Abstract
Introduction:
While cigarette smoking rates have been steadily decreasing over the past decade, there has been a dramatic increase in nicotine use via e-cigarettes, especially during adolescence. Adolescent e-cigarette use is associated with a greater risk of future cigarette smoking, and increased rates of cigarette smoking in individuals who may have otherwise never tried cigarettes. In humans and rodents, early initiation of nicotine use has been associated with greater consumption, dependence, and persistent nicotine use. The present study sought to investigate the long-lasting effect of daily high-dose nicotine exposure during adolescence on nicotine consumption in adulthood.
Method:
Male Sprague-Dawley rats were exposed daily to nicotine (1.0 mg/kg, subcutaneous), or vehicle (1 ml/kg saline, subcutaneous) during adolescence (post-natal day [P] 28–41). Adult nicotine self-administration (0.02 mg/kg/infusion, intravenous) was assessed beginning on P75 on fixed-ratio 1 (FR1), fixed-interval 1min (FI1), and progressive ratio (PR) schedules of reinforcement.
Results:
Adolescent nicotine pre-exposure did not affect adult nicotine self-administration on the simple FR1 schedule, however increased intake and responding for nicotine was observed when a short delay was implemented on an FI1 schedule of reinforcement.
Conclusions:
Adolescence is a critical period when the brain is especially vulnerable to the effects of nicotine. Nicotine exposure in adolescence enhances susceptibility to increased nicotine intake in adulthood on a reinforcement schedule more reflective of human nicotine intake patterns, and this effect can extend into adulthood even after termination of nicotine exposure during adolescence.
Keywords: adolescent, nicotine, self-administration, addiction, vaping, smoking
1. Introduction
Despite the decline in cigarette smoking in recent years, it remains the leading cause of preventable death, and there are over 1 billion users worldwide (World Health Organization, 2017). Nicotine, the primary psychoactive compound found in tobacco (Rose, 2006), can also be administered via non-combustible routes of administration including ‘vaping’ nicotine via electronic-cigarettes (Pearson et al., 2012). This route is becoming increasingly popular, particularly in adolescents. In 2019, nearly 12% of 12th graders reported vaping nicotine daily, while approximately 25% reported vaping nicotine in the last month – a 4.5% increase since 2018 (Miech et al., 2019). Furthermore, nicotine vaping prevalence in 8th graders is almost as high now as the prevalence in 12th graders in 2017 (Miech et al., 2019). In short, adolescent vaping is rising with a decline in the age of onset. This is concerning, as some evidence suggests that vaping during adolescence is associated with a greater prevalence of combustible tobacco use later in life (Bunnell et al., 2015; Chaffee et al., 2018; Leventhal et al., 2015; Loukas et al., 2018), and may put those who would have otherwise never tried tobacco products at a greater risk of use (Barrington-Trimis et al., 2016). Initiation of tobacco use in adolescence is also associated with increases in tobacco dependence later in life (Cullen et al., 2018; Sharapova et al., 2018). Given that nicotine consumption via e-cigarettes has become extremely popular in adolescents in recent years, the potential for long-lasting vulnerability to adult dependency requires further investigation.
Adolescence is a critical developmental period, during which nicotinic acetylcholinergic neurotransmission undergoes considerable fluctuation and exogenous exposure to nicotine can produce long-lasting changes (Natividad et al., 2013; O’Dell et al., 2007; Thorpe et al., 2020; Torres et al., 2008), including increased nicotine-seeking behaviour in adulthood (Adriani et al., 2006, 2003; Natividad et al., 2013). Adolescent nicotine exposure (0.4 mg/kg, intraperitoneally, once daily, P34–44) increased intravenous self-administration (IVSA) of nicotine and enhanced motivation on a between-sessions PR schedule in adulthood, compared to post-adolescent (P60–69) nicotine exposure with the same abstinence period (Adriani et al., 2003). Similarly, adolescent nicotine pretreatment (P28–42; 4.7 mg/kg/day; osmotic minipump) increased nicotine IVSA at P75 (0.03, 0.06, 0.09mg/kg/infusion) on an FR1 schedule compared to drug naïve adult rats (Natividad et al., 2013). However, no studies to date have looked at the long-term effects of a high-dose nicotine (Craig et al., 2014; Matta et al., 2007) pre-exposure in adolescence (importantly, 1 mg/kg dose produces plasma nicotine levels (Craig et al., 2014) similar to those achieved by popular pod e-cigarette devices (Rao et al., 2020)) on later adult nicotine IVSA and motivation.
2. Methods
2.1. Subjects
Adolescent male Sprague-Dawley rats (Charles River Lab, St. Constant, Canada, P25) were pair-housed in standard cages and kept on a 12:12 light-dark cycle (lights on: 8am) in a colony maintained at 21°C. Water and food (Envigo, Madison, Wisconsin, USA, Rodent Diet, 14% protein) were available ad libitum until after surgery, when rats were maintained at ~90% of each rat’s pre-surgical free-feeding weight. Procedures were approved by the University of Guelph Animal Care Committee according to the Canadian Council of Animal Care guidelines.
2.2. Drug Preparation
Nicotine ditartrate dihydrate (Fisher Scientific, Ottawa, ON, Canada) was dissolved in 0.9% saline (1mg base/ml concentration; 1ml/kg volume) and administered subcutaneously. For adult intravenous infusions, nicotine was dissolved in 0.9% saline (0.02 mg base/kg per 0.04 ml/sec infusion (Charntikov et al., 2020). Precision infusion pumps automatically adjusted infusion duration for each rat’s weight on a given day to keep dosing consistent. Nicotine pH was adjusted to 7.0–7.2 (Charntikov et al., 2020; Murray et al., 2011; Palmatier et al., 2006; Schassburger et al., 2015; Swalve et al., 2016) using NaOH and was prepared fresh weekly.
2.3. Nicotine Pre-Exposure
During adolescence (P28–41), pair-housed rats with free-access to food and water were randomly assigned to a daily injection of 1mg/kg nicotine (n=10) or saline (n=6) pre-treatment (Craig et al., 2014; Matta et al., 2007). Rats received nicotine treatments over a period of 14 days. This period is thought to conservatively encapsulate adolescence and is commonly used as a time frame for adolescent nicotine treatment (Natividad et al., 2013; Spear, 2000).
2.4. Apparatus
We used 10 conditioning chambers (30.5×24.1×21.0 cm; LxWxH; ENV-018, MedAssociates, Georgia, VT, USA) enclosed in sound- and light-attenuating cubicles. Chamber front and back walls and ceiling were transparent polycarbonate, and sidewalls and floor were aluminum. Chambers had two retractable levers on the right wall with a single cue light directly above each lever, and a house light on the opposite wall. An emitter-detector unit to measure locomotor activity was located 5.5 cm from the lever-containing sidewall and 6 cm from the grid floor. A precision pump with nicotine syringe provided deliveries of IV nicotine via PE50 tygon tubing attached to a swivel then threaded through metal tether within the chamber. MedAssociates interface and software-controlled stimulus outputs and recorded inputs.
2.5. Surgical Procedures
Rats were surgically implanted with jugular catheters as adults (P68–130); groups were run in parallel and at the same ages for each adolescent pretreatment condition (Saline vs Nicotine). Under isoflurane, a silastic catheter (RJVR-40; SAI, Lake Villa, IL, USA) was secured into the right jugular vein; the other end was threaded subcutaneously over the shoulder and attached to a back-mount cannula (313–000BM-20–5up/spc; PlasticsOne, Anjou, QC, Canada) exiting between the shoulder blades. Rats recovered for 7 days, receiving carprofen (5mg/kg; subcutaneous) for 3 days following surgery. Rats were flushed daily with 0.1 ml flushing solution [heparin (30U/ml)/baytril (5 mg/ml)/saline (0.9% sterile)] and were single housed from surgery onward. Patency was confirmed with IV xylazine at the end of the experiment (Charntikov et al., 2020). Only animals with patent catheters are included in the analyses. All surgical drugs were provided by the Ontario Veterinary College.
2.6. Self-Administration
There was no lever pre-training. All sessions were 1hr, 6–7 days/week. Rats were connected to the tether and allowed to self-administer nicotine on a fixed-ratio 1 (FR1) schedule. Each active lever press resulted in a 1 sec infusion of nicotine, retraction of both levers, and onset of the discrete cue light located above the active lever. After 16 sessions, rats were shifted to a fixed-interval 1-minute (FI1) schedule, wherein the first active lever press following a 1m interval was reinforced with associated stimuli. After 8 sessions, rats were shifted to a within-session progressive ratio (PR) schedule with gradual increase in the number of presses necessary to deliver each successive infusion (i.e., 1, 2, 3, 4, etc.). The experiment concluded following 8 PR sessions. Each of these reinforcement schedules were chosen to evaluate a particular question of interest. FR1 was chosen as it has been suggested to best compare the hedonic effects of a drug (Mendrek et al., 1998). FI1 was chosen to more closely model nicotine use parameters where the availability is limited to certain periods in the day (i.e. smoke breaks) and to remove the constraint of a fixed number of presses in order to allow for more behavioural variability (Murray et al., 2012). PR was chosen as a of measure of incentive motivation (Arnold and Roberts, 1997).
2.7. Data Analysis
Infusions, active lever presses, inactive lever presses, and locomotor activity were analyzed using two-way mixed analyses of variance (ANOVAs) comparing Group (nicotine pretreated versus saline pretreated) across Session (self-administration sessions) for each reinforcement schedule. Within-group active versus inactive Lever pressing across Session at each schedule was examined with two-way within-subject ANOVAs. Significant interactions were followed by planned post hoc comparisons using Fisher’s least significant difference assessments. Only animals that remained patent for the entirety of experiment were used in analyses.
3. Results
3.1. Total infusions
3.1.1: Fixed-Ratio 1 (FR1).
Rats increased nicotine intake across sessions regardless of group (Fig. 1a). There was a main effect of Session (F15,210=4.194, p<.001, ηp22=.231), no main effect of Group (F1,14=1.867, p=.193, ηp2=.118), and no Session by Group interaction (F15,210=0.623, p=.854, ηp2=.043).
Figure 1.
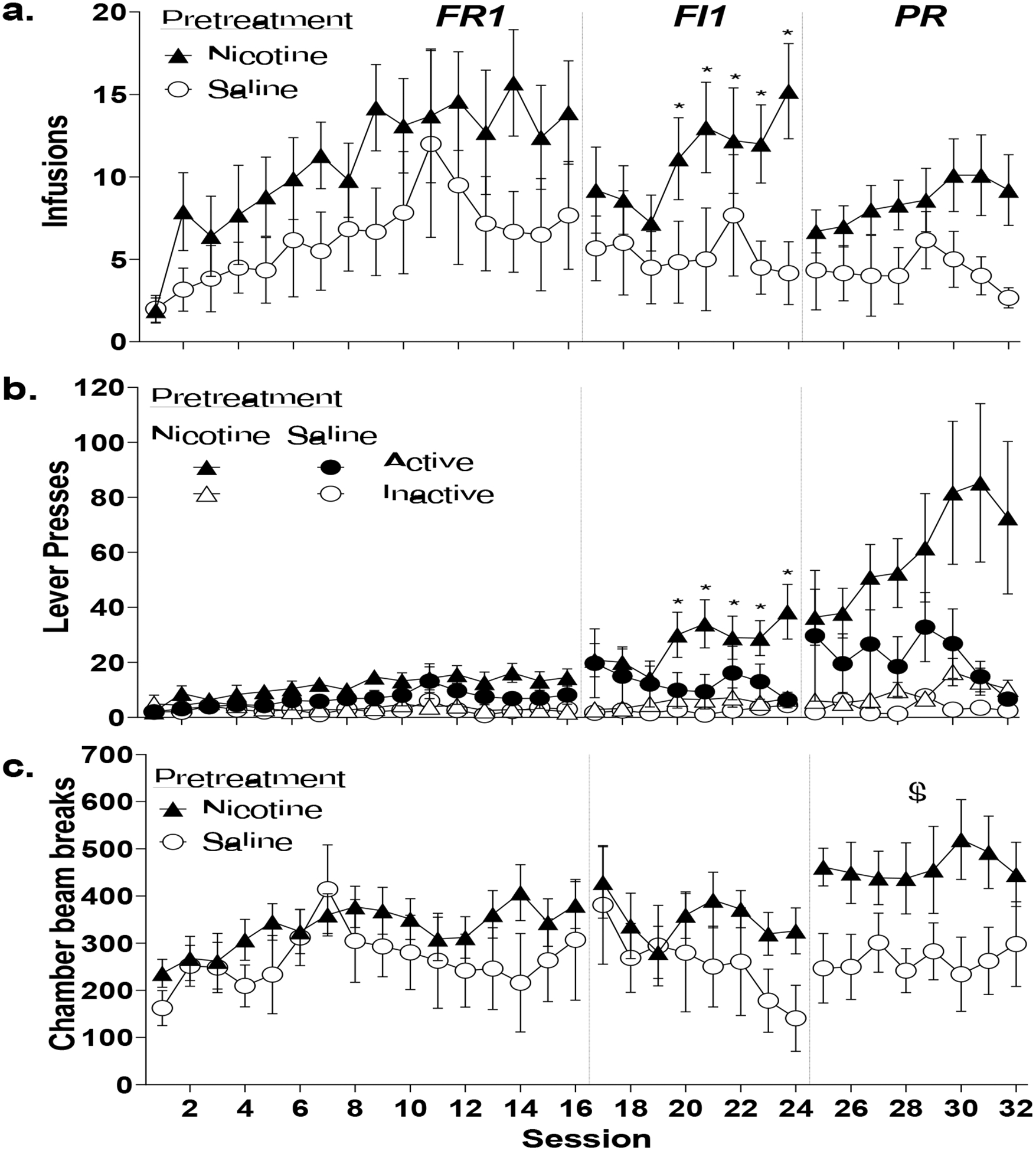
Adolescent nicotine exposure increases fixed-interval nicotine IVSA in adult male Sprague-Dawley rats. All data are presented as mean±standard error of mean. (a) Total infusions on an FR1, FI1 and PR schedule of reinforcement. (b) Active and inactive lever presses on an FR, FI1, and PR schedule. (c) Locomotion (number of chamber photobeam breaks) during the FR1, FI1, and PR schedules. * indicates significant difference from saline pre-exposure controls following significant interaction (p <0.05). $ indicates significant main effect of group in the absence of an interaction.
3.1.2: Fixed-Interval 1 (FI1).
Nicotine pretreatment increased nicotine infusions across sessions compared to saline controls. There was no effect of Session (F7, 98=1.918, p=.075, ηp2=.121) or Group (F1,14=2.858, p=.113, ηp2=.170), but there was a significant Group by Session interaction (F7, 98=2.317, p=.031, ηp2=.142). Nicotine pretreated rats took significantly more nicotine infusions than saline pretreated controls from session 4 onward (p<.05).
3.1.3: Progressive-Ratio (PR).
Nicotine intake did not significantly differ across sessions or groups. There was no effect of Session (F7,98=1.235, p=.291, ηp2=.081) or Group (F1,14=3.112, p=.100, ηp2=.182), and no significant Group by Session interaction (F7,98=1.226, p=.296,ηp2=.081).
3.2. Lever presses
3.2.1: FR1.
Active lever presses increased across sessions regardless of group (Fig. 1b). There was a main effect of Session (F15,210=4.047, p<.001, ηp2=.224), but no effect of Group (F1,14=1.904, p=.189, ηp2=.120) or Session by Group interaction, (F15,210=0.608, p=.867,ηp2=.042). Inactive lever pressing increased across sessions, with only a main effect of Session (F15,210=1.890, p=.026, ηp2=.119) and no effect of Group, (F1,14=0.582, p=.458, ηp2=.040) or Session by Group interaction (F15,210=0.738, p=.744, ηp2=.050).
3.2.2: FI1.
Nicotine pretreatment increased active lever presses across sessions. There was no effect of Session (F7,98=0.990, p=.443, ηp2=.066) or Group (F1,14=2.066, p=.173,ηp2=.129), but a significant Session by Group interaction (F7,98=3.183, p=.004, ηp2=.185). Nicotine pretreated animals made significantly more active lever presses compared to saline pretreated rats from session 4 onward (p<.05). Inactive lever pressing did not change across Session (F7,98=1.042, p=.407, ηp2=.069) or Group (F1,14=3.056, p=.102, ηp2=.179) with no interaction (F7,98=0.579, p =.771, ηp2=.040).
3.2.3: PR.
Active lever presses did not significantly differ across sessions or group. There was no effect of Session (F7,98=0.998, p=.438, ηp2=.067), Group (F1,14=2.766, p=.119, ηp2=.165), or Group by Session interaction (F7,98=1.712, p=.115, ηp2=.109). However, nicotine rats increased inactive lever pressing across PR sessions compared to saline pretreated controls. There was no effect of Session (F7,98=1.315, p=251, ηp2=.086), but there was a main effect of Group (F1,14=4.624, p=.049, ηp2=.248) and a significant Session by Group interaction (F7,98=2.123, p=.048, ηp2=.132). Nicotine rats made significantly more inactive lever presses compared to saline rats on sessions 4, 6, 7, and 8 (p<.05).
3.3. Locomotion
3.3.1: FR1.
Locomotion increased across sessions regardless of group (Fig 1c) with a significant effect of Session (F15,210=2.340, p=.004, ηp2=.143), but no effect of Group (F1,14=1.004, p=.333, ηp2=.067) or Session by Group interaction (F15,210=0.895, p=.570, ηp2=.060).
3.3.2: FI1.
Locomotion increased across sessions regardless of group with a main effect of Session (F7,98=2.711, p=.013, ηp2=.162) but no effect of Group (F1,14=1.255, p=.281, ηp2=.082) or Session by Group interaction (F7,98=0.983, p=.448, ηp2=.066).
3.3.3: PR.
Nicotine pretreatment caused higher locomotion than saline pretreated controls. There was a main effect of Group (F1,14=4.811, p=.046, ηp2=.256) but no effect of Session (F7,98=0.198, p=.985, ηp2=.014) or Session by Group interaction (F7,98=0.574, p=.775, ηp2=.039).
3.4. Active and inactive lever discrimination: Saline controls
3.4.1: FR1.
Saline controls increased lever pressing across sessions regardless of lever (Fig. 2a). There was a main effect of Session (F15,150=2.096, p=.013, ηp2=.173) but no effect of Lever (F1,10=2.158, p=.173, ηp2=.178) or Session by Lever interaction (F15,150=0.966, p=.494, ηp2=.088).
Figure 2.
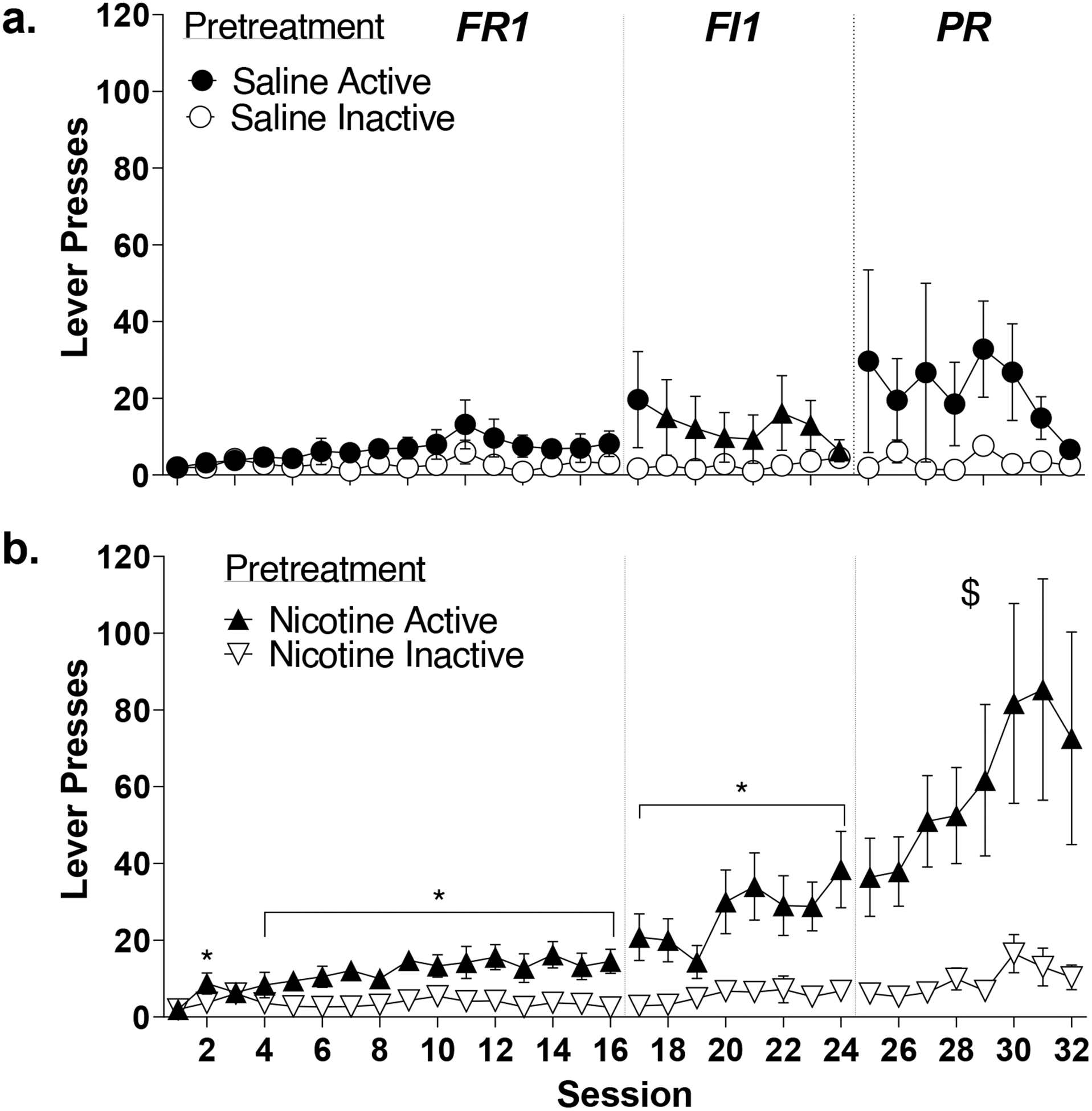
Impact of adolescent nicotine exposure on active and inactive lever pressing during nicotine IVSA in adult male Sprague-Dawley rats. All data are presented as mean±standard error of mean. (a) Saline pre-exposed rats do not significantly differ on active compared to inactive lever presses on an FR1, FI1, and PR schedule of reinforcement. (b) Nicotine pre-exposed rats learn to significantly discriminate between active and inactive levers on an FR1, FI1, and PR schedule of reinforcement. * indicates significant post hoc differences following significant interaction (p<0.05). $ indicates significant effect of group in the absence of an interaction.
3.4.2: FI1.
Saline controls maintained stable pressing. There were no effects of Session (F7,70=1.134, p=.352, ηp2=.102) or Lever (F1,10=1.772, p=.213, ηp2=.151), and no significant Session by Lever interaction (F7,70=1.705, p=.122, ηp22=.146).
3.4.3: PR.
Saline controls maintained stable pressing. There were no effects of Session (F7,70=0.748, p=.632, ηp2=.070) or Lever (F1,10=3.172, p=.105, ηp2=.241) and no Session by Lever type interaction (F7,70=0.579, p=.771, ηp2=.055).
3.5. Active and inactive lever discrimination: Nicotine pretreatment
3.5.1. FR1.
Nicotine pretreatment evoked lever discrimination (Fig. 2b). There were main effects of Session (F15,270=3.589, p<.001, ηp2=.166) and Lever (F1,18=10.678, p=.004, ηp2=.372), and a Session by Lever interaction (F15,270=3.036, p<.001, ηp2=.144). Rats had significantly greater active lever presses on all sessions except 1 and 3 (p<.05).
3.5.2. FI1.
Nicotine pretreated rats continued increasing active lever pressing across sessions. There were effects of Session (F7,126=4.050, p<.001, ηp2=.184) and Lever (F1,18=11.744, p=.003, ηp2=.395) and a Session by Lever interaction (F7,126=2.206, p=.038, ηp2=.109) with more active lever pressing on all 8 sessions (p<.05).
3.5.3. PR.
Nicotine pretreated rats maintained higher active lever presses. There were main effects of Session (F7,126=3.287, p=.003, ηp2=.154) and Lever (F1,18=9.411, p=.007,ηp2=.343), and no Session by Lever interaction (F7,126=.1.596, p=.142, ηp2=.081).
4. Discussion
High-dose nicotine pre-exposure in male adolescence permitted spontaneous nicotine self-administration in adulthood. While nicotine- and saline-pretreated rats do not differ in the amount of nicotine consumed on an FR1 schedule, only nicotine-pretreated rats significantly discriminated between active and inactive levers. When the schedule shifted such that a 1 min delay was implemented for each infusion, nicotine-pretreated rats took more nicotine and made more active lever presses; however, saline rats still failed to discriminate levers. When PR was implemented, nicotine pretreatment continued a trend of more infusions and active lever presses; saline-pretreated controls still failed to reliably self-administer nicotine. Therefore, when given parameters that do not normally result in nicotine IVSA, nicotine pre-exposure in adolescence lowers the threshold for nicotine to act as a reinforcer.
Conditions were chosen to bring the baseline responding in the control animals (saline pre-treatment) sufficiently low to prevent potential ceiling effects on the impact of adolescent nicotine exposure on self-administration in adulthood (especially considering the higher nicotine dose administered during adolescence). A lower dose of 0.02 mg/kg/infusion was used as it is slightly below the 0.03 mg/kg that usually produces robust SA (Donny et al., 1995), though even this dose is not always successful (de la Peña et al., 2014; Shram et al., 2008). Furthermore, no food nor sucrose lever training was given prior to nicotine SA as this has been shown to increase the number of rats that produce robust SA and artificially inflates operant behaviour (Garcia et al., 2014). Additionally, a recent study revealed a complex relationship between age, nicotine exposure, and oral nicotine and oral saccharin self-administration, suggesting an interaction between food reward and nicotine reward that we wanted to avoid (Cole et al., 2019). When adolescent mice (P33–35) receive 2 weeks of nicotine exposure via osmotic minipump (3 mg/kg/day) followed by a 2-week washout period, they show enhanced responding for non-drug reward (saccharin solution) in adulthood (~P90) on PR schedule. However, when mice receive the same adolescent nicotine exposure and a second exposure in adulthood (P61–63) for 2 weeks via osmotic minipump (6.3 mg/kg/day), this exposure preferentially enhanced nicotine reinforcement but not saccharin (non-drug) on a PR schedule. Given the chosen parameters, we were able to successfully bring our control group just under significant nicotine discrimination, thus permitting the expression of robust nicotine self-administration in the animals exposed to nicotine during adolescence.
While our adolescent exposure regimen of 1.0 mg/kg SC nicotine injections does not directly model vaping, it does attempt to model the blood nicotine levels that are achieved by modern nicotine salt-containing pod devices (Rao et al., 2020). Currently, several labs are working to validate models of nicotine vapour exposures (including our own); however, several limitations in the current methods available led us to use SC injections instead. Though vapour exposure models have more face validity than daily bolus injections, these models suffer from the stress associated with long periods of restraint during nose only exposures (Nguyen et al., 2016), or confounding absorption through the skin, nasal cavity, and mouth when licked from fur during whole body exposures (Montanari et al., 2020). Additionally, the use of injections allows for exact dosing accuracy that is currently not possible with vapour methods.
The results of this experiment demonstrating greater motivational potential for nicotine following adolescent nicotine pre-exposure are consistent with findings obtained via minipump (Natividad et al., 2013), as well as once-daily systemic injections (0.4 mg/kg, IP) (Adriani et al., 2003). The current study extends those with high-dose once-daily systemic nicotine (1.0 mg/kg, SC) in adolescence for two weeks. Additionally, our finding that nicotine pretreated rats increased inactive lever pressing is consistent with Cole et al., (2019) which found that both adolescent and adult nicotine exposure increased inactive lever pressing during PR, with this effect being more pronounced in the adolescent pretreated group; the authors suggested that this increased inactive responding may be a result of altered components of impulse control as it could be suggestive of non-specific behavioural activation which is known to drive impulsivity.
The exposure pattern chosen in our study may be more representative of the higher nicotine levels frequently found in pod e-cigarettes (Rao et al., 2020) and with the brief intermittent smoking patterns that still lead to the perception of lost autonomy over consumption (DiFranza et al., 2007) in adolescents. Given the large period of abstinence between adolescence and adulthood, the results of the present experiment are most translationally relevant to those that experiment with nicotine in adolescents when peer pressure is most significant, but do not form an addiction until nicotine is more legally accessible to them later on. The current study reports that adolescent nicotine exposure enhances vulnerability for future nicotine consumption, highlighting the need for understanding the long-term impacts of adolescent nicotine exposure, even if nicotine is only used for a brief period during adolescence. The high rates of adolescent nicotine vaping (Miech et al., 2019), combined with the higher nicotine availability from pod devices(Rao et al., 2020), may contribute to increased risk for future nicotine and tobacco use (Barrington-Trimis et al., 2016; Leventhal et al., 2015).
Highlights.
Electronic cigarette use is rapidly increasing amongst adolescents
High-dose nicotine exposure models this use in adolescent rats
Adult rats with high-dose nicotine exposure in adolescence take more nicotine
They also show higher rates of nicotine seeking
This effect occurs even after a prolonged ‘abstinence’ period
Acknowledgments
Funding for this research was provided by the National Institute on Drug Abuse of the United States to JEM [DA045740] and Natural Sciences and Engineering Research Council of Canada to JYK [RGPIN-2019-05121]. Funding sources had no role in study-design; data collection, analysis, or interpretation; writing; or decision to submit article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PVV, 2006. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology (Berl). 184, 382–390. 10.1007/s00213-005-0125-1 [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV, 2003. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J. Neurosci 23, 4712–4716. 10.1523/jneurosci.23-11-04712.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC., 1997. A Critique of Fixed and Progressive Ratio Schedules Used to Examine the Neural Substrates of Drug Reinforcement. Pharmacol. Biochem. Behav 57, 441–447. 10.1016/S0091-3057(96)00445-5 [DOI] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Urman R, Berhane K, Unger JB, Boley Cruz T, Ann Pentz M, Samet JM, Leventhal AM, Mcconnell R, 2016. E-Cigarettes and Future Cigarette Use. Pediatrics 138 10.1542/peds.2016-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnell RE, Agaku IT, Arrazola RA, Apelberg BJ, Caraballo RS, Corey CG, Coleman BN, Dube SR, King BA, 2015. Intentions to Smoke Cigarettes Among Never-Smoking US Middle and High School Electronic Cigarette Users: National Youth Tobacco Survey, 2011–2013. Nicotine Tob. Res 17, 228–235. 10.1093/ntr/ntu166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee BW, Watkins SL, Glantz SA, 2018. Electronic Cigarette Use and Progression From Experimentation to Established Smoking. Pediatrics 141, e20173594 10.1542/peds.2017-3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Swalve N, Barrett ST, Bevins RA, 2020. Conditioned Enhancement of the Nicotine Reinforcer. Exp. Clin. Psychopharmacol 10.1037/pha0000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RD, Wolsh C, Zimmerman M, Harrington E, Gould TJ, Parikh V, 2019. Adolescent and adult nicotine exposure differentially impacts oral nicotine and oral saccharin self-administration in mice. Behav. Brain Res 359, 836–844. 10.1016/j.bbr.2018.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EL, Zhao B, Cui JZ, Novalen M, Miksys S, Tyndale RF, 2014. Nicotine Pharmacokinetics in Rats Is Altered as a Function of Age, Impacting the Interpretation of Animal Model Data. Drug Metab. Dispos 42, 1447 10.1124/DMD.114.058719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA, 2018. Morbidity and Mortality Weekly Report Notes from the Field Use of Electronic Cigarettes and Any Tobacco Product Among Middle and High School Students-United States, 2011–2018. 10.15585/mmwr.mm6722a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Peña JB, Ahsan HM, Botanas CJ, Sohn A, Yu GY, Cheong JH, 2014. Adolescent nicotine or cigarette smoke exposure changes subsequent response to nicotine conditioned place preference and self-administration. Behav. Brain Res 272, 156–164. 10.1016/j.bbr.2014.06.044 [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, O’Loughlin J, Pbert L, Ockene JK, McNeill AD, Hazelton J, Friedman K, Dussault G, Wood C, Wellman RJ, 2007. Symptoms of Tobacco Dependence After Brief Intermittent Use. Arch. Pediatr. Adolesc. Med 161, 704 10.1001/archpedi.161.7.704 [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C, 1995. Nicotine self-administration in rats. Psychopharmacology (Berl). 122, 390–394. 10.1007/BF02246272 [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Strong DR, Kirkpatrick MG, Unger JB, Sussman S, Riggs NR, Stone MD, Khoddam R, Samet JM, Audrain-McGovern J, 2015. Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. JAMA 314, 700 10.1001/jama.2015.8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, Marti CN, Cooper M, Pasch KE, Perry CL, 2018. Exclusive e-cigarette use predicts cigarette initiation among college students. Addict. Behav 76, 343–347. 10.1016/J.ADDBEH.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM, 2007. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl). 190, 269–319. 10.1007/s00213-006-0441-0 [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG, 1998. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl). 135, 416–422. 10.1007/s002130050530 [DOI] [PubMed] [Google Scholar]
- Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME, 2019. Trends in Adolescent Vaping, 2017–2019. N. Engl. J. Med 381, 1490–1491. 10.1056/NEJMc1910739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanari C, Kelley LK, Kerr TM, Cole M, Gilpin NW, 2020. Nicotine e-cigarette vapor inhalation effects on nicotine & cotinine plasma levels and somatic withdrawal signs in adult male Wistar rats. Psychopharmacology (Berl). 237, 613–625. 10.1007/s00213-019-05400-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ, 2012. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology 37, 2456–2466. 10.1038/npp.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Wells NR, Bevins RA, 2011. Nicotine competes with a visual stimulus for control of conditioned responding. Addict. Biol 16, 152–162. 10.1111/j.1369-1600.2010.00228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Torres OV, Friedman TC, O’Dell LE, 2013. Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behav. Brain Res 257, 275–285. 10.1016/j.bbr.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA, 2016. Inhaled delivery of Δ9-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109, 112–120. 10.1016/j.neuropharm.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA, 2007. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol. Teratol 29, 17–22. 10.1016/j.ntt.2006.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF, 2006. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl). 184, 391–400. 10.1007/s00213-005-0183-4 [DOI] [PubMed] [Google Scholar]
- Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB, 2012. e-Cigarette Awareness, Use, and Harm Perceptions in US Adults. Am. J. Public Health 102, 1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Liu J, Springer ML, 2020. JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tob. Regul. Sci 6, 30–37. 10.18001/TRS.6.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, 2006. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology (Berl). 184, 274–285. 10.1007/s00213-005-0250-x [DOI] [PubMed] [Google Scholar]
- Schassburger RL, Levin ME, Weaver MT, Palmatier MI, Caggiula AR, Donny EC, Sved AF, 2015. Differentiating the primary reinforcing and reinforcement-enhancing effects of varenicline. Psychopharmacology (Berl). 232, 975–983. 10.1007/s00213-014-3732-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharapova S, Reyes-Guzman C, Singh T, Phillips E, Marynak KL, Agaku I, 2018. Age of tobacco use initiation and association with current use and nicotine dependence among US middle and high school students, 2014–2016. Tob. Control tobaccocontrol-2018–054593 10.1136/TOBACCOCONTROL-2018-054593 [DOI] [PubMed] [Google Scholar]
- Shram MJ, Li Z, Lê AD, 2008. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology (Berl). 197, 45–58. 10.1007/s00213-007-1003-9 [DOI] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev 24, 417–463. 10.1016/S0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Swalve N, Smethells JR, Carroll ME, 2016. Sex differences in the acquisition and maintenance of cocaine and nicotine self-administration in rats. Psychopharmacology (Berl). 233, 1005–13. 10.1007/s00213-015-4183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe HHA, Hamidullah S, Jenkins BW, Khokhar JY, 2020. Adolescent neurodevelopment and substance use: Receptor expression and behavioral consequences. Pharmacol. Ther 10.1016/j.pharmthera.2019.107431 [DOI] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE, 2008. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol. Biochem. Behav 90, 658–663. 10.1016/J.PBB.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2017. WHO Report on the Global Tobacco Epidemic: Monitoring tobacco use and prevention policies.


