Abstract
Objective:
To examine the health-related quality-of-life (HRQoL) of persons with opioid use disorder (OUD) seeking treatment in an inpatient detoxification or short-term residential setting; continuing treatment as outpatients.
Methods:
We conducted a secondary analysis of data from a clinical trial (N=508) where participants were randomized to extended-release naltrexone or buprenorphine-naloxone for the prevention of opioid relapse. We used a generalized structural equation regression mixture model to identify associations of HRQoL (EQ-5D) trajectories, including latent characteristics, over the 24-week trial and 36-week follow-up period, among participants who reported HRQoL beyond baseline. This novel framework accounted for baseline and time-varying characteristics, while simultaneously identifying latent classes.
Results:
We identified two subpopulations: HRQoL “pharmacotherapy responsive” (82.3%) and HRQoL “characteristic sensitive” (17.7%). The pharmacotherapy responsive subpopulation was characterized by a shortterm HRQoL improvement and then stable HRQoL over time, and by a positive association between HRQoL and receiving pharmacotherapy in the past 30 days. The characteristic sensitive subpopulation was characterized by an initial improvement in HRQoL with a gradual decline over time, and no significant HRQoL response to pharmacotherapy. HRQoL changes over time in this subpopulation were more influenced by baseline demographic, socioeconomic, and psychosocial characteristics.
Conclusion:
Our findings suggest that while HRQoL may be improved and sustained through targeted efforts to promote use of pharmacotherapy for many persons with OUD, an identifiable subpopulation may require additional services that address socioeconomic and psychosocial issues to achieve HRQoL benefits. Our analysis provides insight for improving individualized care for persons with opioid use disorder seeking treatment.
Keywords: Health-Related Quality-of-life, Opioid Use Disorder, Medications for Opioid Use Disorder, Regression Mixture Modeling, Latent Class Analysis
1. Introduction
Measuring health-related quality of life (HRQoL) represents an opportunity to consider outcomes of opioid use disorder (OUD) treatment that are more person-centered and more relevant to overall health than abstinence alone (Bray et al., 2017). Characterizing subpopulations of persons with OUD can also provide useful insights for developing person-centered approaches to care (Muthén and Muthén, 2000). Recent studies analyzing HRQoL of persons with OUD have utilized latent class, finite or growth mixture models, to identify unobserved subpopulations (i.e., latent classes) whose HRQoL varies over time (De Maeyer et al., 2013; Krebs et al, 2016; Nosyk et al., 2011). These statistical models can also capture variable responses to treatment and evolution of health outcomes in clinical trials (Nagin and Odgers, 2010; Kim et al., 2016). Yet few HRQoL studies of this type have been conducted using data from comparative clinical trials of evidence-based pharmacotherapies for OUD (Nosyk et al., 2011), and, in general, OUD treatment program evaluations rarely collect HRQoL measures even though collecting these measures is encouraged (Bray et al., 2017; Jalali et al., 2020; Murphy and Polsky, 2016).
The NIDA Clinical Trials Network CTN-0051 (X:BOT) trial was a US-based, 8-site, two-arm, open-label, randomized effectiveness trial that compared extended-release naltrexone (XR-NTX) to buprenorphine-naloxone (BUP-NX) for the treatment of OUD over 24 weeks in participants recruited from inpatient or short-term residential OUD treatment programs (Lee et al., 2016; Lee et al., 2018). The opioid relapse rate was significantly higher in the XR-NTX arm among the intent-to-treat sample with this higher relapse rate accounted for by those who failed to initiate treatment. The two pharmacotherapies were equally safe and effective for participants who successfully initiated their assigned medication; i.e., the per-protocol sample (Lee et al., 2018).
Average quality-adjusted life-years (QALYs) gained did not differ significantly between treatment arms among either the intent-to-treat or the per-protocol sample (Murphy et al., 2019). The QALY is a longitudinal measure of effectiveness that combines a person’s HRQoL at a given point in time with the amount of time spent in that health-state (Drummond et al., 2015; Neumann et al., 2017). Despite the lack of a significant difference in average QALYs gained between study arms, questions persist regarding the factors that influenced HRQoL over time among persons seeking or receiving treatment for OUD, and the responsiveness of HRQoL to pharmacotherapy for OUD (Wittenberg et al., 2016). We analyzed self-reported HRQoL data from the X:BOT trial using an innovative latent class analysis to identify clinically relevant characteristics of HRQoL improvement.
2. Methods
2.1. Measures
The outcome measure of interest in our analysis was time-varying HRQoL among all X:BOT participants who were randomized to either XR-NTX or BUP-NX for prevention of OUD relapse (Lee et al., 2016; Lee et al., 2018; Murphy et al., 2019). HRQoL was assessed monthly over the 24-week intervention period, and at the 28- and 36-week follow-up visits using the EuroQol-5D (EQ-5D)-3L, a widely-used, generic, preference-based instrument that measures HRQoL across 5 domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) according to 3 levels: no problems, some problems, and extreme problems (Dolan, 1997; EuroQol, 2017; Richardson et al., 2014; The EuroQol Group, 1990). The participant’s scores from each domain are then mapped to a single health utility index value that represents the general population’s preference for the respondent’s current health state. The EQ-5D-3L health-utility value can range from −0.594 to 1, where 0 represents death, 1 represents perfect health, and values below 0 represent states perceived to be worse than death (a single observation in this study). The Weill Cornell Medical College institutional review board (IRB) approved this health economic analysis; all sites obtained local IRB-approval.
Time-varying indicators of HRQoL included shape parameters of class trajectories (constant, linear trend, and quadratic terms), the pharmacotherapy received for OUD in the past 30 days (XR-NTX, BUP-NX, or none; observed monthly), and binary variables indicating the participant’s baseline self-reported preference for the pharmacotherapy that was received in the past 30 days, operationalized as “preferred”, “indifferent”, or “not preferred”. The pharmacotherapy variables were derived from study case report forms and self-report.
Time-invariant variables included the participant’s baseline HRQoL; demographic, socioeconomic, and psychosocial characteristics; history of intravenous drug use; treatment site fixed effects; and baseline motivation for treatment, measured from 1 to 4 with greater values indicating greater motivation based on a motivation and preference survey instrument used in the X:BOT trial (Lee et al., 2016). Composite measures from the Addiction Severity Index (ASI) comprising baseline medical, employment, alcohol, drug, legal, social, and psychiatric problems, ranging from 0 to 1, were also included in the model, with higher values indicating greater severity (McLellan et al., 1992).
2.2. Statistical Analysis
We identified the number of unobserved latent classes (subpopulations of HRQoL developmental trajectories) among participants offered medication in the X:BOT trial, and estimated the heterogenous associations of time-varying and baseline variables on HRQoL using maximum likelihood estimation in a generalized structural equation regression mixture model (GSE-RM) (Kim et al., 2016). GSE-RM allows for a data-driven approach to identify how an outcome variable evolves over time, given a finite number of classes, by estimating the probability of class assignment and determinants of the outcome variable of interest, by class, simultaneously. In such statistical models, the probability of belonging to a class is continuous instead of discrete. Therefore, participants do not belong to a particular class, but are rather fractional members assigned an estimated probability of membership at each time point. This approach has the advantage of retaining the maximum degrees of freedom in the regressions as opposed to an approach where participants are classified according to time-invariant characteristics prior to sub-regressions being conducted among those classes, sometimes referred to as a “classify-then-analyze” approach (Kamata et al. 2018), that was previously used to evaluate opioid use trajectories among X:BOT trial participants (Ruglass et al., 2019).
The most appropriate mean and variance functions for the GSE-RM were chosen according to established recommendations, and our model accounted for the upper bound of HRQoL index at 1 using censored regression (Glick et al., 2014). Participants with only baseline HRQoL responses available were not included in the model since we were interested in HRQoL trajectories. Our previous work indicated that HRQoL was missing at random (Murphy et al., 2019); therefore, inverse probability weighting was applied to address missing values. Standard errors were clustered at the participant level to account for within-subject correlation across time (Cameron & Miller, 2015). Statistical significance was assessed at the standard 5% level.
No standardized procedure of identifying the optimal number of latent classes in a regression mixture model has been proposed (Nylund et al., 2007); therefore, we developed a decision algorithm synthesized from approaches reported in recently published studies that conducted latent class analyses of HRQoL and patterns of drug use among similar populations (Larance et al., 2014; Dowsey et al., 2015; De Maeyer et al., 2013; Green et al., 2010; Krebs et al., 2011; Nosyk et al., 2011). Details on the selection algorithm can be found in the online supplement, but are briefly described here. Combinations of both the number of latent classes and shape parameters of HRQoL trajectories in GSE-RM models were assessed using parsimony indices; i.e., Akaike’s information criteria (AIC), Bayes information criteria (BIC), Bozdogan’s consistent AIC (CAIC), and the sample-size-adjusted BIC (ABIC) (Tien et al., 2013). The posterior probability of belonging to each latent class was estimated for every observation, and the maximum probability value was used to assign observations to a particular class. The proportion of individuals assigned to each class was then assessed to confirm discovery of class proportion with an a priori threshold of 5% (varied to 10% for sensitivity analysis) to ensure well-defined classes. The quality of the chosen candidate models was then evaluated by posterior probability diagnostics, model validity, and interpretability.
Coefficient estimates from the chosen GSE-RM were compared, and predicted mean HRQoL values were estimated by class and time period, using the method of recycled predictions (Glick et al., 2014). The mean and 95% confidence range of the adjusted predictions were plotted by class to describe trajectory patterns of HRQoL. We also conducted a sensitivity analysis of the pharmacotherapy parameters in the model by excluding participants who failed to initiate treatment. All analyses were performed using Stata, version 16.1 (StataCorp, College Station, TX).
3. Results
Sixty-two participants were dropped from the intent-to-treat sample due to nonresponse on their HRQoL beyond baseline, the primary measure of interest, resulting in a final sample of 508 participants with 2,622 observations over follow-up. Of the included participants, 67% reported 4 or more separate HRQoL assessments beyond baseline (See supplemental Table S1). Participants excluded from the model were similar across the ASI indices except for lower average drug problem severity. Excluded participants also reported higher initial HRQoL values, older age, were more likely to be covered by Medicaid, were less treatment-motivated, and were more likely to be white and female (see Supplemental Table S2). Unadjusted average HRQoL overall and by treatment assignment for participants successfully initiating treatment (per protocol), is plotted in Figure 1, which demonstrates the similarity in HRQoL trajectories by treatment arm. The unadjusted means of HRQoL of all participants who initiated treatment increased by 8.7% in month 1 compared to baseline, increased by approximately 4.0% between months 1–3, and remained stable in months 4–6.
Figure 1.
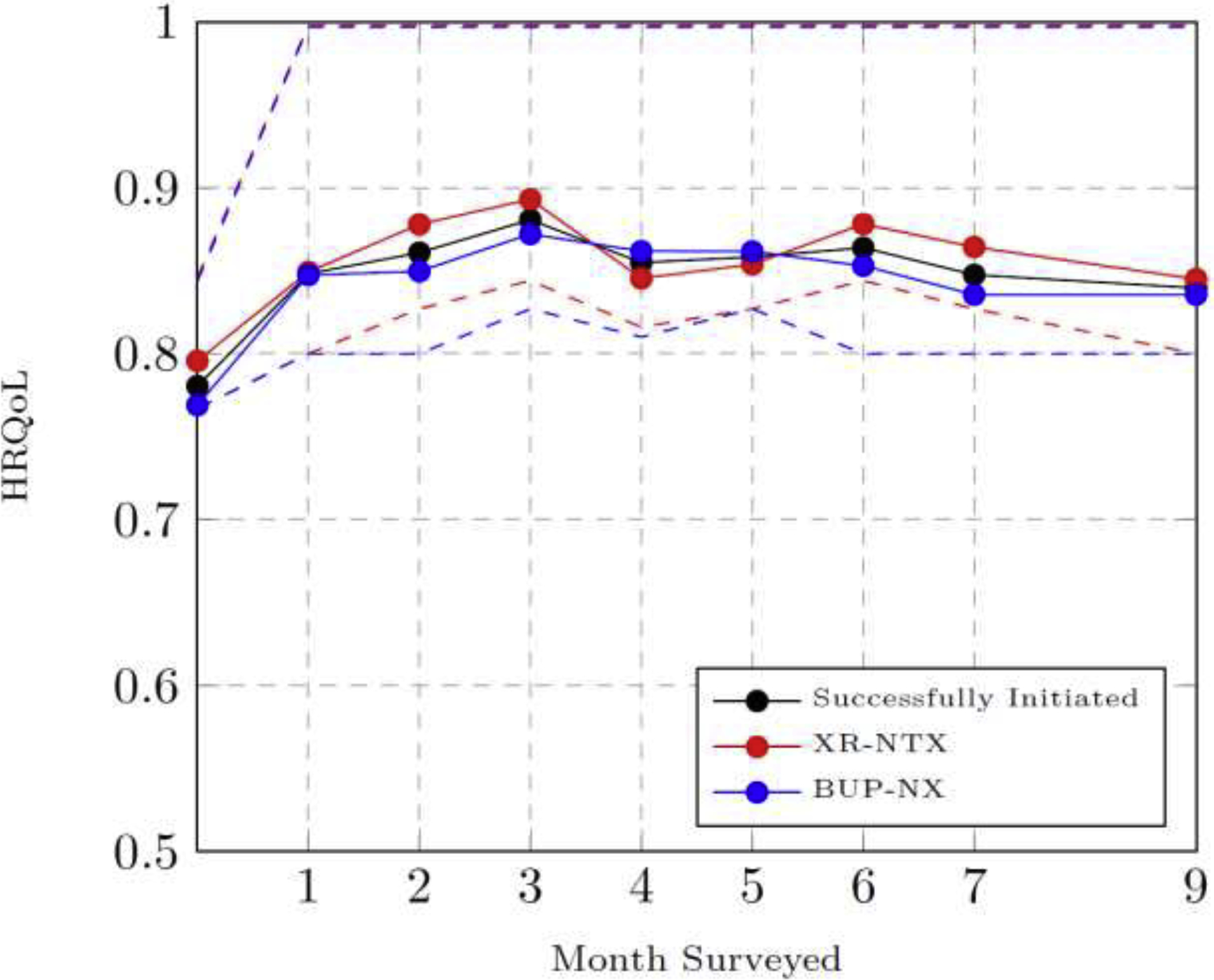
Mean HRQoL Overall and by Treatment Arm Stratified by Successful Initiation
HRQoL = Health Related Quality-of-life
XR-NTX = Extended-Release Naltrexone
BUP-NX = Buprenorphine-Naloxone
Notes: The plot shows unadjusted means of HRQoL at baseline and over the study period for those participants that successfully initiated treatment (per protocol N=474) and also stratified by pharmacotherapy randomization (XR-NTX, N=204; BUP-NX, N=270). Dashed lines indicate the 25th and 75th percentiles. Sample sizes for each observation period are reported in Table S4 in the supplemental appendix.
The quadratic regression mixture model with 2 latent classes was determined to be optimal based on our decision algorithm (see Supplemental Figure S1 and Table S3). Two main differences between the classes were observed. First, HRQoL over time was responsive to pharmacotherapy (XR-NTX or BUP-NX) received in the past 30 days in one class, while no statistically significant relationship was found in the other class. Second, HRQoL in the class that was not responsive to pharmacotherapy was more sensitive to patient characteristics. Therefore, we define the two classes as “pharmacotherapy responsive,” and “characteristic responsive.”
Each participant’s maximum posterior probability was used to assign the participant to one of the classes. According to this method, the majority of the study participants (82.3%) were assigned to the pharmacotherapy responsive class, while the characteristic responsive class represent a minority (17.7%) of trial participants. Table 1 displays characteristics for all participants and by assigned class.
Table 1.
Participant Characteristics
| All n = 2622 |
Characteristic responsive N = 459 |
Pharmacotherapy Responsive N = 2163 |
||
|---|---|---|---|---|
| Average HRQoL | 0.857 | 0.894 | 0.849 | <0.001 |
| Pharmacotherapy received (past 30 days) | ||||
| Buprenorphine-Naloxone | 35.7% | 26.8% | 37.7% | <0.001 |
| Extended-Release Naltrexone | 20.5% | 14.6% | 21.7% | 0.001 |
| Preference for Received Pharmacotherapy | ||||
| Preferred | 16.0% | 11.1% | 17.0% | 0.002 |
| Not Preferred | 12.2% | 9.6% | 12.8% | 0.059 |
| Neutral | 28.0% | 20.7% | 29.6% | <0.001 |
| Baseline Variables | ||||
| Intravenous Drug Use | 63.3% | 60.1% | 64.0% | 0.120 |
| Average Age (years) | 34.5 | 34.6 | 34.4 | 0.755 |
| Female | 31.3% | 37.5% | 30.0% | 0.002 |
| White Race | 75.3% | 82.8% | 73.7% | 0.000 |
| Medicaid | 48.9% | 47.1% | 49.3% | 0.377 |
| High School Education | 79.1% | 73.2% | 80.3% | 0.001 |
| Married | 9.5% | 16.3% | 8.0% | <0.001 |
| Initial HRQoL Value | 0.777 | 0.723 | 0.789 | <0.001 |
| Motivation Index | 3.64 | 3.47 | 3.67 | <0.001 |
| Baseline Addiction Severity Index Measures | ||||
| Medical | 0.176 | 0.226 | 0.165 | <0.001 |
| Employment | 0.672 | 0.670 | 0.672 | 0.917 |
| Alcohol | 0.105 | 0.096 | 0.107 | 0.370 |
| Drug | 0.308 | 0.325 | 0.305 | <0.001 |
| Legal | 0.182 | 0.208 | 0.176 | 0.004 |
| Family/Social | 0.425 | 0.431 | 0.424 | 0.719 |
| Psychiatric | 0.299 | 0.394 | 0.279 | <0.001 |
HRQoL = Health Related Quality-of-life
Notes: Average HRQoL spans months 1 through 9 and does not include the initial baseline HRQoL. All values are based on within-sample observations. Descriptive statistics are based on within-sample observations of the statistical model. Descriptive statistics of excluded participants from the statistical model are reported in Table S3 in the supplemental appendix. Latent class and pooled standard deviations used in performing t-tests are reported in Table S5.
3.1. Class Characteristics
The overall average HRQoL over the study period was 0.857, but was higher for the characteristic responsive class compared to the pharmacotherapy responsive class (0.894 vs 0.849, p < 0.001). The characteristic responsive class had a lower baseline HRQoL (0.723 vs. 0.789, p<0.001). On average, a greater percentage of participants reported receiving BUP-NX than XR-NTX in the past 30 days (35.7% vs. 20.5%). Participants in the pharmacotherapy responsive class were more likely to have received pharmacotherapy in the past 30 days and, conditional on receiving pharmacotherapy in the past 30 days, more likely to have received their pharmacotherapy of choice, or to have been neutral with regard to their medication preference. The characteristic responsive class included a higher proportion of participants who were female, white, and married. Participants in the characteristic responsive class also reported lower average levels of completed education and treatment motivation at baseline compared to the pharmacotherapy responsive class, and these participants also had more severe medical, drug, legal, and psychiatric problems according to the ASI.
3.2. Patterns of HRQoL
Figure 2 plots the adjusted baseline HRQoL and the predicted HRQoL means over time, by class, with 95% confidence levels based on the statistical model. We found an initial improvement in HRQoL among both classes in the first month of treatment. The “characteristic responsive” class is, however, predicted to have stable HRQoL over time, while the pharmacotherapy responsive class exhibits statistically significant improvement in HRQoL within the first three months of treatment and then stable HRQoL over time. Predicted HRQoL values for both classes converge in the follow-up periods, which may be partially due to decline in receipt of either BUP-NX or XR-NTX in the later observations among all trial participants (Murphy et al., 2019).
Figure 2.
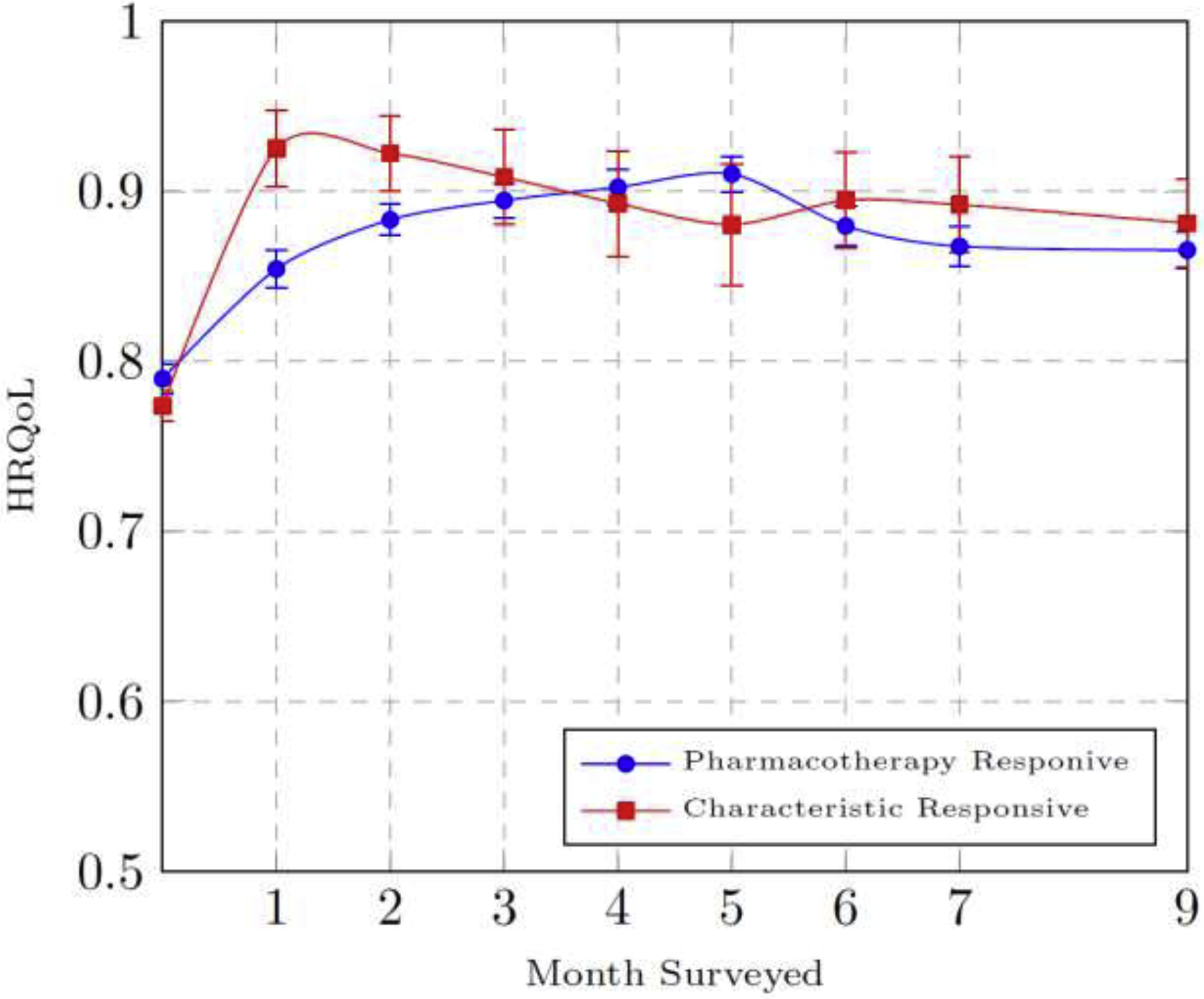
Adjusted Baseline HRQoL and Predicted Means Over Time by Latent Class
HRQoL = Health Related Quality-of-life
Notes: Initial HRQoL was adjusted for baseline variables based on class assignment in month 1. Predicted means of HRQoL in months 1–7, and 9 are derived from the statistical model using the method of recycled predictions.
3.3. Comparison of HRQoL Determinants
We found substantial differences between the two latent classes with regard to statistical associations of HRQoL over the study period (Table 2). As noted above, having received either BUP-NX or XR-NTX in the past 30 days was associated with increasing HRQoL over time for the pharmacotherapy responsive class, while no statistically significant relationship with HRQoL was found among those in the characteristic responsive class. Whether the pharmacotherapy received in the past 30 days was the participant’s preferred medication at baseline, was not associated with greater improvement of HRQoL in either class. Excluding induction failures produced qualitatively similar results for the pharmacotherapy parameters; that is, a statistically significant and positive association between HRQoL and pharmacotherapy in one latent class and no statistical relationship in the other latent class.
Table 2.
Generalized Structural Equation Regression Mixture Model Results of HRQoL
| Characteristic Responsive | Pharmacotherapy Responsive | |||||
|---|---|---|---|---|---|---|
| Variables | β | se | p-value | β | se | p-value |
| Shape Parameters | ||||||
| Constant | 2.642 | 0.847 | 0.002 | 0.820 | 0.102 | <0.001 |
| Linear Trend | −0.055 | 0.048 | 0.251 | 0.018 | 0.008 | 0.039 |
| Quadratic Term | 0.001 | 0.004 | 0.740 | −0.001 | 0.001 | 0.172 |
| Pharmacotherapy received | ||||||
| Buprenorphine-Naloxone | −0.041 | 0.196 | 0.835 | 0.058 | 0.022 | 0.009 |
| Extended-Release Naltrexone | 0.091 | 0.180 | 0.613 | 0.067 | 0.022 | 0.003 |
| Preference for Received Pharmacotherapy | ||||||
| Preferred | −0.307 | 0.167 | 0.066 | −0.021 | 0.023 | 0.363 |
| Neutral | −0.106 | 0.234 | 0.650 | 0.008 | 0.021 | 0.702 |
| Baseline Variables | ||||||
| Intravenous Drug Use | −0.188 | 0.231 | 0.415 | 0.013 | 0.022 | 0.556 |
| Average Age | −0.022 | 0.008 | 0.008 | 0.000 | 0.001 | 0.953 |
| Female | 0.098 | 0.131 | 0.454 | −0.009 | 0.019 | 0.629 |
| White Race | 0.611 | 0.161 | <0.001 | −0.026 | 0.019 | 0.176 |
| Medicaid | 1.323 | 0.191 | <0.001 | −0.039 | 0.019 | 0.040 |
| High School Education | −0.384 | 0.262 | 0.143 | 0.031 | 0.020 | 0.115 |
| Married | 1.132 | 0.125 | <0.001 | −0.066 | 0.040 | 0.099 |
| Initial HRQoL Value | 0.564 | 0.195 | 0.004 | 0.173 | 0.063 | 0.006 |
| Motivation Index | −0.449 | 0.092 | <0.001 | 0.007 | 0.010 | 0.469 |
| Addiction Severity Index Measures | ||||||
| Medical | −0.063 | 0.176 | 0.718 | −0.080 | 0.033 | 0.015 |
| Employment | 0.185 | 0.196 | 0.346 | −0.069 | 0.029 | 0.016 |
| Alcohol | −0.244 | 0.191 | 0.202 | 0.004 | 0.041 | 0.931 |
| Drug | 1.269 | 0.885 | 0.152 | −0.294 | 0.105 | 0.005 |
| Legal | 0.662 | 0.378 | 0.080 | −0.022 | 0.041 | 0.586 |
| Family/Social | −0.656 | 0.228 | 0.004 | 0.037 | 0.030 | 0.229 |
| Psychiatric | −0.183 | 0.038 | <0.001 | −0.157 | 0.045 | 0.001 |
| N (total) | 2,622 | |||||
| N(censored) | 1,508 | |||||
| N (uncensored) | 1,114 | |||||
HRQoL = Health Related Quality-of-life
Notes: Site fixed effects included in model but not reported in table. Standard errors are clustered at the participant level. Pharmacotherapy received is dichotomous and refers to the past 30 days.
Baseline HRQoL was found to have a positive association with HRQoL over time for both the pharmacotherapy responsive and characteristic responsive classes. Medicaid insurance coverage was associated with increased HRQoL over time in the characteristic responsive class, but had a negative (albeit minor) effect on HRQoL over time in the pharmacotherapy responsive class. Being Caucasian, married, and of younger age were all positively associated with HRQoL over time among those in the characteristic responsive class. In addition, we found that higher treatment motivation at baseline was negatively associated with HRQoL over time in the characteristic responsive class.
Nearly all composite measures from the ASI were found to be associated with HRQoL in at least one class. The psychiatric composite measure was negatively associated with HRQoL over time in both classes, but other composite measures had a heterogenous relationship. Medical, employment, and drug problems were all negatively associated with HRQoL over time in the pharmacotherapy responsive class, but not among those in the characteristic responsive class. Greater severity in family/social problems was negatively associated with HRQoL over time in the characteristic responsive class, but not the pharmacotherapy responsive class.
4. Discussion
We examined trajectories and statistical associations of HRQoL among persons seeking or receiving treatment for opioid use disorder using longitudinally-defined latent classes. Defining latent classes using the approach employed in this study, as opposed to the more common “classify-then-analyze” approach, has the advantage of incorporating both baseline and time-varying factors, as well as retaining the maximum degrees of freedom in the statistical model, thereby increasing statistical power.
The largest latent class of participants experienced improvement in HRQoL following receipt of pharmacotherapy, had an HRQoL trajectory that increased initially, and then stabilized over time. The other latent class represented participants whose HRQoL was not significantly associated with pharmacotherapy, but was more heavily influenced by demographic, socioeconomic, and psychosocial characteristics. The pharmacotherapy responsive class consisted of a higher proportion of individuals who had recently received pharmacotherapy, at each time point (see Table S6); combined with the small amount of participant crossover between classes over time (16%), this finding indicates that not only is pharmacotherapy initiation important for improved HRQoL, but also adherence. The characteristic responsive class was at baseline significantly less motivated for treatment and had more severe medical, drug use, legal, and psychiatric issues; increases in their HRQoL were associated with structural and social support issues such as being married, having Medicaid insurance, and having less sever family/social issues reported at baseline. We also found that higher motivation at baseline was negatively associated with HRQoL over time in the characteristic responsive class, but was not significantly associated with the HRQoL trajectory in the pharmacotherapy responsive class.
Nosyk et al. (2011) found differences in HRQoL class characteristics among a per-protocol sample of persons receiving either injectable diacetylmorphine or oral methadone for OUD treatment, including gender, medical, drug use severity, and social variables such as having stable housing (Nosyk et al., 2011). In contrast, we found psychiatric problems at baseline and recent receipt of pharmacotherapy for OUD treatment to be associated with HRQoL trajectories in at least one class. Discontinuation of pharmacotherapy for OUD was not associated with HRQoL in Nosyk (2011); Krebs et al. (2016) found that pharmacotherapy for OUD was not a factor in developmental trajectories of HRQoL, while Nosyk et al. (2015) found that pharmacotherapy had a positive impact on HRQoL across all sub-groups. Our results do not help to clarify these associations as we found pharmacotherapy to have a heterogenous association on HRQoL over time.
Our unadjusted HRQoL and adjusted HRQoL trajectories by class were consistent with HRQoL trajectories reported in Nosyk et al. (2015), which found an initial moderate increase followed by stable or deteriorating HRQoL over time for three of the four sub-groups identified within their analysis. HRQoL trajectories reported in Nosyk et al. (2011) where either consistently high, consistently low, or moderate and increasing. HRQoL trajectories of participants included in the Nosyk et al. (2011) study were less similar to the results reported in this study.
There are several sources of possible variation between our results and the previous studies noted above. First, in contrast to Nosyk et al. (2011; 2015) and Krebs et al. (2016), we statistically defined latent class and estimated model parameters simultaneously in the GSE-RM framework. Nosyk et al. (2015) relied on sub-group selection based on treatment modalities and not based on statistical methods of class assignment. A second difference pertains to data sources. Krebs et al. (2016) analyzed pooled data from multiple cohort studies of persons with OUD who had ever accessed opioid agonist therapy, and Nosyk et al. (2015) analyzed data from two clinical trials that enrolled heroin or prescription opioid dependent persons; our analysis is based on a single clinical trial of BUP-NX versus XR-NTX among persons seeking treatment in an inpatient or residential setting, continuing treatment as outpatients. Finally, we were able to take advantage of more frequently collected HRQoL assessments over time than Nosyk et al. (2015); and more frequently collected HRQoL assessments from a greater number of participants than Nosyk et al. (2011) and Krebs et al. (2016).
Receipt of preferred pharmacotherapy in the past 30 days was not associated with HRQoL in either class; however, it is possible that preferences change as the trial progresses and participants actually begin receiving treatment. The lack of data to account for variation over time in the preference for pharmacotherapy and other baseline measures included in our analysis, such as motivation for treatment, is a limitation. Similarly, receipt of unobserved addiction health services could possibly confound the treatment effect of pharmacotherapy since adherence to pharmacotherapy is likely correlated with adherence to other services. Other limitations include the trial length, which, if it were extended, could allow for greater variation in developmental trajectories to be observed. In addition, trial participants were recruited while seeking treatment in an inpatient or residential facility and their HRQoL trajectories may not be representative of all persons with OUD who are offered pharmacotherapy treatment outside of this setting. Our study did not address the potential for response shift bias or “scale recalibration” in self-reported HRQoL over time (Sprangers and Schwartz, 1999). Further work in integrating response shift in eliciting HRQoL of participants engaged in substance use disorder treatment will allow for a better understanding of the developmental trajectory of HRQoL among this population.
5. Conclusion
This study identified otherwise-unobserved heterogeneity in HRQoL trajectories among participants seeking treatment for OUD in an inpatient or residential setting, continuing treatment as outpatients. Two classes of participants were identified according to both baseline and time-varying factors. Between-class differences in associations of HRQoL over time included response to pharmacotherapy, and demographic, socioeconomic, and psychosocial characteristics.
Prior HRQoL studies of individuals receiving pharmacotherapy for OUD have employed a “classify-then-analyze” approach, where participants are assigned to latent classes that were determined using only baseline time-invariant characteristics, before determinants of HRQoL are assessed by class. We used a GSE-RM framework that accounted for baseline and time-varying characteristics, while simultaneously determining latent classes. Additionally, we were able to account for unique baseline measures such as, treatment motivation and preference for pharmacotherapy type.
Our findings suggest that while HRQoL can be improved and sustained through targeted efforts to promote access and adherence to pharmacotherapy for many persons with OUD, an identifiable subpopulation consisting of younger, less educated women, with more severe medical, substance, and psychiatric problems may require additional services to achieve HRQoL benefits of pharmacotherapy.
Supplementary Material
Highlights:
Health-Related Quality of Life (HRQoL) can be classified into two longitudinal subpopulations.
HRQoL may be improved through the use of pharmacotherapy for many persons with Opioid Use Disorder.
An identifiable minority may require additional services to achieve HRQoL benefits of pharmacotherapy.
Author Disclosures and Acknowledgements
This research was supported in part by the National Institute on Drug Abuse (P30DA040500, R01DA035808, R01DA046721). U10DA013046, UG1/U10DA013035, UG1/U10DA013034, U10DA013045, UG1/U10DA013720, UG1/U10DA013732, UG1/U10DA013714, UG1/U10DA015831, U10DA015833, HHSN271201200017C and HHSN271201500065C from the NIDA National Drug Abuse Treatment Clinical Trials Network; and K24DA022412 (to Dr. Nunes). The authors gratefully acknowledge the NIDA CTN Publications Committee members for their feedback on a previous version of this manuscript. An earlier version of this work was accepted for poster presentation at the 2020 International Society for Pharmacoeconomics and Outcomes Research (cancelled) in Orlando, FL on May 16 – 20, 2020. The content of this article is solely the responsibility of the authors and does not necessarily represent the official view of the funding agency or the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
Dr. Murphy reports having consulted for Sandoz Inc. for work unrelated to the investigation reported here. Ms. Novo reports grants from NIDA during the conduct of the study and outside the submitted work. Dr. Rotrosen reports grants from NIDA/NIH, medication for the present study from Indivior, and medication and/or funds for other studies (as principal investigator or investigator) from Indivior and Alkermes; he serves as a non-paid member of an Alkermes study steering committee. Authors not named here have disclosed no conflicts of interest.
Clinical Trial Registration: NCT02032433
References
- Aden B, Dunning A, Nosyk B, Wittenberg E, Bray JW, Schackman BR, 2015. Impact of Illicit Drug Use on Health-Related Quality of Life in Opioid-Dependent Patients Undergoing HIV Treatment. J Acquir Immune Defic Syndr 70(3), 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangdiwala SI, Bhargava A, O’Connor DP, Robinson TN, Michie S, Murray DM, Stevens J, Belle SH, Templin TN, Pratt CA, 2016. Statistical methodologies to pool across multiple intervention studies. Transl Behav Med 6(2), 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JW, Aden B, Eggman AA, Hellerstein L, Wittenberg E, Nosyk B, … & Schackman BR (2017). Quality of life as an outcome of opioid use disorder treatment: a systematic review. Journal of substance abuse treatment, 76, 88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AC, & Miller DL, 2015. A Practitioner’s Guide to Cluster-Robust Inference. Journal of Human Resources 50(2), 317–372. [Google Scholar]
- De Maeyer J, van Nieuwenhuizen C, Bongers IL, Broekaert E, Vanderplasschen W, 2013. Profiles of quality of life in opiate-dependent individuals after starting methadone treatment: a latent class analysis. Int J Drug Policy 24(4), 342–350. [DOI] [PubMed] [Google Scholar]
- De Maeyer J, Vanderplasschen W, & Broekaert E, 2010. Quality of life among opiate-dependent individuals: A review of the literature. International journal of drug policy, 21(5), 364–380. [DOI] [PubMed] [Google Scholar]
- Dolan P, 1997. Modeling valuations for EuroQol health states. Med Care 35(11), 1095–1108. [DOI] [PubMed] [Google Scholar]
- Dowsey MM, Smith AJ, Choong PFM, 2015. Latent Class Growth Analysis predicts long term pain and function trajectories in total knee arthroplasty: a study of 689 patients. Osteoarthritis Cartilage 23(12), 2141–2149. [DOI] [PubMed] [Google Scholar]
- Drummond M, 2015. Methods for the economic evaluation of health care programmes, Fourth edition. ed. Oxford University Press, Oxford, United Kingdom; New York, NY, USA. [Google Scholar]
- EuroQol, 2017. EQ-5D. https://euroqol.org/. (Accessed February 6 2020).
- Garcia-Sempere A, Hurtado I, Bejarano D, Santa-Ana Y, Rodriguez-Bernal C, Peiro S, Sanfelix-Gimeno G, 2019. Group-based Trajectory Models to Assess Quality of INR Control and its Association with Clinical Outcomes. Med Care 58(4): e23–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick H, Doshi JA, Sonnad SS, Polsky D, 2015. Economic evaluation in clinical trials, Second edition. ed. Oxford University Press, Oxford. [Google Scholar]
- Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, Gordon AJ, Maisto SA, Day NL, Bryant K, Fiellin DA, Justice AC, 2010. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US Veteran cohort. Drug Alcohol Depend 110(3), 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, T.E., 1990. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy 16(3), 199–208. [DOI] [PubMed] [Google Scholar]
- Jalali A, Ryan DA, McCollister KE, Marsch LA, Schackman BR and Murphy SM, 2020. Economic evaluation in the National Drug Abuse Treatment Clinical Trials Network: Past, present, and future. Journal of Substance Abuse Treatment. 2020;112:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata A, Kara Y, Patarapichayatham C, Lan P, 2018. Evaluation of Analysis Approaches for Latent Class Analysis with Auxiliary Linear Growth Model. Front Psychol 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E, Kerr T, Wood E, Nosyk B, 2016. Characterizing Long-Term Health Related Quality of Life Trajectories of Individuals with Opioid Use Disorder. J Subst Abuse Treat 67, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larance B, Carragher N, Mattick RP, Lintzeris N, Ali R, Degenhardt L, 2014. A latent class analysis of self-reported clinical indicators of psychosocial stability and adherence among opioid substitution therapy patients: do stable patients receive more unsupervised doses? Drug Alcohol Depend 142, 46–55. [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, Farkas S, Fishman M, Gauthier P, Hodgkins CC, King J, Lindblad R, Liu D, Matthews AG, May J, Peavy KM, Ross S, Salazar D, Schkolnik P, Shmueli-Blumberg D, Stablein D, Subramaniam G, Rotrosen J, 2018. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet 391(10118), 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JD, Nunes EV, Mpa PN, Bailey GL, Brigham GS, Cohen AJ, Fishman M, Ling W, Lindblad R, Shmueli-Blumberg D, Stablein D, May J, Salazar D, Liu D, Rotrosen J, 2016. NIDA Clinical Trials Network CTN-0051, Extended-Release Naltrexone vs. Buprenorphine for Opioid Treatment (X:BOT): Study design and rationale. Contemp Clin Trials 50, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 9(3), 199–213. [DOI] [PubMed] [Google Scholar]
- Minjung K, Jeroen V, Zsuzsa B, Thomas J, Lee VHM, 2016. Modeling predictors of latent classes in regression mixture models. Struct Equ Modeling 23(4), 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SM, McCollister KE, Leff JA, Yang X, Jeng PJ, Lee JD, Nunes EV, Novo P, Rotrosen J, Schackman BR, 2019. Cost-Effectiveness of Buprenorphine-Naloxone Versus Extended-Release Naltrexone to Prevent Opioid Relapse. Ann Intern Med 170(2), 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen B, Muthen LK, 2000. Integrating person-centered and variable-centered analyses: growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res 24(6), 882–891. [PubMed] [Google Scholar]
- Nagin DS, Odgers CL, 2010. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol 6, 109–138. [DOI] [PubMed] [Google Scholar]
- Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, 2017. Cost effectiveness in health and medicine, Second edition. ed. Oxford University Press, Oxford; New York. [Google Scholar]
- Nosyk B, Bray JW, Wittenberg E, Aden B, Eggman AA, Weiss RD, Potter J, Ang A, Hser YI, Ling W, Schackman BR, 2015. Short term health-related quality of life improvement during opioid agonist treatment. Drug Alcohol Depend 157, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Guh DP, Sun H, Oviedo-Joekes E, Brissette S, Marsh DC, Schechter MT, Anis AH, 2011. Health related quality of life trajectories of patients in opioid substitution treatment. Drug Alcohol Depend 118(2–3), 259–264. [DOI] [PubMed] [Google Scholar]
- Nylund KL, Asparouhov T, & Muthen BO, 2007. Deciding on the Number of Classes In Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study. Structural Equation Modeling A Multidisciplinary Journal, 535–569. [Google Scholar]
- Oviedo-Joekes E, Brissette S, Marsh DC, Lauzon P, Guh D, Anis A, Schechter MT, 2009. Diacetylmorphine versus methadone for the treatment of opioid addiction. N Engl J Med 361(8), 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JRJ, Mckie JR, & Bariola EJ, 2014. Multiattribute utility instruments and their use., Encylopedia of Health Economics, 1st ed. Elsevier, San Diego CA USA. [Google Scholar]
- Ruglass LM, Scodes J, Pavlicova M, Campbell ANC, Fitzpatrick S, Barbosa-Leiker C, Burlew K, Greenfield SF, Rotrosen J, Nunes EV Jr., 2019. Trajectory classes of opioid use among individuals in a randomized controlled trial comparing extended-release naltrexone and buprenorphine-naloxone. Drug Alcohol Depend 205, 107649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprangers MAG, Schhwartz CE, 1999. Integrating response shift into health-related quality of life research: A theoretical model. Soc Sci Med 48(11), 1507–1515. [DOI] [PubMed] [Google Scholar]
- Tein JY, Coxe S, & Cham H, 2013. Statistical power to detect the correct number of classes in latent profile analysis. Structural equation modeling: a multidisciplinary journal, 20(4), 640–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg E, Bray JW, Aden B, Gebremariam A, Nosyk B, & Schackman BR, 2016. Measuring benefits of opioid misuse treatment for economic evaluation: health-related quality of life of opioid-dependent individuals and their spouses as assessed by a sample of the US population. Addiction, 111(4), 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


