comparablitily. Good quality, 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in oucome/exposure domain. Fair quality, 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in oucome/exposure domain. Poor quality, 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in oucome/exposure domain.
3. Outcome findings
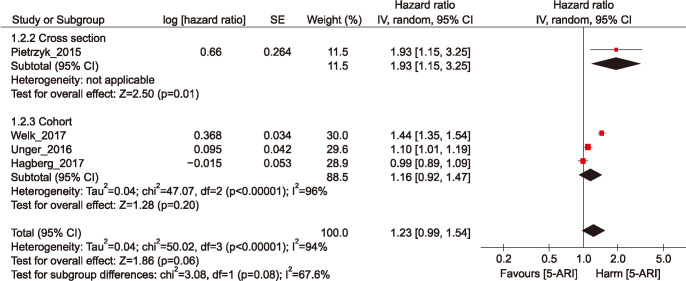
The pooled overall HRs for the 5-ARI medication was 1.23 (95% confidence interval [CI], 0.99–1.54) in random effects model. The Cochran's Q test p-value and the I2 statistic were p<0.001 and 94%. In subgroup analysis of study types, the pooled overall HRs of the cohort studies were 1.16 (95% CI, 0.92–1.47) in random effects model than the cohort studies (Fig. 2).
Fig. 2. Hazard ratio of depression among men exposed to five-alpha reductase inhibitor (5-ARI) medication. SE: standard error, CI: confidence interval, df: degree of freedom.
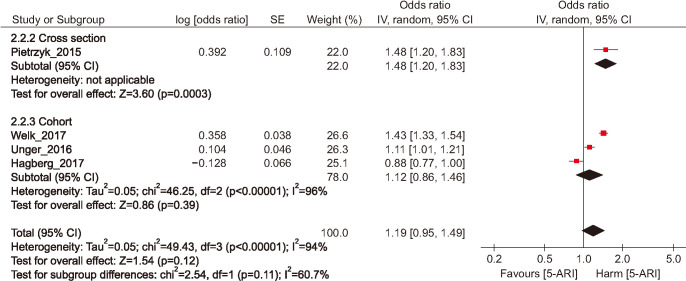
The pooled overall ORs for the 5-ARI versus the control group were 1.19 (95% CI, 0.95–1.49) in the random effects model. The Cochran's Q test p-value and the I2 statistic were p<0.001 and 94%. In subgroup analysis of study types, the pooled overall ORs of cohort studies were 1.12 (95% CI, 0.86–1.46) in the random effects model (Fig. 3).
Fig. 3. Odds ratio of depression among mean exposed to five-alpha reductase inhibitor (5-ARI) medication. SE: standard error, CI: confidence interval, df: degree of freedom.
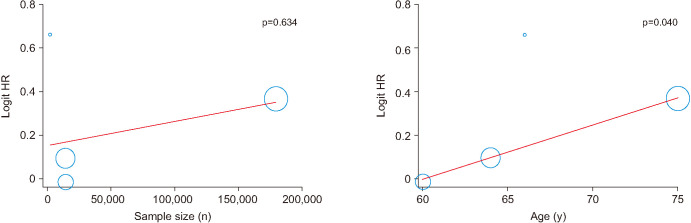
Table 3 provided an overview of meta-regression analysis results to identify the moderator effects. The regression analysis with all continuous variables (e.g., number of patients, age, study type, and control type) did not show a significant difference in HR and OR outcomes. However meta-regression analysis showed that HR was significantly increased by age in cases of inclusion of Irwig at al [9] (Fig. 4). In sensitivity analysis (Supplement 1, 2), both HR and OR was affected by the results of Irwig et al [9].
Table 3. Effects of moderators on HR and OR.
| Variable | HR | OR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | Coef.a | HR | 95% CI | p-valueb | k | Coef.a | OR | 95% CI | p-valueb | |
| No. of patients | 5 | 0.999 | - | 0.999–1.000 | 0.711 | 5 | 0.999 | - | 0.999–1.000 | 0.736 |
| Age | 5 | 0.962 | - | 0.914–1.013 | 0.099 | 5 | 0.936 | - | 0.862–1.017 | 0.085 |
| Study type | 0.103 | 0.254 | ||||||||
| Non-cohort | 2 | 3.722 | 1.015–13.653 | 2 | 5.803 | 0.344–97.961 | ||||
| Cohort | 3 | 1.164 | 0.923–1.468 | 3 | 1.123 | 0.862–1.462 | ||||
| Control group | 0.599 | 0.499 | ||||||||
| Alpha blocker | 2 | 1.314 | 0.683–2.529 | 2 | 1.133 | 0.681–1.885 | ||||
| Non-expose | 3 | 1.757 | 1.234–2.502 | 3 | 1.507 | 1.069–2.122 | ||||
HR: hazard ratio, OR: odds ratio, k: number of effect sizes, Coef.: coefficient, CI: confidence interval.
aExponential regression coefficient. bp-values from random effect meta-regression using restricted maximum likelihood.
Fig. 4. Meta-regression analysis. HR: hazard ratio.
4. Publication bias
The statistical approaches for the detection of publication bias or small-study effect is shown in Supplement 3. Both funnel plots of HR and OR seemed to be asymmetry graphs because of one skewed study: Irwig et al [9]. In the analysis by HR, however, Begg and Mazumdar's correlation was −0.24 (p=1.000), Egger's regression coefficient was 2.907 (p=0.510), suggesting that there was no evidence of publication bias or small-study effect in this meta-analysis. We also did an analysis of OR, by which we found that there is no significant evidence of publication bias in this meta-analysis.
DISCUSSION
Despite its characterization on the internet as a hot focused social issue due to “life-altering” and “devastating” effects, academic evidence on the use of 5-ARI, especially, finasteride, is not sufficient to show the crucial adverse effects of using 5-ARI medication. Interestingly, a relatively high rate of discontinuation of 5-ARI has been reported compared to other urologic medication [21]. Several patients have a fear of the persistent adverse events which have been publicized on the internet despite evidence to the contrary, which has proven the efficacy of 5-ARI in treating BPH/LUTS and alopecia.
Unfortunately, there is only limited evidence about this issue. Moreover, those studies are not intention to treat trial designs to investigate the risk of depression or suicide. Although designing an randomized controlled trial (RCT) regarding this crucial issue is almost impossible, several limitations of observational studies about drug-related adverse events have to be clarified for better understanding of each study. In addition to the fact that our study has a primary goal to suggest a quantitative risk of depression using meta-analysis, it could clarify the characteristics of each of the observational studies that were included during systematic review. This enables us to give critical information for designing future prospective trials.
Using a systematic review of included observational studies, this study is suggesting two important aspects for better understanding of this issue. First, in observational studies, it is possible that patients with adverse events could discontinue 5-ARI by themselves, which could result in a lower incidence rate of depression or suicide attempts. In the large observational study of Welk et al [16], stratified HR of 5-ARI showed decreasing pattern according to time exposure for both self-harm and depression. Second, self-discontinuation of 5-ARI could prevent future events, including depression or suicide attempts, which also could result in a lower incidence rate of depression or suicide attempts. Using these evidences from observational studies, it is hardly to interpret whether long term medication of 5-ARI has decreasing trend of depression or self-harm or this phenomenon is just originated form limited nature of observational studies. Hence, our study is suggesting the quantitative outcome of future depression risk to design further scientific investigation.
Although our study supported null hypothesis of no association between 5-ARI and subsequent depression risk, our meta-analysis showed a borderline statistical significance with its direction to depression risk. Among the studies included in qualitative analysis, one study [9] has not been considered for final quantitative analysis due to its different indication. Moreover, this study is including young population, who are far away from indicated population for this meta-analysis. If this study is included for quantitative analysis, both HR and OR are turned to be statistically significant as 1.52 (95% CI, 1.17–1.98) and 1.33 (95% CI, 1.02–1.72) (Supplement 2, 3).
To date, only limited evidence exists, with conflicting results, about the association between the use of 5-ARI and depression or suicide attempts. Recently, Welk et al [16] reported the association between the use of 5-ARI and depression or suicide attempts using their large retrospective cohort, including approximately 186,000 men who were more than 66 years old, which showed different results according to follow-up periods. The risk of suicide attempts was significantly elevated only until 18 months of follow-up and not significant thereafter. However, risk of depression was significantly elevated both before 18 months or after 18 months but it showed decreasing trend after 18 months [16]. There was no difference according to type of 5-ARI. Moinpour et al [22] also demonstrated that there was no difference in risk of negative mental health using the 36-item Short Form health survey.
A secondary analysis of a large cross sectional study showed that there was a 1.5 fold increased risk of depression after 5-ARI medication [14]. Unger et al [15] used secondary analysis among RCT trials and found that there was a significant risk of depression after finasteride medication, of which HR was 1.10 (95% CI, 1.01–1.19). Irwig [9,10] reported that there were significantly higher rates of depression and suicidal aspect among patients with persistent sexual adverse events after discontinuation of finasteride than among controls. In our study, pooled overall HR for depression risk was 1.23 (95% CI, 0.99–1.54) in random effect model, and pooled overall OR for depression risk was 1.19 (95% CI, 0.95–1.49) in random effect model.
The pathophysiology to explain the association between 5-ARI and depression has been introduced previously, including the role of 5-ARI in synthesis of several important endogenous regulating neuroactive steroids and in modulation of the neuroendocrine stress response [23] Depression is associated with dysregulation of those important bioactive regulation steroids and with decreased androgen activity [7]. In several population-based studies, positive association between cognitive function and androgen have been revealed in both genders [24,25].
Our study has several limitations. First, most of the included studies are of observational design; hence, our meta-analysis result could also be affected by the limitative nature of the observational studies. In observational studies, defining primary outcomes, including depression or suicide attempts could be over diagnosed or underdiagnosed. Moreover, important confounders, including pre-existing psychological disorders or family history or sociodemographic features could not be adjusted [9,26]. However, most included studies are large scaled data from administrative dataset which could yield accurate power and methodological quality. Second, during meta-analysis, there was a high heterogeneity due to the inconsistent definition of depression according to each study. Third, this study could not provide quantitative risk of suicide outcomes including suicidal thought or suicidal evet in association with the use of 5-ARI medication due to limitative data synthesis. Fourth, men with BPH are given a dose 5 times higher, tend to be older and may have their own confounding reasons to become depressed as they age. Moreover, this study could not determine cumulative effect of 5-ARI due to limited information from included studies.
CONCLUSIONS
Our study shows the quantitative risk of depression after 5-ARI medication using meta-analysis for first time, by which we determined the risk to be not significantly high. However, due to limited included studies, this result has to be interpreted with caution for clinical importance in real practice. However, with this quantitative risk and its direction for depression risk, patients could be better informed during the process of shared decision making. It could also provide useful information to design a future prospective study about this issue.
ACKNOWLEDGEMENTS
This study was supported by Soonchunhyang University Research Fund (20190616).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: JHK, BIC.
- Funding acquisition: JHK.
- Methodology: SRS, YK.
- Supervision: BIC.
- Writing—original draft: JHK.
- Writing—review & editing: all authors.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.190046.
Sensitivity analysis for HR.
Sensitivity analysis for OR.
Funnel plot for publication bias.
References
- 1.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803. doi: 10.1016/j.juro.2011.01.074. [DOI] [PubMed] [Google Scholar]
- 2.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–140. doi: 10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Füllhase C, Hakenberg O. New concepts for the treatment of male lower urinary tract symptoms. Curr Opin Urol. 2015;25:19–26. doi: 10.1097/MOU.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 4.McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, et al. Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 5.Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 6.McElwee KJ, Shapiro JS. Promising therapies for treating and/or preventing androgenic alopecia. Skin Therapy Lett. 2012;17:1–4. [PubMed] [Google Scholar]
- 7.Traish AM. 5α-reductases in human physiology: an unfolding story. Endocr Pract. 2012;18:965–975. doi: 10.4158/EP12108.RA. [DOI] [PubMed] [Google Scholar]
- 8.Nickel JC, Gilling P, Tammela TL, Morrill B, Wilson TH, Rittmaster RS. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS) BJU Int. 2011;108:388–394. doi: 10.1111/j.1464-410X.2011.10195.x. [DOI] [PubMed] [Google Scholar]
- 9.Irwig MS. Depressive symptoms and suicidal thoughts among former users of finasteride with persistent sexual side effects. J Clin Psychiatry. 2012;73:1220–1223. doi: 10.4088/JCP.12m07887. [DOI] [PubMed] [Google Scholar]
- 10.Irwig MS. Persistent sexual side effects of finasteride: could they be permanent? J Sex Med. 2012;9:2927–2932. doi: 10.1111/j.1743-6109.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- 11.Post-Finasteride Syndrome Foundation [Internet] Somerset: Post-Finasteride Syndrome Foundation; [cited 2018 Aug 3]. Available from: http://www.pfsfoundation.org/ [Google Scholar]
- 12.Government of Canada. Health product InfoWatch - December 2015 [Internet] Ottawa: Government of Canada; c2015. [cited 2018 Aug 3]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/health-product-infowatch/health-product-infowatch-december-2015.html. [Google Scholar]
- 13.U.S. Food and Drug Administration. Propecia (finasteride) [Internet] Silver Spring: U.S. Food and Drug Administration; c2014. [cited 2018 Aug 3]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/020788Orig1s017.pdf. [Google Scholar]
- 14.Pietrzyk B, Olszanecka-Glinianowicz M, Owczarek A, Gabryelewicz T, Almgren-Rachtan A, Prajsner A, et al. Depressive symptoms in patients diagnosed with benign prostatic hyperplasia. Int Urol Nephrol. 2015;47:431–440. doi: 10.1007/s11255-015-0920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unger JM, Till C, Thompson IM, Jr, Tangen CM, Goodman PJ, Wright JD, et al. Long-term consequences of finasteride vs placebo in the prostate cancer prevention trial. J Natl Cancer Inst. 2016;108:djw168. doi: 10.1093/jnci/djw168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welk B, McArthur E, Ordon M, Anderson KK, Hayward J, Dixon S. Association of Suicidality and Depression with 5α-reductase inhibitors. JAMA Intern Med. 2017;177:683–691. doi: 10.1001/jamainternmed.2017.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole MG, Bellavance F, Mansour A. Prognosis of depression in elderly community and primary care populations: a systematic review and meta-analysis. Am J Psychiatry. 1999;156:1182–1189. doi: 10.1176/ajp.156.8.1182. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and metaanalyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagberg KW, Divan HA, Nickel JC, Jick SS. Risk of incident antidepressant-treated depression associated with use of 5α-reductase inhibitors compared with use of α-blockers in men with benign prostatic hyperplasia: a population-based study using the clinical practice research datalink. Pharmacotherapy. 2017;37:517–527. doi: 10.1002/phar.1925. [DOI] [PubMed] [Google Scholar]
- 21.Kruep EJ, Phillips E, Hogue S, Eaddy M. Early symptom improvement and discontinuation of 5-α-reductase inhibitor (5ARI) therapy in patients with benign prostatic hyperplasia (BPH) Ann Pharmacother. 2014;48:343–348. doi: 10.1177/1060028013514213. [DOI] [PubMed] [Google Scholar]
- 22.Moinpour CM, Darke AK, Donaldson GW, Cespedes D, Johnson CR, Ganz PA, et al. Health-related quality-of-life findings for the prostate cancer prevention trial. J Natl Cancer Inst. 2012;104:1373–1385. doi: 10.1093/jnci/djs359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handa RJ, Kudwa AE, Donner NC, McGivern RF, Brown R. Central 5-alpha reduction of testosterone is required for testosterone's inhibition of the hypothalamo-pituitary-adrenal axis response to restraint stress in adult male rats. Brain Res. 2013;1529:74–82. doi: 10.1016/j.brainres.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giagulli VA, Guastamacchia E, Licchelli B, Triggiani V. Serum testosterone and cognitive function in ageing male: updating the evidence. Recent Pat Endocr Metab Immune Drug Discov. 2016;10:22–30. doi: 10.2174/1872214810999160603213743. [DOI] [PubMed] [Google Scholar]
- 25.Thilers PP, Macdonald SW, Herlitz A. The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Ganzer CA, Jacobs AR, Iqbal F. Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. Am J Mens Health. 2015;9:222–228. doi: 10.1177/1557988314538445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis for HR.
Sensitivity analysis for OR.
Funnel plot for publication bias.