Abstract
Adverse childhood experiences (ACE) are associated with greater neuroendocrine responses to social stress in substance users. The neuropeptide oxytocin might attenuate this relationship. Given sex differences in ACE exposure and neuroendocrine stress reactivity, it is unknown whether this association is similar for males and females. Therefore, this secondary analysis evaluated the interactive effect of sex, ACE, and acute oxytocin administration on neuroendocrine stress responses in adult cigarette smokers (N=144). Participants completed the Adverse Childhood Experiences Questionnaire at screening and were randomized to receive intranasal oxytocin or placebo before undergoing the Trier Social Stress Task (TSST). Cortisol levels were assessed at pre- and post-medication administration and at 20 and 40 minutes following the TSST. Generalized linear mixed models were developed to predict post-TSST cortisol levels. Predictors included treatment assignment (placebo vs. oxytocin), sex (male vs. female), ACE (0-10 total score), pre-medication cortisol levels, and minutes since medication administration. The hypothesized three-way interaction between sex, oxytocin, and ACE scores was significant. Linear associations between ACE scores and cortisol reactivity indicated higher ACE scores were associated with attenuated cortisol response in females, regardless of treatment condition. For males, higher ACE scores were associated with heightened cortisol response, an effect that was attenuated by oxytocin. Results indicate that the association between ACE and neuroendocrine reactivity to social stress, as well as the attenuating effect of oxytocin, is differentially impacted by sex. Males with greater childhood adversity may be more likely to benefit from oxytocin’s anxiolytic properties.
Keywords: Adverse childhood experiences, Intranasal oxytocin, Social stress reactivity, Sex differences, Smoking
1. Introduction
Adverse childhood experiences (ACE) are a risk factor for both lifetime and current smoking status (Hughes et al., 2017), as well as early smoking initiation and heavy cigarette consumption in adulthood (Anda et al., 1999). Disturbances in hypothalamic-pituitary-adrenal (HPA) axis regulation may serve as a mechanistic link between adverse childhood experiences and smoking. More specifically, adults with histories of ACE have greater HPA responses to stressors (Danese & McEwen, 2012), and neuroendocrine stress-reactivity has been shown to predict smoking relapse (McKee et al., 2011). However, there are known sex differences in rates of exposure to ACE, HPA axis activity, and stress-related smoking. Compared to men, women are more likely to be exposed to ACE, and, once exposed, are at higher risk for developing mental health and substance use disorders in adulthood (Cavanaugh et al., 2015). Although smokers generally demonstrate blunted neuroendocrine reactivity to acute stress compared to non-smokers (Ginty et al., 2014), this effect may be more pronounced in female smokers than in male smokers and non-smokers (Back et al., 2008). Female smokers may also be more likely to crave cigarettes in response to acute stress (Saladin et al., 2012). Therefore, sex differences in ACE and neuroendocrine responses may play a role in smoking maintenance and relapse.
Oxytocin administration has been shown to attenuate neuroendocrine reactivity to stress in some samples and is thus considered as a potential pharmacotherapy for substance use disorders (Lee & Weerts, 2016). Oxytocin acts on stress-specific pathways, such as the HPA axis, and may normalize both blunted and heightened neuroendocrine responses to stress (Lee & Weerts, 2016). However, the oxytocin literature has also demonstrated important individual and contextual variants (such as ACE) influencing its behavioral and neurobiological effects (Shamay-Tsoory & Abu-Akel, 2016), which may have contributed to mixed findings regarding oxytocin’s ability to reduce cigarette craving (McClure et al., 2020; Miller et al., 2016). Preliminary evidence suggests that oxytocin may attenuate the relations among ACE, stress-reactivity, and substance use. In a study of predominantly male cocaine users, a higher number of ACE was positively associated with greater neuroendocrine reactivity following a stressor, and this relation was attenuated by oxytocin administration (Flanagan et al., 2015). Given the notable sex differences in oxytocin outcomes, ACE, HPA axis reactivity, and stress-related smoking, it is unknown whether oxytocin attenuates neuroendocrine reactivity to stressors similarly for male and female smokers.
Thus, the overall aim of this exploratory analysis is to examine if the interaction between ACE and acute oxytocin administration differentially predicts neuroendocrine stress responses for male and female smokers. For female smokers, ACE were expected to negatively correlate with neuroendocrine reactivity in the placebo group (Hypothesis #1); this effect would be attenuated (i.e., a more pronounced cortisol response) in the oxytocin group (Hypothesis #2) (Back et al., 2008). We hypothesized that for male smokers ACE would be positively correlated with neuroendocrine reactivity in the placebo group (Hypothesis #3), and that this effect would be attenuated in the oxytocin group (Hypothesis #4) (Flanagan et al., 2015).
2. Material and methods
2.1. Participants
Male (n= 53) and female (n= 91) adult cigarette smokers were recruited from the community, and eligible if they were between 18 – 45 years old, smoked an average of ≥ 5 cigarettes/day for at least the past 6 months and submitted a breath carbon monoxide (CO) sample at screening of at least 5 parts per million (ppm). Because of the aims of the parent project (McClure et al., 2020), female participants (over-recruited 2:1) were required to have regular menstrual cycles and could not be on hormonal contraceptives. Individuals were excluded from study participation if they met criteria for past-month posttraumatic stress disorder (PTSD).
Written informed consent was obtained prior to study participation. All procedures were approved by the Institutional Review Board at the Medical University of South Carolina. Other detailed study and participant information is described elsewhere (McClure et al., 2020; Tomko et al., 2020). The trial was registered on clinicaltrials.gov (NCT 01576874).
2.2. Procedures
This study was part of a larger project involving a screening visit, a 14-day cue reactivity ecological momentary assessment (CREMA) monitoring phase (Tomko et al., 2020), and an in-person laboratory visit scheduled for the 15th day of participation (McClure et al., 2020). Results herein are based on this lab visit, preceded by overnight abstinence and verified via breath CO.
Upon arrival to the lab visit and following collection of salivary cortisol, participants were administered an intranasal dose of 40 IUs of oxytocin or matching placebo (between-subjects design) nasal spray under research staff supervision at approximately 12:00pm. Oxytocin dosing was based on previous work using similar doses of 40 IUs in human laboratory studies (Ditzen et al., 2009; McRae-Clark, Baker, Maria, & Brady, 2013). The social stressor used in this study was the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993). The TSST began at approximately 12:40pm and included preparation for a speech (5 minutes), public speaking (5 minutes), and a mental arithmetic (5 minutes) tasks that were completed in front of an audience of three confederates unknown to the participant. Post-medication cortisol levels were assessed ten minutes prior to the start of the TSST (TSST-10), concurrent with TSST (TSST+0), and again at +20 and +40 minutes following the TSST (TSST+20, TSST+40). Although not the focus of the present study, a smoking resistance task began immediately following completion of the TSST and overlapped with collection of cortisol samples. Participants were given the option to smoke during the task but received financial incentives to delay smoking. A figure depicting study procedures during the lab visit relevant to the current analyses is included in the supplemental materials.
2.3. Measures
2.3.1. Screening Assessments
Standard demographic, medical and psychiatric histories, previous smoking history, and current smoking patterns were gathered at the screening visit (McClure et al., 2020).1
2.3.2. Self-Report Measures
The Adverse Childhood Experiences (ACE) Survey is a 10-item questionnaire used to assess experiences of childhood abuse (emotional, physical, sexual), neglect (emotional and physical), and exposure to household dysfunction (divorce, intimate partner violence, substance use, mental illness, incarceration; Felitti et al., 1998). Participants endorse (yes/no) any exposure to each item. The total ACE score reflects a sum of all items endorsed (range 0-10; Cronbach’s α= 0.78). ACE scores were then dichotomized for post hoc analyses (0 = no items endorsed or ACE- and 1 = one or more items endorsed or ACE+).
2.3.3. Neuroendocrine Assays
Cortisol levels (ug/dl) were assessed via passive drool salivary samples collected at five time points: pre-medication and surrounding TSST administration (−10, 0, +20, +40 minutes).
2.4. Data Analytic Plan
Descriptive statistics were used to summarize baseline sample demographic and clinical characteristics. Group differences in continuous characteristics were assessed using a Wilcoxon Rank-Sum test and differences in categorical characteristics were assessed using Pearson chi-square tests. To assess the associations between individual baseline characteristics (sex, treatment condition, ACE) and post-medication cortisol levels, longitudinal generalized linear mixed effect regression models (GLMM) were developed; characteristics associated with the outcome were considered as initial covariates in adjusted model development. A GLMM was used to test the primary aim (Hypotheses #1-4) that oxytocin moderates the relationship between ACE and cortisol response differentially by sex. Predictors in initial models included main effects of sex (male=1, female=0), treatment condition (oxytocin=1, placebo=0), total ACE score, study visit timing (TSST-10, TSST+0, TSST+20, TSST+40), pre-medication cortisol level, and all relevant interactions. Non-significant higher-order interactions were dropped from the model. GLMMs can account for the fact that the data are nested within individuals (i.e., multiple time points per individual) and that individuals had varying numbers of observations. Residual Maximum Likelihood was used for variance estimation and an unstructured covariance was used as determined by best fit (versus Toeplitz, compound symmetry, or autoregressive, as determined by Akaike’s information criterion). Cortisol was right-skewed and, therefore, was log10-transformed to ensure model assumptions of normally distributed residuals and homoscedasticity were met. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA). Study data collection and management were performed via REDCap electronic data capture platform (Harris et al., 2009). Significance was assessed at α = .05 and adjustments for multiple testing were not applied to the reported p-values. All presented parameter estimates are unstandardized.
If a participant smoked a cigarette following the TSST and before the TSST+40 time point, subsequent data points from that participant were excluded (11.5% of all data points) as to not confound smoking and TSST response (McClure et al., 2020).
3. Results
3.1. Participants
Participants included 144 daily smokers (Mage=31.0; SDage=7.4; 63.2% female). Detailed information regarding differences in demographic, smoking, and clinical characteristics by medication assignment and sex are included in the supplemental materials and summarized elsewhere (McClure et al., 2020). Briefly, more females than males in the oxytocin condition reported their race as white. Females who received oxytocin also reported a longer history of regular smoking than males. In the placebo condition, males reported fewer ACE and demonstrated higher baseline cortisol levels than females. ACE scores and baseline cortisol levels and did not significantly differ for males and females in the oxytocin condition. Condition and sex differences were not observed past-month average cigarettes per day.
3.2. Univariate Associations of Sex, Oxytocin, and Adverse Childhood Experiences on Cortisol Response
Cortisol levels significantly increased following completion of the TSST (TSST+0: b= −0.01, SE= 0.02, t= −0.49, p= .63; TSST+20: b= 0.06, SE= 0.02, t= 3.94, p< .001; TSST+40: b= 0.08, SE= 0.01, t= 6.14, p< .001). Consistent with McClure et al. (2020), cortisol responses following the TSST significantly differed by sex (b= −0.09, SE= 0.03, t= −3.38, p= .001), with males demonstrating higher post-TSST cortisol levels than females when controlling for pre-medication cortisol levels. Cortisol reactivity post-TSST did not differ by medication administration (b= −0.01, SE= 0.03, t= −0.42, p= .68) and was not associated with ACE scores (b= −0.01, SE= 0.01, t= −1.63, p= .11).2
3.3. Interactive Effect of Sex, Oxytocin, and ACE on Cortisol Response
The overarching hypothesis that oxytocin moderates the relationship between ACE and cortisol response differentially by sex was tested using a three-way interaction (sex, treatment condition, and ACE). The interaction term was significantly associated with post-TSST cortisol reactivity (b= −0.07, SE= 0.03, t= −2.14, p= .03). To assist in visualization of the interaction, linear associations between ACE scores and model-predicted, time naïve cortisol responses are shown in Figure 1A for males and 1B for females. For females, higher ACE scores were associated with attenuated cortisol response to the TSST, regardless of treatment condition (Hypotheses #1, 2). The pattern for males was in the opposite direction; higher ACE scores were associated with heightened cortisol response, particularly in the placebo group (Hypothesis #3). This effect was attenuated among males randomized to the oxytocin group (Hypothesis #4). No other higher-order interactions were statistically significant.
Figure 1.
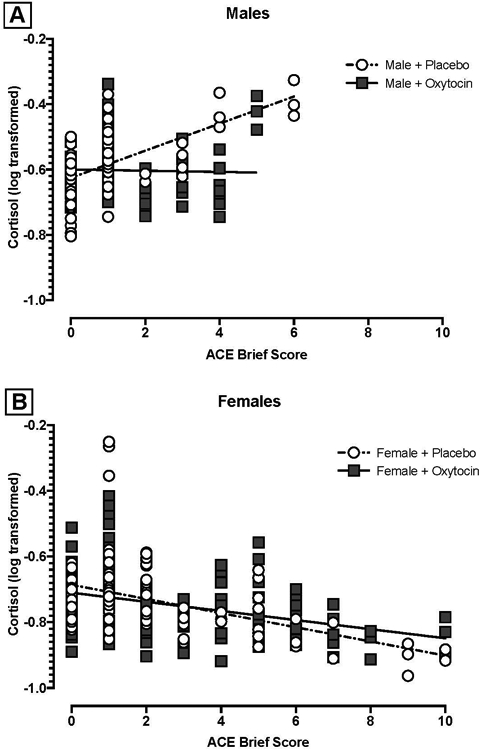
ACE Score and Post-TSST Cortisol Response by Treatment Condition for Males (Panel A) and Females (Panel B)
To further aid in interpretation of the three-way association among sex, treatment condition, and ACE on post-TSST cortisol reactivity, we conducted a separate post-hoc model with ACE score as a binary predictor (0 vs. ≥1 items endorsed, see above). Figure 2 shows the model adjusted means for males (Panel A) and females (Panel B) with all higher-order interaction terms between sex, ACE score, treatment, and time in the model. Though the three-way and four-way higher-order interactions were not significant in the model with dichotomized ACE scores (ACE−/+; all ps> 0.20), at TSST+20 and TSST+40 there was a trend such that males with ACE exposure (ACE+) showed attenuated cortisol response when administered oxytocin relative to placebo (TSST+20: b=0.16, SE= 0.09, t= 1.78, p= 0.08; TSST+40: b= 0.15, SE= 0.08, t= 1.97, p= 0.051).
Figure 2.
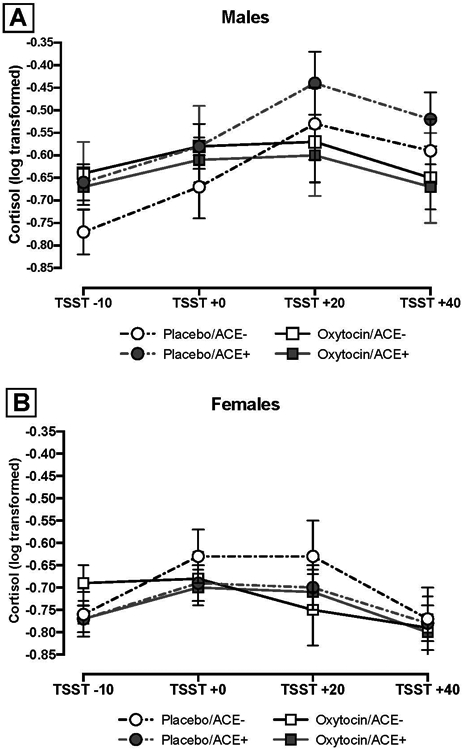
ACE Exposure and Cortisol Response by Treatment Condition for Males (Panel A) and Females (Panel B)
4. Discussion
This exploratory analysis examined if the relation between ACE and oxytocin administration differentially predicted neuroendocrine stress responses for male and female smokers. In line with findings from past studies (Gilmore et al., 2019; Liu et al., 2017), our results suggest that males demonstrated higher post-TSST cortisol levels than females. Further, greater ACE were related to reduced neuroendocrine reactivity in female smokers and increased reactivity in male smokers, consistent with our hypotheses and prior literature (Back et al., 2008; Flanagan et al., 2015). Oxytocin’s attenuating effect on neuroendocrine reactivity was only evident for male smokers and was more pronounced among males with higher ACE scores. A similar pattern of results has been demonstrated for oxytocin’s effect on drug cue reactivity among cocaine-dependent men with childhood trauma exposure (Joseph et al., 2020).
The current findings have important clinical implications for the use of oxytocin as a potential pharmacotherapy for tobacco use. Individuals with specific vulnerability factors (i.e., male smokers with ACE) that alter neurobiological processes and increase neuroendocrine reactivity to acute stress may benefit from oxytocin administration. Moreover, discrepant findings from past studies on oxytocin’s effects on cigarette craving and smoking may have been related to sex differences in neuroendocrine responses and/or variability in responses due to unmeasured adversity histories (McClure et al., 2020; Miller et al., 2016). Future research on oxytocin’s effectiveness as a potential pharmacotherapy for smoking and other substance use disorders should consider the differential influence of sex and ACE (Shamay-Tsoory & Abu-Akel, 2016).
Although this study used a double-blind, placebo-controlled design and was adequately powered to examine sex differences (McClure et al., 2020), a notable limitation to the current analysis is that current PTSD diagnoses were an exclusionary criterion and only 8.3% of the participants met criteria for lifetime PTSD, potentially limiting ACE score variability in our sample. Future studies are needed to extend these findings to an adversity-exposed sample with PTSD symptoms or other forms of ACE-related psychopathology. Moreover, future studies should consider evaluating the relations among sex, ACE, and oxytocin on other markers of neuroendocrine reactivity (e.g., dehydroepiandrosterone, adrenocorticotropic hormone), as well as physiological, subjective, and behavioral responses to stress in smokers.
5. Conclusions
The present study contributes to the existing literature by indicating that the relationship between ACE and smokers’ neuroendocrine reactivity to social stress, as well as the effects of oxytocin, is differentially impacted by sex. Although preliminary, results suggest that males with greater ACE may be more likely to benefit from oxytocin administration.
Supplementary Material
Acknowledgments:
We would like to thank the study participants for their time and effort in completing study procedures. We would also like to thank the medical and research staff at the Medical University of South Carolina; specifically, Lori Ann Ueberroth, Laruen Beech, Jessica Hinton, Jaclyn Condo, Patrick Cato, Casy Johnson, Christine Horne, Danielle Paquette, Priscilla Muldrow, Elhaam Borhanian and Elizabeth Kryway.
Funding: This work was supported by National Institutes of Health grants from the National Institute on Drug Abuse (NIDA P50DA016511) and National Center for Advancing Translational Sciences (NCATS UL1TR001450). Effort on this project was provided by grants from the National Institute of Drug Abuse (NIDA U01DA031779, NIDA R01DA042114, NIDA K01DA036739, NIDA T32DA035200, NIDA R25DA020537), the National Institute on Alcohol Abuse and Alcoholism (NIAAA K23AA023845), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD K12HD055885).
Footnotes
Disclosures: KMG and MJC have provided consultation for Pfizer, Inc. All other authors have no disclosures to declare.
As described in McClure et al., 2020, the MINI International Neuropsychiatric Interview (Sheehan et al., 1998) was used at screening to assess psychiatric disorders per DSM-IV criteria. Twenty individuals were excluded from participation due to meeting DSM-IV criteria for serious or unstable medical or psychiatric disorders that, in the judgment of the study physician, may have interfered with study completion.
In addition to cortisol responses, subjective measures of self-reported stress and craving were assessed throughout the lab visit (McClure et al., 2020). When examined by treatment condition, sex differences in the oxytocin and placebo groups failed to achieve statistical significance for both self-reported craving (p= .49) and stress (p= .30) following the TSST.
References
- Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, & Giovino GA (1999). Adverse childhood experiences and smoking during adolescence and adulthood. JAMA, 282, 1652–1658. [DOI] [PubMed] [Google Scholar]
- Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, … Brady KT (2008). Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology, 33, 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh CE, Petras H, & Martins SS (2015). Gender-specific profiles of adverse childhood experiences, past year mental and substance use disorders, and their associations among a national sample of adults in the United States. Social Psychiatry and Psychiatric Epidemiology, 50, 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106, 29–39. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, & Heinrichs M (2009). Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry, 65, 728–731. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. American Journal of Preventive Medicine, 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Flanagan JC, Baker NL, McRae-Clark AL, Brady KT, & Moran-Santa Maria MM (2015). Effects of adverse childhood experiences on the association between intranasal oxytocin and social stress reactivity among individuals with cocaine dependence. Psychiatry Research, 229, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AK, Guille C, Baker NL, Brady KT, Hahn CK, Davis CM, … & Back SE (2019). Gender differences in subjective stress and neuroendocrine response to a stress task among individuals with opioid dependence: A pilot study. Addictive Behaviors, 92, 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Jones A, Carroll D, Roseboom TJ, Phillips AC, Painter R, & de Rooij SR (2014). Neuroendocrine and cardiovascular reactions to acute psychological stress are attenuated in smokers. Psychoneuroendocrinology, 48, 87–97. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, … Dunne MP (2017). The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health, 2, e356–e366. [DOI] [PubMed] [Google Scholar]
- Joseph JE, McRae-Clark A, Sherman BJ, Baker NL, Moran-Santa Maria M, & Brady KT (2020). Neural correlates of oxytocin and cue reactivity in cocaine-dependent men and women with and without childhood trauma. Psychopharmacology, 237, 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, & Hellhammer DH (1993). The ‘Trier Social Stress Test’ – A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Lee MR, & Weerts EM (2016). Oxytocin for the treatment of drug and alcohol use disorders. Behavioural Pharmacology, 27, 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Ein N, Peck K, Huang V, Pruessner JC, & Vickers K (2017). Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology, 82, 26–37. [DOI] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Gray KM, Hood CO, Tomko RL, Carpenter MJ, … Saladin ME (2020). The influence of gender and oxytocin on stress reactivity, cigarette craving, and smoking a randomized, placebo-controlled laboratory relapse paradigm. Psychopharmacology, 237, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, & Wanzer J (2011). Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of Psychopharmacology, 25, 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM, & Brady KT (2013). Effect of oxytocin on craving and stress response in marijuana-dependent individuals: A pilot study. Psychopharmacology, 228, 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Bershad A, King A, Lee R, & de Wit H (2016). Intranasal oxytocin dampens cue-elicited cigarette craving in daily smokers: A pilot study. Behavioural Pharmacology, 27, 697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, & Upadhyaya HP (2012). Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. The American Journal on Addictions, 21, 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59, 22–57. [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79, 194–202. 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Tomko RL, Saladin ME, Baker NL, McClure EA, Carpenter MJ, Ramakrishnan VR, … Gray KM (2020). Sex differences in subjective and behavioral responses to stressful and smoking cues presented in the natural environment of smokers. Nicotine and Tobacco Research, 22, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


