Abstract
Diabetic peripheral neuropathy (DPN) is one of the most common complications in diabetic patients. Though the exact mechanism for DPN is unknown, it clearly involves metabolic dysfunction and energy failure in multiple cells within the peripheral nervous system. Lactate is an alternate source of metabolic energy that is increasingly recognized for its role in supporting neurons. The primary transporter for lactate in the nervous system, monocarboxylate transporter-1 (MCT1), has been shown to be critical for peripheral nerve regeneration and metabolic support to neurons/axons. In this study, MCT1 was reduced in both sciatic nerve and dorsal root ganglia in wild-type mice treated with streptozotocin (STZ), a common model of type-1 diabetes. Heterozygous MCT1 null mice that developed hyperglycemia following STZ treatment developed a more severe DPN compared to wild-type mice, as measured by greater axonal demyelination, decreased peripheral nerve function, and increased numbness to innocuous low-threshold mechanical stimulation. Given that MCT1 inhibitors are being developed as both immunosuppressive and chemotherapeutic medications, our results suggest that clinical development in patients with diabetes should proceed with caution. Collectively, our findings uncover an important role for MCT1 in DPN and provide a potential lead toward developing novel treatments for this currently untreatable disease.
Keywords: Diabetic peripheral neuropathy, Peripheral nerve, Dorsal root ganglion, Metabolism, Monocarboxylate transporter
Introduction
Diabetic peripheral neuropathy (DPN), which results from the downstream metabolic cascade of longstanding hyperglycemia leading to peripheral nerve injury, is one of the most common complications in diabetic patients (Tesfaye et al., 2010). Sensory, autonomic, and motor nerves can all be affected, most frequently in a distal-to-proximal gradient of severity. The most common initial symptoms of DPN are hyperalgesia, dysesthesia and allodynia, and the disease frequently progresses to numbness and hypoalgesia (Vinik et al., 2000). Though DPN most severely impacts sensory nerves, motor nerve dysfunction can also manifest over the course of the disease (Feldman et al., 2019). The specific mechanism is unknown, but clearly involves metabolic dysfunction and energy failure in multiple cells within the peripheral nerve and dorsal root ganglion (DRG) (Feldman et al., 2017; Hinder et al., 2012).
Growing evidence suggests that lactate is an alternate and effective energy source for the peripheral nerves, and the lactate shuttle between glial and neurons has been demonstrated in the peripheral nervous system (PNS) (Domenech–Estevez et al., 2015; Jha and Morrison, 2018). The primary transporter for lactate in the PNS, monocarboxylate transporter–1 (MCT1), has been shown to be critical for the response of peripheral nerves to injury (Morrison et al., 2015), Schwann cell metabolism, and maintenance of sensory nerve myelination during aging (Jha. et al. , 2020). However, the role of MCT1 in the pathogenesis of neurological manifestations of diabetes remains to be explored. Here, we investigate the contribution of MCT1 to the pathogenesis of DPN using the streptozotocin (STZ)–induced diabetes model. We found that deficiency of MCT1 in mice worsens experimental DPN.
Material and methods
Animals
All animal experiments were carried out in compliance with the protocols approved by the Johns Hopkins University Institutional Animal Care and Use Committee (IACUC). Wildtype male mice aged 8–10 weeks were purchased (C57BL6/J male mice, Jackson Laboratory) and used for the evaluation of MCT1 expression in sciatic nerves and DRG after diabetes induction. Breeding colonies of heterozygous MCT1 null (Het MCT1-null) mice on a C57B16J background were maintained at the Johns Hopkins University (Lee et al., 2012; Lengacher et al., 2013; Morrison et al., 2015). Het MCT1-null mice were used since full knockout of MCT1 is embryonically lethal. Both male and female Het MCT1-null mice and control littermates (wild-type) aged 8–10 weeks were used. Het MCT1-null mice develop normally and show no neurological symptoms at this age (Morrison et al., 2015).
Diabetes induction with or without insulin treatment
As described previously (Lennertz et al., 2011; Wang et al., 1993), type-1 diabetes was induced in age-matched Het MCT1-null mice and control littermates by an intraperitoneal injection of STZ (Sigma-Aldrich; 180 mg/kg body weight) in 0.1 M citrate buffer (pH 4.5). Blood samples were collected from the tail vein three days following injection and then at regular intervals throughout experiment, and glycemia was determined with a OneTouch Ultra 2 glucometer (LifeScan Inc., Milpitas, CA). If hyperglycemia (blood glucose >300 g/dl) was not obtained after a single injection, a second STZ injection (180 mg/kg body weight if no effect and 90 mg/kg body weight if partial effect) was administered after one week. Mice with sustained hyperglycemia were used for diabetes experiments, while those unable to achieve sustained hyperglycemia were used as STZ control mice. 85% of STZ-injected mice survived up to the end of the study with no difference between genotypes.
As an additional control for direct STZ toxicity, a subset of mice who achieved hyperglycemia after STZ were treated with insulin, by subcutaneous implantation of LinBit insulin pellets (LinShin; Toronto Canada) under the mid dorsal skin, to normalize their blood sugars. The number of pellets used was dependent on body weight (1 pellet <15g, 2 pellets 15-24g, 3 pellets >24g). Following implantation, blood sugars were measured weekly to assure normoglycemia.
RNA preparation and quantitative real-time RT-PCR
Deeply anesthetized mice were transcardially perfused with 0.1 M PBS to remove the blood, and the sciatic nerves rapidly dissected. RNA was isolated by an RNeasy Mini Kit (Qiagen), reverse transcribed to cDNA with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems), and quantified by real-time RT PCR using Taqman probes (Applied Biosystems) for MCT1 (Thermo Fisher Scientific; Catalog # 4351372) or GAPDH (Thermo Fisher Scientific; Catalog # 4352339E) on a StepOne Plus RT-PCR System (Applied Biosystems).
Nerve conduction studies
Electrophysiologic recordings were performed to measure sensory nerve action potentials (SNAPs) from the tail nerve while maintaining tail temperature at 32–34°C (measured with the Digi-Sense Infrared Thermometer [Model 20250-05]), and compound muscle action potentials (CMAPs) by using a Neurosoft-Evidence 3102evo electromyograph system (Schreiber & Tholen Medizintechnik, Stade, Germany) as described previously (Jha et al., 2020; Xia et al., 2010).
Behavioral studies
Mechanical and thermal sensitivity was assessed by the frequency method using two calibrated von Frey monofilaments (low force, 0.07 g; high force, 0.45 g) and Hargreaves test, respectively, as described previously .
Histologic analyses
Toluidine blue-stained sections were used for quantification of myelinated axon number and diameter, myelin thickness, or g ratio, as described previously (Jha et al., 2020). Analysis of footpads for intra-epidermal nerve fiber density (IENFD) was measured by PGP9.5-immunoreactive nerve counts normalized to epidermal area, as previously described (Jha et al., 2020).
Quantification and statistical analysis
Statistical analyses were performed with GraphPad Prism 8. The number of animals per group (n), statistical test used for comparison, and statistical significance were included in the figure legends. All data were presented as mean ± SEM. Differences in the p values of <0.05 were considered statistically significant. The investigators performing electrophysiologic recordings, behavioral assessments, and quantification of histology were blinded to genotypes throughout the study.
Results
MCT1 is reduced early in the sciatic nerve and DRG following induction of STZ diabetes in wild-type mice
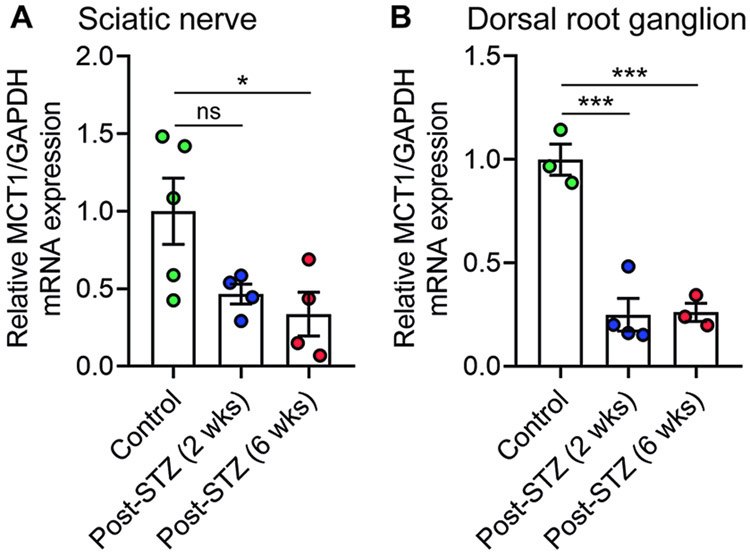
To better understand the function of MCT1 in DPN, we first evaluated whether MCT1 expression in the sciatic nerves and DRG is altered in the STZ model of diabetic neuropathy using quantitative real-time RT-PCR. MCT1 expression was substantially reduced by 2 weeks in both sciatic nerves (Fig. 1A) and DRG (Fig. 1B) of wild-type mice. These experiments provided the first suggestion that MCT1 may contribute to DPN.
Figure 1. Expression of MCT1 in sciatic nerve and DRG of diabetic mice.
The relative expression of MCT1 mRNA in sciatic nerve (A) and DRG (B) after 2 and 6 weeks of STZ treatment, depicted as fold change compared with wild-type mice normalized to their corresponding GAPDH mRNA levels. Mean ± SEM, n = 3–5 per group, *p < 0.05, ***p < 0.001; ns = not significant, one-way ANOVA with Bonferroni's multiple comparisons test.
Het MCT1-null mice show intact nerve function and develop STZ-induced diabetes
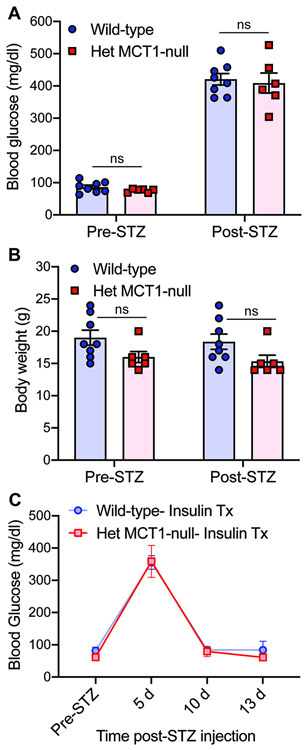
To investigate whether reduced MCT1 plays a role in DPN, we investigated the response to diabetes of Het MCT1-null mice, which express approximately 50% MCT1 of wild-type mice (Morrison et al., 2015). Het MCT1-null mice do not show any notable differences compared to control littermates in their sensory and motor nerve conductions or mechanical and thermal sensitivities at 16 weeks of age, which is the age of the mice used in this study, suggesting a normal nerve biology in matured Het MCT1-null mice (Supplementary Fig. 1). Since a prior publication showed that these mice do not develop diet-induced obesity and insulin resistance after treatment with high fat diet (Lengacher et al., 2013), we confirmed that diabetes induction following STZ administration, as measured by hyperglycemia and body weight, was not altered in Het MCT1-null mice (Fig. 2A,B) and that insulin administration normalized elevated blood sugars (Fig. 2C).
Figure 2. Blood glucose levels and body weight pre- and post-STZ and insulin treatment.
Blood glucose levels (A) and body weight (B) were measure before and 3 days after STZ administration in Het MCT1-null mice and Wild-type littermates. Both groups of mice showed identical extent of hyperglycemia and body weight pre- and post-STZ treatment, n = 6–8 per group. Blood glucose levels (C) were measured in Het MCT1-null mice and Wild-type littermates pre- and post-STZ, as well as following treatment with insulin pellets. n= 4-5 per group. Mean ± SEM, ns = not significant, two-way ANOVA with Bonferroni's multiple comparisons test.
Reducing MCT1 impairs sensory and motor nerve conductions and reduces mechanical sensitivity
To evaluate the impact of reducing MCT1 on DPN, we measured sensory and motor nerve conduction velocities (NCV), SNAP and CMAP amplitudes before and for 9 weeks after STZ administration (Fig. 3). NCV reflects the myelination state of nerves, while SNAP and CMAP amplitudes are indicators of axonal integrity. Before diabetes induction, both genotypes showed identical electrophysiologic measurements, suggesting that reducing MCT1 has no impact on baseline nerve biology, as published previously (Morrison et al., 2015). Following STZ treatment, there was a clear and significant decline in NCV over time in wild-type mice (2-way ANOVA overall by time F[4.1,82.3],p<0.0001; p<0.05 at weeks 1, 3, and 6 by Bonferroni’s multiple comparison). The decline in sensory NCV (Fig. 3A) and SNAP (Fig. 3B) were significantly greater within 3 weeks in Het MCT1-null mice compared to control littermates. Furthermore, Het MCT1-null mice also had impaired motor NCV and CMAP by 6 and 9 weeks, respectively, after diabetes induction (Fig. 3C, D). Importantly, these differences were due to hyperglycemia since mice treated with STZ without hyperglycemia had no electrophysiologic abnormalities (Fig. 3E-H).
Figure 3. Impact of MCT1 deficiency on nerve conductions after diabetes induction.
Sensory (A) and motor (C) nerve conduction velocities and SNAP (B) and CMAP (D) amplitudes in Het MCT1-null mice and control littermates before and after STZ administration with hyperglycemia, n = 7–15 per group. Sensory (E) and motor (G) nerve conduction velocities and SNAP (F) and CMAP (H) amplitudes in Het MCT1-null mice and control littermates before and after STZ administration without hyperglycemia, n = 5-8 per group. Mean ± SEM, *p < 0.05, **p < 0.01; two-way ANOVA with Bonferroni's multiple comparisons test. CMAP, compound muscle action potential; NCV, nerve conduction velocity; SNAP, sensory nerve action potential; N1 Glucose, STZ administration without hyperglycemia.
We next investigated whether Het-MCT1 null mice developed behavioral deficits for 10 weeks following STZ. Prior to STZ administration, there was no difference in mechanical and thermal sensitives between Het-MCT1 null mice and littermate controls (Fig. 4). Following STZ administration, there was a significant increase in paw withdrawal frequency, reflecting allodynia, in wild-type mice at both 0.07g (2-way ANOVA overall by time, f[2.5,43.8], p=0.0006; p<0.05 at week 8 by Bonferroni’s multiple comparison) and 0.45g (2-way ANOVA overall by time, f[3.8,65.4], p<0.0001; p<0.05 at week 6 by Bonferroni’s multiple comparison) tensile strengths. Comparing Het MCT1-null with wild-type mice, there was significantly reduced mechanical sensitivity in Het MCT1-null mice by 6 weeks post-STZ, as indicated by decreased paw withdrawal frequency to repetitive low or high force von Frey filament stimulation (Fig. 4A,B). The experiments were stopped at 10 weeks due to concern for animal death from STZ treatment. In contrast to mechanical sensitivity, no alteration in paw withdrawal latency to thermal stimulation was observed in wild-type or Het MCT1-null mice after diabetes induction (Fig. 4C), as reported previously prior to 12 weeks in STZ-treated mice (Aghanoori et al., 2019; Rojas et al., 2019). Similar to electrophysiology (Fig. 3E-H), reducing MCT1 did not alter behavior in mice treated with STZ without hyperglycemia (Fig. 4D-F).
Figure 4. Impact of MCT1 deficiency on sensory behavioral testing after diabetes induction.
Paw withdrawal frequency to mechanical stimulation by calibrated von Frey monofilaments of forces 0.07 g (A) and 0.45 g (B) and paw withdrawal latency (C) to thermal stimulation by radiant paw-heating assay in Het MCT1-null mice and Wild-type littermates before and after STZ administration with hyperglycemia, n = 6–13 per group. Paw withdrawal frequency to mechanical stimulation by calibrated von Frey monofilaments of forces 0.07 g (D) and 0.45 g (E) and paw withdrawal latency (F) to thermal stimulation by radiant paw-heating assay in Het MCT1-null mice and Wild-type littermates before and after STZ administration without hyperglycemia, n = 5-8 per group. Current set at baseline level: 20%, 10–12 s; cut off time; 30 s. Mean ± SEM, *p < 0.05; **p <0 .01; ***p <0 .001; ****p <0 .0001, two-way ANOVA with Bonferroni's multiple comparisons test. N1 Glucose, STZ administration without hyperglycemia
Reduction in MCT1 results in sural nerve demyelination after diabetes induction
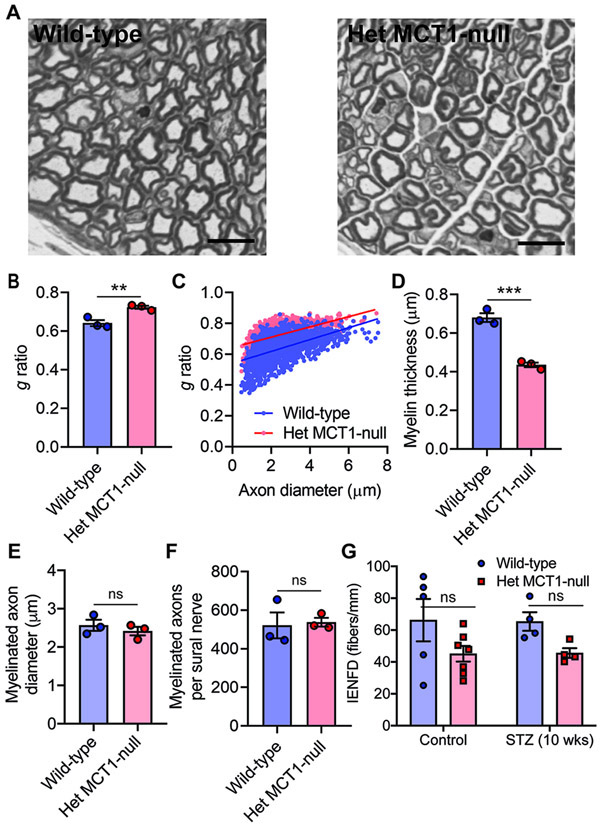
NCV depends primarily on the myelination status and axon diameter of nerve. Thus, we investigated the morphology of sural nerves isolated from Het MCT1-null and control littermate diabetic mice. Consistent with reduced sensory NCV, we observed thinner myelin (Fig. 5A) in Het MCT1-null mice, measured both by g ratio (Fig. 5B and C) and myelin thickness (Fig. 5D), without a change in axon diameter (Fig. 5E), confirming that NCV slowing was due to thinner myelin. Interestingly, despite reduced SNAP amplitude of tail sensory nerves, there was no change in the number of myelinated axons per sural nerve (Fig. 5F). Additionally, there was no change in IENFD, which measures unmyelinated nociceptive nerve fibers in the skin, in Het MCT1 null mice before or after STZ (Fig. 5G), which is consistent with unchanged thermal sensitivities (Fig. 4C),
Figure 5. Impact of reduced MCT1 on sural nerve myelination and integrity in diabetes.
(A) Light microscope photomicrographs of toluidine blue-stained sections of sural nerves from Het MCT1-null mice and Wild-type littermates with hyperglycemia 10 weeks after STZ treatment. These images were analyzed for g ratio (B), scatter plot graph displaying g ratio (y-axis) in relation to axon diameter (x-axis) of individual fiber (C), myelin thickness (D), and the diameter (E) and number (F) of myelinated axons. Mean ± SEM, n = 3 per group, **p < 0.01; ***p < .001; ns = not significant, unpaired t test. Scale bar, 20 μm. The g ratio between wild-type (blue line) and Het MCT1-null (red line) mice (C) was significantly different (p < 0.0001; t = 30.75, df = 3,177, unpaired t test). (H) IENFD obtained from the footpads of control or diabetic mice at 10 weeks after STZ administration following immunohistochemical staining for PGP9.5. Mean ± SEM, n = 4–7 per group, ns = not significant, two-way ANOVA with Bonferroni's multiple comparisons test. IENFD, intraepidermal nerve fiber density.
Discussion
In this study, we evaluate, for the first time, the potential role of MCT1 in the pathogenesis of DPN. Our results suggest that chronic hyperglycemia decreases the expression of MCT1 in PNS, and mice with reduced expression of MCT1 exhibit progressive NCV slowing and reduced SNAP as well as CMAP amplitudes, electrophysiological features of DPN in animal models and humans (Chung et al., 2014; Tankisi et al., 2007). These abnormalities were not due to direct STZ toxicity since STZ treatment without hyperglycemia did not lead to any electrophysiologic or behavioral abnormalities. In addition, this study reveals that these electrophysiological deficits are associated with greater demyelination in Het MCT1-null mice. This study further demonstrates that mice with reduced expression of MCT1 develop depressed sensitivity to mechanical stimulation.
Converging evidence suggests that DPN is at least partly due to energy failure in the peripheral nerve (Feldman et al., 2017; Hinder et al., 2012). MCT1 is the predominant lactate transporter throughout the body (Halestrap, 2012), and its expression is altered in adipocytes, muscle, and brain (Juel et al., 2004; Pierre et al., 2007; Py et al., 2002) of patients and animal models with diabetes. Despite its critical role in nerve regeneration after injury (Morrison et al., 2015) and nerve myelination during aging (Jha et al., 2020), MCT1 has not previously been investigated in DPN. This study shows that in STZ-treated diabetic wild-type mice, MCT1 is reduced early in both sciatic nerves and DRG, prior to any axon or Schwann cell degeneration (Lennertz et al., 2011; Murakami et al., 2013). Interpretation of these studies should be made cautiously, however, since only completed in male mice, measured mRNA expression and not protein, and many different cell types in peripheral nerve and DRG express MCT1. Additionally, we cannot rule out that the loss of MCT1 is a direct impact of STZ and not due to hyperglycemia. Despite these limitations, the reduced MCT1 mRNA expression suggests a possible contribution of lactate pathways and metabolic dysfunction in diabetic sciatic nerves and DRG.
MCT1 deficiency accelerates DPN and sensory loss in Het MCT1-null mice, and the worsening of neuropathy occurs without any change in the degree of hyperglycemia, suggesting that MCT1 does not contribute to these abnormalities directly by modulating the extent of hyperglycemia. MCT1 potentially modulates the metabolism of diverse cells associated with the pathophysiology of DPN. The increased demyelination in sural nerves from diabetic Het MCT1-null mice suggests that MCT1 is critical for the maintenance of myelin integrity during DPN, potentially due to an important role in Schwann cells. This finding is consistent with our recent study, which demonstrated a role for Schwann cell-specific MCT1 in the maintenance of sensory nerve myelination during aging by modulating lipid metabolism in peripheral nerves (Jha et al., 2020). Importantly, however, the changes in myelination following STZ treatment of Het MCT1-null mice is not merely due to aging, since sensory NCV reductions were seen in STZ-treated mice before 3 months of age, which is prior to the previously published hypomyelination observed with aging. Additionally, there were no changes in SNAP amplitude, motor NCV, or motor CMAP amplitude, at any timepoint, following Schwann cell-selective knockdown of MCT1 (Jha et al., 2020).
DPN in patients preferentially targets distal components of sensory axons, and only later, and usually to a lesser extent, motor axons (Feldman et al., 2019; Toth et al., 2004). In this study, reduced expression of MCT1 causes NCV deficits in sensory nerves within 3 weeks and motor nerves within 6 weeks of diabetes induction. Furthermore, Het MCT1-null mice have progressive declines in SNAP and CMAP amplitudes without any remarkable change in the number of myelinated sural nerve axons. There are two possible explanations for these apparently contradictory findings. First, severe demyelination may be causing conduction block, in which action potentials being conducted along a demyelinated nerve are unable to propagate due to a large gap in myelin (Hu et al., 2018). Second, since the axon degeneration in DPN is thought to be primarily a “dying back” process that impacts the most distal components of the nerve (Callaghan et al., 2012), perhaps the sural nerve counts are unchanged because they are measured from more proximal nerve segments than those measured by tail SNAPs. Further confirmation of distal sensory nerve injury comes from behavioral testing, as Het MCT1-null mice treated with STZ have reduced response to mechanosensitivity with von Frey monofilaments.
Conclusions
In summary, our findings demonstrate that MCT1 plays an important role in DPN pathogenesis. MCT1 expression is reduced in both sciatic nerve and DRG following STZ-induced diabetes, and reducing MCT1 worsens DPN in this model of type 1 diabetes. The mechanism by which MCT1 contributes to DPN is not clear, but may be due to reduced capacity of cells without MCT1 to process elevated glucose levels, which would be expected to generate higher amounts of lactate, or impaired PNS cell metabolism leading to deficits in metabolic support to Schwann cells or neurons. Regardless of mechanism, our results suggest that manipulating this transporter may be a potential new avenue for DPN treatments. Additionally, the results of our paper suggest that clinical development of MCT1 inhibitors, either as immunosuppressive agents (Murray et al., 2005) or chemotherapies (Perez-Escuredo et al., 2016), should proceed cautiously, as there may be unexpected side effects in patients with diabetes.
Supplementary Material
Supplementary Figure 1. Reducing MCT1 has no impact on nerve conductions or sensory behavioral testing without streptozotocin treatment. Sensory (A) and motor (C) nerve conduction velocities and SNAP (B) and CMAP (D) amplitudes in Het MCT1-null mice and control littermates are unaltered at 16 weeks of age. Paw withdrawal frequency to mechanical stimulation by calibrated von Frey monofilaments of forces 0.07 g (A) and 0.45 g (B) and paw withdrawal latency (C) to thermal stimulation by radiant paw-heating assay is unaltered in Het MCT1-null mice and Wild-type littermates at 16 weeks of age. Current set at baseline level: 20%, 10–12 s; cut off time; 30 s. n = 4-5 per group. Mean ± SEM, ns = not significant; two-way ANOVA with Bonferroni's multiple comparisons test. CMAP, compound muscle action potential; NCV, nerve conduction velocity; SNAP, sensory nerve action potential.
Highlights.
MCT1 is reduced in sciatic nerve and DRG of streptozotocin-induced diabetic
Reducing MCT1 worsens experimental diabetic neuropathy in mice
Reducing MCT1 led to greater nerve demyelination after induction of diabetes
Acknowledgements
The authors would like to thank Ms. Kimberly Brown and the Johns Hopkins Neurology Electron Microscopy Core for their assistance in processing embedded nerve tissue for toluidine blue staining. We would also like to thank the Pain Research Core funded by the Blaustein Fund and the Neurosurgery Pain Research Institute at The Johns Hopkins University for providing facilities for behavioral studies. Financial support was provided by NIH-NS086818-01 (B.M.M.). B.M.M. is the guarantor of this work and, as such, had full access to all the date in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no competing financial interests.
References
- Aghanoori MR, Smith DR, Shariati-Ievari S, Ajisebutu A, Nguyen A, Desmond F, Jesus CHA, Zhou X, Calcutt NA, Aliani M, Fernyhough P, 2019. Insulin-like growth factor-1 activates AMPK to augment mitochondrial function and correct neuronal metabolism in sensory neurons in type 1 diabetes. Mol Metab 20, 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BC, Little AA, Feldman EL, Hughes RA, 2012. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev, CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Prasad K, Lloyd TE, 2014. Peripheral neuropathy: clinical and electrophysiological considerations. Neuroimaging Clin N Am 24, 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech-Estevez E, Baloui H, Repond C, Rosafio K, Medard JJ, Tricaud N, Pellerin L, Chrast R, 2015. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J Neurosci 35, 4151–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V, 2019. Diabetic neuropathy. Nat Rev Dis Primers 5, 41. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Nave KA, Jensen TS, Bennett DLH, 2017. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 93, 1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, 2012. The monocarboxylate transporter family--Structure and functional characterization. IUBMB Life 64, 1–9. [DOI] [PubMed] [Google Scholar]
- Hinder LM, Vincent AM, Burant CF, Pennathur S, Feldman EL, 2012. Bioenergetics in diabetic neuropathy: what we need to know. J Peripher Nerv Syst 17 Suppl 2, 10–14.22548617 [Google Scholar]
- Hu B, McCollum M, Ravi V, Arpag S, Moiseev D, Castoro R, Mobley B, Burnette B, Siskind C, Day J, Yawn R, Feely S, Li Y, Yan Q, Shy M, Li J, 2018. Myelin abnormality in Charcot-Marie-Tooth type 4J recapitulates features of acquired demyelination. Ann Neurol 83, 756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Lee Y, Russell KA, Yang F, Dastgheyb RM, Deme P, Ament XH, Chen W, Liu Y, Guan Y, Polydefkis MJ, Hoke A, Haughey NJ, Rothstein JD, Morrison BM, 2020. Monocarboxylate transporter 1 in Schwann cells contributes to maintenance of sensory nerve myelination during aging. Glia 68, 161–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Morrison BM, 2018. Glia-neuron energy metabolism in health and diseases: New insights into the role of nervous system metabolic transporters. Exp Neurol 309, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Holten MK, Dela F, 2004. Effects of strength training on muscle lactate release and MCT1 and MCT4 content in healthy and type 2 diabetic humans. J Physiol 556, 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD, 2012. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengacher S, Nehiri-Sitayeb T, Steiner N, Carneiro L, Favrod C, Preitner F, Thorens B, Stehle JC, Dix L, Pralong F, Magistretti PJ, Pellerin L, 2013. Resistance to diet-induced obesity and associated metabolic perturbations in haploinsufficient monocarboxylate transporter 1 mice. PLoS One 8, e82505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennertz RC, Medler KA, Bain JL, Wright DE, Stucky CL, 2011. Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. J Neurophysiol 106, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BM, Tsingalia A, Vidensky S, Lee Y, Jin L, Farah MH, Lengacher S, Magistretti PJ, Pellerin L, Rothstein JD, 2015. Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Exp Neurol 263, 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Iwanaga T, Ogawa Y, Fujita Y, Sato E, Yoshitomi H, Sunada Y, Nakamura A, 2013. Development of sensory neuropathy in streptozotocin-induced diabetic mice. Brain Behav 3, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CM, Hutchinson R, Bantick JR, Belfield GP, Benjamin AD, Brazma D, Bundick RV, Cook ID, Craggs RI, Edwards S, Evans LR, Harrison R, Holness E, Jackson AP, Jackson CG, Kingston LP, Perry MW, Ross AR, Rugman PA, Sidhu SS, Sullivan M, Taylor-Fishwick DA, Walker PC, Whitehead YM, Wilkinson DJ, Wright A, Donald DK, 2005. Monocarboxylate transporter MCT1 is a target for immunosuppression. Nat Chem Biol 1, 371–376. [DOI] [PubMed] [Google Scholar]
- Perez-Escuredo J, Van Hee VF, Sboarina M, Falces J, Payen VL, Pellerin L, Sonveaux P, 2016. Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta 1863, 2481–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Parent A, Jayet PY, Halestrap AP, Scherrer U, Pellerin L, 2007. Enhanced expression of three monocarboxylate transporter isoforms in the brain of obese mice. J Physiol 583, 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py G, Lambert K, Milhavet O, Eydoux N, Prefaut C, Mercier J, 2002. Effects of streptozotocin-induced diabetes on markers of skeletal muscle metabolism and monocarboxylate transporter 1 to monocarboxylate transporter 4 transporters. Metabolism 51, 807–813. [DOI] [PubMed] [Google Scholar]
- Rojas DR, Kuner R, Agarwal N, 2019. Metabolomic signature of type 1 diabetes-induced sensory loss and nerve damage in diabetic neuropathy. J Mol Med (Berl) 97, 845–854. [DOI] [PubMed] [Google Scholar]
- Tankisi H, Pugdahl K, Johnsen B, Fuglsang-Frederiksen A, 2007. Correlations of nerve conduction measures in axonal and demyelinating polyneuropathies. Clin Neurophysiol 118, 2383–2392. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P, Toronto Diabetic Neuropathy Expert, G., 2010. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33, 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C, Brussee V, Cheng C, Zochodne DW, 2004. Diabetes mellitus and the sensory neuron. J Neuropathol Exp Neurol 63, 561–573. [DOI] [PubMed] [Google Scholar]
- Vinik A, Casellini C, Nevoret ML, 2000. Diabetic Neuropathies, in: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP (Eds.), Endotext, South Dartmouth (MA). [Google Scholar]
- Wang Z, Dohle C, Friemann J, Green BS, Gleichmann H, 1993. Prevention of high- and low-dose STZ-induced diabetes with D-glucose and 5-thio-D-glucose. Diabetes 42, 420–428. [DOI] [PubMed] [Google Scholar]
- Xia RH, Yosef N, Ubogu EE, 2010. Dorsal caudal tail and sciatic motor nerve conduction studies in adult mice: technical aspects and normative data. Muscle Nerve 41, 850–856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Reducing MCT1 has no impact on nerve conductions or sensory behavioral testing without streptozotocin treatment. Sensory (A) and motor (C) nerve conduction velocities and SNAP (B) and CMAP (D) amplitudes in Het MCT1-null mice and control littermates are unaltered at 16 weeks of age. Paw withdrawal frequency to mechanical stimulation by calibrated von Frey monofilaments of forces 0.07 g (A) and 0.45 g (B) and paw withdrawal latency (C) to thermal stimulation by radiant paw-heating assay is unaltered in Het MCT1-null mice and Wild-type littermates at 16 weeks of age. Current set at baseline level: 20%, 10–12 s; cut off time; 30 s. n = 4-5 per group. Mean ± SEM, ns = not significant; two-way ANOVA with Bonferroni's multiple comparisons test. CMAP, compound muscle action potential; NCV, nerve conduction velocity; SNAP, sensory nerve action potential.