Abstract
Objective:
Osteogenesis imperfecta (OI) is commonly associated with short stature, but it is unclear whether this is exclusively secondary to fractures and bone deformities or whether there is a primary defect in longitudinal bone growth. As metacarpal and phalangeal bones are rarely affected by fractures and deformities, any length deficits in these bones should reflect a direct disease effect on longitudinal growth. This study therefore assessed the relationship of hand bone length with clinical OI type and genotype.
Study Design:
Prospective study.
Results:
The length of all 19 tubular hand bones were measured in 144 individuals (age 6 to 57 years; 68 female) who had OI caused by COL1A1 or COL1A2 variants. Measurements of bone length were converted to z-scores using published reference data. Bone length was mostly normal in OI type I but was significantly decreased in OI types III and IV. Mean hand bone length z-score (i.e., the average length z-score of all 19 bones of a hand) was −0.2 for OI type I, −2.9 for OI type III and −1.2 for OI type IV. Mean hand bone length z-score was positively associated with height z-score (r2 = 0.65, P < 0.001). Regarding genotype-phenotype correlations, mean hand bone length z-score was close to 0 in individuals with COL1A1 mutations leading to haploinsufficiency but were significantly lower in the presence of mutations leading to triple-helical glycine substitutions in either the alpha 1 or alpha 2 chain of collagen type I.
Conclusion:
COL1A1 and COL1A2 mutations affect bone growth not only by inducing fractures and bone deformities, but also through longitudinal growth deficits in bones that do not fracture or deform.
Keywords: Children, COL1A1, COL1A2, Growth, Osteogenesis imperfecta, Short Stature
1. Introduction
Osteogenesis imperfecta (OI) is a heritable condition that is associated with frequent fractures, scoliosis and many other skeletal and extraskeletal manifestations (1). The clinical severity varies widely, from lack of symptoms to perinatal lethality (1, 2). OI is usually caused by dominant mutations in one of the two genes that code for collagen type I, COL1A1 and COL1A2 (1, 3). The clinical phenotype caused by such mutations is typically separated into four types (4). OI type I represents the milder end of the spectrum, OI type II is the lethal form, OI type III is the most severe type of OI in survivors of the neonatal period, and OI type IV is intermediate in severity between OI types I and III. Presently there is no cure for OI, but intravenous bisphosphonates are widely used in children with OI to increase bone mineral density (BMD) and decrease bone fragility (3).
Short stature is a hallmark of OI, especially in OI types III and IV. A North American multicenter study found that in children with OI, the median height z-score was −0.7 for OI type I, −6.9 for OI type III, and −2.8 for OI type IV (5). The cause of short stature in OI is not entirely clear. In severe OI, short stature might be explained by lower extremity fractures and deformities, as well as vertebral compression fractures and scoliosis, as these manifestations are highly prevalent in OI type III and IV. However, it is also conceivable that mutations in collagen type I encoding genes directly affect growth plate activity during bone development and thereby lead to shorter bones (6).
Determining whether short stature in OI is entirely caused by the complications of bone fragility or is also due to a direct disease effect on growth plates is a clinically relevant question. If the disease-causing mutations in OI have a direct effect on longitudinal bone growth, it might be possible to develop therapies that target the involved pathways.
Analyses of hand radiographs offer the opportunity to evaluate the scope of systemic OI-associated growth deficits, as hand bones are rarely affected by fractures and deformities in OI. Length deficits in tubular hand bones therefore likely result from a direct effect of OI on longitudinal growth. The analysis of hand bone lengths is a well-established method for which detailed reference data are available (7, 8). As there are 19 tubular bones in the hand, it is easy to identify unusual differences in bone length that might point to a local problem, such as a fracture affecting a growth plate. Length measurements of all 19 tubular hand bones, a technique called metacarpophalangeal pattern profile analysis, has been applied to many genetic disorders, but data on the various OI types are lacking (8–11).
A few studies have measured the length of the second metacarpal in individuals with OI and reported normal results for most study participants (12–14). However, these studies were small (42 participants or less), did not report genotype information and either exclusively focused on OI type I (13) or did not compare results between different OI types (12, 14). It remains therefore unknown whether metacarpal length differs between OI types or is related to genotype.
In the present study we therefore aimed at evaluating the systemic effect of OI on longitudinal bone growth by using metacarpophalangeal pattern profile analysis. We investigated hand radiographs from 144 individuals with OI who had known pathogenic variants in either COL1A1 or COL1A2 and assessed the relationship between bone length and both clinical OI type and genotype.
2. Subjects and Methods
2.1. Study Population
The study population was comprised of individuals with a diagnosis of OI who were assessed in the context of the Brittle Bone Disease Consortium study (https://www.rarediseasesnetwork.org/cms/BBD). The consortium is a Rare Disease Clinical Research Network that is funded by the National Institutes of Health. One of the projects conducted by the consortium is a natural history study to assess the clinical features of OI. Patients with a diagnosis of OI of any type and any age are eligible to participate. Hand radiographs were systematically obtained in participants who were six years of age or older. The present evaluation includes data that had been collected on 144 participants at the Shriners Hospital for Children – Canada, who had been diagnosed with OI type I, III or IV as assessed by one of the authors (FHG or FR) and who had disease-causing variants in either COL1A1 (n = 95) or COL1A2 (n = 49). All study participants or their legal guardians provided informed consent. The study was approved by the Institutional Review Board of McGill University.
The majority of study participants had a history of intravenous bisphosphonate treatment (n = 114). These individuals had either received only pamidronate (n = 26), only zoledronate (n = 42) or had first been treated with pamidronate, followed by zoledronate (n = 46), using protocols as described (15, 16). The study cohort did not undergo testing for endocrine abnormalities, as the genetically confirmed diagnosis of OI was considered a sufficient explanation for short stature. Previous studies had shown that children with OI types III and IV and severe short stature had normal serum levels of insulin-like growth factor I and insulin-like growth factor binding protein 3 (17). None of the study participants had signs of rickets or other known concomitant disorders or treatments affecting growth.
2.2. Sequencing and Genotype Classification
Sequence analyses of COL1A1 and COL1A2 were performed by next-generation sequencing using an Ion Torrent PGM device (Life Technologies), as described (18). Results were compared to RefSeq sequences NM_000088.3 for COL1A1 and NM_000089.3 for COL1A2. As per study design, all individuals included in the present study were positive for a disease-causing variant in COL1A1 or COL1A2.
The following genotypic groups were distinguished: Mutations in COL1A1 that introduce stop codons or lead to frameshifts were classified as haploinsufficiency mutations. Mutations in either COL1A1 or COL1A2 that lead to glycine substitutions in the triple helical domains of the collagen type I alpha 1 or alpha 2 chains were regarded as glycine substitutions. Mutations close to exon/intron boundaries that were predicted or proven to affect splicing were considered splice mutations. Mutations affecting the C-propeptide of either the alpha 1 or the alpha 2 chain of collagen type I were classified as C-propeptide mutations. Other types of mutations were too rare for statistical analysis (n = 3) and therefore were excluded from the evaluation of genotype-phenotype associations.
2.3. Anthropometry
Height was measured using a Harpenden stadiometer (Holtain, Crymych, UK). Height measurements were converted to age- and sex-specific z-scores based on reference data for the general population, as reported by the Centers for Disease Control and Prevention (19).
2.4. Metacarpophalangeal Pattern Profile Analyses
Posteroanterior digital radiographs of the hand were taken at a uniform tube-to-film distance of 122 cm. The lengths of the 19 tubular bones of the hand (metacarpals, proximal phalanges, middle phalanges, distal phalanges) were measured on postero-anterior hand radiographs as described by Garn (7). Measurements were performed using a DICOM (RadiAnt Dicom Viewer, version 5.5.1) reader that recorded length measurements with a precision of a tenth of a millimeter. Raw measures of bone length were converted to age- and sex-specific z-scores using published reference data that are based on results from more than 1000 apparently healthy American individuals of European ancestry (7, 8). To assess the inter-rater variability of the bone length measurements, 15 hand radiographs were independently assessed by three of the authors (DR, CS, MER). The coefficient of variation for results from the three evaluators ranged from 0.7% to 1.6% for metacarpal length, from 0.5% to 1.1% for the length of proximal phalanges, from 1.6% to 2.7% for middle phalanges, and from 1.3% to 2.2% for distal phalanges.
Results for all 19 bones expressed as z-scores were arranged in a graphical format, the metacarpophalangeal pattern profile (20). To generate a single descriptor of bone length for each individual, the mean of all bone length z-scores for each hand x-ray was calculated. We called this parameter ‘mean hand bone length z-score’. To quantify the z-score variability between bones of the same hand, the pattern variability index was calculated as the variance of all bone length z-scores of each hand, as described by Garn (8).
2.5. Statistical Analyses
Group differences in dichotomous variables were tested for significance using the chi-square test. Analysis of variance (ANOVA) was used to examine differences in continuous variables between OI types and between genotype groups; Bonferroni’s adjustment was used for post-hoc analyses. The difference of z-score results to 0 (i.e., the mean result expected in the reference population) was tested for significance using the one-sample t-test.
Stepwise multiple regression analysis was used to assess potential predictors of mean hand bone length z-score results. Univariate analysis of variance was used to assess the significance of the difference in mean hand bone length z-scores between genotypes, after adjustment for sex, age and height z-score. For the purpose of the multiple regression analysis and the univariate ANOVA, gender was coded as male = 0 and female = 1, and bisphosphonate treatment history was coded as no = 0, yes = 1. In addition, the age of study participants aged 19 years or older was fixed at 19 years for these regression analyses, as no significant bone length changes are expected beyond 19 years of age (7).
All tests were two-tailed and throughout the study P-values <0.05 were considered significant. Calculations were performed using SPSS Software Version 24 for Windows (SPSS, Inc., Chicago, IL, USA).
3. Results
The study population comprised 144 individuals who had OI and pathogenic variants in either COL1A1 or COL1A2 (Table 1). Close to 80% of patients had received intravenous bisphosphonates prior to the time when the hand x-ray was obtained. As expected, height z-scores were low in all types of OI, but short stature was mild in OI type I, moderate in OI type IV and extreme in OI type III.
Table 1.
Study population by clinical OI type
| All OI types | OI-I | OI-III | OI-IV | P | |
|---|---|---|---|---|---|
| N (m/f) | 144 (76/68) | 55 (34/21) | 28 (12/16) | 61 (30/31) | 0.20 |
| Age (years) | 18.3 (10.7) | 19.6 (14.2) | 18.5 (6.1) | 17.0 (8.3) | 0.44 |
| History of Bisphosphonate Treatment (N, %) | 114 (79%) | 29 (53%) | 27 (96%) | 58 (95%) | <0.001 |
| Height z-score | −3.3 (3.0) | −0.8 (1.0)b,c | −8.0 (1.8)a,c | −3.5 (1.7)a,b | <0.001 |
| Mean Hand Bone Length z-score | −1.1 (1.4) | −0.2 (1.0)b,c | −2.9 (1.2)a,c | −1.2 (1.0)a,b | <0.001 |
| Pattern Variability Index | 0.46 (0.32) | 0.42 (0.32) | 0.52 (0.36) | 0.47 (0.31) | 0.36 |
Results are shown as N or mean (SD). P values indicate significance of the difference between OI types (ANOVA or chi square tests, as appropriate). Superscripts indicate significant differences (Bonferroni’s adjustment) to
OI type I,
OI type III,
OI type IV.
Evaluation of metacarpal phalangeal pattern profiles showed that length z-scores were significantly below zero for all bones in OI type III and IV, indicating that bones were shorter than in the reference population of the same age and sex (Figure 1). In OI type I, bone length z-scores were significantly below zero only for middle phalanx 2 and distal phalanges 2 to 5. Comparisons between OI types showed that z-scores for all 19 bones were significantly lower in OI type III than in either OI type I or OI type IV (p < 0.001 for each comparison). In OI type IV, the z-scores for all bones except for distal phalanx 5 were significantly lower than in OI type I.
Figure 1.
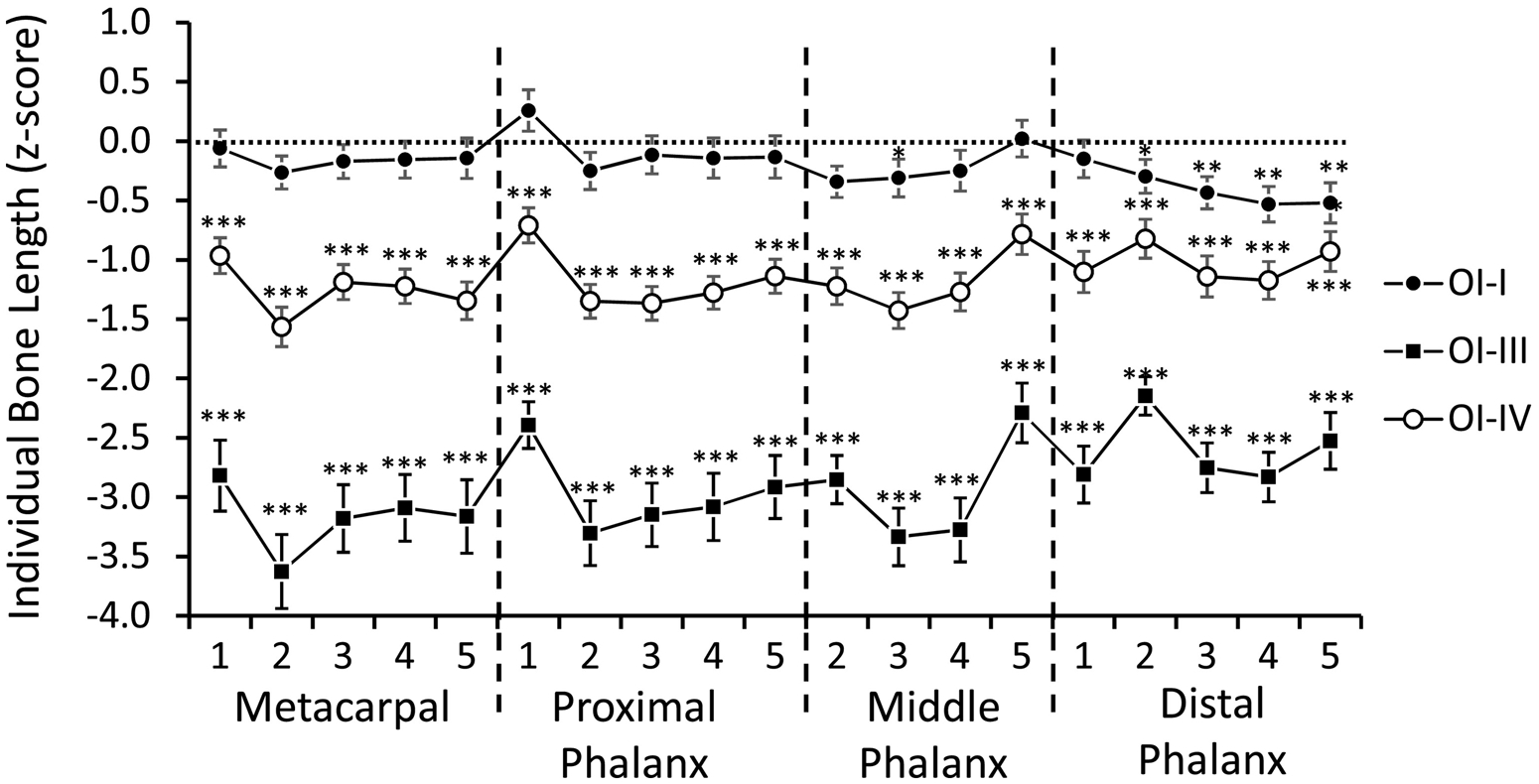
Metacarpophalangeal pattern profile (1 = thumb; 2 = index finger; 3 = middle finger; 4 = ring finger; 5 = little finger) in OI types I (n = 55), III (n = 28) and IV (n = 61). The length of each bone is expressed as age- and sex-specific z-scores. Error bars indicate the standard error of the mean. Results that are significantly different from zero are marked by asterisks: * p < 0.05; ** p < 0.01; *** p < 0.001.
We next evaluated mean hand bone length z-scores, defined as the average of all bone length z-scores of a hand (Figure 2A). Mean hand bone length z-score was highest in OI type I, lowest in OI type III and intermediate in OI type IV, and in the entire study population was closely correlated with height z-score (Table 1, Figure 2B). To assess the determinants of mean hand bone length z-score, we performed a stepwise multiple regression analysis, where age, sex, height z-score, and history of bisphosphonate treatment were assessed as independent variables. This revealed that age, sex, height z-score but not history of bisphosphonate treatment were significant predictors of mean bone length z-score. The final equation (P < 0.001, adjusted r2 = 0.68) was:
Figure 2.
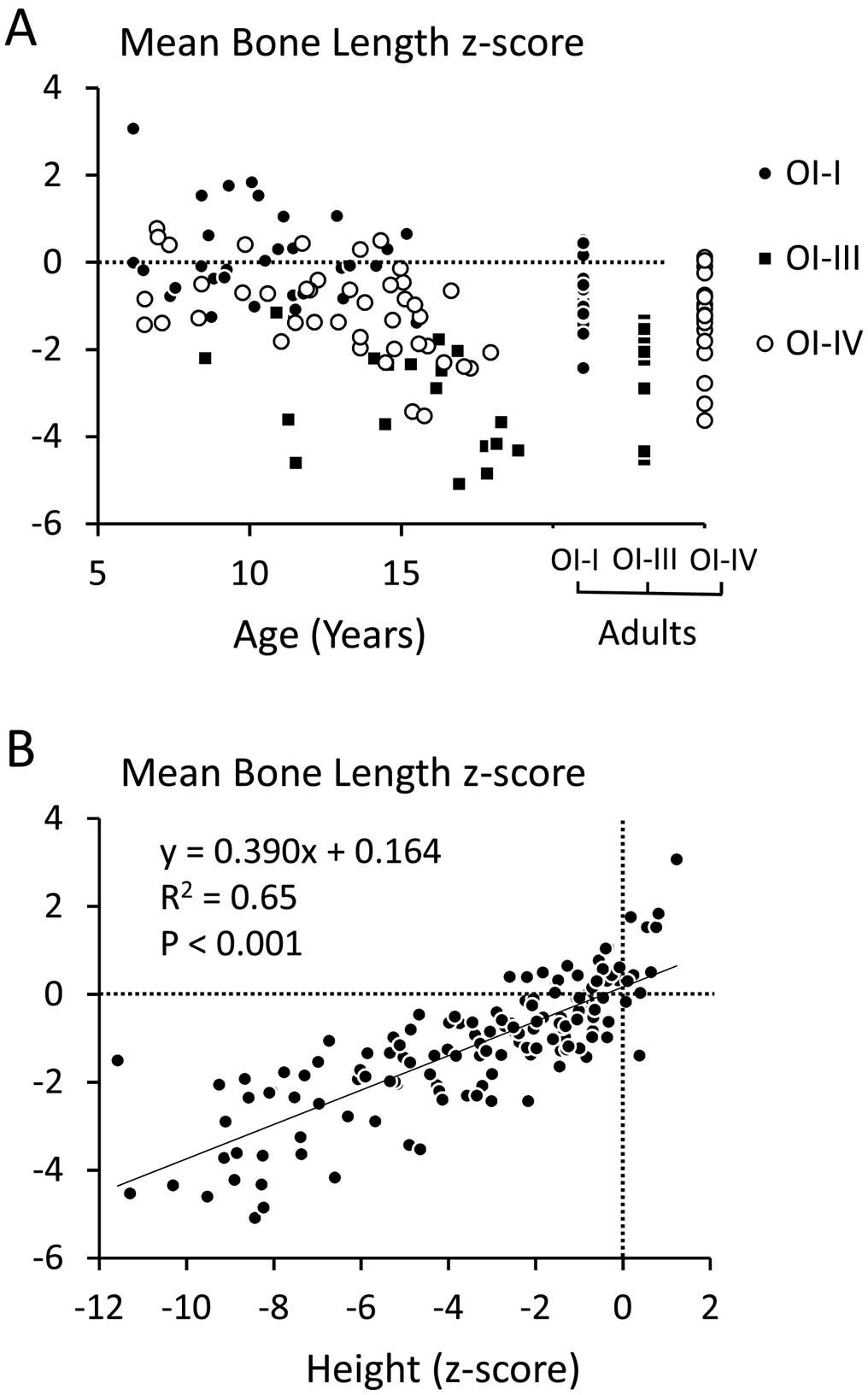
Assessment of mean bone length z-scores. (A) Age-dependency of mean bone length z-score in each study participant. (B) Relationship between height z-scores and mean bone length z-scores in the entire study population.
The mean pattern variability index in the entire study cohort was 0.46 (SD: 0.32) (Table 1), which is close to the published median value of the reference population (0.50) and well within the reference range (0.30 to 0.83) (8). The pattern variability index was similar between OI types.
When the study cohort was grouped according to genotype, significant group differences in mean hand bone length z-score were found (Table 2). Mean hand bone length z-score was close to 0 in individuals with COL1A1 mutations leading to haploinsufficiency but were significantly lower in the group with mutations leading to triple-helical glycine substitutions in either the alpha 1 or alpha 2 chain. The cohort with alpha 1 glycine substitutions had a significantly lower mean hand bone length z-score than the group with alpha 2 glycine substitutions or any other genotypic group. However, univariate ANOVA revealed that these differences between genotypes were no longer significant (P = 0.14) after adjustment for age, sex and height z-score.
Table 2.
Study population by genotype
| Haplo-insufficiency | Alpha 1 Glycine | Alpha 2 Glycine | Splice | C-Pro-peptide | P | |
|---|---|---|---|---|---|---|
| N (m/f) | 38 (23/15) | 37 (18/19) | 40 (17/23) | 18 (10/8) | 8 (5/3) | 0.53 |
| Age (years) | 20.6 (13.8) | 17.5 (8.2) | 18.6 (9.8) | 16.6 (11.4) | 13.8 (6.3) | 0.43 |
| History of Bisphosphonate Treatment (N, %) | 21 (55%) | 35 (95%) | 33 (83%) | 15 (83) | 8 (100%) | <0.001 |
| Height z-score | −0.8 (1.0)b,c,e | −5.8 (2.7)a,c,d | −3.9 (2.8)a,b | −2.4 (2.4)b | −3.5 (2.6)a | <0.001 |
| Mean Hand Bone Length z-score | 0.02 (1.0)b,c | −2.2 (1.3)a,c,d,e | −1.4 (1.1)a,b | −0.7 (1.3)b | −0.9 (1.0)b | <0.001 |
| Pattern Variability Index | 0.41 (0.32) | 0.46 (0.25) | 0.46 (0.34) | 0.55 (0.36) | 0.52 (0.45) | 0.65 |
Results are shown as N or mean (SD). P values indicate significance of the difference between genotypes (ANOVA or chi square tests, as appropriate). Superscripts indicate significant differences (Bonferroni’s adjustment) to
Haploinsufficiency,
Alpha 1 glycine,
Alpha 2 glycine,
Splice,
C-Propeptide
4. Discussion
In this study on 144 individuals with OI caused by COL1A1 or COL1A2 mutations, we found that hand bone length varied significantly between OI types. Hand bone length was mostly normal in OI type I but was significantly lower in OI type IV and in OI type III. Mean hand bone length z-score was closely related to height z-score, but age and sex were also significant independent predictors. In addition, we observed significant genotype – phenotype correlations. Hand bone length was normal in individuals with alpha1(I) haploinsufficiency, but other types of mutations were associated with decreased bone length.
The present study confirms and extends previous reports on hand bone length in OI. A study on 24 adults with OI found that the length of the second metacarpal was normal in 22 of these individuals, but no information about OI types was provided (12). In an analysis of 39 children and adults with OI, the length of the second metacarpal was lower than in age- and sex-matched controls, but results were not broken down by OI type (14). We had previously studied the length of the second metacarpal in a group of 42 children with OI type I and found a mean z-score of +0.1 (13). The present data confirm that hand bone length is mostly normal in OI type I, but in addition we made the novel observation that the more severe OI types are associated with shorter hand bones.
Our regression analyses showed that mean hand bone length z-score was negatively associated with age, suggesting that the growth of hand bones is slower in children and adolescents with OI than in their healthy peers. Female sex was associated with slightly higher mean hand bone length z-score. However, the main determinant of mean hand bone length z-score in these regression analyses was height z-score. This is in line with the hypothesis that bone length in OI is affected by a systemic growth defect. The close correlation between mean hand bone length z-score and height z-score suggests that the height deficit in OI is to a large extent driven by slow growth plate activity rather than by bone deformities or fractures.
It appears that this growth defect affects all hand bones to a similar extent, as the pattern variability index, a measure of the variation in bone length z-scores within a hand, was normal in OI and did not differ between OI types. This is different from some other bone dysplasias that result in short hand bones, such as achondroplasia or Morquio syndrome, where mean bone length z-scores are similarly low as in OI type III, but where the pattern variability index is much higher (8). The normal pattern variability index even in severe OI indicates that the shortness of hand bones is unlikely to be caused by bone fragility, as fractures would presumably lead to some bones being clearly shorter than others, which would lead to a high pattern variability index.
We observed in the present study that mean hand bone length z-scores varied with the type of the underlying mutation. This is not surprising, as alpha1(I) haploinsufficiency mutations consistently lead to mild OI, whereas other types of mutation frequently lead to a more severe phenotype. This genotype-phenotype correlation is reflected not only in clinical indicators of disease severity such as the prevalence of scoliosis or of craniocervical junction abnormalities (21, 22), but also in quantitative measures of bone cell function as determined by bone histomorphometry (23).
From a mechanistic perspective, it is not clear how abnormalities in collagen type I can directly lead to shorter hand bones, which presumably reflect a defect in growth plate function. Growth plate chondrocytes mostly express collagen types II and X (24), and therefore should not be directly affected by collagen type I abnormalities. However, a recent study in mice raised the possibility that some hypertrophic chondrocytes also express collagen type I (6). The same study found in an OI mouse model that hypertrophic chondrocytes showed signs of increased endoplasmic reticulum stress, which might affect growth plate function. Abnormalities in growth plate histology have been observed in a few individuals with OI (25, 26). Collagen type I is also expressed in the perichondrium, the tissue surrounding growth plates that contributes to the control of growth plate activity (27). Thus, the effect of collagen type I abnormalities on growth plates will be an important topic for further mechanistic studies.
We did not observe a significant effect of bisphosphonate treatment on hand bone length. The large majority of study participants with OI type III or IV had received intravenous bisphosphonate treatment, so this study was not well positioned to elucidate bisphosphonate effects on growth. Nevertheless, our data are in accordance with a detailed mouse study that did not detect an effect of either pamidronate or zoledronate on bone length in rapidly growing mice (28).
The present study is limited by its cross-sectional design. It was therefore not possible to precisely evaluate the changes of longitudinal bone growth activity during childhood and adolescence. It is therefore unclear from these data whether children with OI have slow but steady growth or rather have a decelerating growth pattern over time. Also, the study does not provide information on the OI types that are not caused by COL1A1 and COL1A2 mutations, which are all very rare but cumulatively include about 20% of individuals with moderate to severe OI (18).
In conclusion, the present study found that COL1A1 and COL1A2 mutations affect bone growth not only by inducing bone fractures and bone deformities, but also through slower growth of bones that do not fracture or deform. Mechanistic studies are required to elucidate how COL1A1 and COL1A2 mutations affect the activity of growth plates.
Highlights.
The length of metacarpal and phalangeal bones is mostly normal in OI type I
Metacarpal and phalangeal bone length is low in OI types III and IV
Mean hand bone length z-score is positively associated with height z-score
OI causes longitudinal growth deficits in bones that do not fracture or deform
Acknowledgements
We thank Michaela Durigova for organizational support. Roles of the authors: DR: data collection and analysis; MER: data collection and interpretation; CS: data collection and interpretation; RS: study organization; BL: study organization; FG: contributed patient information and revised manuscript content; FR conceptualized the project, contributed patient information, completed the report and accepts responsibility for the integrity of the data analysis. All authors have read and approved of the final version of the manuscript.
The authors are grateful to the patients and their families for participation in the study. This study was supported by the Brittle Bone Disease Consortium (BBDC). The BBDC (1U54AR068069-0) is a part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN), and is funded through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute of Dental and Craniofacial Research (NIDCR), and the Eunice Kennedy Shriver National Institutes of Child Health and Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.” The BBDC is also supported by the Osteogenesis Imperfecta Foundation. The study was also supported by the Shriners of North America.
Grant support: This study was supported by the National Institutes of Health, the Osteogenesis Imperfecta Foundation and the Shriners of North America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors state that they have the following disclosures:
Damian Rauch: None
Marie-Eve Robinson: None
Cristian Seiltgens: None
V. Reid Sutton: None
Brendan Lee: None
Francis H. Glorieux: Novartis, Amgen and Mereo Biopharma: consulting fees and research grants. Frank Rauch: Novartis and Mereo BioPharma: consulting fees. Ultragenyx, Catabasis, PreciThera: study grant to institution
Conflict of Interest
The authors state that they have the following disclosures: Frank Rauch: PreciThera Inc: Study grant to institution; Ultragenyx Inc: Study grant to institution. FG: Study grants from Mereo Biopharma, Amgen, Ultragenyx and Kyowa-Kirin. V. Reid Sutton: unpaid advisor, Mereo Biopharma.
References
- 1.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet 2016;387:1657–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson ME, Rauch F. Mendelian bone fragility disorders. Bone 2019;126:11–7. [DOI] [PubMed] [Google Scholar]
- 3.Tauer JT, Robinson ME, Rauch F. Osteogenesis Imperfecta: New Perspectives From Clinical and Translational Research. JBMR Plus 2019;3:e10174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortier GR, Cohn DH, Cormier-Daire V, Hall C, Krakow D, Mundlos S, Nishimura G, Robertson S, Sangiorgi L, Savarirayan R, Sillence D, Superti-Furga A, Unger S, Warman ML. Nosology and classification of genetic skeletal disorders: 2019 revision. Am J Med Genet A 2019;179:2393–419. [DOI] [PubMed] [Google Scholar]
- 5.Jain M, Tam A, Shapiro JR, Steiner RD, Smith PA, Bober MB, Hart T, Cuthbertson D, Krischer J, Mullins M, Bellur S, Byers PH, Pepin M, Durigova M, Glorieux FH, Rauch F, Lee B, Sutton VR, Consortium MotBBD, Nagamani SCS. Growth characteristics in individuals with osteogenesis imperfecta in North America: results from a multicenter study. Genet Med 2019;21:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheiber AL, Guess AJ, Kaito T, Abzug JM, Enomoto-Iwamoto M, Leikin S, Iwamoto M, Otsuru S. Endoplasmic reticulum stress is induced in growth plate hypertrophic chondrocytes in G610C mouse model of osteogenesis imperfecta. Biochem Biophys Res Commun 2019;509:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garn SM, Hertzog KP, Poznanski AK, Nagy JM. Metacarpophalangeal length in the evaluation of skeletal malformation. Radiology 1972;105:375–81. [DOI] [PubMed] [Google Scholar]
- 8.Garn SM, Leonard WR, Poznanski AK. Applications of the pattern variability index (sigma z) to the quantification of dysmorphogenesis in the hand. Am J Med Genet 1987;27:143–52. [DOI] [PubMed] [Google Scholar]
- 9.Bicknell LS, Farrington-Rock C, Shafeghati Y, Rump P, Alanay Y, Alembik Y, Al-Madani N, Firth H, Karimi-Nejad MH, Kim CA, Leask K, Maisenbacher M, Moran E, Pappas JG, Prontera P, de Ravel T, Fryns JP, Sweeney E, Fryer A, Unger S, Wilson LC, Lachman RS, Rimoin DL, Cohn DH, Krakow D, Robertson SP. A molecular and clinical study of Larsen syndrome caused by mutations in FLNB. J Med Genet 2007;44:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Sanctis L, Vai S, Andreo MR, Romagnolo D, Silvestro L, de Sanctis C. Brachydactyly in 14 genetically characterized pseudohypoparathyroidism type Ia patients. J Clin Endocrinol Metab 2004;89:1650–5. [DOI] [PubMed] [Google Scholar]
- 11.Ludecke HJ, Schaper J, Meinecke P, Momeni P, Gross S, von Holtum D, Hirche H, Abramowicz MJ, Albrecht B, Apacik C, Christen HJ, Claussen U, Devriendt K, Fastnacht E, Forderer A, Friedrich U, Goodship TH, Greiwe M, Hamm H, Hennekam RC, Hinkel GK, Hoeltzenbein M, Kayserili H, Majewski F, Mathieu M, McLeod R, Midro AT, Moog U, Nagai T, Niikawa N, Orstavik KH, Plochl E, Seitz C, Schmidtke J, Tranebjaerg L, Tsukahara M, Wittwer B, Zabel B, Gillessen-Kaesbach G, Horsthemke B. Genotypic and phenotypic spectrum in tricho-rhino-phalangeal syndrome types I and III. Am J Hum Genet 2001;68:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson CR. Metacarpal morphometry in adults with osteogenesis imperfecta. Br Med J 1978;1:213–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauch F, Land C, Cornibert S, Schoenau E, Glorieux FH. High and low density in the same bone: a study on children and adolescents with mild osteogenesis imperfecta. Bone 2005;37:634–41. [DOI] [PubMed] [Google Scholar]
- 14.Citron K, Veneziale C, Marino J, Carter EM, Jepsen KJ, Raggio C. Bone robusticity in two distinct skeletal dysplasias diverges from established patterns. J Orthop Res 2017;35:2392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet 2004;363:1377–85. [DOI] [PubMed] [Google Scholar]
- 16.Trejo P, Rauch F. Osteogenesis imperfecta in children and adolescents-new developments in diagnosis and treatment. Osteoporos Int 2016;27:3427–37. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer-Kuhn H, Hobing L, Cassens J, Schoenau E, Semler O. Children with severe Osteogenesis imperfecta and short stature present on average with normal IGF-I and IGFBP-3 levels. J Pediatr Endocrinol Metab 2016;29:813–8. [DOI] [PubMed] [Google Scholar]
- 18.Bardai G, Moffatt P, Glorieux FH, Rauch F. DNA sequence analysis in 598 individuals with a clinical diagnosis of osteogenesis imperfecta: diagnostic yield and mutation spectrum. Osteoporos Int 2016;27:3607–13. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002;109:45–60. [DOI] [PubMed] [Google Scholar]
- 20.Poznanski AK, Garn SM, Nagy JM, Gall JC Jr., Metacarpophalangeal pattern profiles in the evaluation of skeletal malformations. Radiology 1972;104:1–11. [DOI] [PubMed] [Google Scholar]
- 21.Sato A, Ouellet J, Muneta T, Glorieux FH, Rauch F. Scoliosis in osteogenesis imperfecta caused by COL1A1/COL1A2 mutations - genotype-phenotype correlations and effect of bisphosphonate treatment. Bone 2016;86:53–7. [DOI] [PubMed] [Google Scholar]
- 22.Cheung MS, Arponen H, Roughley P, Azouz ME, Glorieux FH, Waltimo-Siren J, Rauch F. Cranial base abnormalities in osteogenesis imperfecta: Phenotypic and genotypic determinants. J Bone Miner Res 2011;26:405–13. [DOI] [PubMed] [Google Scholar]
- 23.Rauch F, Lalic L, Roughley P, Glorieux FH. Relationship between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfecta. J Bone Miner Res 2010;25:1367–74. [DOI] [PubMed] [Google Scholar]
- 24.Melrose J, Shu C, Whitelock JM, Lord MS. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol 2016;52–54:363–83. [DOI] [PubMed] [Google Scholar]
- 25.Sanguinetti C, Greco F, De Palma L, Specchia N, Falciglia F. Morphological changes in growth-plate cartilage in osteogenesis imperfecta. J Bone Joint Surg Br 1990;72:475–9. [DOI] [PubMed] [Google Scholar]
- 26.Sarathchandra P, Cassella JP, Ali SY. Enzyme histochemical localisation of alkaline phosphatase activity in osteogenesis imperfecta bone and growth plate: a preliminary study. Micron 2005;36:715–20. [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg HM. Developmental regulation of the growth plate. Nature 2003;423:332–6. [DOI] [PubMed] [Google Scholar]
- 28.Zhu ED, Louis L, Brooks DJ, Bouxsein ML, Demay MB. Effect of bisphosphonates on the rapidly growing male murine skeleton. Endocrinology 2014;155:1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


