Abstract
Brains from persons with Alzheimer disease (AD) and its earlier stage, amnestic mild cognitive impairment (MCI), exhibit high levels of oxidative damage, including that to phospholipids. One type of oxidative damage is lipid peroxidation, the most important index of which is protein-bound 4-hydroxy-2-trans-nonenal (HNE). This highly reactive alkenal changes the conformations and lowers the activities of brain proteins to which HNE is covalently bound. Evidence exists that suggests that lipid peroxidation is the first type of oxidative damage associated with amyloid β-peptide (Aβ), a 38-42 amino acid peptide that is highly neurotoxic and critical to the pathophysiology of AD. The Butterfield laboratory is one of, if not the, first research group to show that Aβ42 oligomers led to lipid peroxidation and to demonstrate this modification in brains of subjects with AD and MCI. The Mattson laboratory, particularly when Dr. Mattson was a faculty member at the University of Kentucky, also showed evidence for lipid peroxidation associated with Aβ peptides, mostly in in vitro systems. Consequently, there is synergy between our two laboratories. Since this special tribute issue of Aging Research Reviews is dedicated to the career of Dr. Mattson, a review of some aspects of this synergy of lipid peroxidation and its relevance to AD, as well as the role of lipid peroxidation in the progression of this dementing disorder seems germane. Accordingly, this review outlines some of the individual and/or complementary research on lipid peroxidation related to AD published from our two laboratories either separately or jointly.
Keywords: Lipid peroxidation, HNE, Alzheimer disease, Amnestic mild cognitive impairment, Brain protein conformational and functional changes
1. Introduction
This review focuses on lipid peroxidation in brains of persons with Alzheimer disease (AD) and its earlier stage, amnestic mild cognitive impairment (MCI). More specifically, this review focuses on the lipid peroxidation product, 4-hydroxy-2-nonenal (HNE), and the changes in structure and function of brain proteins in AD and MCI relative to those control brains that this highly reactive and neurotoxic alkenal engenders. This review also focuses on the roles of HNE associated with amyloid β-peptide (Aβ) oligomers in AD-relevant in vitro, ex vivo, and in vivo systems reported by the Butterfield and/or Mattson laboratories. This emphasis is apropos given that this paper is part of a Special Issue of the journal, Ageing Research Reviews honoring Mark P. Mattson for his distinguished scientific career in these fields.
2.0. Alzheimer Disease and Mild Cognitive Impairment
2.1. Epidemiology, Pathophysiology, and Pathogenesis
An authoritative pathological exposition of AD and MCI has been reported by Nelson and Markesbery (Nelson and Markesbery, 2010; Markesbery, 2007; 2009) or other neuropathologists (Landau and Frosch,,2014; Malek-Ahmadi et al., 2018). The sixth leading cause of death in the United States, AD presents as a disorder associated with progressive loss of cognitive function finally ending in dementia. In later stages of AD, aphasia often occurs. AD patients often are restless, unsettled, and require nearly constant attention for safety, making AD a truly family disorder. The costs of caring for AD patients in the USA is enormous and will become more so as the Baby Boomer population group of approximately 70-80 million Americans ages, as age is the single greatest risk factor of sporadic AD. In addition, there are several genes or gene alleles that increase risk of developing AD, but do not directly cause this disorder (Strittmatter et al., 1993; Tanzi and Bertram, 2001; Butterfield and Mattson, 2020). Presenting as a condition first characterized by diminishing memory, MCI arguably is a prodromal stage of AD in which patients have normal activities of daily living.
Both AD and, to a lesser degree, MCI brains are pathologically characterized by extracellular deposits of amyloid β-peptide (Aβ) coupled with dystrophic neurites and other moieties. These key pathological hallmarks of AD are named senile plaques (SP) or neuritic plaques. In addition, brains of both AD and MCI have intra-neuronal deposits of hyperphosphorylated tau, called neurofibrillary tangles (NFT), with MCI brain exhibiting these neuropathological alterations to a lesser degree. Loss of synapses and perhaps neurons characterize both conditions, with MCI having fewer synapses lost compared to AD.
Aβ is produced by proteolytic processing of amyloid precursor protein (APP), coded on chromosome 21, by two proteases: beta-secretase (BACE) and gamma-secretase (Yuksel and Tacal, 2019). BACE cleaves APP on the N-terminal side of the type I transmembrane protein, APP, while gamma-secretase cleaves APP at a site within the lipid bilayer.
2.2. Involvement of Oxidative Stress in AD
Aβ is postulated to be central to the pathogenesis of AD and its earlier stages, based mostly of genetics of familial AD, which accounts for 1-4 percent of AD subjects (Selkoe and Hardy, 2016; Butterfield et al., 2001). Persons with mutations in presenilin-1 or presenilin-2 genes develop AD. Moreover, our laboratory, along with others (Butterfield et al., 2001; Butterfield et al., 2002; Keller et al., 2005; Markesbery, 1997; Nunomura al., 2001; Butterfield and Halliwell, 2019), reported oxidative damage to brain proteins (indexed by elevated levels of protein resident protein carbonyls or 3-nitrotyrosine (3-NT)), brain DNA or RNA oxidation (indexed by elevated levels of 8-hydroxy-2-deoxyguanine or 8-hydroxyguanine, respectively), or brain lipid peroxidation (indexed principally by protein-bound HNE [Figure 1] (Di Domenico et al., 2017a; Butterfield and Boyd-Kimball, 2019; Sultana et al., 2013). Others used iso- or neuro-prostanes detected by mass spectrometry as markers of lipid peroxidation (Montine et al., 2007; Pratico, 2010).
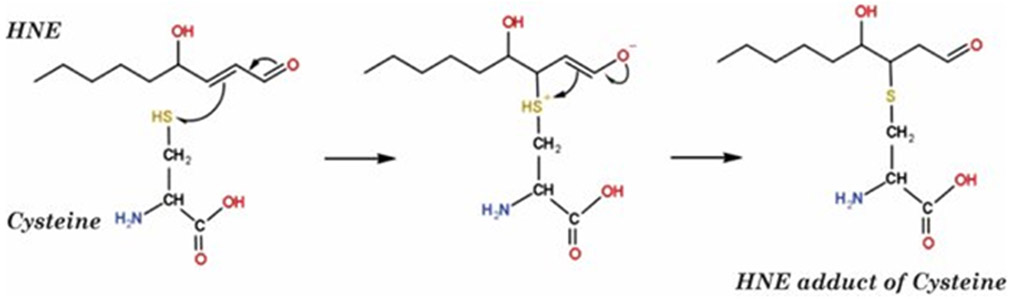
Figure 1.
Mechanism of Michael addition reaction of HNE with Cysteine to form a covalent adduct with this amino acid. Such reactions on proteins, including also potentially with His and Lys residues, changes the conformation of such proteins with consequent diminution or loss of function.
Aβ is central to the pathogenesis of AD on the one hand, and the AD brain is rich in indices of oxidative damage on the other hand. In 1994, our laboratory developed a model for AD based on these two characteristics (Hensley et al., 1994). The model [Figure 2] posits the following:
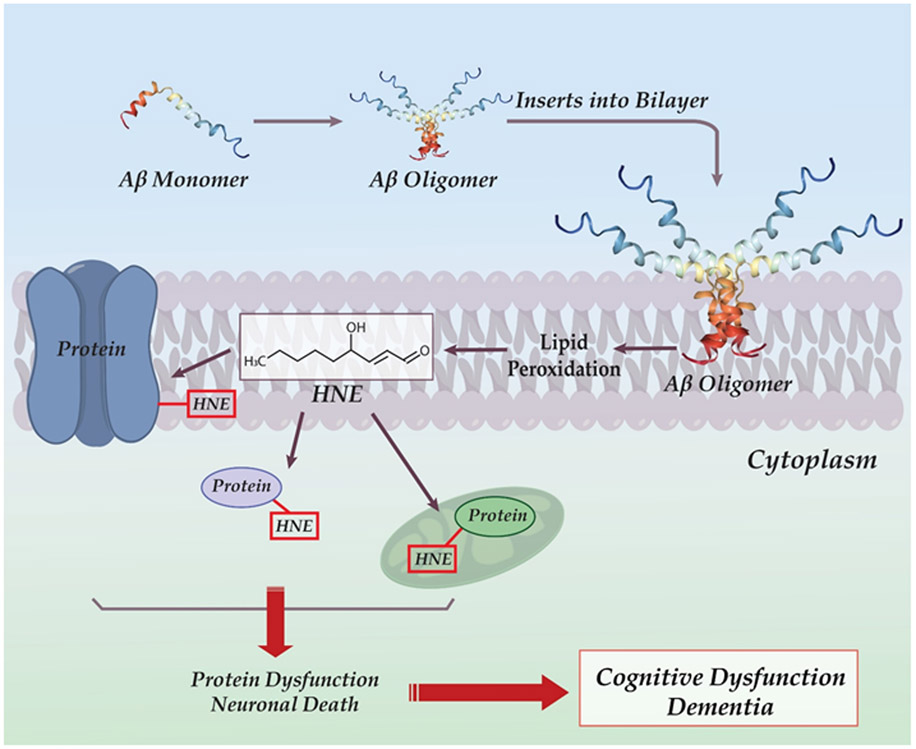
Figure 2.
Schematic diagram of hydrophobic Aβ oligomer inserting into the neuronal lipid bilayer, where lipid peroxidation takes place leading to formation of HNE. This highly reactive moiety then forms covalent adducts with membrane, cytosolic, and mitochondrial proteins, resulting in protein dysfunction and neuronal death. Cognitive loss is a consequence of neuronal death in key brain regions.
Oxidative damage underlying AD is in major part due to the effects of Aβ oligomers, which being of relatively low molecular weight and highly hydrophobic (Butterfield et al., 2001), would enter lipid bilayers as small oligomers, i.e., dimers, trimers, tetramers, etc.
We discovered Aβ was associated with lipid peroxidation, the major and highly reactive product of which is HNE (Butterfield et al., 1994; Butterfield et al., 2001 Butterfield and Lauderback, 2002; Butterfield et al., 2002; Di Domenico et al., 2017a; Butterfield and Boyd-Kimball, 2019; Sultana et al., 2013; Montine et al., 2007; Pratico, 2010).
HNE is known to covalently bind to and modify the structure of key neuronal membrane, cytosolic, and mitochondrial proteins (Esterbauer, 1991; Butterfield and Halliwell, 2019; Subramanian et al., 1997; Di Domenico et al., 2017a; Butterfield and Boyd-Kimball, 2019; Sultana et al., 2013); Halliwell and Gutteridge, 2015). Covalent binding of HNE to key neuronal proteins causes their dysfunction, leading to neuronal death.
When enough neurons have died, cognitive dysfunction and later dementia becomes apparent.
This model is discussed in more biochemical and neurochemical detail later in this paper.
3.0. Studies of Lipid Peroxidation and Aβ
3.1. Aβ and lipid peroxidation: first evidence
Arguably, the first demonstration of lipid peroxidation induced by Aβ employed electron paramagnetic resonance (EPR) methods (Butterfield et al., 1994). A lipid bilayer-soluble stearic acid spin label, which contains a sterically protected nitroxide moiety from which the EPR spectrum arises, was added to a gerbil synaptosomal preparation. Addition of Aβ to synaptosomes prepared from gerbils led to diminution of the intensity of this EPR spectrum. This phenomenon arises because a lipid centered free radical associated with lipid peroxidation induced by Aβ reacted with the unpaired electron on the nitroxide moiety of the spin label to form the hydroxylamine moiety that has no unpaired electron and therefore led to decreased intensity of the EPR spectrum. Kinetics analyses of this process showed that most of the loss of the EPR signal was completed by 10 min after Aβ addition, which is much faster than typically one sees for protein oxidation reactions. Dr. Mattson was a co-author of this paper. Likewise, using a murine model of mutant presenilin-1 (PS-1, M146V), oxidative damage in brain was observed in collaborative studies with Dr. Mattson (LaFontaine et al., 2002).
Dr. Mattson and his laboratory team at the University of Kentucky demonstrated production of HNE using high performance liquid chromatography (HPLC) after addition of Aβ to primary neuronal cultures (Mark et al., 1997). Rather high concentrations of HNE were detected.
3.2. Proposed mechanism
Aβ42 is a relatively hydrophobic peptide as discerned from its amino acid sequence [Figure 3]. In oligomeric form, Aβ leads to insoluble protofibrils and then fibrils external to the neuronal plasma membrane. In addition, these Aβ42 oligomers also solubilize into the hydrophobic milieu of the lipid bilayer of the neuronal membrane. In the latter case, this peptide, like nearly all proteins that solubilize in a lipid bilayer, would adopt an α-helical secondary structure in the membrane-spanning region of the peptide. The i+4 rule of helices in proteins states that every amino acid at position i would interact with an amino acid four residues away (Garrett and Grisham, 2017). Based on the importance of residue 35 (Met) of Aβ42 in oxidative damage associated with this peptide both in vitro and in vivo (Varadarajan et al., 2001; Yatin et al., 1999; Butterfield et al., 2010), and employing the I + 4 rule of protein helices, residue 31 (Ile) was substituted by Pro, a helical-breaking amino acid, and oxidative stress and neurotoxic measures were determined (Kanski et al., 2002). No elevated oxidative damage nor neurotoxicity were observed in primary neuronal cultures with Aβ(1-42)I35P, in marked contrast to the results with native oligomeric Aβ(1-42). These results suggest that the secondary structure of helical Aβ42 in the lipid bilayer contributes to the oxidative damage and neurotoxic properties of this AD- and MCI-relevant peptide. How this α-helix conformation within the lipid bilayer likely participates in the oxidative damage associated with Aβ oligomers is outlined in the following [Figure 4]:
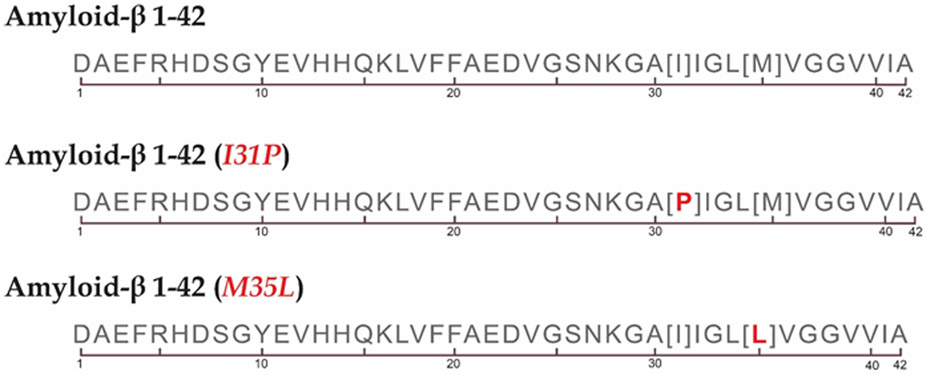
Figure 3.
Amino acid sequence of Aβ42 showing the generally hydrophobic nature of this peptide, which drives oligomers to solubilize in the lipid bilayer. Also shown are Aβ42 variants that were used to establish the critical importance of the helical conformation of the peptide in the lipid bilayer and the single methionine residue for the oxidative stress produced by Aβ(1-42) in in vitro [Aβ(1-42)I31P] (Kanski et al., 2002) and in in vivo [Aβ(1-42)M35L] (Butterfield et al., 2010) studies.
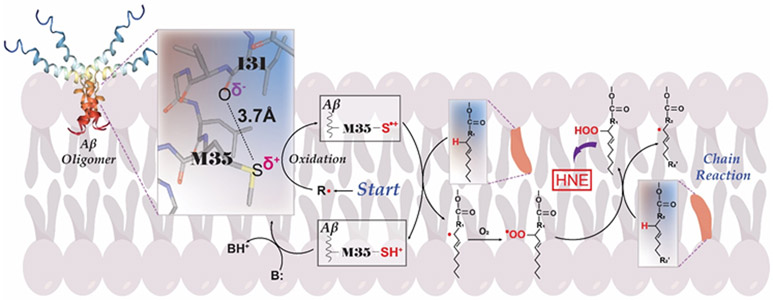
Figure 4.
Role of Methionine residue 35 in Aβ42-associated lipid peroxidation and HNE formation. After insertion into the lipid bilayer, Aβ42 oligomer, with its Met-35 residue, adopts an alpha-helical secondary structure with the latter’s i + 4 rule of amino acid interactions (highlighted as expanded view) showing that the O-atom of the peptide bond of Ile-31 is within a 3.7 Angstrom distance of the S-atom of Met-35. The greater electronegativity of O vs. S leads to one of the lone pairs of electrons on the S-atom being pulled toward the Ile-31’s O-atom, which causes less attraction of these electron to the protons in the S-atom nucleus and thereby making these electrons vulnerable to a one-electron oxidation by a radical R. shown near the word “Start.” The resulting S.+ radical immediately abstracts a nearby labile allylic H-atom from an unsaturated acyl chain of a lipid in the bilayer, leading to SH+ on Met and a C. radical on the allylic carbon atom. The SH+, being an acid with pKa of −5, immediately loses the H+ to any base B, to regenerate Met. That is, this is a catalytic reaction in which Met starts and ends the reaction on the peptide. Meanwhile, because radical-radical reactions are among the fastest reactions known, the C. radical on the allylic carbon atom immediately binds a paramagnetic oxygen molecule (there are 2 unpaired electrons on molecular oxygen), which has zero dipole moment and is therefore highly soluble in a hydrophobic environment like a lipid bilayer. The resulting COO. lipid peroxyl radical abstracts another nearby labile allylic H-atom from an unsaturated acyl chain of a phospholipid to form the lipid hydroperoxide, COOH, and another C. radical on the allylic carbon atom from which the second allylic H-atom was obtained. That is, this becomes a chain reaction that constantly repeats as long as there are present oxygen and sufficient number of unsaturated lipid acyl chains with allylic H-atoms. The lipid hydroperoxide is the moiety from which HNE is derived. Note that only a small amount of the original S.+ radical needs to be formed, since a large amount of HNE will be formed from the chain reaction associated with lipid peroxidation. The nature of the initiating radical or other species are not known. This author speculates that either molecular oxygen (which results in superoxide formation after obtaining an electron from the S-atom of Met) or weakly-associated Cu2+ on Met is reduced to Cu+ , which would react by Fenton Chemistry with hydrogen peroxide to form the highly reactive .OH radical, are the most likely possibilities for formation of the S.+ radical.
The O-atom in the peptide bond of Ile in Aβ42 was shown to be within a van der Waals distance of the S-atom of Met (Shao et al., 1999).
The O atom is more electronegative than the S atom, so a lone pair of electrons on the Met S-atom will be drawn away from the S-atom nuclear attraction towards the O-atom of Ile peptide bond. Being less tightly held to the S atom than normal would make these electrons subject to a one-electron oxidation by an appropriate oxidant, R..
Such one-electron oxidation leads to a S.+ sulfuranyl free radical, that in the bilayer would immediately abstract a nearby labile allylic H-atom of the acyl chain of a phospholipid, forming a SH+ species. This latter moiety is an acid with a pKa of minus 5 (Schöneich et al., 2003). Hence, any base would immediately abstract the H+ from SH+, forming methionine again. That is, Met acts as a catalyst, starting as Met and ending as Met within Aβ42 when this peptide in oligomeric form is solubilized with helical secondary structure within the lipid bilayer.
The abstraction of the labile allylic H atom from the C-atom on the phospholipid acyl chain results in a lipid-resident C. radical. Oxygen [O2] is a paramagnetic molecule [two unpaired electrons], and as a homonuclear diatomic molecule oxygen has zero dipole moment. Consequently, O2 is non-polar, and, therefore, highly soluble in the hydrophobic environment of the lipid bilayer. One of the two unpaired electrons on O2 would immediately react with the unpaired electron on the C. forming the lipid peroxyl radical, COO.. This radical, in turn, abstracts another nearby labile allylic H-atom from the unsaturated β-acyl chain of a phospholipid to form the lipid hydroperoxide with chemical structure COOH.
Note that, because of [d] above, another C. radical results on a lipid β-acyl chain in the bilayer. That is, this reaction scheme becomes a propagating chain reaction, forming large amounts of lipid hydroperoxides. It is from lipid hydroperoxides that HNE is formed (Esterbauer et al., 1991; Butterfield et al., 2010; Butterfield and Halliwell, 2019).
Moreover, only a small amount of S.+ on Aβ42 is necessary to initiate the chain reaction leading to lipid peroxidation, and because there is such a relatively large amount of labile H-atoms, production of copious amounts of highly damaging and neurotoxic HNE occurs. Lastly on this topic, an α-helix has an intrinsic dipole moment directed along the helical axis. This dipole moment in helical Aβ42 in the lipid bilayer conceivably could stabilize the S.+ radical long enough to permit the radical to abstract a labile allylic H-atom from the unsaturated β-chain of a phospholipid in the bilayer to initiate the chain reaction discussed above (Butterfield, 2014). Related to this discussion, the greater dipole moment of Aβ42 compared to Aβ40 plausibly may be relevant to the greater neurotoxicity of the former compared to the latter due to the greater stabilization of the S.+ radical by the Aβ42 dipole moment compared to the dipole moment of Aβ40.
3.3. HNE-induced Protein Modifications
That HNE could alter the conformation of proteins was demonstrated by our laboratory using EPR spin labeling (Subramaniam et al., 1997). Proteins in synaptic membrane preparations that contained both pre- and post-synaptic membranes and other brain cells were covalently spin labeled by the predominantly Cys-binding N-(2,2,6,6-tertramethylpiperidine-N-1 oxyl) maleimide [MAL-6]. Following HNE addition, the observed decreased heights and increased linewidths of the EPR spectrum of MAL-6 were indicative of slower motion of the spin label because of increased steric interference of components of the protein, i.e., reflective of conformational changes in the protein (Butterfield, 1982). Consistent with the principle that functions of proteins are directed by their structures, altered structures of proteins would be expected to lead to decreased function. We demonstrated that the vast majority of HNE-modified proteins from AD brain are characterized by oxidative dysfunction (Butterfield and Halliwell, 2019; Butterfield and Boyd-Kimball, 2019; Sultana et al., 2013b; Di Domenico et al., 2017a; Sultana et al., 2006; Aksenov et al., 1997; 2000; Castegna et al., 2002a,b; Butterfield, 2014).
As noted above, the lipid peroxidation mechanism involves a chain reaction that leads to significant amounts of HNE produced. Vitamin E is a chain-breaking antioxidant, and supportive of the mechanism for lipid peroxidation is the observation that vitamin E addition to neurons in culture blocks formation of reaction oxygen species (Yatin et al., 2000). Moreover, vitamin E blocks polyamine uptake (Yatin et al., 2001), and blocks oxidative damage (Butterfield et al., 1999; Yatin et al., 2000; Mark et al., 1995). In collaborative research with the Mattson laboratory, overexpression of the anti-apoptotic protein, Bcl-2, protected neurons from lipid peroxidation-mediated cell death (Bruce-Keller et al., 1998).
The glutamate transporter EAAT2 (also called Glt-1), located principally in astrocyte membranes but also on neuronal membranes, was probed for HNE binding to Glt-1 in hippocampal specimens from subjects with AD (Lauderback et al., 2001). An approximately 70 percent elevation of HNE binding of Glt-1 in AD compared to that in normal control specimens was found. In the same study, synaptic membrane preparations from gerbil brains were treated with Aβ42; Glt-1 pulldown followed by probing for HNE showed about a 70 percent elevation of HNE binding to this glutamate transporter (Lauderback et al., 2001). That is, the HNE elevation in AD brain and specifically on Glt-1 (EAAT2) was replicated in rodent brain by the addition of Aβ42. Coupled with the knowledge that HNE binding changes the conformation of proteins as discussed above, this result provides an explanation for the loss of activity of Glt-1 in AD brain reported by the Masliah and Cook laboratories (Li et al., 1997; Scimemi et al., 2013). Moreover, given the inactivation of glutamine synthetase in AD brain due to oxidative modification (Butterfield et al. 1997; Castegna et al., 2002), the oxidative dysfunction of Glt-1 suggests two mechanisms by which excess glutamate accrues in the synapse, leading to the likelihood of repetitive depolarization of post-synatpic neurons and consequent intracellular Ca2+, free radicals, and neuronal death, i.e., excitotoxicity [Figure 5] (Butterfield and Pocernich, 2003). This phenomenon was further described in papers by Mark Mattson (Bezprozvanny and Mattson, 2008; Mattson and Chan, 2003).
Figure 5.
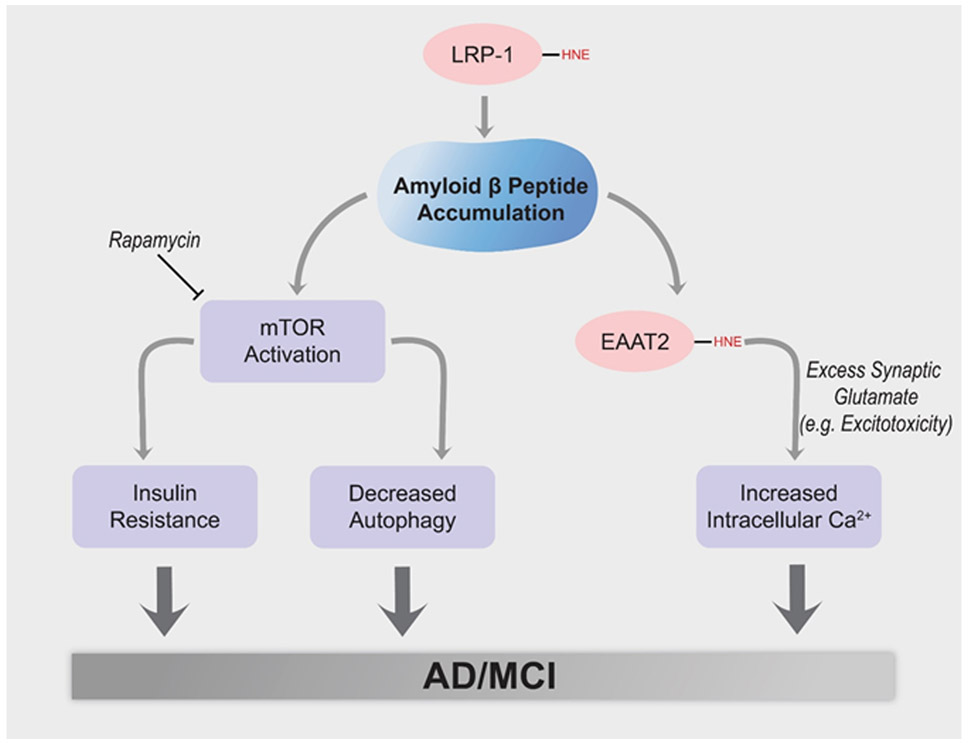
Aβ42 oligomer-associated lipid peroxidation leads to inhibition of the low density-like receptor protein-1 (LRP-1), whose loss of function could contribute to brain accumulation of Aβ42 and resulting oxidative damage. In addition, Aβ42 oligomers are reportedly capable of initiating the PI3K/Akt/mTOR axis, with resulting insulin resistance and inhibition of autophagy. Similarly, Aβ42 oligomer-associated lipid peroxidation leads to HNE binding to and inhibition of the glutamate transporter, EAAT2 (also called Glt-1) in AD brain, likely contributing to excitotoxicity and neuronal death.
A similar examination of HNE binding to the low density-like receptor protein-1 (LRP-1), a key efflux protein from brain to blood of Aβ42 in AD hippocampus, showed an approximately 70 percent elevation of HNE binding to this efflux protein (Owen et al., 2010). This oxidative modification of LRP-1 by HNE, which likely decreased its function, conceivably could contribute to Aβ42 accumulation in AD brain.
A prediction of our model of Aβ42 oligomer mediated oxidative damage and subsequent neurotoxicity in AD (Butterfield et al., 2001: Butterfield and Halliwell, 2019; Butterfield and Boyd-Kimball, 2019) is elevation of intracellular Ca2+. Collaboration of the Butterfield and Mattson laboratories showed that in AD brain the ion-motive ATPases, Na+,K+-ATPase and Ca2+-ATPase, were oxidatively modified and dysfunctional, and separately these ion-motive ATPases were made dysfunctional by addition of Aβ to preparations containing these transporters (Mark et al., 1995; 1997). Oxidative dysfunction would have the effect of decreasing the neuronal cell potential, opening voltage gated Ca2+ channels. Since there is 104 more Ca2+ outside the neuron than inside this cell, such Ca2+ channel opening would lead to massive increased intracellular levels of this ion that can overwhelm intracellular Ca2+ stores in endoplasmic reticulum (ER) and mitochondria. For ER, excessive Ca2+ can lead to alterations in the unfolded protein response, also leading to neuronal death (Butterfield and Halliwell, 2019). In the case of mitochondria, elevated mitochondrial Ca2+ can lead to opening of the mitochondrial permeability transition pore (MPTP), with subsequent release of cytochrome C and induction of apoptosis. Investigations of the role of Aβ in Ca2+ accumulation in neuronal cultures in the Mattson laboratory at UK showed that this neurotoxic peptide led to elevated Ca2+, and the neurons with excessive Ca2+ also were positive to anti-Aβ antibody, consistent with the scenario described above.
Alzheimer disease and MCI are characterized by decreased autophagy and other components of the proteostasis network and by insulin resistance, both associated with elevated lipid peroxidation and activation of the mechanistic target of rapamycin (Di Domenico et al., 2017b; Tramutola et al., 2015; Nixon, 2017). Mattson also published on these aspects of neuronal function (Mattson et al., 1999). Decreased autophagy leads to diminution of removal of cellular detritus via the lysosome (Nixon, 2017; Tramutola et al., 2015), ultimately leading to neuronal death. Insulin resistance greatly deceases the brain’s utilization of its primary energy source, glucose, ending in neuronal damage and death [Figure 5] (Butterfield et al., 2014a; Tramutola et al., 2015, 2020; Butterfield and Halliwell, 2019).
Noting from the above discussion that lipid peroxidation is initiated by abstraction of an acyl chain allylic hydrogen atom, several studies by others showed that if allylic H-atoms were replaced by allylic deuterium-atoms, and reasoning that since C-D bonds are much stronger than C-H bonds, less or no lipid peroxidation would occur. Some in vivo oxidative stress models were examined (Beaudoin-Chabot et al., 2019; Chistryakov et al., 2018). Indeed, essentially no lipid peroxidation occurred, and better clinical outcomes were observed in a human trial of D-polyunsaturated fatty acid (D-PUFA) in Friedreich’s ataxia (Zesiewicz at al., 2018). The Mattson laboratory reasoned that similar considerations could be applied to an AD mouse model (APP/PS1 transgenic model). As predicted, there was less lipid peroxidation found assessed by F2-isoprostanes and neuroprostanes (Raefsky et al., 2018). However, although there was an apparent early protection against loss of cognition in these mice, by the end of the experiment, no significant differences in cognitive loss were observed between mice treated with D-PUFA and these transgenic mice with no treatment. The authors suggested that had they used an APP/PS1 human double-mutant knock-in mouse model of AD (Abdul et al., 2008), cognitive protection may have been observed. This mouse is characterized by the correct amount and location of the two human mutant genes and associated proteins and not the unlimited amount and undirected protein location of the transgenic mouse.
4.0. AD-related In Vivo or Ex Vivo Studies Involving Aβ and Lipid Peroxidation
Table 1 shows selected examples of AD-related in vivo or ex vivo studies involving Aβ from the Butterfield and Mattson laboratories. The main takeaways from these studies are:
Table 1.
In Vivo and Ex Vivo AD-related Studies Involving Aβ from the Butterfield and Mattson Laboratories
| Selected Studies from the Butterfield Laboratory | |||
|---|---|---|---|
| Reference | System | In vivo or Ex Vivo |
Comments |
| Hensley et al. (1995) | AD brain | In vivo | Compared to control brain, oxidative damage occurs in AD brain regions rich in Aβ, but not in Aβ-poor cerebellum |
| Yatin et al. (1999); Drake et al. (2003); Boyd-Kimball et al. (2005a) | C. elgans expressing human Aβ(142) | In vivo | Demonstrated that protein oxidation occurred prior to Aβ42 deposition, i.e., consistent with the role of Aβ42 oligomers. |
| Boyd-Kimball et al. (2005b) | Human Aβ42 injected into rat nucleus baysalis of Mynert | In vivo | Demonstrated elevated HNE-bound proteins in hippocampus |
| Abdul et al. (2008) | Human double mutant APP/PS1 knock-in mice as a function of age | In vivo | Elevated oxidative damage in brain that correlated with levels of soluble Aβ42 and prior to deposition of this peptide, i.e., consistent with the role of Aβ42 oligomers. |
| Lauderback et al. (2001);Castegna et al. (2002a,b); Butterfield et al. (1997);Aksenov et al. (1997) | AD brain or rodent synaptosomal preparations to which Aβ42 was added | In vivo Ex vivo | In AD brain, HNE covalently binds to and causes dysfunction of the major astrocyte glutamate transporter, Glt-1, also known as EAAT2, which coupled to oxidative dysfunction of glutamine synthetase, leads to excess synaptic levels of glutamate that can contribute to excitotoxic neuronal death. Addition of Aβ42 to synaptosomal preparations replicates the HNE binding to Glt-1 (EAAT2). |
| Reed et al. (2009a) | Early AD brain | In vivo | HNE-modified proteins found in brain of early AD subjects, a condition in the progression of AD between MCI and late-stage AD. |
| Sultana et al. (2011b);Butterfield et al. (2012);Butterfield and Boyd-Kimball (2019) | Human double mutant APP/PS1 knock-in mice as a function of age. AD and MCI brains | In vivo | Redox proteomics demonstrated specific brain proteins, including proteins involved in glucose metabolism, were oxidatively modified and dysfunctional. Similar results were found in MCI and AD brain. |
| Butterfield et al. (2010);Robinson et al. (2011a) | Human double mutant APP(Swe/Ind) J20 mice with third mutation [M631L] = Met35 of Aβ42 replaced by Leucine. | In vivo | At one year of age, AD mice lacking Met-35 of Aβ42 had no brain oxidative damage, in marked contrast to the APP(Swe/Ind) J20 mice. Demonstrates the critical role of Met35 in Aβ42-associated oxidative damage. |
| Butterfield and Halliwell, (2019);Butterfield and Boyd-Kimball (2019) | AD and MCI brain | In vivo | Demonstrates relationships among oxidative damage, glucose dysmetabolism, and AD |
| Butterfield and Boyd-Kimball, (2019) | C.elegans, neuronal cell cultures, brain from AD mice models, and AD and MCI brain | In vivo, Ex vivo, In vitro | Compendium of redox proteomics results in relation to brains from subjects with AD and its earlier stages, and ex vivo and in vitro studies of Aβ. |
| Selected Studies from the Mattson Laboratory | |||
| Reference | System | In vivo, Ex Vivo, or In vitro | Comments |
| Mark et al., 1995; Mark et al., 1997 | Neuronal cell cultures, AD brain | In vitro, In vivo | Demonstrated that Aβ disrupts ion channels and ion-motive ATPases. |
| Smith-Swintosky et al., 1994 | Neuronal Cultures | In vitro | Reviews relationships among glutamate, Aβ and neurotoxicity. |
| Mattson, 2007 | Neuronal cultures, AD animal models, AD brain | In vivo In vitro | Solidifies Mattson’s role as a major leader in dysregulation of Ca2+ signaling in AD. |
| Wang and Mattson, 2014 | 3 X Tg AD Mice | Ex vivo | Demonstrated age-dependent increased Ca2+ currents of L-type Ca2+ channels at neuronal synapses in in CA1 region of hippocampus, but not in CA3 or dendate granule regions. |
| Guo et al (1999a,b) | PC6 Neuronal cultures, AD animal models, | In vitro; In vivo | Superoxide radical anion facilitates lipid peroxidation and neuronal death in human PS-1 mutations following Aβ42 addition. Similar conclusions by Butterfield study on PS-1 mutations. |
Aβ42 and lipid peroxidation are intimately associated. This association makes sense, as this hydrophobic, oligomeric peptide intercalates into the lipid bilayer, in which there are copious amounts of labile lipid allylic H-atoms that can be xtracted by a radical, leading to a cascade of events that culminates in production of HNE. Being a highly neurotoxic product of lipid peroxidation, HNE causes dysfunction of glucose metabolism, and coupled with consequent decreased ATP production, HNE modification disrupts ion-motive ATPases and Ca2+ signaling. These HNE-mediated alterations lead to neuronal death and are of critical importance to the pathogenesis and progression of AD. (See further discussion on this topic below).
Methionine residue 35 of Aβ42 is critical to the in vitro and in vivo oxidative damage associated with this neurotoxic peptide.
Neuronal Ca2+ dyshomeostasis is a key factor in neurodegeneration in AD and associated with Aβ42, as are dysfunctions in glutamate signaling.
Synaptic remodeling, key to learning and memory, is highly disrupted in AD and by Aβ42 oligomers.
Generally, investigations of brains isolated from animal models of AD replicate what is observed in AD brain.
4.1. Small Molecule Antioxidants and Studies Involving Aβ
Table 2 shows selected examples from the Butterfield and Mattson laboratories of small molecules and their relationship to oxidative damage associated with Aβ or in AD and MCI brain. Antioxidants have different mechanisms of action. Some directly scavenge radicals, while others induced a hormetic response. For the latter antioxidants, their model of action often is to induce a small stress to cells, to which the cells respond by upregulating protective genes, often employing the transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2). This, in turn, leads to upregulation of antioxidant proteins such as γ-glutamylcysteine ligase, Mn-superoxide dismutase, heme oxygenase-1, etc. (Raefsky and Mattson, 2017; Calabrese et al., 2007). Mark Mattson has been a pioneer of this concept along with Vittorio Calabrese and Cesare Mancuso, among others (Mattson, 2008; Calabrese et al., 2007; Mancuso et al., 2007).
Table 2.
Selected Studies of Small Molecule Antioxidants and AD or MCI or Involving Aβ from the Butterfield and Mattson Laboratories
| A. Selected Studies from the Butterfield Laboratory | |||
|---|---|---|---|
| Reference | System | In vivo or Ex Vivo |
Comments |
| Sultana et al. (2011a); Sultana et al. (2013a) | Mitochondria isolated from lymphocytes from persons with AD or MCI | Ex Vivo | Elevated oxidative damage that correlated inversely both with cognitive performance and in vivo plasma levels of small molecule antioxidants |
| Pocernich et al. (2001); Drake et al. (2002); Drake et al. (2003a); Boyd-Kimball et al. (2005c,d); Reed et al. (2009b); Pocernich et al. (2012) | Rodent brain; Mitochondria; Primary neuronal cultures; Brain from traumatic brain-injured rats; AD brain; | In vitro, Ex vivo | Glutathione Ethyl Ester or γ-Glutamylcysteine Ethyl Ester (GCEE) |
| Farr et al. (2003); Huang et al. (2010); Robinson et al. (2011b); | Aged-accelerated SAMP8 Mice; APP/PS-1 human double mutant knock-in mice; | Ex vivo; In vivo | N-Acetylcysteine (NAC) in drinking water or injected s.q. protected brain against oxidative damage by raising glutathione levels, consistent with the notion that inhibition of free radical oxidative stress is critical to improved cognition. |
| Poon et al. (2005a); Fiorini et al. (2013); Farr et al. (2014) | APP/PS-1 human double mutant knock-in mice; SAMP8 Mice | Ex vivo; In vivo | Antisense oligonucleotides against the Aβ region of APP, presenilin-1, or GSK-3β improved cognition and reduced oxidative damage, consistent with decreased Aβ and hyper-phosphorylated tau. |
| Farr et al. (2003); Poon et al. (2005b); Opii et al., (2010) | Brain from SAMP8 age-accelerated mice, and beagle dogs | In vivo | Lipoic Acid (LA) sq. led to improved cognition and decreased oxidative stress in SAMP8 mice. LA led to changes in the brain proteome reflecting increased brain function and decreased oxidative damage. LA as part of high antioxidant diet and a program of social enrichment over 3-year program led to much improved learning and memory in 15 year-old beagle dogs, who deposited less Aβ42 (of same amino acid sequence as humans) in brain than dog-chow controls. |
| Sultana et al. (2005); Mohmmad-Abdul et al. (2005); Perluigi et al. (2006); | Primary neuronal cultures treated with FAEE then Aβ42; synaptic membranes from rodents following i.p. administration of FAEE. Synaptic membranes were treated with Aβ42 | In vitro; Ex vivo | Ferulic Acid Ethyl Ester (FAEE) protected against added Aβ42-induced oxidative damage in neurons. FAEE was administered i.p. to rodents. Synaptic membranes were isolated and then exposed to Aβ42. Decreased oxidative damage was associated with hormetic elevation of protective genes and downregulation of a potentially damaging gene in both neurons and synaptic cultures. Insights into polyphenols in neuroprotection were gained, even though polyphenols do not easily cross the BBB. |
| Ansari et al., 2006; Sultana et al. (2004; 2006); Perluigi et al. (2006); Mohmmad-Abdul et al. (2005);Lauderback et al. (2003) | Primary neuronal cultures; synaptic membranes or brain mitochondria isolated from D609-treated rodents | In vitro; Ex vivo | D609, a xanthate, protected neurons, synaptic membranes, and brain mitochondria against Aβ42. D609 has a free thiol that when oxidized forms a disulfide bond that is reducible by glutathione reductase and NADPH |
| Ansari et al. (2009) | Primary neurons | In vitro | Quercetin, at low concentrations, protects neurons against Aβ42-mediated oxidative damage. However, at a sufficient and still relatively small concentration, this polyphenol is toxic to neurons. |
| B. Selected Studies from the Mattson Laboratory | |||
| Reference | System |
In vivo or Ex Vivo |
Comments |
| Zhang et al. (2019) | AD and AD rodent models | Ex Vivo and In Vitro | Senescent oligodendrocyte progenitor cells (OPC) were found in AD brain in areas rich in Aβ42. Aβ was shown to cause senescent characteristics of oligodendrocyte progenitor cells, which then become sensitive to being killed by desatnib and quercetin. |
| Liu et al. (2019) | AD mouse model | In vivo | The mitochondrial deacetylase, SIRT 3, mediates benefits of intermittent fasting on improved cognition in AD mice. May be an important intervention in AD. |
| Cutler et al. (2015); Yu et al. (1998) | AD and AD rodent models; primary neuronal cultures | In vitro and Ex vivo | Uric acid shown to be preventative of age-related neurodegenerative disorders. Uric acid is a good scavenger of peroxynitrite, preventing 3-NT formation. |
| Kennedy et al., 2016 | AD animal models and AD patients | In vivo and Ex vivo | A specific BACE inhibitor inhibits Aβ production in the CNS. Therefore, less neurotoxic Aβ. |
| Lee et al. (2014) | Plants | Ex vivo | Plants produce anti-feedants that discourage their being eaten. These phytochemicals appear to protect the CNS by inducing a hormetic response to produce anti-oxidant proteins. |
The main takeaways from these above-mentioned studies and those of others (Mecocci et al., 2018; Picco et al., 2014) are:
Low molecular weight antioxidants often are of lower concentrations in plasma of persons with AD and MCI.
When elevated in brain, thiol-based antioxidants such as N-acetylcysteine, lipoic acid, D609, or glutathione, act as protective moieties against Aβ42.
Polyphenols are highly effective in scavenging radicals in the periphery when at low concentration, less so in the CNS since BBB permeability is marginal, and cellular toxicity can occur when concentrations of these fruit-based phytochemicals are elevated.
Intercepting Aβ42-associated oxidative damage prior to damaging cellular components by such low molecular weight antioxidants conceivably could be part of a multi-pronged therapeutic strategy to slow progression of AD.
5.0. Redox Proteomics, Glucose Dysmetabolism, and AD
Redox proteomics is that branch of proteomics that is used to identify oxidatively modified proteins (Butterfield et al., 2012; Butterfield et al., 2014b; Butterfield and Boyd-Kimball, 2019). In essentially all oxidatively modified brain proteins, loss of activity of such proteins was observed. Loss of activity likely results from either oxidative modification of active site amino acids (e.g., cysteine; serine) and/or structural changes to oxidized proteins caused by oxidative modification of hydrophobic amino acids. In the latter case, the 3-dimenisional structure of proteins becomes altered because the polar dipole introduced on these hydrophobic amino acids by carbonyl formation from HNE or protein carbonyls forces these normally internally located amino acids to the protein-water interface (Butterfield and Stadtman, 1997).
A recent paper (Butterfield and Boyd-Kimball, 2019) summarizes redox proteomics-mediated identification of Aβ-associated, and therefore AD-relevant, proteins in in vitro, ex vivo and in vivo systems. Identification of oxidatively modified proteins following in the following systems are listed in this paper:
addition of Aβ42 oligomers to primary neuronal cultures;
expression of Aβ42 in C.elegans and in brains of rodent models of AD;
brains obtained at very short post-mortem intervals from subjects with AD, early AD, amnestic mild cognitive impairment (MCI), and preclinical AD, along with brains from corresponding non-disease age- and gender-matched controls.
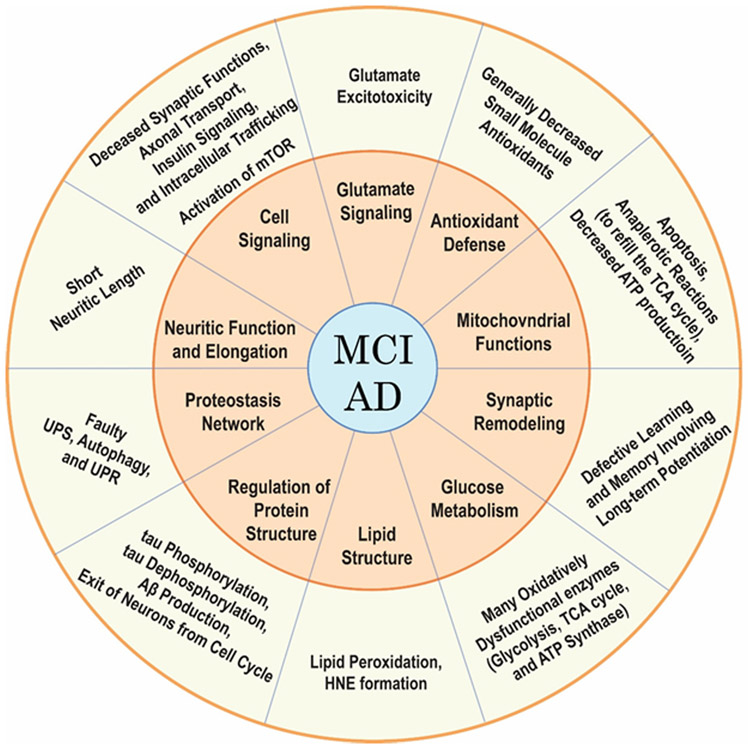
Redox proteomics-mediated identification of oxidatively-modified proteins, whose dysfunctions are consistent with biochemical and pathological alterations and clinical presentations in MCI and AD, included proteins associated with altered: 1) glutamate signaling and glutamate excitotoxicity; 2) proteostasis network; 3) synaptic remodeling for learning and memory involving long-term potentiation; 4) antioxidant defense; 5) lipid structure; 6) regulation of protein structure, particularly proteins involved in tau phosphorylation and dephosphorylation, Aβ production, and exit of neurons from the cell cycle; 7) neuritic function and elongation to form synaptic connections; 8) cell signaling; 9) glucose metabolism; and 10) mitochondrial functions, including proteins associated with apoptosis, anaplerotic reactions to refill the TCA cycle, and, of particular importance, ATP production [Figure 6].
Figure 6.
Brains in persons with AD or MCI have elevated HNE-bound proteins compared to aged-matched controls. In the middle ring, various functional pathways are noted that are damaged secondary to covalent HNE binding to Cys, His, or Lys residues. Other processes and pathways also are affected. The outer ring indicates the resulting dysfunctions that are seen in AD and MCI brain as a consequence of HNE-bound proteins in the various pathways noted. See text for more details.
It is this last altered function in MCI and AD brains, glucose dysmetabolism associated with HNE-modified proteins that is discussed in the following. PET scanning employing [18F]-2-deoxyglucose [FDG] demonstrated decreased glucose metabolism in MCI and significantly greater loss of glucose metabolism in AD brain (Cohen and Klunk, 2014; Brown et al., 2014). Redox proteomics studies from our laboratory identified several oxidatively dysfunctional glycolytic, TCA enzymes, and components of ATPase synthase as oxidatively modified by the lipid peroxidation product, HNE, that are proposed to significantly contribute to the diminished glucose metabolism in MCI and AD brains (Butterfield and Boyd-Kimball, 2019; Butterfield and Halliwell, 2019).
The consequences of decreased glucose metabolism are profound for neuronal survival. Specifically, not only is ATP essential for axonal transport and neurotransmission, but ATP also is essential for maintenance of neuronal cell potentials largely maintained by ion-motive ATPases, such as Na+/K+-ATPase and Ca2+-ATPase. Loss of ATP secondary to oxidative modification of proteins involved in glucose metabolism would lead to changes of neuronal cell potential with consequent opening of voltage gated Ca2+ channels with massive influx of this dipositive cations in neurons. This, in turn, would lead to saturation of endoplasmic reticulum and mitochondria stores of Ca2+. In the case of mitochondria, the response to this large increase in Ca2+ would lead to large influx of water in an osmotic process that would greatly swell the mitochondria, opening the mitochondrial permeability transition pore (MPTP). Once opened, cytochrome c, a peripheral membrane protein held to the membrane by electrostatic interactions, would exit the mitochondria via the MPTP. Apoptosis would ensue and the neuron would die. When enough neurons die, cognitive dysfunction would occur. As this process continues, one would see decreased glucose metabolism manifested in FDG-PET scans, and MRI imaging would demonstrate decreased volumes of hippocampus and frontal cortex, two regions highly involved in Aβ-mediated loss of glucose utilization and neurodegeneration (Kato et al., 2016).
When redox proteomics identification of HNE-modified brain proteins was applied to different stages of the progression of AD, i.e., MCI, early AD, late-stage AD, two proteins were prominently modified in each of these stages: enolase and ATP-synthase. That is, two proteins directly involved in processes associated with production of ATP were modified by the lipid peroxidation product HNE at each stage of the progression of AD from memory loss though the development of dementia (Butterfield and Halliwell, 2019; Butterfield and Boyd-Kimball, 2019). These results are consistent with the notion that enolase [known to be multifunctional (Butterfield and Lange, 2009)] and ATP synthase are damaged by HNE in AD brain are central to the progression of AD. More research is required to test if this concept is sustained.
The following scenario of events proposed from our laboratory (Butterfield et al., 2001; Butterfield and Halliwell, 2019) has been validated by multiple imaging methods performed on asymptomatic persons with inherited AD (Gordon et al., 2018) as described below:
oligomeric Aβ42 insertion into the neuronal lipid bilayer;
initiation of lipid peroxidation and subsequent formation of large amounts of HNE in the lipid bilayer but also diffusible into the cytosol or mitochondria (Butterfield and Stadtman, 1997; Sultana et al., 2013; Di Domenico et al., 2017a);
covalent modification of key proteins associated with glucose metabolism resulting in their dysfunction with consequent decreased ATP production, massive influx of Ca2+ into neurons and subsequent neuronal death, which would lead to shrinkage of hippocampus and frontal cortex and cognitive dysfunction resulting in dementia [Figure 6] (Butterfield and Halliwell, 2019).
In the study reported by Gordon et al., 2018, persons in the Dominantly Inherited Alzheimer Network (DIAN), comprised of thousands of individuals from multiple continents who are positive for development of autosomal-dominant inheritance of AD, agreed to be imaged for Aβ42 deposition (by PET scanning for Aβ42 fibrils), glucose metabolism (FDG-PET), and for thickness of hippocampus and frontal cortex (MRI) repeatedly over a period of up to 22 years prior to the development of clinical symptoms of AD and for three years following onset of symptoms, i.e., over a 25-year period. The principal questions addressed by this study were: what imaging-detected pathology occurred first, what occurred second, and what occurred last? As reported, Aβ42 deposition [which implicates the presence of oligomers, since Aβ42 fibrils are formed from precursor aggregates that include oligomeric Aβ42] occurred first. At some time later, glucose dysmetabolism was found, and lastly decreased hippocampal and frontal cortical thicknesses were observed (Gordon et al., 2018). This order of events for inherited AD follows those predicted from our model of sporadic AD and enumerated above (Butterfield et al., 2001; Butterfield and Halliwell, 2019).
6.0. Synergy of Studies between the Butterfield and Mattson Laboratories.
The highly reactive and neurotoxic product of lipid peroxidation, HNE, by covalently binding to and modifying the structure and function of target proteins in brains of persons with AD, MCI, or animal models thereof, contributes significantly to the pathology, biochemistry, and clinical presentation of AD and its earlier stages, as described above. The Butterfield laboratory for the most part has focused on oxidative damage, with emphasis on lipid peroxidation and its sequale, in AD and MCI brains and additional studies of mice and canine models thereof (Lauderback et al., 2001; Butterfield and Lauderback, 2002; Butterfield et al., 2002; Sultana et al., 2013; Di Domenico et al., 2017a; Butterfield and Halliwell, 2019; Butterfield and Boyd-Kimball, 2019; Abdul et al., 2008; Sultana et al., 2011b; Opii et al., 2008). In a complementary approach, the Mattson laboratory has focused for the most part on cellular adaptations to oxidative damage in mice models and neuronal culture models of AD, with additional studies of human brain specimens of AD (Texel and Mattson, 2011; Kapogiannis and Mattson, 2011; Mattson and Arumugam, 2018). In essentially every case, major conclusions and predictions reached by one laboratory have been confirmed by the other laboratory. In this sense, there is synergy between our two laboratories. It is my hope and trust that the reader of this paper will appreciate this synergy and the resultant intellectual satisfaction and increased understanding of AD that this synergy has provided the author and Dr. Mattson, as well as the AD research community.
7.0. Brief Reflections on Mark Mattson and His Outstanding Career.
While he was at the University of Kentucky, Mark Mattson and I became very good friends and colleagues, and we remain so. We were both recruited to be part of the Sanders-Brown Center on Aging by the founder of this Center, Dr. William R. Markesbery, who also was a gifted Alzheimer disease researcher, clinician, and neuropathologist. Mark and I talked often of research ideas and plans, and we were separate Project Leaders together on two NIA-funded P01 Program Project Grants headed by Dr. Markesbery and Dr. Philip Landfield, respectively, for more than 10 years.
Mark always kept the highest ethical standards with respect to research. And his penchant for getting the fruition of research ideas published in good journals and with some regularity was noteworthy. Indeed, at a recent celebration of Mark Mattson’s career in Baltimore held at Johns Hopkins University, it was announced that Dr. Mattson had reached the 1000 published paper milestone, an extraordinary accomplishment. This amazing achievement is not surprising: Mark got to his lab at UK early in the morning darkness, oftentimes jogging to work and jogging back to his home in the darkness at the end of the workday for him.
We worked together on specific projects and published 11 papers jointly [Table 3]. His departure to the NIA Intramural Research Program was a great professional loss for the University and for me personally, since we had developed a very good personal and professional relationship. To Mark’s credit, he kept in touch with Sanders-Brown personnel at UK, and he continued to publish with Sanders-Brown faculty, including me.
Table 3.
Joint Publications of D. Allan Butterfield and Mark P. Mattson
| Hensley K, Carney J, Mattson M, Aksenova M, Harris M, Wu JF, Floyd R,. Butterfield DA (1994) A Model for β-Amyloid aggregation and neurotoxicity based on free radical generating capacity of the peptide: Insights into Alzheimer disease. Proc Nat Acad Sci USA 91, 3270-3274. |
| Butterfield DA, Hensley K, Harris M, Mattson M, Carney J. (1994) beta-Amyloid peptide free radical fragments initiate synaptosomal lipoperoxidation in a sequence-specific fashion: implications to Alzheimer’s disease. Biochem Biophys Res Commun 200, 710-715. |
| M.P. Mattson, J.M. Carney, and D.A. Butterfield (1995) Glycation: A Tombstone in AD? Nature 373, 481. |
| Mark RJ, Hensley K, Butterfield DA, Mattson MP (1995) Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci 15, 6239-6249. |
| Hensley K, Butterfield DA, Mattson M, Aksenova M, Harris M, Wu JF, Floyd R, Carney J. (1995) A model for beta-amyloid aggregation and neurotoxicity based on the free radical generating capacity of the peptide: implications of "molecular shrapnel" for Alzheimer's disease. Proc West Pharmacol Soc 38, 113-120. |
| Hensley K, Butterfield DA, Hall N, Cole P, Subramaniam R, Mark R, Mattson MP, Markesbery WR, Harris ME, Aksenov M, Aksenova, M, Wu JF, Carney JM (1996) Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer’s disease-associated amyloid beta peptide. Ann N Y Acad Sci 786, 120-134. |
| Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg, G..Butterfield DA (1997) The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J Neurochem 69, 1161-1169. |
| Begley JG, Butterfield DA, Keller JN, Koppal T, Drake J, Mattson MP (1998) Cryopreservation of rat cortical synaptosomes and analysis of glucose and glutamate transporter activities, and mitochondrial function. Brain Res Brain Res Protoc 3, 76-82. |
| Bruce-Keller AJ, Begley JG, Fu W, Butterfield DA, Bredesen DE, Hutchins JB, Hensley K, Mattson MP (1998) Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid beta-peptide. J Neurochem 70, 31-39. |
| Guo Z, Ersoz A, Butterfield DA, Mattson MP (2000) Beneficial effects of dietary restriction on cerebral cortical synaptic terminals: preservation of glucose and glutamate transport and mitochondrial function after exposure to amyloid beta-peptide, iron, and 3-nitropropionic acid. J Neurochem 75, 314-320. |
| LaFontaine MA, Mattson MP, Butterfield DA (2002) Oxidative stress in synaptosomal proteins from mutant presenilin-1 knock-in mice: Implications for familial Alzheimer’s disease. Neurochem Res 27, 417-421. |
When not in the lab at UK, Mark enjoyed the horse environment that is famous in Lexington, Kentucky. He regularly raised and raced trotter horses at the Red Mile Racetrack and found a stress-relieving avocation in trotter-horse racing.
It is a great pleasure to dedicate this paper to Mark Mattson in honor of his extraordinary scientific career, and I wish him continued good health, good retirement from NIA, and a continued productive involvement in life’s new adventures for him.
Acknowledgements
The author thanks the faculty of Sanders-Brown Center on Aging at the University of Kentucky for providing well-characterized specimens from AD, MCI, preclinical AD and brains and those from corresponding aged-matched controls, obtained at a quite short post-mortem interval for studies in our laboratory that are referenced in the current paper. The author thanks Dr. Xiaojia Ren for preparation of figures for this paper. This work was supported in part by grants from the National Institutes of Health’s National Institute on Aging [AG060056; AG055596].
REFERENCES
- Aksenov M, Aksenova M, Butterfield DA, Markesbery WR (2000) Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. J Neurochem 74, 2520–2527. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Carney JM, Butterfield DA (1997) Oxidative modification of glutamine synthetase by amyloid beta peptide. Free Radic Res 27, 267–281. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Joshi G, Huang Q, Opii WO, Abdul HM, Sultana R, Butterfield DA. (2006) In vivo administration of D609 leads to protection of subsequently isolated gerbil brain mitochondria subjected to in vitro oxidative stress induced by amyloid beta-peptide and other oxidative stressors: relevance to Alzheimer’s disease and other oxidative stress-related neurodegenerative disorders. Free Radic Biol Med 41, 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Mohmmad Abdul H, Joshi G, Opii WO, Butterfield DA (2009) Protective effect of quercetin in primary neruons against Ab(1-42): relevance to Alzheimer’s disease. J Nutr Biochem 20, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul HM, Sultana R, St Clair DK, Markesbery WR, Butterfield DA. (2008) Oxidative damage in brain from human mutant APP/PS-1double knock-in mice as a function of age. Free Radic Biol Med 45,1420–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin-Chabot C, Wang L, Smarun AV, Vidović D, Shchepinov MS, Thibault G (2019) Deuterated Polyunsaturated Fatty Acids Reduce Oxidative Stress and Extend the Lifespan of C. elegans. Front Physiol 10, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley JG, Butterfield DA, Keller JN, Koppal T, Drake J, Mattson MP (1998) Cryopreservation of rat cortical synaptosomes and analysis of glucose and glutamate transporter activities, and mitochondrial function. Brain Res Brain Res Protoc 3, 76–82. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Mattson MP (2008) Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci 31, 454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd-Kimball D, Poon HF, Lynn BC, Cai J, Pierce WM Jr, Klein JB, Ferguson J, Link CD, Butterfield DA (2005a) Proteomic identification of proteins specifically oxidized in Caenorhabditis elegans expressing human Abeta(1-42): implications for Alzheimer’s disease. Neurobiol Aging 27, 1239–1249. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Poon HF, Lynn BC, Casamenti F, Pepeu G, Klein JB, Butterfield DA (2005b) Proteomic identification of proteins specifically oxidized by intracerebral injection of amyloid beta-peptide (1-42) into rat brain: implications for Alzheimer’s disease. Neuroscience 132, 313–324. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Abdul HM, Butterfield DA (2005c) Gamma-glutamylcysteine ethyl ester-induced up-regulation of glutathione protects neurons against Abeta(1-42)-mediated oxidative stress and neurotoxicity: implications for Alzheimer's disease. J Neurosci Res 79, 700–706. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Poon HF, Mohmmad-Abdul H, Lynn BC, Klein JB, Butterfield DA (2005d) Gamma-glutamylcysteine ethyl ester protection of proteins from Abeta(1-42)-mediated oxidative stress in neuronal cell culture: a proteomics approach. J Neurosci Res 79, 707–713. [DOI] [PubMed] [Google Scholar]
- Brown RK, Bohnen NI, Wong KK, Minoshima S, Frey KA (2014) Brain PET in suspected dementia: patterns of altered FDG metabolism. Radiographics 34, 684–701. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Begley JG, Fu W, Butterfield DA, Bredesen DE, Hutchins JB, Hensley K, Mattson MP (1998) Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid beta-peptide. J Neurochem 70, 31–39. [DOI] [PubMed] [Google Scholar]
- Butterfield DA. (1982) Spin labeling in disease in Biological Magnetic Resonance Vol. IV, (Berliner LJ; Reuben J, Eds.), Plenum Press, New York, pp. 1–78. [Google Scholar]
- Butterfield DA (2014) The 2013 SFRBM Discovery Award: selected discoveries from the Butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radic Biol Med 74, 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Boyd-Kimball D (2019) Redox proteomics and amyloid β-peptide: Insights into Alzheimer disease. J Neurochem 151, 459–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. (2002) Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging 23, 655–664. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Di Domenico F, Barone E (2014a) Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta 1842, 1693–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A. (2001) Evidence of oxidative damage in Alzheimer's disease brain: Central role of amyloid β-peptide. Trends Mol Med 7, 548–554. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Galvan V, Lange MB, Tang H, Sowell RA, Spilman P, Fombonne J, Gorostiza O, Zhang J, Sultana R, Bredesen DE (2010) In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic Biol Med 48, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Gu L, Di Domenico F, Robinson RA (2014b) Mass spectrometry and redox proteomics: applications in disease. Mass Spectrom Rev 33, 277–301. [DOI] [PubMed] [Google Scholar]
- Butterfield DA; Halliwell B (2019) Oxidative stress, glucose dysmetabolism and Alzheimer disease. Nature Rev Neurosci 20, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Harris M, Mattson M, Carney J. (1994) beta-Amyloid peptide free radical fragments initiate synaptosomal lipoperoxidation in a sequence-specific fashion: implications to Alzheimer's disease. Biochem Biophys Res Commun 200, 710–715. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Hensley K, Cole P, Subramaniam R, Aksenov M, Aksenova M, Bummer PM, Haley BE, Carney JM (1997) Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: relevance to Alzheimer's disease. J Neurochem 68, 2451–2457. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Koppal T, Subramaniam R, Yatin S. (1999) Vitamin E as an antioxidant/free radical scavenger against amyloid beta-peptide-induced oxidative stress in neocortical synaptosomal membranes and hippocampal neurons in culture: insights into Alzheimer's disease. Rev Neurosci 10, 141–149. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Lange ML (2009) Multifunctional roles of enolase in Alzheimer’s disease brain: beyong altered glucose metabolism. J Neurochem 111, 915–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Lauderback CM. (2002) Lipid peroxidation and protein oxidation in Alzheimer's disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med 32, 1050–1060. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Mattson MP (2020) Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer disease. Neurobiol Dis, in press. doi: 10.1016/j.nbd.2020.104795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Perluigi M, Reed T, Muharib T, Hughes CP, Robinson RA, Sultana R (2012) Redox proteomics in selected neurodegenerative disorders: from its infancy to future applications. Antioxid Redox Signal 17, 1610–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Pocernich CB (2003) The glutamatergic system and Alzheimer's disease: therapeutic implications. CNS Drugs 17, 641–652. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Stadtman ER (1997) Protein oxidation processes in aging brain. Adv Cell Aging Gerontol 2, 161–191. [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8, 766–775. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield DA, Stella AG (2007) Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Res 32, 757–773. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA (2002a) Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med 33, 562–571. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA (2002b) Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: Dihydropyrimidinase-related protein 2, a-enolase, and heat shock cognate 71. J Neurochem 82, 1524–1532. [DOI] [PubMed] [Google Scholar]
- Chistyakov DV, Filimonov IS, Azbukina NV, Goriainov SV, Chistyakov VV, Fomich MA, Bekish AV, Shmanai VV, Sergeeva MG, Shchepinov MS (2018) Deuterated Arachidonic Acids Library for Regulation of Inflammation and Controlled Synthesis of Eicosanoids: An In Vitro Study. Molecules 23, E3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AD, Klunk WE (2014) Early detection of Alzheimer's disease using PiB and FDG PET. Neurobiol Dis 72 PtA, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Camandola S, Malott KF, Edelhauser MA, Mattson MP (2015) The Role of Uric Acid and Methyl Derivatives in the Prevention of Age-Related Neurodegenerative Disorders. Curr Top Med Chem 15, 2233–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F, Tramutola A, Butterfield DA (2017a) Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of Alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic Biol Med 111, 253–261. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Barone E, Perluigi M, Butterfield DA (2017b) The triangle of death in Alzheimer's disease brain: the aberrant cross-talk among energy metabolism, mammalian target of rapamycin signaling, and protein homeostasis revealed by redox proteomics. Antioxid Redox Signal 26, 364–387. [DOI] [PubMed] [Google Scholar]
- Drake J, Link CD, Butterfield DA (2003a) Oxidative stress precedes fibrillar deposition of Alzheimer's disease amyloid beta-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol Aging 24, 415–420. [DOI] [PubMed] [Google Scholar]
- Drake J, Sultana R, Aksenova M, Calabrese V, Butterfield DA (2003b) Elevation of mitochondrial glutathione by gamma-glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J Neurosci Res 74, 917–927. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11, 81–128. [DOI] [PubMed] [Google Scholar]
- Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE (2003) The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 84, 1173–1183. [DOI] [PubMed] [Google Scholar]
- Farr SA, Ripley JL, Sultana R, Zhang Z, Niehoff ML, Platt TL, Murphy MP, Morley JE, Kumar V, Butterfield DA (2014) Antisense oligonucleotide against GSK-3β in brain of SAMP8 mice improves learning and memory and decreases oxidative stress: Involvement of transcription factor Nrf2 and implications for Alzheimer disease. Free Radic Biol Med 67, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini A, Sultana R, Förster S, Perluigi M, Cenini G, Cini C, Cai J, Klein JB, Farr SA, Niehoff ML, Morley JE, Kumar VB, Butterfield DA (2013) Antisense directed against PS-1 gene decreases brain oxidative markers in aged senescence accelerated mice (SAMP8) and reverses learning and memory impairment: a proteomics study. Free Radic Biol Med 65, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett RH, Grisham CM (2017) Biochemistry, 6th Ed., Cengage Learning, Boston. [Google Scholar]
- Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, Christensen J, McDade E, Wang G, Xiong C, Cairns NJ, Hassenstab J, Marcus DS, Fagan AM, Jack CR Jr, Hornbeck RC, Paumier KL, Ances BM, Berman SB, Brickman AM, Cash DM, Chhatwal JP, Correia S, Förster S, Fox NC, Graff-Radford NR, la Fougère C, Levin J, Masters CL, Rossor MN, Salloway S, Saykin AJ, Schofield PR, Thompson PM, Weiner MM, Holtzman DM, Raichle ME, Morris JC, Bateman RJ, Benzinger TLS (2018) Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer's disease: a longitudinal study. Lancet Neurol 17, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Fu W, Holtsberg FW, Steiner SM, Mattson MP (1999a) Superoxide mediates the cell-death-enhancing action of presenilin-1 mutations. J Neurosci Res 56, 457–470. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sebastian L, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP (1999b) Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: central roles of superoxide production and caspase activation. J Neurochem 72,1019–1029. [DOI] [PubMed] [Google Scholar]
- Guo Z, Ersoz A, Butterfield DA, Mattson MP (2000) Beneficial effects of dietary restriction on cerebral cortical synaptic terminals: preservation of glucose and glutamate transport and mitochondrial function after exposure to amyloid beta-peptide, iron, and 3-nitropropionic acid. J Neurochem 75, 314–320. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (2015) Free Radicals in Biology and Medicine, 5th Ed., Oxford University Press, New York. [Google Scholar]
- Hensley K, Butterfield DA, Mattson M, Aksenova M, Harris M, Wu JF, Floyd R, Carney J. (1995) A model for beta-amyloid aggregation and neurotoxicity based on the free radical generating capacity of the peptide: implications of "molecular shrapnel" for Alzheimer's disease. Proc West Pharmacol Soc 38, 113–120. [PubMed] [Google Scholar]
- Hensley K, Butterfield DA, Hall N, Cole P, Subramaniam R, Mark R, Mattson MP, Markesbery WR, Harris ME, Aksenov M, Aksenova M, Wu JF, Carney JM (1996) Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer's disease-associated amyloid beta peptide. Ann N Y Acad Sci 786, 120–134. [DOI] [PubMed] [Google Scholar]
- Hensley K, Carney J, Mattson M, Aksenova M, Harris M, Wu JF, Floyd R,. Butterfield DA (1994) A Model for β-Amyloid aggregation and neurotoxicity based on free radical generating capacity of the peptide: Insights into Alzheimer disease. Proc Nat Acad Sci USA 91, 3270–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM, Lovell M, Markesbery WR, Butterfield DA (1995) Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J Neurochem 65, 2146–2156. [DOI] [PubMed] [Google Scholar]
- Huang Q, Aluise CD, Joshi G, Sultana R, St Clair DK, Markesbery WR, Butterfield DA (2010) Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: toward therapeutic modulation of mild cognitive impairment. J Neurosci Res 88, 2618–2629. [DOI] [PubMed] [Google Scholar]
- Kanski J, Aksenova M, Schöneich C, Butterfield DA (2002) Substitution of isoleucine-31 by helical-breaking proline abolishes oxidative stress and neurotoxic properties of Alzheimer's amyloid beta-peptide. Free Radic Biol Med 32, 1205–1211. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Mattson MP (2011) Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer's disease. Lancet Neurol 10, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Inui Y, Nakamura A, Ito K (2016) Brain fluorodeoxyglucose (FDG) PET in dementia. Ageing Res Rev 30, 73–84. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR (2005) Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 64, 1152–1156. [DOI] [PubMed] [Google Scholar]
- Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, Egan M, Ereshefsky L, Hodgson RA, Hyde LA, Jhee S, Kleijn HJ, Kuvelkar R, Li W, Mattson BA, Mei H, Palcza J, Scott JD, Tanen M, Troyer MD, Tseng JL, Stone JA, Parker EM, Forman MS (2016) The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer's disease patients. Sci Transl Med. 8(363), 363ra150. [DOI] [PubMed] [Google Scholar]
- LaFontaine MA, Mattson MP, Butterfield DA (2002) Oxidative stress in synaptosomal proteins from mutant presenilin-1 knock-in mice: Implications for familial Alzheimer’s disease. Neurochem Res 27, 417–421. [DOI] [PubMed] [Google Scholar]
- Landau SM & Frosch MP (2014) Tracking the earliest pathological changes in Alzheimer disease. Neurology 82, 878–883. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA (2001) The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer's disease brain: the role of Abeta1-42. J Neurochem 78, 413–416. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Drake J, Zhou D, Hackett JM, Castegna A, Kanski J, Tsoras M, Varadarajan S, Butterfield DA (2003) Derivatives of xanthic acid are novel antioxidants: application to synaptosomes. Free Radic Res 37, 355–365. [DOI] [PubMed] [Google Scholar]
- Lee J, Jo DG, Park D, Chung HY, Mattson MP (2014) Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system. Pharmacol Rev 66, 815–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mallory M, Alford M, Tanaka S, Masliah E (1997) Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol 56, 901–911. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cheng A, Li YJ, Yang Y, Kishimoto Y, Zhang S, Wang Y, Wan R, Raefsky SM, Lu D, Saito T, Saido T, Zhu J, Wu LJ, Mattson MP (2019) SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun 10, 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, Banks WA, Butterfield DA (2010) Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Aβ accumulation in AD brain. Free Radic Biol Med 49, 1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek-Ahmadi M, Chen K, Perez SE, He A, Mufson EJ (2018) Cognitive composite score association with Alzheimer's disease plaque and tangle pathology. Alzheimers Res Ther 10, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso C, Scapagini G, Currò D, Giuffrida Stella AM, De Marco C, Butterfield DA, Calabrese V (2007) Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci 12, 1107–1123. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Hensley K, Butterfield DA, Mattson MP (1995) Amyloid beta-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci 15, 6239–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP (1997). A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem 68, 255–264. [DOI] [PubMed] [Google Scholar]
- Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6, 337–350. [DOI] [PubMed] [Google Scholar]
- Mattson MP (2008) Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol 27, 155–162. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Arumugam TV (2018) Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab 27, 1176–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Chan SL (2003) Neuronal and glial calcium signaling in Alzheimer's disease. Cell Calcium 34, 385–397. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA, Duan W, Culmsee C, Camandola S. (1999) Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer's and Parkinson's diseases. Ann N Y Acad Sci 893, 154–175. [DOI] [PubMed] [Google Scholar]
- Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med 23, 134–147. [DOI] [PubMed] [Google Scholar]
- Markesbery WR (2010) Neuropathologic alterations in mild cognitive impairment: a review. J. Alzheimers Dis. 19, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR (1997) Neuropathological criteria for the diagnosis of Alzheimer’s disease. Neurobiol Aging 18 (Suppl) S13–19. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Boccardi V, Cecchetti R, Bastiani P, Scamosci M, Ruggiero C, Baroni M (2018) A Long Journey into Aging, Brain Aging, and Alzheimer's Disease Following the Oxidative Stress Tracks. J Alzheimers Dis 62, 1319–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohmmad Abdul H, Butterfield DA (2005) Protection against amyloid beta-peptide (1-42)-induced loss of phospholipid asymmetry in synaptosomal membranes by tricyclodecan-9-xanthogenate (D609) and ferulic acid ethyl ester: implications for Alzheimer's disease. Biochim Biophys Acta 1741, 140–148. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Quinn J, Kaye J, Morrow JD (2007) F(2)-isoprostanes as biomarkers of late-onset Alzheimer's disease. J Mol Neurosc 33, 114–119. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Braak H & Markesbery WR (2009) Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropathol. Exp. Neurol 68, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA (2017) Amyloid precursor protein and endosomal-lysosomal dysfunction in Alzheimer's disease: inseparable partners in a multifactorial disease. FASEB J 31, 2729–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S Atwood CS, Petersen RB, Smith MA (2001). Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol 60, 759–767. [DOI] [PubMed] [Google Scholar]
- Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB, Pierce WM, Cotman CW, Butterfield DA (2008) Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer's disease. Neurobiol Aging 29, 51–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perluigi M, Joshi G, Sultana R, Calabrese V, De Marco C, Coccia R, Butterfield DA (2006) In vivo protection by the xanthate tricyclodecan-9-yl-xanthogenate against amyloid beta-peptide (1-42)-induced oxidative stress. Neuroscience 138, 1161–1170. [DOI] [PubMed] [Google Scholar]
- Picco A, Polidori MC, Ferrara M, Cecchetti R, Arnaldi D, Baglioni M, Morbelli S, Bastiani P, Bossert I, Fiorucci G, Brugnolo A, Dottorini ME, Nobili F, Mecocci P (2014) Plasma antioxidants and brain glucose metabolism in elderly subjects with cognitive complaints. Eur J Nucl Med Mol Imaging 41, 764–75. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Cardin AL, Racine CL, Lauderback CM, Butterfield DA (2001) Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem Int 39, 141–149. [DOI] [PubMed] [Google Scholar]
- Poon HF, Farr SA, Banks WA, Pierce WM, Klein JB, Morley JE, Butterfield DA (2005a) Proteomic identification of less oxidized brain proteins in aged senescence-accelerated mice following administration of antisense oligonucleotide directed at the Abeta region of amyloid precursor protein. Brain Res Mol Brain Res 138, 8–16. [DOI] [PubMed] [Google Scholar]
- Poon HF, Farr SA, Thongboonkerd V, Lynn BC, Banks WA, Morley JE, Klein JB, Butterfield DA (2005b) Proteomic analysis of specific brain proteins in aged SAMP8 mice treated with alpha-lipoic acid: implications for aging and age-related neurodegenerative disorders. Neurochem Int 46, 159–168. [DOI] [PubMed] [Google Scholar]
- Pratico D (2010) The neurobiology of isoprostanes and Alzheimer disease. Biochim Biophys Acta 1801, 930–933. [DOI] [PubMed] [Google Scholar]
- Raefsky SM, Furman R, Milne G, Pollock E, Axelsen P, Mattson MP, Shchepinov MS (2018) Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid β-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer's disease. Neurobiol Aging 66, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raefsky SM, Mattson MP (2017) Adaptive responses of neuronal mitochondria to bioenergetic challenges: Roles in neuroplasticity and disease resistance. Free Radic Biol Med 102, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed TT, Pierce WM, Markesbery WR, Butterfield DA (2009a) Proteomic identification of HNE-bound proteins in early Alzheimer disease: Insights into the role of lipid peroxidation in the progression of AD. Brain Res 1274, 66–76. [DOI] [PubMed] [Google Scholar]
- Reed TT, Pierce WM Jr, Turner DM, Markesbery WR, Butterfield DA (2009b) Proteomic identification of nitrated brain proteins in early Alzheimer's disease inferior parietal lobule. J Cell Mol J Cell Mol Med Med 13(8B), 2019–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RA, Lange MB, Sultana R, Galvan V, Fombonne J, Gorostiza O, Zhang J, Warrier G, Cai J, Pierce WM, Bredesen DE, Butterfield DA (2011a) Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: effects of the amyloid-β peptide of amyloid precursor protein. Neuroscience 177, 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RA, Joshi G, Huang Q, Sultana R, Baker AS, Cai J, Pierce W, St Clair DK, Markesbery WR, Butterfield DA (2011b) Proteomic analysis of brain proteins in APP/PS-1 human double mutant knock-in mice with increasing amyloid β-peptide deposition: insights into the effects of in vivo treatment with N-acetylcysteine as a potential therapeutic intervention in mild cognitive impairment and Alzheimer's disease. Proteomics 11, 4243–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneich C, Pogocki D, Hug GL, Bobrowski K. (2003) Free radical reactions of methionine in peptides: mechanisms relevant to beta-amyloid oxidation and Alzheimer's disease. J Am Chem Soc 125, 13700–13713. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Meabon JS, Woltjer RL, Sullivan JM, Diamond JS, Cook DG (2013) Amyloid-β1-42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. J Neurosci 33, 5312–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. (2016) The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8, 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao H, Jao S, Ma K, Zagorski MG (1999) Solution structures of micelle-bound amyloid beta-(1-40) and beta-(1-42) peptides of Alzheimer's disease. J Mol Biol 285, 755–773. [DOI] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Mattson MP (1994) Glutamate, beta-amyloid precursor proteins, and calcium mediated neurofibrillary degeneration. J Neural Transm Suppl. 44, 29–45. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D., Pericak-Vance M, Enghild J, Salvesen GS, Roses AD (1993) Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Nat Acad Sci USA 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G..Butterfield DA (1997) The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J Neurochem 69, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Sultana R, Baglioni M, Cecchetti R, Cai J, Klein JB, Bastiani P, Ruggiero C, Mecocci P, Butterfield DA (2013a) Lymphocyte mitochondria: toward identification of peripheral biomarkers in the progression of Alzheimer disease. Free Radic Biol Med 65, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu KP, Butterfield DA (2006) Oxidative modification and down-regulation of Pin 1 in Alzheimer’s disease hippocampus: a redox proteomics analysis. Neurolbiol Aging 27, 918–925. [DOI] [PubMed] [Google Scholar]
- Sultana R, Mecocci P, Mangialasche F, Cecchetti R, Baglioni M, Butterfield DA (2011a) Increased protein and lipid oxidative damage in mitochondria isolated from lymphocytes from patients with Alzheimer's disease: insights into the role of oxidative stress in Alzheimer's disease and initial investigations into a potential biomarker for this dementing disorder. J Alzheimers Dis 24, 77–84. [DOI] [PubMed] [Google Scholar]
- Sultana R, Newman SF, Abdul HM, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA (2006) Protective effect of D609 against amyloid-beta1-42-induced oxidative modification of neuronal proteins: redox proteomics study. J Neurosci Res 84, 409–417. [DOI] [PubMed] [Google Scholar]
- Sultana R, Newman S, Mohmmad-Abdul H, Keller JN, Butterfield DA (2004) Protective effect of the xanthate, D609, on Alzheimer's amyloid beta-peptide (1-42)-induced oxidative stress in primary neuronal cells. Free Radic Res 38, 449–458. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA (2013b) Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med. 62, 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R, Robinson RA, Di Domenico F, Abdul HM, St Clair DK, Markesbery WR, Cai J, Pierce WM, Butterfield DA (2011b) Proteomic identification of specifically carbonylated brain proteins in APP(NLh)/APP(NLh) × PS-1(P264L)/PS-1(P264L) human double mutant knock-in mice model of Alzheimer disease as a function of age. J Proteomics 74, 2430–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L (2001) New frontiers in Alzheimer’s genetics. Neuron 32, 181–184. [DOI] [PubMed] [Google Scholar]
- Texel SJ, Mattson MP (2011) Impaired adaptive cellular responses to oxidative stress and the pathogenesis of Alzheimer's disease. Antioxid Redox Signal 14, 1519–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramutola A, Lanzillotta C, Di Domenico F, Head E, Butterfield DA, Perluigi M, Barone E (2020) Brain insulin resistance triggers early onset Alzheimer disease in Down syndrome. Neurobiol Dis 137, 104772. [DOI] [PubMed] [Google Scholar]
- Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA (2015) Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 133, 739–349. [DOI] [PubMed] [Google Scholar]
- Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA (2001) Different mechanisms of oxidative stress and neurotoxicity for Alzheimer's A beta(1--42) and A beta(25--35). J Am Chem Soc 123, 5625–5631. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mattson MP (2014) L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol Aging 35, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatin SM, Varadarajan S, Link CD, Butterfield DA.(1999) In vitro and in vivo oxidative stress associated with Alzheimer's amyloid beta-peptide (1-42). Neurobiol Aging 20, 325–330. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Varadarajan S, Butterfield DA (2000) Vitamin E prevents Alzheimer's amyloid beta-peptide (1-42)-induced neuronal protein oxidation and reactive oxygen species production. J Alzheimer’s Dis 2, 123–131. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Yatin M, Varadarajan S, Ain KB, Butterfield DA (2001) Role of spermine in amyloid beta-peptide-associated free radical-induced neurotoxicity. J Neurosci Res 63, 395–401. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP (1998) Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res 53, 613–625. [DOI] [PubMed] [Google Scholar]
- Yuksel M, Tacal O (2019) Trafficking and proteolytic processing of amyloid precursor protein and secretases in Alzheimer’s disease development: An up-to-date review. Eur J Pharmacol 856, 172415. [DOI] [PubMed] [Google Scholar]
- Zesiewicz T, Heerinckx F, De Jager R, Omidvar O, Kilpatrick M, Shaw J, Shchepinov MS (2018) Randomized, clinical trial of RT001: Early signals of efficacy in Friedreich's ataxia. Mov Disord 33, 1000–1005. [DOI] [PubMed] [Google Scholar]
- Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, Mattson MP (2019) Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer's disease model. Nat Neurosci 22, 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]