Abstract
Background
The combination of antibacterial and mucolytic actions makes nitric oxide (NO) an attractive dual-action cystic fibrosis (CF) therapeutic. The delivery of any therapeutic agent through pathological mucus is difficult, and the use of inhaled NO gas is inherently limited by toxicity concerns. Herein, we directly compare the ability of NO to eradicate infection and decrease mucus viscoelastic moduli as a function of delivery method (i.e., as a gas or water-soluble chitosan donor).
Methods
To compare bactericidal action in tissue, an ex vivo porcine lung model was infected and treated with either gaseous NO or NO-releasing chitosan for 5 h. In vitro Pseudomonas aeruginosa biofilm viability was quantified after NO treatment. Human bronchial epithelial mucus and CF sputum were exposed to NO and their viscoelastic moduli measured with parallel plate macrorheology.
Results
Larger NO concentrations were achieved in solution when delivered by chitosan relative to gas exposure. The bactericidal action in tissue of the NO-releasing chitosan was greater compared to NO gas in the infected tissue model. Chitosan delivery also resulted in improved antibiofilm action and reduced biofilm viability (2-log) while gaseous delivery had no impact at an equivalent dose (∼0.8 μmol/mL). At equivalent NO doses, mucus and sputum rheology were significantly reduced after treatment with NO-releasing chitosan with NO gas having no significant effect.
Conclusions
Delivery of NO by chitosan allows for larger in-solution concentrations than achievable via direct gas with superior bactericidal and mucolytic action.
Keywords: cystic fibrosis, nitric oxide, Pseudomonas aeruginosa, biofilm, mucolytic; mucus
1. Introduction
Cystic fibrosis (CF) is characterized by chronic respiratory infection leading to severe lung degradation.[1,2] The pathological mucus in the lungs of CF patients has altered biophysical properties (e.g., viscosity, elasticity) and results in decreased or failed mucociliary clearance, impairment of the immune response, and decreased antibiotic efficacy.[3–5] The altered biophysical characteristics of CF mucus are due in large part to elevated concentrations of macromolecules (e.g., mucins, DNA) and decreased volume of the airway surface liquid.[2,4–6] Mucus abnormalities precede chronic infection, which further exacerbates the inflammatory response.[2,7] Pathological mucus coupled with chronic infection results in a vicious cycle that causes severe lung degradation and mortality.[4,8,9] Pseudomonas aeruginosa is the leading cause of infection-related mortality in CF patients, hypermutable, and frequently multidrug-resistant.[10,11] Additionally, mucus with high elastic and viscous moduli promotes biofilm formation due in part to restricted bacterial motility and small molecule diffusion, thus inhibiting bactericidal action by both the host defense system and administered antibiotics.[6,12,13] Therapies designed to reduce mucus concentration or alter rheology are currently being developed, but no commercially available mucolytic agent is also capable of mitigating infection.[14] Chronic infection directly contributes to decreased mucociliary clearance by further concentrating already pathological mucus and increasing mucus elasticity.[4–6] As such, new therapies that target both mucus viscoelasticity and biofilm infection are urgently needed.
Nitric oxide (NO) has demonstrated potential as a CF therapeutic due to its mucolytic and bactericidal properties.[15,16] However, the delivery of gaseous NO (gNO) to the airway is limited by toxicity concerns (i.e., methemoglobinemia).[17–19] While multiple clinical trials with inhaled NO have shown reduced bacterial load and improved overall lung function, the clinical utility of inhaled NO is limited (i.e., confined to hospital setting, cost-prohibitive) with systemic effects not fully appreciated.[17–21] Nitric oxide-releasing macromolecular scaffolds have been developed for NO delivery into solution and for lowered potential of systemic toxicity.[15,16,22,23] Related to CF, concentrated pathological mucus may inhibit the efficacy of NO, particularly when delivered to the airway as a gas, but this barrier may be addressed with a macromolecular scaffold that allows for localized delivery.[4,15,16]
Previously, we compared the bactericidal efficacy of gNO and NO-releasing chitosan oligosaccharides (COS/NO).[24] The release of NO in solution allowed for superior planktonic killing under static conditions (i.e., in buffer).[24] However, no direct comparison of antibiofilm and mucolytic action of these two NO delivery methods has been performed in conditions relevant to CF. The exposure chamber used in previous reports is an attractive comparison method because it allows for facile delivery of gNO into solution that is easily measured with an endpoint colorimetric assay.[24] Herein, we focused on evaluating the ability of NO to eradicate infection, disrupt biofilms, and affect human bronchial epithelial (HBE) mucus and CF sputum rheology concomitantly as a function of delivery method (i.e., when delivered as a gas or with a chitosan donor) under CF-relevant conditions. The bactericidal action of gNO and COS/NO in tissue was assessed using an ex vivo infected porcine lung model. Additionally, P. aeruginosa biofilm viability and rheology, and both HBE mucus and CF sputum rheology were evaluated after exposure to either gNO or COS/NO.
2. Materials and Methods
2.1. Materials
A detailed list of materials and the synthesis and characterization of COS/NO are available in the SI.
2.2. Ex vivo porcine lung model infection and treatment with NO
Frozen stocks of P. aeruginosa strain K (PAK) were reconstituted in tryptic soy broth (TSB, 3 mL) and incubated with gentle shaking overnight. The solution was reinoculated into fresh TSB, grown to a concentration of 108 CFU/mL and subsequently diluted to 106 CFU/mL in TSB. Sections of alveolar tissue (∼5 mm3) from two sets of pig lungs were dissected and infected as previously described.[25] Sections were placed in 24-well plates containing 400 μL artificial sputum media (ASM, recipe in SI) that was supplemented with 0.8% w/v agarose. The ASM used in these experiments was sterilized by autoclaving and not by filtration, due to the loss of significant mucin content when filtering through a 0.22 μm filter. The sections were subsequently inoculated with 50 μL of P. aeruginosa in TSB (106 CFU/mL) using a 30G needle attached to a 1 mL syringe. The well plates were incubated with gentle shaking for 24 h at 37 °C. After incubation, sections were gently rinsed with 1 mL PBS to remove loosely adherent bacteria and then transferred to a new 24-well plate containing 400 μL ASM with 0.8% w/v agarose. Tissue sections were exposed in a volume of 500 μL PBS to fully submerge the section.[25]
Tissue sections were exposed to gNO or COS/NO over several concentrations. As the concentration of NO delivered into PBS via gas is limited by the diffusion rate of NO, the concentration of NO in PBS after exposure was measured via the Griess assay (Supplementary Information). A range of COS/NO concentrations, including one that matched the concentration of NO delivered via gNO, were employed to assess concentration-dependent effects on bacterial viability (Table S1). Exposure to NO was performed for 5 h in keeping with previous work.[24] For gNO exposure, 500 μL PBS was added to the tissue-containing wells, and the well plate was exposed to gNO at 500 ppm for 5 h. For COS/NO exposure, COS/NO in a range of concentrations was dissolved in PBS (500 μL) and added to wells containing tissue. Two tissue sections were used per exposure for each condition (technical duplicates). After treatment with NO, each tissue section was homogenized individually in PBS (500 μL) with vortexing and bead beating. Homogenates were serially diluted (10 to 100,000x) in MilliQ water and spiral plated on Pseudomonas isolation agar. Viability was assessed by colony counting. Bacteriostatic and bactericidal action were defined as a ≥2-log or ≥3-log reduction in viability compared to the PBS-treated control, respectively. Of note, the limit of detection for this method is 2.5 × 103 CFU/mL. Each exposure condition was evaluated on lung tissue sections infected with three separately grown cultures of P. aeruginosa (biological replicates, n=3).
2.3. Biofilm growth and eradication
P. aeruginosa biofilms were grown in vitro as previously described.[26] Briefly, frozen stocks of PAK were reconstituted in tryptic soy broth (TSB, 3 mL) and incubated with gentle shaking overnight. The solution was reinoculated into fresh TSB, grown to a concentration of 108 CFU/mL and subsequently diluted to 106 CFU/mL in 1.8 mL TSB in a 24-well plate. The well plate was incubated at 37 °C for 3 d while gently shaking until a macrocolony formed that was easily separated from growth media. The biofilm (250 μL) was removed, washed gently in PBS (1 mL, pH 6.5), and added to 750 μL fresh PBS or COS/NO dissolved in PBS in a 24-well plate. Of note, biofilms grew to volumes larger than 250 μL, but only 250 μL was used for each experiment. Biofilms were exposed for 5 h to COS/NO or PBS inside a separate incubator or to gNO in the exposure chamber as described previously.[24] After exposure, a portion of the biofilm (100 μL) was removed with a positive displacement pipette and added to 900 μL sterile MilliQ water. The biofilm was disrupted with pipetting and vortexing, serially diluted in MilliQ, and spiral plated on TSA. Colonies were enumerated using a Flash & Go colony counter (IUL, Farmingdale, NY). Each exposure condition was evaluated on biofilms grown on n≥3 separate days.
2.4. Biofilm rheology
Separately exposed biofilms were assessed for changes in rheology with parallel plate macrorheology. Stress amplitude and frequency sweeps were performed on a TA Discovery Hybrid Rheometer 3 (TA Instruments DHR-3; New Castle, Delaware) as previously described.[4,16] All analyses were performed at 23°C with a 40 mm diameter sandblasted parallel plate and solvent trap to minimize sample dehydration at a gap of 100 μm. Macroscopic rheological data were analyzed via TA Trios software. Five technical replicates were measured for each biofilm. Each exposure condition was evaluated on biofilms grown on n≥3 separate days.
2.5. Mucolytic action of NO
Human bronchial epithelial (HBE) mucus with 4% wt solids was prepared and characterized as previously described (cell culture, mucus collection, and characterization are included in the SI).[3,4,6] Sputum collected from two CF patients was homogenized by pipetting and preserved with a protease inhibitor (sputum collection and preservation are detailed in the SI). HBE mucus or CF sputum (200 μL) was added to a 24-well plate with an additional 50 μL PBS added prior to 500 ppm gNO exposure for 5 h. In a separate well plate, HBE mucus or CF sputum was treated with COS/NO dissolved in 50 μL PBS for 5 h. Macrorheological properties were assessed immediately using the DHR-3 instrument using a 20 mm smooth stainless-steel parallel plate with solvent trap at a 50 μm gap. Five technical replicates were measured for each sample. Each exposure condition was evaluated on n≥3 separate days.
2.6. Statistical analysis
Statistical significance was calculated using a two-tailed Student’s t-test for biological replicates (i.e., means of technical replicates) for n≥3 samples (i.e., tissue sections, biofilms, HBE mucus, CF sputum) for each exposure condition.
3. Results
3.1. Comparison of COS/NO and gNO concentrations in solution
The concentration of NO released into solution from the chitosan oligosaccharide was easily controlled by adjusting the mass of chitosan added. For example, a 10 mg/mL solution of COS/NO will deliver 10x more NO than a 1mg/mL solution while release kinetics remain unchanged. In contrast, the concentration of NO in solution when delivered as a gas depends upon the diffusion rate of NO into and through the solution.[24] For simplicity, exposure media and volumes were varied according to the reference protocol while the concentration of gNO in the headspace and the exposure duration were held constant (500 ppm for 5 h). In addition, the concentrations of NO in each solution after exposure varied depending upon the medium (Table S2). Concentrations of NO in PBS were inversely related to the volume in the well, consistent with previous work.[24] Greater NO concentrations were measured in HBE mucus compared to PBS after exposure to gNO at 500 ppm for 5 h. The largest NO concentration was measured in CF sputum.
In order to directly compare the antibiofilm and mucolytic action of COS/NO and gNO, the concentration of COS/NO was tuned to deliver equivalent NO doses achieved by gaseous delivery for each volume and medium tested (Table S1). Nitric oxide doses delivered by the chitosan scaffold were measured in PBS using chemiluminescence (Detailed instrumentation and measurement is included in the SI). The COS/NO dose described is representative of the largest possible concentration of NO in solution at 5 h, assuming no scavenging (Figure S1). As scavenging in proteinaceous media is likely, actual NO concentrations delivered via chitosan to HBE mucus or CF sputum will be lower than projected. Unpublished work in our lab indicates that NO-release in nutrient broth decreases NO payload by ∼20%.
3.2. Treatment of infected lung tissue
To assess the ability of each delivery system to eradicate infection in lung tissue, ex vivo porcine lungs were sectioned, infected with P. aeruginosa, and then exposed to gNO or COS/NO. Infected lung tissue sections supported an average of 109 CFU/mL, consistent with previous work.[25] Some native bacteria were present in the uninfected sections (~104 CFU/mL) but did not grow on Pseudomonas isolation agar. As such, isolation agar was used to quantify P. aeruginosa killing for these ex vivo experiments.
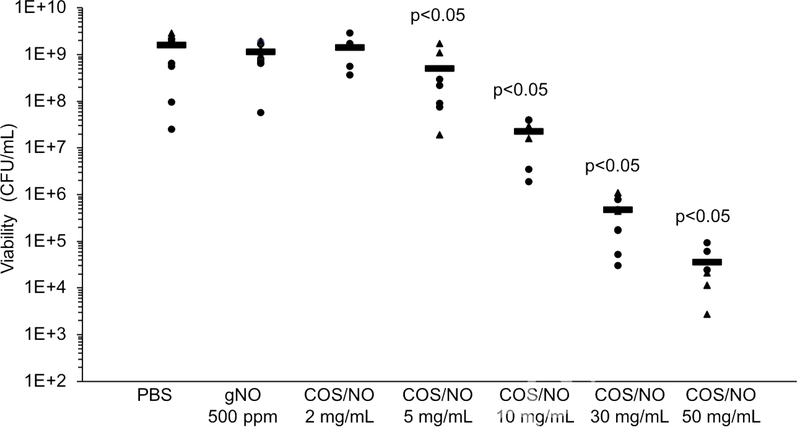
Tissue sections were exposed to gNO at 500 ppm for 5 h in 500 μL PBS.[25] Approximately 2.3 μmol/mL NO accumulated in solution but with no observed change in bacterial viability in tissue (Fig. 1). An equivalent dose of NO (2.3 μmol/mL) released from COS/NO (5 mg/mL) elicited a significant (p<0.05) reduction in P. aeruginosa viability in the tissue sections. Larger doses of COS/NO (e.g., 10 and 30 mg/mL) led to both bacteriostatic (≥2-log reduction) and bactericidal action (≥3-log reduction). A 5-log reduction in P. aeruginosa viability was achieved at 50 mg/mL COS/NO. Control (i.e., non-NO-releasing) chitosan oligosaccharides were previously shown to have no effect on bacterial viability;[26] thus, all antibacterial action is attributable to NO.
Figure 1.
P. aeruginosa viability in the ex vivo porcine lung model after exposure to NO. Two sets of lungs were used, indicated by the circles and triangles. Black bars indicate average viability across exposures and lungs. Significance was calculated with a two-tailed students t-test compared to the PBS-treated control.
3.3. Antibiofilm action of COS/NO vs gNO
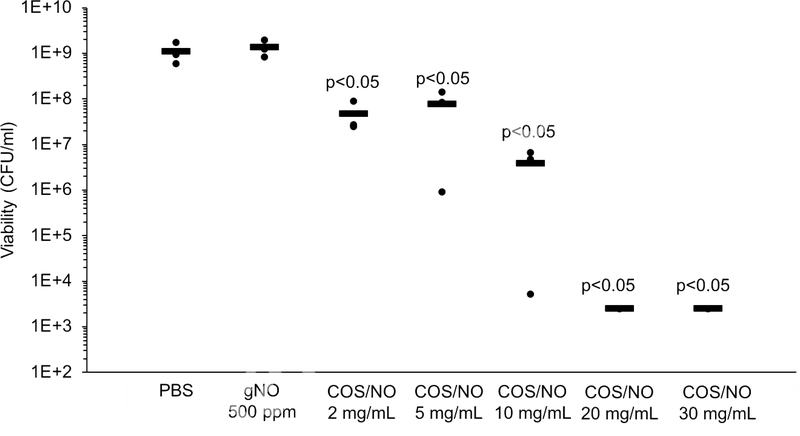
Biofilm studies were performed in 750 μL PBS wherein 250 μL was added to 750 μL PBS with or without dissolved COS/NO.[26] Exposure to gNO at 500 ppm for 5 h resulted in a NO solution concentration of 0.86 μmol/mL (Table S2), with no significant change in biofilm viability following treatment (Fig. 2). An equivalent NO dose from 2 mg/mL COS/NO (0.92 μmol/mL NO, Table S1) resulted in a 2-log reduction (∼107 CFU/mL) in biofilm viability (p<0.05). Biofilm eradication (i.e., 5-log reduction in viability) was not possible with NO gas at 500 ppm for 5 h, and could only be achieved with a significantly greater one-time dose of COS/NO (20 mg/mL, 9.2 μmol/mL NO).
Figure 2.
Viability of in vitro P. aeruginosa biofilms after exposure to NO. Average viability is indicated by black bars. Significance was calculated with a two-tailed students t-test compared to the PBS-treated control.
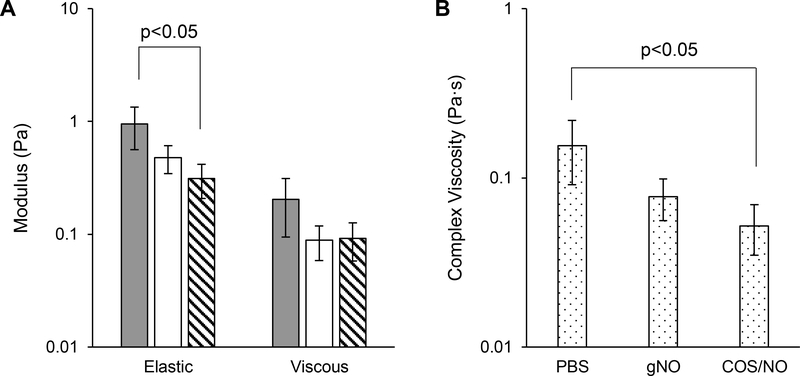
Previous studies by Gloag et al. and Peterson et al. found that biofilm viscoelasticity is related to antibiotic susceptibility.[26,27] Thus, the effects of NO delivery method on the physical biofilm structure were assessed with macrorheological analysis. Doses as low as 2 mg/mL COS/NO (0.92 μmol/mL NO) caused significant (p<0.05) decreases in both the elastic modulus (G’) and complex viscosity (η*) of P. aeruginosa biofilms (65% and 67%, respectively; Fig. 3). Though an apparent reduction in viscoelastic moduli was noted, no significant changes were observed after gNO exposure. In this manner, rheological analysis showed that an equivalent dose of NO from chitosan was superior at disrupting biofilm structure and reducing viability compared to NO delivered as a gas. The ability of COS/NO to simultaneously affect biofilm rheology and reduce bacteria viability suggests NO delivered into solution is superior as an antibiofilm agent compared to gNO.
Fig. 3.
Antibiofilm action of NO delivered as a gas or by a chitosan donor at equivalent NO doses. (A) Elastic (G’) and viscous (G”) moduli of P. aeruginosa biofilms after exposure to PBS (gray), gNO (white), or COS/NO (stripes) for 5 h. (B) Complex viscosity (η*) of P. aeruginosa biofilms after exposure to NO. Data is presented as the mean of n≥3 biofilms from separately grown batches at 1% strain and 1 Hz. Error is representative of the standard deviation of the mean.
A major benefit to oligosaccharide delivery of NO is the capacity for greater in-solution NO concentrations. To demonstrate the maximum therapeutic potential of COS/NO as an antibiofilm agent, larger in-solution NO concentrations were delivered by the chitosan donor than were possible via gaseous delivery under the current exposure conditions. As might be anticipated, biofilm eradication (5-log reduction) was achieved at 20 mg/mL COS/NO (Fig. 2), equating to 9.2 μmol/mL NO in solution. This concentration of COS/NO also significantly decreased all viscoelastic moduli (i.e., elastic and viscous (G”) moduli and η*, Fig. S2). While both elastic and viscous moduli decreased after exposure to COS/NO, no significant change in tanδ (G”/G’) was observed, suggesting unaltered elastic-dominant behavior of the biofilm (Table S3). Complete biofilm eradication was achieved by COS/NO due to its capacity to release greater concentrations of NO into solution than were possible by gaseous delivery.
3.4. Mucolytic action of COS/NO vs gNO
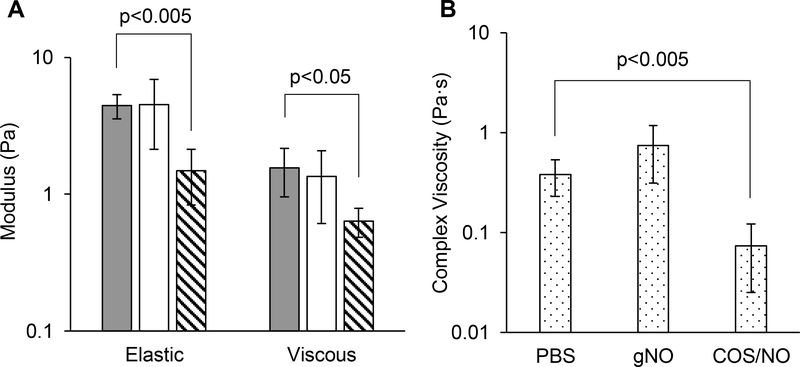
Larger concentrations of NO were measured in 4% wt HBE mucus after exposure to gNO compared to PBS (1.55 and 0.86 μmol/mL, respectively). Treatment with gNO increased the numerical mean of G’ and G”, but not with statistical significance due to high heterogeneity (Fig. 4). In contrast, an equivalent one-time dose of NO (1.55 μmol/mL) delivered by COS/NO (4 mg/mL) decreased G’ and η* by an order of magnitude. As with biofilms, no significant change in tanδ was observed (Table S3). Both G’ and G” increased as a function of frequency (Fig. S3).
Figure 4.
Mucolytic action of NO in 4% wt HBE mucus when delivered via gas or chitosan at equivalent doses. (A) Elastic (G’) and viscous moduli (G”) and of HBE mucus after exposure to PBS (gray), gNO (white), or COS/NO (stripes) at equivalent NO doses for 5 h. (B) Complex viscosity (η*) of HBE mucus after exposure to NO. Data presented was measured at 1% strain and 1 Hz. Error bars represent the standard deviation of the mean for n≥3 separate exposures.
As shown in Fig. 5, no significant change (p>0.1) in G’, G”, or η* was observed in CF sputum exposed to gNO. In contrast, an equivalent NO dose delivered by COS/NO resulted in a significant (p<0.05) decreases in G’, G”, and η*. No significant change in tanδ was observed (Table S3). Both G’ and G” increased as a function of frequency (Fig. S4). Superior mucolytic action was achieved using COS/NO in both HBE mucus and CF sputum relative to gNO at equivalent in-solution concentrations of NO, further demonstrating the utility of COS/NO for CF therapy.
Fig. 5.
Mucolytic action of NO in CF sputum when delivered via gas or chitosan at equivalent doses. (A) Elastic (G’) and viscous (G”) moduli of CF sputum after exposure to PBS (gray), gNO (white), or COS/NO (stripes) at equivalent NO doses for 5 h. (B) Complex viscosity (η*) of CF sputum after NO exposure. Data presented was measured at 1% strain and 1 Hz. Error bars represent the standard deviation of the mean for n≥3 separate exposures.
4. Discussion
The potential of inhaled NO as an antibacterial CF therapy has proven effective in reducing overall bacterial load in clinical trials.[19,28] In response to potential adverse side effects associated with extended exposures at high doses (required for antibacterial activity), water-soluble macromolecular scaffolds that can be nebulized and made to release NO in solution have been developed as an alternative delivery modality.[15,16,26,29] The capacity of NO to eradicate infection, disrupt biofilms, and reduce HBE mucus and CF sputum viscoelastic moduli was assessed as a function of delivery method. By evaluating the antibacterial and mucolytic action of gas versus chitosan NO donor at equivalent exposure conditions and NO solution concentrations, the utility of each system could be compared and assessed with respect to potential therapeutic utility.
The bactericidal action of inhaled NO has previously been reported, including results in clinical trials.[19,28] However, patients were allowed to continue using mucomodulating therapies (i.e., mannitol, Pulmozyme, hypertonic saline)[28] and antibiotics.[19] In this regard, the isolated antibiofilm and mucolytic activity of gNO has yet to be elucidated. We thus set out to directly compare the bactericidal action of gNO and COS/NO in infected lung tissue using an ex vivo porcine model. As shown in Fig. 1, gNO treatment was largely ineffective. Treatment with an equivalent dose of NO (2.30 μmol/mL) from NO donor-modified chitosan (5 mg/mL) resulted in an observable 95% reduction in P. aeruginosa viability. As reported previously, delivery by COS/NO has the advantage of releasing NO into the solution that surrounds the tissue, removing the need for NO gas diffusion from the headspace into solution.[24] Due to its mucoadhesive properties, the COS/NO may associate with the tissue, improving NO delivery locally.[15] The ability of NO to significantly reduce bacterial load in lung tissue progressed in a dose-dependent manner, with a 5-log reduction in viability achieved at 50 mg/mL COS/NO (20.5 μmol/mL NO). Delivery of NO by the chitosan also resulted in improved bactericidal action in the infected porcine lung tissue relative to NO gas exposure, which would require larger NO solution concentrations to achieve antibacterial activity.[22] The ability of COS/NO to deliver greater in-solution concentrations of NO allowed for significant eradication (5-log reduction) of the P. aeruginosa infected tissue.
At equivalent NO doses, the in vitro antibiofilm action of COS/NO also proved superior to that of gNO.[24] Modest bactericidal action (2-log reduction) was elicited by COS/NO at 0.92 μmol/mL NO (p<0.05), while no change in viability (0.15-log increase, p>0.5) was observed using equivalent gNO exposure. Likewise, rheological analysis elucidated a physical impact on biofilm rheology depending on delivery method. While gNO treatment had no effect on biofilm rheology, COS/NO exposure led to significant decreases in both the elastic modulus (G’) and complex viscosity (η*), indicating that the release of NO in solution and chitosan-biofilm association contributed to enhanced disruption compared to gNO. The chitosan NO donor was able to facilitate greater in-solution concentrations of NO (9.2 μmol/mL NO) than gas with complete eradication (5-log reduction) of viable colonies. The viscoelastic moduli of biofilms treated with COS/NO at 20 mg/mL decreased further compared to 5 mg/mL, indicating a likely dose-dependent impact of NO on P. aeruginosa biofilm viability and mechanical integrity (Fig. 3 and Fig. S2). While few general studies concerning biofilm rheology and lung function have been performed, it has been proposed that decreasing biofilm viscoelastic moduli could improve pathogen clearance from the lungs and contribute to improved antibiotic action.[26,27] The ability of COS/NO to decrease the viscoelastic moduli of P. aeruginosa biofilms while simultaneously eradicating colonies suggests that NO-releasing chitosan oligosaccharides may be a superior antibiofilm agent compared to inhaled NO gas.
The mucolytic actions of COS/NO and gNO were compared to further probe the suitability of NO for CF therapy. Prior research has indicated that the mucolytic action of NO is likely the result of breaking the disulfide bonds holding mucins together.[15,16,30] At equivalent NO doses (1.8 μmol/mL), COS/NO decreased HBE mucus G’ and η* by an order of magnitude while gNO-treated HBE mucus proved heterogeneous with no statistically significant effect. In CF sputum, 10 mg/mL COS/NO significantly decreased G’, G”, and η* while gNO had no effect at an equivalent in-solution NO concentration (4 μmol/mL). Of note, no significant change in tanδ (i.e., G”/G’) was measured (Table S3) in either mucus or sputum, suggesting that COS/NO similarly reduced both G’ and G”. The focused delivery of NO made possible by COS/NO may contribute to superior mucolytic action compared to gas.
5. Conclusions
Nitric oxide-releasing chitosan displayed superior antibiofilm and mucolytic activity compared to gaseous NO when delivered at equivalent NO doses. Complete biofilm eradication with concomitant reduction of HBE mucus and CF sputum viscoelastic moduli were measured with NO concentrations that were only achieved using a chitosan donor (9.2–23 μmol/mL NO). The benefit of chitosan delivery is the capacity for large in-solution concentrations of NO in a format (i.e., localized) that should diminish any systemic toxicity, making COS/NO a powerful potential CF therapeutic. Currently, work is being planned to determine the most effective delivery mechanism for COS/NO to deep lung regions to eradicate bacteria within mucus.
Supplementary Material
Highlights.
Delivery of nitric oxide by a chitosan donor allowed for higher in-solution concentrations of nitric oxide compared to gaseous delivery.
Gaseous delivery resulted in solution nitric oxide concentrations that were both medium- and volume-dependent.
Nitric oxide-releasing chitosan was a superior antibiofilm and mucolytic agent over nitric oxide gas.
Localized, in-solution delivery via nitric oxide-releasing chitosan allowed for greater bactericidal action.
Acknowledgements
We thank William Kissner for preparing HBE mucus, and Scott Randell and the UNC Hospitals Pulmonary Specialty Clinic for providing CF sputum. We also thank Camille Ehre for providing pig lungs organs and Cameron Morrison for assisting with dissection. Funding for this research was provided by the Cystic Fibrosis Foundation (Schoen18G0, Hill16XX0, and Bouche15R0), the National Institute of Health (P30DK065988) and KnowBIO, LLC.
Footnotes
Conflicts of Interest
The corresponding author declares competing financial interest. Mark Schoenfisch is a co-founder, member of the board of directors, and maintains a financial interest in Vast Therapeutics and KnowBIO, LLC. Vast Therapeutics is commercializing macromolecular nitric oxide storage and release scaffolds for the treatment of respiratory infections.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Høiby N, et al. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012; 10: 841–51. [DOI] [PubMed] [Google Scholar]
- 2.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007; 261: 5–16. [DOI] [PubMed] [Google Scholar]
- 3.Hill DB, Vasquez PA, Mellnik J, McKinley SA, Vose A, Mu F, et al. A biophysical basis for mucus solids concentration as a candidate biomarker for airways disease. PLoS One. 2014; 9: e87681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill DB, Long RF, Kissner WJ, Atieh E, Garbarine IC, Markovetz MR, et al. Pathological mucus and impaired mucus clearance in cystic fibrosis patients result from increased concentration, not altered pH. Eur Respir J. 2018; 52: 1801297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma JT, Tang C, Kang L, Voynow JA, Rubin BK. Cystic fibrosis sputum rheology correlates with both acute and longitudinal changes in lung function. Chest. 2018; 154: 370–7. [DOI] [PubMed] [Google Scholar]
- 6.Matsui H, Wagner VE, Hill DB, Schwab UE, Rogers TD, Button B, et al. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci U S A. 2006; 103: 18131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esther CR, Muhlebach MS, Ehre C, Hill DB, Wolfgang MC, Kesimer M, et al. Mucus accumulation in the lungs precedes structural changes and infection in children with cystic fibrosis. Sci Transl Med. 2019; 11: eaav3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sriramulu DD, Lünsdorf H, Lam JS, Römling U, Lunsdorf H, Lam JS, et al. Microcolony formation: A novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol. 2005; 54: 667–76. [DOI] [PubMed] [Google Scholar]
- 9.Birket SE, Rowe SM. Revealing the molecular signaling pathways of mucus stasis in cystic fibrosis. J Clin Invest. 2019; 129: 4089–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovach K, Davis-Fields M, Irie Y, Jain K, Doorwar S, Vuong K, et al. Evolutionary adaptations of biofilms infecting cystic fibrosis lungs promote mechanical toughness by adjusting polysaccharide production. npj Biofilms Microbiomes. 2017; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sousa A, Pereira M. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs—a review. Pathogens. 2014; 3: 680–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staudinger BJ, Muller JF, Halldórsson S, Boles B, Angermeyer A, Nguyen D, et al. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2014; 189: 812–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci Transl Med. 2015; 7: 276ra27–276ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin BK. Aerosol medications for treatment of mucus clearance disorders. Respir Care. 2015; 60: 825–32. [DOI] [PubMed] [Google Scholar]
- 15.Reighard KP, Ehre C, Rushton ZL, Ahonen MJR, Hill DB, Schoenfisch MH. Role of nitric oxide-releasing chitosan oligosacchardies on mucus viscoelasticity. ACS Biomater Sci Eng. 2017; 3: 1017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahonen MJR, Hill DB, Schoenfisch MH. Nitric Oxide-Releasing Alginates as Mucolytic Agents. ACS Biomater Sci Eng. 2019. July 8; 5: 3409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salguero KL, Cummings JJ. Inhaled nitric oxide and methemoglobin in full-term infants with persistent pulmonary hypertension of the newborn. Pulm Pharmacol Ther. 2002; 15: 1–5. [DOI] [PubMed] [Google Scholar]
- 18.Quezado ZMN, Natanson C, Karzai W, Danner RL, Koev CA, Fitz Y, et al. Cardiopulmonary effects of inhaled nitric oxide in normal dogs and during E. coli pneumonia and sepsis. J Appl Physiol. 1998; 84: 107–15. [DOI] [PubMed] [Google Scholar]
- 19.Bentur L, Gur M, Ashkenazi M, Livnat-Levanon G, Mizrahi M, Tal A, et al. Pilot study to test inhaled nitric oxide in cystic fibrosis patients with refractory Mycobacterium abscessus lung infection. J Cyst Fibros. 2019; In press. [DOI] [PubMed] [Google Scholar]
- 20.Angus DC, Clermont G, Watson RS, Linde-Zwirble WT, Clark RH, Roberts MS. Cost-effectiveness of inhaled nitric oxide in the treatment of neonatal respiratory failure in the United States. Pediatrics. 2003; 112: 1351–60. [DOI] [PubMed] [Google Scholar]
- 21.Weinberger B The toxicology of inhaled nitric oxide. Toxicol Sci. 2001; 59: 5–16. [DOI] [PubMed] [Google Scholar]
- 22.Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, et al. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano. 2008; 2: 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schairer DO, Chouake JS, Nosanchuk JD, Friedman AJ. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence. 2012; 3: 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall JR, Rouillard KR, Suchyta DJ, Brown MD, Ahonen MJR, Schoenfisch MH. Mode of Nitric Oxide Delivery Affects Antibacterial Action. ACS Biomater Sci Eng. 2020; 6: 433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison F, Muruli A, Higgins S, Diggle SP. Development of an ex vivo porcine lung model for studying growth Virulence, And signaling of pseudomonas aeruginosa. Infect Immun. 2014; 82: 3312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reighard KP, Hill DB, Dixon GA, Worley BV, Schoenfisch MH. Disruption and eradication of P. aeruginosa biofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling. 2015; 31: 775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozenbaum RT, Van Der Mei HC, Woudstra W, De Jong ED, Busscher HJ, van der Mei HC, et al. Role of viscoelasticity in bacterial killing by antimicrobials in differently grown P. aeruginosa biofilms. Antimicrob Agents Chemother. 2019; 63: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deppisch C, Herrmann G, Graepler-Mainka U, Wirtz H, Heyder S, Engel C, et al. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study. Infection. 2016; 44: 513–20. [DOI] [PubMed] [Google Scholar]
- 29.Ahonen MJR, Dorrier JM, Schoenfisch MH. Antibiofilm efficacy of nitric oxide-releasing alginates against cystic fibrosis bacterial pathogens. ACS Infect Dis. 2019; 5: 1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsutsumi N, Itoh T, Ohsawa A. Cleavage of S-S bond by nitric oxide (NO) in the presence of oxygen: A disproportionation reaction of two disulfides. Chem Pharm Bull. 2000; 48: 1524–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.