Abstract
Human amniotic fluid mesenchymal stem cells (hAF-MSCs) have been shown to be effective in the treatment of many diseases. Platelet lysate (PL) contains multiple growth and differentiation factors; therefore, it can be used as a differentiation inducer. In this study, we attempted to evaluate the efficiency of human platelet lysate (hPL) on cell viability and the effects on cardiomyogenic differentiation of hAF-MSCs. When treating the cells with hPL, the result showed an increase in cell viability. Expressions of cardiomyogenic specific genes, including GATA4, cTnT, Cx43 and Nkx2.5, were higher in the combined treatment groups of 5-azacytidine (5-aza) and hPL than the expressions of cardiomyogenic specific genes in the control group and in the 5-aza treatment group. In terms of the results of immunofluorescence and immunoenzymatic staining, the highest expressions of cardiomyogenic specific proteins were revealed in combined treatment groups. It can be summarized that hPL may be an effective supporting cardiomyogenic supplementary factor for cardiomyogenic differentiation in hAF-MSCs.
Keywords: Cell biology, Biomedical engineering, Molecular biology, Regenerative medicine, Stem cell research, Cardiomyogenic differentiation, Cardiomyocyte-like cells, Human amniotic fluid mesenchymal stem cells, Human platelet lysate, 5-azacytidine
Cell biology; Biomedical engineering; Molecular biology; Regenerative medicine; Stem cell research; Cardiomyogenic differentiation; Cardiomyocyte-like cells; Human amniotic fluid mesenchymal stem cells; Human platelet lysate; 5-azacytidine.
1. Introduction
Coronary artery disease (CAD) is not only a significant health problem that has led to a high mortality rate in Thailand, but it is also known to be a leading cause of death world-wide [1]. Increases in occurrences of CAD across the globe are largely due to poor lifestyle choices [2]. Myocardial infarction (MI) is one of the most common presentations of CAD. Since adult cardiomyocytes are unable to regenerate necrotic tissue to compensate for cardiac dysfunction, massive loss of functional cardiomyocytes can lead to myocardial disorders [3, 4]. For many centuries, a number of therapeutic methods have been developed that are intended to improve the quality of life of patients. Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue and immune disorders. There can be enormous potential for embryonic stem cells (ESCs); however, there are also a number of ethical and political issues associated with ESCs [5]. At present, several cell types are being studied for their potential in the treatment of heart disease [6]. Using stem cells such as MSCs for regenerative medicine has also become an interesting therapeutic mission in the treatment of various incurable diseases [7] including MI [8]. MSCs are multipotent stem cells that have the capability of being differentiated into cardiomyocytes when they have been induced by certain factors such as 5-azacytidine (5-aza) [9, 10]. MSCs can be isolated from adipose tissue, amniotic fluid, bone marrow, placenta, peripheral blood and umbilical cord [11, 12, 13]. They have displayed plastic adherent properties when maintained in standard culture conditions. They have also been found to express MSCs surface markers and can be differentiated into multiple mesenchymal linages [12, 14]. However, adult stem cells (ASCs), such as bone marrow MSCs, have been associated with a range of challenges and obstacles including pain, morbidity, low cell numbers upon harvesting [15] and high levels of immunoreactivity [16]. For these reasons, high yields of cells and favorable growth kinetics of in vitro cultures are practical requirements for a clinically useful cell source that can be used for tissue engineering [17]. Thus, different fetal tissues have been studied for their potential as an alternative source of stem cells.
Human amniotic fluid represents a rich source of MSCs and contains a heterogeneous cell population derived from placental membranes and fetal origins [14]. The hAF-MSCs obtained from the second-trimester of a pregnancy possess a self-renewal capacity and multilineage differentiation potential [18, 19, 20]. They can differentiate into chondrocytes [21, 22], endothelial cells [23], osteocytes [24] and cardiomyocytes [25]. Furthermore, they have been shown to be effective in the treatment of many diseases such as MI [26].
Specifically, 5-aza is a DNA demethylating chemical compound that can induce MSCs into cardiomyocytes. Previous studies have proven that 10 μM of 5-aza can induce cardiomyogenic differentiation in vitro [27, 28, 29]. Notably, hPL is classified as a cell-free, low level protein. It is produced from highly concentrated human platelets in the plasma. The preparation is highly enriched in thrombocytic growth factors, but exhibits a low content of plasma proteins [30, 31]. It is known to contain multiple growth factors such as platelet-derived growth factor (PDGF), basic fibroblast growth factor (b-FGF), insulin-like growth factor (IGF) and transforming growth factor beta (TGF-β) [32, 33]. Interestingly, hPL possesses most of the characteristics that are involved with cardiomyocyte differentiation. Thus, hPL was selected for this study from an extensive review of literature. Based on relevant data, this present study is focused on how hPL could improve the efficiency of hAF-MSCs to be differentiated into cardiomyocyte-like cells. Importantly, hPL was used as an inducing factor when combined with 5-aza for hAF-MSCs without retroviral transduction and reprogramming. The aim of this study was to evaluate the optimal dose of hPL that effects on cell viability and the differentiation potential of hAF-MSCs toward cardiomyocyte-like cells.
2. Materials and methods
2.1. Cell samples
Back-up flask of human amniotic fluid cell (hAF cell) samples with normal karyotype (46, XX/46, XY) (17 samples) were obtained from the 16th-22 nd weeks of gestation by amniocentesis after prenatal diagnosis from the Human Genetics Laboratory, Department of Anatomy, Faculty of Medicine, Chiang Mai University. This study was approved and allowed by the Ethics Committee from the Faculty of Medicine, Chiang Mai University, 13th March 2018, No. ANA-2561-05344.
2.2. Cell preparation and cultivation
The direct adherent method was used to separate hAF-MSCs [34]. In brief, hAF cells that were cultured in 25 cm2 flasks (Corning Incorporated, NY, USA) with expansion medium (BIOAMF-3TM Complete Medium) (Biological Industries, Kibbutz Beit Haemek, Israel) at 37 °C, 5% CO2 and 95% humidity were changed to culture with the basal growth medium comprised of Dulbecco's Modified Eagle Medium (DMEM)–high glucose (Gibco, USA) with a supplement of 10% fetal bovine serum (FBS) (Gibco, South America), gentamycin 40 mg/ml and Pen Strep (penicillin and streptomycin) 10,000 U/ml (Gibco, USA). The medium was changed every 3 days. After the cells reached 80% confluence, they were sub-cultured using 0.25% trypsin-EDTA (Gibco, USA). The cell samples that were collected from the 2nd passage were used in all of our experiments.
The hAF cell samples were observed under a DMi1 inverted phase contrast microscope (Leica Microsystems, USA). The cell samples that were collected from the 2nd passage were washed twice with sterile phosphate-buffered saline (PBS) (Amresco, Ohio, USA) and were trypsinized with 0.25% trypsin-EDTA. Subsequently, hAF cells were suspended in basal growth medium and centrifuged (C2 Series, Centurion Scientific Ltd, UK) at 2,035 g for 6 min at room temperature. After that, the supernatant was removed and the hAF cells were used in the experiments.
2.3. Flow cytometry analysis
The MSCs population in the hAF cell samples was examined by observing the expression of MSC specific cell surface proteins, 3 hAF cell samples with duplicate were incubated with monoclonal antibodies; phycoerythrin (PE)-conjugated mouse anti-human CD31, CD117, HLA-DR (Immuno Tools GmbH, Friesoythe, Germany), mouse anti-human CD44 (Pierce Biotechnology, Rockford, USA), mouse anti-CD45 (Biolegend, San Diego, USA) and mouse anti-human CD73 (Life Technologies, California, USA), as well as fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD34, CD90 (Biolegend, San Diego, USA) and mouse anti-human HLA-ABC (Immuno Tools GmbH, Friesoythe, Germany). PE mouse isotype control and FITC mouse isotype control (Biolegend, San Diego, USA) were used as negative controls. Cell fluorescence was evaluated using FACscan (Becton Dickinson, Lincon Park, NJ) and analyzed using CellQuest Pro 9.0 software (Becton Dickinson).
2.4. Alamar blue cell proliferation assay
Alamar blue cell proliferation assay was used to measure living cell proliferation from the oxidation–reduction of the living cell metabolism. In order to confirm one of the properties of MSCs that can involve cell proliferation, 3 hAF cell samples with duplicate were cultured in a 24-well culture plate with basal growth medium at a concentration of 2 × 103 cells/well for 24 h. Thereafter, the basal growth medium was removed and 100 μl of 10% alamar blue in DMEM was added. The samples were then incubated at 37 °C, 5% CO2 with 95% humidity for 4 h. After that, the supernatant was evaluated using the colorimetric change from each well, while hAF cells were continuously cultivated under the same conditions. Absorbance measurements were taken every other day until day 21 of the cell-culturing procedure using a spectrophotometer plate reader (Original Multiskan EK, Thermoscientific, UK) at 540 and 630 nm. The control solution was performed by 10% alamar blue in pure DMEM aspirated from wells that were without the cells. The % reduction of each case was calculated using the formula
Sx = the alamar blue fluorescence signal of the sample at day x
Scontrol = the signal from the control
S100% = reduced form of alamar blue was produced by autoclaving control
2.5. Human platelet lysate preparation
Human platelet concentrates (hPCs) were obtained from the laboratory of the Blood Bank Section of Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University using apheresis methods after positive red blood cell antibody screening. This study was approved of by the Ethics Committee from the Faculty of Medicine, Chiang Mai University on the 13th of March 2018, No. ANA-2561-05344.
The hPCs were used to prepare the hPL as follows [35, 36]. The hPL analysis was performed by three cycles of freezing (-80 °C) and thawing (37 °C) of the pooled 15 hPC samples. These samples were then centrifuged at 2,200 g for 20 min at room temperature to remove membrane fragments. After that, the supernatant was filtered using 0.2 μm filters (Corning Incorporated, NY, USA). Finally, 4U/ml of heparin (Bellerup, Denmark) was added to prevent clot formation and aliquots of the hPL were stored at -20 °C for 6 months.
2.6. Enzyme-linked immunosorbent assay (ELISAs)
For further characterization of the hPL, the concentrations of the basic fibroblast growth factor (b-FGF), insulin-like growth factor 1 (IGF-1), transforming growth factor β1 (TGF-β1) and vascular endothelial growth factor (VEGF) were determined using commercially available sandwich ELISAs (Sigma-Aldrich, St. Louis, MO, USA). Frozen hPL products were thawed and ELISAs were performed with triplicate according to the manufacturer's instructions. The degree of absorbance was determined with a spectrophotometer plate reader at 450 nm.
2.7. Cell viability
Methylthiazole tetrazolium (MTT) assay was used to evaluate the optimal concentrations of hPL and the viability of the hAF cells after hPL treatment. The 3 hAF cell samples with triplicate were plated in a 96-well culture plate at a density of 5×103 cells for 24 h. After cell plating, cells were exposed with hPL (0.3125–40%) [37, 38] for 24, 48 and 72 h. The basal growth medium was then discarded, replaced with MTT solution (0.5 mg/ml of Thiazolyl Blue Tetrazolium Bromide in DMEM) (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 4 h. After that, the MTT solution was removed and 100 μl of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) was added to dissolve the formazan crystals. Absorbance measurement of the samples was performed using a spectrophotometer plate reader at 540 nm. The control solution was performed by pure DMEM. The percentage of the viable cells was calculated using the following formula.
2.8. Cardiomyogenic specific gene expression analysis
The 3 hAF cell samples with triplicate were cultivated in a 24-well culture plate at a density of 5×104 cells and were incubated with basal growth medium for 24 h. Afterwards, the cells were exposed to 2.5, 5, 10 and 20% of hPL for 21 days, 10μM of 5-aza and 2.5, 5, 10 and 20% of hPL combined with 10μM of 5-aza for 24 h. After 24 h of 5-aza treatment, the medium was changed to basal growth medium until day 21. The concentrations of hPL in this method were determined based on the results of the MTT assay. The concentrations at 2.5–20% were selected as the cells showed the high viability. Total ribonucleic acid (RNA) was extracted by utilizing an Illutra RNAspin Mini RNA Isolation Kit (GE Healthcare, UK), and the complementary DNA (cDNA) was then synthesized from 0.5 μg RNA using the iScript™ cDNA Synthesis kit (Bioline, USA) according to the manufacturer's instructions. Reverse transcriptase − quantitative PCR (RT-qPCR) was carried out using a SensiFAST™ SYBR® No-ROX Kit (Bioline, USA) on a Chromo4™ Real-Time PCR Detector (Bio-Rad, United States). Gene specific primer sequences (GATA4, cTnT, Cx43, Nkx2.5 and GAPDH) are shown (Table 1). GAPDH was used as the internal control gene for the normalization of the relative gene expression level using the 2−ΔΔct method.
Table 1.
The primers for RT–qPCR and products size.
| Genes | Primer sequences | Sizes (bp) |
|---|---|---|
| GATA4 | F5′-CTGTGCCAACTGCCACACCA-3′ | 437 |
| R5′-GGCTGACCGAAGATGCGTAG-3′ | ||
| cTnT | F5′-GGCAGCGGAAGAGGATGCTGAA-3′ | 150 |
| R5′-GAGGCACCAAGTTGGGCATGAACGA-3′ | ||
| Cx43 | F5′-GAATCCTGCTCCTGG-3′ | 380 |
| R5′-GATGCTGATGATGTAG-3′ | ||
| Nkx2.5 | F5′-CTGCCGCCGCCAACAAC-3′ | 136 |
| R5′-CGCGGGTCCCTTCCCTACCA-3′ | ||
| GAPDH | F5′-ATGGGGAAG GTGAAGGTCG-3′ | 70 |
| R5′-TAAAAGCAGCCCTGGTGACC-3′ |
2.9. Differentiation of hAF-MSCs to cardiomyocyte-like cells
The 5 hAF cell samples with duplicate were plated in a 24-well culture plate at a density of 1×105 cells and incubated with basal growth medium for 24 h. After that cells were divided into three groups under different culture medium conditions. The control group was cultured with basal growth medium and the cardiomyogenic induced group was cultured with cardiomyogenic induced medium (10 μM 5-aza) combined with and without 20% of hPL. After 24 h, the cardiomyogenic induced medium was changed to basal growth medium and the culture medium was changed every 3 days for 21 days. Cardiomyogenic specific gene expression (GATA4, cTnT, Nkx2.5 and Cx43) was evaluated by RT-qPCR and the degree of expression of GAPDH was used as the internal control gene for the normalization of the relative gene expression level using the 2−ΔΔct method. Cardiomyogenic specific protein expression was evaluated using immunofluorescence and immunoenzymatic staining.
2.10. Immunohistochemistry staining
2.10.1. Immunofluorescence staining
The control and cardiomyogenic induced groups were cultured on coverslips (Thermo scientific, UK) for 21 days. After fixation for 30 min with 4% paraformaldehyde, the cell membranes were permeabilized with 0.2% triton X-100 (Amresco, Ohio, USA) in PBS and blocked in 10% AB-serum in 1% bovine serum albumin in PBS (BSA-PBS) for 30 min at room temperature. The cells were incubated with mouse monoclonal primary antibodies against human GATA4, cTnT and Nkx2.5 (Sigma-Aldrich, St. Louis, MO, USA) for 2 h at 37 °C. After being washed with PBS, cells were incubated with goat anti-mouse secondary antibody conjugated with FITC (Thermo Scientific, UK) (495 nm/519 nm) for 1 h at 37 °C. Subsequently, the nuclei and cover-slips were mounted onto the microscopic slides using anti-fade reagent with 4′-6-diamidino-2-phenylindole (DAPI) (Invitrogen, USA). Cells were visualized using a fluorescence microscope Olympus AX70. Photographs were taken with a DP manager and DP controller (Olympus Life Science, USA). The degree of expression of the fluorescent signal was assessed using imageJ 1.50i software and calculated by the corrected total cell fluorescence (CTCF).
2.10.2. Immunoenzymatic staining
The control and cardiomyogenic induced groups were cultured on coverslips (Thermo scientific, UK) for 21 days. After fixation, the cells were blocked in 10% AB-serum in 1% BSA-PBS for 30 min at room temperature, and then incubated with mouse anti-human Cx43 primary antibodies (Sigma-aldrich, USA) for 2 h at 37 °C. After being washed with PBS, the cells were incubated with goat anti-mouse horseradish peroxidase secondary antibody (Immuno Tools GmbH, Germany) for 1 h at 37 °C. Finally, the immunoreaction was detected by using 3, 3 -diaminobenzidine (DAB) substrate (Sigma-aldrich, USA). The cells were visualized under Axiostar plus light microscope (Carl Zeiss, Germany). Photographs were taken with Canon pc 1049 PowershotG5 (Canon, USA). The signal expression was analyzed using imageJ 1.50i software and calculated by CTCF.
2.11. Statistical analysis
The data were analyzed with descriptive analysis and presented as the mean ± standard error (S.E.) of the mean. Statistical comparisons were performed three times using the Kruskal Wallis test with a post hoc Dunn's test using SPSS version 22.0 software (IBM Corp). A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Cell preparation and cultivation
A microscopic examination revealed that the hAF cells in the 2nd passage densely adhered to the flask in a monolayer. The homogenous fibroblast-like morphology was then identified (Figure 1).
Figure 1.
Morphology of hAF cells in the 2nd passage displaying homogenous fibroblast-like morphology with 100% confluence.
3.2. Flow cytometry analysis
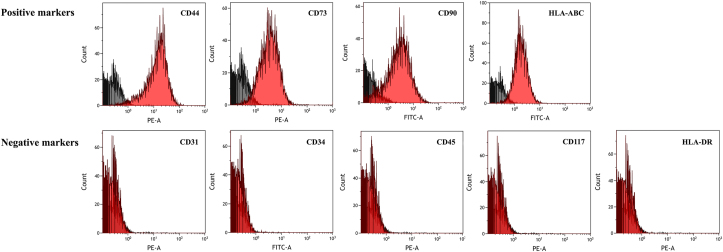
The results revealed that hAF cells positively expressed typical MSCs surface markers including CD44 (75.9 ± 2.3%), CD73 (77.8 ± 2%), CD90 (66.5 ± 2.9%) and HLA-ABC (72.1 ± 7.7%). Additionally, they were negatively stained for CD31 (0.29 ± 0.1%), CD34 (0.25 ± 0.1%), CD45 (0.23 ± 0.2%), CD117 (0.3 ± 0.2%) and HLA-DR (0.32 ± 0.2%) (Figure 2).
Figure 2.
Expression of cell surface markers by flow cytometry histrogram of hAF cells in the 2nd passage showing positive results for CD44, CD73, CD90 and HLA-ABC markers while showing negative results for CD31, CD34 and CD45, CD117 and HLA-DR markers.
3.3. Alamar blue cell proliferation analysis
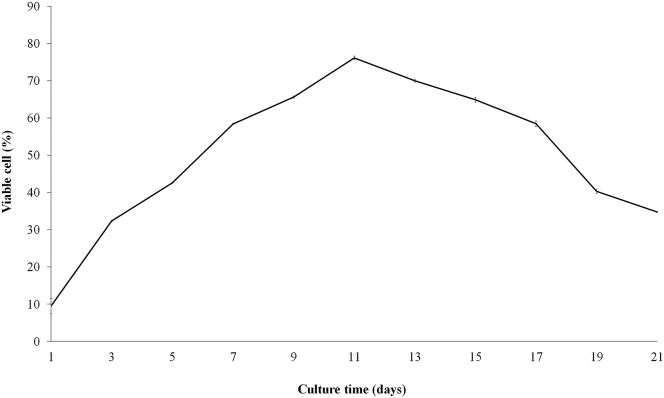
The growth curve continuously increased from the early stage of cultivation (day 1 - day 11). The highest degree of cell proliferation was observed on day 11 by an increase of 8-fold from first day. The continually cultured cells seemed to decrease in cell number and cell-replicating ability as the cell culture process came to an end (Figure 3).
Figure 3.
Results of alamar blue cell proliferation assay showing the growth characteristics of hAF-MSCs culture in the basal growth medium for 21 days. Data are presented as mean ± S.E. values.
3.4. Growth factor quantification
The hPL presented an overview of relevant growth factors including b-FGF, IGF-1, TGF-β1 and VEGF (Table 2).
Table 2.
The concentration of growth factors in hPL.
| Growth factors | Concentration |
|---|---|
| b-FGF | 39.62 ± 0.69 pg/ml |
| IGF-1 | 4.32 ± 0.17 ng/ml |
| TGF-β1 | 100 ± 1.56 pg/ml |
| VEGF | 44.78 ± 1.46 pg/ml |
3.5. Cell viability
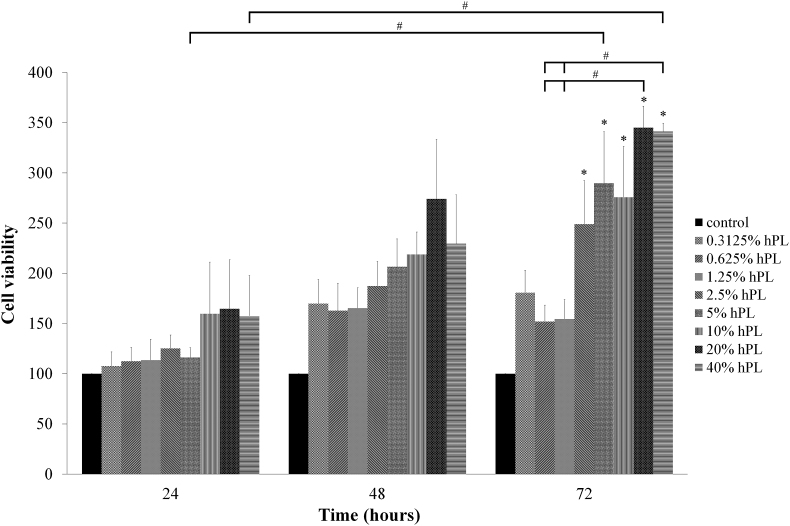
The hAF-MSCs were exposed to different concentrations of hPL (0.3125–40%). The results indicated an increase in cell viability higher than the control at all-time points. At 72 h, the hPL treated cells at concentrations of 2.5, 5, 10, 20 and 40% displayed higher statistically significance compared to the control and at concentrations of 20 and 40% displayed higher statistically significance compared to 0.625 and 1.25%. Moreover, at concentration of 5 and 40% displayed higher statistically significance on hAF-MSCs viability compared to 24 h. Interestingly, at a concentration of 20% hPL displayed the highest degree of cell viability at all-time points (24, 48 and 72 h) (Figure 4).
Figure 4.
Viability of hAF-MSCs exposed to hPL (0.3125–40%) in DMEM for 24, 48, and 72 h. The cells cultured in pure DMEM were used as the control. Data are presented as mean ± S.E. values. ∗ statistically significant versus control. # statistically significant between group.
3.6. Human platelet lysate-regulating effect on cardiomyogenic specific gene expression
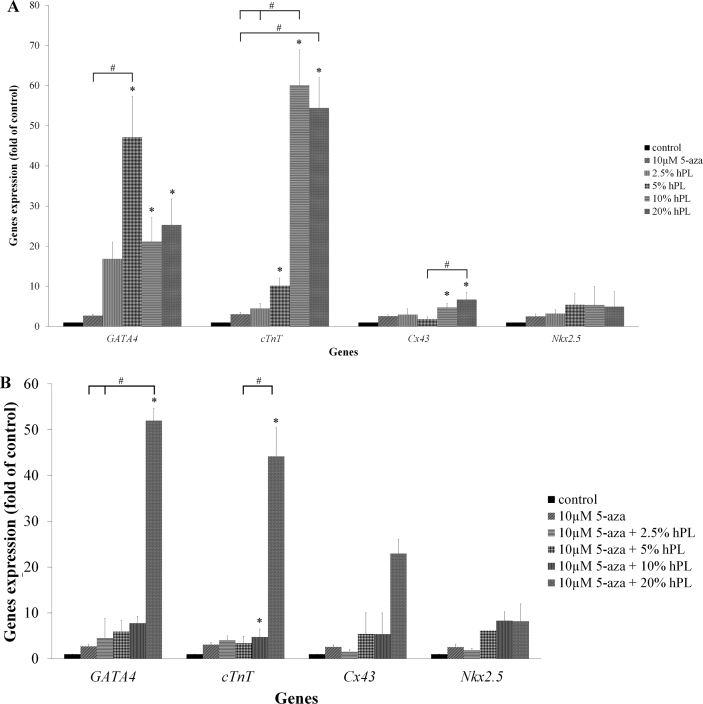
The results revealed that the expression levels of GATA4, cTnT, Cx43 and Nkx2.5 were increased in hPL treatment group when compared to control and 10 μM 5-aza treatment group. GATA4 level was up-regulated through the highest expressions with 47-fold relative to the control group and 17-fold relative to 5-aza treatment group at a concentration of 5% hPL as well as the expression of Nkx2.5 showed 5.5-fold and 2-fold relative to the control group and 10 μM 5-aza treatment group, respectively. cTnT level was up-regulated through the highest expressions with 60-fold relative to the control group and 19.5-fold relative to 10 μM 5-aza treatment group at a concentration of 10% hPL. Cx43 level was up-regulated through the highest expressions with 6.7-fold relative to the control group and 2.6-fold relative to 10 μM 5-aza treatment group at a concentration of 20% hPL (Figure 5A).
Figure 5.
A: hPL ability to up-regulate cardiomyogenic specific genes expression. Each graph displays the expression levels of GATA4, cTnT, Cx43 and Nkx2.5, which were normalized to GAPDH and were relative to the control group. Data are presented as mean ± S.E. values. ∗ statistically significant versus control. # statistically significant between group. B: hPL combined with 5-aza ability to up-regulate cardiomyogenic specific genes expression. Each graph displays the expression levels of GATA4, cTnT, Cx43 and Nkx2.5, which were normalized to GAPDH and were relative to the control group. Data are presented as mean ± S.E. values. ∗ statistically significant versus control. # statistically significant between group.
Furthermore, the results of combined treatment group showed the increased of genes expression levels in combined treatment group when compared to control and 10 μM 5-aza treatment group. Strikingly, at a concentration of 10μM 5-aza with 20% hPL revealed the highest expressions of GATA4 (52-fold), cTnT (44-fold), Cx43 (23-fold) and Nkx2.5 (8-fold) compared to control group. Similarly, the levels of these genes were higher than 10 μM 5-aza treatment group (19-fold, 34-fold, 10-fold and 3-fold, respectively) (Figure 5B).
3.7. Effect of hPL on cardiomyogenic differentiation
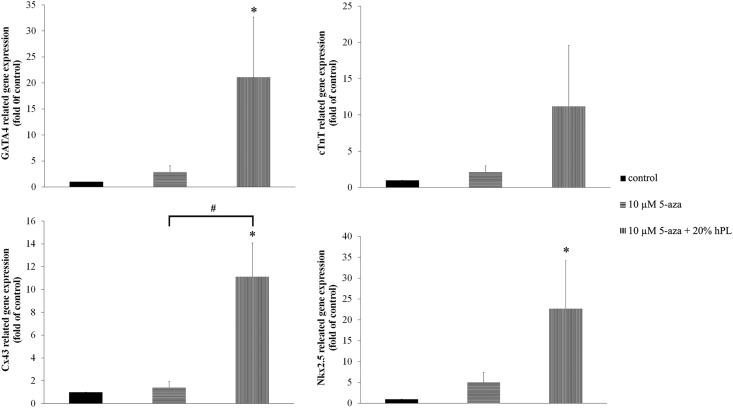
A suitable dose (10 μM 5-aza with 20% hPL) was used in this experiment. The results revealed that the expression levels of GATA4, cTnT, Cx43 and Nkx2.5 were increased in combined treatment group when compared to control and 10 μM 5-aza treatment group. Gene levels could be up-regulated through the highest expressions of GATA4 (21.08-fold), cTnT (11.2-fold), Cx43 (11.12-fold) and Nkx2.5 (22.68-fold) relative to the control. Similarly, the levels of these genes were higher than 10 μM 5-aza treatment group (7-fold, 5-fold, 8-fold and 4.5-fold, respectively) (Figure 6).
Figure 6.
Ability of hPL to promote the differentiation of hAF-MSCs into cardiomyocyte-like cells. Each graph displays the expression levels of GATA4, cTnT, Cx43 and Nkx2.5 that were normalized to GAPDH and were relative to the control group. Data are presented as mean ± S.E. values. ∗ statistically significant versus control. # statistically significant between group.
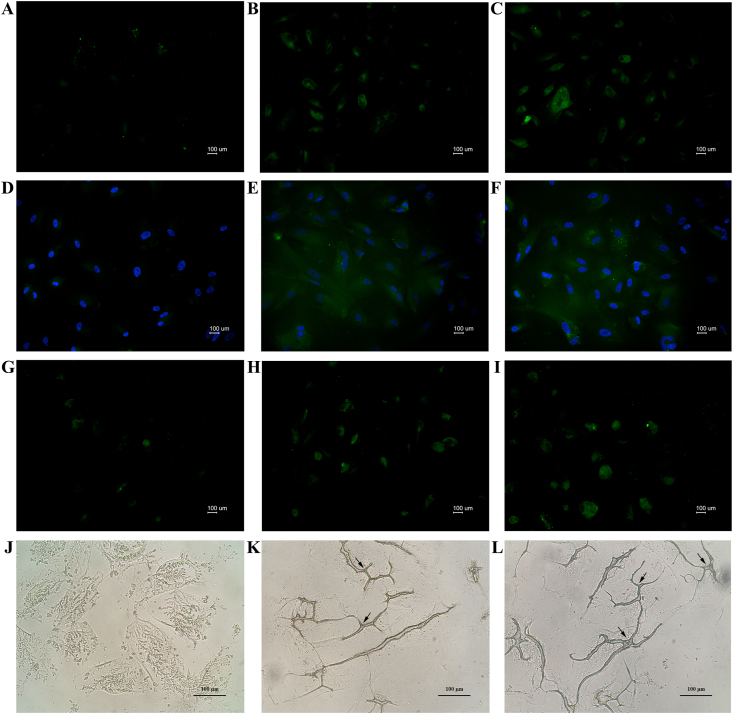
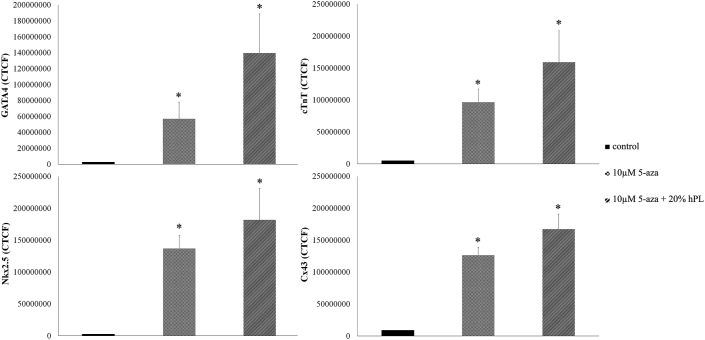
Moreover, cells were investigated for cardiomyogenic specific proteins. The results of immunohistochemistry staining indicated that the combined treatment group was strongly positive for cardiomyogenic specific proteins. Cardiac transcription factors GATA4 and Nkx2.5 proteins are localized in the nucleus. cTnT proteins are localized in the cytoplasm and Cx43 proteins are localized in the cell membrane (Figure 7). The expression levels of proteins signal (CTCF) showed GATA4 (2-fold), cTnT (1.5-fold), Nkx2.5 (1.3-fold) and Cx43 (2-fold) relative to 10 μM 5-aza treatment (Figure 8).
Figure 7.
Detection of cardiomyogenic specific proteins; immunofluorescence staining FITC (495 nm/519 nm), green color for all cardiomyogenic specific proteins (A–I) and immunoenzymatic staining (J–L); GATA4 (localized in nucleus) staining (A) control group, (B) 10 μM 5-aza induced group, (C) 10 μM 5-aza with 20% hPL induced group; cTnT (localized in cytoplasm) staining (D) control group, (E) 10 μM 5-aza induced group, (F) 10 μM 5-aza 20% hPL induced group; Nkx2.5 (localized in nucleus) staining (G) control group, (H) 10 μM 5-aza induced group, (I) 10 μM 5-aza with 20% hPL induced group; Cx43 (localized in cell membrane) staining (J) control group, (K) 10 μM 5-aza induced group (black arrow), (L) 10 μM 5-aza with 20% hPL induced group (black arrow). (A-C and G-I) insets without nuclear counterstain showing no nuclear staining of two key core cardiac transcription factors. Scale bar = 100 μm.
Figure 8.
Image J analysis showing the results of CTCF (the expression levels of GATA4, cTnT, Nkx2.5 and Cx43 proteins signal). Data are presented as mean ± S.E. values. ∗ statistically significant versus control.
4. Discussion
Non-communicable diseases (NCDs) are also known as chronic diseases. A person's lifestyles, along with genetic, physiological and environmental factors, increase the possibility of contracting certain NCDs [39]. CAD is just one NCDs of particular interest. It can weaken the cardiac muscle, cause the death of cardiac muscle cells and contribute to heart failure [40]. Unlike organs such as the skin and liver, after birth most cardiomyocytes stop dividing and become terminally differentiated [4, 41, 42]. Currently, many cell types are being considered for the treatment of various incurable diseases including MI [6, 7, 8]. MSCs have been applied in the treatment or restoration of damaged tissue [26]. Previous studies have reported that MSCs obtained from different sources are associated with different differentiation tendencies [43,44]; moreover, proper cells and the efficiency of differentiation are considered highly important. Previous studies have demonstrated that AF-MSCs represent the intermediate stage between ESCs and ASCs [18, 45]. The advantages of obtaining hAF-MSCs are that they are considered easy, safe and are known to be associated with a low risk of destroying embryos. Moreover, AF-MSCs are capable of self-renewal and high expansion rates, while they possess a high number of isolated cells [46].
In this study, the morphology of hAF cells in the 2nd passage displayed fibroblast-like morphology that adhered to the plastic culture flask. This outcome is in accordance with previous studies which reported that these cells can be classified into three types based on their morphology and growth characteristics, namely amniotic fluid type, epitheloid type and fibroblast type cells. Mesenchymal tissue is considered an origin of fibroblast type cells. At the 1st passage cells has revealed the presence of heterogeneous populations. After the process of sub-culturing, the adherent fibroblast type cells remained during the 2nd passage. The fibroblast cell types are easier to subculture, more selective and display the highest degree of growth potential [46, 47, 48]. Furthermore, several published studies have reported that hAF-MSCs positively expressed MSCs surface markers including CD44, CD73 and CD90 [48, 49, 50, 51]. On the other hand, there were no expressions of the platelet endothelial cells marker (CD31), the HSCs marker (CD34 and CD45) and the AFSCs marker (CD117 or c-kit) [46, 52]. CD117 or c-kit can be used to distinguish between AF-MSCs and amniotic fluid stem cells [48]. In addition, the hAF-MSCs displayed a high degree of expression of HLA-ABC (MHC class I) and a low level of expression of HLA-DR (MHC class II). MHC class I is the antigen that was found on the cell surface of all nucleated cells, while MHC class II is normally found only under inflammatory cell conditions and in activated leukocytes [48]. The data of MSCs surface proteins and the immune-compatibility of hAF-MSCs indicated the possibility of immunosuppression agent reduction in hAF-MSCs for use in clinical applications [53]. Consequently, the findings of this study indicate that the hAF-cells displayed AF-MSCs characteristics after applying the isolation method and have the potential for clinical applications. The growth characteristics of MSCs indicate that these cells had a proliferation capacity that is related to MSCs growth and proliferation pattern. Previous studies have demonstrated that, the growth curve of the proliferation assay recorded in the previous study showed a similar pattern to the MSCs growth pattern, which indicated a short lag-phase at the early stage of proliferation. The log-phase was initiated until reaching the highest peak and then plateaued for a couple of days afterward [21, 23, 24, 54, 55].
The hPL is a growth factor-rich supplement. It was obtained from hPCs via a standardized platelet apheresis technique. This technique is thought to be significantly advantageous owing to the high concentration of platelets and low level of leukocyte contamination that are associated with it [56]. Freezing and thawing is known to be the simplest method, resulting in a mechanical disruption of hPCs. This method presents no impurities of chemical activators; moreover, it is acknowledged as being fast and effective [31, 36, 57]. Notably, hPL contains the most of multiple growth factors [32, 33] that are involved in cardiomyocyte differentiation including IGF-1, b-FGF and TGF-β1. Previous studies have reported that IGF-1 and TGF-β1 could affect the proliferation of embryonic cardiomyocytes; moreover, b-FGF and TGF-β1 could be involved the development of cardiomyocytes [58, 59]. Additionally, the combination of multiple growth factors and induction factors can improve cardiomyogenic differentiation and enhance the cardiomyogenic specific gene expression levels of MSCs in vitro [60, 61]. In 2016, hPL was reported to be able to induce the differentiation of rat BMSCs into cardiomyocytes [37]. In 2017, Jiang and Zhang reported that a combination of TGF-β1 and 5-aza could improve the efficiency of AF-MSCs differentiation into cardiomyocytes [62]. Interestingly, hPL contains most of these growth factors. Consequently, it was selected to be used in this study. TGF-β is an important signaling pathway in the regulation of cardiomyogenic differentiation. It showed an improvement in the ability of human cardiomyocyte progenitor cells to differentiate into functional cardiomyocytes in vitro through the TGF-β signaling pathway [63]. The FGF signaling axis plays an important role in heart development and is essential for normal heart development. Previous studies have reported that cardiac remodeling can be promoted by activating the RAS-MAPK and PI3K pathways through the activation of FGFR1c [64]. Moreover, IGF-1 was found to promote cardiac lineage induction in vitro via activation of PI3K, Akt and mTOR [65].
As has been mentioned previously, 5-aza is also known as a cardiomyogenic inducing factor for different cell types. It can be used in traditional methods of cardiomyogenic induction as a well-known demethylating agent. The expression of the cardiomyogenic specific markers can then be evaluated [66]. It is a synthetic analog of cytosine that changes the expression pattern of a group of genes involved in differentiation. This is likely achieved by suppressing DNA methylation [67, 68]. Although 5-aza-induced differentiation of MSCs into cardiomyocytes has been widely studied, several studies have revealed that 5-aza induced effectively the up-regulation of phosphorylated cardiomyogenic specific genes such as cTnT and Nkx2.5 [69, 70] through the ERK pathway. These results suggest that the sustained activation of ERK by 5-aza contributed to the induction of the differentiation of MSCs into cardiomyocytes in vitro [70].
In the current study, a combination of 10 μM 5-aza with 20% hPL was able to up-regulate the cardiac specific genes and protein levels that play an important role in cardiomyogenesis. GATA4 functions as a critical regulator of cardiac differentiation [71] which expressed in the nucleus. It is known to be highly expressed in cardiac muscle cells throughout the stages of development [11]. Furthermore, it controls the expression of genes involved in the cardiovascular system and cardiac structure [72]. Importantly, cTnT is considered a dependable biomarker that can function as a critical part of the troponin complex of myofibrils only in cardiac muscles and express in cell cytoplasm [73, 74]. Cx43 represents just one variety of gap junction proteins [75]. In 2005, Thimm and colleagues reported that Cx43 functioned along with calcium [76] which then maintained electrical activity (open/closed conformations) [77]. Moreover, it is an important component of ventricles and the cardiac muscle [78]. It is found in cell membrane. Nkx2.5 is essential for normal cardiac development. It is a transcription factor for cardiac development and plays an important role in early cardiac development [74] which is localized in the nucleus. These results indicate that the combined induction may improve the differentiation efficiency of hAF-MSCs into cardiomyocyte-like cells. However, at present, the sample size is limited due to the source of MSCs. Therefore, further studies are required to increase sample sizes to improve the confidence intervals and significant difference in the data. In addition, the proper use of hPL that would be suitable for cardiomyogenic induction is dependent upon the individual source of the MSCs.
5. Conclusion
This study demonstrated that hAF-MSCs the characteristics of MSCs; express the MSCs positive markers, the ability of increased proliferation and could be potentially differentiated into cardiomyocyte-like cells. Furthermore, the results of cell viability demonstrated that 20% hPL displayed the highest degree of cell viability and the combined treatment revealed some encouraging effects on cardiomyogenic differentiation. These results indicated that combined induction could improve the cardiomyogenic differentiation of hAF-MSCs. In conclusion, it has been suggested that 10 μM5-aza with 20% hPL could be considered an effective supporting cardiomyogenic supplementary factor for cardiomyogenic differentiation in hAF-MSCs.
Declarations
Author contribution statement
R. Markmee: Conceived and designed the experiments; Wrote the paper.
S. Aungsuchawan: Conceived and designed the experiments.
P. Pothacharoen, W. Tancharoen and S. Narakornsak: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Faculty of Medicine, Chiang Mai University (NO.ANA-2561-05344).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kiatchoosakun S., Sutra S., Thepsuthammarat K. Coronary artery disease in the Thai population: data from health situation analysis 2010. J. Med. Assoc. Thai. 2012;95(Suppl 7):S149–S155. [PubMed] [Google Scholar]
- 2.Anand S.S., Islam S., Rosengren A., Franzosi M.G., Steyn K., Yusufali A.H., Keltai M., Diaz R., Rangarajan S., Yusuf S. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur. Heart J. 2008;29:932–940. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 3.Hare J.M., Chaparro S.V. Cardiac regeneration and stem cell therapy. Curr. Opin. Organ Transplant. 2008;13:536–542. doi: 10.1097/MOT.0b013e32830fdfc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mescher A.L. McGraw-Hill; New York: 2010. Junqueira's Basic Histology; p. 184. [Google Scholar]
- 5.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 6.Sanganalmath S.K., Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karikkineth B.C., Zimmermann W.H. Myocardial tissue engineering and heart muscle repair. Curr. Pharmaceut. Biotechnol. 2013;14:4–11. doi: 10.2174/138920113804805322. [DOI] [PubMed] [Google Scholar]
- 8.Dimmeler S., Burchfield J., Zeiher A.M. Cell-based therapy of myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 9.Kadivar M., Khatami S., Mortazavi Y., Shokrgozar M.A., Taghikhani M., Soleimani M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2006;340:639–647. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Singh A., Singh A., Sen D. Mesenchymal stem cells in cardiac regeneration: a detailed progress report of the last 6 years (2010-2015) Stem Cell Res. Ther. 2006;7:82. doi: 10.1186/s13287-016-0341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai J., Hu Y., Wang Y.R., Lui L.F., Chen J., Su S.P. Comparison of human amniotic fluid-derived and umbilical cord Wharton's Jelly-derived mesenchymal stromal cells: characterization and myocardial differentiation capacity. J. Geriatr. Cardiol. 2012;9:166–171. doi: 10.3724/SP.J.1263.2011.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominici M., Le Blanc K., Muller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D.J., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Kim J., Lee Y., Kim H., Hwang K., Kwon H., Kim S. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savickiene J., Treigyte G., Baronaite S., Valiuliene G., Kaupinis A., Valius M., Arlauskiene A., Navakauskiene R. Human amniotic fluid mesenchymal stem cells from second- and third-trimester amniocentesis: differentiation potential, molecular signature, and proteome analysis. Stem Cell. Int. 2015:319238. doi: 10.1155/2015/319238. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macias M.I., Grande J., Moreno A., Dominguez I., Bornstein R., Flores A.I. Isolation and characteriazation of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am. J. Obstet. Gynecol. 2010;203:495. doi: 10.1016/j.ajog.2010.06.045. e9-e23. [DOI] [PubMed] [Google Scholar]
- 16.Editorial: Myocardial regeneration by human amniotic fluid stem cells: challenges to be outcome. J. Mol. Cell. Cardiol. 2007;42:730–732. doi: 10.1016/j.yjmcc.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Talkhabi M., Pahlavan S., Aghdami N., Baharvand H. Ascorbic acid promotes the direct conversion of mouse fibroblasts into beating cardiomyocytes. Biochem. Biophys. Res. Commun. 2015;463:699–705. doi: 10.1016/j.bbrc.2015.05.127. [DOI] [PubMed] [Google Scholar]
- 18.Prusa A.R., Marton E., Rosner M., Bernaschek G., Hengstschläger M. Oct-4 expressing cells in human amniotic fluid: a new source for stem cell research. Hum. Reprod. 2003;18:1489–1493. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- 19.Kolambkar Y.M., Peister A., Soker S., Atala A., Guldberg R.E. Chondrogenic differentiation of amniotic fluid-derived stem cells. J. Mol. Histol. 2007;38:405–413. doi: 10.1007/s10735-007-9118-1. [DOI] [PubMed] [Google Scholar]
- 20.Vadasz S., Jensen T., Moncada C., Girard E., Zhang F., Blanchette A., Finck C. Second and third trimester amniotic fluid mesenchymal stem cells can repopulate a de-cellularized lung scaffold and express lung markers. J. Pediatr. Surg. 2014;49:1554–1563. doi: 10.1016/j.jpedsurg.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Narakornsak S., Poovachiranon N., Peerapapong L., Pothacharoen P., Aungsuchawan S. Mesenchymal stem cells differentiated into chondrocyte-Like cells. Acta Histochem. 2016;118:418–429. doi: 10.1016/j.acthis.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Narakornsak S., Aungsuchawan S., Pothacharoen P., Markmee R., Tancharoen W., Laowanitwattana T., Thaojamnong C., Peerapapong L., Boonma N., Tasuya W., Keawdee J., Poovachiranon N. Sesamin encouraging effects on chondrogenic differentiation of human amniotic fluid-derived mesenchymal stem cells. Acta Histochem. 2017;119:451–461. doi: 10.1016/j.acthis.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Tancharoen W., Aungsuchawan S., Pothacharoen P., Markmee R., Narakornsak S., Kieodee J., Boonma N., Tasuya W. Differentiation of mesenchymal stem cells from human amniotic fluid to vascular endothelial cells. Acta Histochem. 2017;119:113–121. doi: 10.1016/j.acthis.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Laowanitwattana T., Aungsuchawan S., Narakornsak S., Markmee R., Tancharoen W., Keawdee J., Boonma N., Tasuya W., Peerapapong L., Pangjaidee N., Pothacharoen P. Osteoblastic differentiation potential of human amniotic fluid-derived mesenchymal stem cells in different culture conditions. Acta Histochem. 2018;120:701–712. doi: 10.1016/j.acthis.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Markmee R., Aungsuchawan S., Narakornsak S., Tancharoen W., Bumrungkit K., Pangchaidee N., Pothacharoen P., Puaninta C. Differentiation of mesenchymal stem cells from human amniotic fluid to cardiomyocyte-like cells. Mol. Med. Rep. 2017;16:6068–6076. doi: 10.3892/mmr.2017.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X., Yang X., Han Z.P., Qu F.F., Shao L., Shi Y.F. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol. Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nartprayut K., U-Pratya Y., Kheolamai P., Manochantr S., Chayosumrit M., Issaragrisil S., Supokawej A. Cardiomyocyte differentiation of perinatally-derived mesenchymal stem cells. Mol. Med. Rep. 2013;7:1465–1469. doi: 10.3892/mmr.2013.1356. [DOI] [PubMed] [Google Scholar]
- 28.Tomita S., Li R.K., Weisel R.D., Mickle D.A.G., Kim E.J., Sakai T., Jia Z.Q. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:247–256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y., Chu Y., Shen W., Dou Z. Effect of 5-azacytidine induction duration on differentiation of human first-trimester fetal mesenchymal stem cells towards cardiomyocyte-like cells. Interact. Cardiovasc. Thorac. Surg. 2009;9:943–946. doi: 10.1510/icvts.2009.211490. [DOI] [PubMed] [Google Scholar]
- 30.Blande I.S., Bassaneze V., Lavini-Ramos C., Fae K.C., Kalil J., Miyakawa A.A., Schettert I.T., Krieger J.E. Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion. 2009;49:2680–2685. doi: 10.1111/j.1537-2995.2009.02346.x. [DOI] [PubMed] [Google Scholar]
- 31.Gstraunthaler G., Rauch C., Feifel E., Lindl T. Preparation of platelet lysates for mesenchymal stem cell culture media. J. Stem Cell. Res. Rev. Rep. 2015;2:1021. [Google Scholar]
- 32.Kilian O., Flesch I., Wenisch S., Taborski B., Jork A., Schnettler R., Jonuleit T. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur. J. Med. Res. 2004;9:337–344. [PubMed] [Google Scholar]
- 33.Van Den Dolder J., Mooren R., Vloon A.P., Stoelinga P.J., Jansen J.A. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12:3067–3073. doi: 10.1089/ten.2006.12.3067. [DOI] [PubMed] [Google Scholar]
- 34.Fei X., Jiang S., Zhang S., Li Y., Ge J., He B., Goldstein S., Ruiz G. Isolation, culture, and identification of amniotic fluid-derived mesenchymal stem cells. Cell Biochem. Biophys. 2013;67:689–694. doi: 10.1007/s12013-013-9558-z. [DOI] [PubMed] [Google Scholar]
- 35.Fekete N., Gadelorge M., Fürst D., Maurer C., Dausend J., Fleury-Cappellesso S., Mailänder V., Lotfi R., Ignatius A., Sensebé L., Bourin P., Schrezenmeier H., Rojewski M.T. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy. 2012;14:540–554. doi: 10.3109/14653249.2012.655420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauch C., Feifel E., Amann E.M., Spöt H.P., Schennach H., Pfaller W., Gstraunthaler G. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011;28:305–316. doi: 10.14573/altex.2011.4.305. [DOI] [PubMed] [Google Scholar]
- 37.Homayouni Moghadam F., Tayebi T., Barzegar K. Differentiation of Rat bone marrow Mesenchymal stem cells into Adipocytes and Cardiomyocytes after treatment with platelet lysate. Int. J. Hematol. Oncol. Stem Cell Res. 2016;10:21–29. [PMC free article] [PubMed] [Google Scholar]
- 38.Naaijkens B.A., Niessen H.W., Prins H.J., Krijnen P.A., Kokhuis T.J., de Jong N., van Hinsbergh V.W., Kamp O., Helder M.N., Musters R.J., van Dijk A., Juffermans L.J. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012;348:119–130. doi: 10.1007/s00441-012-1360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett J.E., Stevens G.A., Mathers C.D., Bonita R., Rehm J., Kruk M.E., Riley L.M., Dain K., Kengne A.P., Chalkidou K., Beagley J., Kishore S.P., Chen W.1, Saxena S., Bettcher D.W., Grove J.T., Beaglehole R., Ezzati M. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet. 2018;392:1072–1088. doi: 10.1016/S0140-6736(18)31992-5. [DOI] [PubMed] [Google Scholar]
- 40.Braun C.A., Anderson C.M. Wolters Kluwer/Lippincott Williams&Wilkins; Philadelphia: 2011. p. 30. (Pathophysiology A Clinical Approach). [Google Scholar]
- 41.Fukuda K. Development of regenerative cardiomyocytes from mesenchymal stem cells for cardiovascular tissue engineering. Artif. Organs. 2001;25:187–193. doi: 10.1046/j.1525-1594.2001.025003187.x. [DOI] [PubMed] [Google Scholar]
- 42.Passier R., Mummery C. Cardiomyocyte differentiation from embryonic and adult stem cells. Curr. Opin. Biotechnol. 2005;16:498–502. doi: 10.1016/j.copbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Struys T., Moreels M., Martens W., Donders R., Wolfs E., Lambrichts I. Ultrastructural and immunocytochemical analysis of multilineage differentiated human dental pulp- and umbilical cord-derived mesenchymal stem cell. Cells Tissues Organs. 2011;193:366–378. doi: 10.1159/000321400. [DOI] [PubMed] [Google Scholar]
- 44.Cui X., Chen L., Xue T., Yu J., Liu J., Ji Y., Cheng L. Human umbilical cord and dental pulp-derived mesenchymal stem cells: biological characteristics and potential roles in vitro and in vivo. Mol. Med. Rep. 2015;11:3269–3278. doi: 10.3892/mmr.2015.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi B., Merlo B., Colleoni S., Iacono E., Tazzari P.L., Ricci F., Lazzari G., Galli C. Isolation and in vitro characterization of bovine amniotic fluid derived stem cells at different trimesters of pregnancy. Stem Cell. Res. Rep. 2014;10:712–724. doi: 10.1007/s12015-014-9525-0. [DOI] [PubMed] [Google Scholar]
- 46.Ferdaos N., Nordin N. Human amniotic fluid cells and their future perspectives. Regener. Res. 2012:14–19. 2012. [Google Scholar]
- 47.Chao K.C., Yang H.T., Chen M.W. Human umbilical cord mesenchymal stem cells suppress breast cancer tumourigenesis through direct cell-cell contact and internalization. J. Cell Mol. Med. 2012;16:1803–1815. doi: 10.1111/j.1582-4934.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Coppi P., G Bartsch, Siddiqui M.M., Xu T., Santos C.C., Perin L., Mostoslavsky G., Serre A.C., Snyder E.Y., Yoo J.J., Furth M.E., Soker S., Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 49.Pievani A., Scagliotti V., Russo F.M., Azario I., Rambaldi B., Sacchetti B., Marzorati S., Erba E., Giudici G., Riminucci M., Biondi A., Vergani P., Serafini M. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy. 2014;16:893–905. doi: 10.1016/j.jcyt.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramos T., Sánchez-Abarca L.I., Muntión S., Preciado S., Puig N., López-Ruano G., Hernández-Hernández Á., Redondo A., Ortega R., Rodríguez C., Sánchez-Guijo F., del Cañizo C. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. 2016;14 doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mareschi K., Rustichelli D., Comunanza V., De Fazio R., Cravero C., Morterra G., Martinoglio B., Medico E., Carbone E., Benedetto C., Fagioli F. Multipotent mesenchymal stem cells from amniotic fluid originate neural precursors with functional voltage-gated sodium channels. Cytotherapy. 2009;11:534–547. doi: 10.1080/14653240902974024. [DOI] [PubMed] [Google Scholar]
- 52.Cananzi M., De Coppi P. CD117 (+) amniotic fluid stem cells: state of the art and future perspectives. Organogenesis. 2012;8:77–88. doi: 10.4161/org.22426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rockett J.C., Darnton S.J., Crocker J., Matthews H.R., Morris A.G. Expression of HLA-ABC, HLA-DR and intercellular adhesion molecule-1 in oesophageal carcinoma. J. Clin. Pathol. 1995;48:539–544. doi: 10.1136/jcp.48.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carraro G., Garcia O.H., Perin L., Filippo R.D., Warburton D. Amniotic fluid stem cells. Amniotic fluid stem cells, Embryonic stem cells-Differentiation and pluripotent alternatives. In: Kallos M.S., editor. Embryonic Stem Cells-Differentiation and Pluripotent Alternatives. 2011. pp. 493–506. (IN TECH). 2011. [Google Scholar]
- 55.Vellasamy S., Sandrasaigaran P., Vidyadaran S., George E., Ramasamy R. Isolation and characterisation of mesenchymal stem cells derived from human placenta tissue. World J. Stem Cell. 2012;4:53–61. doi: 10.4252/wjsc.v4.i6.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naskou M.C., Sumner S.M., Chocallo A., Kemelmakher H., Thoresen M., Copland I., Galipeau J., Peroni J.F. Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018;9 doi: 10.1186/s13287-018-0823-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus. Med. Hemotherapy. 2013;40:326–335. doi: 10.1159/000354061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kardami E. Stimulation and inhibition of cardiac myocyte proliferation in vitro. Mol. Cell. Biochem. 1990;92:129–135. doi: 10.1007/BF00218130. [DOI] [PubMed] [Google Scholar]
- 59.Choi W.Y., Gemberling M., Wang J., Holdway J.E., Shen M.C., Karlstrom R.O., Poss K.D. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development. 2013;140:660–666. doi: 10.1242/dev.088526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Behfar A., Yamada S., Crespo-Diaz R., Nesbitt J.J., Rowe L.A., Perez-Terzic C., Gaussin V., Homsy C., Bartunek J., Terzic A. Guided cardiopoiesis enhances therapeutic benefit of bone marrow human mesenchymal stem cells in chronic myocardial infarction. J. Am. Coll. Cardiol. 2010;56:721–734. doi: 10.1016/j.jacc.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sachinidis A., Gissel C., Nierhoff D., Hippler-Altenburg R., Sauer H., Wartenberg M., Hescheler J. Identification of plateled-derived growth factor-BB as cardiogenesis-inducing factor in mouse embryonic stem cells under serum-free conditions. Cell. Physiol. Biochem. 2003;13:423–429. doi: 10.1159/000075130. [DOI] [PubMed] [Google Scholar]
- 62.Jiang S., Zhang S. Differentiation of cardiomyocytes from amniotic fluid-derived mesenchymal stem cells by combined induction with transforming growth factor β1 and 5-azacytidine. Mol. Med. Rep. 2017;16:5887–5893. doi: 10.3892/mmr.2017.7373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goumans M.J., de Boer T.P., Smits A.M., van Laake L.W., van Vliet P., Metz C.H., Korfage T.H., Kats K.P., Hochstenbach R., Pasterkamp G., Verhaar M.C., van der Heyden M.A., de Kleijn D., Mummery C.L., van Veen T.A., Sluijter J.P., Doevendans P.A. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–149. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Itoh N., Ohta H., Nakayama Y., Konishi M. Roles of FGF signals in heart development, health, and disease. Front. Cell Dev. Biol. 2016;4:110. doi: 10.3389/fcell.2016.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engels M.C., Rajarajan K., Feistritzer R., Sharma A., Nielsen U.B., Schalij M.J., de Vries A.A., Pijnappels D.A., Wu S.M. Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm. Stem Cell. 2014;32:1493–1502. doi: 10.1002/stem.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoon B.S., Yoo S.J., Lee J.E., You S., Lee H.T., Yoon H.S. Enhanced differentiation of human embryonic stem cells into cardiomyocytes by combining hanging drop culture and 5-azacytidine treatment. Differentiation. 2006;74:149–159. doi: 10.1111/j.1432-0436.2006.00063.x. [DOI] [PubMed] [Google Scholar]
- 67.Rosenblatt-Velin N., Lepore M.G., Cartoni C., Beermann F., Pedrazzini T. FGF-2 controls the differentiation of resident cardiac precursors into functional cardiomyocytes. J. Clin. Invest. 2005;115:1724–1733. doi: 10.1172/JCI23418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hupkes M., Jonsson M.K., Scheenen W.J., van Rotterdam W., Sotoca A.M., van Someren E.P., van der Heyden M.A., van Veen T.A., van Ravestein-van Os R.I., Bauerschmidt S., Piek E., Ypey D.L., van Zoelen E.J., Dechering K.J. Epigenetics: DNA demethylation promotes skeletal myotube maturation. Faseb. J. 2011;25:3861–3872. doi: 10.1096/fj.11-186122. [DOI] [PubMed] [Google Scholar]
- 69.Khajeniazi S., Solati M., Yazdani Y., Soleimani M., Kianmehr A. Synergistic induction of cardiomyocyte differentiation from human bone marrow mesenchymal stem cells by interleukin 1β and 5-azacytidine. Biol. Chem. 2016;397:1355–1364. doi: 10.1515/hsz-2016-0151. [DOI] [PubMed] [Google Scholar]
- 70.Qian Q., Qian H., Zhang X., Zhu W., Yan Y., Ye S., Peng X., Li W., Xu Z., Sun L., Xu W. 5-Azacytidine induces cardiac differentiation of human umbilical cord-derived mesenchymal stem cells by activating extracellular regulated kinase. Stem Cell. Dev. 2012;21:67–75. doi: 10.1089/scd.2010.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosca A.M., Burlacu A. Effect of 5-azacytidine: evidence for alteration of the multipotent ability of mesenchymal stem cells. Stem Cell. Dev. 2011;20:1213–1221. doi: 10.1089/scd.2010.0433. [DOI] [PubMed] [Google Scholar]
- 72.Xu M., Millard R.W., Ashraf M. Role of GATA-4 in differentiation and survival of bone marrow mesenchymal stem cells. Prog. Mol. Biol. Transl. Sci. 2012;111:217–241. doi: 10.1016/B978-0-12-398459-3.00010-1. [DOI] [PubMed] [Google Scholar]
- 73.Oma Y., Harata M. Actin-related proteins localized in the nucleus: from discovery to novel roles in nuclear organization. Nucleus. 2011;2:38–46. doi: 10.4161/nucl.2.1.14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stennard F.A., Costa M.W., Elliott D.A., Rankin S., Haast S.J., Lai D., McDonald L.P., Niederreither K., Dolle P., Bruneau B.G., Zorn A.M., Harvey R.P. Cardiac T-box factor Tbx20 directly interacts with Nkx2-5, GATA4, and GATA5 in regulation of gene expression in the developing heart. Dev. Biol. 2003;262:206–224. doi: 10.1016/s0012-1606(03)00385-3. [DOI] [PubMed] [Google Scholar]
- 75.Laird D.W. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Thimm J., Mechler A., Lin H., Rhee S., Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J. Biol. Chem. 2005;280:10646–10654. doi: 10.1074/jbc.M412749200. [DOI] [PubMed] [Google Scholar]
- 77.Shen H., Wang Y., Zhang Z., Yang J., Hu S., Shen Z. Mesenchymal stem cells for cardiac regenerative therapy: optimization of cell differentiation strategy. Stem Cell. Int. 2015 doi: 10.1155/2015/524756. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Antunes E., Borrecho G., Oliveira P., Brito J., Águas A., Martins dos Santos J. Immunohistochemical evaluation of cardiac connexin43 in rats exposed to low-frequency noise. Int. J. Clin. Exp. Pathol. 2013;6:1874–1879. [PMC free article] [PubMed] [Google Scholar]