Graphical abstract
Abbreviations: BIF, biological impact factor; CELSD, causal chain of events leading to disease; CHTP, carbon heated tobacco product; CS, cigarette smoke; CVD, cardiovascular disease; GVP, gas/vapor phase; HPHC, harmful and potentially harmful constituents; MRTP, modified risk tobacco product; NPA, network perturbation amplitude; PMI, Philip Morris International; RBIF, relative BIF; THS, Tobacco Heating System; TPM, total particulate matter
Keywords: Modified risk tobacco product (MRTP), Tobacco harm reduction, Systems toxicology, Substantial equivalence
Highlights
-
•
Heated tobacco products tested for reduced exposure and reduced risk properties.
-
•
Bridging opportunities for nonclinical results from two heated tobacco products.
-
•
Similarly reduced impact on apical and molecular endpoints relative to cigarettes.
-
•
Evidence evaluated along a “causal chain of events leading to disease” (CELSD).
-
•
Representative assays along CELSD could support nonclinical substantial equivalence.
Abstract
Cigarette smoking causes major preventable diseases, morbidity, and mortality worldwide. Smoking cessation and prevention of smoking initiation are the preferred means for reducing these risks. Less harmful tobacco products, termed modified-risk tobacco products (MRTP), are being developed as a potential alternative for current adult smokers who would otherwise continue smoking. According to a regulatory framework issued by the US Food and Drug Administration, a manufacturer must provide comprehensive scientific evidence that the product significantly reduces harm and the risk of tobacco-related diseases, in order to obtain marketing authorization for a new MRTP. For new tobacco products similar to an already approved predicate product, the FDA has foreseen a simplified procedure for assessing “substantial equivalence”. In this article, we present a use case that bridges the nonclinical evidence from previous studies demonstrating the relatively reduced harm potential of two heat-not-burn products based on different tobacco heating principles. The nonclinical evidence was collected along a “causal chain of events leading to disease” (CELSD) to systematically follow the consequences of reduced exposure to toxicants (relative to cigarette smoke) through increasing levels of biological complexity up to disease manifestation in animal models of human disease. This approach leverages the principles of systems biology and toxicology as a basis for further extrapolation to human studies. The experimental results demonstrate a similarly reduced impact of both products on apical and molecular endpoints, no novel effects not seen with cigarette smoke exposure, and an effect of switching from cigarettes to either MRTP that is comparable to that of complete smoking cessation. Ideally, a subset of representative assays from the presented sequence along the CELSD could be sufficient for predicting similarity or substantial equivalence in the nonclinical impact of novel products; this would require further validation, for which the present use case could serve as a starting point.
1. Introduction
The concept of bioequivalence testing in the pharmaceutical industry has been successful in marketing high-quality branded and generic drugs at reduced costs, because it eases the requirements for authorization of new products [1]. The safety and efficacy of the new drug have to be demonstrated to be equivalent to those of an approved reference drug, for example, by assessment of the interchangeability of the products in accordance with a catalog of regulatory requirements, such as those defined in the Code of Federal Regulations applied by the United States Food and Drug Administration (US FDA; for details, see [1]).
The US Family Smoking Prevention and Tobacco Control Act defines a modified risk tobacco product (MRTP) as “any tobacco product that is sold or distributed for use to reduce harm or risk of tobacco-related diseases associated with commercially marketed tobacco products” [18]. Concerning the marketing authorization of new tobacco products, particularly MRTPs, the FDA has issued a regulatory framework for assessing “substantial equivalence” for tobacco products [2]. As with the bioequivalence testing of drugs, the aim here is to avoid repetition of the full set of assessment studies for products that have comparable biological effects at comparable doses; however, unlike in drug testing, a fully defined catalog of regulatory requirements is not yet available.
As of June 2020, the FDA had only issued modified risk orders for snus products from a single manufacturer (Swedish Match USA, Inc.) [3]. At the same time, MRTP applications for four other novel tobacco products were under substantive review by the FDA, including one for a heat-not-burn tobacco product, the Tobacco Heating System (THS) 2.2 [4], from Philip Morris Products S.A. (branded as “IQOS System with Marlboro Heatsticks”). In fact, the MRTP application for THS 2.2 represents the first submitted MRTP application for an electronic nicotine delivery device (submitted on November 18, 2016)2 [5]. Given the expectation of additional modified risk orders in the future, it is worth considering how these might shape the assessment approaches for novel tobacco products that are in the same category as an approved MRTP in the future.
Specifically, regarding future assessment of the similarity of novel tobacco products to a fully characterized predicate MRTP, we propose that a systematic weight-of-evidence approach, grounded in the “causal chain of events leading to disease” (CELSD), can guide robust decision-making. Within this rigorous causal framework, a similar yield of harmful and potentially harmful constituents (HPHC), a similar exposure profile, and the same type of molecular response profiles engaging the same or similar disease-relevant mechanisms—in addition to a few well-selected apical endpoints along the CELSD—should be sufficient to plausibly demonstrate a similar harm reduction potential (relative to cigarette smoke [CS]) of a novel tobacco product.
Here, we present a use case of such a systematic bridging strategy. Specifically, we compare the effect and risk-reduction potential of two well-characterized candidate and potential candidate MRTPs, the abovementioned THS 2.2 and the Carbon Heated Tobacco Product (CHTP) 1.2, respectively [6,7], along the CELSD—from toxicant emission to exposure to (stress) responses to primary toxic effects to disease manifestations—which would finally constitute the population health impact/harm potential [[8], [9], [10]].
By integrating results across multiple in vitro and in vivo preclinical studies, the data demonstrate that the two distinct candidate and potential candidate MRTPs, both based on the heat-not-burn principle, achieve a similar reduction in HPHC yield and are associated with corresponding profiles of exposure, uptake, biological response, and disease-relevant effects. The findings from this use case can be leveraged to inform a strategy for developing a concise set of parameters and studies that will be sufficient to demonstrate the “substantial equivalence” of other potential MRTPs.
2. Background
2.1. Tobacco harm reduction
Cigarette smoking has been causally linked to major preventable diseases, morbidity, and mortality worldwide. Prevention of smoking initiation and smoking cessation are the best means of harm reduction [[11], [12], [13]]. Tobacco control policies have contributed to a worldwide decrease in smoking prevalence by approximately 30 % between 1990 and 2015 [14] – in the United States, the prevalence of smoking is at an all-time low of 14 % [15]. As a potential alternative for decreasing the risk of smoking-related diseases in current smokers, the National Academy of Medicine (formerly, Institute of Medicine, USA) has developed and defined the concept of tobacco harm reduction and suggested a regulatory and scientific framework for developing less harmful tobacco products, termed MRTPs [16,17,4].
The US Family Smoking Prevention and Tobacco Control Act granted the FDA the authority to regulate the manufacture, distribution, and marketing of tobacco products, including MRTPs, which are defined as any tobacco product that is sold or distributed for use to reduce harm or the risk of tobacco-related diseases associated with commercially marketed tobacco products [18,19]. MRTP applications must provide scientific evidence that the product significantly reduces harm and the risk of tobacco-related diseases to individual users and that it benefits the health of the population as a whole, taking into account both users and nonusers of tobacco products [20]. In the United Kingdom, the Royal College of Physicians concluded that the health and life expectancy of smokers could be improved if they switched to a smoke-free source of nicotine, although the ultimate goal should be complete cessation [21].
Although nicotine might not be absolutely harmless, it is relatively safe at concentrations typically found in tobacco products [22]; it is not a carcinogen [23] and does not contribute to respiratory diseases or cardiovascular diseases (CVD) [12].
2.2. Potential reduced-risk products
Nicotine delivery systems, which include potential MRTPs, utilize various principles of nicotine administration. There are non-heated pure nicotine-based products intended for nicotine replacement therapy (e.g., nicotine gum, nicotine patches), heated pure nicotine-based products (e.g., e-cigarettes), non-heated tobacco-based products (e.g., chewing tobacco), and heated tobacco-based products (e.g., vaporizers and heat-not-burn products). A “harm minimization continuum” has been proposed: Tobacco- and/or nicotine-containing products, including oral smokeless tobacco and e-cigarettes, were ranked on a continuum of harm, with cigarettes positioned at the highest level of harm and nicotine replacement therapies at the lowest level of harm [[24], [25], [26]].
In the current paper, to exemplify the potential of bridging between candidate and potential candidate MRTPs, we focus on two inhalable heat-not-burn products developed by Philip Morris International (PMI), the Tobacco Heating System 2.2 (THS 2.2) and Carbon Heated Tobacco Product 1.2 (CHTP 1.2). The CHTP is a disposable tobacco product that uses a fast-lighting carbon heat source to heat a tobacco plug in a specially designed stick to produce an aerosol that contains nicotine and tobacco flavor. THS 2.2 is based on a heat-not-burn technology that electrically heats specially designed tobacco sticks instead of burning them. The stick is inserted into a holder with a battery and a heater blade, which carefully heats the tobacco to produce an aerosol. In both products, during use, the tobacco in the tobacco stick does not exceed a well-defined temperature threshold, which prevents combustion of the tobacco and, consequently, significantly limits the generation and delivery of harmful smoke constituents into the aerosol. Detailed descriptions of both product platforms have been published previously: THS 2.2 [4] and CHTP 1.2 [6].
2.3. MRTP assessment
As outlined in detail elsewhere [4], the approaches for assessing the risk of MRTPs relative to cigarettes have been described by the National Academy of Medicine (formerly, Institute of Medicine, USA) [16] and reviewed recently [27]. The assessment approach should leverage the best available science, including short- or long-term epidemiological studies, which can be initiated once the product is in the market and under actual use conditions [4]. The aim is to assess to which extent a potential candidate MRTP (i) reduces harm and the risk of tobacco-related diseases to individual tobacco users and (ii) benefits the health of the population as a whole, taking into account both users and nonusers of tobacco products.
For generating such a robust scientific evidence base for potential candidate MRTPs, PMI has designed a seven-step assessment program, which was first applied for assessment of THS 2.2 [4]:
-
1
Product design and specifications: ensure robust and well-controlled product quality
-
2
Aerosol chemistry and physics: assess reduced formation of HPHCs relative to a cigarette
-
3
Standard toxicology assessment: battery of in vitro and in vivo models to determine toxicity reduction in laboratory models.
-
4
Systems toxicology assessment: in-depth mechanistic toxicology assessment to derive reduced-risk estimates in nonclinical models.
-
5
Clinical exposure studies: assess markers of reduced exposure and risk in humans.
-
6
Consumer perception and behavior: ensure accurate, non-misleading, scientifically substantiated product information and communication to adult smokers to provide them with an incentive to switch from cigarettes to an MRTP.
-
7
Post-market studies and surveillance: toward assessment of reduced population harm.
The potential benefit of switching to MRTPs is compared against the well-documented increasing risks of developing smoking-related diseases when continuing to smoke by using the effects of complete cessation as the gold standard/maximum achievable risk reduction, in accordance with the requirement that, after switching to an MRTP, the risk of developing smoking-related diseases should approach the risk profile of cessation [16].
To assess the risk-reduction potential of a potential candidate MRTP before long-term clinical and epidemiological data become available, a weight-of-evidence approach can be used that integrates and evaluates the available preclinical and short-term clinical data in the conceptual framework of the CELSD [4,28].
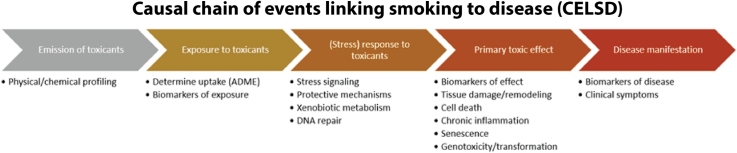
For cigarette smoking, the causal chain of events originates from the emission of toxicants from cigarettes, leading to exposure of the organism and uptake of the toxicants with the inhaled smoke, leading to initial responses such as stress signaling, detoxification, xenobiotic metabolism, and DNA repair, which eventually become saturated/exhausted, leading to primary toxic effects such as tissue damage/remodeling and dysfunction, cell death, chronic inflammation, senescence, genotoxicity, and neoplastic transformation, leading to disease manifestation with morbidity and clinical symptoms (Fig. 1).
Fig. 1.
Causal chain of events linking smoking to disease (CELSD) (modified from [28]). ADME: absorption, distribution, metabolism, and excretion.
The first step in the CELSD concerns the emission of toxicants from a tobacco product under conditions of its intended use. Because the risk of smoking-related diseases is dose-dependent [[29], [30], [31], [32], [33], [34], [35], [36]], a significant reduction in toxicant emission can be expected to result in a significant reduction in toxicant exposure in human subjects who switch from cigarette smoking to a candidate MRTP in clinical studies. Ideally, this exposure reduction should approach the effect of complete smoking abstinence [[37], [38], [39], [40], [41], [42]].
While exposure reduction can be measured in clinical studies, the development of smoking-related diseases, which commonly develop over decades rather than years, cannot. Instead, within a reasonable timeframe, the magnitude of reduction in disease manifestation—the last step in the CELSD—can be inferred from its preceding steps on the basis of relevant measurable and quantifiable endpoints. This approach leverages the principles of “21st century toxicology”, including systems toxicology, and integrates multiple lines of evidence derived from in vitro, in vivo, and clinical studies, enabling the shortcomings of each experimental system to be addressed with data derived from other systems to compare the effects of a candidate MRTP aerosol with those of CS along the key steps of the CELSD. It is the totality of this evidence that should be considered when evaluating the risk reduction potential of a candidate MRTP [43,4,28,[44], [45], [46]]. Similarly, adverse outcome pathways have been suggested to reduce the uncertainty in extrapolating in vitro data in a causal chain of events, leveraging validated high-confidence links between the assay end points and mechanisms of disease [[47], [48], [49]].
In essence, the CELSD approach for assessing a candidate MRTP addresses the complexity in exposure levels, organisms, tissues, time frames, and endpoints by exploring a broad array of indicators of exposure, effect, and disease to demonstrate that the use of the candidate MRTP has a reduced impact relative to cigarettes on mechanisms leading to tobacco smoking-related diseases.
2.4. Bridging & substantial equivalence testing
The bridging strategy aims to demonstrate the essential similarity in tobacco harm reduction potential between two or more products by using a sufficiently stringent correlation of exposure and disease outcome [50] along the CELSD on the basis of a minimal number of key studies in order to minimize the need for complex long-term animal and human exposure studies. The success of this strategy depends on appropriate measurements and endpoints that provide a framework of sufficiently stringent causal relationships from exposure via molecular changes to the final biological responses and disease symptoms. This has been achieved for a few adverse outcome pathways that are also relevant to CS exposure [47,48,51,52]. A regulatory set of rules has been suggested by the FDA to demonstrate substantial equivalence between a new tobacco product and an already-marketed “predicate product” in the process of premarket application [2]. According to this Federal Food, Drug, and Cosmetic Act, substantial equivalence can be assigned to a new product either if it has the same characteristics as the predicate product or if it has different characteristics, but the submitted data are deemed sufficient by the FDA to demonstrate that the product does not raise different questions of public health [2]. For example, the above-mentioned candidate and potential candidate MRTPs, THS 2.2 and CHTP 1.2 respectively, are both based on the heat-not-burn principle; however, they demonstrate different characteristics, including different ways of heating the tobacco. Thus, it is likely that the assignment of substantial equivalence needs to demonstrate that no different public health questions will be raised.
Adequate dosing and dosimetry are essential for bridging studies. Epidemiological studies indicate that the risk for many smoking-related diseases, including lung cancer, chronic obstructive pulmonary disease (COPD), and CVD, is dose-dependent in general [32,11,53], although saturation might occur in the high pack-year3 range, as shown for the incidence of emphysema, which plateaus around 40 % for 50 pack-years and above [50]. Likewise, biomarkers of exposure and harm increase with the number of cigarettes smoked per day [54,55]. Because of these dose dependencies, the similar effects of the new and predicate products need to be demonstrated at comparable effective doses.
In clinical studies, exposure doses are usually measured as the number of cigarettes or, for heat-not-burn products, sticks used per day, and the reference biomarkers of exposure are the levels of nicotine and its metabolites in blood and urine. Exposure doses in non-clinical studies should use a range that spans realistic human equivalent doses. However, some guidelines require that markedly toxic doses are included. This toxicological requirement might lead to unrealistically high concentrations of constituents (compared with those achievable in an exposed organism) if the assay endpoint has low sensitivity and/or the test items have low biological activity, such as low cytotoxicity or genotoxicity. In vitro data must be interpreted very carefully with regard to in vivo health risks if the effects were observed at concentrations far beyond the systemic or local concentrations that can be achieved in an organism — for example, in vitro nicotine concentrations in the millimolar range vs. those below 600 nM in blood plasma or 50 μM in the saliva of cigarette smokers [54].
In summary, assessment of similarity in harm reduction potential between products has to ensure that the comparisons are made at doses that are (i) relevant to human exposure when using these products and (ii) equivalent for the predicate and new products in the various test systems/models applied. Of note, although the proposed strategy, exemplified here with THS 2.2 and CHTP 1.2 assessments as a use case, aims at “functional equivalence” to be granted by the FDA, the term “similarity” will be used for the purpose of this publication, not excluding the possibility that these principles might fulfill the criteria for “substantial equivalence” as well.
3. Material and methods
3.1. Studies evaluated for bridging use case
To compare the risk-reduction potential (on the basis of preclinical studies) of the two heat-not-burn tobacco products, CHTP 1.2 and THS 2.2, we integrated the relevant data along the CELSD. The data for this bridging use case have been published previously, and the reader is referred to the respective publications for additional details (Table 1). For the comparisons, data from exposure groups with matching nicotine concentrations in the CS/MRTP aerosol exposure groups have been selected.
Table 1.
Design parameters of four inhalation toxicology studies.
| CELSD level | Designation | Type | Products tested | References |
|---|---|---|---|---|
| 1 | Aerosol chemistry | THS 2.2 | [56] | |
| 1 | Aerosol chemistry | CHTP 1.2 | [6] | |
| 1 | Ultra-fine carbon-based particles | THS 2.2 | [57] | |
| 1 | Ultra-fine carbon-based particles | CHTP 1.2 | this work | |
| 3 | Standard genotoxicity and cytotoxicity | In vitro neutral red, Ames, and mouse lymphoma assays. | THS 2.2 | [56] |
| 3 | Standard genotoxicity and cytotoxicity | In vitro neutral red, Ames, and mouse lymphoma assays. | CHTP 1.2 | |
| 2,3,4 | OECD rat THS 2.2 | Nose-only 90-day OECD rat study | 3R4F, THS 2.2 | [58] |
| 2,3,4 | OECD rat CHTP 1.2 | Nose-only 90-day OECD rat study | 3R4F, CHTP 1.2 | (Phillips et al.; Titz et al.) |
| 2,3,4,5 | ApoE−/− mice #1 | Whole-body 8-month ApoE−/− study (+ switch/cessation after 2 months) | 3R4F, THS 2.2 | [[59], [60], [61], [62], [63]] |
| 2,3,4,5 | ApoE−/− mice #2 | Whole-body 6-month ApoE−/− study (+ switch/cessation after 3 months) | 3R4F, THS 2.2, CHTP 1.2 | [64] |
| 3,4 | Organotypic nasal THS 2.2 | In vitro exposure of organotypic nasal cultures at the air–liquid interface | THS 2.2 | [65] |
| 3,4 | Organotypic nasal CHTP 1.2 | In vitro exposure of organotypic nasal cultures at the air–liquid interface | CHTP 1.2 | [66] |
| 3,4 | Organotypic small airway THS 2.2 | In vitro exposure of organotypic small airway cultures at the air–liquid interface | THS 2.2 | [67] |
| 3,4 | Organotypic small airway CHTP 1.2 | In vitro exposure of organotypic small airway cultures at the air–liquid interface | CHTP 1.2 | [66] |
| 3,4 | Monocyte-to-endothelial adhesion | In vitro adhesion of human MM6 monocytic cells to human coronary artery endothelial cells | THS 2.2 | [68] |
| 3,4 | Monocyte-to-endothelial adhesion | In vitro adhesion of human MM6 monocytic cells to human coronary artery endothelial cells | CHTP 1.2 | [69] |
| 3,4 | Transendothelial migration | In vitro transendothelial migration with THP-1 and human coronary artery endothelial cells | THS 2.2 | [70] |
| 3,4 | Transendothelial migration | In vitro transendothelial migration with THP-1 and human coronary artery endothelial cells | CHTP 1.2 | [71] |
CELSD levels: 1, emission of toxicants; 2, exposure to toxicants; 3, (stress) response to toxicants; 4, primary toxic effect; 5, disease manifestations. THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product; OECD, Organisation for Economic Co-operation and Development.
3.2. Characterization of ultra-fine carbon-based particles in CHTP 1.2 aerosol
A previous study comparatively characterized and quantified the ultra-fine carbon-based particles released by CHTP 1.2 and those found in CS from the 3R4F reference cigarette, as described for assessment of THS 2.2 [57]. Briefly, CHTP 1.2 aerosol and 3R4F CS were generated in accordance with the Health Canada regimen, and the diluted aerosol/smoke was passed through a thermo-denuder, after which the particles were counted by using a TSI condensation particle counter. In addition, a two-stage impactor trap was used to deposit the aerosols on a collection substrate for further scanning electron microscopy/energy dispersive X-ray analyses.
4. Results and discussion
As a use case for illustrating the potential of bridging two heat-not-burn tobacco products, THS 2.2 and CHTP 1.2, we summarize the assessment results of chemical analyses, biomarkers, toxicological endpoints, and molecular mechanisms from four published inhalation studies and a number of in vitro studies to illustrate the similarity in reduced exposure and risk outcomes between these two products. Comparative evaluation of effects across the CELSD [4,28]—which was focused on preclinical assessment in this manuscript—can then provide a solid basis for evaluating whether a candidate MRTP is substantially equivalent to a predicate MRTP and does not raise different questions of public health.
4.1. Similarly reduced emission of HPHCs in CHTP 1.2 and THS 2.2 aerosols relative to CS
Reduced emission of HPHCs is the basis of tobacco harm reduction. Standardized machine-smoking regimens with fixed puffing profiles, volumes, and frequencies are essential for analytical chemical comparison among tobacco products. Additionally, a reference product—such as the standard reference cigarette 3R4F [72]—is included to directly assess HPHC reduction and serve as a control across studies. As described previously [73], PMI primarily evaluates a profile of 58 analytes and ISO parameters to characterize the yields of cigarettes and MRTPs, including carcinogens and other HPHCs from various panels issued by regulatory bodies or suggested by authorities. Relevant MRTP-related compounds, such as glycerol, can be added. The standard puffing regimen follows the Health Canada Intense protocol: 55-mL puff volume, 30-s puff interval, and all ventilation holes blocked [74]. Previous results have shown that the reduction in the yield of aerosol constituents relative to the constituents of CS is preserved when THS 2.2 aerosol is produced under extreme puffing regimens [73].
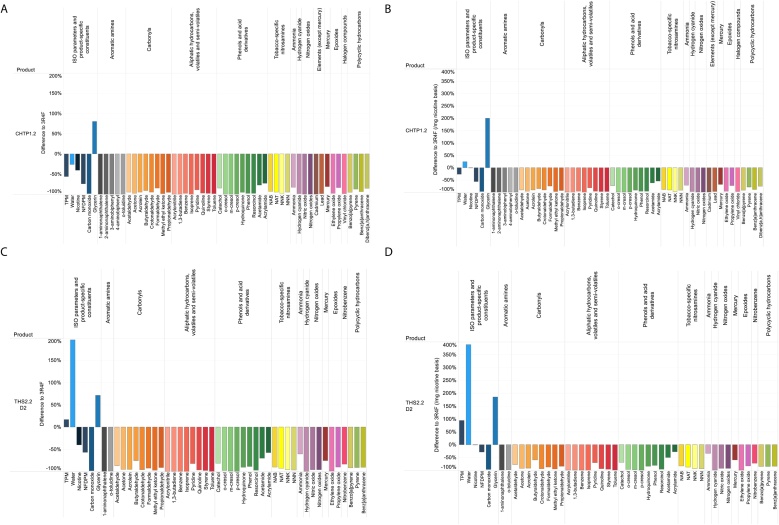
Fig. 2 shows the reduction in HPHC yields, relative to CS, in the aerosol from CHTP 1.2 (Fig. 2A) and THS 2.2 (Fig. 2C). In addition to comparisons on a per stick basis, normalization of the yields per milligram nicotine is meaningful because of possible self-titration by users for achieving similar nicotine levels (see ‘Background’ and Fig. 2B and D).
Fig. 2.
Aerosol chemistry. A. Reduction in the yields of HPHCs in CHTP 1.2 aerosol relative to 3R4F mainstream smoke (per stick) [6]. B. As in A, but on a per mg nicotine basis. C. Reduction in the yields of HPHCs in THS 2.2 aerosol relative to 3R4F mainstream smoke (per stick, THS 2.2 D2) [56]. D. As in C, but on a per mg nicotine basis. HPHC, harmful and potentially harmful constituents; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product.
In general, the HPHC yields of one CHTP 1.2 stick are less than 30 % of those in CS (70 % reduction), with most of the compounds exhibiting more than 90 % reduction. For THS 2.2, these compound yields are reduced by at least 58 %, with most values below 10 % of those in 3R4F CS. The HPHCs with the least reduction in relative yield per stick were, in both products, ammonia, acrylamide, and acetamide as well as various heavy metals. The somewhat lower yield reduction efficiency in THS 2.2 than in CHTP 1.2 is less pronounced when the yields are normalized per milligram nicotine, given the lower nicotine yield per CHTP stick (Fig. 2B and D).
In addition to HPHCs, mainstream CS contains solid carbon-based nanoparticles, which are generated through incomplete combustion processes [75,76]. Several studies have linked nanoparticles to lung inflammation and lung disease [[77], [78], [79]]. In THS 2.2 and CHTP 1.2, tobacco is heated at temperatures below 350℃ rather than being burnt. At this relatively low temperature, distillation processes form an aerosol consisting of suspended liquid droplets via a homogeneous nucleation process; however, because combustion does not occur, the release and transfer of solid carbon particles in the aerosol is not expected. To verify that this is indeed the case, we detected and quantified ultrafine solid particles in the aerosol of THS 2.2 and CHTP 1.2, including 3R4F CS for comparison (Table 2). While 6 × 1011 ultra-fine combustion-related carbon-based solid particles were detected in 3R4F CS under the test conditions, no such ultra-fine particles could be detected in THS 2.2 or CHTP 1.2 aerosol [56,57].
Table 2.
Smoke and THS 2.2/CHTP 1.2 aerosol characteristics.
| 3R4F | THS 2.2 | CHTP 1.2 | |
|---|---|---|---|
| MMAD [μm] | 0.81 | 0.71 | 0.93 |
| GSD | 1.31 | 1.51 | 1.33 |
| Mainstream ultra-fine combustion-related carbon-based solid particles [11 puffs]2 | 6 × 1011 | ND | ND |
The physical properties of the inhaled aerosol affect HPHC exposure: The size distribution of the particles/droplets determines which fraction of the smoke/aerosol passes through the upper respiratory tract and reaches the lungs [[80], [81], [82]]. In this context, the mass median aerodynamic diameter (MMAD), which is calculated from the measured size distribution, is an important parameter, with an MMAD below 2.5 μm indicating a respirable aerosol [83]. The MMAD values for 3R4F CS and THS 2.2 and CHTP 1.2 aerosol were found to be similar and well below 2.5 μm (Table 2).
Therefore, THS 2.2 and CHTP 1.2 aerosols both demonstrate respirable properties that are similar to those of 3R4F CS, but both carry substantially reduced levels of HPHCs and ultra-fine carbon-based solid particles.
4.2. Similar reduction in HPHC exposure and uptake
Aerosol composition and concentration of toxicants are key to downstream biological responses in the CELSD. Thus, it is important to demonstrate that reduced emission results in reduced uptake of HPHCs by an organism.
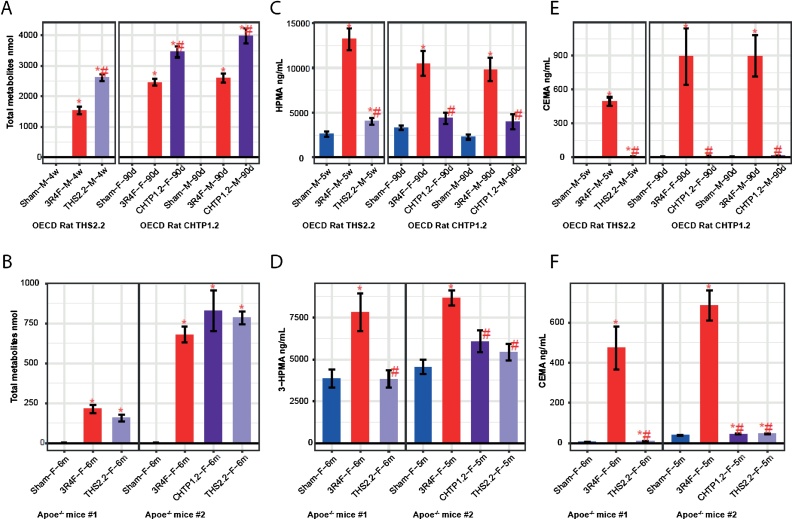
In rats exposed to aerosol from THS 2.2 or CHTP 1.2, the levels of total urinary nicotine metabolites were consistently higher than those in 3R4F CS-exposed rats, although the nicotine exposure concentrations (23 μg nicotine/L) were identical (Fig. 3A). This can be explained by the lower respiratory minute volume in CS-exposed rats, resulting from the irritancy of the smoke. In the THS 2.2 study, we observed a 42 % reduction in respiratory minute volume in the CS group, corresponding to a 41 % reduction in total urinary nicotine metabolites, while we observed no effect on respiratory minute volume (relative to fresh air inhalation) in the comparable (i.e., 23 μg nicotine/L) THS 2.2 aerosol exposure group [84]. Although the uptake of inhaled aerosol was higher than the uptake of CS, there was a strong reduction in the urine levels of the HPHC exposure biomarkers hydroxypropyl mercapturic acid (HPMA, a metabolite of acrolein) and 2-cyanoethylmercapturic acid (CEMA, a metabolite of acrylonitrile) (Fig. 3B and C) in THS-exposed rats, relative to the CS-exposed ones. The elevated background levels of HPMA in the urine of rats exposed to THS 2.2 aerosol and fresh air (sham group) are attributable to the normal metabolism of endogenously formed acrolein.
Fig. 3.
Exposure markers in aerosol and smoke. A, B. Levels of total nicotine metabolites in urine. C, D. 3-HPMA concentrations in urine. E, F. CEMA concentrations in urine. Data are mean ± SEM. *p value vs. sham < 0.05; #p value vs. 3R4F < 0.05. ApoE−/− mouse study #1 refers to [61], and ApoE−/− mouse study #2 refers to [64]. OECD rat THS 2.2 study refers to [84] and OECD rat CHTP 1.2 study refers to [6]. Groups and group vs. sham comparisons are labeled as Exposure–Sex (F, female; M, male)–Time. 3-HPMA, 3-hydroxypropyl mercapturic acid; CEMA, 2-cyanoethylmercapturic acid; SEM, standard error of the mean; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product; OECD, Organisation for Economic Co-operation and Development.
Similar outcomes for HPMA and CEMA were observed in the mouse studies (Fig. 3E and F). Remarkably, there is good reproducibility of biomarker levels between both sexes and also between the studies, which took place several years apart; an exception can be seen in the generally very low nicotine metabolite levels in the first mouse study, which, however, can be explained by the shorter urine collection period (18 h, excluding the 6-h exposure period). This effect appears to have less influence on HPMA and CEMA levels, possibly owing to the faster metabolism of nicotine than that of inhaled acrolein and acrylonitrile [[85], [86], [87]], the urinary biomarker levels of which were comparable with those in study #2.
4.3. Similar reductions in cytotoxic and mutagenic potential
Regulatory in vitro assays are used to characterize the toxic potential of products (e.g., their cytotoxicity and mutagenicity). These assays determine exposure hazards by using a specific design (e.g., Ames assay and mouse lymphoma assay for mutagenicity) that cannot be directly extrapolated to in vivo exposure effects but provide essential data for formal risk assessment.
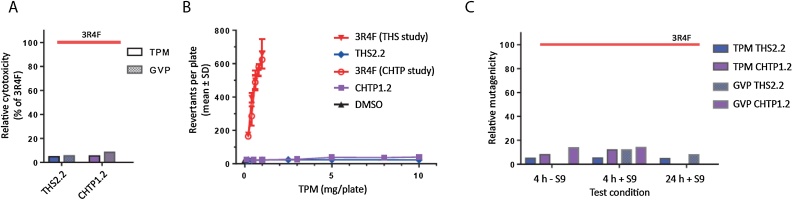
Fig. 4shows the comparative cytotoxicity and mutagenicity results obtained from classical regulatory in vitro assays. Cytotoxicity was determined by using the neutral red uptake assay separately for the total particulate matter (TPM) and gas/vapor phase (GVP) fractions (Fig. 4A). Relative to 3R4F CS, the relative cytotoxicity of THS 2.2 aerosol reached approximately 5 % (TPM) and 6 % (GVP) and that for CHTP 1.2 reached 5 % (TPM) and approximately 10 % (GVP). The higher cytotoxicity of CHTP 1.2 GVP than that of THS 2.2 GVP can be explained by the somewhat lesser reduction resulting in higher concentrations of carbonyls, such as formaldehyde and crotonaldehyde, driving the GVP cytotoxicity (Fig. 2).
Fig. 4.
Standard genotoxicty and cytotoxicity assays. (A) Neutral red cytotoxicity assay. Relative cytotoxicity compared with 3R4F for the TPM and GVP fractions. (B) Ames genotoxicity assay with the Salmonella TA98 strain for TPM. (C) Mouse lymphoma mutagenicity assay. Relative mutagenicity compared with 3R4F. Assessed at two time points (4 h and 24 h) and with and without the S9 microsomal fraction. TPM, total particulate matter; GVP, gas/vapor phase; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product.
In the Salmonella reverse mutation (Ames) assay, 3R4F CS TPM was positive for mutagenicity in five of the routinely tested strains, whereas THS 2.2 and CHTP 1.2 were not mutagenic in any of the conditions/bacterial strains tested; Fig. 4B shows an example of the results for the Salmonella TA98 strain. Mutagenicity in mammalian cells was determined by the mouse lymphoma assay, which yielded relative mutagenicity values between zero and 20 % of the mutagenicity of 3R4F CS, with CHTP 1.2 being more active (genotoxic) than THS 2.2, especially in the GVP, similar to the findings of the bacterial mutation (Ames) assay (Fig. 4C).
Overall, the observed reduction in the cytotoxicity and mutagenicity of both MRTP aerosols relative to CS reflected the degree of toxicant reduction in these aerosols (Fig. 2).
4.4. Similar reduction in effects in nasal epithelium of rats and mice
Inhalation studies in rodents are routinely conducted to assess the general toxicity of inhaled materials such as gases and aerosols (e.g., 90-day rat inhalation studies in accordance with Organisation for Economic Co-operation and Development Test Guideline [OECD TG] 413), or focus on specific disease-related endpoints following more extended exposure periods (e.g., atherosclerosis-related aortic plaque formation in ApoE−/− mice or inflammatory and emphysematous lung changes in C57BL/6 mice). Because rodents, unlike humans, are obligate nose breathers, nasal epithelium is the initial site at which inhaled substances come into contact with the respiratory tract. The established effects of CS in nasal passages are typically basal cell hyperplasia and squamous metaplasia of respiratory epithelium and, less regularly, atrophy of olfactory epithelium and inflammatory infiltration in the submucosa.
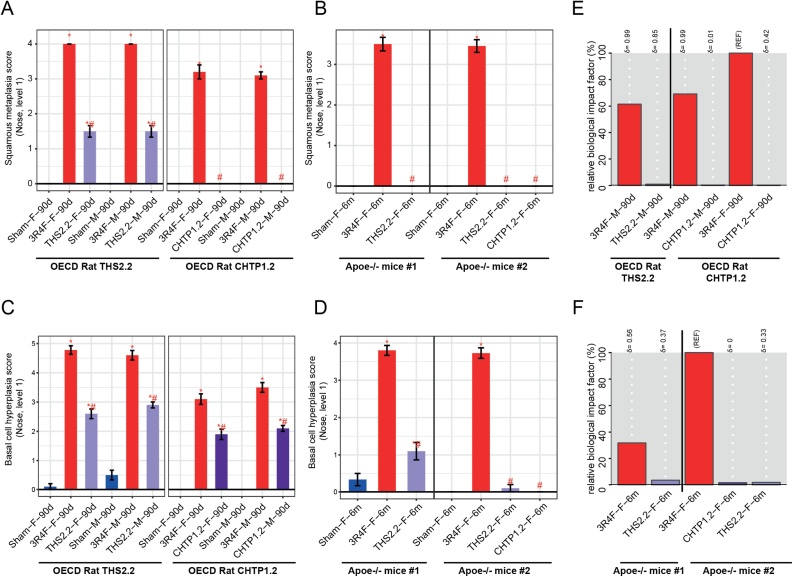
The histopathological scores for basal cell hyperplasia and squamous metaplasia in both rat inhalation studies reproducibly followed the same pattern of response to 3R4F CS inhalation, reaching typical mean values of 3–5 for the high concentration (23 μg nicotine/L) for both endpoints and in both sexes (Fig. 5A, C). In rats exposed to THS 2.2 and CHTP 1.2 aerosols, the mean scores in the comparable concentration groups were significantly lower than those in the CS group, especially for squamous metaplasia.
Fig. 5.
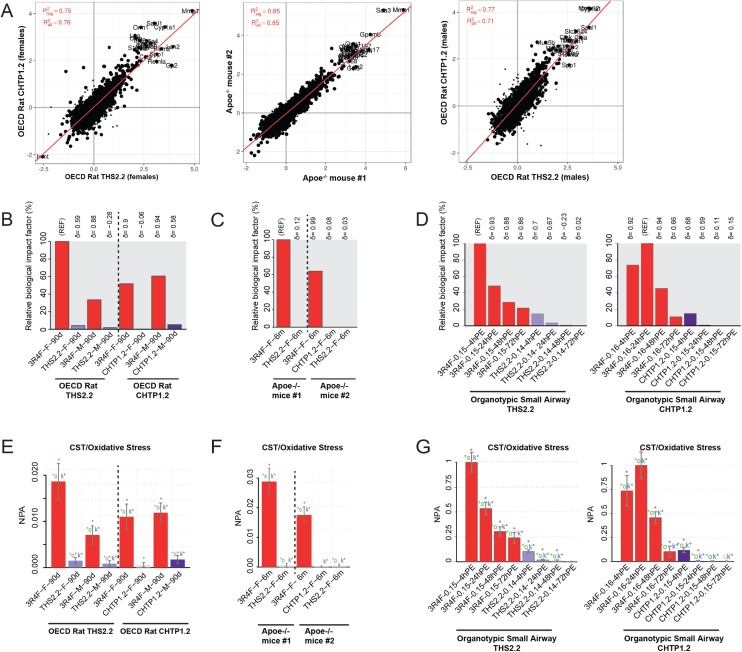
Effects on rodent nasal epithelia. A, B. Histological evaluation at nose level 1: squamous epithelial metaplasia. C, D. Histological evaluation at nose level 1: basal cell hyperplasia. Data are mean severity scores ± SEM. *p value vs. sham < 0.05; #p value vs. 3R4F < 0.05. E, F. Evaluation of biological impact on nose tissue by using the causal network enrichment approach based on transcriptomics data. Relative biological impact factors are represented for each group vs. sham. SEM, standard error of the mean; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product; OECD, Organisation for Economic Co-operation and Development Test Guideline.
In both mouse inhalation studies, following chronic (8- or 6-month) exposure, the lower severity of hyperplasia and metaplasia in THS 2.2 and CHTP 1.2 aerosol-exposed mice (compared with CS-exposed mice at a nicotine-matched concentration) was even more pronounced than that in the rat studies: No squamous metaplasia was observed after 6 months of exposure, while the mean scores in CS-exposed mice ranged from approximately 3.5–4 (Fig. 5B, D).
Based on measurement of global gene changes by using systems toxicology methods, the global molecular perturbations underlying the observed histopathological changes can be expressed as the biological impact factor (BIF), which integrates exposure-related gene expression changes along important pathways of cellular stress and toxicity responses (Fig. 5E, F).
In both rat inhalation studies, the effects of 3R4F CS on the molecular networks were pronounced. Considering the BIF value for female rats in the CHTP 1.2 study as the reference (100 %), the exposure responses of male rats were approximately 40 % lower, while their quality (i.e., the affected mechanisms) was the same, as indicated by the delta value of 0.99 in both studies. For both MRTPs, the BIF values were lower than 1 % (Fig. 5E).
In the mouse studies, the effect of 3R4F was stronger in ApoE−/− study #1; compared with this value, the BIF for the 3R4F group was only one third, and yet the responses to the MRTP aerosols were much lower (Fig. 5F). As in the rat studies, the reproducibility of THS 2.2-related BIFs in both studies was good.
4.5. Similar reduction in effects on human organotypic nasal epithelium in vitro
Short-term in vitro studies on organotypic human nasal epithelial cultures allow mimicking of the human exposure situation by exposing cells directly to CS or MRTP aerosol at the apical surface (air–liquid interface).
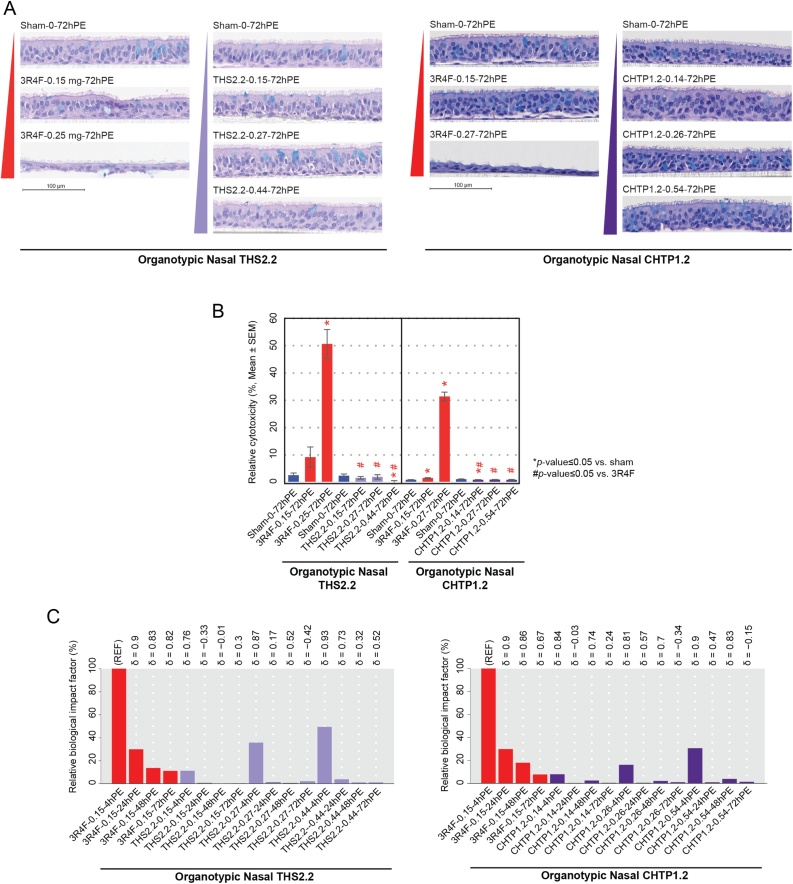
Fig. 6A shows cross-sections of cultured human epithelia following exposure to fresh air (sham), CS from 3R4F, or aerosol from the MRTPs. The sham-exposed epithelia exhibit the normal, pseudostratified morphology of human nasal respiratory epithelium, with a continuous ciliated surface and occasional goblet cells. A single exposure to a low concentration of 3R4F smoke (0.15 mg nicotine/L) caused discrete changes in the epithelial structure 72 h post-exposure, particularly in the THS 2.2 study; exposure to 0.25 mg nicotine/L 3R4F CS led to massive damage and cell loss. In the CHTP 1.2 study, the effect of the high 3R4F smoke concentration was similarly strong, while the lower concentration elicited less obvious damage, mainly detachment from basal cells. The MRTP exposures, in contrast, did not result in these effects either at comparable nicotine concentrations or at a high aerosol concentration that exceeded the 3R4F-comparable level by 60 % (for THS 2.2) or 100 % (for CHTP 1.2). Likewise, there was a sharp increase in the measured cytotoxicity from the low to high 3R4F CS concentrations, while no increase in cytotoxicity was observed in either MRTP aerosol-exposed group at any of the tested concentrations (Fig. 6B).
Fig. 6.
Effects on organotypic nasal epithelial cultures. A. Morphology of organotypic nasal epithelial cultures following a 28-min exposure at the air–liquid interface. B. Relative cytotoxicity in the exposed organotypic nasal cultures evaluated by the adenylate kinase release assay. C. Relative biological impact factors for the exposed nasal organotypic cultures. See Fig. 3 legend for details on the study and group labeling in the in vivo studies. Group labeling for the in vitro cultures is a follows: Exposure type–Nicotine concentration in aerosol/cigarette smoke (milligrams nicotine per liter)–PE duration. PE, post-exposure; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product.
The biological impact of smoke or aerosol exposure on nasal epithelium was evaluated by using the causal network enrichment approach based on transcriptomics data. The BIF peaked 4 h post-exposure and rapidly declined until 72 h post-exposure (Fig. 6C). At comparable concentrations, the relative BIF (RBIF) values for THS 2.2 and CHTP 1.2 aerosols (4 h) were 10 % and <10 %, respectively, of the CS BIF (4 h). Even at 3-fold higher aerosol concentrations, the RBIF values reached only 50 % (THS 2.2) and 30 % (CHTP 1.2) of the CS reference BIF.
4.6. Similar reduction in lung inflammatory processes in rats and mice
Bronchoalveolar lavage recovers free lung cells — that is, cells (mostly immune cells) that are loosely attached to the luminal surfaces of the alveolar region and pulmonary airways. Their abundance and profile reflect and distinguish initial activation of the innate immune system and inflammatory responses as well as non-resolving, chronic inflammatory processes typical of prolonged CS exposure.
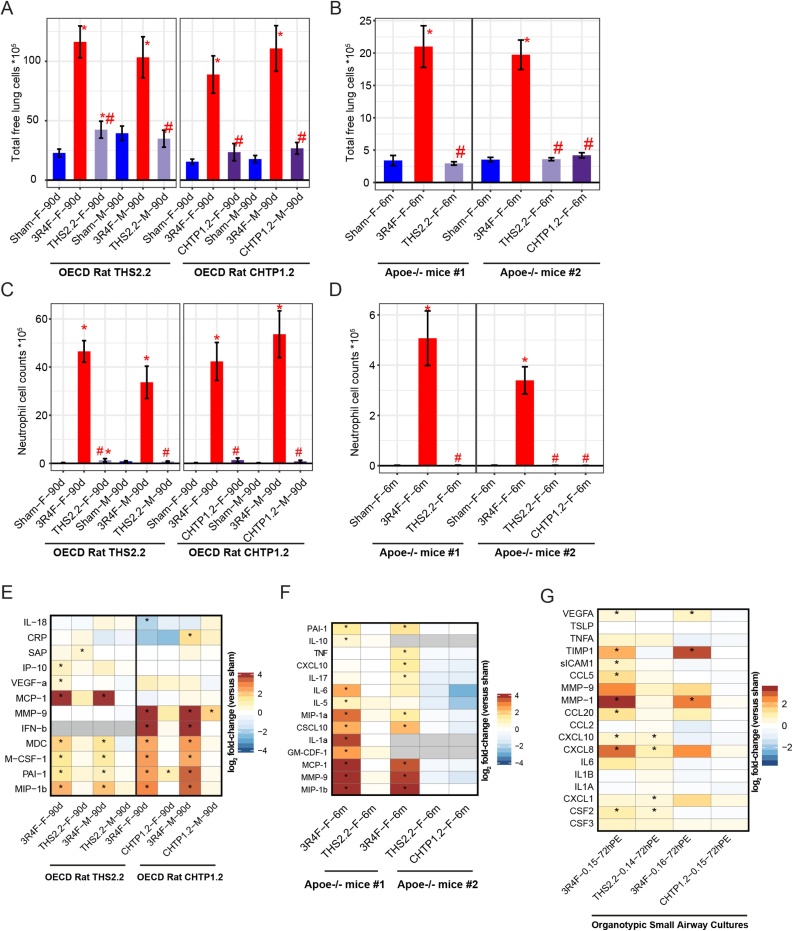
Exposure to 3R4F CS caused a massive increase in the number of free lung cells, while only a weak increase was seen in female rats exposed to THS 2.2 aerosol, and no effect was seen in male rats in the THS 2.2 group (Fig. 7A). Fig. 7B shows the corresponding results in the murine studies, again with an approximate 5-fold increase in the number of free lung cells following exposure to 3R4F CS and no increase following a 6-month exposure to THS 2.2 or CHTP 1.2 aerosol. Among the free lung cell populations, the most pronounced response to CS exposure was exerted by neutrophils (Fig. 7C and D), accounting for 30–50 % of the total free lung cells in CS-exposed rat lungs (17–25 % in mouse lungs). These neutrophil data reflect the reduction in total inflammatory cell numbers following MRTP aerosol exposure relative to their numbers following CS exposure.
Fig. 7.
Lung inflammation. A, B. Total free lung cells in bronchoalveolar lavage fluid, measured by flow cytometry. C, D. Total neutrophils in bronchoalveolar lavage fluid. Data are mean ± SEM. *p value vs. sham < 0.05; #p value vs. 3R4F < 0.05. E. Inflammatory mediators in cell-free supernatants from bronchoalveolar lavage fluid in rat studies. Changes in inflammatory mediator levels relative to the levels in the sham group are color-coded. F. Inflammatory mediators in cell-free supernatants from bronchoalveolar lavage fluid in mouse studies. G. Changes in inflammatory mediator levels in in vitro assessment of organotypic small airway cultures. *p value < 0.05. See Fig. 3 legend for details on the study and group labeling. SEM, standard error of the mean; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product.
In addition, we measured soluble inflammatory mediators in BALF by multianalyte profiling. Fig. 7E shows the inflammatory mediators measured in BALF in both rat studies. 3R4F CS caused a pronounced increase in the levels of the proteins MDC, PAI-1, M-CSF-1, and MIP-1B in both studies; in addition, while study #1 found a strong increase in MCP-1 levels which was not observed in study #2, only study #2 found higher levels of MMP-9 and IFN-β. These differences might be attributable to technical differences in the immunoassay platform. In both studies, both MRTPs caused only weak sporadic changes, such as a very weak increase in SAP (in THS 2.2 aerosol-exposed female rats), PAI-1 (in CHTP 1.2-exposed female rats), and MMP-9 (in CHTP 1.2-exposed male rats). Fig. 7F shows the corresponding inflammatory mediator profiles for both mouse studies. While a significant increase in the abundance of several inflammatory mediators (e.g., MMP-9 and MCP-1) was observed upon 3R4F CS exposure, no significant changes were detected in either mouse study with either the candidate or the potential candidate MRTP.
While BALF contains secreted inflammatory mediators from free and resident (mostly epithelial) lung cells, the culture medium in organotypic small airway epithelial cultures contains only the mediators secreted by this cell type (Fig. 7G). The most prominent response to 3R4F CS in both studies was an increase in the levels of the metalloproteinase MMP-1, the protease inhibitor TIMP-1, and the interleukin CXCL-8; the increase in CXCL-8 levels, however, did not reach statistical significance in study #2. Exposure to THS 2.2 aerosol, like exposure to CS, elicited a very weak but statistically significant increase in CXCL-10 levels, weaker responses than CS exposure in case of CXCL-8 and CSF1, and a weak increase in CXCL-1 levels, which was numerically similar but not statistically significant relative to that observed after CS exposure.
4.7. Similar reductions in molecular effects in the lungs
The biological impact on lung tissue was also evaluated by using a systems toxicology approach based on transcriptomics data. In this regard, a high consistency and study-to-study reproducibility was observed in the molecular effects induced by CS exposure in the lungs (Fig. 8A). The results of the causal network enrichment approach are presented in Fig. 8B and C. In the rat studies (Fig. 8B), the highest BIF (set as 100 % of the reference, RBIF) was observed in CS-exposed female rats in the THS 2.2 study, while the male rat RBIF upon CS exposure was only approximately 35 % of this value. In the CHTP 1.2 study, the RBIFs in female and male rats upon CS exposure were approximately 50 % and 60 %, respectively. In contrast, the RBIFs of both MRTPs were approximately 5 % or lower, indicating a pronounced reduction in their biological impact relative to CS.
Fig. 8.
Molecular effects in the lungs (in vivo and in vitro), including cell stress. A. Consistency in the molecular effects of cigarette smoke in the lungs. Correlation of gene expression responses as fold changes in 3R4F exposure vs. sham in the rat and mouse in vivo studies. Red lines represent fit from linear model. and are the coefficients of determination for all or the significantly affected genes, respectively. B–D. Evaluation of biological impact on lung tissue by using a causal network enrichment approach based on transcriptomics data. Relative biological impact factors are represented for each group vs. sham. E–G. Network enrichment analysis of the oxidative stress network. Bars show the overall NPA on the basis of transcriptomic data; error bars indicate 95 % confidence intervals. Three statistical measures are shown: The red star indicates statistical significance with respect to biological replicatesl the green star (o statistic) indicates significance with respect to permutation of genes downstream of the network nodes; and the blue star (k statistic) indicates significance with respect to permutation of the network topology (p < 0.05). THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product; NPA, network perturbation amplitude; OECD, Organisation for Economic Co-operation and Development Test Guideline.
A similar outcome was observed in both mouse studies (Fig. 8C): The RBIF in CS-exposed mice in study #2 was approximately 65 % of the BIF in study #1, and the BIF values for THS 2.2 and CHTP 1.2 exposure were close to zero. As an example, the response in the oxidative stress network model is shown in Fig. 8E and F. The network perturbation amplitudes (NPA) were pronounced in CS-exposed rats and mice but reached only a small fraction of these values in the MRTP aerosol-exposed animals, indicating a strong reduction in MRTP-related oxidative stress in the latter groups.
Similarly, the biological impact of exposure on in vitro organotypic small airway epithelial cell models exposed at the air–liquid interface was evaluated with the causal network approach (Fig. 8D and G). In small airway epithelial organotypic cultures, CS exposure elicited a pronounced response, which peaked 4 h post-exposure in the first (THS 2.2) study. In the second (CHTP 1.2) study, the maximum BIF was reached 24 h post-exposure, indicating somewhat delayed kinetics; for THS 2.2 and CHTP 1.2 aerosol exposure, the RBIF values at comparable aerosol concentrations reached only about 10 % of the CS reference BIF at 4 h post-exposure, and their RBIF values declined to zero at the 48-h and 72-h time points. On the single-network model level for oxidative stress (Fig. 8G), the same kinetics for CS exposure was evident, and, again, the maximum NPA in the MRTP aerosol-exposed cultures was only about 10 % of that of the CS reference.
4.8. Similar reductions in lung disease-associated endpoints in rats and mice
The ApoE−/− mouse model also allows assessment of more advanced, disease-related endpoints—representing the 5th stage in the CELSD—that resemble the features associated with human disease, such as emphysematous changes mimicking human emphysema in COPD patients.
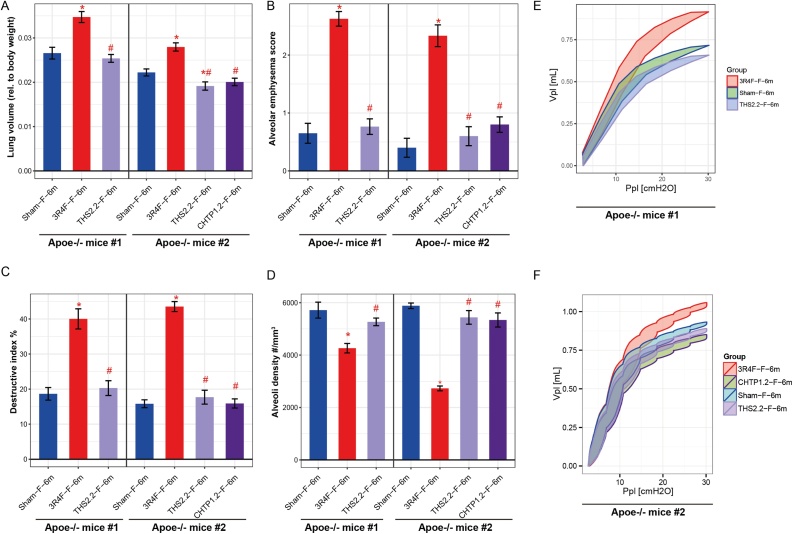
Fig. 9 shows the corresponding characteristic changes in CS-exposed mice: The plethysmographic pressure–volume loops recorded in living, anesthetized mice represent the loss of elastic recoil and increase in alveolar volume; in contrast, THS 2.2 and CHTP 1.2 exposure did not cause any significant change relative to fresh air (sham) exposure (Fig. 9E and F). Likewise, an increase in CS-induced lung volume was seen in isolated lungs after dissection, while no increase in lung volume was observed following exposure to CHTP 1.2 or THS 2.2 (Fig. 9A). The histological emphysema scores assigned by the pathologist demonstrate a clear and significant increase in emphysema in CS-exposed mice, while there was no significant difference in scores between MRTP aerosol-exposed mice and the sham-exposed controls (Fig. 9B). These semiquantitative results were confirmed by morphometric evaluation of histological sections (Fig. 9C and D). The destructive index (Fig. 9C) and volume-independent parameter of alveolar density (Fig. 9D) both indicate emphysematous changes in the lungs of CS-exposed mice and their absence in the lungs of mice exposed to aerosol from THS 2.2 and CHTP 1.2.
Fig. 9.
Disease-associated changes in the lungs. A. Lung volume relative to body weight. The lungs were removed, and their volume was determined by displacement of fixative under hydrostatic pressure. Data are mean ± SEM. *p value vs. sham < 0.05; #p value vs. 3R4F < 0.05. Histopathological/morphometric evaluation of lung tissue: B. Alveolar emphysema (severity score 1–5). C. Destructive index (%). D. alveolar density. E, F. Pressure–volume loops. Relationship between pressure (Ppl, pressure plethysmography) and the resultant volume (Vpl, volume plethysmography) over an inflation/deflation cycle is shown. See Fig. 3 legend for details on the study and group labeling. SEM, standard error of the mean; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product.
4.9. Similar reductions in acceleration of atherosclerotic plaque formation
CS is causally linked to the development of CVDs through different pathophysiologic pathways, including endothelial injury and dysfunction, oxidative stress, a procoagulatory status, inflammation, and an abnormal lipid profile, all contributing to the development of atherosclerosis. While most laboratory rodent strains are relatively resistant to experimental induction of atherosclerosis, ApoE−/− mice have been designed for massive hyperlipidemia, which can lead to spontaneous development of atherosclerosis, making them particularly useful for testing anti-atherosclerotic drugs [88]. CS exposure accelerates atherosclerotic processes, and mechanisms of plaque formation have been investigated by using this model [89].
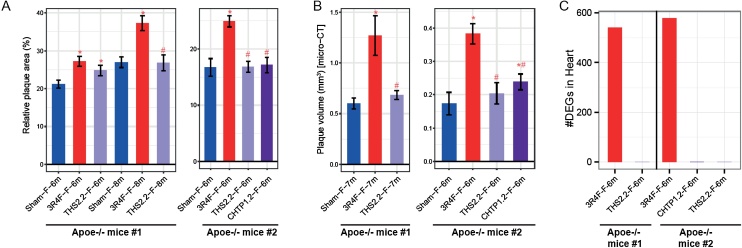
In both studies with ApoE−/− mice, we observed significantly higher relative plaque areas in CS-exposed mice than in sham-exposed mice, as determined by planimetry of excised aortic arches (at 6 and 8 months in study #1 and at 6 months in study #2) (Fig. 10A). THS 2.2 aerosol did not cause an increase in plaque size over the sham values after 8 months of exposure in study #1 and after 6 months in study #2; however, there was a significant increase after 6 months in study #1. In this regard, the CHTP 1.2 value in study #2 did not differ from the sham value.
Fig. 10.
Cardiovascular effects in vivo. A. Percentage of atherosclerotic plaque area (planimetry): atherosclerotic plaque area as percentage of the total aortic arch area. Data are mean ± SEM. *p value vs. sham < 0.05; #p value vs. 3R4F < 0.05. B. Atherosclerotic plaque volume measured by micro-CT. Note: The sites of measurements might differ between the two studies (two different CROs). C. Number of DEGs in the left heart ventricle tissue (false discovery rate-adjusted p value < 0.05). See Fig. 3 legend for details on the study and group labeling. CT, computed tomography; DEG, differentially expressed gene; SEM, standard error of the mean; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product.
In both studies, additional cohorts of mice were submitted to micro-computed tomography (micro-CT) for alternative assessment of plaque size (Fig. 10 B). In both studies, the effects of THS 2.2 on plaque volume were significantly smaller than those of 3R4F CS and did not differ from the sham values after 7 or 6 months of exposure (study #1 and #2, respectively). In the CHTP 1.2 group, the plaque volume was also significantly smaller than that in the 3R4F group, but there was a small but statistically significant increase over the volume in the sham group. The relatively moderate reduction can be explained, at least in part, by the high background levels and rapid increase in the size of spontaneous atherosclerotic plaques in the genetically engineered ApoE−/− mouse model; CS exposure can only accelerate the spontaneous rate of plaque growth [90].
Taken together, these complementary endpoints from independent mouse groups in each study suggest a smaller effect of both MRTP aerosols compared with the effects of CS on acceleration of plaque formation in ApoE−/− mice.
4.10. Similar reductions in gene expression effects in the heart
In both studies with ApoE−/− mice, we also analyzed the effects of exposure on the heart. Exposure to 3R4F CS, in both studies, induced differential expression of approximately 550–580 genes in the myocardial tissue (Fig. 10C); many of these differentially expressed genes were downregulated, including genes related to inflammatory responses and cytoskeletal and structural integrity, while those related to amino acids and xenobiotic metabolism were upregulated [62,91]. In contrast, no significant differential gene expression was observed in the heart in mice exposed to THS 2.2 or CHTP 1.2 aerosol (Fig. 10C).
This lack of significant differential gene expression following exposure to either MRTP aerosol points to a strong reduction in their cardiac effects in ApoE−/− mice relative to the effects of CS.
4.11. Similar reductions in cardiovascular in vitro effects: adhesion and transmigration
Endothelial dysfunction, associated with adhesion of monocytes to the endothelial surface and their subsequent transendothelial migration into the arterial intima, is an early inflammatory process necessary for initiation of plaque formation [92]. Both monocyte-to-endothelial adhesion and monocyte transmigration can be investigated with in vitro models. Here, we summarize the corresponding results from our previously published studies [[68], [69], [70], [71]].
The monocyte adhesion assay is based on an indirect exposure mode designed to mimic the in vivo situation: Human monocytic MM6 cells were treated with aqueous extracts of CS or MRTP aerosol, and the conditioned medium resulting from this treatment—containing smoke/aerosol constituents and inflammatory mediators (mostly TNFα)—was used to expose primary human coronary artery endothelial cells. Untreated MM6 cells were co-incubated with the endothelial cells, and the rate of monocyte adhesion to the endothelial cells was determined by image cytometry [68,69].
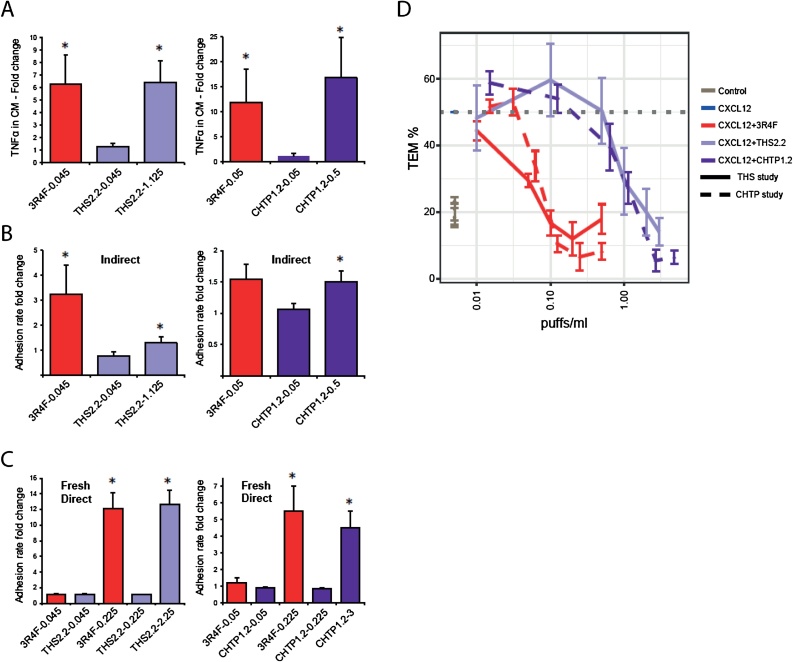
Fig. 11A depicts the induction of TNFα secretion in MM6 cells. In study #1, treatment with aqueous 3R4F smoke extract (0.045 puffs/mL) induced a 6-fold increase in secreted TNFα levels, while treatment with the extract from THS 2.2 aerosol at this concentration did not increase TNFα levels. Only a 25-fold higher concentration of the THS 2.2 extract induced the same response as CS. In study #2, treatment with the CS extract (0.05 puffs/mL) induced a 12-fold increase in TNFα levels, while treatment with the CHTP 1.2 extract at the same concentration failed to induce a significant increase over the levels induced in the saline-treated control; a 10-fold higher concentration of CHTP 1.2 aerosol extract was required to induce a similar level of TNFα secretion (approximately 16-fold higher than the levels in the control). The adhesion of monocytic cells to coronary arterial endothelial cells treated with TNFα-containing conditioned medium from 3R4F-treated monocytes increased by approximately a factor of 3 (Fig. 11B, left panel), while the extract from THS 2.2 aerosol was inactive at the same concentration, and a 25-fold higher concentration induced a statistically significant increase of only 1.5-fold in monocyte adhesion. In the CHTP 1.2 study (Fig. 10B, right panel), the extract from CS (0.05 puffs/mL) induced a 1.5-fold increase in MM6 adhesion (not statistically significant because of high variability), while a 10-fold higher concentration of the CHTP 1.2 extract was required to elicit the same 1.5-fold response (statistically significant because of lower variability of the measured values).
Fig. 11.
Cardiovascular effects in vitro. A. Induction of TNFα secretion in human MM6 cells treated with extracts from 3R4F, THS 2.2, and CHTP 1.2. B. Adhesion of monocytic cells to coronary arterial endothelial cells treated with the TNFα-containing CM from monocytes treated with extracts from 3R4F, THS 2.2, and CHTP 1.2 (indirect exposure). C. Adhesion of monocytic cells to coronary arterial endothelial cells treated with extracts from 3R4F, THS 2.2, and CHTP 1.2 (direct exposure). D. Effect of extracts from 3R4F, THS 2.2, and CHTP 1.2 on real-time impedance-based TEM. Real-time impedance-based TEM of THP-1 cells migrating across a layer of human coronary artery endothelial cells and exposed to increasing concentrations of extracts from 3R4F, THS 2.2, or CHTP 1.2 in cell invasion/migration chambers. The CXCL12 concentration that induced 50 % migration was used in these assays. Data are expressed as the mean ± SEM of at least three independent experiments (*p value < 0.05). TNF, tumor necrosis factor; CM, conditioned medium; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product; TEM, transendothelial migration; CXCL12, C-X-C motif chemokine 12; SEM, standard error of the mean.
In addition, an alternative exposure mode (fresh direct) was applied to test the pro-adhesive effect of direct treatment of endothelial cells without influence from smoke/aerosol-treated monocyte secretions (Fig. 11C). The CS concentrations used for indirect exposure did not enhance monocyte adhesion following direct treatment; however, an approximately 5-fold higher concentration of the CS extract induced a 12-fold increase in MM6 cell adhesion in the THS 2.2 study (Fig. 11C, left panel) and a 5.5-fold increase in the CHTP 1.2 study (Fig. 11C, right panel). In case of THS 2.2 and CHTP 1.2 extracts, 10- and 13-fold higher concentrations, respectively, were required to achieve a similar response.
It should be noted that the exposure modalities, indirect and fresh direct, address different mechanisms of inducing adhesive (dysfunctional) properties in aortic endothelial cells. Indirect exposure drives predominantly inflammatory pathways, while the effects of fresh direct exposure are mediated by cytotoxicity pathways [68,69], with the results supporting a substantial reduction in both mechanisms with MRTP aerosol exposure, relative to CS.
The ability of monocytes/macrophages to cross the endothelial barrier from the vascular lumen to subendothelial intima (extravasation) and vice versa (intravasation) plays an important role in plaque formation, and treatment with CS fractions can modulate monocyte chemotaxis and transendothelial migration [70,71]. The data presented here are related to impairment of intravasation — that is, the ability to clear material from an emerging plaque back into systemic circulation. Of note, with an alternative exposure design, mechanisms of extravasation could be shown to increase in the same in vitro model, but with less sensitivity [71].
Exposure of the human monocyte-like THP-1 cells to 3R4F CS extracts inhibited their transendothelial migration in a concentration-dependent manner, with a half-maximal inhibitory concentration (IC50) of around 0.06 puffs/mL. In contrast, higher concentrations of MRTP aerosol extracts were required for achieving the same effect (18-fold higher for THS 2.2 and 15-fold higher for CHTP 1.2) (Fig. 11 and Table 3). Table 3 also lists some endpoints related to transendothelial migration. The IC50 values for chemotaxis were 50-fold higher for THS 2.2 and 13-fold higher for CHTP 1.2 than for 3R4F CS exposure. For the release of proinflammatory cytokines (TNFα and interleukin 8), the IC50 values of both THS 2.2 and CHTP 1.2 were 10- to 20-fold higher than that for 3R4F CS. Endothelial barrier function was impaired by the 3R4F CS extract in a similar concentration range as that required to disrupt transendothelial migration, and both MRTP aerosol extracts were approximately 17 times less potent. Likewise, cytotoxicity towards THP-1 cells was at least one order of magnitude lower with THS 2.2 and CHTP 1.2 than with the 3R4F CS extract (Table 3).
Table 3.
Reduced activity in assay parameters related to chemotaxis and TEM of monocytes. Effects of extracts from 3R4F, THS 2.2, and CHTP 1.2 on functional endpoints such as TEM, THP-1 cytotoxicity (7AAD), inflammation (IL-8 and TNFα release by THP-1 cells), barrier function, and THP-1 cell chemotaxis.
| THS study |
CHTP study |
|||
|---|---|---|---|---|
| 3R4F (puff/mL) | THS 2.2 (puff/mL) | 3R4F (puff/mL) | CHTP 1.2 (puff/mL) | |
| TEM IC50 | 0.049 | 0.87 | 0.067 | 0.98 |
| 7AAD IC50 | 0.085 | >3.0 | >0.5 | >4.8 |
| IL-8 peak | 0.05 | 1.0 | 0.125 | 1.52 |
| TNF peak | 0.1 | 1.0 | 0.125 | 2.03 |
| Barrier function IC50 | 0.047 | 0.79 | 0.040 | 0.68 |
| Chemotaxis IC50 | 0.016 | 0.86 | 0.055 | 0.74 |
IC50 or peak values are calculated from at least three independent experiments. TEM, transendothelial migration; 7AAD, 7-aminoactinomycin D; IL, interleukin; TNF, tumor necrosis factor; IC50, half-maximal inhibitory concentrations; THS, Tobacco Heating System; CHTP, Carbon Heated Tobacco Product.
4.12. Similar effects in the liver
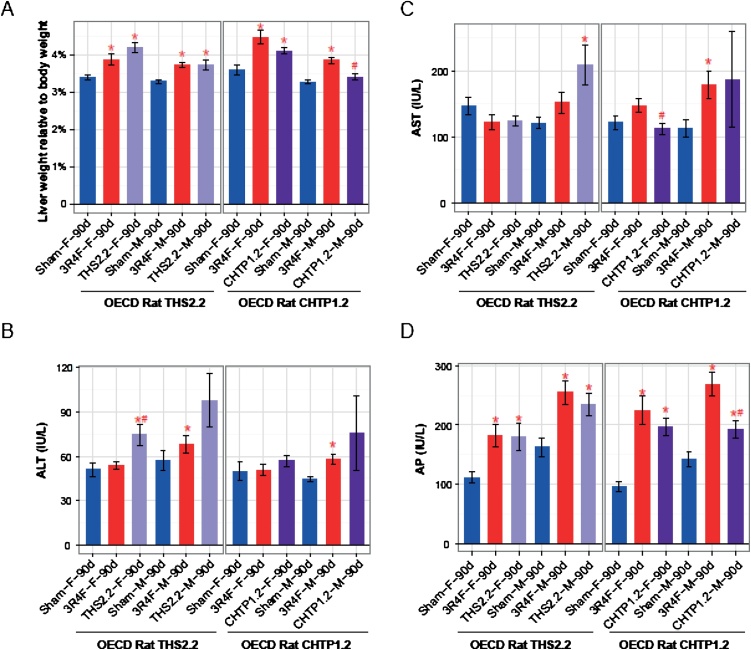
Inhalation exposure of rats to 3R4F CS consistently induced an increase in liver weight (relative to body weight) and alkaline phosphatase activity in blood, relative to sham exposure (Fig. 12A, D). The 3R4F-related increases in two other markers of hepatic stress, alanine aminotransferase and aspartate aminotransferase, were less consistent: Alanine aminotransferase levels were only increased in male rats, and aspartate aminotransferase levels were only increased in male rats in study #2 (Fig. 12B, C). Exposure to aerosol from THS 2.2 or CHTP 1.2 caused a similar increase in liver weight as exposure to CS (Fig. 12A); both MRTPs also elicited a similar increase in alkaline phosphatase activity in blood. For THS 2.2, only male rats showed an increase in aspartate aminotransferase activity (Fig. 12C), and only female rats showed an increase in alanine aminotransferase activity (Fig. 12B). In previous studies, similar effects were observed in rats exposed to nicotine aerosols with various vehicles; in particular, liver weight and alkaline phosphatase and alanine aminotransferase values were dependent on the nicotine concentration [93,94]. Therefore, these changes can be attributed to nicotine exposure, and no reduction in these effects can be expected in studies where MRTP aerosol and CS concentrations are matched for nicotine concentration. The persistent effect of nicotine was predominantly seen in the blood levels of liver enzymes and in the histological changes in hepatic tissues, while considerable reductions were evident in the global transcriptomics changes in the rat liver, indicating here a partial effect of nicotine.
Fig. 12.
Effects on the liver. A. Liver weight relative to body weight. Data are mean ± SEM. Activities of ALT (B), AST (C), and AP (D) in blood. *p value vs. sham < 0.05; #p value vs. 3R4F < 0.05. See Fig. 1 legend for details on the study and group labeling. SEM, standard error of the mean; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AP, alkaline phosphatase.
In contrast to the findings in rats, CS exposure-related histological changes in the murine liver were only weak, while the corresponding effects were completely absent following exposure to THS 2.2 and CHTP 1.2 aerosols. Similarly, global transcriptomics changes in the mouse liver were mostly observed upon 3R4F CS exposure, whereas the effects upon MRTP exposure were much more limited or absent [60].
4.13. Similar effects of switching and cessation
Consistent with the requirement that, after switching to an MRTP, the risk of developing smoking-related diseases should approach the risk profile of cessation [16], we included switching and cessation scenarios as additional arms in our chronic inhalation studies in ApoE−/− mice. In study #1, a 2-month inhalation exposure to 3R4F CS was followed by switching to THS 2.2 aerosol or fresh air (cessation) for up to 6 months [61], and, in study #2, a 3-month CS exposure was followed by switching to CHTP 1.2 aerosol or cessation for up to 3 months [64].
In both studies, switching to MRTP aerosol or cessation led to complete recovery from squamous metaplasia in the nasal respiratory epithelium (Fig. 13A). Nasal respiratory epithelial hyperplasia also reverted to sham levels within 6 months of cessation as well as after switching to THS 2.2 (Fig. 13B, left panel). In the CHTP 1.2 study (Fig. 13B, right panel), the 3 months of cessation were not sufficient for complete regression; however, switching to CHTP 1.2 aerosol had the same effect as cessation.
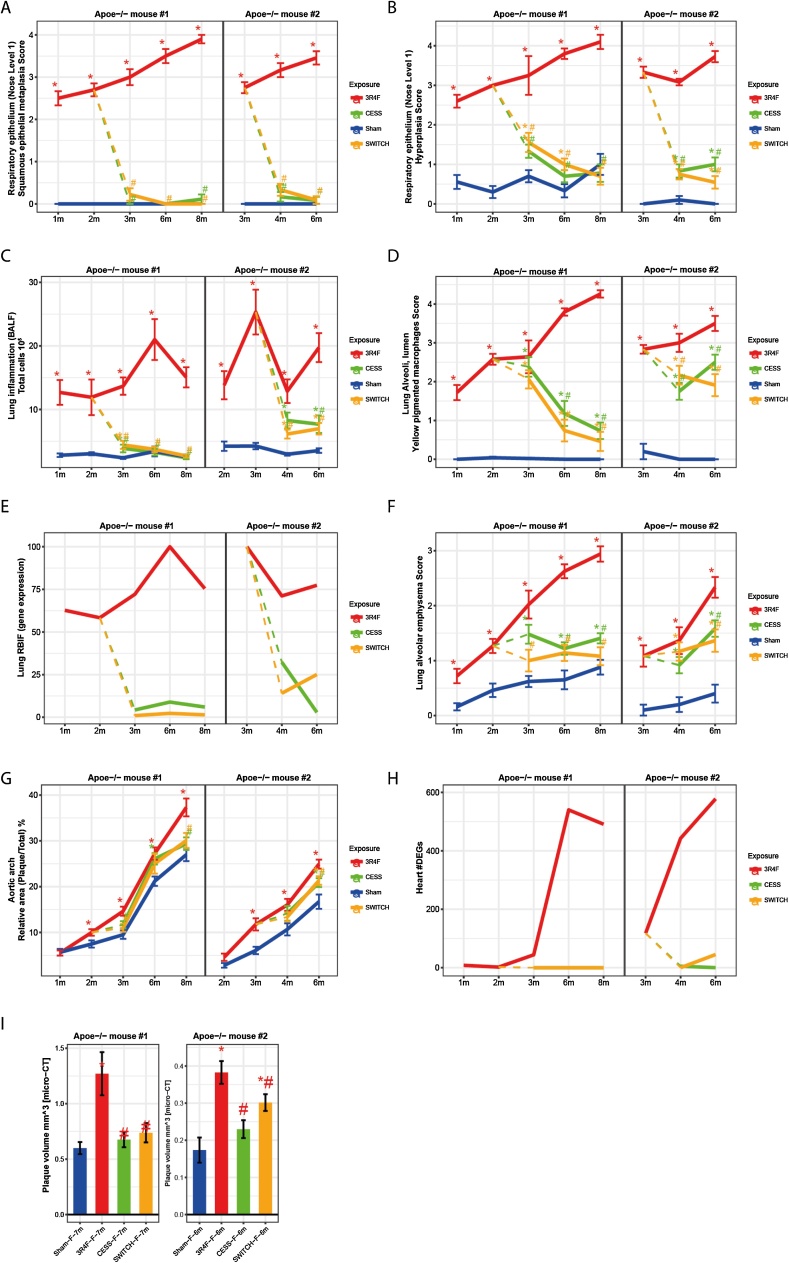
Fig. 13.
Effects of switching or cessation in ApoE−/− mice. Following 2 (study #1) or 3 (study #2) months of exposure to 3R4F cigarette smoke, mice were switched to fresh air (sham) or aerosol from THS 2.2 (study #1) or CHTP 1.2 (study #2). A 3R4F reference group was continued on cigarette smoke exposure. Selected groups and endpoints are shown; for the full data, see the original publications [61,64]. A. Adaptive change in response to irritation: squamous metaplasia of respiratory epithelium, nose level 1. B. Adaptive change in response to irritation: hyperplasia of respiratory epithelium, nose level 1. C. Lung inflammation: number of free lung cells in bronchoalveolar lavage fluid. D. Lung inflammation: yellow pigmented macrophages, left lung. E. Biological impact on the lungs: causal network models interpreting transcriptomics data. F. Lung disease: emphysematous changes (histopathology scores). G. Atherosclerosis: relative plaque size (morphometry) in the aortic arch. H. Heart response profiles: number of DEGs. I. Atherosclerosis: plaque volume (micro-CT data) in the aorta. DEG, differentially expressed gene; RBIF, relative biological impact factor; micro-CT, micro-computed tomography.
These relationships were also seen with free inflammatory cell counts in the lungs (Fig. 13C): full recovery to sham-exposure levels within 6 months of cessation or switching to THS 2.2; incomplete recovery within 3 months of cessation or switching to CHTP 1.2; and, most importantly, no difference between the effects of cessation and switching.
The number of yellow-pigmented macrophages in the lungs steadily increased with the duration of CS exposure and decreased markedly over time upon cessation or switching to either MRTP; however, even the 6-month cessation/switching period was not sufficient to completely clear the yellow-pigmented macrophages (Fig. 13D). Of note, in a previous study with a different mouse strain (A/J), even a 13-month cessation period did not suffice to remove all pigmented macrophages [95]. There was no difference in the effects of cessation and switching on the decrease in yellow-pigmented macrophages in either study (Fig. 13D).
Alveolar emphysematous changes also increased with the duration of 3R4F CS exposure; but, we observed a weaker steady increase in emphysema score over time in sham-exposed mice, reflecting an age-related background effect (Fig. 13F). Cessation and switching to THS 2.2 halted the progression of emphysema over a period of 6 months, and the switching group scores matched the sham group scores at the 8-month time point, while the cessation scores remained slightly higher than the sham group scores (Fig. 13F). In the CHTP 1.2 study, cessation and switching to CHTP 1.2 arrested the emphysema scores equally. In both groups, the scores were significantly lower than those in the 3R4F group 3 months after ending CS exposure but still higher than the scores in the sham group.
The aggregated gene expression changes in the lungs, expressed as the RBIF, followed the apical endpoint kinetics: A sharp drop from the level achieved by 3R4F CS exposure was observed after cessation or switching to THS 2.2 or CHTP 1.2, and switching reduced the impact on gene expression in the same way as cessation (Fig. 13E).
Fig. 13G depicts the CS-induced acceleration in atherosclerotic plaque size, which was significantly greater than the rapid increase in spontaneous plaque size in the sham group in both studies and at all time points following the second or third month of exposure; this is a typical feature of this genetically engineered mouse model. Despite the narrow dynamic range between the background and exposure effects, the plaque size increased after switching, with a smaller slope than the 3R4F curve, paralleling the sham group curve and reaching a significantly lower level than the 3R4F effect 6 or 3 months after cessation/switching to THS 2.2 or CHTP 1.2 (Fig. 13G). Again, for both MRTPs, the effect of switching was indistinguishable from that of cessation. In both studies, a separate cohort of mice were subjected to complementary evaluation of plaque volume by micro-CT at only one time point (after 7 and 6 months in study #1 and #2, respectively; Fig. 13I). Switching to THS 2.2 or CHTP 1.2, like cessation, led to statistically significant lower plaque volumes than those observed after 3R4F CS exposure; however, in the 3-month switching period of study #2 (CHTP 1.2), only the cessation group values reached those of the sham group, while, during the 5-month period of study #1 (THS 2.2), the plaque volumes after cessation and switching reached those of the sham group (Fig. 13I). The effects of CS exposure on the number of differentially expressed genes are shown in Fig. 13H; in both studies, the maximum numbers of differentially expressed genes (approximately 550 and 580, respectively, in study #1 and #2) were observed after 6 months of 3R4F CS exposure. Cessation and switching to THS 2.2 after 2 months of 3R4F exposure reduced the number of differentially expressed genes to zero (Fig. 13H) at all time points. In contrast, in study #2, cessation and switching after 3 months of 3R4F exposure resulted in a complete absence of differentially expressed genes after only 1 month of cessation (at the 4-month time point), while a few genes (<50) were still differentially expressed at the 6-month time point upon switching (Fig. 13H).
Taken together, these examples of apical and molecular endpoints demonstrate that switching to THS 2.2 or CHTP 1.2 following a 2- or 3-month 3R4F CS exposure consistently and significantly decreased or halted (emphysema) CS-related adverse effects in the respiratory and cardiovascular systems in ApoE−/− mice and that the switching effects approached those of cessation in this disease model. Depending on the degree of reversibility of the various endpoints, sham exposure levels were not always reached, particularly in study #2, which had a longer CS exposure period and a shorter switching/cessation period. Nevertheless, the results for both MRTPs support their potential for risk reduction in the respiratory and cardiovascular systems.
5. Summary and conclusion
We propose that, for future assessment of the similarity of novel tobacco products to a fully characterized predicate MRTP, a systematic weight-of-evidence approach, grounded in the CELSD, can guide robust decision-making.
As a use case of the CELSD-based assessment strategy, we have systematically compared candidate and potential candidate MRTPs, THS 2.2 and CHTP 1.2 respectively, along the CELSD, integrating (preclinical) results across several published studies (Table 1). While both products are based on the heat-not-burn principle—that is, prevention of tobacco combustion—they employ distinct approaches: electrical heating in THS 2.2 and heat transfer from a carbon tip in CHTP 1.2. Despite these design differences, we show that, along the CELSD, THS 2.2 and CHTP 1.2 achieve a similar overall reduction in HPHC emission, exposure biomarkers, and exposure-related effects relative to CS (Table 4). In the ApoE−/− mouse studies, switching from CS exposure to THS 2.2 or CHTP 1.2 aerosol exposure led to attenuation of effects at a comparable degree and in a manner similar to that of complete cessation.
Table 4.
Summary of THS 2.2 and CHTP 1.2 assessment along the CELSD.
| CELSD level 1: Similar reduction in emission of toxicants |
| The levels of the measured HPHCs were substantially lower in THS 2.2 and CHTP 1.2 aerosol than in smoke from 3R4F reference cigarettes. |
| CELSD level 2: Similar reduction in exposure to toxicants |
| At a comparable level of nicotine exposure, the lower emission of HPHCs translated into reduced uptake and internal exposure to selected smoke/aerosol constituents, as measured by the levels of biomarkers of exposure. The reduction rates relative to those observed with cigarette smoke were comparable for THS 2.2 and CHTP 1.2. |
| CELSD level 3: Similar reduction in (stress) response to toxicants |
| In the respiratory tract and cardiovascular system, activation of cell stress responses—including mechanisms of adaptive tissue changes (nasal epithelium), xenobiotic metabolism, and antioxidant protection—was similarly reduced following exposure THS 2.2 and CHPT 1.2 aerosols relative to that following cigarette smoke exposure. This reduction was shown in a number of in vivo and in vitro investigations by using causal network modeling of transcriptomics data targeting cigarette smoke-related mechanisms of response. Moreover, in non-targeted analyses of genome-wide transcriptomics data, no effect was observed with the candidate MRTP aerosols that was absent in cigarette smoke exposure. In liver tissue, where several of the effects of cigarette smoke exposure were nicotine-driven, the reduction in stress responses was less marked than that in the respiratory and cardiovascular systems; however, the differences relative to the effects of cigarette smoke exposure were very similar for THS 2.2 and CHTP 1.2 aerosol exposure. |
| CELSD level 4: Similar reduction in primary toxic effects |
| The genotoxicity and cytotoxicity of aerosols from THS 2.2 and CHTP 1.2 were much lower than those of cigarette smoke, when assessing their relative potencies in regulatory short-term in vitro assays. Likewise, the toxic effects of both THS 2.2 and CHPT 1.2 aerosols were equally lower following subchronic inhalation exposure in rats, chronic exposure in mice, and short-term exposure in organotypic in vitro systems. |
| CELSD level 5: Similar reduction in disease manifestation |
| To complete the evidence of similar reductions in the adverse effects of THS 2.2 and CHTP 1.2 exposure vs. cigarette smoke exposure along the CELSD, analyses in disease models and related human in vitro models demonstrated the significantly lower potential of the THS 2.2 and CHPT 1.2 to induce or accelerate lung inflammation, emphysema, atherosclerosis, and cardiac hypertrophy on the histological, molecular, and functional levels. |
Beyond establishing the similarity of the specific candidate and potential candidate MRTPs compared here, this use case, more generally, exemplifies the potential of a systematic CELSD-based approach for establishing substantial equivalence between the risk-reduction potential of a novel tobacco product and a predicate product. In this context, within the CELSD framework, absence of disease effects can be causally linked to a reduction in the preceding steps, overall, supporting causal evidence-based decision-making. Moreover, the findings from this use case can be leveraged to inform a strategy for developing a concise set of parameters and studies that will be sufficient to demonstrate the “substantial equivalence” of other potential MRTPs.
Funding
Philip Morris International is the sole source of funding and sponsor of this research.
CRediT authorship contribution statement
Walter K. Schlage: Conceptualization, Writing - original draft, Writing - review & editing. Bjoern Titz: Conceptualization, Software, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Anita Iskandar: Investigation, Resources, Writing - review & editing, Supervision. Carine Poussin: Investigation, Resources, Writing - review & editing, Visualization. Marco Van der Toorn: Investigation, Resources, Writing - review & editing. Ee Tsin Wong: Investigation, Resources, Writing - review & editing, Supervision. Pascal Pratte: Investigation, Resources, Writing - review & editing. Serge Maeder: Resources, Writing - review & editing, Supervision. Jean-Pierre Schaller: Resources, Writing - review & editing. Pavel Pospisil: Resources, Supervision. Stephanie Boue: Data curation, Visualization. Grégory Vuillaume: Software, Formal analysis, Visualization, Supervision. Patrice Leroy: Software, Formal analysis, Visualization. Florian Martin: Conceptualization, Software, Formal analysis, Writing - review & editing, Supervision. Nikolai V. Ivanov: Resources, Writing - review & editing, Supervision, Funding acquisition. Manuel C. Peitsch: Conceptualization, Writing - review & editing, Supervision, Funding acquisition. Julia Hoeng: Conceptualization, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The work reported in this publication involved candidate/potential modified risk tobacco products developed by Philip Morris International. Philip Morris International is the sole source of funding and sponsor of this research. All authors are employees of PMI R&D or had worked for PMI R&D under contractual agreements.
Acknowledgements
The authors would like to thank the study team and especially acknowledge the technical assistance and support of the Bioresearch and Aerosol Teams at PMI R&D, Philip Morris International Research Laboratories Pte. Ltd., Singapore, and PMI R&D, Philip Morris Products S.A., Neuchâtel, Switzerland. The authors thank Sam Ansari for managing the biobanking and acknowledge the support of Nick Karoglou and Sindhoora Bhargavi Gopala Reddy for editing a draft of the manuscript.
Footnotes
Note added in proof: “On July 7, 2020, FDA issued exposure modification orders to Philip Morris Product S.A. for its IQOS Tobacco Heating System.”
Pack-years: the number of years of smoking multiplied by the number of packs of cigarettes smoked per day.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.09.004.
Contributor Information
Bjoern Titz, Email: bjorn.titz@pmi.com.
Julia Hoeng, Email: julia.hoeng@pmi.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Midha K.K., McKay G. Springer; 2009. Bioequivalence; Its History, Practice, and Future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(FDA), F.a.D.A . U.S.D.O.H.A.H. Services, F.A.D. Administration, C.F.T. Products (Eds.) 2011. Section 905(j) reports: demonstrating substantial equivalence for tobacco products. [Google Scholar]
- 3.FDA . 2020. Modified Risk Orders. [Google Scholar]
- 4.Smith M.R., Clark B., Luedicke F., Schaller J.P., Vanscheeuwijck P., Hoeng J., Peitsch M.C. Evaluation of the Tobacco Heating System 2.2. Part 1: description of the system and the scientific assessment program. Regulatory toxicology and pharmacology: RTP. 2016 doi: 10.1016/j.yrtph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 5.FDA . 2020. Philip Morris Products S.A. Modified Risk Tobacco Product (MRTP) Applications. [Google Scholar]
- 6.Phillips B.W., Schlage W.K., Titz B., Kogel U., Sciuscio D., Martin F., Leroy P., Vuillaume G., Krishnan S., Lee T., Veljkovic E., Elamin A., Merg C., Ivanov N.V., Peitsch M.C., Hoeng J., Vanscheeuwijck P. A 90-day OECD TG 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects of the aerosol from the carbon heated tobacco product version 1.2 (CHTP1.2) compared with cigarette smoke. I. Inhalation exposure, clinical pathology and histopathology. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2018;116:388–413. doi: 10.1016/j.fct.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Titz B., Kogel U., Martin F., Schlage W.K., Xiang Y., Nury C., Dijon S., Baumer K., Peric D., Bornand D., Dulize R., Phillips B., Leroy P., Vuillaume G., Lebrun S., Elamin A., Guedj E., Trivedi K., Ivanov N.V., Vanscheeuwijck P., Peitsch M.C., Hoeng J. A 90-day OECD TG 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects of the aerosol from the carbon heated tobacco product version 1.2 (CHTP1.2) compared with cigarette smoke. II. Systems toxicology assessment. Food Chem. Toxicol. 2018;115:284–301. doi: 10.1016/j.fct.2018.02.058. [DOI] [PubMed] [Google Scholar]
- 8.Djurdjevic S., Lee P.N., Weitkunat R., Sponsiello-Wang Z., Ludicke F., Baker G. Modeling the population health impact of introducing a modified risk tobacco product into the U.S. Market. Healthcare (Basel) 2018:6. doi: 10.3390/healthcare6020047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee P.N., Djurdjevic S., Weitkunat R., Baker G. Estimating the population health impact of introducing a reduced-risk tobacco product into Japan. The effect of differing assumptions, and some comparisons with the U.S. Regulatory toxicology and pharmacology : RTP. 2018;100:92–104. doi: 10.1016/j.yrtph.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Weitkunat R., Lee P.N., Baker G., Sponsiello-Wang Z., Gonzalez-Zuloeta Ladd A.M., Ludicke F. A novel approach to assess the population health impact of introducing a Modified Risk Tobacco Product. Regul. Toxicol. Pharmacol. 2015;72:87–93. doi: 10.1016/j.yrtph.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Office of the Surgeon General, U.S . Centers for Disease Control and Prevention (US); Atlanta (GA): 2004. The Health Consequences of Smoking: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 12.Office of the Surgeon General, U.S . Centers for Disease Control and Prevention (US); Atlanta GA: 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. [PubMed] [Google Scholar]
- 13.Surgeon_General . 2014. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General, Atlanta GA. [Google Scholar]
- 14.GBD 2015 Tobacco Collaborators . 2017. Smoking Prevalence and Attributable Disease Burden in 195 Countries and Territories, 1990-2015: a Systematic Analysis From the Global Burden of Disease Study 2015. Lancet (London, England) pp. 1885–1906. 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services . U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2020. Smoking Cessation: A Report of the Surgeon General; p. 700. [Google Scholar]
- 16.Institute of Medicine, I . The National Academies Press; Washington, DC: 2012. Scientific Standards for Studies on Modified Risk Tobacco Products. [Google Scholar]
- 17.Stratton K., Shetty P., St Claire A.W., Martinez R.M., Pellmar T. In: Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. I. Institute of Medicine, editor. National Academy Press; Washington, DC: 2001. p. 636. [Google Scholar]
- 18.Family Smoking Prevention and Tobacco Control Act . Public Law No. 111–131; 2009. Family Smoking Prevention and Tobacco Control Act (FSPTCA) [Google Scholar]
- 19.Food and Drug Administration, F . Federal food, drug, and cosmetic act. In: F. Food, Drug Administration, editors. 18 FDC Act as Amended by the Family Smoking Prevention and Tobacco Control Act (Public Law 111-31) 2012. p. 21 U.S.C 387 k. [Google Scholar]
- 20.Food and Drug Administration, F . 2012. Modified Risk Tobacco Product Applications: Draft Guidance for Industry. [Google Scholar]
- 21.Tobacco Advisory Group of the Royal College of Physicians . Royal College of Physicians; 2016. Nicotine without Smoke—tobacco Harm Reduction. [Google Scholar]
- 22.Greenland S., Satterfield M.H., Lanes S.F. A meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Saf. 1998;18:297–308. doi: 10.2165/00002018-199818040-00005. [DOI] [PubMed] [Google Scholar]
- 23.International Agency for Research on Cancer . International Agency for Research on Cancer; Lyon: 2004. Tobacco Smoke and Involuntary Smoking. [Google Scholar]
- 24.Abrams D.B., Glasser A.M., Pearson J.L., Villanti A.C., Collins L.K., Niaura R.S. Harm minimization and tobacco control: reframing societal views of nicotine use to rapidly save lives. Annu. Rev. Public Health. 2018;39:193–213. doi: 10.1146/annurev-publhealth-040617-013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeill A., Munafo M.R. Reducing harm from tobacco use. J. Psychopharmacol. (Oxford) 2013;27:13–18. doi: 10.1177/0269881112458731. [DOI] [PubMed] [Google Scholar]
- 26.Nutt D.J., Phillips L.D., Balfour D., Curran H.V., Dockrell M., Foulds J., Fagerstrom K., Letlape K., Milton A., Polosa R. Estimating the harms of nicotine-containing products using the MCDA approach. Eur. Addict. Res. 2014;20:218–225. doi: 10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- 27.Berman M.L., Connolly G., Cummings K.M., Djordjevic M.V., Hatsukami D.K., Henningfield J.E., Myers M., O’Connor R.J., Parascandola M., Rees V., Rice J.M., Shields P.G. Providing a science base for the evaluation of tobacco products. Tob. Regul. Sci. 2015;1:76–93. doi: 10.18001/TRS.1.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturla S.J., Boobis A.R., Fitzgerald R.E., Hoeng J., Kavlock R.J., Schirmer K., Whelan M., Wilks M.F., Peitsch M.C. Systems toxicology: from basic research to risk assessment. Chem. Res. Toxicol. 2014;27:314–329. doi: 10.1021/tx400410s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baba S., Iso H., Mannami T., Sasaki S., Okada K., Konishi M. Cigarette smoking and risk of coronary heart disease incidence among middle-aged Japanese men and women: the JPHC Study Cohort I. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology. Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2006;13:207–213. doi: 10.1097/01.hjr.0000194417.16638.3d. [DOI] [PubMed] [Google Scholar]
- 30.Charvat H., Sasazuki S., Shimazu T., Budhathoki S., Inoue M., Iwasaki M., Sawada N., Yamaji T., Tsugane S. Development of a risk prediction model for lung cancer: the japan public health center-based prospective study. Cancer Sci. 2018;109:854–862. doi: 10.1111/cas.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doll R., Peto R. Cigarette smoking and bronchial carcinoma: dose and time relationships among regular smokers and lifelong non-smokers. J. Epidemiol. Community Health. 1978;32:303–313. doi: 10.1136/jech.32.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forey B.A., Thornton A.J., Lee P.N. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm. Med. 2011;11:36. doi: 10.1186/1471-2466-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann D., Hoffmann I., El-Bayoumy K. The less harmful cigarette: a controversial issue. A tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001;14:767–790. doi: 10.1021/tx000260u. [DOI] [PubMed] [Google Scholar]
- 34.Holahan C.K., Holahan C.J., North R.J., Hayes R.B., Powers D.A., Ockene J.K. Smoking status, physical health-related quality of life, and mortality in middle-aged and older women. Nicotine Tob. Res. 2013;15:662–669. doi: 10.1093/ntr/nts182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lubin J.H., Albanes D., Hoppin J.A., Chen H., Lerro C.C., Weinstein S.J., Sandler D.P., Beane Freeman L.E. Greater coronary heart disease risk with lower intensity and longer duration smoking compared with higher intensity and shorter duration smoking: congruent results across diverse cohorts. Nicotine Tob. Res. 2017;19:817–825. doi: 10.1093/ntr/ntw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynder E.L., Hoffmann D. Tobacco and tobacco smoke. Semin. Oncol. 1976;3:5–15. [PubMed] [Google Scholar]
- 37.Gale N., McEwan M., Eldridge A.C., Fearon I.M., Sherwood N., Bowen E., McDermott S., Holmes E., Hedge A., Hossack S., Wakenshaw L., Glew J., Camacho O.M., Errington G., McAughey J., Murphy J., Liu C., Proctor C.J. Changes in Biomarkers of Exposure on Switching From a Conventional Cigarette to Tobacco Heating Products: A Randomized, Controlled Study in Healthy Japanese Subjects. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2018 doi: 10.1093/ntr/nty104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goniewicz M.L., Gawron M., Smith D.M., Peng M., Jacob P., 3rd, Benowitz N.L. Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine Tob. Res. 2017;19:160–167. doi: 10.1093/ntr/ntw160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haziza C., de La Bourdonnaye G., Skiada D., Ancerewicz J., Baker G., Picavet P., Ludicke F. Evaluation of the Tobacco Heating System 2.2. Part 8: 5-Day randomized reduced exposure clinical study in Poland. Regul. Toxicol. Pharmacol. 2016;81(Suppl 2):S139–S150. doi: 10.1016/j.yrtph.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Ludicke F., Picavet P., Baker G., Haziza C., Poux V., Lama N., Weitkunat R. Effects of Switching to the Tobacco Heating System 2.2 Menthol, Smoking Abstinence, or Continued Cigarette Smoking on Biomarkers of Exposure: A Randomized, Controlled, Open-Label, Multicenter Study in Sequential Confinement and Ambulatory Settings (Part 1) Nicotine Tob. Res. 2018;20:161–172. doi: 10.1093/ntr/ntw287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connell G., Graff D.W., D’Ruiz C.D. Reductions in biomarkers of exposure (BoE) to harmful or potentially harmful constituents (HPHCs) following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Toxicol. Mech. Methods. 2016;26:443–454. doi: 10.1080/15376516.2016.1196282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuki D., Takeshige Y., Nakaya K., Futamura Y. Assessment of the exposure to harmful and potentially harmful constituents in healthy Japanese smokers using a novel tobacco vapor product compared with conventional cigarettes and smoking abstinence. Regul. Toxicol. Pharmacol. 2018;96:127–134. doi: 10.1016/j.yrtph.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Committee on Toxicity Testing and Assessment of Environmental Agents, C . In: Toxicity Testing in the Twenty-First Century: A Vision and a Strategy. Council N.R., editor. National Academies Press; Washington, DC: 2007. [Google Scholar]
- 44.Hartung T. Lessons learned from alternative methods and their validation for a new toxicology in the 21st century. J. Toxicol. Environ. Health B Crit. Rev. 2010;13:277–290. doi: 10.1080/10937404.2010.483945. [DOI] [PubMed] [Google Scholar]
- 45.Hartung T., FitzGerald R.E., Jennings P., Mirams G.R., Peitsch M.C., Rostami-Hodjegan A., Shah I., Wilks M.F., Sturla S.J. Systems toxicology: real world applications and opportunities. Chem. Res. Toxicol. 2017;30:870–882. doi: 10.1021/acs.chemrestox.7b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauer J.M., Kleensang A., Peitsch M.C., Hayes A.W. Advancing risk assessment through the application of systems toxicology. Toxicol. Res. 2016;32:5–8. doi: 10.5487/TR.2016.32.1.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clippinger A.J., Allen D., Behrsing H., BeruBe K.A., Bolger M.B., Casey W., DeLorme M., Gaca M., Gehen S.C., Glover K., Hayden P., Hinderliter P., Hotchkiss J.A., Iskandar A., Keyser B., Luettich K., Ma-Hock L., Maione A.G., Makena P., Melbourne J., Milchak L., Ng S.P., Paini A., Page K., Patlewicz G., Prieto P., Raabe H., Reinke E.N., Roper C., Rose J., Sharma M., Spoo W., Thorne P.S., Wilson D.M., Jarabek A.M. Pathway-based predictive approaches for non-animal assessment of acute inhalation toxicity. Toxicology in vitro: an international journal published in association with BIBRA. 2018;52:131–145. doi: 10.1016/j.tiv.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowe F.J., Luettich K., Talikka M., Hoang V., Haswell L.E., Hoeng J., Gaca M.D. Development of an adverse outcome pathway for the onset of hypertension by oxidative stress-mediated perturbation of endothelial nitric oxide bioavailability. Appl. In Vitro Toxicol. 2017;3:131–148. [Google Scholar]
- 49.Rovida C., Alepee N., Api A.M., Basketter D.A., Bois F.Y., Caloni F., Corsini E., Daneshian M., Eskes C., Ezendam J., Fuchs H., Hayden P., Hegele-Hartung C., Hoffmann S., Hubesch B., Jacobs M.N., Jaworska J., Kleensang A., Kleinstreuer N., Lalko J., Landsiedel R., Lebreux F., Luechtefeld T., Locatelli M., Mehling A., Natsch A., Pitchford J.W., Prater D., Prieto P., Schepky A., Schuurmann G., Smirnova L., Toole C., van Vliet E., Weisensee D., Hartung T. Integrated Testing Strategies (ITS) for safety assessment. Altex. 2015;32:25–40. doi: 10.14573/altex.1411011. [DOI] [PubMed] [Google Scholar]
- 50.Hogg J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet (London, England) 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 51.Clippinger A.J., Allen D., Jarabek A.M., Corvaro M., Gaca M., Gehen S., Hotchkiss J.A., Patlewicz G., Melbourne J., Hinderliter P., Yoon M., Huh D., Lowit A., Buckley B., Bartels M., BeruBe K., Wilson D.M., Indans I., Vinken M. Alternative approaches for acute inhalation toxicity testing to address global regulatory and non-regulatory data requirements: an international workshop report. Toxicol. In Vitro. 2018;48:53–70. doi: 10.1016/j.tiv.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wikoff D.S., Rager J.E., Chappell G.A., Fitch S., Haws L., Borghoff S.J. A framework for systematic evaluation and quantitative integration of mechanistic data in assessments of potential human carcinogens. Toxicol. Sci. 2018 doi: 10.1093/toxsci/kfy279. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz A.G., Cote M.L. Epidemiology of lung Cancer. Adv. Exp. Med. Biol. 2016;893:21–41. doi: 10.1007/978-3-319-24223-1_2. [DOI] [PubMed] [Google Scholar]
- 54.Benowitz N.L., Hukkanen J., Jacob P., 3rd . Handbook of Experimental Pharmacology. 2009. Nicotine chemistry, metabolism, kinetics and biomarkers; pp. 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology. 1999;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- 56.Schaller J.-P., Keller D., Poget L., Pratte P., Kaelin E., McHugh D., Cudazzo G., Smart D., Tricker A.R., Gautier L. Evaluation of the Tobacco Heating System 2.2. Part 2: chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016;81:S27–S47. doi: 10.1016/j.yrtph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Pratte P., Cosandey S., Goujon Ginglinger C. Investigation of solid particles in the mainstream aerosol of the Tobacco Heating System THS2. 2 and mainstream smoke of a 3R4F reference cigarette. Hum. Exp. Toxicol. 2017;36:1115–1120. doi: 10.1177/0960327116681653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sewer A., Kogel U., Talikka M., Wong E.T., Martin F., Xiang Y., Guedj E., Ivanov N.V., Hoeng J., Peitsch M.C. Evaluation of the Tobacco Heating System 2.2 (THS2.2). Part 5: microRNA expression from a 90-day rat inhalation study indicates that exposure to THS2.2 aerosol causes reduced effects on lung tissue compared with cigarette smoke. Regul. Toxicol. Pharmacol. 2016;81(Suppl 2):S82–S92. doi: 10.1016/j.yrtph.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 59.Elamin A., Titz B., Dijon S., Merg C., Geertz M., Schneider T., Martin F., Schlage W.K., Frentzel S., Talamo F., Phillips B., Veljkovic E., Ivanov N.V., Vanscheeuwijck P., Peitsch M.C., Hoeng J. Quantitative proteomics analysis using 2D-PAGE to investigate the effects of cigarette smoke and aerosol of a prototypic modified risk tobacco product on the lung proteome in C57BL/6 mice. J. Proteomics. 2016 doi: 10.1016/j.jprot.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 60.Lo Sasso G., Titz B., Nury C., Boue S., Phillips B., Belcastro V., Schneider T., Dijon S., Baumer K., Peric D., Dulize R., Elamin A., Guedj E., Buettner A., Leroy P., Kleinhans S., Vuillaume G., Veljkovic E., Ivanov N.V., Martin F., Vanscheeuwijck P., Peitsch M.C., Hoeng J. Effects of cigarette smoke, cessation and switching to a candidate modified risk tobacco product on the liver in Apoe mice - a systems toxicology analysis. Inhal. Toxicol. 2016:1–15. doi: 10.3109/08958378.2016.1150368. [DOI] [PubMed] [Google Scholar]
- 61.Phillips B., Veljkovic E., Boue S., Schlage W.K., Vuillaume G., Martin F., Titz B., Leroy P., Buettner A., Elamin A., Oviedo A., Cabanski M., De Leon H., Guedj E., Schneider T., Talikka M., Ivanov N.V., Vanscheeuwijck P., Peitsch M.C., Hoeng J. An 8-Month systems toxicology Inhalation/Cessation study in apoe-/- mice to investigate cardiovascular and respiratory exposure effects of a candidate modified risk tobacco product, THS 2.2, compared with conventional cigarettes. Toxicological sciences: an official journal of the Society of Toxicology. 2016;149:411–432. doi: 10.1093/toxsci/kfv243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szostak J., Boue S., Talikka M., Guedj E., Martin F., Phillips B., Ivanov N.V., Peitsch M.C., Hoeng J. Aerosol from Tobacco Heating System 2.2 has reduced impact on mouse heart gene expression compared with cigarette smoke. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2017 doi: 10.1016/j.fct.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Titz B., Boue S., Phillips B., Talikka M., Vihervaara T., Schneider T., Nury C., Elamin A., Guedj E., Peck M.J., Schlage W.K., Cabanski M., Leroy P., Vuillaume G., Martin F., Ivanov N.V., Veljkovic E., Ekroos K., Laaksonen R., Vanscheeuwijck P., Peitsch M.C., Hoeng J. Effects of cigarette smoke, cessation and switching to two heat-not-burn tobacco products on lung lipid metabolism in C57BL/6 and Apoe-/- mice - an integrative systems toxicology analysis. Toxicol. Sci. 2016;149(441):457. doi: 10.1093/toxsci/kfv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phillips B., Szostak J., Titz B., Schlage W.K., Guedj E., Leroy P., Vuillaume G., Martin F., Buettner A., Elamin A., Sewer A., Sierro N., Choukrallah M.A., Schneider T., Ivanov N.V., Teng C., Tung C.K., Lim W.T., Yeo Y.S., Vanscheeuwijck P., Peitsch M.C., Hoeng J. A six-month systems toxicology inhalation/cessation study in ApoE(-/-) mice to investigate cardiovascular and respiratory exposure effects of modified risk tobacco products, CHTP 1.2 and THS 2.2, compared with conventional cigarettes. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2019;126:113–141. doi: 10.1016/j.fct.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Iskandar A.R., Mathis C., Martin F., Leroy P., Sewer A., Majeed S., Kuehn D., Trivedi K., Grandolfo D., Cabanski M., Guedj E., Merg C., Frentzel S., Ivanov N.V., Peitsch M.C., Hoeng J. 3-D nasal cultures: systems toxicological assessment of a candidate modified-risk tobacco product. Altex. 2016 doi: 10.14573/altex.1605041. [DOI] [PubMed] [Google Scholar]
- 66.Iskandar A.R., Martin F., Leroy P., Schlage W.K., Mathis C., Titz B., Kondylis A., Schneider T., Vuillaume G., Sewer A., Guedj E., Trivedi K., Elamin A., Frentzel S., Ivanov N.V., Peitsch M.C., Hoeng J. Comparative biological impacts of an aerosol from carbon-heated tobacco and smoke from cigarettes on human respiratory epithelial cultures: a systems toxicology assessment. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2018;115:109–126. doi: 10.1016/j.fct.2018.02.063. [DOI] [PubMed] [Google Scholar]
- 67.Iskandar A.R., Martinez Y., Martin F., Schlage W.K., Leroy P., Sewer A., Torres L.O., Majeed S., Merg C., Trivedi K. Comparative effects of a candidate modified-risk tobacco product Aerosol and cigarette smoke on human organotypic small airway cultures: a systems toxicology approach. Toxicol. Res. 2017;6:930–946. doi: 10.1039/c7tx00152e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poussin C., Laurent A., Peitsch M.C., Hoeng J., De Leon H. Systems toxicology-based assessment of the candidate modified risk tobacco product THS2.2 for the adhesion of monocytic cells to human coronary arterial endothelial cells. Toxicology. 2016;339:73–86. doi: 10.1016/j.tox.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Poussin C., Laurent A., Kondylis A., Marescotti D., van der Toorn M., Guedj E., Goedertier D., Acali S., Pak C., Dulize R., Baumer K., Peric D., Maluenda E., Bornand D., Suarez I.G., Schlage W.K., Ivanov N.V., Peitsch M.C., Hoeng J. In vitro systems toxicology-based assessment of the potential modified risk tobacco product CHTP 1.2 for vascular inflammation- and cytotoxicity-associated mechanisms promoting adhesion of monocytic cells to human coronary arterial endothelial cells. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2018;120:390–406. doi: 10.1016/j.fct.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 70.van der Toorn M., Frentzel S., De Leon H., Goedertier D., Peitsch M.C., Hoeng J. Aerosol from a candidate modified risk tobacco product has reduced effects on chemotaxis and transendothelial migration compared to combustion of conventional cigarettes. Food Chem. Toxicol. 2015;86:81–87. doi: 10.1016/j.fct.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 71.van der Toorn M., Frentzel S., Goedertier D., Peitsch M., Hoeng J., De Leon H. A prototypic modified risk tobacco product exhibits reduced effects on chemotaxis and transendothelial migration of monocytes compared with a reference cigarette. Food Chem. Toxicol. 2015;80:277–286. doi: 10.1016/j.fct.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 72.University of Kentucky - Kentucky Tobacco Research and Development Center . The University of Kentucky Printing Services; Lexington: 2003. The Reference Cigarette. [Google Scholar]
- 73.Schaller J.-P., Keller D., Poget L., Pratte P., Kaelin E., McHugh D., Cudazzo G., Smart D., Tricker A.R., Gautier L., Yerly M., Pires R.R., Le Bouhellec S., Ghosh D., Hofer I., Garcia E., Vanscheeuwijck P., Maeder S. Evaluation of the Tobacco Heating System 2.2. Part 2: chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016 doi: 10.1016/j.yrtph.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 74.Health Canada . In: Tobacco Products Information Regulations. Regulation June 2000. G.o. Canada, editor. 2000. [Google Scholar]
- 75.Fariss M.W., Gilmour M.I., Reilly C.A., Liedtke W., Ghio A.J. Emerging mechanistic targets in lung injury induced by combustion-generated particles. Toxicol. Sci. 2013;132:253–267. doi: 10.1093/toxsci/kft001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lighty J.S., Veranth J.M., Sarofim A.F. Combustion aerosols: factors governing their size and composition and implications to human health. J. Air Waste Manage. Assoc. 2000;50:1565–1618. doi: 10.1080/10473289.2000.10464197. [DOI] [PubMed] [Google Scholar]
- 77.Sahu D., Kannan G., Vijayaraghavan R. Carbon black particle exhibits size dependent toxicity in human monocytes. Int. J. Inflam. 2014;2014 doi: 10.1155/2014/827019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.You R., Lu W., Shan M., Berlin J.M., Samuel E.L., Marcano D.C., Sun Z., Sikkema W.K., Yuan X., Song L. Nanoparticulate carbon black in cigarette smoke induces DNA cleavage and Th17-mediated emphysema. elife. 2015;4 doi: 10.7554/eLife.09623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang R., Dai Y., Zhang X., Niu Y., Meng T., Li Y., Duan H., Bin P., Ye M., Jia X. Reduced pulmonary function and increased pro-inflammatory cytokines in nanoscale carbon black-exposed workers. Part. Fibre Toxicol. 2014;11:73. doi: 10.1186/s12989-014-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bernstein D.M. A review of the influence of particle size, puff volume, and inhalation pattern on the deposition of cigarette smoke particles in the respiratory tract. Inhal. Toxicol. 2004;16:675–689. doi: 10.1080/08958370490476587. [DOI] [PubMed] [Google Scholar]
- 81.Kane D.B., Asgharian B., Price O.T., Rostami A., Oldham M.J. Effect of smoking parameters on the particle size distribution and predicted airway deposition of mainstream cigarette smoke. Inhal. Toxicol. 2010;22:199–209. doi: 10.3109/08958370903161224. [DOI] [PubMed] [Google Scholar]
- 82.Robinson R.J., Yu C. Deposition of cigarette smoke particles in the human respiratory tract. Aerosol Sci. Technol. 2001;34:202–215. [Google Scholar]
- 83.Hinds W.C. John Wiley & Sons; 1999. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. [Google Scholar]
- 84.Wong E.T., Kogel U., Veljkovic E., Martin F., Xiang Y., Boue S., Vuillaume G., Leroy P., Guedj E., Rodrigo G., Ivanov N.V., Hoeng J., Peitsch M.C., Vanscheeuwijck P. Evaluation of the Tobacco Heating System 2.2. Part 4: 90-day OECD 413 rat inhalation study with systems toxicology endpoints demonstrates reduced exposure effects compared with cigarette smoke. Regulatory toxicology and pharmacology: RTP. 2016;81(Suppl 2):S59–S81. doi: 10.1016/j.yrtph.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 85.Fennell T.R., Kedderis G.L., Sumner S.C. Urinary metabolites of [1,2,3-13C]acrylonitrile in rats and mice detected by 13C nuclear magnetic resonance spectroscopy. Chem. Res. Toxicol. 1991;4:678–687. doi: 10.1021/tx00024a013. [DOI] [PubMed] [Google Scholar]
- 86.Parent R.A., Caravello H.E., Sharp D.E. Metabolism and distribution of [2,3-14C]acrolein in Sprague-Dawley rats. Journal of applied toxicology: JAT. 1996;16:449–457. doi: 10.1002/(SICI)1099-1263(199609)16:5<449::AID-JAT369>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 87.Raunio H., Pokela N., Puhakainen K., Rahnasto M., Mauriala T., Auriola S., Juvonen R.O. Nicotine metabolism and urinary elimination in mouse: in vitro and in vivo. Xenobiotica. 2008;38:34–47. doi: 10.1080/00498250701708539. [DOI] [PubMed] [Google Scholar]
- 88.Smith J.D., Breslow J.L. The emergence of mouse models of atherosclerosis and their relevance to clinical research. J. Intern. Med. 1997;242:99–109. doi: 10.1046/j.1365-2796.1997.00197.x. [DOI] [PubMed] [Google Scholar]
- 89.Lo Sasso G., Schlage W.K., Boue S., Veljkovic E., Peitsch M.C., Hoeng J. The Apoe(-/-) mouse model: a suitable model to study cardiovascular and respiratory diseases in the context of cigarette smoke exposure and harm reduction. J. Transl. Med. 2016;14:146. doi: 10.1186/s12967-016-0901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von Holt K., Lebrun S., Stinn W., Conroy L., Wallerath T., Schleef R. Progression of atherosclerosis in the Apo E-/- model: 12-month exposure to cigarette mainstream smoke combined with high-cholesterol/fat diet. Atherosclerosis. 2009;205:135–143. doi: 10.1016/j.atherosclerosis.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 91.Szostak J., Titz B., Schlage W.K., Guedj E., Sewer A., Phillips B., Leroy P., Buettner A., Neau L., Trivedi K., Martin F., Ivanov N.V., Vanscheeuwijck P., Peitsch M.C., Hoeng J. Structural, functional, and molecular impact on the cardiovascular system in ApoE(-/-) mice exposed to aerosol from candidate modified risk tobacco products, Carbon Heated Tobacco Product 1.2 and Tobacco Heating System 2.2, compared with cigarette smoke. Chem. Biol. Interact. 2019;315 doi: 10.1016/j.cbi.2019.108887. [DOI] [PubMed] [Google Scholar]
- 92.Libby P., Okamoto Y., Rocha V.Z., Folco E. Inflammation in atherosclerosis. Circ. J. 2010;74:213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 93.Phillips B., Esposito M., Verbeeck J., Boue S., Iskandar A., Vuillaume G., Leroy P., Krishnan S., Kogel U., Utan A., Schlage W.K., Bera M., Veljkovic E., Hoeng J., Peitsch M.C., Vanscheeuwijck P. Toxicity of aerosols of nicotine and pyruvic acid (separate and combined) in Sprague–Dawley rats in a 28-day OECD 412 inhalation study and assessment of systems toxicology. Inhal. Toxicol. 2015;9:1–27. doi: 10.3109/08958378.2015.1046000. [DOI] [PubMed] [Google Scholar]
- 94.Phillips B., Titz B., Kogel U., Sharma D., Leroy P., Xiang Y., Vuillaume G., Lebrun S., Sciuscio D., Ho J., Nury C., Guedj E., Elamin A., Esposito M., Krishnan S., Schlage W.K., Veljkovic E., Ivanov N.V., Martin F., Peitsch M.C., Hoeng J., Vanscheeuwijck P. Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague-Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2017;109:315–332. doi: 10.1016/j.fct.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 95.Stinn W., Buettner A., Weiler H., Friedrichs B., Luetjen S., van Overveld F., Meurrens K., Janssens K., Gebel S., Stabbert R., Haussmann H.J. Lung inflammatory effects, tumorigenesis, and emphysema development in a long-term inhalation study with cigarette mainstream smoke in mice. Toxicol. Sci. 2013;131:596–611. doi: 10.1093/toxsci/kfs312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.