Abstract
Benign prostatic hyperplasia (BPH), a common cause of lower urinary tract symptoms in the elderly male population, has conventionally treated by transurethral resection of the prostate (TURP). During recent years, newer minimally invasive therapies (MITs) have entered the playing field and challenged TURP with their convenience, lack of sexual side effects, and overall safety. The present paper provides an update on the more heavily studied and most recent MITs, analyzing their mechanism of action, tolerability, and efficacy in clinical practice. Particularly, robust clinical data have propelled UroLift and Rezuum to the forefront in the armamentarium of minimally invasive BPH treatment. Newer mechanical therapies such as the temporary implantable nitinol device, ClearRing, ZenFlow Spring, and Butterfly are appealing options as they forego cutting, ablation, heating, or removing prostatic tissue. It is obvious that there is wide variation in the degree of clinical readiness of each modality and only time and long-term, multicenter studies will decide which of these therapies are accepted by the patient and urologist.
Keywords: Benign prostatic hyperplasia, Prostate, Surgery, Urination
INTRODUCTION
Benign prostatic hyperplasia (BPH) is the most common cause of lower urinary tract symptoms (LUTS) in aging men [1]. LUTS is categorized as any combination of symptoms related to storage, voiding, or post-micturition including, but not limited to, frequency, urgency, and nocturia [2]. The prevalence of both BPH and LUTS markedly increases with age: about 8% in men age 31–40 to over 80% of men older than 80 are affected [3]. From an healthcare economics perspective, BPH has accounted for 4.4 million office visits, 105,000 hospitalizations, and 21 to 38 million hours lost in productivity in the United States according to data from 2003 [4].
For decades, the gold standards of treatment for LUTS due to BPH were transurethral resection of the prostate (TURP) and open simple prostatectomy (OSP) for prostates <80 g and >80 g, respectively [5]. While highly effective, these procedures are not without their morbidities. TURP, for example, commands a recurrence rate of 15% over five to ten years. In a multicenter evaluation of over 10,000 patients undergoing TURP, the most common immediate complications included surgical reoperation (5.6% of patients), transfusions (2.9%), and transurethral resection syndrome (1.4%) [6]. In the long term, many patients experience retrograde ejaculation and erectile dysfunction as a result of this procedure. Close to 8% of patients undergoing OSP require transfusions and close to 4% require intervention for severe bleeding, making this a less popular option today [1].
Several new technologies have been developed in recent years in an effort to limit surgical morbidity of TURP and OSP. These minimally invasive surgical therapies (MIST) are typically done with minimal anesthesia as an outpatient procedure or reduced hospital stay and involve a quicker recovery period. They are generally well tolerated and have improved complication profiles (including less effects on sexual dysfunction) but these benefits are countered by higher rates of failure and less overall improvement to LUTS [7]. The purpose of this review is to briefly review the methodology, efficacy, and safety of varieties of MIST and their current use in patients with BPH.
MECHANICAL STRATEGIES
Mechanical MIST generally boil down to a physical retraction of obstructing prostatic lobes. This type of strategy involves no cutting, ablation, heating or removal of prostatic tissue and preserves ejaculatory function, making it one option for therapy.
1. Prostatic urethral lift (UroLift™)
Prostatic urethral lift or UroLift™, manufactured by NeoTract (Pleasanton, CA, USA), is a novel technique that can be performed in the outpatient setting. The UroLift delivery device is a disposable cartridge consisting of nonabsorbable monofilament sutures placed through the urethra to access the enlarged prostate [7]. The device retracts the obstructing prostatic lobes and delivers a small transprostatic permanent implant via a needle, creating a continuous anterior channel through the prostatic lumen from the bladder neck to the verumontanum or seminal colliculus. This keeps the lateral lobes under traction and establishes a more open prostatic urethra. Because there is no tissue removal or ablation involved, this method theoretically avoids damage to the primary neurovascular bundle and dorsal venous complex [8].
Most studies have outlined common inclusion and exclusion criteria for use of this new technique. Table 1 from Jones et al' 2016 article [8] summarizes this. Of note, the most important contraindicatory factors to this surgical option include men with obstructing median lobes, men with large prostate burdens (>100 mL) and patients with a history of urinary retention [8].
Table 1. Inclusion and exclusion criteria for the UroLift technique.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Age>50 y | • Obstructive median lobe |
| • Prostate volume 20–70 mL (on ultrasound) | • Active urinary tract infection |
| • IPSS>12 | • PSA>10 ng/mL (unless negative biopsy) |
| • Qmax<15 mL/s | • Prostatitis within past 1 year |
| • PVR<350 mL | • History of urinary retention |
| • Previous BPH surgery |
IPSS: international prostate symptom score, Qmax: maximum urinary flow rate, PVR: postvoid residual, PSA: prostate specific antigen, BPH: benign prostatic hyperplasia.
Adopted from Jones et al (Ther Adv Urol 2016;8:372–6) [8].
The advantages of this procedure make it a very attractive option. UroLift can be done under local or general anesthesia, is a short and very safe procedure, and has a short learning curve for health care providers. Unlike TURP, which can cause retrograde ejaculation in up to 75% of men, there are no reported cases of sexual dysfunction resulting from UroLift [9]. The implants also hold the prostatic urethra open during the period of expected postoperative edema, so urinary catheterization rates have been shown to be as low as 20% for an overall mean duration of one day [10]. Side effects are due to the irritation induced by the mechanical implant, causing mild dysuria, hematuria, pelvic discomfort and urgency.
The biggest limitation to this procedure is by far the exclusion criteria noted above, which eliminate a large proportion of patients from trying this procedure. The lack of sufficient long term results is also a significant disadvantage (only two studies have been published with follow-up data of over two years, with the most recent data by Roehrborn et al [10,11] showing improvement in quality of life (QoL), flow rate, and symptoms durable up to five years). Most of the main cohort studies have outcomes at one year [12,13,14,15]. And while data has shown improvement in subjective outcomes, such as international prostate symptom score (IPSS) and QoL, only marginal improvements were seen in more objective parameters, such as maximum urinary flow rate (Qmax) and postvoid residual (PVR) [9]. The 5-year LIFT trial mentioned above also noted a slightly higher retreatment rate for the UroLift at 2% to 3% per year compared with a retreatment rate of 1% to 2% per year for TURP [10].
The most recent systematic review included 5 cases series and one clinical trial and reported improvements in LUTS, as measured by IPSS, BPH Impact Index, Qmax, and PVR [7,16]. These differences were significant (p<0.05).
2. Intraprostatic stents
Intraprostatic stents come in either a temporary flavor or more permanent type (UroLume). Briefly, these stents are positioned endoscopically and placed into the prostatic urethra to open up the bladder outlet [7].
Permanent stents promote epithelialization, fasten onto the prostatic stroma, and can be placed in less than 15 minutes under regional anesthesia [17]. However, the disadvantages of this procedure include possible infection (making the stent hard to remove) and possible dislodging of the stent, which can lead to urinary obstruction or total incontinence. The risk of urethral injury and stent migration is also higher compared to a temporary stent [18].
Temporary stents are non-epithelializing, prohibitive of tissue ingrowth, and are either retrievable or biodegradable [17]. They can also be placed rather quickly and are preferred in patients who cannot undergo general anesthesia. In a systematic review in 2007, stent failure rates approached 16% in 606 patients, mostly due to stent migration [19]. Removal of these stents requires undergoing general anesthesia which can be dangerous in patients who cannot handle these procedures.
Outcomes for the permanent stents have been underwhelming. Masood et al's 12 year analysis [20] reported that only 18% of patients had their stents placed at the end of the follow-up and in 40% of cases, the stent was removed due to malpositioning, dislodgement, and irritation symptoms. Limited long-term studies and high complication rates have reduced the utility of this device as a long-term durable option in BPH management.
The dearth of robust data on intraprostatic stents has led the European Association of Urology to recommend their use as an alternative to catheterization in men unfit for invasive procedures requiring spinal or general anesthesia.
3. Temporary implantable nitinol device
A nickel-titanium alloy, or nitinol, device aims to relieve LUTS due to BPH by using expanded struts that exert a radial force [21]. The radial force compresses obstructive prostatic lobes causing local ischemic necrosis of the urethral mucosa [22]. Five days after placement, the nitinol wires expand and sink into the periurethral tissues, allowing a decrease of bladder neck tension, reducing urinary flow obstruction. To date, the 12-month and 36-month follow-up results of the first prospective in-human clinical trial have been reported by Porpiglia et al [23,24].
Device related side effects include discomfort, mild dysuria and perineal pressure, increased urgency and mild hematuria [22]. Inclusion and exclusion criteria used in the initial study are listed in Table 2 [23].
Table 2. Inclusion and exclusion criteria for temporary implantable nitinol device.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Age>50 y | • Previous prostate surgery |
| • IPSS≥10 | • Prostate cancer |
| • Qmax≤12 mL/s | • Urethral stricture |
| • Prostate volume assessed by TRUS of <60 mL | • Bladder stones |
| • Obstructing median lobe |
IPSS: international prostate symptom score, Qmax: maximum urinary flow rate, TRUS: transrectal ultrasonography.
In the initial study with 12-month follow-up, temporary implantable nitinol device (TIND) was implanted within the bladder neck and prostatic urethra using a rigid cystoscope in 32 patients and removed five days later in the outpatient setting [23]. Four complications were reported: one urinary retention, one transient incontinence due to device displacement, one prostatic abscess and one urinary tract infection. After 12 months, the median IPSS was 9 (down from a preoperative median IPSS of 19), the mean improvement in IPSS compared with baseline was 45%, and the mean improvement in Qmax versus preoperatively was 67%, all statistically significant changes [23]. No additional medical or surgical therapy was required at the time of the patients' last follow-up visit 12 months post procedure.
The extended 3-year follow-up results corroborated the initial 1-year results [24]. No further complications were recorded during the 36-month follow-up. The change from baseline in IPSS (median score of 12 at 36 months), Qmax (41% rise from baseline), and QoL (median score of 2) were all statistically significant. This limited data has suggested that TIND implantation is well-tolerated, though more studies need to be done.
4. Emerging mechanical therapies
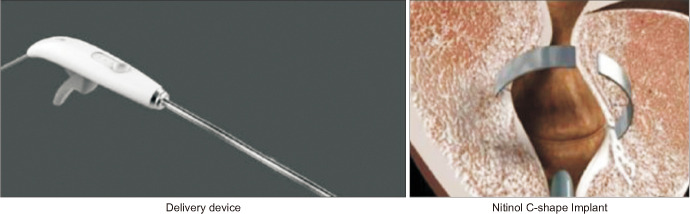
The ClearRing™ device (manufactured by ProArc Medical; Fig. 1) is an implantable C-shaped ring that is deployed in a circular incision in the prostatic tissue, compressing the prostatic transition zone tissue [25]. A recently published multicenter single-arm study in October 2018 treated 29 patients with mean IPSS 21.6, mean Qmax 8 mL/s and prostates between 35 and 50 mL using this device [25]. Ejaculation was preserved and there was no reported effect on erectile function. By 12 months, mean IPSS and Qmax improved by 53% and 49%, respectively.
Fig. 1. ClearRing (with permission from ProArc Medical).
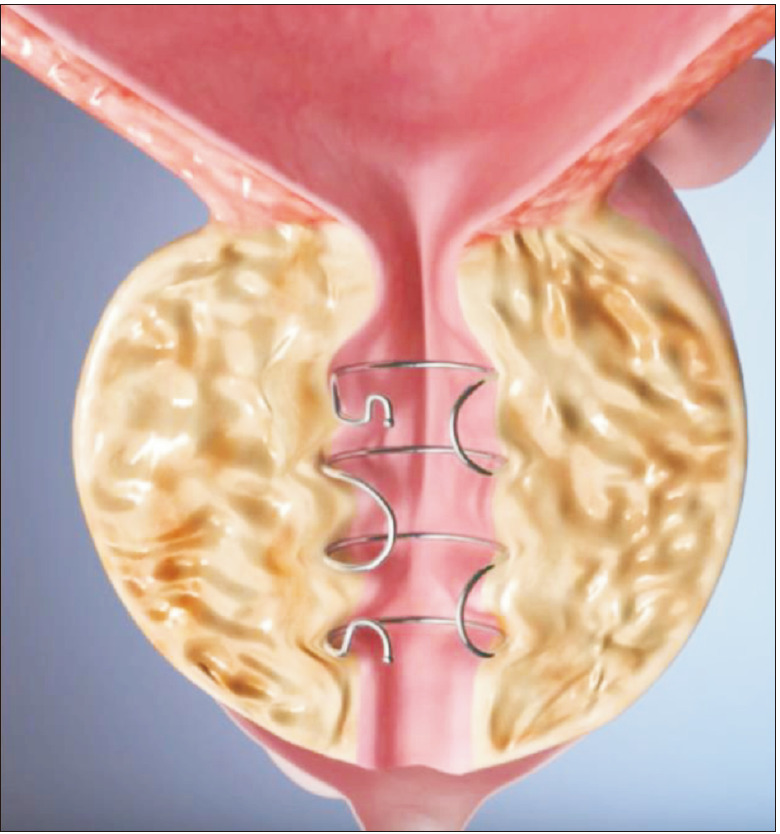
Currently undergoing clinical trials, the ZenFlow™ Spring (Fig. 2) is a small nitinol implant deployed through a flexible cystoscope in the outpatient setting used to treat BPH symptoms [22]. Designed to be a permanent device, the spring creates an internal tension that imbeds it into the wall of the prostatic urethra [26]. Patients have been followed out for 12 months and so far, early clinical cases have shown a low rate of side effects, fast recovery and durable results for this period.
Fig. 2. ZenFlow Spring (with permission from ZenFlow).
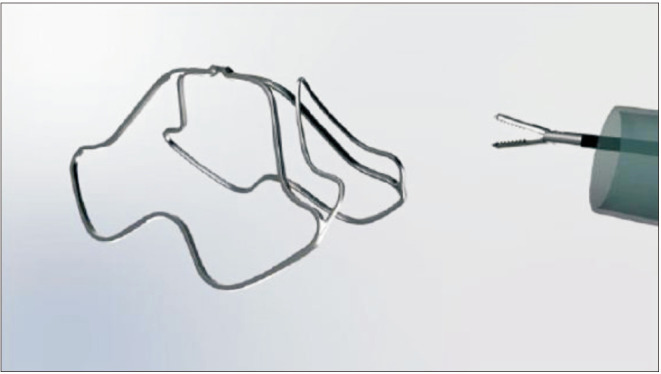
The Butterfly™ device (Fig. 3) is another upcoming metallic implant that functions by retracting the lateral lobes of the prostate and is delivered with either a rigid or flexible cystoscope. It is designed to be permanent but can easily be extracted. A multicenter trial to assess safety and efficacy is underway [22].
Fig. 3. Butterfly device (with permission from Butterfly Medical).
BOTULINUM TOXIN INTRAPROSTATIC INJECTIONS
A variety of pharmaceutical or chemical compounds may be injected deep into the prostate to elicit either cellular apoptotic pathways or module neurotransmitter activity. While transurethral ethanol ablation of the prostate and Topsalysin (PRX302, a pore-forming ablative agent) are treatments falling under the category of intraprostatic injections, this review article will focus on botulinum toxin.
Botulinum neurotoxin-A (BoNT-A) acts by binding presynaptically to high-affinity recognition sites on the cholinergic nerve terminals and decreases the release of acetylcholine at the neuromuscular junction. Parasympathetic stimulation affects the growth and secretion of the prostate epithelium. While the mechanism has not been extensively studied, in canine models, there has been marked atrophy and diffuse apoptosis of the prostate following the blocked release of acetylcholine [27,28]. This treatment has also been shown to decrease prostatic outflow obstruction by decreasing smooth muscle tone [17].
In large scale randomized controlled trials (RCTs), BoNT-A has not shown superiority to placebo [29,30]. Marberger et al's randomized double-blind placebo-controlled study [29] in 2013 found no significant difference between the placebo group and the Botox group in improvement of LUTS and BPH symptoms. McVary et al's multicenter, RCT [30] in 2014 also showed no significant difference in placebo or treatment groups in terms of IPSS, Qmax, total prostate volume, PVR or increase in prostate specific antigen (PSA) levels. While other studies have shown small benefits to PVR and increased peak flow rates, the results are overall mixed. Therefore, the utility of BoNT-A in the treatment of LUTS is, although promising, limited to select patients who are not able to undergo more invasive procedures or adhere to oral medications long-term.
PROSTATIC ARTERY EMBOLIZATION
Prostatic artery embolization (PAE) is a procedure carried out by interventional radiology where the vessels feeding the prostate (prostatic arteries) are blocked to impede prostatic growth and decrease urinary tract symptoms. In brief, embolization is performed by first advancing a catheter through the common femoral artery to the internal iliac artery and then through the inferior vesicle artery before arriving at the prostatic artery [17]. Prior to this, a Foley catheter is inserted to serve as a reference point for surrounding structures. Then, after an arteriogram is done, microspheres (tiny particles) are injected through the catheter and into the prostatic arteries. Ideally, the prostate should begin to reduce in size over the next several days, relieving symptoms. This effect is a result of the ischemia of intraprostatic vessels and subsequent inflammatory response along with a decrease in dihydrotesterone and intraprostatic testosterone, all of which initiate and aid the shrinking process [31,32].
Inclusion and exclusion criteria for this procedure are listed in Table 3 [31,32]. Approximately 33% of patients seen in a pre-procedural evaluation satisfy the criteria to be selected for PAE [31]. Indications for PAE as a first choice therapy for BPH include patients with special risks regarding surgery or anesthesia, sexually active men (as retrograde ejaculation is a common complication of TURP), prostate volume>65 mL, permanent bladder cathether, and recurrent bleeding caused by BPH [32].
Table 3. Inclusion and exclusion criteria for prostatic artery embolization.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Age>40 y | • Malignancy |
| • Prostate volume≥30 mL | • Bladder anomalies |
| • IPSS≥18 and/or QoL≥3 | • Chronic renal failure |
| • Acute urinary retention | • Acute urinary infection |
| • BPH refractory to medical therapy | • Renal insufficiency (eGFR<60 mL/min) |
| • Qmax≤15 mL/s at micturition volume of minimum 150 mL | • Advanced atherosclerosis of the iliac or prostatic arteries |
| • Urethral strictures |
IPSS: international prostate symptom score, QoL: quality of life, BPH: benign prostatic hyperplasia, Qmax: maximum urinary flow rate, eGFR: estimated glomerular filtration rate.
PAE is a very safe method overall that is still accompanied by certain complications. Dysuria, hematuria, hematospermia, urinary retention, and rectal bleeding with the procedure have been noted in the literature [7,17,32]. ‘Post-prostatic artery embolization syndrome’ is another complication which involves the mixture of small amounts of blood with urine and stool [33]. Patients can also experience pelvic and perineal pain and pressure for up to a few days after the procedure.
Failure rates are relatively high in PAE. A systematic review done in 2014 by Schreuder et al [34] identified up to a 19% failure rate with 15% of patients requiring TURP within the first year after treatment. Overall, there is growing evidence for the efficacy of this treatment. The previously mentioned systematic review found short term improvements in prostate volume, PVR and PSA but these improvements did not persist after 30 months [34]. In 2017, Shim et al [35] published a systematic review encompassing sixteen studies and found that PAE significantly improved outcomes with regards to IPSS, maximal urinary flow rates, and prostate volume in comparison with control groups. The dearth of long term data for this procedure impedes progression into a forerunner in BPH treatment.
WATER VAPOR THERAPY (REZUM™)
The Rezum™ system, manufactured by Boston Scientific, transforms sterile water into stored thermal energy (in the form of vapor or steam) to ablate prostatic tissue primarily in the outpatient setting [7,21]. Briefly, water vapor is transported transurethrally via cystoscopy. The thermal energy generated travels through the transition zone of the prostate, disrupts cell membranes and causes instant cell necrosis.
Inclusion and exclusion criteria for the Rezum thermal therapy are mentioned as follows in Table 4 [36,37]. In contrast with some of the other techniques mentioned above, patients with a median lobe obstruction can still qualify to receive this therapy.
Table 4. Inclusion and exclusion criteria for Rezum.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Age≥45 y with symptomatic BPH | • PVR>300 mL |
| • Prostate volume<120 mL | • PSA>2.5 ng/mL |
| • IPSS≥13 | • Recurrent or active UTI |
| • PVR<250 mL | • Prior prostate intervention/surgery |
| • 5≤Qmax≤15 mL/s |
BPH: benign prostatic hyperplasia, IPSS: international prostate symptom score, PVR: postvoid residual, Qmax: maximum urinary flow rate, PSA: prostate specific antigen, UTI: urinary tract infection.
Adopted from Westwood et al (Ther Adv Urol 2018;10:327–33) [37].
This procedure is quite safe with a minimal perioperative effects. The most important advantages are ease (day procedure with no general anesthesia needed) and no reports of sexual dysfunction [7,36,37]. Other advantages include significant improvements in Qmax, IPSS, QoL, and PVR and cost-effectiveness. The most significant disadvantages include limited long-term data and inability to ascertain incidental cases of prostate cancer [7,36,37]. TURP, in contrast, provides benefit by collecting tissue specimens which can detect prostate cancer. Another disadvantage is post-procedure catheterization in over half of patients.
Since approval in 2015 by the US Food and Drug Administration, a three year prospective, multicenter RCT has been published that found a significant (160%, p<0.0001) improvement in IPSS and at least a 50% improvement in QoL, Qmax, and BPH impact index that persisted for at least 3 years (p<0.001) in 197 men over the age of 50 meeting the inclusion criteria mentioned above [38]. This RCT also found applicability to treatment of median lobe tissue with overall preservation of sexual function. A crossover trial published by Roehrborn et al [39] in 2017 found significant mean improvements in IPSS, Qmax, and PVR at 12 months (p<0.001); this type of study has participants serving as self-controls which eradicates the placebo effects in other studies. In other pilot trials, similar results have been found which demonstrates the substantial, prolonged symptomatic relief derived from the Rezum procedure. To date, there have been no reports of effects on ejaculatory function unlike the relatively high incidence seen in most surgical treatments of BPH.
The most common adverse events are dysuria, hematuria, urinary tract infections (UTIs) and symptoms of urgency that typically resolve within weeks after the procedure [40]. In the literature, more serious adverse events, such as urosepsis, extended urinary retention, and nausea and vomiting have been reported but these complications are not the norm. The clinical effectiveness, long-term positive benefits, and applicability to the outpatient setting make Rezum a versatile and novel treatment for TURP.
HISTOTRIPSY
The least studied technique thus far, histotripsy uses high intensity ultrasound technology to vaporize fluid in prostatic tissues and release gaseous microbubbles which cause tissue destruction [7,21,41]. In animal studies with canines by Darnell et al [42] in 2015 found that histotripsy caused 31% decrease in prostate volume with limited inflammation and fibrosis. The prostatic urethra was well preserved in these models.
An in-human prospective single-arm clinical trial was conducted from July 2013 to June 2017 and investigated the safety and efficacy in men aged 50 or older with BPH and LUTS [41]. Results showed a significant increase in IPSS (p<0.001) of 12.5 points at 1 month and 10.4 points at 6 months but no clinical improvement in uroflow or PVR. Of the 25 men who underwent histotripsy treatment, there were no serious adverse events intraoperatively and postoperatively, transient urinary retention, a minor anal abrasion, and microscopic hematuria were the only noted adverse events [41].
Although prostate histotripsy has shown safety in this pilot human trial, its marginal improvement in LUTS warrants exploration with more studies in the future.
AQUABLATION
A robotic-assisted novel technique, Aquablation utilizes high-pressure saline to dissect prostatic tissue under transrectal ultrasound guidance [7,21,43]. Once resection is complete, cauterization can be used to initiate hemostasis.
Because careful patient selection is a necessary ingredient for successful therapy, patients should be assessed for inclusion and exclusion criteria, listed in Table 5 [43]. Inclusion criteria include LUTS refractory to medical therapy, age over 50, IPSS>12, Qmax≤12 mL/s, and prostate size 25 to 80 mL. Specific exclusion criteria include active UTI, large prostate size (>100 mL), PVR>400 mL and abnormal renal function.
Table 5. Inclusion and exclusion criteria for Aquablation.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age≥50 y | Active UTI |
| IPSS>12 | Large prostate size>100 mL |
| LUTS refractory to medical therapy | PVR>400 mL |
| Qmax≤12 mL/s | Abnormal renal function |
| Prostate size 25–80 mL |
IPSS: international prostate symptom score, LUTS: lower urinary tract symptoms, Qmax: maximum urinary flow rate, UTI: urinary tract infection, PVR: postvoid residual.
Adopted from Taktak et al (Ther Adv Urol 2018;10:183–8) [43].
The primary advantages of Aquablation include a resection time of under 10 minutes, preservation of sexual function and urinary continence, and avoidance of thermal damage (which seems to be the reason for the reduction in urinary symptoms) [21,43,44]. With only one double blind RCT to date, lack of consistent Level 1 evidence and long-term follow-up pose the biggest disadvantages to this procedure [45]. This procedure is also not suitable for large prostates, large median lobes and requires general anesthesia and inpatient admission. Many of the previously reviewed techniques were outpatient procedures such as PAE and UroLift.
Only one double-blind RCT has been done comparing Aquablation to the gold standard TURP, and its results were published recently in June 2018 [45]. In 181 patients with BPH and moderate to severe LUTS, Aquablation therapy was found to produce large IPSS improvements at six months and demonstrated non-inferiority to TURP (p<0.0001). The rate of ejaculatory dysfunction was also significantly lower (p=0.0003) than of TURP.
There have been no reports of negative effects on sexual function, major adverse events, or any cases where blood transfusion have been required. The most common complications to date have been dysuria, catheter insertion for urinary retention, and medically treated UTIs.
RECOMMENDATIONS AND SUMMARY
The MIST, though many are under study, are a unique set of procedures that represent a shift in the treatment of BPH. These personalized approaches allow the most efficacious treatment for a select group of patients. Table 6 lists a summary of the types of MIST discussed above in this article and factors specific to each type [7]. Histotripsy is not included in this table because only one pilot human trial has been done. The emerging mechanical therapies have also not been included because of a lack of data. American Urological Association guidelines suggest surgical intervention for patients with moderate to severe symptoms of BPH and for patients who have experienced BPH-related acute urinary retention and other complications. The wide variety of options available now tailor therapies to a patient's own anatomy, comorbidities, and other risk factors.
Table 6. Types of MIST and factors specific to each type.
| Type of MIST | Mechanism of action | Prostate size | Anesthetic | Relative contraindication | Major advantage |
|---|---|---|---|---|---|
| UroLift | Transprostatic perma- nent implant widens prostatic urethra | <100 mL | Local anesthesia and sedation | Previous prostate surgery, obstructive median lobe, renal insufficiency | No known cases of sexual dysfunction; can be done in clinic |
| Intraprostatic stents | Endoscopic stents open up the bladder outlet | <100 mL | Local or regional anesthesia | Penile or artificial urinary sphincters; acute UTI | Can be placed fairly quickly |
| TIND | Local ischemic necrosis remodels bladder neck and prostatic urethra | <60 mL | Local anesthesia | Previous prostate surgery, prostate cancer, urethral stricture, obstructing median lobe | Placed quickly and in the outpatient setting, no sexual side effects |
| BoNT-A intraprostatic inejctions | AcH inhibition decreases growth of prostatic tissue | N/A | Local anesthesia and sedation | Urethral stricture, neurogenic bladder | Short-term symptomatic relief in patients who cannot undergo invasive procedures |
| PAE | Injection of microspheres impede prostatic arteries | >30 mL | Local anesthesia and sedation | Prostate malignancy, urethral strictures, coagulation disorders, chronic renal failure | Useful in patients who cannot undergo more invasive procedures |
| Aquablation | High pressure saline dissects prostatic tissue | 25–80 mL | General anesthesia | Urinary retention, large prostate size (>100 mL) | Can be performed in under 10 minutes; preservation of urinary and ejaculatory function |
| Rezum | Thermal energy generated by water vapor causes prostatic cell necrosis | <120 mL | Local anesthesia and sedation | PSA>2.5 ng/mL, active UTI, urinary retention, prior prostate surgery | Day procedure; no effects on sexual function |
MIST: minimally invasive surgical therapies, TIND: temporary implantable nitinol device, BoNT-A: botulinum neurotoxin-A, PAE: prostatic artery embolization, N/A: not available, UTI: urinary tract infection, PSA: prostate specific antigen.
Footnotes
Conflict Interest: The authors have nothing to disclose.
- Conceptualization: AS, RW.
- Data curation: AS, RW.
- Formal analysis: AS, RW.
- Investigation: AS, RW.
- Methodology: AS, RW.
- Project administration: AS, RW.
- Resources: AS, RW.
- Software: AS, RW.
- Supervision: AS, RW.
- Validation: AS, RW.
- Visualizaton: AS, RW.
- Writing—original draft: AS, RW.
- Writing—reviewing & editing: AS, RW.
References
- 1.Sivarajan G, Borofsky MS, Shah O, Lingeman JE, Lepor H. The role of minimally invasive surgical techniques in the management of large-gland benign prostatic hypertrophy. Rev Urol. 2015;17:140–149. [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J International Scientific Committee and members of the committees, 6th International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol. 2009;181:1779–1787. doi: 10.1016/j.juro.2008.11.127. [DOI] [PubMed] [Google Scholar]
- 3.Parsons JK. Benign prostatic hyperplasia and male lower urinary tract symptoms: epidemiology and risk factors. Curr Bladder Dysfunct Rep. 2010;5:212–218. doi: 10.1007/s11884-010-0067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel ND, Parsons JK. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J Urol. 2014;30:170–176. doi: 10.4103/0970-1591.126900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. European Association of Urology. European Association of Urology. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–140. doi: 10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol. 2008;180:246–249. doi: 10.1016/j.juro.2008.03.058. [DOI] [PubMed] [Google Scholar]
- 7.Christidis D, McGrath S, Perera M, Manning T, Bolton D, Lawrentschuk N. Minimally invasive surgical therapies for benign prostatic hypertrophy: the rise in minimally invasive surgical therapies. Prostate Int. 2017;5:41–46. doi: 10.1016/j.prnil.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones P, Rai BP, Aboumarzouk O, Somani BK. UroLift: a new minimally-invasive treatment for benign prostatic hyperplasia. Ther Adv Urol. 2016;8:372–376. doi: 10.1177/1756287216671497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones P, Rajkumar GN, Rai BP, Aboumarzouk OM, Cleaveland P, Srirangam SJ, et al. Medium-term outcomes of urolift (minimum 12 months follow-up): evidence from a systematic review. Urology. 2016;97:20–24. doi: 10.1016/j.urology.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Roehrborn CG, Rukstalis DB, Barkin J, Gange SN, Shore ND, Giddens JL, et al. Three year results of the prostatic urethral L.I.F.T. study. Can J Urol. 2015;22:7772–7782. [PubMed] [Google Scholar]
- 11.Roehrborn CG, Barkin J, Gange SN, Shore ND, Giddens JL, Bolton DM, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol. 2017;24:8802–8813. [PubMed] [Google Scholar]
- 12.Woo HH, Chin PT, McNicholas TA, Gill HS, Plante MK, Bruskewitz RC, et al. Safety and feasibility of the prostatic urethral lift: a novel, minimally invasive treatment for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) BJU Int. 2011;108:82–88. doi: 10.1111/j.1464-410X.2011.10342.x. [DOI] [PubMed] [Google Scholar]
- 13.McNicholas TA, Woo HH, Chin PT, Bolton D, Fernández Arjona M, Sievert KD, et al. Minimally invasive prostatic urethral lift: surgical technique and multinational experience. Eur Urol. 2013;64:292–299. doi: 10.1016/j.eururo.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Chin PT, Bolton DM, Jack G, Rashid P, Thavaseelan J, Yu RJ, et al. Prostatic urethral lift: two-year results after treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology. 2012;79:5–11. doi: 10.1016/j.urology.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Bozkurt A, Karabakan M, Keskin E, Hirik E, Balci MB, Nuhoglu B. Prostatic urethral lift: a new minimally invasive treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urol Int. 2016;96:202–206. doi: 10.1159/000441850. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Gómez LM, Polo-deSantos M, Gómez-Sancha F, Luengo-Matos S. Efficacy and safety of the urolift® system for the treatment of benign prostate hyperplasia symptoms: systematic review. Actas Urol Esp. 2015;39:311–319. doi: 10.1016/j.acuro.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Nimeh T, Magnan B, Almallah YZ. Benign prostatic hyperplasia: review of modern minimally invasive surgical treatments. Semin Intervent Radiol. 2016;33:244–250. doi: 10.1055/s-0036-1586148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papatsoris AG, Junaid I, Zachou A, Kachrilas S, Zaman F, Masood J, et al. New developments in the use of prostatic stents. Open Access J Urol. 2011;3:63–68. doi: 10.2147/OAJU.S11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armitage JN, Cathcart PJ, Rashidian A, De Nigris E, Emberton M, van der Meulen JH. Epithelializing stent for benign prostatic hyperplasia: a systematic review of the literature. J Urol. 2007;177:1619–1624. doi: 10.1016/j.juro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Masood S, Djaladat H, Kouriefs C, Keen M, Palmer JH. The 12-year outcome analysis of an endourethral wallstent for treating benign prostatic hyperplasia. BJU Int. 2004;94:1271–1274. doi: 10.1111/j.1464-410X.2004.05155.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung ASJ, Woo HH. Update on minimally invasive surgery and benign prostatic hyperplasia. Asian J Urol. 2018;5:22–27. doi: 10.1016/j.ajur.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sountoulides P, Karatzas A, Gravas S. Current and emerging mechanical minimally invasive therapies for benign prostatic obstruction. Ther Adv Urol. 2019;11:1756287219828971. doi: 10.1177/1756287219828971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porpiglia F, Fiori C, Bertolo R, Garrou D, Cattaneo G, Amparore D. Temporary implantable nitinol device (TIND): a novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): feasibility, safety and functional results at 1 year of follow-up. BJU Int. 2015;116:278–287. doi: 10.1111/bju.12982. [DOI] [PubMed] [Google Scholar]
- 24.Porpiglia F, Fiori C, Bertolo R, Giordano A, Checcucci E, Garrou D, et al. 3-year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU Int. 2018;122:106–112. doi: 10.1111/bju.14141. [DOI] [PubMed] [Google Scholar]
- 25.Vjaters E, Nitsan D, Mullerad M, Engelstein D, Leibovitch I, Feld Y. First-in-man safety and efficacy of the ClearRing implant for the treatment of benign prostatic hyperplasia. Eur Urol Focus. 2020;6:131–136. doi: 10.1016/j.euf.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Zenflow, Inc. Zenflow [Internet] South San Francisco: Zenflow, Inc.; c2014. [cited 2019 May 17]. Available from: http://www.zenflow.com/ [Google Scholar]
- 27.Chuang YC, Giannantoni A, Chancellor MB. The potential and promise of using botulinum toxin in the prostate gland. BJU Int. 2006;98:28–32. doi: 10.1111/j.1464-410X.2006.06184.x. [DOI] [PubMed] [Google Scholar]
- 28.Ng LG. Botulinum toxin and benign prostatic hyperplasia. Asian J Urol. 2018;5:33–36. doi: 10.1016/j.ajur.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marberger M, Chartier-Kastler E, Egerdie B, Lee KS, Grosse J, Bugarin D, et al. A randomized double-blind placebo-controlled phase 2 dose-ranging study of onabotulinumtoxinA in men with benign prostatic hyperplasia. Eur Urol. 2013;63:496–503. doi: 10.1016/j.eururo.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 30.McVary KT, Roehrborn CG, Chartier-Kastler E, Efros M, Bugarin D, Chen R, et al. A multicenter, randomized, double-blind, placebo controlled study of onabotulinumtoxinA 200 U to treat lower urinary tract symptoms in men with benign prostatic hyperplasia. J Urol. 2014;192:150–156. doi: 10.1016/j.juro.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 31.A Pereira J, Bilhim T, Duarte M, Rio Tinto H, Fernandes L, Martins Pisco J. Patient selection and counseling before prostatic arterial embolization. Tech Vasc Interv Radiol. 2012;15:270–275. doi: 10.1053/j.tvir.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Teichgräber U, Aschenbach R, Diamantis I, von Rundstedt FC, Grimm MO, Franiel T. Prostate artery embolization: indication, technique and clinical results. Rofo. 2018;190:847–855. doi: 10.1055/a-0612-8067. [DOI] [PubMed] [Google Scholar]
- 33.Carnevale FC, Antunes AA. Prostatic artery embolization for enlarged prostates due to benign prostatic hyperplasia. How I do it. Cardiovasc Intervent Radiol. 2013;36:1452–1463. doi: 10.1007/s00270-013-0680-5. [DOI] [PubMed] [Google Scholar]
- 34.Schreuder SM, Scholtens AE, Reekers JA, Bipat S. The role of prostatic arterial embolization in patients with benign prostatic hyperplasia: a systematic review. Cardiovasc Intervent Radiol. 2014;37:1198–1219. doi: 10.1007/s00270-014-0948-4. [DOI] [PubMed] [Google Scholar]
- 35.Shim SR, Kanhai KJ, Ko YM, Kim JH. Efficacy and safety of prostatic arterial embolization: systematic review with meta-analysis and meta-regression. J Urol. 2017;197:465–479. doi: 10.1016/j.juro.2016.08.100. [DOI] [PubMed] [Google Scholar]
- 36.Woo HH, Gonzalez RR. Perspective on the Rezūm® system: a minimally invasive treatment strategy for benign prostatic hyperplasia using convective radiofrequency water vapor thermal therapy. Med Devices (Auckl) 2017;10:71–80. doi: 10.2147/MDER.S135378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westwood J, Geraghty R, Jones P, Rai BP, Somani BK. Rezum: a new transurethral water vapour therapy for benign prostatic hyperplasia. Ther Adv Urol. 2018;10:327–333. doi: 10.1177/1756287218793084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McVary KT, Roehrborn CG. Three-year outcomes of the prospective, randomized controlled Rezūm system study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology. 2018;111:1–9. doi: 10.1016/j.urology.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Roehrborn C, Gange S, Gittelman M. PD23-10. Convective radiofrequency thermal therapy: durable two-year outcomes of a randomized controlled and prospective crossover study to relieve lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol. 2017;197 Suppl:e450–e451. doi: 10.1016/j.juro.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 40.Dixon C, Cedano ER, Pacik D, Vit V, Varga G, Wagrell L, et al. Efficacy and safety of Rezūm system water vapor treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology. 2015;86:1042–1047. doi: 10.1016/j.urology.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 41.Schuster TG, Wei JT, Hendlin K, Jahnke R, Roberts WW. Histotripsy treatment of benign prostatic enlargement using the Vortx Rx system: initial human safety and efficacy outcomes. Urology. 2018;114:184–187. doi: 10.1016/j.urology.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Darnell SE, Hall TL, Tomlins SA, Cheng X, Ives KA, Roberts WW. Histotripsy of the prostate in a canine model: characterization of post-therapy inflammation and fibrosis. J Endourol. 2015;29:810–815. doi: 10.1089/end.2014.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taktak S, Jones P, Haq A, Rai BP, Somani BK. Aquablation: a novel and minimally invasive surgery for benign prostate enlargement. Ther Adv Urol. 2018;10:183–188. doi: 10.1177/1756287218760518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilling P, Reuther R, Kahokehr A, Fraundorfer M. Aquablation - image-guided robot-assisted waterjet ablation of the prostate: initial clinical experience. BJU Int. 2016;117:923–929. doi: 10.1111/bju.13358. [DOI] [PubMed] [Google Scholar]
- 45.Gilling P, Barber N, Bidair M, Anderson P, Sutton M, Aho T, et al. WATER: a double-blind, randomized, controlled trial of Aquablation® vs transurethral resection of the prostate in benign prostatic hyperplasia. J Urol. 2018;199:1252–1261. doi: 10.1016/j.juro.2017.12.065. [DOI] [PubMed] [Google Scholar]