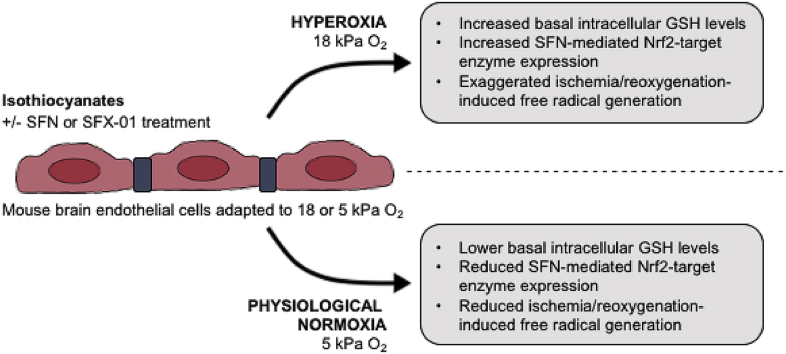
Abstract
Ischemic stroke is associated with a surge in reactive oxygen species generation during reperfusion. The narrow therapeutic window for the delivery of intravenous thrombolysis and endovascular thrombectomy limits therapeutic options for patients. Thus, understanding the mechanisms regulating neurovascular redox defenses are key for improved clinical translation. Our previous studies in a rodent model of ischemic stroke established that activation of Nrf2 defense enzymes by pretreatment with sulforaphane (SFN) affords protection against neurovascular and neurological deficits. We here further investigate SFN mediated protection in mouse brain microvascular endothelial cells (bEnd.3) adapted long-term (5 days) to hyperoxic (18 kPa) and normoxic (5 kPa) O2 levels. Using an O2-sensitive phosphorescent nanoparticle probe, we measured an intracellular O2 level of 3.4 ± 0.1 kPa in bEnd 3 cells cultured under 5 kPa O2. Induction of HO-1 and GCLM by SFN (2.5 μM) was significantly attenuated in cells adapted to 5 kPa O2, despite nuclear accumulation of Nrf2. To simulate ischemic stroke, bEnd.3 cells were adapted to 18 or 5 kPa O2 and subjected to hypoxia (1 kPa O2, 1 h) and reoxygenation. In cells adapted to 18 kPa O2, reoxygenation induced free radical generation was abrogated by PEG-SOD and significantly attenuated by pretreatment with SFN (2.5 μM). Silencing Nrf2 transcription abrogated HO-1 and NQO1 induction and led to a significant increase in reoxygenation induced free radical generation. Notably, reoxygenation induced oxidative stress, assayed using the luminescence probe L-012 and fluorescence probes MitoSOX™ Red and FeRhoNox™-1, was diminished in cells cultured under 5 kPa O2, indicating an altered redox phenotype in brain microvascular cells adapted to physiological normoxia. As redox and other intracellular signaling pathways are critically affected by O2, the development of antioxidant therapies targeting the Keap1-Nrf2 defense pathway in treatment of ischemia-reperfusion injury in stroke, coronary and renal disease will require in vitro studies conducted under well-defined O2 levels.
Keywords: Brain endothelial cells, Keap1-Nrf2, Heme oxygenase-1, Glutamate cysteine ligase, Ischemia-reoxygenation, Redox signaling, Sulforaphane, Oxygen, Physiological normoxia
Graphical abstract
Highlights
-
•
Physiological normoxia alters the redox phenotype of murine microvascular brain endothelial cells.
-
•
Intracellular GSH levels are lower in bEnd.3 cells adapted to 5 kPa versus 18 kPa O2.
-
•
Nrf2 activated HO-1 and GCLM expression is attenuated under physiological normoxia.
-
•
Sulforaphane protects against reoxygenation induced reactive oxygen species generation via Nrf2.
1. Introduction
Ischemic stroke is a leading cause of death and adult morbidity worldwide [1]. The critical reduction of blood flow within a major cerebral artery leads to reduced oxygen and nutrient delivery to the brain [2], time-dependent neuronal cell death and the development of neurological deficits [3]. The initiation of a pathophysiological cascade, involving oxidative stress and inflammation [4], is further exacerbated by the generation of reactive oxygen species (ROS) and mitochondrial dysfunction during reperfusion [5,6]. Timely restoration of cerebral blood flow is currently the only effective pharmacological treatment for acute ischemic stroke. Treatment with tissue plasminogen activator (rt-PA) improves reperfusion and functional outcomes, yet is limited to a ~4.5 h window after the onset of stroke due an increased risk of hemorrhagic transformation [7,8]. Recent trials of endovascular thrombectomy in stroke patients with large vessel occlusion report significant improvements in functional outcomes [1]. However, increased generation of reactive oxygen species in ischemic brain regions may compromise potentially rescuable penumbral tissue surrounding the infarct core [5,9].
Disruption of the blood-brain barrier (BBB) in ischemic stroke leads to extravasation of blood-borne inflammatory cells and fluid into the brain parenchyma which underlies dysregulation of neurovascular function [[10], [11], [12], [13]]. Our previous studies in a rodent model of ischemic stroke established that pretreatment of animals with the dietary isothiocyanate sulforaphane (SFN) [14], an electrophilic activator of the redox sensitive transcription factor Nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) [15,16], significantly reduces BBB permeability, infarct volume and behavioral deficits [17,18]. Our MRI studies further demonstrated that prophylactic SFN delivery reduced lesion volume, consistent with reduced BBB permeability to IgG and improved neurological outcome [17]. Notably, SFN rapidly enters the brain [19] and upregulates Nrf2 and HO-1 expression in brain perivascular astrocytes and endothelial cells [17,18].
The majority of studies in endothelial and other cell types are conducted during culture under atmospheric O2 (18 kPa), whereas most cells experience much lower levels in vivo, with brain endothelial cells exposed to ~3–7 kPa [20]. Hyperoxic conditions create a pro-oxidation environment, reducing replicative lifespan [21] and enhancing cellular antioxidant defenses [22,23], thereby potentially limiting the clinical relevance of in vitro findings. We recently reported that SFN mediated induction of select Nrf2 target genes in umbilical vein endothelial cells (HUVEC) is attenuated under physiological normoxia (5 kPa O2) compared to atmospheric O2 levels [22]. Moreover, we reported that adaptation of HUVEC to 5 kPa O2 enhances nitric oxide bioavailability, modulates agonist-induced Ca2+ signaling [24] and protects against Ca2+ overload due to increased SERCA activity [25].
In this study, we further explore the mechanisms underlying SFN afforded protection in ischemic stroke by investigating redox signaling in mouse brain microvascular endothelial cells (bEnd.3) subjected to hypoxia-reoxygenation following adaptation to defined O2 levels. Our findings demonstrate that SFN induces Nrf2-regulated defense enzymes in bEnd.3 cells to protect against reoxygenation induced reactive oxygen species generation. These findings together with our study in of ischemic stroke in vivo [17,18] suggest that SFN may be a prophylactic therapeutic for targeting the Keap1-Nrf2 defense pathway in stroke and potentially coronary and renal disease.
2. Methods and materials
2.1. Culture and adaptation of bEnd.3 cells under defined O2 levels
Endothelialpolyoma middle T antigen transformed mouse brain microvascular endothelial cells (bEnd.3) were obtained from ATCC-LGC (Teddington, UK). Cells were cultured in phenol red free DMEM (Sigma, UK), supplemented with fetal calf serum (10%), l-glutamine (4 mM) and penicillin (100U/ml)/streptomycin (100 μg/ml). Cell monolayers were maintained for at least 5 days (d) in an O2-regulated dual workstation (Scitive, Baker-Ruskinn, USA), gassed to 18 kPa (hyperoxia), 5 kPa (physiological normoxia) or 1 kPa (hypoxia) O2 under 5% CO2 at 37 °C. This experimental protocol ensures adaptation of the cell proteome [20] and obviates re-exposure of cells to room air, as all cell culture, treatments and experiments are conducted within the O2-regulated workstation and/or plate reader (CLARIOstar, BMG Labtech, Germany). All experiments were conducted using bEnd.3 cells in passages 7–15.
2.2. Phosphorescence lifetime measurements of O2 levels in bEnd.3 cell cytosol and medium
Intracellular O2 levels were monitored in live cells using a cell-penetrating phosphorescent platinum–porphyrin based nanoparticle probe, MitoXpress®-INTRA (Agilent, USA) [26]. A time-resolved fluorescence plate reader (CLARIOstar, BMG Labtech), equipped with an atmospheric control unit, enabled us to measure cytosolic O2 levels under defined ambient O2 levels. bEnd.3 cells were seeded into 96-well black microtitre plates and loaded with MitoXpress®-INTRA (10 μg/ml) for 16 h in complete DMEM. The probe emits a phosphorescence signal at 655 ± 55 nm when excited at 355 ± 55 nm [22,24]. Molecular oxygen quenches the phosphorescence signal, and the signal decay is inversely proportional to the concentration of O2. Phosphoresence intensity after excitation was measured after 30 μs (t1) and 70 μs (t2) with a 30μs window and converted to probe lifetime (τ) using the formula: τ=(t2–t1)/ln (f1/f2), where f1 and f2 represent phosphorescence intensities at respective timepoints [27]. Averaged lifetime measured at 7 ambient O2 tensions was plotted against the known O2 tension and subjected to an exponential fit analysis. Lifetime values were then interpolated from this curve (see Fig. 2B) to give the dissolved intracellular O2 level in live bEnd.3 cells. Dissolved O2 culture medium was also measured in parallel by diluting MitoXpress®-INTRA (2.5 μg/ml) in DMEM medium.
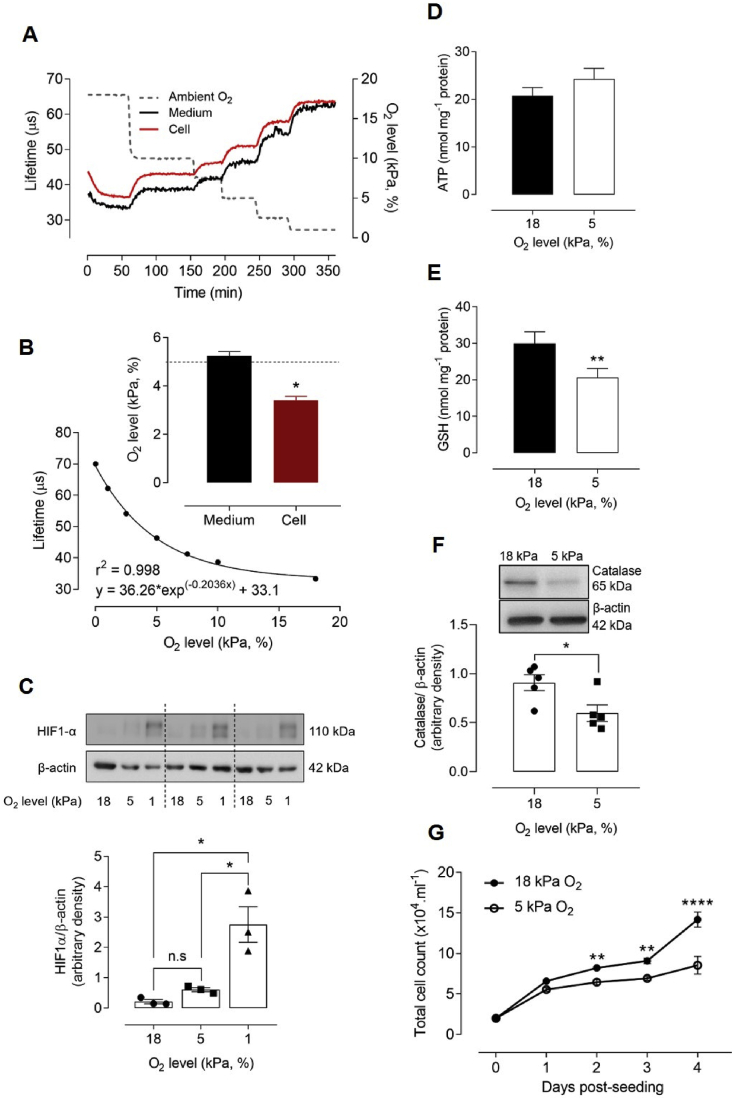
Fig. 2.
Adaptation to 5 kPa O2alters the redox phenotype of bEnd.3 cells in the absence of HIF-1α stabilization
bEnd.3 cells adapted to 18 kPa O2 were loaded with MitoXpress®-INTRA for 16 h, transferred rapidly to an O2-regulated plate reader and exposed to stepwise reductions in O2 (dotted line, right axis). (A) Phosphorescence lifetime measurements (see Methods) in cells and dissolved O2 in medium. (B) Averaged phosphorescence lifetime versus ambient O2 levels in the plate reader were fit by exponential analysis. Inset: Interpolated O2 content in bEnd.3 cell cytosol and medium under 5 kPa O2 (dashed line). (C) Immunoblots of HIF-1α expression relative to β-actin and densitometric analysis of 3 cultures (separated by dashed lines). (D–E) Intracellular ATP and GSH levels in cells adapted for 5 d to 18 or 5 kPa O2. (F) Immunoblot and densitometric analysis of catalase expression relative to β-actin under 18 or 5 kPa O2. (G) Differential rate of bEnd.3 cell proliferation under 18 or 5 kPa O2. Data denote mean ± S.E.M., n = 3–5 independent bEnd.3 cell cultures, *P < 0.05, **P < 0.01, ****P < 0.0001, n.s. non-significant.
2.3. Immunoblotting
Cell lysates were extracted with SDS lysis buffer containing protease and phosphatase inhibitors (pH 6.8) on ice for 10 min. Denatured samples (10 μg) were separated by gel electrophoresis, electro-transferred onto polyvinylidene difluoride membranes and then probed with primary and HRP-conjugated secondary antibodies, using Lamin A/C (Santa Cruz, USA), α-tubulin (Millipore, UK) or β-actin (Sigma-Aldrich, USA) as reference proteins for nuclear and cell protein, respectively [22,28]. Nuclear protein was extracted using a nuclear extraction kit (Active Motif). Membranes were probed for HO-1 (Cell Signaling Technology), GCLM (gift from Prof. T. Kavanagh, University of Washington, WA, USA), NQO1 (Santa Cruz, USA), HIF-1α (Abcam, UK), catalase (Calbiochem, UK) and Nrf2 (Santa Cruz, USA). Protein expression was determined by enhanced chemiluminescence with images captured in a gel documentation system (G-BOX, Syngene Ingenius Bioimaging) and analysed by densitometry using Image J software (NIH, USA).
2.4. Quantitative RT-PCR
bEnd.3 cell RNA was isolated using a Nucleospin RNA Kit (Macherey-Nagel) and RNA content and purity assessed using a spectrophotometer (NanoDrop, Thermo Scientific, UK). Total RNA was reverse–transcribed using a high capacity cDNA conversion kit (Applied Biosystems) and HO-1, NQO1, GCLM, Bach1 and Keap1mRNA assessed by real-time qPCR (Corbett Rotorgene) [22,28] and normalized to the geometric mean of β-2-microglobulin (B2M), ribosomal protein L13a (RPL13A) and succinate dehydrogenase unit complex A (SDHA) (see primer sequences in Supplementary Table S1).
2.5. siRNA Nrf2 silencing
bEnd.3 cells were seeded at 30,000 cells/well and transfected with 40 pmol/well of either scrambled siRNA or Nrf2 siRNA (Santa Cruz, USA) [28] for 24 h using Dharmafect 4 transfection reagent (GE Healthcare, USA), as previously described [29].
2.6. Measurement of intracellular glutathione and ATP levels and cell viability
bEnd.3 cells were adapted to 18 or 5 kPa O2 for 5 days, and intracellular ATP and GSH extracted using 6.5% trichloroacetic acid (TCA, Sigma, UK). For ATP measurements, extracts were incubated with firefly lantern extract (Sigma, UK) containing both luciferase and luciferin, while total GSH levels were determined using a fluorometric assay [22,30]. Fluorescence and luminescence were measured in a plate reader (CLARIOstar, BMG Labtech). Cell viability was determined assaying mitochondrial dehydrogenase activity with 3-[4,5-dimethylthiazol-2-yl]2,5-diphenyl tetrazolium bromide (Sigma, UK) [29].
2.7. L-012 luminescence measurements of reactive oxygen species generation
bEnd.3 cells were seeded into white-walled, clear-bottomed 96-well plates and adapted for 5 d under 18 or 5 kPa O2. Confluent monolayers were incubated in low-serum medium (1% FCS) for 24 h prior to incubation in Krebs buffer in the absence or presence of superoxide dismutase (SOD, 100U/ml, Sigma, UK), polyethylene glycol SOD (PSOD, 50U/ml, Sigma, UK), polyethylene glycol catalase (PCAT, 200U/ml, Sigma, UK) or the NADPH oxidase inhibitor VAS2870 (VAS, 5 μM) [31] and the chemiluminescent luminol analogue L-012 (8-amino-5-chloro-7-phenyl-pyridol [3,4-d] pyridazine-1,4-(2H, 3H)dione sodium salt, 10 μM, Wako Chemicals) [32,33]. Cells adapted to either 18 or 5 kPa O2 were then rapidly (<30 s) transferred to an O2-regulated plate reader (CLARIOstar, BMG Labtech) at 37 °C and exposed to hypoxia (1 kPa O2) for 1 h and reoxygenation under 18 or 5 kPa O2, respectively. Luminescence was measured at 60 s intervals over 3 h and data expressed as mean light units/mg protein.
2.8. Mitochondrial reactive oxygen species measured using MitoSOX™ Red
Mitochondrial reactive oxygen species generation was measured using a mitochondrial targeted fluorogenic probe MitoSOX™ Red [34], and we previously confirmed that MitoSOX fluorescence in endothelial cells is attenuated by scavenging superoxide [28,35]. bEnd.3 cells seeded in black-walled, clear-bottomed 96-well plates were cultured under 18 or 5 kPa O2 for 5 d and then incubated in serum-free DMEM in the absence or presence of rotenone (1 μM, complex 1 inhibitor) or l-NAME (100 μM, eNOS inhibitor). Cells were exposed to hypoxia (1 kPa O2, 1 h) and loaded with MitoSOX™ Red (5 μM, Invitrogen) for 5 min before the start of reoxygenation under 18 or 5 kPa O2, respectively. Cells were washed twice with ice-cold PBS and fixed with 4% paraformaldehyde for 10 min before staining nuclei with DAPI (2 μg/ml, Sigma). MitoSOX™ Red fluorescence (Ex 545 nm/Em 602 nm) was detected using a Nikon Diaphot microscope, with images captured using an ORCA-03G (Hamamatsu, Japan) camera with 0.89 s exposure. Fluorescence quantification was conducted using image analysis software (ImageJ, NIH, USA), measuring the integrated intensity of fluorescence, area of field of view and mean grey value.
2.9. Intracellular free iron levels in bEnd.3 cells measured using FeRhoNox™-1
Intracellular iron release was measured using FeRhoNox™-1 (Goryo Chemical, Japan), a free iron turn-on fluorescent indicator specific for the detection of labile iron Fe(II) [36,37]. Cells in black, clear-bottomed 96-well plates were adapted to 18 or 5 kPa O2 and then incubated with FeRhoNox (5 μM) for 1 h, washed twice with PBS and incubated for 30 min with Hank's Balanced Saline Solution (HBSS, Gibco) containing either vehicle (DMSO, 0.01%), PEG-SOD (PSOD, 50U/ml) or the SOD inhibitor (ammonium tetrathiomolybdate, 4 μM) [38] before an assay. Fluorescence (Ex 540 nm/Em 575 nm) was measured in an O2-regulated plate reader (CLARIOstar, BMG Labtech).
2.10. Statistics
Data denote the mean ± S.E.M. of at least 3–5 different bEnd.3 cell cultures and were processed using Graphpad Prism 8, with some preliminary handling steps performed using MARS data analysis software (BMG Labtech). Significance was assessed using either a paired Student's t-test or one- or two-way ANOVA followed by a Bonferroni Post Hoc test where appropriate, with significance confirmed by P < 0.05.
3. Results
3.1. Sulforaphane induces Nrf2 nuclear translocation and antioxidant enzymes in bEnd.3 cells
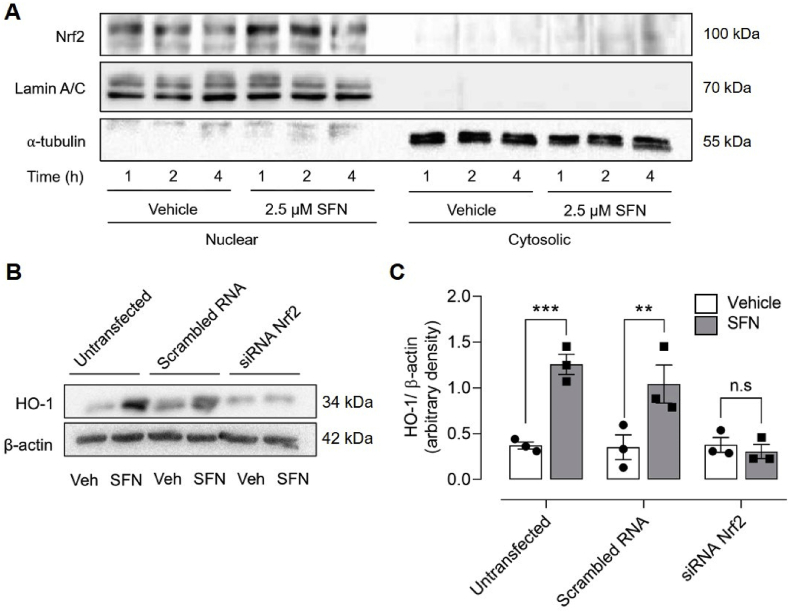
Treatment of bEnd.3 cells under 18 kPa O2 with the Nrf2 inducer sulforaphane (SFN, 2.5 μM) increased nuclear accumulation of Nrf2 over 1–2 h (Fig. 1A). Nrf2 gene silencing had negligible effects on basal HO-1 protein levels but abrogated SFN-induced upregulation of HO-1 (Fig. 1B and C) and NQO1 (data not shown) expression. In initial experiments, we established that physiological concentrations of SFN (0.5–2.5 μM) [14] significantly upregulated Nrf2 mediated HO-1 protein levels (12–24 h, Supplementary Fig. S1A) and mRNA expression of HO-1, NQO1, Bach1 and Keap1 (4 h, Supplementary Fig. S1B).
Fig. 1.
Silencing Nrf2 abrogates induction of HO-1 by sulforaphane in bEnd.3 endothelial cells
(A) Representative immunoblot of Nrf2 in nuclear and cytosolic fractions isolated from bEnd.3 cells treated for 1, 2 and 4 h with vehicle (0.01% DMSO) or sulforaphane (SFN, 2.5 μM). Lamin A/C and α-tubulin are loading controls for nuclear and cytosolic fractions, respectively. (B–C) bEnd.3 cells were transfected with scrambled or Nrf2 siRNA 24 h post-seeding to silence Nrf2 transcriptional activity and then challenged with vehicle (0.01% DMSO) or SFN (2.5 μM) for 24 h. Cell lysates immunoblotted for HO-1 expression relative β-actin (B) and analysed by densitometry (C). Data denote mean ± S.E.M., n = 3 independent bEnd.3 cultures, two-way ANOVA, **P < 0.01, ***P < 0.001, n.s. non-significant.
3.2. Real-time measurement of intracellular O2 level in bEnd.3 cells
We and others have emphasized the importance of monitoring O2 gradients between culture medium and cell cytosol [20,22,39,40], and here report the first real-time measurement of intracellular O2 in brain microvascular endothelial cells (bEnd.3) using the cell-penetrating phosphorescent nanoparticle probe MitoXpress®-INTRA [26]. Phosphorescence lifetime in bEnd.3 cells and medium was measured during stepwise reductions of O2 (18 kPa–0 kPa) within an O2-regulated, time-resolved fluorescence plate reader (Fig. 2A). The relationship between ambient O2 levels in the plate reader and phosphorescence lifetime is illustrated in Fig. 2B. An intracellular O2 level of 3.4 ± 0.1 kPa (inset, Fig. 2B) was measured in cells cultured under 5 kPa O2, recapitulating levels in the cortex of awake mice [41], with dissolved O2 in the medium (5.2 ± 0.2 kPa) similar to the O2 level (5 kPa) in the plate reader.
3.3. Adaptation of bEnd.3 cells to 5 kPa O2 does not induce a hypoxic phenotype
To determine whether adaptation of bEnd.3 cells under 5 kPa O2 induces hypoxic responses, we examined stabilization of HIF-1α, a key modulator of transcriptional responses to hypoxia. In the presence of oxygen, HIF-1α is degraded via prolyl hydroxylation [42], involving HIF-1α association with von Hippel-Lindau protein E3 ubiquitin ligase complex to promote degradation [42,43]. As intracellular O2 availability decreases, these enzymes are no longer able to hydroxylate HIF-1α subunits, resulting in stabilization and upregulation of HIF-1α protein levels. When bEnd.3 cells were adapted to 18, 5 or 1 kPa O2, HIF-1α stabilization was only detected under hypoxia (Fig. 2C), confirming the absence of a hypoxic phenotype in cells maintained long-term under physiological normoxia (5 kPa O2).
3.4. Effects of ambient O2 levels on cell viability, ATP and GSH content and proliferation
Adaptation of bEnd.3 cells to 5 kPa O2 had no effect on cell viability, as evidenced by negligible changes in mitochondrial dehydrogenase activity (data not shown) or intracellular ATP levels (5 kPa O2: 24.3 ± 2.2 vs 18 kPa O2: 20.7 ± 1.7 nmol/mg.protein) (Fig. 2D). Intracellular GSH (Fig. 2E) and catalase (Fig. 2F) levels were significantly lower in bEnd.3 cells adapted to 5 kPa O2, consistent with our previous findings in airway epithelial cells [23] and other studies in epidermoid carcinoma cells [40]. Total intracellular GSH levels were similar in bEnd.3 cells in passages 7–15 (data not shown). Moreover, bEnd.3 cell proliferation was decreased under 5 kPa O2 compared to 18 kPa O2 (Fig. 2G). The implications of these findings are that the enhanced oxidative stress during standard cell culture under hyperoxia (18 kPa O2) is attenuated in cells adapted to physiological normoxia 5 kPa O2).
3.5. Physiological normoxia attenuates sulforaphane induced Nrf2 regulated enzyme expression
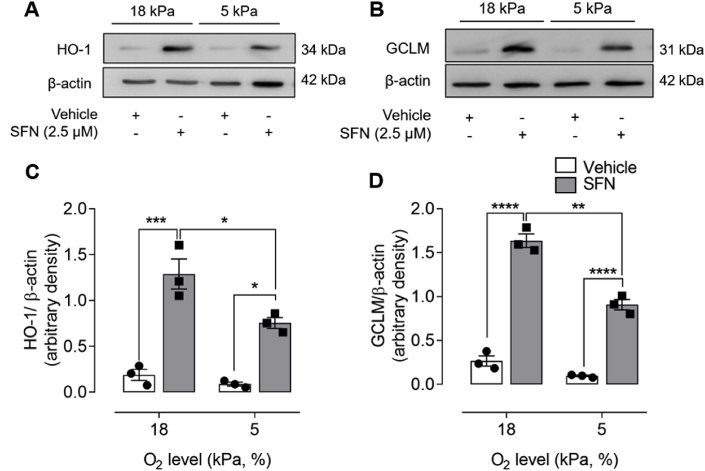
To determine whether Nrf2 redox signaling was affected by changes in ambient O2 levels, bEnd.3 cells were adapted to 18 or 5 kPa O2 and Nrf2 induced antioxidant enzyme expression determined by immunoblotting. Although basal levels of HO-1 and GCLM expression were affected negligibly following adaptation to physiological normoxia (5 kPa O2), upregulation of Nrf2 regulated enzyme expression by SFN (2.5 μM, 24 h) was significantly attenuated in cells adapted to 5 kPa O2 (Fig. 3). These findings are consistent with reports of diminished induction of antioxidant enzymes by Nrf2 in HUVEC [22], airway epithelial cells [23], RAW264.7 macrophages [44] and epidermoid carcinoma cells [40] under physiological normoxia.
Fig. 3.
Adaptation to physiological normoxia diminishes sulforaphane induced HO-1 and GCLM protein expression
bEnd 3 cells were cultured under either 18 or 5 kPa O2 for 5 d and then treated with vehicle (0.01% DMSO) or sulforaphane (SFN, 2.5 μM) for 24 h. Cell lysates were immunoblotted for HO-1 (A) and GCLM (B) expression relative to β-actin and analysed by densitometry (C–D). Data denote mean ± S.E.M., n = 3 independent bEnd.3 cultures, two-way ANOVA, *P < 0.05. **P < 0.01,***P < 0.001, ****P < 0.0001.
3.6. Reoxygenation induced superoxide production in bEnd.3 cells under 18 kPa O2
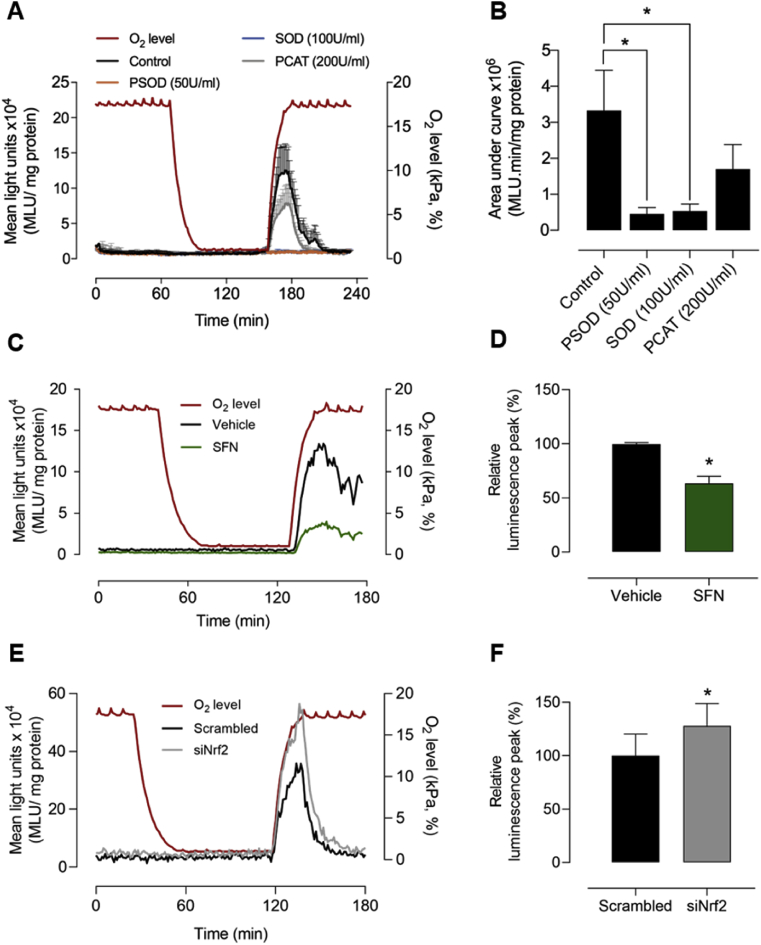
bEnd.3 cells adapted to 18 kPa O2 were incubated with the chemiluminescent probe L-012 to investigate reactive oxygen species generation during hypoxia-reoxygenation. As shown in Fig. 4A, reoxygenation induced free radical generation was significantly inhibited by SOD (100U/ml) and PEG-SOD (PSOD, 50U/ml), whereas PEG-CAT (PCAT, 200U/ml) led to a non-significant decrease in L-012 luminescence, suggesting that reoxygenation most likely increases superoxide generation. Fig. 4B summarizes the changes in luminescence induced by reoxygenation in the absence and presence of scavengers of reactive oxygen species.
Fig. 4.
Sulforaphane reduces and Nrf2 gene silencing enhances reoxygenation induced superoxide production in bEnd.3 cells under 18 kPa O2
bEnd.3 cells were cultured under 18 kPa O2 for 5 d. (A) Cells were treated with vehicle (0.01% DMSO), PEG-superoxide dismutase (PSOD, 50U/ml), superoxide dismutase (SOD, 100U/ml) or PEG-catalase (PCAT, 200U/ml) for 30 min prior to incubation with L-012 (see Methods). Cells were rapidly transferred to an O2-regulated plate reader and subjected to hypoxia (1 h) and reoxygenation under 18 kPa O2, with L-012 luminescence measured in the absence (control) or presence of inhibitors. O2 levels inside the plate reader are indicated by the red line (right axis) and mean light units (MLU/mg protein) on the left axis. (B) Area under L-012 traces during reoxygenation (160–190 min) for each treatment. (C) Representative L-012 traces in cells pre-treated with vehicle (0.01% DMSO) or SFN (2.5 μM, 24 h) and (D) relative peak luminescence (120–140 min, % vehicle) on reoxygenation. (E) Representative L-012 traces in cells transfected with scrambled or Nrf2 siRNA and (F) relative peak luminescence (125–140 min, % scrambled siRNA). Data denote mean ± S.E.M., n = 3–4 independent bEnd.3 cultures, one-way ANOVA, *P < 0.05.
As NADP(H) oxidases (NOX) have been implicated as a source of free radical generation in cerebral ischemia-reperfusion [45,46], bEnd.3 cells adapted to 18 kPa O2 were pre-treated with the NOX inhibitor VAS2870 (5 μM) for 30 min, incubated with L-012 and then exposed to hypoxia (1 kPa O2, 1 h) and reoxygenation. As shown in Supplementary Fig. S2, reoxygenation induced increases in L-012 luminescence were unaffected by VAS2870, suggesting that NADPH oxidases are an unlikely source of acute reoxygenation induced free radical generation in bEnd.3 cells.
3.7. Sulforaphane pretreatment protects against reoxygenation induced superoxide generation
To determine whether upregulation of Nrf2 target genes attenuates reoxygenation induced free radical generation, bEnd.3 cells were adapted to 18 kPa O2 and pre-treated with either vehicle (DMSO 0.01%) or SFN (2.5 μM) for 24 h. SFN significantly diminished reoxygenation induced L-012 luminescence (Fig. 4C and D) and moreover, in cells transfected with scrambled or Nrf2 siRNA, we confirmed that silencing Nrf2 significantly enhances the L-012 luminescence signal during reoxygenation (Fig. 4E and F).
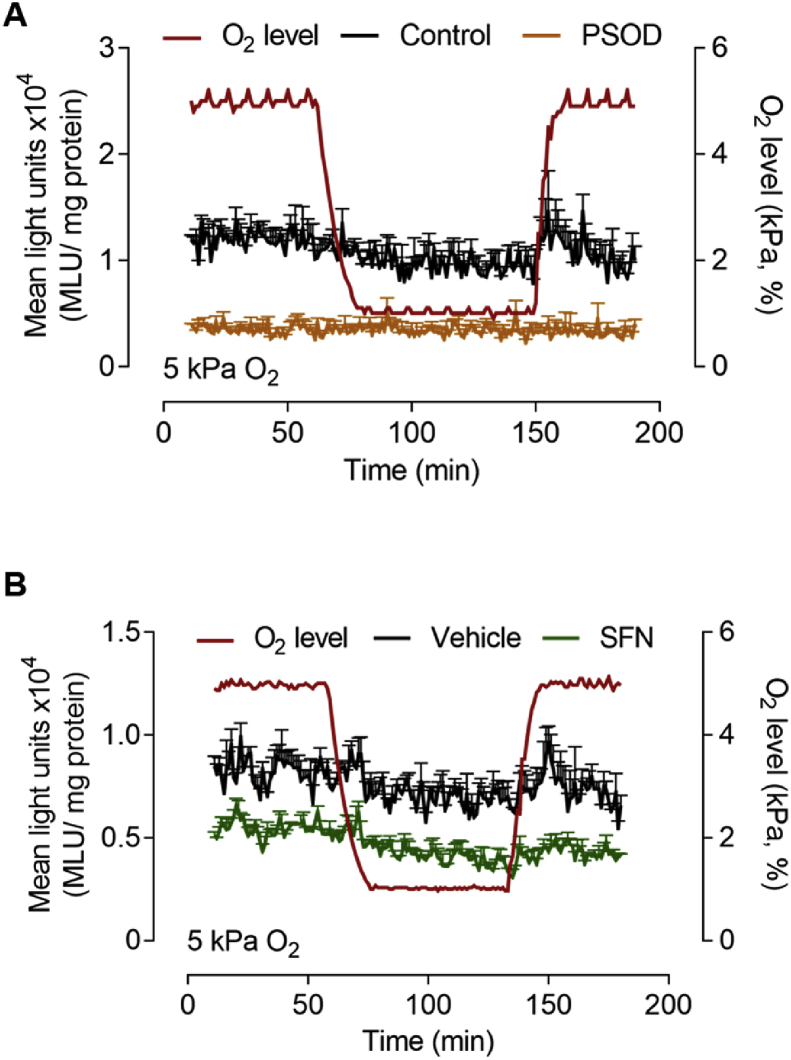
3.8. Reoxygenation induced free radical generation is diminished in bEnd.3 cells under 5 kPa O2
Basal and reoxygenation induced L-012 luminescence was significantly lower in bEnd.3 cells adapted to 5 kPa O2 (Fig. 5A) compared to 18 kPa O2 (Fig. 4A). Although reoxygenation induced changes in L-012 luminescence were not significant, the signal appeared decreased in the presence of PSOD (Fig. 5A) or following pretreatment of cells with SFN (2.5 μM, 24 h) (Fig. 5A and B). This attenuated intracellular free radical production in bEnd.3 cells under 5 kPa O2 is consistent with previous studies in RAW264.7 macrophages [44], epidermoid carcinoma cells [40], SH-SY5Y neuronal cells [47] and skeletal myoblasts/myotubes [48].
Fig. 5.
Adaptation to 5 kPa O2diminishes reoxygenation induced reactive oxygen species generation in bEnd.3 cells
bEnd.3 cells were cultured under 5 kPa O2 for 5 d. (A) Cells were treated with vehicle (0.01% DMSO, black line, control) or PEG-superoxide dismutase (PSOD, 50 U/ml, orange line) for 30 min prior to incubation with L-012. Cells were transferred to an O2-regulated plate reader and subjected to hypoxia (1 h) and reoxygenation under 5 kPa O2. O2 levels inside the plate reader are indicated by the red line (right axis) and mean light units (MLU/mg protein) on the left axis. (B) bEnd.3 cells pre-treated with vehicle (0.01% DMSO) or SFN (2.5 μM, 24 h) prior to hypoxia (1 h) and reoxygenation under 5 kPa O2. Data denote mean ± S.E.M, n = 3 independent bEnd.3 cultures. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
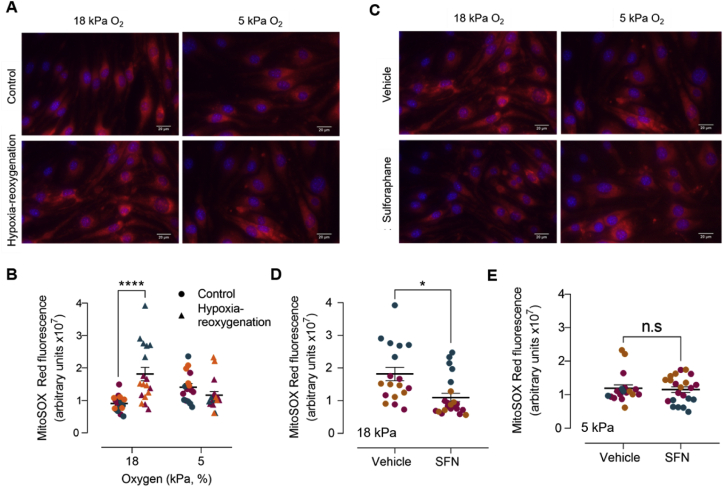
3.9. Reoxygenation-induced increases in MitoSOX red fluorescence
To further validate reoxygenation mediated changes in L-012 luminescence, we examined mitochondrial reactive oxygen species generation in bEnd.3 cells adapted to 18 or 5 kPa O2. Hypoxia-reoxygenation increased MitoSOX fluorescence in cells under 18 kPa O2 with negligible changes detectable under 5 kPa O2 (Fig. 6A and B). As shown in Supplementary Fig. S3, reoxygenation induced increases in MitoSOX fluorescence were unaffected by inhibition of complex I (rotenone, 1 μM) or eNOS (l-NAME, 100 μM). Notably, pretreatment of cells with SFN (2.5 μM, 12 h) significantly attenuated reoxygenation induced MitoSOX fluorescence in cells under 18 but not 5 kPa O2 (Fig. 6C–E), consistent with the negligible changes in L-012 luminescence observed in bEnd.3 cells during acute reoxygenation under 5 kPa. To exclude the possibility that changes in ambient O2 levels affected MitoSOX fluorescence, the probe was dissolved in 1% FCS medium and a 96-well plate transferred to the plate reader under 18 or 5 kPa O2. When H2O2 (10 μM) was injected using on-board injector units in the plate reader, MitoSOX fluorescence was similar under 18 and 5 kPa O2 (data not shown).
Fig. 6.
Effects of sulforaphane pretreatment on reoxygenation induced mitochondrial reactive oxygen species generation in bEnd.3 cells adapted to 18 kPa or 5 kPa O2
bEnd.3 cells seeded in Ibidi μ-Slide 8-well chambers were cultured under 18 or 5 kPa O2 for 5 d. Cells were subjected to hypoxia (1 kPa O2, 1 h) and loaded with MitoSOX™ Red for 5 min before the start of 30 min reoxygenation under 18 or 5 kPa O2, respectively. Control cells were loaded with MitoSOX™ Red during the last 30 min of an experiment. Cells were fixed with 4% paraformaldehyde and images acquired using a Nikon Diaphot microscope with a 40× objective. (A) Representative images of MitoSOX fluorescence and DAPI stained nuclei after 30 min reoxygenation and (B) quantification of MitoSOX fluorescence. (C) bEnd.3 cells were pre-treated with vehicle (0.01% DMSO) or SFN (2.5 μM) for 24 h before exposure to hypoxia (1 h) and reoxygenation under 18 or 5 kPa O2, respectively. Representative images of MitoSOX fluorescence and DAPI stained nuclei after 30 min reoxygenation and (D–E) quantitation of MitoSOX fluorescence. Each symbol in panels B, D and E represents the mean fluorescence from at least 10 cells in a field of view, with each color denoting a different bEnd.3 experiment with at least 6 different fields of view. Data denote mean ± S.E.M., n = 18–20 fields of view in each of 3 independent bEnd.3 cell cultures, two-way ANOVA followed by Bonferroni post-hoc analysis, ****P < 0.0001. Scale bar = 20 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
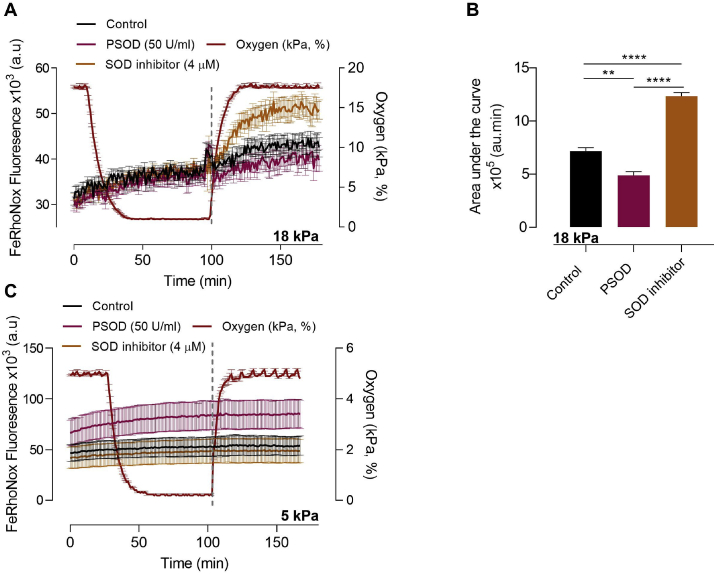
3.10. Reoxygenation-induced increases in FeRhoNox fluorescence
Based on caveats associated with the specificity of L-012 and MitoSOX Red, such as non-specific oxidation of the probes [49,50], further indirect measurements of reoxygenation induced superoxide production were conducted using an Fe2+-specific fluorescent indicator, FeRhoNox™-1, in bEnd.3 adapted to 18 or 5 kPa O2. Inhibition of superoxide dismutase by ammonium tetrathiomolybdate has been reported to prolong the cytosolic Fe2+ signal in dermal fibroblasts and endothelial cells challenged with UVA radiation [37]. As shown in Fig. 7A, increases in FeRhoNox-1 fluorescence during reoxygenation under 18 kPa O2 were inhibited by PEG-SOD and potentiated by inhibition of SOD with ammonium tetrathiomolybdate. Previous studies have shown that mitochondria exposed to superoxide release iron from iron-sulphur clusters, indicating that increased radical production will lead to an increase in free iron [51,52]. In the present study, we exploited the fact that increases in free iron would increase FeRhoNox™-1 fluorescence, and thus changes in fluorescence served as an indirect measure of superoxide generation in bEnd.3 cells subjected to reoxygenation. Notably, reoxygenation induced FeRhoNox-1 fluorescence was attenuated in bEnd.3 cells adapted to 5 kPa O2 (Fig. 7B). Together with our findings of reoxygenation induced changes in L-012 luminescence and MitoSOX fluorescence, FeRhoNox-1 measurements suggest that superoxide is the most likely free radical generated during acute reoxygenation induced oxidative stress in bEnd.3 cells.
Fig. 7.
Reoxygenation induces intracellular Fe2+release in bEnd.3 cells adapted to 18 kPa O2but not 5 kPa O2.
bEnd.3 cells were cultured under 18 or 5 kPa O2 for 5 d. Cells were then incubated with the Fe2+-selective probe FeRhoNox™-1 (5 μM, 1 h) in HBSS in the presence of vehicle (0.01% DMSO, control, black line), PEG-superoxide dismutase (PSOD, 50U/ml, pink line) or a SOD inhibitor (4 μM ammonium tetrathiomolybdate, orange line). (A-B) Mean FeRhoNox fluorescence traces in cells exposed to hyperoxia (18 kPa O2), hypoxia (1 kPa O2, 1 h) and reoxygenation under 18 kPa O2 and area under the curve following reoxygenation (dashed line indicates reoxyenation period 100–160 min) for each treatment. (C) Mean FeRhoNox fluorescence traces in cells adapted to physiological normoxia (5 kPa O2), hypoxia (1 kPa O2, 1 h) and reoxygenation under 5 kPa O2. Data denote mean ± S.E.M from 3 to 4 independent bEnd.3 cell cultures, one-way ANOVA followed by Bonferroni post-hoc analysis, **P < 0.001, ****P < 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Changes in ambient O2 levels during cell culture in vitro alter (i) ion channel and kinase activities [[53], [54], [55]], (ii) endothelial Ca2+ signaling, nitric oxide bioavailability and their sensitivity to Ca2+ overload [24,25], and (iii) induction of Nrf2-targeted antioxidant defenses [22,23,56]. We here further demonstrate that the redox phenotype of mouse brain microvascular endothelial cells is critically affected by ambient oxygen levels. Endothelial cells lining the blood-brain barrier in vivo are exposed to O2 levels ranging between ~3 and 7 kPa, yet the majority of studies in brain endothelial and other cell types in vitro have employed standard culture conditions in which cells are exposed to hyperoxia (18 kPa O2) and therefore sustained oxidative stress [20].
Using the O2-sensitive nanoparticle probe MitoXpress®-INTRA, we obtained the first measurements of intracellular O2 (3.4 kPa) in bEnd.3 endothelial cells, recapitulating O2 levels measured in brain endothelium in vivo. Importantly, long-term adaptation of bEnd.3 cells to 5 kPa O2 was not associated with HIF-1α stabilization, confirming the absence of a hypoxic phenotype under physiological normoxia. Moreover, as gradients exist between ambient O2 levels in a Scitive workstation, medium and cytosol, it is critical that medium and intracellular O2 levels are measured simultaneously [20,22]. MitoXpress®-INTRA has been used to measure intracellular O2 in umbilical vein endothelial cells [22,24], mouse embryonic fibroblasts [26], cortical neurons [57] and now in brain microvascular endothelial cells.
Adaptation of bEnd.3 cells under 5 kPa O2 did not affect cell viability or intracellular ATP levels, but significantly decreased levels of intracellular GSH and catalase, suggesting that cells under physiological normoxia experience less oxidative stress [20,58]. Basal expression of Nrf2-regulated antioxidant enzymes was similar in bEnd.3 cells cultured under 18 or 5 kPa O2, however SFN mediated induction of HO-1 and GCLM was significantly attenuated in cells adapted to 5 kPa O2. Our finding of diminished HO-1 induction is in agreement with our previous studies in human umbilical vein and coronary artery endothelial cells [22] and other studies in lung epithelial cells [23] and human dental pulp stem cells [56] cultured under relevant physiological O2 levels. Notably, electrophile and nitric oxide mediated induction of HO-1 in human endothelial cells adapted 5 kPa O2 is attenuated, but reversible on re-exposure of cells to 18 kPa O2 or following silencing of the Nrf2 repressor Bach1 [22].
Our previous studies of reperfusion injury in a rodent model of transient ischemic stroke established that activation of Nrf2 antioxidant defenses by SFN affords neurovascular and neurological protection [17,18]. To mimic ischemia-reperfusion injury in stroke at a cellular level, bEnd.3 cells were adapted to either 18 or 5 kPa O2 and subjected to hypoxia (1 kPa O2) and reoxygenation under 18 or 5 kPa O2, respectively. Reoxygenation-induced increases in L-012 luminescence in cells adapted to 18 kPa O2 was abrogated by SOD and polyethylene glycol SOD, implicating superoxide as the most likely free radical species generated during reoxygenation. Although polyethylene glycol catalase led to a non-significant decrease in reoxygenation-induced free radical production, we cannot exclude that inhibition of L-012 luminescence signal by SOD or polyethylene glycol SOD may be due to generation of superoxide from molecular oxygen during L-012 oxidation by H2O2/peroxidase [59]. We further demonstrated that upregulation of Nrf2-regulated antioxidant enzymes by SFN led to a significant decrease in reoxygenation induced free radicals (Fig. 4C and D), whilst silencing Nrf2 transcriptional activity enhanced reoxygenation induced free radical generation (Fig. 4E and F).
Reoxygenation induced changes in L-012 luminescence were lower in bEnd.3 cells adapted to 5 kPa O2 (Fig. 5), although L-012 signals trended to decrease in the presence of PEG-SOD or following SFN pretreatment. In this context, studies in macrophages [44], epidermoid carcinoma cells [40] and dental pulp stem cells [60], as well as, our experiments in human endothelial cells (data not shown) confirm that oxidative stress is lower in cells adapted to physiological normoxia. Thus, under standard, hyperoxic cell culture conditions, the redox phenotype of cells is characterized by an upregulation of Nrf2-regulated gene transcription to counteract enhanced reactive oxygen species generation and sustained oxidative stress [20].
We further investigated the redox status of bEnd.3 cells exposed to hypoxia-reoxygenation by assaying MitoSOX fluorescence as an index of mitochondrial reactive oxygen species generation. Reoxygenation significantly increased MitoSOX fluorescence in bEnd.3 cells adapted to 18 but not 5 kPa O2, and notably SFN pretreatment only attenuated reoxygenation-induced MitoSOX fluorescence in cells adapted to 18 kPa O2, further supporting our finding that SFN inhibits acute reoxygenation induced increases in L-012 luminescence. Reoxygenation induced increases MitoSOX fluorescence were unaffected by l-NAME or a pan-NADPH oxidase inhibitor (VAS2870), suggesting that free radical generation in bEnd.3 cells was unlikely due to eNOS or NOX. Although VAS2870 had no effect on cell viability or reoxygenation induced free radical generation, we cannot exclude that VAS2870 and other NOX inhibitors may have off-target effects via thiol alkylation, inhibition of mitochondrial respiration and cytotoxicity [61]. Furthermore, although undetectable in our study, we cannot exclude the possibility of enhanced reactive oxygen species generation during hypoxia, as it has recently been suggested that acute hypoxia drives the import of Na+ into the mitochondrial matrix, reducing inner mitochondrial fluidity and consequently concentrating the production of superoxide at complex III [62].
To further characterize reoxygenation-induced free radical generation in bEnd.3 cells, release of intracellular Fe2+ was monitored as an indirect measure of intracellular superoxide generation. By using PEG-SOD and a SOD inhibitor, we demonstrated for the first time that changes in FeRhoNox-1 fluorescence provide a useful measure of reoxygenation-induced superoxide generation in brain microvascular endothelial cells. Release of labile iron is closely associated with reactive oxygen species generation, and iron accumulation occurs in stroke [63], traumatic brain injury [64] and neurodegenerative disorders [65,66]. Furthermore, mitochondria exposed to superoxide anions release iron from iron-sulphur clusters, such that increased free radical generation will result in increased free iron [51,52]. Increases in superoxide in the presence of SOD inhibition reduces Fe3+ in the ferritin core to Fe2+, releasing Fe2+ into the cytoplasm [37,67].
Our study establishes that bEnd.3 cells adapted to long-term to hyperoxia (18 kPa O2) exhibit heightened sensitivity to hypoxia-reoxygenation, resulting in increased reactive oxygen species generation on reoxygenation. Studies in vivo have reported that following ischemia-reperfusion injury in the heart and brain, accumulation of succinate in mitochondria drives reactive oxygen species generation via reverse electron transport at mitochondrial complex I, and that oxidative damage can be decreased by reducing succinate accumulation [68]. In the present study, activation of Nrf2 by SFN significantly diminished reoxygenation induced free radical generation while silencing of Nrf2 exacerbated free radical generation, implicating Nrf2 in protection against reoxygenation/reperfusion injury. In this context, Nrf2 has been shown to significantly affect the mitochondrial membrane potential, fatty acid oxidation and the availability of substrates including succinate [69,70]. As Nrf2 deficient cells and mice in vivo are more sensitive to oxidative damage [71,72], activation of Nrf2 by SFN not only upregulates antioxidant defense enzymes but importantly also influences mitochondrial substrate utilization and respiration [69,70].
As generation of reactive oxygen species was attenuated in bEnd.3 cells adapted to physiological normoxia, it is possible that the probes L-012 and MitoSOX Red used in this study and other studies lack sufficient sensitivity to monitor low levels of radical generation in response to acute reoxygenation. Although recent advances in multiphoton redox and pO2 imaging have enabled elegant quantification of metabolic processes under different ambient O2 levels [73], we consider it important to ensure that decreasing ambient oxygen levels from 18 kPa O2 does not result in HIF-1α stabilization and activation hypoxic signaling pathways. In this context, we previously reported that long-term (~5 d) culture of vascular cells under physiological O2 levels is required to exclude a hypoxic phenotype [22,24].
In view of the caveats concerning luminescence and fluorescence indicators [49,50], further studies are warranted using novel genetic biosensors for high-resolution, real-time imaging of reactive oxygen and nitrogen species in single cells and subcellular compartments [74,75]. Conducting such experiments in cells adapted long-term under controlled and physiologically relevant O2 levels will prove challenging, but we are convinced that such in vitro cell culture models, in particular targeting biosensors to mitochondria in live cells, will provide insights for the design of novel therapeutics for treatment cerebral, coronary, renal and hepatic ischemia-perfusion injury.
Author contributions
G.W., P.A.F. and G.E.M. conceptualized the study; G.W. developed the methodology, T.P.K. assisted with MitoXpress®-Intra experiments and R.C.M.S. with FeRhoNox-1 experiments; G.W., S.S. and M.J.S. performed the experiments; G.W. and G.E.M. wrote the manuscript which was reviewed by all authors. G.E.M. is the guarantor of this study, with responsibility for the integrity of the data and accuracy of the data analysis.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
Supported by British Heart Foundation (FS/16/67/32548, G.E.M.), Heart Research UK (RG2673, G.E.M.), and EVGEN R&D Grant (G.E.M.). We gratefully acknowledge Dr David Begley (King's College London) for supply of bEnd.3 endothelial cells for initial experiments and Prof. T. Kavanagh (University of Washington, WA, USA) for the GCLM antibody. We thank Dr Ron Jacob (King's College London) and Dr Sarah J. Chapple (King's College London) for their helpful discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101708.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Phipps M.S., Cronin C.A. Management of acute ischemic stroke. BMJ. 2020;368:l6983. doi: 10.1136/bmj.l6983. [DOI] [PubMed] [Google Scholar]
- 2.Campbell B.C.V., De Silva D.A., Macleod M.R., Coutts S.B., Schwamm L.H., Davis S.M., Donnan G.A. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 3.Endres M., Dirnagl U. Ischemia and stroke. Adv. Exp. Med. Biol. 2002;513:455–473. doi: 10.1007/978-1-4615-0123-7_17. [DOI] [PubMed] [Google Scholar]
- 4.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 5.Peters O., Back T., Lindauer U., Busch C., Megow D., Dreier J., Dirnagl U. Increased formation of reactive oxygen species after permanent and reversible middle cerebral artery occlusion in the rat. J. Cerebr. Blood Flow Metabol. 1998;18:196–205. doi: 10.1097/00004647-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Yang J.L., Mukda S., Chen S.D. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018;16:263–275. doi: 10.1016/j.redox.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacke W., Kaste M., Bluhmki E., Brozman M., Davalos A., Guidetti D., Larrue V., Lees K.R., Medeghri Z., Machnig T., Schneider D., von Kummer R., Wahlgren N., Toni D., Investigators E. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 8.Emberson J., Lees K.R., Lyden P., Blackwell L., Albers G., Bluhmki E., Brott T., Cohen G., Davis S., Donnan G., Grotta J., Howard G., Kaste M., Koga M., von Kummer R., Lansberg M., Lindley R.I., Murray G., Olivot J.M., Parsons M., Tilley B., Toni D., Toyoda K., Wahlgren N., Wardlaw J., Whiteley W., del Zoppo G.J., Baigent C., Sandercock P., Hacke W., Stroke Thrombolysis Trialists' Collaborative G. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spratt N.J., Donnan G.A., McLeod D.D., Howells D.W. Salvaged' stroke ischaemic penumbra shows significant injury: studies with the hypoxia tracer FMISO. J. Cerebr. Blood Flow Metabol. 2011;31:934–943. doi: 10.1038/jcbfm.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser P.A. The role of free radical generation in increasing cerebrovascular permeability. Free Radical Biol. Med. 2011;51:967–977. doi: 10.1016/j.freeradbiomed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney M.D., Zhao Z., Montagne A., Nelson A.R., Zlokovic B.V. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 2019;99:21–78. doi: 10.1152/physrev.00050.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen C.L., Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 13.Abbott N.J., Ronnback L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 14.Dinkova-Kostova A.T., Kostov R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012;18:337–347. doi: 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T., Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radical Biol. Med. 2015;88:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Ishii T., Itoh K., Ruiz E., Leake D.S., Unoki H., Yamamoto M., Mann G.E. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 17.Alfieri A., Srivastava S., Siow R.C., Cash D., Modo M., Duchen M.R., Fraser P.A., Williams S.C., Mann G.E. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radical Biol. Med. 2013;65:1012–1022. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava S., Alfieri A., Siow R.C., Mann G.E., Fraser P.A. Temporal and spatial distribution of Nrf2 in rat brain following stroke: quantification of nuclear to cytoplasmic Nrf2 content using a novel immunohistochemical technique. J. Physiol. 2013;591:3525–3538. doi: 10.1113/jphysiol.2013.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jazwa A., Rojo A.I., Innamorato N.G., Hesse M., Fernandez-Ruiz J., Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxidants Redox Signal. 2011;14:2347–2360. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
- 20.Keeley T.P., Mann G.E. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol. Rev. 2019;99:161–234. doi: 10.1152/physrev.00041.2017. [DOI] [PubMed] [Google Scholar]
- 21.Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S., Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapple S.J., Keeley T.P., Mastronicola D., Arno M., Vizcay-Barrena G., Fleck R., Siow R.C., Mann G.E. Bach1 differentially regulates distinct Nrf2-dependent genes in human venous and coronary artery endothelial cells adapted to physiological oxygen levels. Free Radical Biol. Med. 2016;92:152–162. doi: 10.1016/j.freeradbiomed.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A., Dailey L.A., Swedrowska M., Siow R., Mann G.E., Vizcay-Barrena G., Arno M., Mudway I.S., Forbes B. Quantifying the magnitude of the oxygen artefact inherent in culturing airway cells under atmospheric oxygen versus physiological levels. FEBS Lett. 2016;590:258–269. doi: 10.1002/1873-3468.12026. [DOI] [PubMed] [Google Scholar]
- 24.Keeley T.P., Siow R.C.M., Jacob R., Mann G.E. A PP2A-mediated feedback mechanism controls Ca2+-dependent NO synthesis under physiological oxygen. Faseb. J. 2017;31:5172–5183. doi: 10.1096/fj.201700211R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keeley T.P., Siow R.C.M., Jacob R., Mann G.E. Reduced SERCA activity underlies dysregulation of Ca(2+) homeostasis under atmospheric O2 levels. Faseb. J. 2018;32:2531–2538. doi: 10.1096/fj.201700685RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fercher A., Borisov S.M., Zhdanov A.V., Klimant I., Papkovsky D.B. Intracellular O2 sensing probe based on cell-penetrating phosphorescent nanoparticles. ACS Nano. 2011;5:5499–5508. doi: 10.1021/nn200807g. [DOI] [PubMed] [Google Scholar]
- 27.Sharman K.K., Periasamy A., Ashworth H., Demas J.N. Error analysis of the rapid lifetime determination method for double-exponential decays and new windowing schemes. Anal. Chem. 1999;71:947–952. doi: 10.1021/ac981050d. [DOI] [PubMed] [Google Scholar]
- 28.Cheng X., Chapple S.J., Patel B., Puszyk W., Sugden D., Yin X., Mayr M., Siow R.C., Mann G.E. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes. 2013;62:4088–4097. doi: 10.2337/db13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava S., Blower P.J., Aubdool A.A., Hider R.C., Mann G.E., Siow R.C. Cardioprotective effects of Cu((II))ATSM in human vascular smooth muscle cells and cardiomyocytes mediated by Nrf2 and DJ-1. Sci. Rep. 2016;6:7. doi: 10.1038/s41598-016-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hissin P.J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976;74:214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 31.Wingler K., Altenhoefer S.A., Kleikers P.W., Radermacher K.A., Kleinschnitz C., Schmidt H.H. VAS2870 is a pan-NADPH oxidase inhibitor. Cell. Mol. Life Sci. 2012;69:3159–3160. doi: 10.1007/s00018-012-1107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daiber A., August M., Baldus S., Wendt M., Oelze M., Sydow K., Kleschyov A.L., Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radical Biol. Med. 2004;36:101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 33.He M., Siow R.C., Sugden D., Gao L., Cheng X., Mann G.E. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr. Metabol. Cardiovasc. Dis. 2011;21:277–285. doi: 10.1016/j.numecd.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Robinson K.M., Janes M.S., Pehar M., Monette J.S., Ross M.F., Hagen T.M., Murphy M.P., Beckman J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowlands D.J., Chapple S., Siow R.C., Mann G.E. Equol-stimulated mitochondrial reactive oxygen species activate endothelial nitric oxide synthase and redox signaling in endothelial cells: roles for F-actin and GPR30. Hypertension. 2011;57:833–840. doi: 10.1161/HYPERTENSIONAHA.110.162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mukaide T., Hattori Y., Misawa N., Funahashi S., Jiang L., Hirayama T., Nagasawa H., Toyokuni S. Histological detection of catalytic ferrous iron with the selective turn-on fluorescent probe RhoNox-1 in a Fenton reaction-based rat renal carcinogenesis model. Free Radic. Res. 2014;48:990–995. doi: 10.3109/10715762.2014.898844. [DOI] [PubMed] [Google Scholar]
- 37.Smith M.J., Fowler M., Naftalin R.J., Siow R.C.M. UVA irradiation increases ferrous iron release from human skin fibroblast and endothelial cell ferritin: consequences for cell senescence and aging. Free Radical Biol. Med. 2020;155:49–57. doi: 10.1016/j.freeradbiomed.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 38.Choi S., Park S., Liang G.H., Kim J.A., Suh S.H. Superoxide generated by lysophosphatidylcholine induces endothelial nitric oxide synthase downregulation in human endothelial cells. Cell. Physiol. Biochem. 2010;25:233–240. doi: 10.1159/000276557. [DOI] [PubMed] [Google Scholar]
- 39.Place T.L., Domann F.E., Case A.J. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radical Biol. Med. 2017;113:311–322. doi: 10.1016/j.freeradbiomed.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferguson D.C.J., Smerdon G.R., Harries L.W., Dodd N.J.F., Murphy M.P., Curnow A., Winyard P.G. Altered cellular redox homeostasis and redox responses under standard oxygen cell culture conditions versus physioxia. Free Radical Biol. Med. 2018;126:322–333. doi: 10.1016/j.freeradbiomed.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 41.Lyons D.G., Parpaleix A., Roche M., Charpak S. Mapping oxygen concentration in the awake mouse brain. Elife. 2016;5 doi: 10.7554/eLife.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratcliffe P.J., O'Rourke J.F., Maxwell P.H., Pugh C.W. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J. Exp. Biol. 1998;201:1153–1162. doi: 10.1242/jeb.201.8.1153. [DOI] [PubMed] [Google Scholar]
- 43.Ratcliffe P.J. HIF-1 and HIF-2: working alone or together in hypoxia? J. Clin. Invest. 2007;117:862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas B., Chrusciel S., Fayad-Kobeissi S., Dubois-Rande J.L., Azuaje F., Boczkowski J., Motterlini R., Foresti R. Permanent culture of macrophages at physiological oxygen attenuates the antioxidant and immunomodulatory properties of dimethyl fumarate. J. Cell. Physiol. 2015;230:1128–1138. doi: 10.1002/jcp.24844. [DOI] [PubMed] [Google Scholar]
- 45.Abramov A.Y., Scorziello A., Duchen M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahles T., Brandes R.P. NADPH oxidases as therapeutic targets in ischemic stroke. Cell. Mol. Life Sci. 2012;69:2345–2363. doi: 10.1007/s00018-012-1011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villeneuve L., Tiede L.M., Morsey B., Fox H.S. Quantitative proteomics reveals oxygen-dependent changes in neuronal mitochondria affecting function and sensitivity to rotenone. J. Proteome Res. 2013;12:4599–4606. doi: 10.1021/pr400758d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormick R., Pearson T., Vasilaki A. Manipulation of environmental oxygen modifies reactive oxygen and nitrogen species generation during myogenesis. Redox Biol. 2016;8:243–251. doi: 10.1016/j.redox.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forman H.J., Augusto O., Brigelius-Flohe R., Dennery P.A., Kalyanaraman B., Ischiropoulos H., Mann G.E., Radi R., Roberts L.J., 2nd, Vina J., Davies K.J. Even free radicals should follow some rules: a guide to free radical research terminology and methodology. Free Radical Biol. Med. 2015;78:233–235. doi: 10.1016/j.freeradbiomed.2014.10.504. [DOI] [PubMed] [Google Scholar]
- 51.Murphy M.P., Echtay K.S., Blaikie F.H., Asin-Cayuela J., Cocheme H.M., Green K., Buckingham J.A., Taylor E.R., Hurrell F., Hughes G., Miwa S., Cooper C.E., Svistunenko D.A., Smith R.A., Brand M.D. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J. Biol. Chem. 2003;278:48534–48545. doi: 10.1074/jbc.M308529200. [DOI] [PubMed] [Google Scholar]
- 52.Imlay J.A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006;59:1073–1082. doi: 10.1111/j.1365-2958.2006.05028.x. [DOI] [PubMed] [Google Scholar]
- 53.Ward J.P. Oxygen sensors in context. Biochim. Biophys. Acta. 2008;1777:1–14. doi: 10.1016/j.bbabio.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Gupte S.A., Wolin M.S. Relationships between vascular oxygen sensing mechanisms and hypertensive disease processes. Hypertension. 2012;60:269–275. doi: 10.1161/HYPERTENSIONAHA.112.190702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weir E.K., Lopez-Barneo J., Buckler K.J., Archer S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Alami M., Vina-Almunia J., Gambini J., Mas-Bargues C., Siow R.C., Penarrocha M., Mann G.E., Borras C., Vina J. Activation of p38, p21, and NRF-2 mediates decreased proliferation of human dental pulp stem cells cultured under 21% O2. Stem Cell Reports. 2014;3:566–573. doi: 10.1016/j.stemcr.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dmitriev R.I., Borisov S.M., Kondrashina A.V., Pakan J.M., Anilkumar U., Prehn J.H., Zhdanov A.V., McDermott K.W., Klimant I., Papkovsky D.B. Imaging oxygen in neural cell and tissue models by means of anionic cell-permeable phosphorescent nanoparticles. Cell. Mol. Life Sci. 2015;72:367–381. doi: 10.1007/s00018-014-1673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishii T., Mann G.E. Redox status in mammalian cells and stem cells during culture in vitro: critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014;2:786–794. doi: 10.1016/j.redox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zielonka J., Lambeth J.D., Kalyanaraman B. On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation. Free Radical Biol. Med. 2013;65:1310–1314. doi: 10.1016/j.freeradbiomed.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu F., Huang X., Luo Z., He J., Haider F., Song C., Peng L., Chen T., Wu B. Hypoxia-activated PI3K/Akt inhibits oxidative stress via the regulation of reactive oxygen species in human dental pulp cells. Oxid. Med. Cell. Longev. 2019;2019:6595189. doi: 10.1155/2019/6595189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Schroder K. NADPH oxidases: current aspects and tools. Redox Biol. 2020;34:101512. doi: 10.1016/j.redox.2020.101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hernansanz-Agustin P., Choya-Foces C., Carregal-Romero S., Ramos E., Oliva T., Villa-Pina T., Moreno L., Izquierdo-Alvarez A., Cabrera-Garcia J.D., Cortes A., Lechuga-Vieco A.V., Jadiya P., Navarro E., Parada E., Palomino-Antolin A., Tello D., Acin-Perez R., Rodriguez-Aguilera J.C., Navas P., Cogolludo A., Lopez-Montero I., Martinez-Del-Pozo A., Egea J., Lopez M.G., Elrod J.W., Ruiz-Cabello J., Bogdanova A., Enriquez J.A., Martinez-Ruiz A. Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature. 2020 doi: 10.1038/s41586-020-2551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almutairi M.M.A., Xu G., Shi H. Iron pathophysiology in stroke. Adv. Exp. Med. Biol. 2019;1173:105–123. doi: 10.1007/978-981-13-9589-5_6. [DOI] [PubMed] [Google Scholar]
- 64.Imai T., Iwata S., Hirayama T., Nagasawa H., Nakamura S., Shimazawa M., Hara H. Intracellular Fe(2+) accumulation in endothelial cells and pericytes induces blood-brain barrier dysfunction in secondary brain injury after brain hemorrhage. Sci. Rep. 2019;9:6228. doi: 10.1038/s41598-019-42370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ashraf A., Jeandriens J., Parkes H.G., So P.W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer's disease: evidence of ferroptosis. Redox Biol. 2020;32:101494. doi: 10.1016/j.redox.2020.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascon S., Hatzios S.K., Kagan V.E., Noel K., Jiang X., Linkermann A., Murphy M.E., Overholtzer M., Oyagi A., Pagnussat G.C., Park J., Ran Q., Rosenfeld C.S., Salnikow K., Tang D., Torti F.M., Torti S.V., Toyokuni S., Woerpel K.A., Zhang D.D. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biemond P., van Eijk H.G., Swaak A.J., Koster J.F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J. Clin. Invest. 1984;73:1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chouchani E.T., Pell V.R., Gaude E., Aksentijević D., Sundier S.Y., Robb E.L., Logan A., Nadtochiy S.M., Ord E.N.J., Smith A.C., Eyassu F., Shirley R., Hu C.-H., Dare A.J., James A.M., Rogatti S., Hartley R.C., Eaton S., Costa A.S.H., Brookes P.S., Davidson S.M., Duchen M.R., Saeb-Parsy K., Shattock M.J., Robinson A.J., Work L.M., Frezza C., Krieg T., Murphy M.P. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmstrom K.M., Baird L., Zhang Y., Hargreaves I., Chalasani A., Land J.M., Stanyer L., Yamamoto M., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radical Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishii T., Itoh K., Takahashi S., Sato H., Yanagawa T., Katoh Y., Bannai S., Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 72.Shah Z.A., Li R.-C., Thimmulappa R.K., Kensler T.W., Yamamoto M., Biswal S., Doré S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Penjweini R., Roarke B., Alspaugh G., Gevorgyan A., Andreoni A., Pasut A., Sackett D.L., Knutson J.R. Single cell-based fluorescence lifetime imaging of intracellular oxygenation and metabolism. Redox Biol. 2020;34:101549. doi: 10.1016/j.redox.2020.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eroglu E., Gottschalk B., Charoensin S., Blass S., Bischof H., Rost R., Madreiter-Sokolowski C.T., Pelzmann B., Bernhart E., Sattler W., Hallstrom S., Malinski T., Waldeck-Weiermair M., Graier W.F., Malli R. Development of novel FP-based probes for live-cell imaging of nitric oxide dynamics. Nat. Commun. 2016;7:10623. doi: 10.1038/ncomms10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pak V.V., Ezerina D., Lyublinskaya O.G., Pedre B., Tyurin-Kuzmin P.A., Mishina N.M., Thauvin M., Young D., Wahni K., Martinez Gache S.A., Demidovich A.D., Ermakova Y.G., Maslova Y.D., Shokhina A.G., Eroglu E., Bilan D.S., Bogeski I., Michel T., Vriz S., Messens J., Belousov V.V. Ultrasensitive genetically encoded indicator for hydrogen peroxide identifies roles for the oxidant in cell migration and mitochondrial function. Cell Metabol. 2020;31:642–653 e6. doi: 10.1016/j.cmet.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.