Abstract
Background and aims:
Calcineurin is a ubiquitously expressed central Ca2+-responsive signaling molecule that mediates acute pancreatitis, but little is known about its effects. We compared the effects of calcineurin expression by hematopoietic cells vs pancreas in mouse models of pancreatitis and pancreatitis-associated lung inflammation.
Methods:
We performed studies with mice with hematopoietic-specific or pancreas-specific deletion of protein phosphatase 3, regulatory subunit B, alpha isoform (PPP3R1, also called CNB1), in mice with deletion of CNB1 (Cnb1UBCΔ/Δ), and in the corresponding controls for each deletion of CNB1. Acute pancreatitis was induced in mice by administration of caerulein or high-pressure infusion of radiocontrast into biliopancreatic ducts; some mice were also given intraductal infusions of an adeno-associated virus vector that expressed NFAT-luciferase into pancreas. Pancreas, bone marrow, liver, kidney, heart, and lung were collected and analyzed by histopathology, immunohistochemistry, and immunoblots; levels of cytokines were measured in serum. Mouse and human primary pancreatic acinar cells were transfected with a vector that expressed NFAT-luciferase and incubated with an agent that blocks interaction of NFAT with calcineurin; cells were analyzed by immunofluorescence. Calcineurin-mediated neutrophil chemotaxis and reactive oxygen species (ROS) production were measured in neutrophils from mice.
Results:
Mice with hematopoietic-specific deletion of CNB1 developed the same level of local pancreatic inflammation as control mice after administration of caerulein or infusion of radiocontrast into biliopancreatic ducts. Cnb1UBCΔ/Δ mice or mice with pancreas-specific deletion of CNB1 developed less severe pancreatitis and reduced pancreatic inflammation after administration of caerulein or infusion of radiocontrast into biliopancreatic ducts compared with control mice. NFAT was activated in pancreas of Swiss Webster mice given caerulein or infusions of radiocontrast into biliopancreatic ducts. Blocking the interaction between calcineurin and NFAT did not reduce pancreatic acinar cell necrosis in response to caerulein or infusions of radiocontrast. Mice with hematopoietic-specific deletion of CNB1 (but not mice with pancreas-specific deletion of CNB1) had reduced infiltration of lung tissues by neutrophils. Neutrophil chemotaxis and production of ROS were decreased following incubation with a calcineurin inhibitor.
Conclusion:
Hematopoietic and neutrophil expression of calcineurin promotes pancreatitis-associated lung inflammation, whereas pancreatic calcineurin promotes local pancreatic inflammation. The findings indicate that the protective effects of blocking or deleting calcineurin on pancreatitis are mediated by the source of its expression. This information should be used in development of strategies to inhibit calcineurin for prevention of pancreatitis and pancreatitis-associated lung inflammation.
Keywords: calcium signaling, targeted calcineurin inhibition, drug development, PEP
INTRODUCTION
Acute pancreatitis is a common, painful, and potentially life-threatening inflammatory disorder of the pancreas that has a rising incidence worldwide.1–3 It accounts for health care costs of $2.5 billion and for 275,000 admissions each year.2, 4 The overall mortality approaches 30% among patients with persistent organ failure, which is driven by excessive immune responses.1, 2, 5 Calcineurin, a calcium-activated serine/threonine phosphatase, is a central calcium-responsive signaling molecule that modulates inflammation and is also an important target of the immunosuppressive drugs cyclosporin A (CsA) and tacrolimus (FK506).6–8 Besides promoting T-cell activation, a recent study demonstrated that calcineurin also modulates the activation of myeloid cells, including macrophages, dendritic cells, and neutrophils.9 These studies highlight the pleiotropic role of calcineurin in regulating broad immune responses. We and others have shown that calcineurin is a novel target of aberrant calcium signal within the main parenchymal cell of the pancreas, the pancreatic acinar cell, and plays a critical role in the development of acute pancreatitis.10–12 Recently, intestinal epithelial calcineurin was shown to control microbiota-dependent colorectal tumor development,13 implying a role of organ-intrinsic calcineurin in mediating organ-specific inflammation. Since calcineurin is ubiquitously expressed and acute pancreatitis is synergistically mediated by intra-pancreatic pathophysiological signals and excessive immune responses, it is unclear what is the relative impact of calcineurin expressed within the hematopoietic compartment versus the pancreas on localized pancreatic inflammation and extra-pancreatic organ involvement.
Calcineurin dephosphorylates several substrates, notably nuclear factor of activated T-cells (NFAT).14 The NFAT protein family consists of five members (NFATc1-c4 and NFAT5). Other than NFAT5, nuclear translocation of NFATc1-c4 is dependent on dephosphorylation by calcineurin.15 Systemic inhibition of NFAT with A-285222 or global deletion of NFATc3 were shown to protect against two models of acute pancreatitis,16 suggesting a role of NFAT in the development of acute pancreatitis. However, during acute pancreatitis, the direct targets of calcineurin which transduce the signals in pancreatic acinar cells remain to be determined.
Acute lung injury (ALI) is a major extra-pancreatic complication of acute pancreatitis.17, 18 ALI is mediated by various factors, including neutrophils, macrophages/monocytes, cytokines/chemokines and intracellular signaling pathways.19 Neutrophils are the earliest immune cells to be recruited to the site of lung inflammation and play a critical role in the progression of ALI.20 Depletion of neutrophils or blockage of neutrophil adhesive functions prevented pancreatitis-associated lung injury and inflammation.21, 22 Cytokines and chemokines, including IL-6, TNF-α, IL-1β, IL-18, CXCL2, have been shown to play a role in the pathogenesis of pancreatitis-associated lung injury.19 Limited studies indicate that calcineurin signaling mediates lung injury either by decreasing the production of CXCL1 and CXCL223 or by regulating chemotaxis of neutrophils or macrophages.24-26
In this study, we sought to firstly examine the role of calcineurin expressed within the hematopoietic compartment and the pancreas using pancreatitis models induced by caerulein hyperstimulation or biliopancreatic duct radiocontrast injection at high pressure. We induced hematopoietic compartment-specific CNB1 deletion using bone-marrow chimeric mice and pancreas-specific CNB1 deletion using intra-pancreatic ductal infusion of viral vectors, and we compared them with Cnb1UBCΔ/Δ mice. We found that hematopoietic-specific calcineurin deletion did not affect localized pancreatic inflammation; by contrast, pancreas-specific or global calcineurin deletion largely protected against localized pancreatic inflammation in two models of acute pancreatitis, suggesting that the protective effects of calcineurin on pancreatitis is differentially mediated by the cellular source of expression. Secondly, we assessed whether pancreatic calcineurin mediates acinar cell injury through its downstream effector NFAT. During acute pancreatitis, pancreatic NFAT was activated both in vivo and ex vivo, and blockade of the interaction between calcineurin and NFAT by INCA-6 markedly reduced acinar cell NFAT in isolated mouse and human pancreatic acinar cells. However, calcineurin-NFAT blockade did not prevent acinar cell necrosis with pancreatitis stimuli. Thirdly, we found that during caerulein hyperstimulation pancreatitis, hematopoietic-specific, but not pancreas-specific CNB1 deletion, abrogated lung neutrophil infiltration, and the effects appeared to be cell intrinsic. Overall, these data imply that the effects of calcineurin in the context of pancreatitis are dependent on the cellular compartment of expression.
METHODS
Reagents and animals
All reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise specified. Mice carrying loxP-flanked (‘floxed’) alleles of Cnb1 (Cnb1f/f) were a kind gift of Dr. Gerald Crabtree.27 C57BL/6J, UBC-CreERT2 and Lox-Stop-Lox (LSL)-tdTomato reporter mice were purchased from the Jackson Laboratory. Eight to ten weeks old male and female mice weighing 22–25 g were used. All mice were housed at 22°C with a 12 h light-dark cycle and maintained on a standard laboratory chow with free access to food and water. All animal experiments were performed using protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee or by the Institutional Animal Care and Research Ethics Committee of Shanghai Jiao Tong University School of Medicine (SYXK 2013–0050, Shanghai, China). The in vivo studies were carried out in Pittsburgh. Some of the processing of tissues was performed in Palo Alto (Stanford). The ex vivo neutrophil studies were performed in Shanghai.
Generation of hematopoietic compartment-specific Cnb1 deletion
To achieve hematopoietic-specific Cnb1 deletion, UBC-CreERT2/Cnb1f/f bone marrow (BM) chimeras were generated. The donor UBC-CreERT2/Cnb1f/f (CD45.1) mice were firstly backcrossed with a congenic C57BL/6J (CD45.1) line for more than 6 generations. To prevent graft-versus-host disease, the purity of the offspring to C57BL/6J background was confirmed by genome scanning. Thereafter, BM cells were collected from the UBC-CreERT2/Cnb1f/f (CD45.1) donor mice by flushing the femur and tibia with standard culture media containing 10% fetal bovine serum. The recipient C57BL/6J wild-type (WT; CD45.2) mice were lethally irradiated with two doses of 5.5 Gy, 4 hours apart, after which 3 × 106 BM cells per mouse were transferred via a tail vein injection. Six weeks after BM transplantation, UBC-CreERT2/Cnb1f/f BM→ irradiated WT mice received intraperitoneal injections of 100 mg/kg tamoxifen daily for 5 days. Before inducing acute pancreatitis, the resulting UBC-CreERT2/Cnb1f/f BM→ irradiated WT mice were allowed to recover for 8 weeks to ensure stable engraftment.
Confirmation of engraftment and Cnb1 deletion
To confirm engraftment, peripheral blood leukocytes were stained with allophycocyanin (APC)-conjugated CD45.1 (eBioscience, Waltham, MA, USA) and phycoerythrin (PE)-conjugated CD45.2 (eBioscience, Waltham, MA, USA) and analyzed by flow cytometry. Six weeks after BM transplantation, UBC-CreERT2/Cnb1f/f → WT mice received intraperitoneal injections of 100 mg/kg tamoxifen daily for 5 days. To confirm hematopoietic-specific deletion of Cnb1, total RNA was extracted from pancreas and BM tissue one week after the last tamoxifen injection 28, 29. Reverse transcription quantitative polymerase chain reaction (RT-qPCR) was performed to determine the relative expression of Cnb1, using the following specific primers: Ppp3r1 forward, 5’-TGGGAAATGAGGCGAGTTACC-3’ and Ppp3r1 reverse, 5’-TTCCACGCTCAAAGAACCAGA-3’. Samples were normalized to β-actin and expressed as fold change relative to control.
Luminex assay
The Luminex assay was performed with serum samples collected from in vivo experiments at the University of Pittsburgh Cancer Institute Luminex Core Facility. The customized mouse 13-plex kit, including granulocyte colony-stimulating factor (G-CSF), interferon gamma (IFNγ), tumor necrosis factor alpha (TNF-α), interleukin (IL)-1α, IL-6, IL-9, IL-13, C-X-C motif chemokine ligand 1(CXCL1), CXCL2, CXCL9, CXCL10, chemokine ligand 2 (CCL2), and CCL11, was purchased from EMD Millipore (Billerica, MA, USA) and used according to the manufacturer’s recommendations with modifications as described below. Briefly, samples were mixed with antibody-linked polystyrene beads on Curiox DA-96 plates and incubated on magnet array overnight at 4°C. Plates were vacuum filtered and washed three times, then incubated with biotinylated detection antibody for 1 h at room temperature, followed by adding streptavidin-R-Phycoerythrin and incubated for 30 min at room temperature. After incubation and two additional vacuum washes, the samples were resuspended in Reading Buffer. Plates were read using a Luminex 100/200™ instrument (Luminex, Austion, TX, USA).
Flow cytometry
Cells were stained with the following surface marker antibodies from eBioscience (Waltham, MA, USA): FITC-conjugated CD11b (Clone M1/70), Percp/Cy5.5-conjugated Ly6c (Clone HK1.4), APC-conjugated Gr-1 (Clone RB6-8C5), eFluor 450-conjugated CD45.2 (Cloe 104), PE-conjugated MHC-II (Clone AF6-120.1), PE/Cy7-conjugated F4/80 (Clone, BM8), BV711-conjugated CD45.1 (Clone A20). Cells were acquired on a Fortessa BD Biosciences and analyzed with FlowJo software.
Statistics
Data were expressed as mean ± SEM, unless otherwise specified. Statistical analysis was performed using GraphPad Prism 6 (GraphPad, La Jolla, CA, USA). Comparisons between groups were performed using ANOVA. A p value <0.05 was considered statistically significant.
RESULTS
Hematopoietic-specific calcineurin deletion does not affect localized pancreatic injury in mouse models of acute pancreatitis.
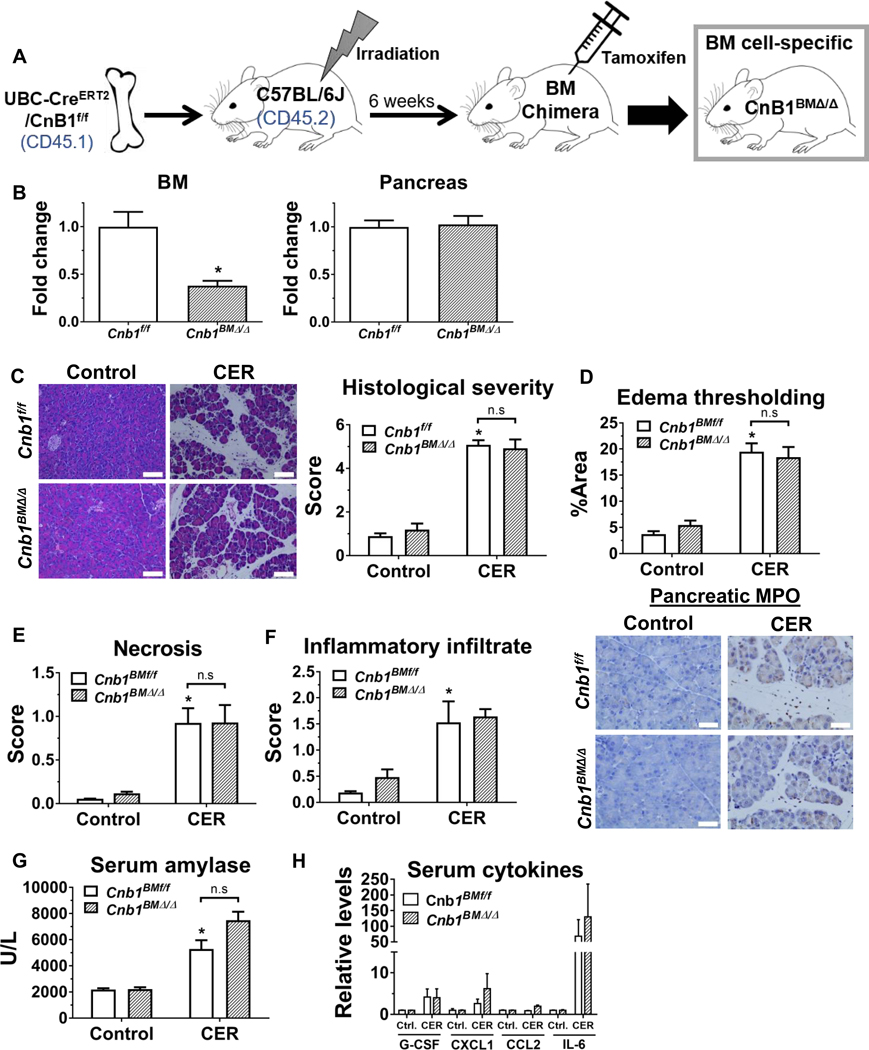
Pancreatic calcineurin signaling plays a crucial role in mediating pancreatitis outcomes.10, 11, 30 However, the specific contribution of hematopoietic calcineurin on the development of acute pancreatitis remains to be elucidated. To pursue this question, we generated hematopoietic-specific CNB1 deletors by adoptive transfer of bone marrow (BM) cells from a UBC-CreERT2/Cnb1f/f mouse line into lethally irradiated wild-type C57BL/6J mice (Figure 1A). To reduce the potential graft versus host response, the BM donor was selected from the UBC-CreERT2/Cnb1f/f mice (with 94% C57BL/6J genetic background assessed through genome scanning) that had been backcrossed to C57BL/6J mice for more than 5 generations (Supplementary Figure 1). Six weeks after BM engraftment, the percentage of host peripheral blood leukocytes (CD45.2) versus donors (CD 45.1), as assessed by flow cytometry, demonstrated successful BM chimera generation (Supplementary Figure 2). The expression of Cnb1 in BM cells, but not in the pancreas, from tamoxifen-treated chimeric mice significantly decreased (Figure 1B). However, during caerulein-induced pancreatitis, the reduction in Cnb1 expression in BM-derived cells did not affect localized pancreatic injury and inflammation, including pancreatic histological damage (Figure 1C), edema thresholding (Figure 1D), subscoring of necrosis (Figure 1E), inflammatory infiltrate, or neutrophil infiltration assessed by MPO immunostaining (Figure 1F). Similarly, serum amylase and cytokines/chemokines remained unaffected (Figure 1G, H and Supplementary Table 1).
Figure 1. During caerulein hyperstimulation pancreatitis (CER), hematopoietic compartment-specific deletion of CNB1 does not affect pancreatic localized injury.
(A) A hematopoietic compartment-specific calcineurin conditional knockout line (Cnb1BMΔ/Δ) was generated by adoptively transferring bone marrow (BM) cells from UBC-CreERT2/Cnb1f/f mice to the irradiated C57BL/6J mice, followed by tamoxifen administration. (B) RT-qPCR for Cnb1 expression in BM cells and the pancreas from Cnb1BMf/f and Cnb1BMΔ/Δ mice. (C) Representative H&E images of the pancreas (200X) from the control and CER conditions from Cnb1BMf/f and Cnb1BMΔ/Δ mice, along with overall histological severity. (D) Edema thresholding by ImageJ. (E) Subscoring for necrosis. (F) Subscoring for inflammatory infiltrate along with immunostaining for pancreatic myeloperoxidase (MPO) (400X). (G) Serum amylase, (H) serum cytokines assessed by Luminex multiplex assay. n=5 animals per conditions from two batches of animals; *p<0.05, compared to the controls; #p<0.05, compared to the Cnb1BMf/f group.
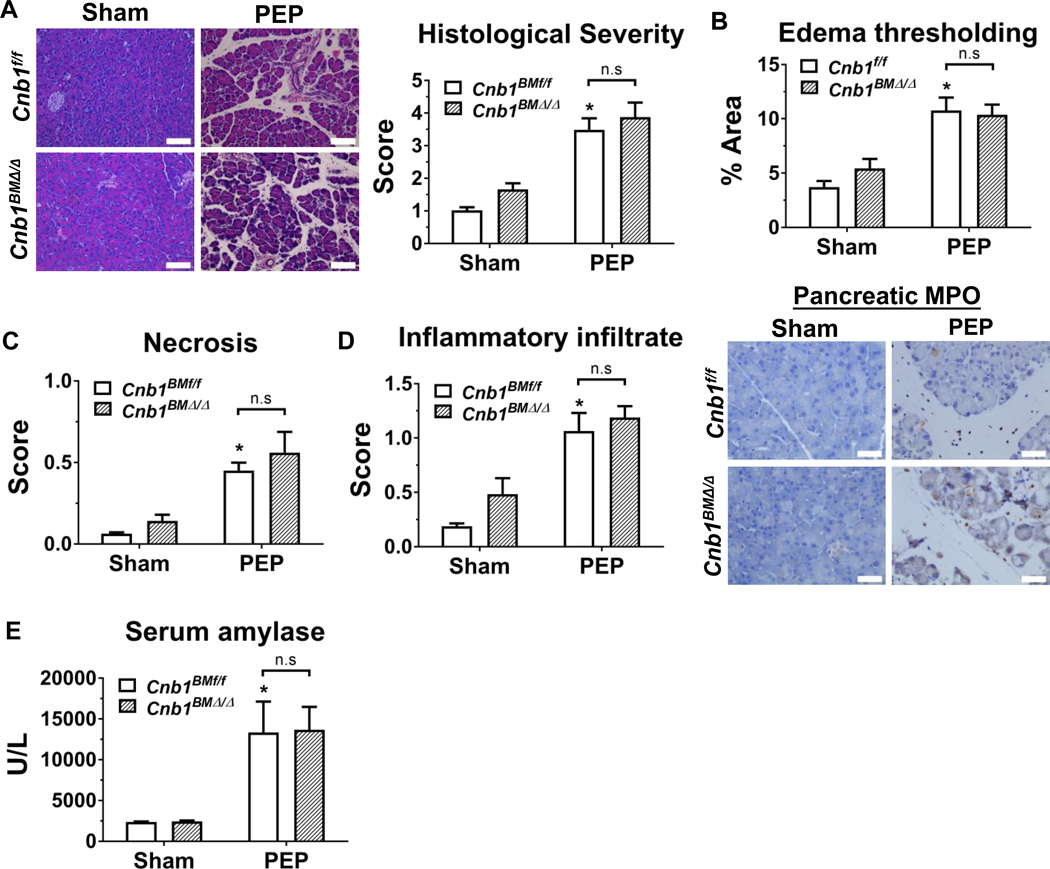
Post-ERCP pancreatitis, or PEP, is a troublesome iatrogenic etiology of pancreatitis.31 A mouse model that mimics PEP by infusing radiocontrast at high pressure into the pancreatic duct is a mildly severe model of pancreatitis, with injury restricted to the head of pancreas and with negligible systemic or distant organ damage.30 We next assessed the impact of hematopoietic-specific calcineurin deletion on PEP outcomes. Similar to caerulein hyperstimulation pancreatitis, during PEP, hematopoietic deletion of CNB1 did not influence localized pancreatic injury/inflammation as assessed by the parameters described above (Figure 2A–E). Here, we examined pancreatic macrophage infiltration (by F4/80 immunostaining) and also found no difference with hematopoietic deletion of CNB1 (Supplementary Figure 5). In contrast to the caerulein model, in the PEP model, the differences in systemic cytokines/chemokines could not be assessed, since there was no appreciable systemic inflammation or extra-pancreatic damage. Taken together, these findings demonstrate that calcineurin expressed within the hematopoietic compartment does not affect localized pancreatic injury and inflammation during acute pancreatitis.
Figure 2. Hematopoietic-specific deletion of CNB1, protects against post-ERCP pancreatitis (PEP).
(A) Representative H&E images of the pancreas (200X) from the sham and PEP conditions from Cnb1BMf/f and Cnb1BMΔ/Δ mice along with overall histological severity scoring. (B) Edema thresholding by ImageJ. (C) Subscoring for necrosis. (D) Subscoring for inflammatory infiltrate and immunostaining for pancreatic myeloperoxidase (MPO) (400X). (E) Serum amylase. n=5 animals per groups; *p<0.05, compared to the controls; #p<0.05, compared to the Cnb1f/f groups.
Pancreas-specific calcineurin deletion mediates localized pancreatic injury in mouse models of acute pancreatitis.
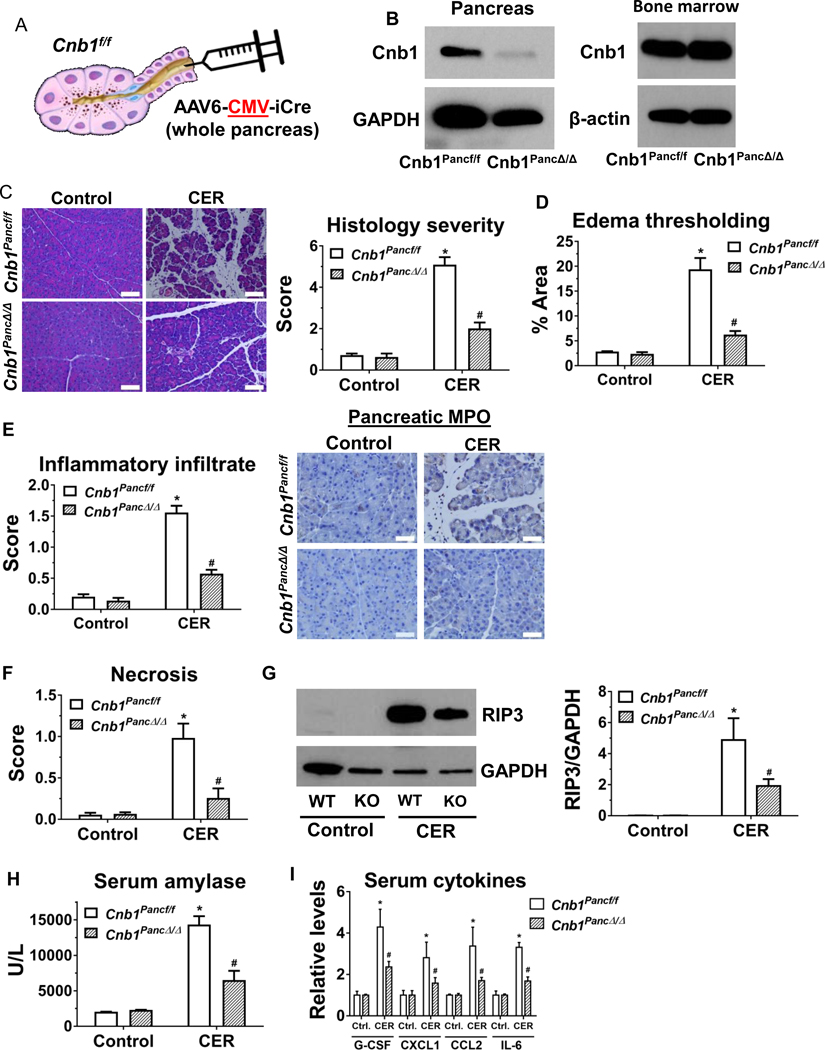
To compare the protective effect of calcineurin expressed within the pancreas, we next examined the impact of pancreas-specific calcineurin deletion on pancreatitis responses. We induced pancreas-specific calcineurin deletion in Cnb1f/f mice by intraductal infusion of an adeno-associated virus 6 (AAV6), which contained an enhanced Cre recombinase (iCre) driven by a ubiquitous cytomegalovirus (CMV) promoter (Figure 3A). As proof-of-principle to ensure that the whole pancreas is targeted through this procedure and, conversely, that there is lack of spillage of virus into the adjacent intestine or liver, we performed intraductal infusion of AAV6-CMV-iCre in LSL-tdTomato reporter mice and observed a robust pancreas-specific red fluorescence throughout the whole pancreas, indicating the widespread expression of iCre (Supplementary Figure 3A). Immunostaining was conducted to further confirm the expression of iCre in three main pancreatic cells--acinar cells, ductal cells, and islets (Supplementary Figure 3B). As expected, the protein level of CNB1 was reduced in the pancreas, but not in the BM cells (Figure 3B).
Figure 3. During caerulein hyperstimulation pancreatitis (CER), pancreas-specific deletion of CNB1 largely prevents localized pancreatic injury and systemic inflammation, but it does not affect distant organ damage.
(A) Schema for intraductal infusion of AAV6-CMV-iCre into Cnb1f/f mice to specifically delete CNB1 in the whole pancreas. (B) Western blot for CNB1 from pancreas and BM lysates. AAV, adeno-associated virus. (C) Representative H&E images of the pancreas (200X) from the control and CER conditions from AAV6 control-infused (Cnb1Pancf/f) and AAV6-CMV-iCre-infused (Cnb1PancΔ/Δ) groups, along with overall histological severity scoring. (D) Edema thresholding by Image. (E) Subscoring for inflammatory infiltrate and immunostaining for pancreatic MPO (400X). (F) Subscoring for necrosis. (G) Immunoblotting and densitometry analysis for RIP3 from pancreas lysates. (I) Serum amylase. (J) Serum cytokines assessed by Luminex multiplex assay. n=5 animals per conditions from two batches of animals; *p<0.05, compared to the controls; #p<0.05, compared to the Cnb1Pancf/f group.
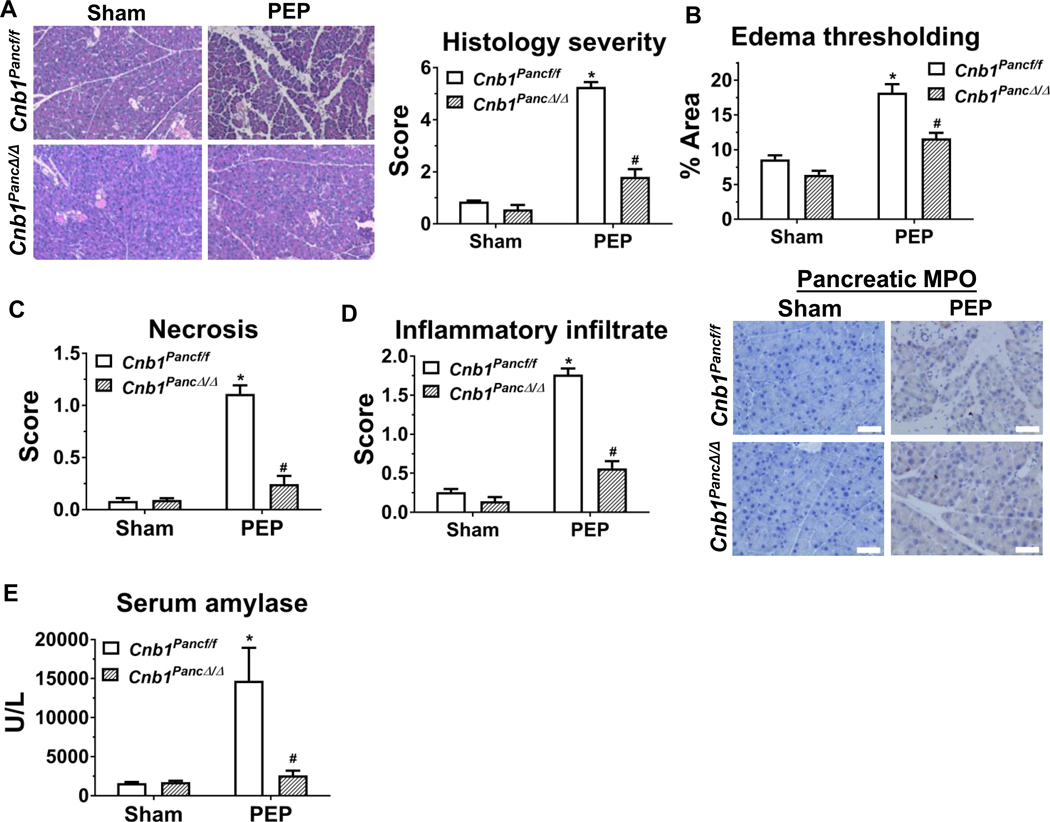
During caerulein hyperstimulation pancreatitis, the mice with pancreas-specific deletion of CNB1 (Cnb1PancΔ/Δ) exhibited a marked reduction in pancreatic histological damage (Figure 3C), edema thresholding (Figure 3D) and inflammatory infiltrate (Figure 3E). We did not observe sex differences in those parameters, with or without pancreas-specific Cnb1 deletion. Neutrophil infiltration, assessed by pancreatic MPO immunostaining, was markedly reduced with pancreas-specific deletion of CNB1 (Figure 3E), whereas macrophage infiltration (by F4/80) was not different (Supplementary Figure 5). The findings suggest that pancreatic calcineurin selectively influences neutrophil infiltration. Pancreas-specific deletion of CNB1 also markedly decreased the score of pancreatic necrosis score (Figure 3F) and the expression of receptor-interacting serine/threonine-protein kinase 3 (RIP3) (Figure 3G), which is a surrogate marker of programmed necrosis.32 Similarly, serum amylase and the pro-inflammatory cytokines, such as G-CSF, CXCL1, CCL2, and IL-6 were markedly reduced in pancreas-specific Cnb1 deletors (Figure 3H and I). However, several serum cytokines (IFN-γ, TNF-α, IL-1α, IL-9, and IL-13) and chemokines (CXCL2, CXCL9, CXCL10, CCL11) at baseline or with caerulein hyperstimulation were not affected by pancreas-specific deletion of CNB1 (Supplementary Table 2), suggesting calcineurin may differentially mediate various chemokine/cytokine responses. Awla et al. showed an upregulation of CXCL2 in pancreatic tissue and acinar cells during pancreatitis, that was dependent on NFATc3.16 However, we did not observe elevations in serum CXCL2 levels during pancreatitis, with or without calcineurin deletion. Next, we examined the impact of pancreas-specific deletion of CNB1 on PEP and found that pancreas-specific deletion of CNB1 caused a marked reduction in pancreatic histological damage (Figure 4A), edema thresholding (Figure 4B), pancreatic necrosis (Figure 4C), inflammatory infiltrate or neutrophil infiltration assessed by MPO immunostaining (Figure 4D), and serum amylase (Figure 4E). Collectively, these findings demonstrate that pancreas-intrinsic calcineurin expression primarily mediates localized pancreatic injury and inflammation.
Figure 4. Pancreas-specific deletion of CNB1 protects against post-ERCP pancreatitis (PEP).
(A) Representative H&E images of the pancreas (200X) from the sham and PEP conditions from Cnb1pancf/f and Cnb1PancΔ/Δ mice along with overall histological severity scoring. (B) Edema thresholding by ImageJ. (C) Subscoring for necrosis. (D) Subscoring for inflammatory infiltrate and immunostaining for pancreatic MPO (400X). (E) Serum amylase. n=5 animals per groups; *p<0.05, compared to the controls; #p<0.05, compared to the Cnb1f/f groups.
Global calcineurin deletion protects against two mouse models of acute pancreatitis.
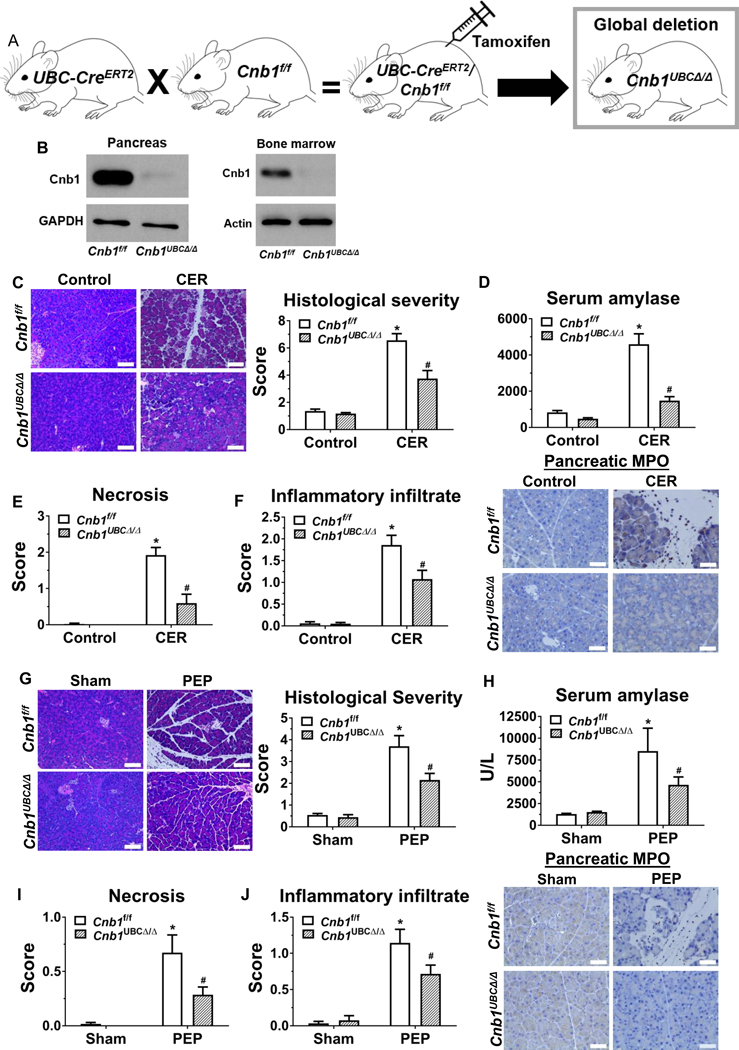
We next examined the impact of global ablation of CNB1 on pancreatitis using an inducible CNB1 deletor, since germline inactivation of the Cnb1 gene results in lethal defects in vascular patterning.33 We generated a UBC-CreERT2/Cnb1f/f line by crossing the Cnb1 line with a UBC-CreERT2 line (Figure 5A). In these mice, expression of Cre recombinase (tethered to the tamoxifen receptor ERT2) is under the control of the ubiquitously expressed human ubiquitin C (UBC) promoter. Two weeks after tamoxifen injection of adult mice, by PCR, no Cnb1 allele was detected in the tissues that were assayed, including pancreas, BM, liver, kidney, heart, and lung (Supplementary Figure 4), and by western blotting, there was minimal CNB1 protein in the pancreas and BM cells (Figure 5B). No gross abnormalities were observed in the global Cnb1-deleted mice. Consistent with previous reports from partial calcineurin inactivation using global CnAβ knockouts11, 30, we found that during caerulein hyperstimulation pancreatitis, acute global deletion of CNB1 significantly reduced pancreatic histological score (Figure 5C), serum amylase (Figure 5D), pancreatic necrosis (Figure 5E), inflammatory infiltrate, and neutrophil infiltration (Figure 5F). Similarly, global deletion of CNB1 markedly reduced PEP outcomes (Figure 5G-J). These data demonstrate that global calcineurin deletion protects against two disparate experimental models of acute pancreatitis.
Figure 5. Global deletion of CNB1 protects against two disparate models of acute pancreatitis.
(A) The global Cnb1 conditional knockout line (Cnb1UBCΔ/Δ) was induced by crossing UBC-CreERT2 mice with Cnb1f/f mice, followed by tamoxifen administration. (B) Western blotting confirms negligible Cnb1 expression in the pancreas and BM cells from the Cnb1UBCΔ/Δ mice. (C) Representative H&E images of the pancreas (200X) from the control and CER conditions from Cnb1f/f and Cnb1UBCΔ/Δ mice and overall histological severity scoring. (D) Serum amylase. (E) Subscoring for necrosis. (F) Subscoring for inflammatory infiltrate and immunostaining for pancreatic myeloperoxidase (MPO) (400X). (G) Representative H&E images of the pancreas (200X) from the sham and PEP conditions from Cnb1f/f and Cnb1UBCΔ/Δ mice and overall histological severity scoring. (H) Serum amylase. (I) Subscoring for necrosis. (J) Subscoring for inflammatory infiltrate and immunostaining for pancreatic MPO (400X). n=5 animals per conditions; *p<0.05, compared to the controls; #p<0.05, compared to the Cnb1f/f condition.
Pancreatic NFAT is activated during pancreatitis, but acinar cell damage is independent of NFAT.
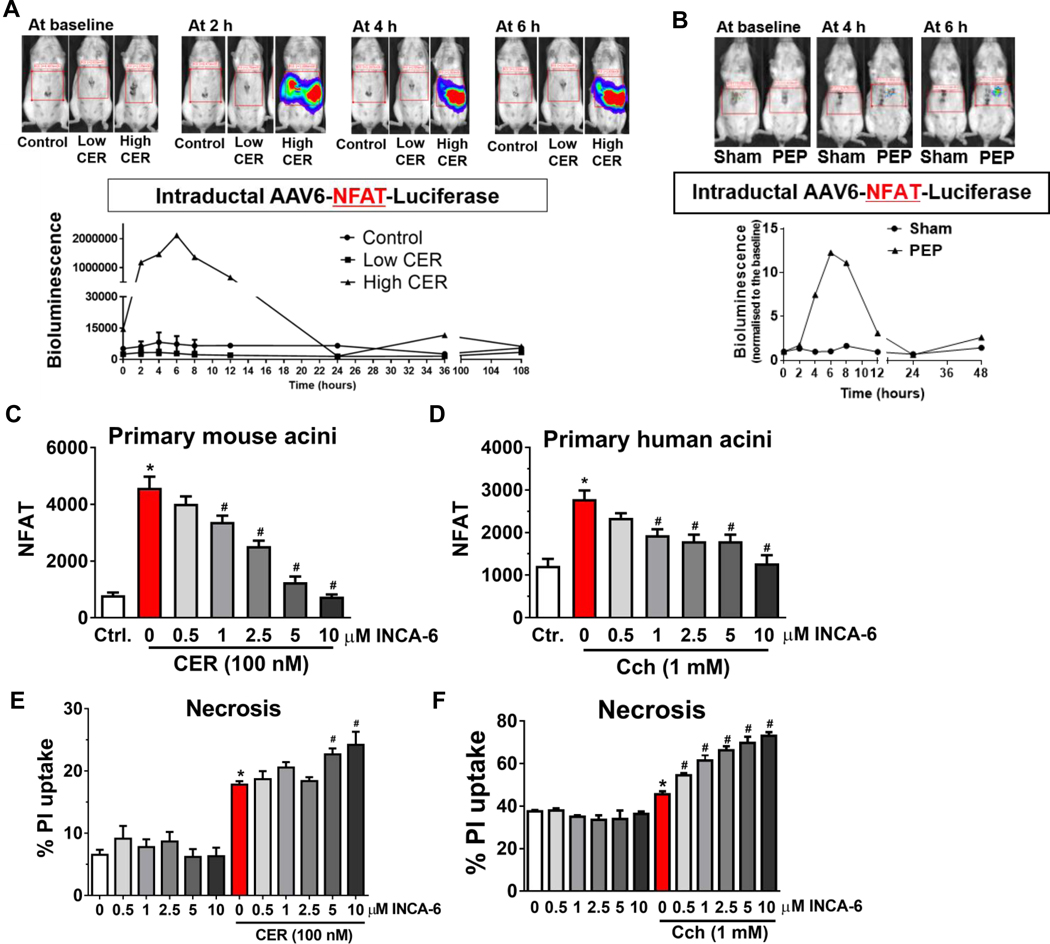
The direct targets of pancreatic calcineurin in pancreatitis are unclear. NFAT is a well-studied substrate, and nuclear translocation of NFATc1-4 is dependent on dephosphorylation by calcineurin.14, 15 Tissue-wide NFATc3 was previously shown to mediate pancreatic trypsinogen activation, inflammation, and tissue damage in acute pancreatitis.16 Therefore, we sought to examine whether pancreas-intrinsic calcineurin transduces its damaging signals during pancreatitis through its classical downstream target, NFAT. To dynamically monitor in vivo pancreatic NFAT activation, we intraductally infused the AAV6-NFAT-luciferase into the pancreas.28 With both caerulein hyperstimulation and PEP induction, pancreatic NFAT bioluminescence signals peaked at 6 h and then returned to baseline by 24 h (Figure 6A and B). Using either mouse or human primary pancreatic acinar cells infected with Ad-NFAT-luciferase construct, we first confirmed that in both isolated mouse and human primary pancreatic acinar cells, supramaximal concentrations of caerulein or carbachol led to an increase in NFAT activity. Inhibitor of NFAT-calcineurin association (INCA)-6 blocks the interaction between calcineurin and NFAT34 and its use ex vivo in acinar cells would serve to examine whether acinar NFAT is a downstream effector of calcineurin with pancreatitis stimuli. Treatment with inhibitor of INCA-6 reduced NFAT activity in a dose-dependent manner (Figure 6C and D). However, in both isolated mouse and human primary pancreatic acinar cells, NFAT inhibition by INCA-6 did not reduce acinar cell necrosis as measured by the propidium iodide (PI) uptake. On the contrary, there was an increase in cell necrosis (Figure 6E and F). The findings suggest that in the ex vivo and in vivo setting, pancreatic NFAT is activated during pancreatitis. However, the damaging signals of pancreatitis on the pancreas are independent of acinar cell NFAT. In fact, acinar NFAT appears to mitigate, rather than mediate acinar damage.
Figure 6. The calcineurin target NFAT is activated in the pancreas during acute pancreatitis, but inhibition of acinar cell NFAT does not reduce acinar necrosis.
(A) Images and quantification of the pancreatic nuclear factor of activated T cells (NFAT) signal at baseline, 2, 4, and 6 h after administration of low or high dose of caerulein. (B) Images and quantification of the pancreatic NFAT signal at baseline, 4, and 6 h after PEP induction. NFAT activity with or without INCA-6 in isolated pancreatic acinar cells from (C) mouse and (D) human. PI uptake with or without INCA-6 in isolated pancreatic acinar cells from (E) mouse and (F) human.
Hematopoietic calcineurin deletion attenuates pancreatitis-associated lung neutrophil infiltration through neutrophil-intrinsic calcineurin.
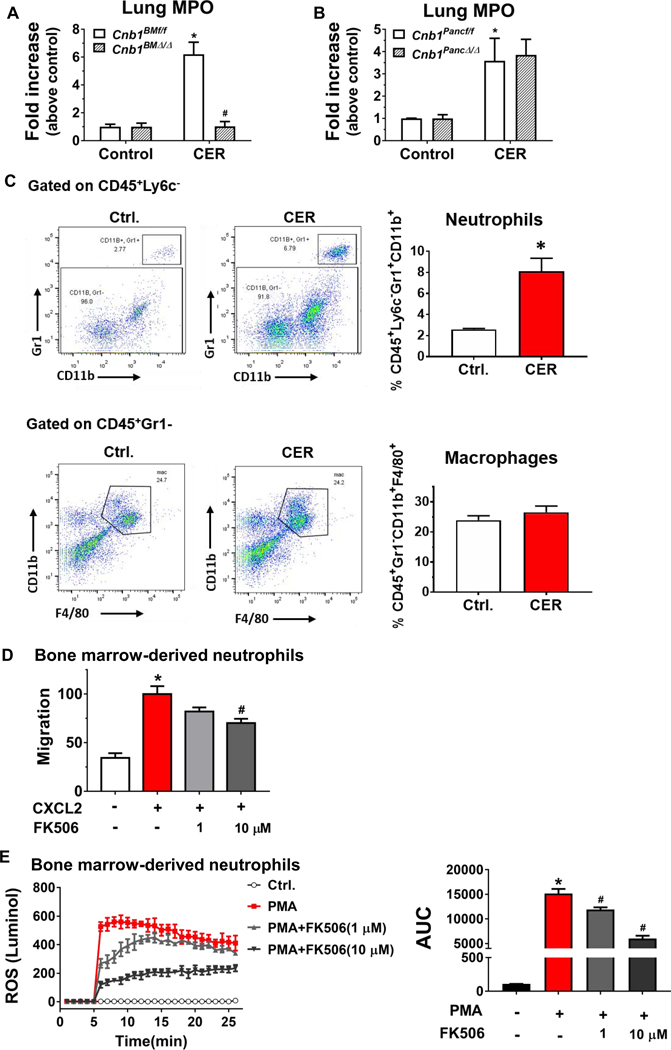
Pancreatitis-associated acute lung injury is a major complication of severe acute pancreatitis and is associated with significantly increased mortality.1, 20 Previous studies have suggested a role for calcineurin in models of lung injury.23, 35, 36 Therefore, we examined the impact of calcineurin on pancreatitis-associated lung injury and inflammation. We found that hematopoietic deletion of CNB1 abrogated lung MPO activity, a surrogate marker of inflammatory infiltration (Figure 7A), suggesting that calcineurin expressed within the hematopoietic compartment plays a role in the protection of pancreatitis-associated lung inflammation. By contrast, pancreas-specific deletion of CNB1 did not affect lung MPO activity (Figure 7B). These data further underscore that the effects of calcineurin on pancreatitis are mediated by its cellular source of expression.
Figure 7. Inactivation of calcineurin modulates lung neutrophil infiltration in vivo and neutrophil migration and ROS production ex vivo.
(A) Lung MPO activity from hematopoietic compartment-specific calcineurin deletion. (B) Lung MPO activity from pancreas-specific calcineurin deletion. (C) Lung leukocytes were isolated from the control and caerulein hyperstimulation pancreatitis (CER) conditions and analyzed by flow cytometry for neutrophil (gated on CD45+Ly6c−, then CD11b+Gr1+) and macrophages (gated on CD45+Gr1−, then CD11b+ F4/80+) numbers. n=5 animals per condition. (D) Percentage of migrated BM derived neutrophil (BMDN) from Transwell migration assay stimulated with CXCL2 in the presence or absence of FK506 pre-treatment. (E) Neutrophil total ROS production in response to phorbol 12-myristate 13-acetate (PMA) with or without FK506 pretreatment was assayed using luminol and further quantified by an area-under-the curve. 3 independent BMDN isolations; *p<0.05, compared to the control; #p<0.05, compared to the CXCL2- or PMA-stimulated condition.
Next, we sought to examine the mechanism for the impact of hematopoietic calcineurin on lung neutrophil infiltration. Previous studies have implicated neutrophil-derived matrix metalloproteinases,37 intercellular adhesion molecule 1,38 and soluble IL-6 receptor signaling39 in the pathogenesis of pancreatitis-associated lung injury. Since we did not observe a reduction in serum IL-6 and the neutrophil chemoattractant CXCL1 or changes in the neutrophil chemoattractant CXCL2 with hematopoietic deletion of CNB1 (Figure 1H and Supplementary Table 1), we, therefore, hypothesized that the protective effect of hematopoietic calcineurin deletion on lung neutrophil infiltration could be mediated directly through neutrophil-intrinsic calcineurin. To confirm that neutrophils are the primary contributor of MPO activity in the lung during pancreatitis, we performed flow cytometry from mouse lung in caerulein hyperstimulation pancreatitis. Ly6c−CD11b+Gr1+ neutrophils, but not CD11b+Gr1−F4/80+ macrophages, were elevated in the lung (Figure 7C). This suggested, as expected, that the neutrophils, but not macrophages, accounted for the lung MPO activity observed with pancreatitis.
We next examined whether calcineurin that is intrinsic to neutrophils has an impact on neutrophil chemotaxis or ROS production. In isolated BM-derived neutrophils, pre-treatment with the highly specific calcineurin inhibitor FK506 inhibited neutrophil migration that was stimulated by the neutrophil attractant CXCL2 (Figure 7D). PMA-stimulated ROS production was also significantly reduced by FK506 (Figure 7E). These data demonstrate that inhibition of neutrophil calcineurin decreases neutrophil chemotaxis and ROS production, suggesting that the effect of hematopoietic calcineurin on lung neutrophil infiltration was directly mediated by neutrophil-intrinsic calcineurin expression.
DISCUSSION
In the current study, using two disparate mouse models of acute pancreatitis, we found that selective deletion of calcineurin within the hematopoietic compartment failed to impact the pathological features of localized pancreatic injury, including even pancreatic immune cell infiltration, or the systemic cytokine profile. However, pancreatic or global deletion of calcineurin markedly both features. Consistent with our previous findings with partial calcineurin inactivation,11, 28, 30, 40 this study confirms that calcineurin plays a crucial role in the development of acute pancreatitis and further demonstrates that the protective effects of calcineurin on pancreatitis are dependent on the cellular source of expression. We had previously shown that deletion of calcineurin in the pancreatic acinar cell or targeted administration of calcineurin inhibitors to the pancreas was sufficient to protect against pancreatitis, which indicated that an epithelial cell source of calcineurin could exert a critical role in localized pancreatic inflammation and injury. In addition, data obtained from patients with acute or recurrent acute pancreatitis or from isolated pancreatic acinar cells indicate that acinar cells are the main source of inflammatory cytokines or chemokines, including TNF-α, IL-1β, and IL-6, MCP-1, CXCL2.41–43 Our current study demonstrates that during caerulein hyperstimulation pancreatitis, serum levels of several cytokines/chemokines, including G-CSF, CXCL1, CCL2, and IL-6 are significantly increased and pancreas-specific, but not hematopoietic deletion of CNB1, decreases the levels of those cytokines/chemokines. The findings add support to the notion that the pancreatic acinar cell is the trigger for initiating the production of these inflammatory mediators.
Calcineurin is activated in the acinar cell during pancreatitis by aberrant acinar cell calcium signals.11, 30 These high amplitude, non-oscillatory, sustained peak-plateau calcium signals are an early and critical mediator of pancreatitis in virtually all of the known experimental models.44 The microdomains or subcellular pools of calcium that activate the calcineurin signaling network responsible for the inflammatory changes in the acinar cell have yet to be determined. What we do know is that calcineurin inhibition does not reciprocally impact the shape of the aberrant acinar cell calcium signal.30, 45 The downstream pathological changes with calcineurin signaling include intra-acinar protease activation, NF-κB, and acinar cell necrosis.11, 12, 30, 40, 45
The direct targets of pancreatic acinar cell calcineurin in pancreatitis are unclear. Several studies have shown a role for NFAT in regulating pancreatic tissue damage during acute pancreatitis,16 pancreatic acinar-to-ductal metaplasia and tissue reconstitution after pancreatitis,46, 47 and pancreatic cancer initiation.48 In this study, we observed that, during acute pancreatitis, pancreatic NFAT is indeed activated in both in vivo models of pancreatitis and in isolated mouse and human pancreatic acinar cells induced with pancreatitis stimuli. Blockade of the interaction of calcineurin and NFAT with INCA-6, however, failed to impact acinar cell necrosis, suggesting that the damaging signals transduced by calcineurin on the pancreas are independent of NFAT. Several other calcineurin substrates have been identified,49 including dynamin related protein 1 (Drp1),50 CREB-regulated transcriptional coactivator-1(CTRC-1),51 B-cell lymphoma 10 (Bcl10),52 and transcription factor EB (TFEB)53. There are recent reports of TFEB in lysosomal biogenesis during pancreatitis.54, 55 Further work is required to investigate the role of calcineurin substrates in transducing calcium-calcineurin signals during pancreatic acinar cell injury.
Pancreatitis-associated ALI is a major complication of severe acute pancreatitis, and leukocyte infiltration plays a critical role in the pathogenesis of ALI.19 In this study, we found that with caerulein hyperstimulation pancreatitis, the absence of calcineurin expression in the hematopoietic compartment led to a significant reduction in lung neutrophil infiltration. By contrast, pancreas-specific calcineurin deletion did not affect lung neutrophil infiltration, further underscoring that the effects of calcineurin on pancreatitis are dependent on the cellular source of expression. Neutrophil-intrinsic calcineurin expression appears to mediate the migration of neutrophils into the lung. It is also possible that neutrophilic calcineurin mediates the recently described phenomenon of the reverse transmigration of neutrophils into lung niches after tissue injury and repair elsewhere in the body.56 Interestingly, deletion of hematopoietic calcineurin was also insufficient to reduce pancreatic neutrophil infiltration. A likely reason for this observation is that the intense local inflammatory cytokine profile overrode the deficiency of hematopoietic calcineurin. Further, we observed that deletion of hematopoietic calcineurin had no influence on the serum levels of cytokines or chemokines, such as IL-6 or CXCL1, indicating that the effect of hematopoietic calcineurin on lung neutrophil infiltration is likely through intrinsic expression of calcineurin on the immune cells. Indeed, we showed that inhibition of calcineurin decreases neutrophil chemotaxis and ROS production, both of which are associated with the phagocytotic function of neutrophil during inflammation.57
In conclusion, we demonstrate here that hematopoietic calcineurin fails to influence localized pancreatic injury during pancreatitis, while pancreas-specific calcineurin mediates both localized pancreatic inflammation and the subsequent production of systemic cytokine/chemokines. Furthermore, calcineurin expressed within the pancreas mediates pancreatic necroptosis, but pancreatic acinar cell necrosis is independent of NFAT. We further showed that hematopoietic calcineurin mediates lung neutrophil infiltration, and the inhibition of neutrophil calcineurin decreases neutrophil chemotaxis and ROS production. The findings indicate that the protective effects of blocking or deleting calcineurin on pancreatitis are mediated by its cellular source of expression, and they aid in devising optimal strategies for the targeted inhibition of calcineurin to prevent pancreatitis and pancreatitis-associated lung inflammation.
Supplementary Material
What you need to know:
Background and Context:
Calcineurin is a ubiquitously expressed Ca2+-responsive signaling molecule that mediates acute pancreatitis, but little is known about its effects.
New Findings:
Hematopoietic and neutrophil expression of calcineurin promotes pancreatitis-associated lung inflammation, whereas pancreatic calcineurin promotes local pancreatic inflammation. The findings indicate that the protective effects of blocking or deleting calcineurin on pancreatitis are mediated by the source of its expression.
Limitations:
This study was performed in mice; further studies are needed in humans.
Impact:
This information should be used in development of strategies to inhibit calcineurin for prevention of pancreatitis and pancreatitis-associated lung inflammation.
Lay Summary:
The authors identified a protein in bone marrow that activate immune cells to localize to the lungs where they cause inflammation in mice with pancreatitis. This protein is also in the pancreas, where it can cause inflammation and initiate a body-wide inflammatory response.
Acknowledgements:
The project used the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory, which is supported in part by NIH award (P30CA047904). We thank Dennis Prosser and Anna Lokshin from the Luminex Core Laboratory at the University of Pittsburgh for their assistance in the mouse Luminex assay.
This work started at the Department of Pediatrics, University of Pittsburgh, and was concluded at the Shanghai General Hospital, Shanghai Jiao Tong University, and Stanford University.
Grant support: This work was supported by National Institutes of Health (NIH) awards (DK093491 and DK103002; to S.Z.H.), a Children’s Hospital of Pittsburgh of UPMC Foundation Grant (to L.W.), National Natural Science Foundation of China award (81900585; to L.W.), and Shanghai Pujiang Program award (19PJ1408400; to L.W.)
Abbreviations:
- AAV6
adeno-associated virus 6
- BM
bone marrow
- CXCL2
C-X-C motif ligand 2
- IL
interleukin
- MPO
myeloperoxidase
- NFAT
nuclear factor of activated T cell
- PEP
post-ERCP pancreatitis
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
Footnotes
Conflict of interest statement: S.Z.H has equity in Prevcon, a startup that has sublicensed a patent on the use of calcineurin inhibitors in pancreatitis. All the other authors have no conflict of interest.
Author names in bold designate shared co-first authorship
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 2.Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med 2016;375:1972–81. [DOI] [PubMed] [Google Scholar]
- 3.Sah RP, Garg P, Saluja AK. Pathogenic mechanisms of acute pancreatitis. Curr Opin Gastroenterol 2012;28:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peery AF, Crockett SD, Barritt AS, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015;149:1731–41.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015;386:85–96. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol 2011;21:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Farmer JD Jr., Lane WS, et al. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991;66:807–15. [DOI] [PubMed] [Google Scholar]
- 8.Fruman DA, Bierer BE, Benes JE, et al. The complex of FK506-binding protein 12 and FK506 inhibits calcineurin phosphatase activity and IgE activation-induced cytokine transcripts, but not exocytosis, in mouse mast cells. J Immunol 1995;154:1846–51. [PubMed] [Google Scholar]
- 9.Fric J, Zelante T, Wong AY, et al. NFAT control of innate immunity. Blood 2012;120:1380–9. [DOI] [PubMed] [Google Scholar]
- 10.Orabi AI, Wen L, Javed TA, et al. Targeted inhibition of pancreatic acinar cell calcineurin is a novel strategy to prevent post-ERCP pancreatitis. Cell Mol Gastroenterol Hepatol 2017;3:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muili KA, Wang D, Orabi AI, et al. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem 2013;288:570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah AU, Sarwar A, Orabi AI, et al. Protease activation during in vivo pancreatitis is dependent on calcineurin activation. Am J Physiol Gastrointest Liver Physiol 2009;297:G967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peuker K, Muff S, Wang J, et al. Epithelial calcineurin controls microbiota-dependent intestinal tumor development. Nat Med 2016;22:506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 2005;5:472–84. [DOI] [PubMed] [Google Scholar]
- 15.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells 2004;18:1–9. [PubMed] [Google Scholar]
- 16.Awla D, Zetterqvist AV, Abdulla A, et al. NFATc3 regulates trypsinogen activation, neutrophil recruitment, and tissue damage in acute pancreatitis in mice. Gastroenterology 2012;143:1352–60 e1–7. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia M, Saluja AK, Hofbauer B, et al. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A 1998;95:4760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranson JH, Turner JW, Roses DF, et al. Respiratory complications in acute pancreatitis. Ann Surg 1974;179:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbarshahi H, Rosendahl AH, Westergren-Thorsson G, et al. Acute lung injury in acute pancreatitis--awaiting the big leap. Respir Med 2012;106:1199–210. [DOI] [PubMed] [Google Scholar]
- 20.Garg PK, Singh VP. Organ Failure due to Systemic Injury in Acute Pancreatitis. Gastroenterology 2019;156:2008–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandoval D, Gukovskaya A, Reavey P, et al. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology 1996;111:1081–91. [DOI] [PubMed] [Google Scholar]
- 22.Hartman H, Abdulla A, Awla D, et al. P-selectin mediates neutrophil rolling and recruitment in acute pancreatitis. Br J Surg 2012;99:246–55. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Luo L, Wang Y, et al. Nuclear factor of activated T cells regulates neutrophil recruitment, systemic inflammation, and T-cell dysfunction in abdominal sepsis. Infect Immun 2014;82:3275–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshmane SL, Kremlev S, Amini S, et al. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009;29:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opalek JM, Ali NA, Lobb JM, et al. Alveolar macrophages lack CCR2 expression and do not migrate to CCL2. J Inflamm (Lond) 2007;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asaduzzaman M, Zhang S, Lavasani S, et al. LFA-1 and MAC-1 mediate pulmonary recruitment of neutrophils and tissue damage in abdominal sepsis. Shock 2008;30:254–9. [DOI] [PubMed] [Google Scholar]
- 27.Neilson JR, Winslow MM, Hur EM, et al. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity 2004;20:255–66. [DOI] [PubMed] [Google Scholar]
- 28.Wen L, Javed TA, Yimlamai D, et al. Transient high pressure in pancreatic ducts promotes inflammation and alters tight junctions via calcineurin signaling in mice. Gastroenterology 2018;155:1250–63.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boggs K, Wang T, Orabi AI, et al. Pancreatic gene expression during recovery after pancreatitis reveals unique transcriptome profiles. Sci Rep 2018;8:1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin S, Orabi AI, Le T, et al. Exposure to radiocontrast agents induces pancreatic inflammation by activation of nuclear factor-kappaB, calcium signaling, and calcineurin. Gastroenterology 2015;149:753–64 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Committee ASoP, Chandrasekhara V, Khashab MA, et al. Adverse events associated with ERCP. Gastrointest Endosc 2016. [DOI] [PubMed] [Google Scholar]
- 32.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 2009;137:1100–11. [DOI] [PubMed] [Google Scholar]
- 33.Graef IA, Chen F, Chen L, et al. Signals transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vasculature. Cell 2001;105:863–75. [DOI] [PubMed] [Google Scholar]
- 34.Roehrl MH, Kang S, Aramburu J, et al. Selective inhibition of calcineurin-NFAT signaling by blocking protein-protein interaction with small organic molecules. Proc Natl Acad Sci U S A 2004;101:7554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Zhang S, Garcia-Vaz E, et al. Streptococcal M1 protein triggers chemokine formation, neutrophil infiltration, and lung injury in an NFAT-dependent manner. J Leukoc Biol 2015;97:1003–10. [DOI] [PubMed] [Google Scholar]
- 36.Karpurapu M, Lee YG, Qian Z, et al. Inhibition of nuclear factor of activated T cells (NFAT) c3 activation attenuates acute lung injury and pulmonary edema in murine models of sepsis. Oncotarget 2018;9:10606–10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keck T, Balcom JHt, Fernandez-del Castillo C, et al. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology 2002;122:188–201. [DOI] [PubMed] [Google Scholar]
- 38.Frossard JL, Lenglet S, Montecucco F, et al. Role of CCL-2, CCR-2 and CCR-4 in cerulein-induced acute pancreatitis and pancreatitis-associated lung injury. J Clin Pathol 2011;64:387–93. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Neuhofer P, Song L, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest 2013;123:1019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muili KA, Ahmad M, Orabi AI, et al. Pharmacological and genetic inhibition of calcineurin protects against carbachol-induced pathological zymogen activation and acinar cell injury. Am J Physiol Gastrointest Liver Physiol 2012;302:G898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu H, Werner J, Bergmann F, et al. Necro-inflammatory response of pancreatic acinar cells in the pathogenesis of acute alcoholic pancreatitis. Cell Death Dis 2013;4:e816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grady T, Liang P, Ernst SA, et al. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology 1997;113:1966–75. [DOI] [PubMed] [Google Scholar]
- 43.Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 2007;292:G143–53. [DOI] [PubMed] [Google Scholar]
- 44.Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci 2006;27:113–20. [DOI] [PubMed] [Google Scholar]
- 45.Husain SZ, Grant WM, Gorelick FS, et al. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol 2007;292:G1594–9. [DOI] [PubMed] [Google Scholar]
- 46.Chen NM, Singh G, Koenig A, et al. NFATc1 Links EGFR Signaling to Induction of Sox9 Transcription and Acinar-Ductal Transdifferentiation in the Pancreas. Gastroenterology 2015;148:1024–1034 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen NM, Neesse A, Dyck ML, et al. Context-dependent Epigenetic Regulation of Nuclear Factor of Activated T Cells 1 in Pancreatic Plasticity. Gastroenterology 2017;152:1507–1520.e.15. [DOI] [PubMed] [Google Scholar]
- 48.Hessmann E, Zhang JS, Chen NM, et al. NFATc4 Regulates Sox9 Gene Expression in Acinar Cell Plasticity and Pancreatic Cancer Initiation. Stem Cells Int 2016;2016:5272498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy J, Cyert MS. Identifying New Substrates and Functions for an Old Enzyme: Calcineurin. Cold Spring Harb Perspect Biol 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cereghetti GM, Stangherlin A, Martins de Brito O, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 2008;105:15803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mair W, Morantte I, Rodrigues AP, et al. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 2011;470:404–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palkowitsch L, Marienfeld U, Brunner C, et al. The Ca2+-dependent phosphatase calcineurin controls the formation of the Carma1-Bcl10-Malt1 complex during T cell receptor-induced NF-kappaB activation. J Biol Chem 2011;286:7522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Medina DL, Di Paola S, Peluso I, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol 2015;17:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang S, Ni HM, Chao X, et al. Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis. Autophagy 2019;15:1954–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, Ni HM, Chao X, et al. Critical Role of TFEB-Mediated Lysosomal Biogenesis in Alcohol-Induced Pancreatitis in Mice and Humans. Cell Mol Gastroenterol Hepatol 2020;January 25 pii: S2352–345X(20)30014-X. doi: 10.1016/j.jcmgh.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest 2019;129:2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nemeth T, Sperandio M, Mocsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov 2020;19:253–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.