Abstract
Mild episodes of breathing low oxygen (O2) (i.e., acute intermittent hypoxia, AIH) elicits rapid mechanisms of neural plasticity that enhance respiratory and non-respiratory motor function after spinal cord injury (SCI). Despite promising outcomes in humans and rodents with SCI, the translational potential of AIH as a clinical therapy remains dependent on a safer and more reliable air delivery system. The purpose of this study is to investigate the performance of a novel AIH delivery system to overcome inconsistencies in human AIH protocols using a hand-operated (manual) delivery system. Specifically, we characterized system performance of AIH delivery in terms of flow rate, O2 concentration, dose timing, and air temperature. Our data show that a novel ‘automated’ delivery system: i) produces reliable AIH with a goodness-of-fit at 98.1% of ‘ideal’; ii) eliminates dose timing errors via programmable solenoid switches; iii) reduces fluctuations in O2 to less than 0.01%; and iv) delivers 62.7% more air flow than the ‘manual’ delivery method. Automated physiological recordings, threshold detection, and visual feedback of the participant’s blood O2 saturation, heart rate, and blood pressure ensures real-time user safety. In summary, the ‘automated’ system outperformed the ‘manual’ delivery method in terms of accuracy, reliability, and safety. The ‘automated’ system offers several design features that move the technology closer to a medically approved treatment for clinical and home use.
Keywords: hypoxia, oxygen, breathing, pressure swing adsorption, spinal cord injury, motor function, rehabilitation
Background
Nearly 17,000 individuals in the United States alone suffer a spinal cord injury (SCI) each year, for which there is no cure (NSCISC, 2016). Most of these injuries result in partial damage to connections to spinal neurons below the injury that consequently limit control of functional movements such as breathing and walking. Developing effective therapeutic technologies to strengthen or re-establish connections to these spared motor neurons may further improve the restoration of movement after SCI paralysis; a major goal for persons living with SCI.
One method to improve motor function after SCI is through mild episodes of breathing low oxygen (i.e., acute intermittent hypoxia, AIH). In rodent SCI models, AIH triggers mechanisms of neural plasticity that enhance respiratory and non-respiratory function (Lovett-Barr et al., 2012). AIH elicits long-term facilitation of phrenic motor neuron activity via a signaling cascade that up-regulates brain-derived neurotropic factor (BDNF) and subsequently initiates downstream cellular events that purportedly strengthen synapses between pre-motor and motor neurons (Baker-Herman et al., 2004; Gonzalez-Rothi et al., 2015). Lovett-Barr et al. exposed SCI rodents to daily (7 consecutive days) AIH via Plexiglas chamber flushed with 10, 5 min episodes of 10.5% O2; 5 min intervals of 21% O2 (Lovett-Barr et al., 2012). They reported that these rodents substantially improved breathing capacity, as well as, locomotor skills. More recently, results from clinical studies showed AIH-induced improvements in motor behaviors in persons with motor-incomplete SCI. In three separate studies, persons with iSCI showed increased ankle strength after a single day of AIH treatment that persisted for hours (Lynch et al., 2017; Sandhu et al., 2019; Trumbower et al., 2012). A set of randomized clinical trials also uncovered substantial benefits of multi-session (up to 14 days) AIH on walking speed and endurance, as well as, hand function (Hayes et al., 2014; Navarrete-Opazo et al., 2017; Trumbower et al., 2017). While these results provide evidence that AIH offers tremendous treatment potential in humans, establishing a safe and reliable delivery method for clinical and home use remains under-developed.
Early clinical trials modified existing air delivery technologies to administer AIH interventions in persons with SCI (Hayes et al., 2014; Lynch et al., 2017; Navarrete-Opazo et al., 2017; Sandhu et al., 2019; Tester et al., 2014; Trumbower et al., 2017; Trumbower et al., 2012). These AIH protocols consisted of 15, 60–90s episodes of 10.0% O2 along with 60s intervals of ambient room air (20.9% O2). These seminal studies involved either modifications to commercially available PSA-dependent generators (HYP-123, Hypoxico Inc., USA) or pressurized gas cylinders with programmable mechanical valve controls (Navarrete-Opazo et al., 2017). Pressurized gas cylinder systems are well calibrated for laboratory application, but they are less feasible for clinic and home use due, in part, to stringent storage requirements and high maintenance costs. The PSA-dependent delivery systems appear more practical since they have a smaller “footprint”, low maintenance costs, and minimal storage requirements; however, their inefficiencies in administering AIH requires further attention.
Considering recent report of variable responses to AIH (Tan et al., 2020), elucidating the factors that may affect AIH-induced plasticity, safety, and personalized dosing requires a precise method for administering AIH protocols. Several design characteristics may make the PSA-dependent delivery systems vulnerable to inconsistencies such as dose timing, flow rate, and fractional inspired oxygen (FiO2) fluctuations. For example, dose timing error is a consequence of pseudo-random switching errors that occur while the human administrator manually connects and disconnects the system’s tubing to the end-user’s facemask during the AIH protocol. Physical switching of air supply lines to the facemask ensures rapid changes in FiO2 required of AIH protocols, but also is susceptible to imprecise delivery and settling times, and delays in FiO2 propagation, and settling times that may contribute to FiO2 target errors. The generators also depend on the PSA output and reservoir bags to provide adequate air flow to the face mask. However, the specifications for these technologies may not provide enough pressure to accommodate greater inspiratory volumes (e.g., deep breathing, sighs, yawns). Inadequate reserves or PSA constraints may substantially limit air flow and compromise stability of FiO2 during episodes of breathing low O2. Yet, the extent of these design limits on AIH delivery performance are not known.
The goal of this study is to advance our efforts toward operationalizing a promising AIH technology to treat SCI paralysis, as well as, potential to treat other neurologic pathologies. To achieve this goal, we conducted a set of experiments to rigorously assess air delivery performance of the ‘manual’ PSA-dependent system used in prior clinical trials (Hayes et al., 2014; Lynch et al., 2017; Sandhu et al., 2019; Trumbower et al., 2017; Trumbower et al., 2012). We then compared this system’s performance to a novel ‘automated’ delivery system designed to improve accuracy and reliability of dose timing, flow rate, and FiO2 during AIH treatment.
Methods
A. Subjects
Three able-bodied individuals (2 males and 1 female) participated in this investigation. The Mass General Brigham Institutional Review Board approved this study protocol. Study participants signed informed consent and could withdraw at any time. They did not have history of neurological, pulmonary, cardiovascular, and/or severe musculoskeletal impairments. Participants completed at least one of the three experimental sessions. Data collection occurred on separate experimental sessions.
B. Equipment
Air delivery system:
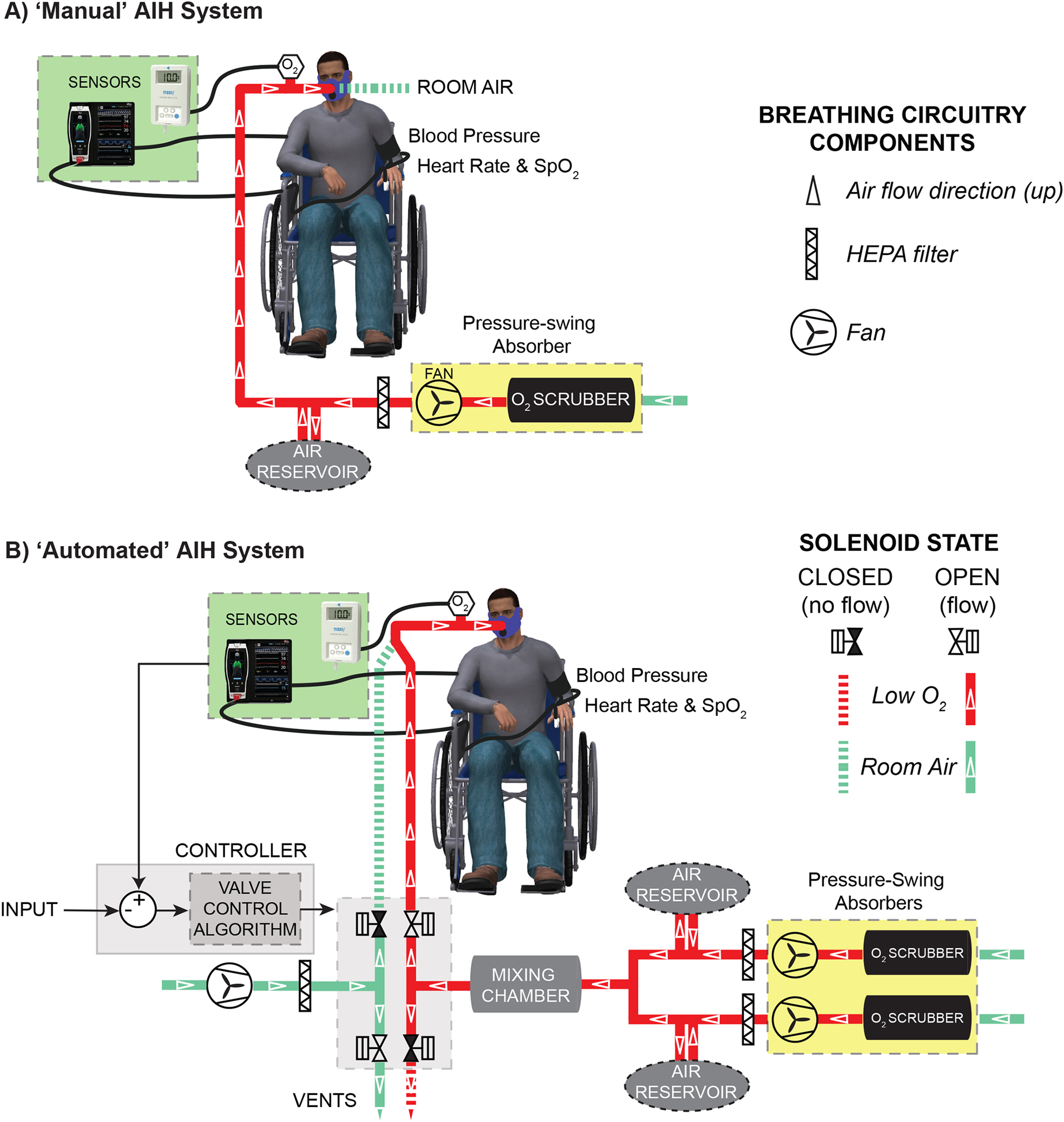
As illustrated in Figure 1, a commercially available pressure-swing absorption (PSA) system [HYP-123; Hypoxico Inc., USA] served as the air source for administering intermittent oxygen-depleted air treatment (Hayes et al., 2014; Lynch et al., 2017; Sandhu et al., 2019; Trumbower et al., 2017; Trumbower et al., 2012). The system included an onboard flow indicator for manual adjustment of FiO2 between 10.0 ± 2.0% (low O2) and 20.9 ± 2.0% (room air). The system also included a closed breathing circuit to a non-rebreather mask [Universal mask; Hypoxico Inc., USA] via a HEPA filter, 3-liter expandable reservoir bags, and approximately 3 m of clear tubing per system. Expandable reservoir bags provided air storage for occasional changes in inspiration rates that exceed output flow from the generator.
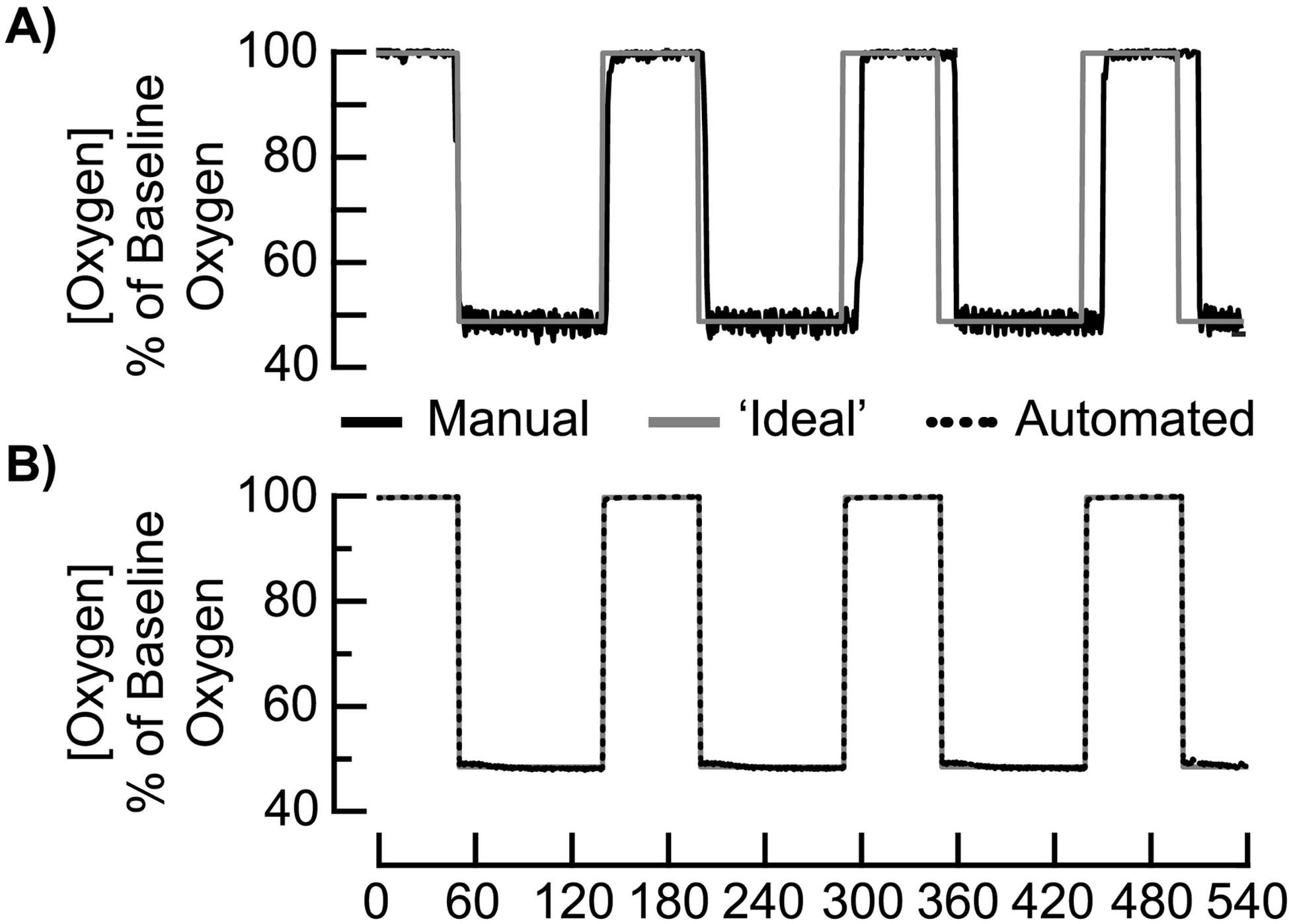
Figure 1.
A block diagram depicts the ‘manual’ acute intermittent hypoxia (AIH) delivery system for humans (A). The system includes a single pressure-swing absorber (yellow box) that generates and distributes low oxygen air through a human breathing circuit (solid red line) manually connected/disconnected to the end-user’s face mask during AIH treatment. The dashed line denotes room air not supplied to humans during low O2 breathing. White open triangles point along the direction of air flow. An O2 sensor near the face mask records the percentage of O2 within the air mixture. A stand-alone patient monitor displays heart rate (HR), blood O2 saturation (SpO2), and blood pressure (BP) for safety. In B, a block diagram depicts the ‘automated’ AIH delivery system. The system includes 1) double pressure-swing absorbers (yellow boxes) that generate and distribute low O2 air (solid, red line) and 2) blower that distributes room air (solid, green line) through the breathing circuit and 3) a gas mixing chamber that reduces fluctuations ins steady state O2 concentration. A microcontroller board controls two pairs of one-way solenoid valves that route air from either blower or absorbers to face mask. As shown, dashed lines denote air not supplied to human. Patient monitoring unit acquires HR, SpO2, and BP for real-time feedback to a microcontroller that maintains AIH protocols within safe limits.
Air flow regulation:
We used a microcontroller [Arduino Uno SMD R3, Arduino® LLC, Italy] to control air delivery intervals during ‘automated’ AIH protocols. During ‘manual’ AIH protocols, switching between air sources often results in random errors in temporal sequencing of AIH. We eliminated this possibility using automated valve-switching where each selected solenoid valve directed air flow using 110 VAC and 10.5 watts power consumption via a microcontroller. The microcontroller timed a solid-state relay [4-channel, 5VDC relay board, SainSmart® Inc, USA] to distribute power to a either pair of four total solenoid valves [K3A462U, ASCO® Inc, USA]. Solenoid valves directed air flow within the breathing circuit. Constant flows from the PSA output (hypoxia or sham) and a fan that supplies room air at positive pressure (blower) are constantly provided. In the case of low O2 (or sham) delivery, one valve delivered PSA output air to the mask and the other valve recirculated blower air to the spacious room. In the case of room air delivery, one valve delivered blower air to the mask and the other valve redirected unused low O2 air to the spacious indoor environment.
Room air blower:
An 18.9 liter, high-flow air blower [PVC01A-1000W, Tacklife, USA] provided continuous positive pressure air to the breathing circuit. Using a 20-ampere variable transformer set to about 20VAC [input: 110 VAC/60 Hz, Model: TDGC2–2KM, Beleeb, USA], we regulated blower operating speed to produce a flow rate of 1.25 liters s−1 to the mask. The room air blower provides continuous positive pressure of air to ease inspiration through the face mask.
Mask:
A non-rebreather mask served as interface between subject and air delivery system [HYP-123; Hypoxico Inc., USA]. The mask included a one-way inlet valve that opened during inspiration of room air or delivery of positive pressure air from the generator. During expiration, the inlet valve closed, and a second one-way outlet valve opened to allow exhaled air to escape to surrounding environment. Air leakage at the mask reduces inspired FiO2 (Goutorbe et al., 2013). To minimize this possibility, we used inflatable padding along the face mask perimeter to enclose the subject’s nose and mouth, and a neoprene sleeve with Velcro® strappings to secure the mask to the subject’s head.
Oxygen analyzers:
We used two in-line O2 analyzers to monitor and record O2 concentration within our breathing circuit. We affixed a MAX-250E galvanic, partial pressure sensor to the breathing circuit tee connector proximal to the non-rebreather mask. The sensor provided estimates of oxygen with a measurement range of 0 to 100%, a response time of 15s, and full-scale linearity ± 1.0%. Using a MaxO ®2 analyzer [OM25-RME, Maxtec Inc., Salt Lake City UT]. we performed a single-point calibration of this sensor at room air and ~23°C prior to each use (Trumbower et al., 2012).
Serial data transmitted from the analyzer (1Hz) corresponded to changes in absolute O2 concentration ± 0.2% error, with about 12-second settling time. To measure O2 levels during rapid transitions in air sources, we used a high-precision fiber oxygen microsensor [PM-Pst7, Presens, Regensburg, Germany]. The PM-Pst7 provided estimates of O2 concentration with a measurement range of 0 to 100%, a temporal resolution of t90 = 1s, a resolution of ± 0.01% at 1.0% O2 and ± 0.05% at 20.9% O2, and an accuracy of ± 0.05% at 20.9 % O2 when properly calibrated. The high-precision O2 analyzer [Oxy1-ST, Presens, Regensberg, Germany] transmitted these digital data to a PC at 1Hz.
Pneumotach flow sensor:
We used a factory-calibrated linear pneumotachometer with amplifier [sensor model: 4700, amplifier model 1100; Hans Rudolph, Shawnee, USA] to measure air flow rate. The sensor has a linear range of ± 800-liter s−1 and accuracy of ± 2.0% at room air. We calibrated flow rate using integration of the analog flow signal to a fixed 3-liter volume (calibration syringe). We acquired flow rate signals using a PC-based acquisition system [Powerlab 16/30; AD Instruments, CO USA] at 1000Hz. The sensor data estimated the magnitude and frequency of variations in air flow within the breathing circuit.
Temperature sensor:
We recorded temperatures of ambient room air and of our breathing circuit before and during AIH and SHAM delivery protocols. We affixed an NTC thermistor [ITC-1000F InkBird Tech Co., USA] adjacent to the O2 analyzer during recordings. The sensor provided a measurement range of −50 to 99°C, a resolution of 0.1°C, and accuracy of ± 2.0°C. We used this sensor to estimate the magnitude of variations in air temperature at the face mask during AIH and SHAM treatments protocols.
Cardiopulmonary Monitor:
To ensure safety, a stand-alone cardiopulmonary monitoring system [Masimo Radical-7 and Root Platform, Masimo Corp., USA] recorded participant blood O2 saturation (SpO2), and heart rate (HR) every second, and blood pressure (BP) every five minutes. During ‘automated’ AIH delivery, we used custom PC-based control software to acquire these parameters at 1Hz via universal serial bus (USB).
C. Protocols
AIH Delivery Protocols
The purpose of our study protocol is to characterize the performance of the ‘manual’ system to administer AIH as compared to a novel ‘automated’ system.
Manual delivery:
The ‘manual’ AIH protocol requires a trained administrator to physically control the supply of air to a participant’s non-rebreather facemask. We refer to “manual” as the use of a protocol administrator to ensure the air delivery system hose is either physically connected to or disconnected from the facemask at alternating time intervals (Figure 1A). When the administrator connected the air generator to the facemask, the generator served as air source. When the administrator disconnected the air generator from the mask, the room served as air source, with flow driven by subject inspiration. An episode of AIH consisted of 60s or 90s bouts of generator air (nominally 10.0% or 20.9% O2 concentration) and 60s interval of room air (nominally 20.9% O2 concentration). A sequence of AIH consisted of 15 episodes of both generator and room air intervals.
Automated delivery:
The ‘automated’ delivery system provides alternating air delivery without the need for a trained administrator (Figure 1B). The system consisted of a timed solenoid-valve system that directed air alternately from the air delivery system or blower to the non-rebreather facemask. A pair of PSA generators served as the low O2 (AIH) or ambient air (SHAM) source. The air blower operated as a source of positive-pressure room air during the 60s intervals. As shown in Figure 1B, these sources suppled air via a common breathing circuit. Similar to the ‘manual’ delivery protocol, the circuit included HEPA filters, a set of 3-liter expandable reservoir bags, and ~3m of clear tubing for each generator. The automated protocol also used a 6-liter mixing chamber to help reduce oscillations in FiO2 during steady-state hypoxic air flow from the generators.
To automate air delivery, we developed and implemented a stand-alone AIH delivery application using the C# programming language. As opposed to ‘manual’ switching between low O2 and room air sources, this software application provided a user interface to command switching protocols to the airflow controller. The control software required USB-interface between the microcontroller and a laptop PC running the Windows 10® operating system. The application provided protocol adjustments, as well as acquired dose timing and physiological data to ensure safety and efficacy. The application enabled users to 1) configure physiological recordings from a patient monitor, 2) adjust duration, and frequency of low O2 intervals, 3) establish safety limits that truncate or terminate low O2 exposure, and 4) save/load person-specific protocol settings. Collectively, these features provide safe, consistent, and flexible AIH delivery options that are not available in current ‘manual’ systems.
Safety Protocols
Safely and consistently administering AIH treatment protocols is of highest priority. Regardless of delivery system, we monitored participant comfort and attention, as well as, their SpO2, HR, and BP before, during, and after AIH delivery. For ‘manual’ AIH delivery protocols, we implemented a 75% SpO2 safety limit that prompted trained personnel to remove low O2 hosing from a participant’s facemask until SpO2 levels returned to baseline levels. This required ‘manual’ switching between air sources based on visually inspected SpO2 readings. The ‘automated’ delivery system used real-time data acquisition and recording of SpO2, HR, and BP to eliminate the possibility of human switching and recording errors. In cases where SpO2 measurements fell below 75%, the ‘automated’ delivery system provided one or more 60s intervals of room air until SpO2 measurements returns to > 90%. In cases where systolic blood pressure exceeded 150 mmHg, the delivery system provided room air.
Experimental Protocols
While brief episodes of AIH appear safe and efficacious in humans (Lynch et al., 2017; Navarrete-Opazo et al., 2016; Tester et al., 2014; Trumbower et al., 2017; Trumbower et al., 2012), there are deficiencies in these delivery systems that may delay or prevent the technology’s translation to clinic and home use. Thus, we performed a set of experiments to examine design issues related to inconsistencies in dose timing and undesired fluctuations in flow rate, and O2 concentration during ‘manual’ AIH delivery protocols. We also performed similar experiments to examine the extent to which an ‘automated’ AIH delivery system may overcome the design challenges inherent to the earlier delivery systems.
Experiment 1: Quantifying dose timing
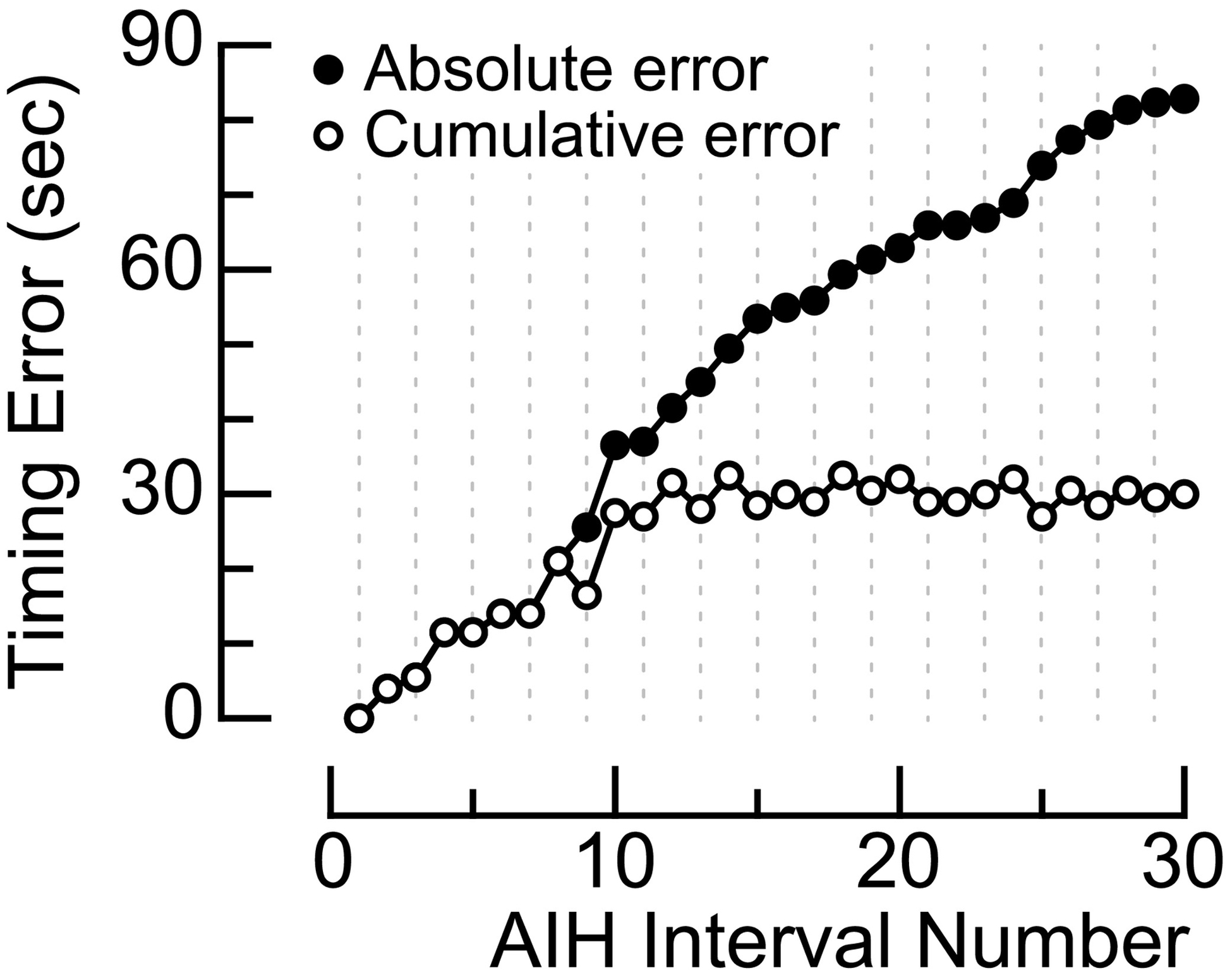
The sequence of ‘manual’ AIH treatment protocols is susceptible to human error, but the extent of this error remains unclear. In brief, the ideal dose timing of AIH consists of 15, 90s breathing episodes of oxygen-deprived air alternating with 60s intervals of room air (Trumbower et al., 2012). To deliver this sequence of concentration intervals to the participant, trained personnel physically attached and detached air-supply tubing to the participant’s face mask. In experiment 1, we measured the cumulative and absolute timing errors while a trained administrator (S1) delivered a single sequence of AIH treatment (Figure 2A).
Figure 2.
Quantifying temporal accuracy during an acute intermittent hypoxia (AIH) delivery protocol of 90s breathing bouts of low O2 with 60s intervals of breathing ambient room air. A) Time-dependent changes in relative O2 (solid, black line) from the ‘manual’ AIH delivery system as compared to the ‘ideal’ AIH protocol (solid, gray line). B) Time-dependent changes in relative O2 from the ‘automated’ AIH delivery system (dashed, black line) as compared to the ‘ideal’ AIH protocol (solid, gray line).
Experiment 2: Characterizing flow rates
Adequate flow to the face mask is important for maintaining safe and comfortable breathing during AIH treatment protocols. The PSA systems rely on pumps (e.g., vacuum) to distribute air mixtures through the breathing circuit. However, air flow from the PSA systems are oscillatory due to alternating pump phases and other mechanisms. The pulsatile air flow propagates from the generator to the face mask and varies according to generator flow settings. In experiment 2, we evaluated the amplitude and frequency of fluctuations in air flow proximal to the face mask via the ‘manual’ delivery system (one PSA with reservoir bags) that delivered 10.0% and 20.9% FiO2. We repeated these air flow measurements using the ‘automated’ system (two PSA with reservoir bags).
Experiment 3: Characterizing fluctuation in oxygen concentrations
An AIH delivery system must provide consistent levels of O2 to the face mask to accommodate variations in breathing (i.e., deep inhales, yawns) during AIH therapy. Breathing in low O2 often triggers episodes of deep breathing to increase tissue oxygenation (Bilo et al., 2012), but also may exceed the available air supplied by the ‘manual’ single PSA system. We first examined consistency in the O2 supply within and between the AIH delivery systems during quiet breathing of a study participant (S2). Similar to Experiment 1, each system delivered a sequence of AIH. We attached an O2 sensor proximal to the face mask and measured O2 concentrations during the AIH sequence. We then instructed S2 to take a series of deep breaths during a single 60s bout of low O2. A similar protocol involved the use of an ‘automated’ AIH system.
D. Data Analyses
AIH dosing:
Precise timing and amplitude of air delivery ensure safe and reliable AIH treatments. Physical switching during ‘manual’ AIH delivery is subject to dosing accuracy and timing errors. Thus, we estimated the accuracy of our delivery systems (manual, automated) to the ‘ideal’ AIH protocol defined above. Specifically, we computed the normalized root mean square error (NRMSE) of the goodness-of-fit (GOF, eq. 1) between delivery systems and the ‘ideal’ delivery protocol (Hyndman and Koehler, 2006).
| (1) |
where ỹ is the ‘ideal’ output, y is the output from either the ‘manual’ or ‘automated’ delivery system, and ȳ is the mean of y. Timing errors corresponded to the differences between the ‘ideal’ and ‘manual’ dose time during a single AIH sequence (n = 15 low O2 episodes, n = 15 room air intervals). We computed the absolute timing error (TEabs) as the absolute incremental sum of the timing errors (eq 2).
| (2) |
Flow rate:
While continuous air flow to the face mask ensures user comfort and stability of AIH treatment, there is potential for fluctuations in air flow within the breathing circuit that may compromise consistency of air delivery. Thus, we quantified the extent to which frequency, amplitude, and range of flow rate change during steady-state room air and low O2 air delivery. We compared the peak amplitudes and ranges in measured flow rate between the AIH systems (‘manual’, ‘automated’) and FiO2 (low O2 at 10.0%, ambient O2 at 20.9%).
Oxygen concentrations:
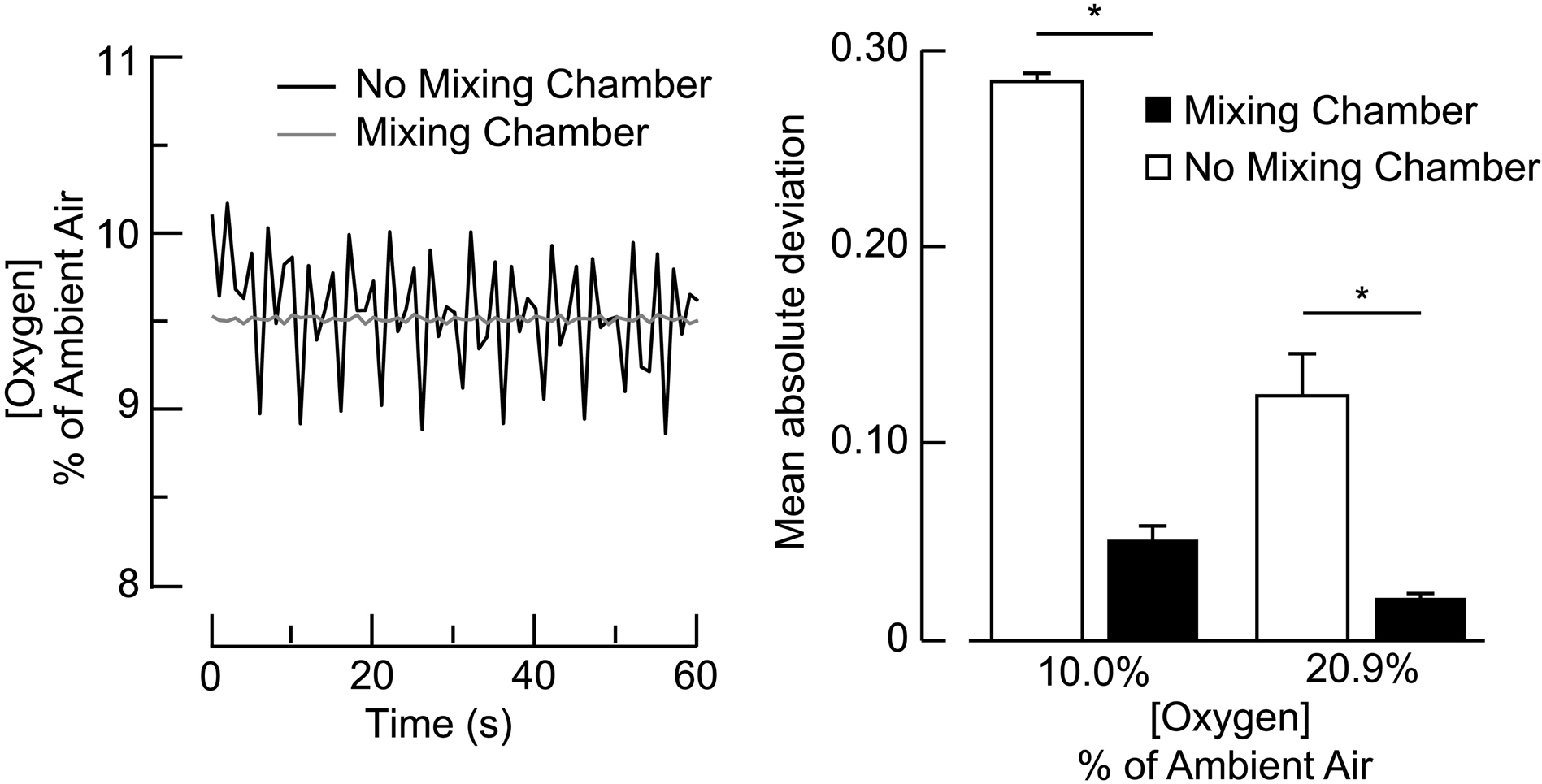
Consistent and reliable FiO2 to the face mask is necessary to maintain safe and accurate AIH treatments. Fluctuations in air flow from the PSA systems contribute to fluctuations in O2 concentrations during AIH protocols, but the magnitude of these fluctuations remains unclear. Thus, we quantified the relationship between air flow to the face mask and oxygen-deprived air generated from the breathing circuit of the ‘manual’ and ‘automated’ delivery systems. A major design feature of the ‘automated’ system is the addition of a mixing chamber air from the HYP-123 generator. To examine the extent to which the mixing chamber reduced fluctuations in constant-resistance FiO2, we compared the mean absolute standard deviation (MAD) in fluctuations between the ‘automated’ delivery system (with and without the mixing chamber) and FiO2 (low O2 at 10.0%, ambient O2 at 20.9%).
During episodes of low O2 delivery, positive pressure from the PSA device ensures the AIH delivery system expels oxygen-rich air to the environment and delivers oxygen-depleted air to the breathing circuit. However, spontaneous deep breaths (e.g., yawn or sighs) and other patient specific ventilation patterns may disrupt this air separation due, in part, to pressure and/or the depletion of the available air supply by the single generator. Limited air flow from the ‘manual’ (single PSA) system during deep breathing contributes to increases in the inspiratory work of breathing and to negative pressure changes within the breathing circuit. The extent to which this vacuum may reduce generation of oxygen-depleted air needed to be characterized. Thus, we compared peak O2 concentration generated from a single versus a double PSA system while a participant (S2) performed five consecutive deep breaths at 10.0% FiO2.
However, spontaneous deep breathes (e.g., yawn) may disrupt this air separation due, in part, to reduction in air volume within the breathing circuit. Limited air flow from the ‘manual’ (single PSA) system during deep breathing contributes to increases in the inspiratory work of breathing and to negative pressure changes within the breathing circuit. The extent to which this vacuum may reduce generation of oxygen-depleted air is not clear. Thus, we compared peak FiO2generated from a single versus a double PSA system while a participant (S2) performed five consecutive deep breaths at 10.0% FiO2.
Air temperature:
Stable air temperature during AIH treatment is important for ensuring breathing comfort, as well as, for preserving SHAM blinding. However, the extent to which changes in O2 during AIH protocols may result in differences in air temperature when administering room air and low O2 is not known. Thus, we measured air temperature within the breathing circuit during two sequences (30 episodes) of AIH and SHAM and quantified air temperature changes and compared these changes between the two air delivery methods.
E. Statistical Analyses
We performed all statistical analyses using SPSS® 26 (IBM SPSS Inc, USA) and reported data as mean ± 1 standard error (SE). Statistical significance corresponded to a p-value < 0.05. We used the Levene Test to determine homogeneity of variances between our independent factors (Levene, 1960). If variances differed (p > 0.05), we used equivalent non-parametric tests. We compared the TEabs to a null expectation of 0 (no error) using a one-sample t-test. We also compared the effects of transitioning between room air and low O2 on TEabs using a two-sample t-test. To compare the effects of AIH delivery systems on amplitude and range of our peak flow rate estimates, we used a linear mixed-model with fixed effects (Cleophas et al., 2012) and Bonferroni corrections for the multiple contrasts. A two-way analysis of variance (ANOVA) model provided comparison between the main effects of AIH delivery system (‘manual’, ‘automated’) and FiO2 (10.0%, 20.9%) and their interactions on fluctuation in O2 and temperature within the breathing circuit.
Results
In this study, we examined the performance of two AIH delivery systems. Our results include between-system comparisons for dose timing, air flow, FiO2, and air temperature constancy.
AIH dose timing
The ‘manual’ delivery system is less accurate in administering an AIH protocol at prescribed timing intervals than the ‘automated’ delivery system. Goodness-of-fit between ‘ideal’ and ‘manual’ AIH delivery equated to 34.8% as compared to 98.1% between ‘ideal’ and ‘automated’ AIH delivery (Figure 2A, 2B). Reduced accuracy in the ‘manual’ system is due, in part, to physical switching errors. Manual attaching/detaching the hosing to the face mask resulted in a mean absolute timing error of 2.9 ± 0.5s (t1,28 = 6.4; p < 0.001) that corresponded to a cumulative timing error of 30s within a single AIH sequence (Figure 3). Transition time resulted in a longer duration of low O2 (93.6 ± 0.7s) than the prescribed duration of 90s (t1,14 = 3.6; p < 0.003) and a shorter duration of room air exposure (58.4 ± 0.5s) than the prescribed duration of 60s (t1,13 = 4.7; p < 0.001). Larger absolute timing errors occurred during transitions to room air (3.6 ± 0.7s) than to low O2 (2.1 ± 0.4s). However, we found no difference in the absolute timing errors between room air and low O2 transitions (t1,27 = −1.8; p = 0.09).
Figure 3.
Cumulative temporal errors from S1 who administered a single sequence of AIH with the ‘manual’ delivery system. The plot with white-filled circles indicate a cumulative positive temporal error that corresponds to overall delay in the trained administrator (S1) who disconnected the tube from the face mask of participant S3. The plot with black-filled circles indicate the cumulative absolute error (secs) over time. There was a significant absolute error in switching times (p<0.001).
Fluctuations in flow rate
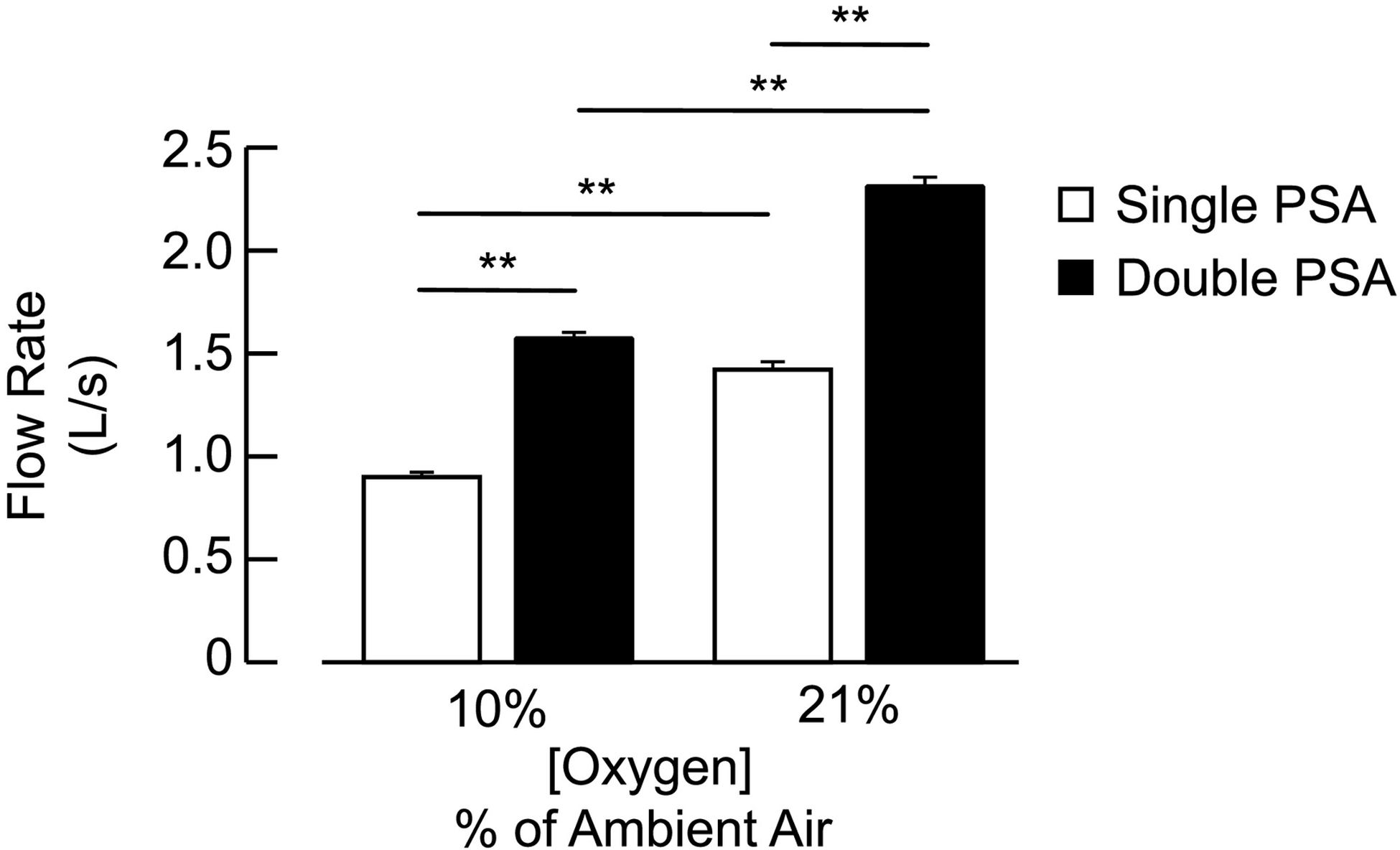
Flow rates differed between the ‘manual’ and ‘automated’ AIH delivery systems (Figure 4). As expected, the ‘automated’ system generated greater peak and average flow rates due to the additional PSA device. At 20.9% FiO2, peak flow rates from the ‘automated’ system reached 2.31 ± 0.05 liters s−1, which corresponded to 62.7% more flow than the ‘manual’ system (p < 0.001). We suspect the higher flow rates reduced one-way valve resistance at the face mask and reduced inspiratory work during breathing ‘automated’ system generated greater flow rate fluctuations at 20.9% FiO2 (1.17 ± 0.05 liters s−1) as compared to 10.0% FiO2 (0.90 ± 0.03 liters s−1; p < 0.001), which exceeded the flow rate fluctuations within the ‘manual’ system (all p-values < 0.001). We found the increased flow rates within the ‘automated’ system also corresponded to increased fluctuations in flow that occurred periodically at 0.4Hz.
Figure 4.
A) Relation between delivered O2 concentrations and pressure-swing absorption (PSA) flow rate. B) Effects of delivery system on flow rate at low O2 (10.0 ± 2.0%) and room air (20.9 ± 2.0%). Bars correspond to mean ± 1 standard error. Black bars correspond to the flow rate for ‘automated’ system with double PSA and white bars indicate the flow rate for ‘manual’ system with single PSA. Asterisk (*) corresponds to statistical significance at p < 0.01.
Variations in FiO2
Consistency in FiO2 is important for ensuring safe and effective AIH treatment. The reduced accuracy of AIH delivery in the ‘manual’ system is due, in part, to inherent fluctuations in FiO2 (Figure 5A) while the participant is not connected to the breathing circuit. During ‘manual’ air delivery, steady state FiO2 varied ± 10.0% at 0.4 Hz. The inclusion of a mixing chamber within the ‘automated’ delivery system resulted in a significant improvement (± 1.0% variation) in stability of air delivery at 10.0% and 20.9% FiO2 (Figure 5B). The mixing chamber reduced MAD to less than 5.0% during 10.0% FiO2 and less than 1.0% during 21.0% FiO2.
Figure 5.
Effects of a mixing chamber on magnitude of fluctuations in steady-state O2 concentration. In A, plots show the air delivery system without the mixing chamber that resulted in ~1% peak-to-peak O2 fluctuations (black trace) as compared to the delivery system with a 6L mixing chamber that resulted in ~0.06% peak-to-peak O2 fluctuation (gray trace). B) Bars represent mean ± 1 standard error in mean absolute deviation of O2 concentration within the breathing circuit. The mixing chamber (black bars) significantly reduced O2 deviations during room air and low O2 as compared to no mixing chamber (white bars). Greater O2 deviations occurred during low O2 as compared to room air. Asterisks indicate significant difference (p<0.05).
Changes in breathing volume imposed by the participants’ ventilation cause breath-by-breath fluctuations in FiO2 during AIH treatment. During quiet breathing, average flow rates are less than 0.2 liters s−1 (Zuurbier et al., 2009). However, deep breathing may exceed the available air supplied by the single PSA system since the required flow capacity is dictated by occasional extremes, rather than averages. Deep breaths in succession, with peak flow rates exceeding 1 liter s−1, depleted the ‘manual’ system’s reservoir bags and reduced the PSA outlet resistance leading to less effective O2 removal. As a result, oxygen concentrations increased 65.7% during deep breathing of low O2 air. However, the ‘automated’ system’s mixing chamber reduced the swing in FiO2 by 28.5% during extreme deep breathing.
Temperature during AIH delivery
We found significant changes in temperature within the breathing circuit during AIH and SHAM protocols. The temperature fluctuated approximately ±2.2°C. The ‘manual’ delivery system produced marginally higher temperatures (26.6 ± 0.1°C) as compared to the ‘automated’ system (26.1 ± 0.1°C; p < 0.001). The ‘manual’ system generated a small, but significant difference (p = 0.001) in temperature between AIH (26.8 ± 0.1°C) and SHAM (26.4 ± 0.1°C). However, we found no significant temperature differences between AIH (26.2 ± 0.1°C) and SHAM (26.0 ± 0.1°C) using the ‘automated’ delivery system (p = 0.6). We observed that greatest fluctuations in air temperature occurred within 45 min of system start-up. We compared the ‘automated’ air temperatures before after a 45 min warm-up. The average air temperature was significantly lower (p<0.001) before warm-up at 26.6 ± 0.1°C versus 27.0 ± 0.1°C after a 45 min warm-up.
Discussion
Mild exposure to breathing low O2 (i.e., AIH) is a novel treatment to enhance motor function after SCI. While the ‘manual’ AIH delivery system enabled several exciting proof-of-principle experiments in humans (Christiansen et al., 2018; Hayes et al., 2014; Lynch et al., 2017; Sandhu et al., 2019; Trumbower et al., 2017; Trumbower et al., 2012), this technology faces design challenges that limit possible translation to clinical and home use. Here, we found significant inconsistencies in dose timing, volume flow rates, FiO2, and to a lesser extent temperature stability of the ‘manual’ AIH delivery system. We pursued development and implementation of an ‘automated’ AIH system to address several of these inconsistencies.
The ‘automated’ AIH delivery system improved dose timing accuracy. The new system outperformed the ‘manual’ system by more than 63%. This is not surprising since the automated system used programmable relay switch circuits to control the state of solenoid valves on the order of milliseconds. The transition time is a major contrast to the ‘manual’ system with an administrator who switched between air sources 100x slower. Manual switching resulted in accumulation of timing errors that equated to an extra dose of low O2 over the course of a daily exposure protocol We suspected fatigue may have resulted in larger errors in manual switching with time, but our results from S1 showed no positive correlation between the absolute timing error size and episode number. We recognize timing errors are likely to vary drastically between and within administrators and treatment days. However, the ‘automated’ delivery system eliminates this variability and affords greater temporal consistency as compared to ‘manual’ AIH delivery protocols.
There are several other air delivery systems to consider, but present with deficiencies that limit their translation potential. Several commercial companies offer stand-alone PSA systems (e.g., HYP123; Hypoxico, Inc.) that transform enclosed rooms into high-altitude training experiences. While these systems are capable of achieving low O2 levels at or near 10%, these enclosures require several minutes for transition between ambient room air and low O2. They also do not ensure a room with uniform concentration of the prescribed air mixture. Alternatively, high-pressure gas cylinders may be a reasonable consideration since they have the capacity to deliver high precision air mixtures (Navarrete-Opazo et al., 2017). Gas companies (e.g., Praxair Technology Inc., USA) provide customized gases, as well as various accessories such as check valves, fittings, regulators, alarms, and gauges. However, these cylinders require administrative operating expenses, gas handling equipment, and storage areas free from liquids, combustibles, and corrosive materials. Clinical facilities routinely accommodate these requirements, but at a premium. There are hidden costs associated with maintaining vendor contracts, inflexible delivery schedules, rental fees, Occupational Safety and Health Administration regulatory requirements, and a hazardous material fees to ensure public safety.
Maintaining a net positive pressure of air flow to the facemask accommodates a broader range of end-users with varying breathing frequencies and tidal volumes. Average resting minute ventilation of healthy adults is between 0.09 to 0.35 liters s−1. While a single PSA meets these ventilatory demands, the required flow rate capacity needs to accommodate breath-by-breath variations in flow rates that exceed this average. A previous report showed that peak inspiratory flow rate can be nearly 4 times the average resting rate (Seheult et al., 2014).
Moreover, participants often yawn during AIH; while the physiological triggers that may induce yawns is debatable (Corey et al., 2012; Marraffa et al., 2017), a yawn induces rapid inspiration ~400% greater than resting tidal volume (Corey et al., 2012). In either case, a second PSA with reservoir bags meets these transient increases volume and flow rate demands, as well as, ensures positive pressure at the mask for comfortable breathing.
While two PSA systems are sufficient to meet a broad range of flow rate demands, they may not be necessary. One alternative strategy to multiple PSAs is to increase the reservoir volume. Elastic reservoir bags reduce negative pressure (i.e., suction) caused when the end user’s respiratory flow variations overcome the positive pressure supplied by the PSA. If the reservoir volume is emptied by several large, rapid breaths, then mask inflow is limited to the PSA flow rate. Any further inhalation is impeded, and PSA output pressure may drop by as much as 25.4 cm H2O, for a moderate increase in flow rate, along with a rise in O2 concentration. As we observed in one study participant (S2), deep inspiration raised the mean ventilation well above 1.0 liters s−1 with peak flow rates 3 times the average, causing breathing difficulty as the inspiration rate was faster than the generators supplied. The resulting FiO2 climbs gently while two reservoir bags are being emptied. When the bags are depleted, O2 briefly jumps to about 17% at each deep breath. The ‘automated’ system doubles the reservoir volume to 12 liters, alleviates the breathing challenge due to higher flow resistance during deep inspiration and mitigates disturbance to O2 concentration. Notably, even when inflated, the reservoir bags do not deliver air with a sustained pressure. If charged to approximately 7.6 cm of water pressure, bag pressure drops to 1.3 cm after delivering 0.02–0.03 liters, requiring the subject to pull the gas through the hose system with noticeable breathing difficulty. One future design opportunity would be to develop an air reservoir that stores considerable air at higher constant pressure (e.g., 5 cm of water) to ensure deep breaths would not destabilize reservoir pressure or PSA FiO2.
The ‘automated’ system also reduced unwanted fluctuations in steady-state FiO2 that were inherent to the ‘manual’ delivery system (Figure 4). The dramatic reduction in FiO2 fluctuations is due, in large part, to the 6-liter air mixing chamber. The chamber provided volume expansion to enable air mixing (e.g., averaging) from two generators. However, the pulsatile flow pattern of each generator is unique to the PSA mechanism and was not eliminated. Changes in flow patterns were perceivable by study participants, but the participants did not report discomfort.
A concern for participants was the slight increases in breathing difficulty when the reservoir bags of the ‘manual’ system neared depletion after deep breathes. Nevertheless, changes in flow patterns that achieve the same average flow rate may be considered in future designs to further ensure that sham deliveries retain similar features as low O2 delivery.
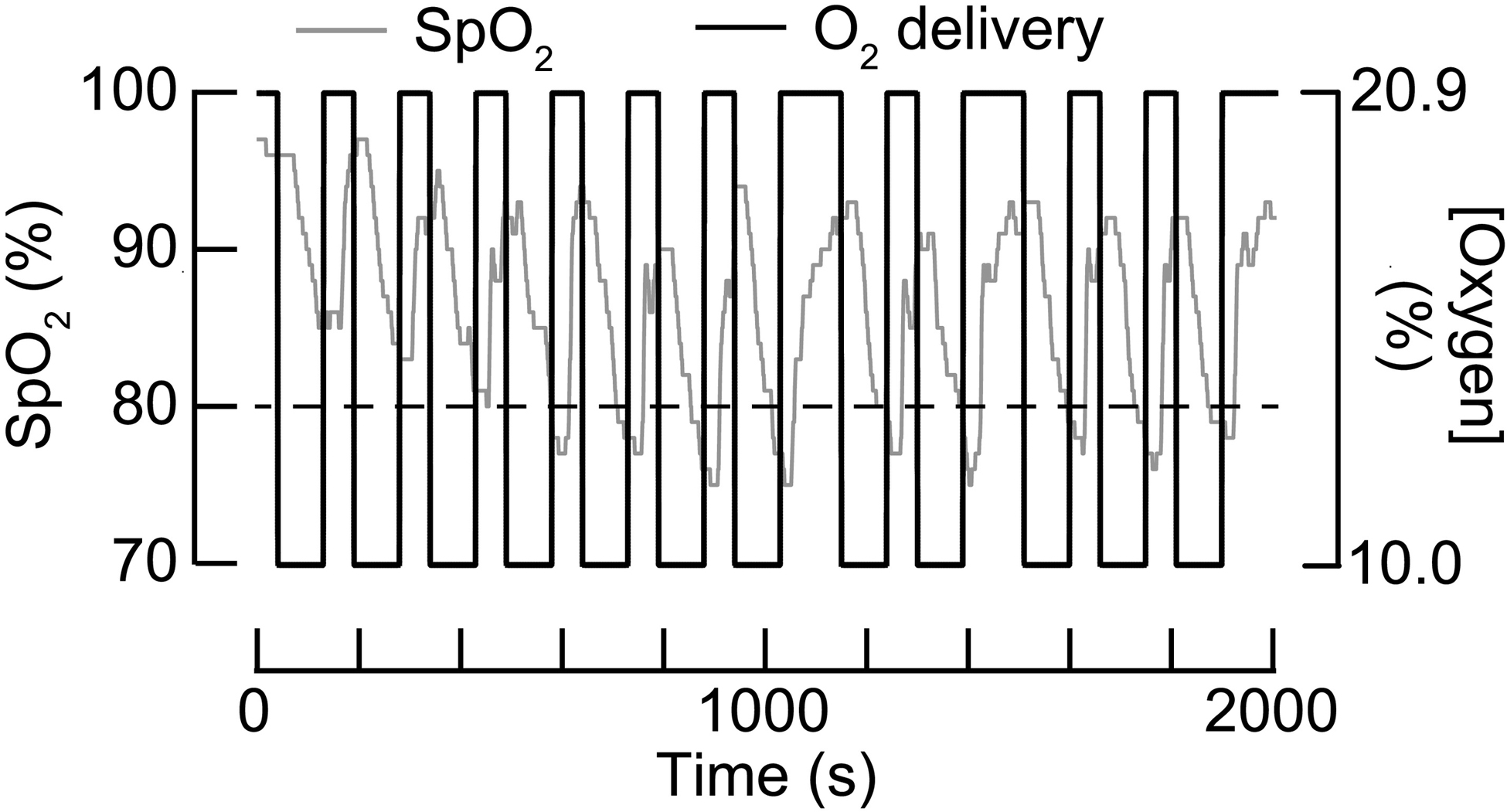
In this study, we implemented a stand-alone graphical user-interface to prescribe, monitor, and log dosing parameters and physiological data during AIH treatments. The user-interface enabled the administrator to preset the level of threshold detection for SPO2, HR, and BP parameters for end-user safety. Thus, the AIH protocol does not require an administrator to disconnect low O2 sources in response to cycle-to-cycle variations in the end-user’s de-saturation and re-saturation rates in addition to keeping track of timing intervals. Further, override commands are implemented for the administrator to immediately stop low oxygen delivery at any point in the cycle if deemed necessary. Automated monitoring and logging of the end-user’s vitals eliminates the possibility of adverse events resulting from prolonged exposures to low O2 even when the administrator fails to override the automated switching. Figure 6 plots representative SPO2 data logged with the automated system from an able-bodied subject (S3) during a full 15 cycle AIH delivery. Automated extensions in room air delivery can be observed in cycle 7 when S3 did not re-saturate passed the 80% SPO2 safety threshold. Further, the user interface allows the flexibility to quickly manipulate the low oxygen and room air interval timing for studies that may require alternative dosing parameters. Together, these user-interface features simplify the experimental protocol setup while supplementing safety monitoring of the end user.
Figure 6.
Temporal changes in blood oxygen saturation (SpO2) of a research participant (S3) during a singles sequence (N=15 episodes) of acute intermittent hypoxia (AIH). Gray trace corresponds to the change in SpO2 levels during repetitive breathing bouts at 10.0% and 20.9% O2 (Black trace). Broken black horizontal trace indicates an 80% SpO2 safety threshold. The ‘automated’ system delivers room air when SpO2 dips below 80% during low O2 and extends room air intervals when SpO2 values remain below 80%.
Despite these improvements, there are several unmet challenges for future system design consideration. To further enable consistent low O2 delivery, future designs need to consider ways to reduce face mask leaks. Improper seating of the inlet valve flap, as sometimes caused by distortion of the flap, may result in discernible outflow during attempts to exhale. It seems likely that such outflow is the cause of observed reduced O2 readings from the inlet line, since exhaled air is always lower O2 than incoming air. Similarly, improper sealing around the end-user’s facial features may cause room air to mix with delivered low O2, resulting in higher FiO2. Given our user’s sensitivity to air flow and breathing comfort, the length of the tubing used, and the volume of the breathing circuit may be further reduced in order to minimize dead space and perceived breathing resistance. Another consideration is the inclusion of permanent sensors for airflow, pressure, temperature, and humidity to provide continuous readout to the administrator. Data from these sensors would enable not only consistent delivery parameters from day to day laboratory environmental variations (e.g. air conditioning), but also minimize detectable differences between sham room air and intermittent hypoxia. Preserving the integrity of protocol blinding is critical for addressing confounds of potential placebo effects. Considering evidence that mild AIH with sustained hypercapnic conditions increased ventilatory long term facilitation and minute ventilation in rodents (Wen et al., 2019) and humans (Tester et al., 2014) with cervical SCI, the regulation and monitoring of CO2 (Georgopoulos et al., 1989; Reynolds et al., 1972) may be an important implementation in future device development. Similarly, applying real time capnography in future versions of the ‘automated’ system will further elucidate AIH-induced ventilatory responses while also enhancing safety. Finally, reducing the footprint of all the system components (user interface + breathing circuit + PSA+ sensors) into a single, integrative form factor may likely facilitate easier clinical integration.
Conclusion
We developed and characterized an ‘automated’ AIH delivery system that confers several advantages over the previous ‘manual’ AIH system. The automated system 1) incorporated digital control to automate precise interval timing for temporal consistency 2) eliminated large fluctuations in delivered O2 concentration to increase accuracy, 3) added physiological monitoring via closed loop feedback to increase safety, 4) added of continuous positive pressure to accommodate end user respiratory variations and breathing comfort, and 4) implemented a stand-alone graphical user interface for prescribing personalized treatment protocols, as well as, logging physiological responses. Together, these new features provide a consistent, safe, and flexible AIH delivery system for the development of new AIH treatment protocols (Naidu et al., 2020).
Acknowledgements
We greatly appreciate our study participants. We thank Stella Barth and Sarah Adamo for their assistance in data collection. This work received support from the National Institutes of Health, National Institute of Child Health and Development (R01 HD081274), U.S. Department of Defense, Spinal Cord Injury Research Program (W81XWH-15-2-0045), Craig H. Neilsen Foundation, and the Wings for Life Foundation (WFL-US-026).
Abbreviations
- AIH
acute intermittent hypoxia
- BDNF
brain-derived neurotrophic factor
- FiO2
fraction of inspired oxygen
- O2
Oxygen
- PSA
pressure-swing absorption
- SCI
spinal cord injury
- SE
standard error
- SHAM
Breathing room air
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All subjects gave written informed consent in accordance with the Declaration of Helsinki prior to participation and the study is approved by the institutional review board of Partners Healthcare in Boston, MA USA.
Competing interests
The authors declare that they do not have competing interests.
References
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, 2004. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7, 48–55. [DOI] [PubMed] [Google Scholar]
- Bilo G, Revera M, Bussotti M, Bonacina D, Styczkiewicz K, Caldara G, Giglio A, Faini A, Giuliano A, Lombardi C, Kawecka-Jaszcz K, Mancia G, Agostoni P, Parati G, 2012. Effects of slow deep breathing at high altitude on oxygen saturation, pulmonary and systemic hemodynamics. PLoS One 7, e49074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Urbin MA, Mitchell GS, Perez MA, 2018. Acute intermittent hypoxia enhances corticospinal synaptic plasticity in humans. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleophas TJ, Zwinderman AH, van Ouwerkerk B, 2012. Clinical research: a novel approach to the analysis of repeated measures. Am J Ther 19, e1–7. [DOI] [PubMed] [Google Scholar]
- Corey TP, Shoup-Knox ML, Gordis EB, Gallup GG Jr., 2012. Changes in Physiology before, during, and after Yawning. Front Evol Neurosci 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos D, Berezanski D, Anthonisen NR, 1989. Effects of CO2 breathing on ventilatory response to sustained hypoxia in normal adults. J Appl Physiol (1985) 66, 1071–1078. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119, 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutorbe P, Daranda E, Asencio Y, Esnault P, Prunet B, Bordes J, Palmier B, Meaudre E, 2013. Leaks can dramatically decrease FiO2 on home ventilators: a bench study. BMC Res Notes 6, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD, 2014. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyndman RJ, Koehler AB, 2006. Another look at measures of forecast accuracy. International Journal of Forecasting 22, 679–688. [Google Scholar]
- Levene H, 1960. Robust tests for equality of variance, in: Olkin (Ed.), Contributions to probability and statistics: Essays in honor of Howard Hotelling. Stanford University Press, Palo Alto, pp. 278–292. [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS, 2012. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32, 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Duffell L, Sandhu M, Srivatsan S, Deatsch K, Kessler A, Mitchell GS, Jayaraman A, Rymer WZ, 2017. Effect of acute intermittent hypoxia on motor function in individuals with chronic spinal cord injury following ibuprofen pretreatment: A pilot study. J Spinal Cord Med 40, 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffa A, Lekander M, Solsjo P, Olsson MJ, Lasselin J, Axelsson J, 2017. Yawning, a thermoregulatory mechanism during fever? A study of yawning frequency and its predictors during experimentally induced sickness. Physiol Behav 182, 27–33. [DOI] [PubMed] [Google Scholar]
- Naidu A, Peters DM, Tan AQ, Barth S, Crane A, Link A, Balakrishnan S, Hayes HB, Slocum C, Zafonte R, Trumbower RD, 2020. Daily acute intermittent hypoxia to improve walking function in persons with subacute spinal cord injury: a randomized clinical trial study protocol. BMC Neurol In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Alcayaga J, Sepulveda O, Rojas E, Astudillo C, 2017. Repetitive Intermittent Hypoxia and Locomotor Training Enhances Walking Function in Incomplete Spinal Cord Injury Subjects: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J Neurotrauma 34, 1803–1812. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Alcayaga J, Testa D, Quinteros AL, 2016. Intermittent Hypoxia Does not Elicit Memory Impairment in Spinal Cord Injury Patients. Arch Clin Neuropsychol 31, 332–342. [DOI] [PubMed] [Google Scholar]
- NSCISC NSCISC, 2016. Spinal Cord Injury (SCI) Facts and Figures at a Glance. J Spinal Cord Med 39, 370–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds WJ, Milhorn HT Jr., Holloman GH Jr., 1972. Transient ventilatory response to graded hypercapnia in man. J Appl Physiol 33, 47–54. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Gray E, Kocherginsky M, Jayaraman A, Mitchell GS, Rymer WZ, 2019. Prednisolone Pretreatment Enhances Intermittent Hypoxia-Induced Plasticity in Persons With Chronic Incomplete Spinal Cord Injury. Neurorehabilitation and neural repair 33, 911–921. [DOI] [PubMed] [Google Scholar]
- Seheult JN, Costello S, Tee KC, Bholah T, Al Bannai H, Sulaiman I, Costello RW, 2014. Investigating the relationship between peak inspiratory flow rate and volume of inhalation from a Diskus Inhaler and baseline spirometric parameters: a cross-sectional study. Springerplus 3, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AQ, Barth S, Trumbower RD, 2020. Acute intermittent hypoxia as a potential adjuvant to improve walking following spinal cord injury: evidence, challenges, and future directions. Current Physical Medicine and Rehabilitation Reports In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH, 2014. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med 189, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Hayes HB, Mitchell GS, Wolf SL, Stahl VA, 2017. Effects of acute intermittent hypoxia on hand use after spinal cord trauma: A preliminary study. Neurology 89, 1904–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ, 2012. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabilitation and neural repair 26, 163–172. [DOI] [PubMed] [Google Scholar]
- Wen MH, Wu MJ, Vinit S, Lee KZ, 2019. Modulation of Serotonin and Adenosine 2A Receptors on Intermittent Hypoxia-Induced Respiratory Recovery following Mid-Cervical Contusion in the Rat. J Neurotrauma 36, 2991–3004. [DOI] [PubMed] [Google Scholar]
- Zuurbier M, Hoek G, van den Hazel P, Brunekreef B, 2009. Minute ventilation of cyclists, car and bus passengers: an experimental study. Environ Health 8, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]


