1. Introduction
The rising number of older patients with cancer is creating a growing patient population with unique health needs. The geriatric assessment (GA) has been pivotal in uncovering vulnerabilities and providing management recommendations for this population [1]. Both the American Society of Clinical Oncology (ASCO) and the International Society of Geriatric Oncology (SIOG) guidelines recommend performing GA in patients 65 and older with cancer, especially those considering chemotherapy [1, 2]. While there is robust data to support the benefits and need for GA in older patients, widespread implementation in clinical practice is still lacking [3]. However, a feasibility study confirmed that the GA could be completed by patients in a timely manner and mostly without assistance [4]. Furthermore, a recent study outlined the resources (e.g., administrative support, proactive infrastructure planning) required to integrate GA into oncologic practices [5]. One systematic review points out that though GA is recommended by SIOG and National Comprehensive Cancer Network (NCCN), in a public health setting with finite resources, the cost-effectiveness of GA improving oncology outcomes is necessary for it to become standard of care [6]. Evidence suggests GA implementation is feasible, but there remains a lack of studies that assess processes of GA implementation in community oncology practices.
In order to better understand the potential for wider dissemination and implementation of the GA through an electronic platform, we participated in the United States National Cancer Institute’s Speeding Research Testing Interventions Program (SPRINT). The program aims to reduce the burden of cancer through a faster translation of scientific knowledge into clinical application. We participated in this program alongside six other teams of researchers. The program lasted 8 weeks and included two webinars (introduction to dissemination and implementation and course expectations), an in-person introductory meeting, weekly online meetings (discussion of team progress and lessons learned), and an in-person close out meeting (presentations and reflections).
As part of this program, we utilized the customer discovery (CD) approach, commonly used in product commercialization to improve customer satisfaction. This iterative and rapid process involves defining and validating assumptions of customer needs by creating, evaluating, and adjusting hypotheses to ultimately meet marketplace demand [7]. Thamjamrassri et al. previously demonstrated the use of CD while developing a tele-rehabilitation system, and discovered other unique needs and opportunities that ultimately altered the original product idea and value proposition [8]. We herein share our experience participating in the SPRINT program.
2. Customer Discovery Approach and Business Canvas Model
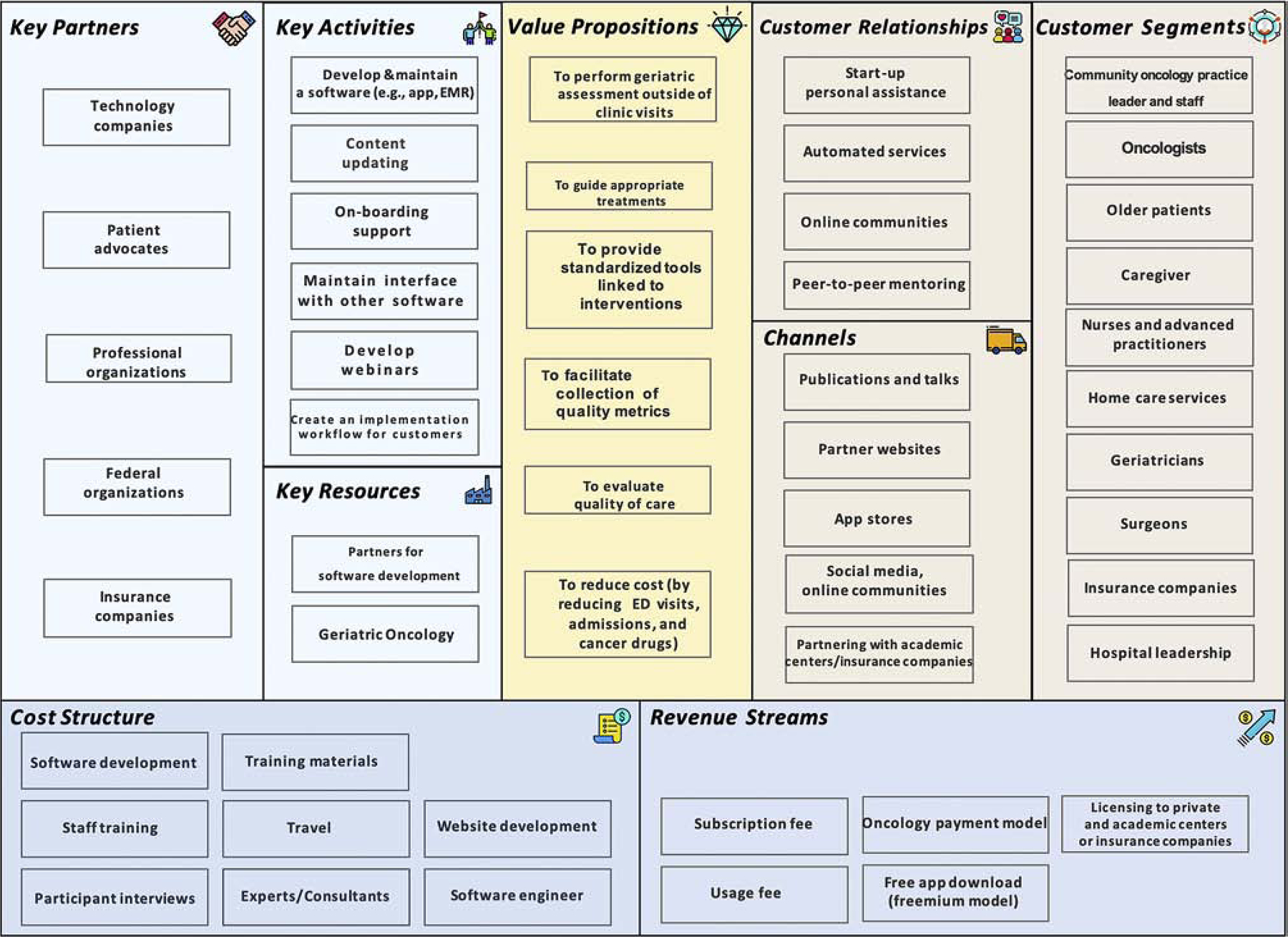
The CD approach includes the following four phases: 1) Stating the hypotheses, 2) Testing the hypotheses, 3) Testing a product concept, and 4) Evaluating customer feedback. Upon completion and analysis, a further round of product development may occur in which the hypotheses are revised, and the four stages are repeated. Another important component of the CD approach is the use of a business canvass model to guide our hypotheses. A business canvas model consists of nine segments that form the building blocks of a business model: 1) Key partners, 2) Key activities, 3) Value propositions, 4) Customer relationship, 5) Customer segment, 6) Key resource, 7) Distribution channel, 8) Cost structure, and 9) Revenue stream. In our case, our business model was the use of an electronic platform to disseminate and implement GA (Table 1). The business model is intended to be dynamic, and change as the hypotheses are altered. Figures 1 and 2 illustrate our business canvas model prior to and at completion of the SPRINT program.
Table 1.
Key learnings from using the customer discovery approach to examine geriatric assessment
| Examples of Hypotheses | Key learnings |
|---|---|
| Oncologists need to be the primary champion for geriatric assessment implementation | Any champion within a practice can implement geriatric assessment |
| Oncologists prefer that interventions (e.g., physical therapy referral) are automatically linked to impairment detected on the assessments |
|
| Time and resources are main barriers for geriatric assessment implementation | Barriers are confirmed; lack of resources varied and included the need for staff, financial support, and infrastructure to implement therapies |
| Health care professionals have difficulty finding information on how to implement the geriatric assessment |
|
| Nurses would find the geriatric assessment information helpful | Nurses are actively monitoring toxixities; information generated from the assessment may help reduce phone calls |
| Payors make the final decision regarding program implementation | Typically, a committee is formed; buy-in from oncologists and other healthcare professionals is needed |
| Payors care about readmission rates as a metric |
|
| Patients are not willing to share technology associated costs | It needs to be paid by the health care system or insurance companies |
| Advocacy groups and governmental agencies are interested in improving outcomes of older adults | Priorities may not be age- or condition-specific; may be based on issues related to patient safety (e.g., increased rate of infection) |
|
|
Figure 1.
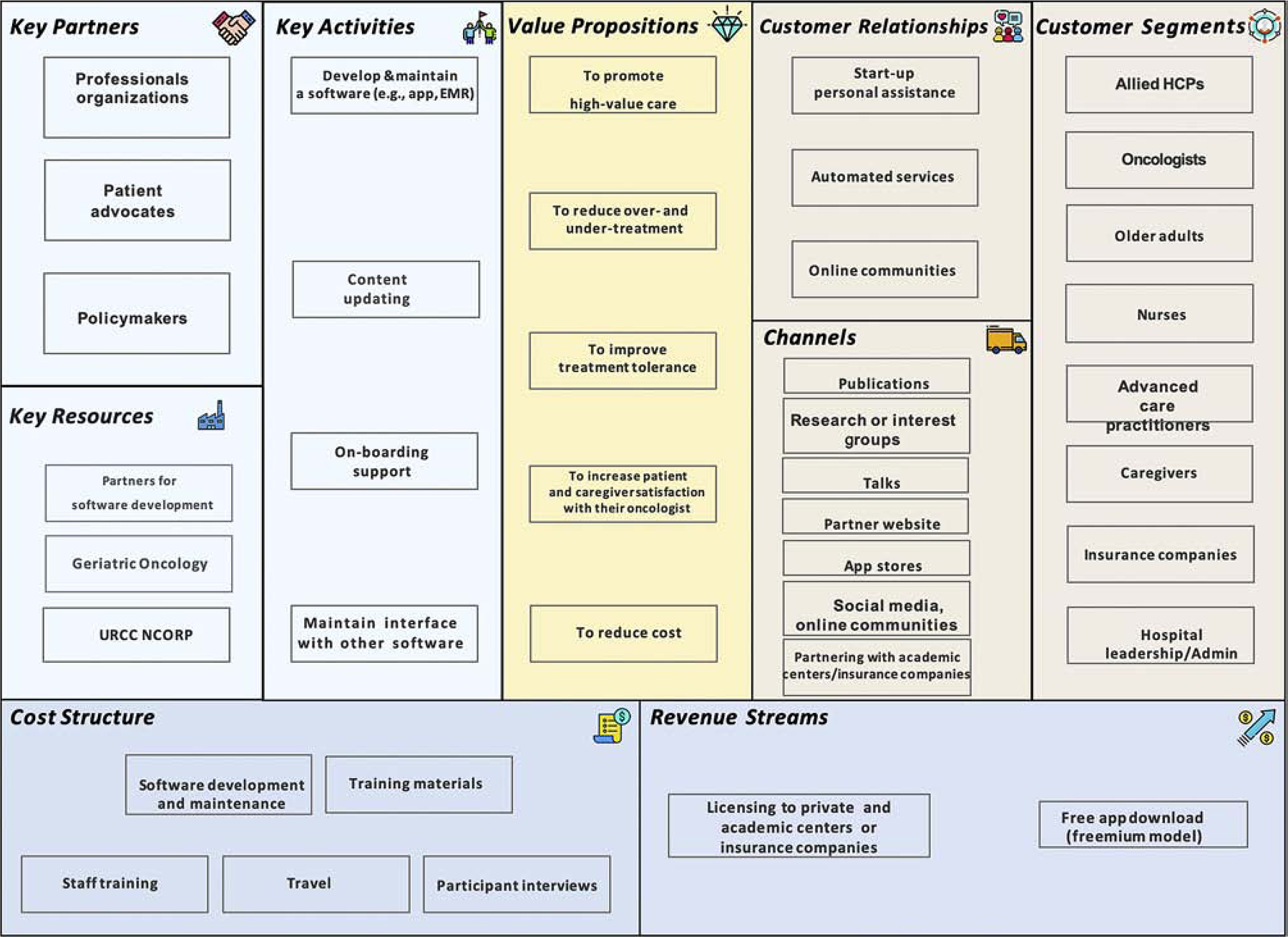
Business Canvas Model before stakeholder interviews
Figure 2. Business Canvas Model after stakeholder interviews.
2.1. Generating Hypotheses
The most important component of the business canvas model is the value proposition. A value proposition is a term used in business world that defines a bundle of benefits that a company can offer its customer segments and guides how businesses organize and frame product development and communication processes with customers and stakeholders [7]. We understood that healthcare professionals are end-users of the GA and their buy-in is crucial. Therefore, they were our initial focus. We hypothesized GA can improve reduce over- and under-treatment and promote patient and caregiver satisfaction with their oncologists. On an institutional level, we hypothesized that GA can promote high-value care and reduce cost. Several of our hypotheses also focused on understanding barriers to implementation of GA. These hypotheses were based on prior studies [9, 10]. For example, we hypothesized that the main barriers of clinical GA implementation would be limited time and resources. In addition, we also tested specific hypotheses about our product concept i.e., electronic platform for GA. For example, we hypothesized that the platform would get more buy-in if it was integrated into the electronic health record.
2.2. Testing the hypotheses
To test our hypotheses, we recruited 49 stakeholders such as community oncologists, nurses, advanced practitioners, patient advocates, mobile application developers, chief executives (insurance companies, hospitals, cancer centers), members of professional organizations, and financial officers. They were recruited through email communications, and interviews were conducted in-person, via telephone, or via video conference. Interviews were intended to be flexible and semi-structured with a focus on stakeholders’ broad views of the unique needs of older patients with cancer and the GA.
As mentioned above, we hypothesized that community oncologists needed to be the primary champion for GA implementation; however, after talking with our stakeholders, it became clear that any member within the clinical practice could champion and implement the GA. Many of the practitioners surveyed believed that there had to be buy-in from all team members for implementation to be feasible and sustainable. One community oncologist shared the following anecdote: “One thing I have noticed is once I actually got buy in from nurses in the clinic is when things started moving a lot faster…I have nurses that are coming to me requesting to do GA on patients that I hadn’t even thought of necessarily doing it on.” Oncologists ultimately preferred to use their time reviewing assessment data rather than performing assessments.
Initially, we had hypothesized that the main barriers of clinical GA implementation would be limited time and resources. We confirmed these hypotheses – these barriers prohibit many community oncologists from integrating GA. Lack of resources greatly varied and included the need for staff, financial support, and infrastructure to implement therapies based on GA results. We originally also believed that health care professionals had difficulty finding information on how to implement the GA. Instead, we discovered that many oncologists are not aware of the literature on how aging related conditions inform outcomes. One community oncologist pointed out, “I think the challenges are hearing the physicians’ reluctance to use the ASCO guideline as it’s intended and hearing that they don’t think that having this score would inform whether they went with a dual therapy or a mono therapy because they think that they would dose reduce anyway if somebody was elderly.”
2.3. Testing a Product Concept
Our product concept was an electronic GA platform where patients can complete questionnaires electronically (via a mobile app or patient portal), and that oncologists and other team members can review this information and provide recommendations. These recommendations may be converted into daily tasks or activities (e.g., if an oncologist recommend a walking exercise program, daily step goal can be generated on the portal). However, before we introduced this product concept, interviewees were asked for potential solutions to problems they may have identified or have proposed. If they were interested in developing or utilizing a GA, we then proposed our product concept. We also inquired specifically about logistics (e.g., how should this be incorporated into the clinical work flow, who should be viewing the questionnaires), support needed for customers (e.g., should we include on-boarding support and develop training webinars), and price (e.g., who will pay for the product?), guided by our business canvas model (specifically the key activities, channels, cost structure, and revenue streams). The customer interviews provided context for the product concept, and shed light on problems and solutions that had not initially been considered.
It was a challenge to decide whether the electronic GA platform should be integrated into electronic medical records (EMR) or function as a standalone application. Our hypothesis was that EMR integration would be laborious during the application development, but would prove to be more user-friendly and more likely to be implemented by health care systems. A stand-alone application would be relatively simple to create, but would be less accessible to users. Stakeholder interviews confirmed our hypotheses. Though we intended to create an application for oncologists, multiple interviewees suggested that a modified electronic GA be used in other specialties to better evaluate an older patient’s status. In addition to product development, further steps needed to be taken to support customer onboarding. This includes developing educational webinars, creating a workflow for customers, and maintaining interface software.
We focused several hypotheses on who and how the electronic GA platform fits into the United States healthcare expenditure, where healthcare coverage is provided through both public health coverage and private health insurance. In addition, there are generally copay and deductible involved. Considering these, we hypothesized that patients are not willing to share technology associated costs. When speaking with patient advocates, while they believe GA is beneficial, they did not want to or did not have the means to financially support the platform. A patient advocate stated, “…it needs to be [free] because it’s not going to be a high priority for things that they have to pay for…but it is very, very important.” The freemium model (basic services are provided free of charge while more advanced features are paid for) was not well-received by patient advocates because of the potential to create a hierarchy and prohibit access to all. We also hypothesized that insurance companies could cover the cost of electronic GA design and implementation. While there is a diagnostic code for GA in the United States, the code is not billable. To that extent, adoption of a GA platform requires a cancer center and practice buy-in, with or without partnership with companies and investors. We hypothesized that investors would only fund health technology that will create profit, but several investors pointed out that initial financial loss is expected with revenue generated a few years late. A potential funder explained, “…we’re not paying for the day to day but for new ideas that may be home runs in the future.” The main priority for one investor was to decrease cancer drug spending, “We’ve got to look at these high-cost patients and see what’s drive it…in oncology it appeared to be high-cost cancer drugs.” This informed our value proposition for the payor. We also learned that that professional organizations may be a potential funder in an initiative that promotes digital health.
It is important to note that the above hypotheses were through solicitation of opinions from stakeholders and not confirmed through conventional scientific experiment. Hypotheses were also focused on the workflow and process in the United States.
2.4. Evaluation of stakeholder feedback
Our team had weekly conference calls with the instructors from the SPRINT program. During these calls, we discussed lessons learned and feedback obtained from our customers during steps 2 and 3. Based on this iterative process, we altered our original hypotheses and repeated the steps above. For example, when we determined that patients and insurance companies would not be able cover the cost of GA adoption, we revised our hypothesis (i.e., To adopt GA, cancer centers and practices would need to bear the cost to allow successful implementation.)
4. Conclusions
Our team’s experience with the CD process to assess our product, an electronic GA, revealed the observations and understandings of our customers. We modified and applied business principles including CD while thinking about GA dissemination. The process was efficient where hypotheses were frequently modified and tested. Based on conversations with stakeholders, we revised our original business canvas model and identified new challenges. We have also summarized key learning from using the CD approach to examining GA (Table 1).
There have been increasing efforts to translate research-tested interventions to clinical practice at a more rapid pace to improve outcomes. CD approach in the business world is akin to Dissemination and Implementation Science in the biomedical field. Researchers may consider using the CD approach when developing and implementing interventions, compared to the traditional medical research model of several phase, multi-year study. There are limitations associated with the use of the CD approach. The opinions provided are dependent on the number, types, and diversity of stakeholders. Like qualitative study, “generalizability” and “theoretical saturation” are important considerations. While this is an established approach in the business world, its use the healthcare setting needs to be further investigated and validated.
Through the NCI SPRINT program, which focuses on cancer prevention and control interventions, we have gained important insights specifically on how not only to design an ideal customer focused tool, but also on how to involve stakeholders based on their priorities and value propositions. This information will guide our future efforts in implementing and disseminating GA.
Financial Support:
The work was funded through US National Cancer Institute (NCI) SPeeding Research-tested INTerventions. The SPRINT curriculum is designed to educate interventionists on how to transform and idea into a market-ready product. Dr. Loh is supported by the Wilmot Research Fellowship Award and National Cancer Institute (K99CA237744). Dr. Mohile is supported by the National Institute of Aging (K24 AG056589).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures and Conflict of Interest Statements:
Dr. Loh has served as a consultant to Pfizer and Seattle Genetics. All other authors have declared no conflicts of interest.
Prior presentation: The abstract was presented at the 2020 International Society of Geriatric Oncology Annual Meeting.
References
- 1.Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, et al. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(22):2326–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(24):2595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohile SG, Magnuson A, Pandya C, Velarde C, Duberstein P, Hurria A, et al. Community Oncologists’ Decision-Making for Treatment of Older Patients With Cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2018;16(3):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCleary NJ, Wigler D, Berry D, Sato K, Abrams T, Chan J, et al. Feasibility of computer-based self-administered cancer-specific geriatric assessment in older patients with gastrointestinal malignancy. The oncologist. 2013;18(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Overcash J, Ford N, Kress E, Ubbing C, Williams N. Comprehensive Geriatric Assessment as a Versatile Tool to Enhance the Care of the Older Person Diagnosed with Cancer. Geriatrics (Basel, Switzerland). 2019;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puts MT, Hardt J, Monette J, Girre V, Springall E, Alibhai SM. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst. 2012;104(15):1133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osterwalder Alexander, et al. Business Model Generation: a Handbook for Visionaries, Game Changers, and Challengers. Wiley, 2010. [Google Scholar]
- 8.Thamjamrassri P, Song Y, Tak J, Kang H, Kong HJ, Hong J. Customer Discovery as the First Essential Step for Successful Health Information Technology System Development. Healthcare informatics research. 2018;24(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenis C, Heeren P, Decoster L, Van Puyvelde K, Conings G, Cornelis F, et al. A Belgian Survey on Geriatric Assessment in Oncology Focusing on Large-Scale Implementation and Related Barriers and Facilitators. The journal of nutrition, health & aging. 2016;20(1):60–70. [DOI] [PubMed] [Google Scholar]
- 10.Ramsdale E, Arastu A, Narlock G, et al. “People are shocked and then they start using it”: Oncology Providers’ Perceived Barriers to Implementation of the Geriatric Assessment. Journal of Geriatric Oncology 2018; 9(6): S123–S4. [Google Scholar]


