Abstract
Background
Anti-depressants, particularly selective serotonin reuptake inhibitors (SSRIs), are associated with an increased risk of fracture. The mechanism is unclear and may be due to effects on bone metabolism, muscle strength, falls or other factors. It is unknown if serotonin norepinephrine reuptake inhibitors (SNRIs) have similar effects.
Methods
We compared musculoskeletal health in current female anti-depressant users and non-users from a population-based multiethnic (35.6% black, 22.3% white and 42.1% mixed) cohort study of adults ≥ 65 years old in New York (N=195) using dual x-ray absorptiometry (DXA), trabecular bone score (TBS), vertebral fracture assessment (VFA), high resolution peripheral quantitative computed tomography (HR-pQCT), body composition, and grip strength.
Results
Current anti-depressant users were more likely to be white than non-white (OR 1.9, 95% CI 1.2–2.9) and were shorter than non-users, but there were no differences in age, weight, BMI, physical activity, calcium/vitamin D intake, falls or self-rated health. There were more pelvic fractures in current vs. non-users (7.1% vs. 0%, p=0.04). Age- and weight-adjusted T-score by DXA was lower in current users at the 1/3-radius (−1.6±1.1 vs. −1.0±1.4, p=0.04) site only. There was no difference in TBS, vertebral fractures or fat/lean mass by DXA. Age- and weight adjusted grip strength was 13.3% lower in current users vs. non-users (p=0.04). By HR-pQCT, age- and weight-adjusted cortical volumetric BMD (Ct. vBMD) was 4.8% lower in users vs. non-users at the 4% radius site (p=0.007). A similar cortical pattern was seen at the proximal (30%) tibia. When assessed by anti-depressant class, deteriorated cortical microstructure was present only in SSRI users at the radius and only in SNRI users at the proximal tibia.
Conclusions
Anti-depressant use is associated with cortical deterioration and reduced physical function, but effects may be class-specific. These findings provide insight into the mechanism by which anti-depressants may contribute to the increased fracture risk in older women.
Keywords: anti-depressants, selective serotonin reuptake inhibitors, microstructure, skeletal, fracture
Introduction
Animal models indicate serotonin is an important endogenous regulator of bone mass [1]. Corollary data in humans regarding the role of serotonin in the skeleton is limited. Indirect supportive evidence, however, comes from recent work indicating anti-depressants, particularly selective serotonin reuptake inhibitors (SSRIs), are associated with an increased risk of fracture [2–5]. Serotonin norepinephrine reuptake inhibitors (SNRIs) and tricyclic anti-depressants (TCA) have also been implicated, while it is unclear if anti-depressants in other categories affect risk [5–7]. Because of their effectiveness and limited side effects, use of anti-depressants has increased in the United States. Estimates suggest that 12.7% of the American public take anti-depressants. Use is more frequent in certain subgroups including older adults, women and non-Hispanic Caucasians, who are also at the highest risk for osteoporotic fracture [8]. Because long-term use of anti-depressants (≥10 years) is common, potential detrimental effects on the skeletal health are important to elucidate [8].
Multiple observational and case-control studies indicate an increased risk of hip and non-vertebral fractures in SSRI-users, ranging from 1.3–2.4 fold when compared to non-users [2, 4, 9]. A similar effect size has been reported in TCA users [10, 11]. The mechanism by which these medications increase fracture risk is not clear. Whether the excess fracture risk associated with anti-depressant use in humans is due to depression or confounders such as reduced physical activity, more co-morbidities, and concomitant medications or postural hypotension caused by anti-depressants leading to falls, is controversial. However, a recent double-blind, randomized placebo-controlled trial (RCT) of fluoxetine in patients with acute stroke showed a 2-fold significant increase in the risk of fractures in those allocated to fluoxetine [12], indicating a causal role of anti-depressants. Unfortunately, bone mineral density (BMD) and other indices of skeletal health were not measured in this study. Additionally, there was a non-significant trend toward more falls in those allocated to fluoxetine, making it unclear if the excess fracture risk was due in part to changes in BMD, bone metabolism or falls or a combination of these factors.
Mechanistic data regarding the skeletal pathophysiological changes that occur with antidepressant use are sparse. Animal models suggest SSRIs reduce bone mass by increasing sympathetic tone [13, 14]. Data regarding the effects of anti-depressants, particularly SSRIs and SNRIs, on the musculoskeletal system in humans are limited. There are conflicting data with regard to the effect of SSRIs on BMD as measured by DXA and few data in SNRI users [15–22]. Skeletal microstructure has only recently begun to be evaluated in those taking anti-depressants. A recent study using high resolution peripheral quantitative computed tomography (HR-pQCT) indicated no association between SSRI use and skeletal microstructure in older women [16]. In contrast, another study assessing bone microstructure using the trabecular bone score (TBS) found SSRI use to be associated with lower TBS but the association was not independent of covariates [23].
Several studies have demonstrated a greater risk of falls in those taking any anti-depressants [24, 25]. Recent work shows this to be true in SSRI users, in particular [24]. Reduced muscular function or mass may play a role in explaining the excess risk of falls and fracture in those taking anti-depressants. A recent study indicated SSRI use was associated with worse physical function including lower grip strength, walking speed and lower extremity proximal muscle strength [16]. It is unclear if SSRIs may also affect muscle mass. Whether the increased risk of fractures related to anti-depressant use is attributable to skeletal effects or effects on muscle, balance and falls or a combination of factors remain unclear. The purpose of this analysis was to compare musculoskeletal health, including areal BMD, skeletal microstructure, bone mechanical competence, body composition, physical function, falls and vertebral fractures in anti-depressant users and non-users. We hypothesized that anti-depressant users would have deteriorated skeletal microstructure compared to non-users.
Methods
Design
This is a cross-sectional analysis comparing skeletal health in elderly female current antidepressant users compared to those not currently taking anti-depressants (non-users) who were participating in a population–based cohort study of aging. The Columbia University Irving Medical Center (CUIMC) Institutional Review Board approved this study and all participants provided written informed consent.
Study Population
The Washington Heights Hamilton Heights Inwood Community Aging Project (WHICAP) is an NIH-funded community-based prospective cohort study of aging among >5,900 elderly, African American, Caribbean Hispanic, and Caucasian urban-dwelling residents (age >65) living in Northern Manhattan. The design and recruitment for the study have previously been reported [26]. Briefly, a probability sample of Medicare recipients, age ≥65 without dementia from 3 zip codes in Northern Manhattan was invited to participate. The original cohort was recruited beginning in 1992 and enriched with further recruitment from 1999–2010 and again from 2009 to present. Returning participants were invited to this ancillary study assessing bone health. Those who agreed to participate underwent evaluation with DXA, HR-pQCT, a dynamometer for grip strength and a questionnaire regarding their health and fracture history. Participants were enrolled to this ancillary study between 1/2019 and 3/2020. Because all but five current anti-depressant users were female, this analysis was limited to women (n=195).
DXA
Areal BMD and body composition were measured with a QDR Discovery or Horizon instrument (Hologic Inc). BMD measurements were obtained at the lumbar spine (LS; L1–L4), femoral neck (FN), and 1/3 radius. T-scores were obtained using the manufacturer’s Caucasian reference norms. Participants were scanned at all three skeletal sites unless hardware precluded the analysis of BMD at a given site, in which case the site(s) with hardware were omitted. We excluded vertebra with hardware or other artifacts from the analysis of BMD at the spine. In vivo precision, determined according to the standard method at this facility, is 1.28% at the LS, 1.36% at the hip, and 0.70% for the distal radius (1/3 site)[27]. Subtotal (excluding head) fat and lean mass were obtained and expressed as percentages. Body composition data were available in 161.
Spine TBS and Lateral VFA was calculated from subjects’ spine DXA image using TBS iNsight software as previously described (version 3.0.3; Medimaps, Geneva, Switzerland) [28]. TBS was available on 192 participants. Lateral VFA was acquired from T4 to L5. Participants were categorized as having VF(s) in the imaged spine based on an International Society for Clinical Densitometry (ISCD)certified densitometrist’s reading of the interpretable image using the Genant semi-quantitative method: mild, moderate and severe compression fractures were defined as a 20–25%, 26–40% or >40% reduction in vertebral height, respectively [29]. The Genant visual semi-quantitative method is the current recommended clinical technique for diagnosing vertebral fracture with VFA. In nineteen participants, VFA could not be interpreted due to poor visualization.
HR-pQCT
HR-pQCT was performed with an XtremeCT II scanner (Scanco Medical, Brüttisellen, Switzerland) which uses a microfocus x-ray source (68 kVp voltage, 900 μA current, 43 sec integration time) scanning a region 10.2 mm long along the axis of the long bone resulting in VOI of 60.7 μm isotropic voxel size. The non-dominant distal radius and tibia were scanned unless there was a contraindication (prior fracture or metal implant), in which case the contralateral limb was scanned. Region of Interest was defined on a 2-D scout view by placing a reference line at the endplate: proximal endplate for radius and distal endplate for tibia. Images were acquired using a relative offset from the reference line; radius scans at 4% of limb length and tibia at 7.3%. We also scanned the tibia at a more proximal diaphyseal region at 30%, which is composed almost entirely of cortical bone. A single highly trained operator acquired and analyzed all scans. Scans were scored for motion on a scale of 1–5 and scans with motion score >3 were excluded from analysis. We used the manufacturer’s standard method to filter and binarize the HR-pQCT images. Automated segmentation algorithm was used to segment the cortical and trabecular regions. We assessed standard HR-pQCT morphological microstructure outcomes, including area; density - total, trabecular (Tb) and cortical (Ct) volumetric BMD (vBMD); microstructure - trabecular number (Tb.N), thickness (Tb.Th), and separation (Tb.Sp), cortical thickness (Ct.Th), and cortical porosity (Ct.Po)[30]. In vivo short term reproducibility (CV) for HR-pQCT measures at our center is between 0–5% for all measures except Ct.Po.
FEA
Bone strength was estimated from the HR-pQCT images using micro-finite element analysis (μFEA) based on a voxel conversion approach. We simulated a uniaxial compression on each radius and tibia model up to 1% strain using a homogeneous Young’s modulus of 10 GPa and Poisson’s ratio of 0.3. We used μFEA solver provided by the manufacturer (Scanco Medical FE-software v1.13, Scanco Medical, Brüttisellen, Switzerland) to solve the models. We estimated whole bone stiffness (N/mm). FEA was available on a subset of participants (N=142).
Grip strength
Grip strength was assessed using a hand-held dynamometer with maximum force using the participant’s dominant hand. Three trials were performed in 178 participants. An average score was recorded for each participant based on all three trials
Questionnaire and Clinical Evaluation
Information regarding past medical history, lifestyle, and medications was collected by questionnaire. Fall recall was assessed by questionnaire by asking participants if they had fallen in the last 12 months and the number of falls they sustained. Daily dietary calcium and vitamin D intake was assessed with a validated standardized food frequency questionnaire as previously described [31]. Physical activity was assessed with the physical activity scale for the elderly (PASE) [32]. Self-rated health was assessed on a scale of 1–5 corresponding to poor, fair, good, very good and excellent. Weight and height were measured by balance beam and a wall-mounted, calibrated Harpenden stadiometer, respectively. Anti-depressants were categorized as SSRIs (escitalopram, citalopram, sertraline), SNRIs (duloxetine, venlafaxine) or other (buproprion, trazodone, mirtazapine, tricyclics, etc.). Women taking buspirone, an anxiolytic, were included in the “other” group as it is a serotonin receptor agonist and it has been shown to have anti-depressant properties [33]. Women taking an SSRI or SNRI plus a medication in the “other” category were classified as SSRI and SNRI-users respectively.
Statistics
Descriptive statistics were expressed as means and standard deviations or absolute (n) and relative (%) frequency. Between- group differences in demographic and skeletal indices were evaluated with Student’s t-test or Fisher’s exact test as appropriate. Adjusted analyses and those assessing the effects of anti-depressants by category were conducted with general linear models (GLM) or logistic regression controlling for age and weight due to their known influence on skeletal outcomes. We adjusted for weight rather than BMI as it tended to have a stronger correlation with bone outcomes. Values are expressed as mean ± standard deviation. Stepwise multiple linear regression was used to assess the independent association between anti-depressant use and bone outcomes that differed between groups: cortical vBMD, T-score at the 1/3 radius and grip strength. The potential predictors in multiple regression models were age, weight, race, anti-depressant use, use of osteoporosis medications, tobacco and alcohol use, physical activity, vitamin D intake and self-rated health. The stepwise selection process criterion for entry to the model was a univariate P=0.3 and the criterion for retention in the model was a multivariate P=0.05. All analyses were performed using SAS Version 9.4 (Cary, NC). A two-tailed p-value <0.05 was considered statistically significant.
Results
The cohort was mean age (±SD) 76.3±6.1 years old and the racial distribution was 35.6% black, 22.3% white and 42.1% mixed race. The mixed group was almost entirely (98.8%) composed of individuals who self-identify as Hispanic. Within the mixed race group, 92.8% were Caribbean Hispanic (i.e. from the Dominican Republic, Puerto Rico, and Cuba). Twenty-eight participants were current antidepressant users. Among current users, 35.7% were taking SSRIs, 17.9% were taking SNRIs and 46.4% were taking other categories of anti-depressants. As shown in Table 1, anti-depressant users were more likely to be white than non-white (OR 1.9, 95% CI 1.2–2.9) and were shorter compared to non-users, but there was no difference in age, weight, BMI, physical activity, calcium or vitamin D intake, falls, self-rated health, alcohol or tobacco use, steroid use or current use of osteoporosis medications (Table 1). Current anti-depressant users were more likely to have had a pelvic fracture (7.1% vs 0%, p=0.02) and the fractures were present in the SSRI (n=1) and SNRI (n=1) groups only (p=006).
Table 1.
Demographic and Clinical Characteristics of Current Anti-Depressant Users vs. Non-Users
| Non-Users N=167 | Current Users N=28 | P-value | |
|---|---|---|---|
| Age (years) | 76.5±6.3 | 75.7±5.2 | 0.52 |
| Race | 0.02 | ||
| White (%) | 18.0 | 42.9 | |
| Black (%) | 37.1 | 25.0 | |
| Mixed Race (%) | 44.9 | 32.1 | |
| Ethnicity (% Hispanic) | 51.5 | 46.4 | 0.69 |
| Weight (pounds) | 158.6±35.8 | 157.3±26.8 | 0.85 |
| Height (inches) | 62.1±2.7 | 60.7±2.5 | 0.02 |
| BMI (kg/m2) | 28.9±5.9 | 30.0±5.5 | 0.34 |
| Physical Activity Score | 86.3±42.3 | 76.5±38.4 | 0.25 |
| Calcium Intake (mg/day) | 1213±608 | 1164±481 | 0.69 |
| Vitamin D Intake (IU/day) | 1551±1829 | 1084±1205 | 0.09 |
| Fall in Last Year (%) | 31.1 | 46.4 | 0.13 |
| Number of Falls in Last year | 1.9±2.7 | 2.5±2.0 | 0.48 |
| Self-rated Health Score | 3.0±1.0 | 2.9±1.0 | 0.70 |
| Historical Clinical Fracture (%) | 43.6 | 59.3 | 0.14 |
| Pelvic Fracture (%) | 0 | 7.1 | 0.02 |
| Fracture Number | 1.5±0.8 | 1.8±1.3 | 0.44 |
| Alcohol Use (drinks/week) | 1.1±3.2 | 1.9±4.3 | 0.36 |
| Tobacco Use (%) | |||
| Current | 4.8 | 3.6 | 0.64 |
| Past | 32.9 | 42.9 | |
| Current Steroid use (%) | 0 | 0 | 1.0 |
| Current Osteoporosis Treatment (%) | 12.0 | 7.1 | 0.78 |
Values represent mean ± SD or percentages
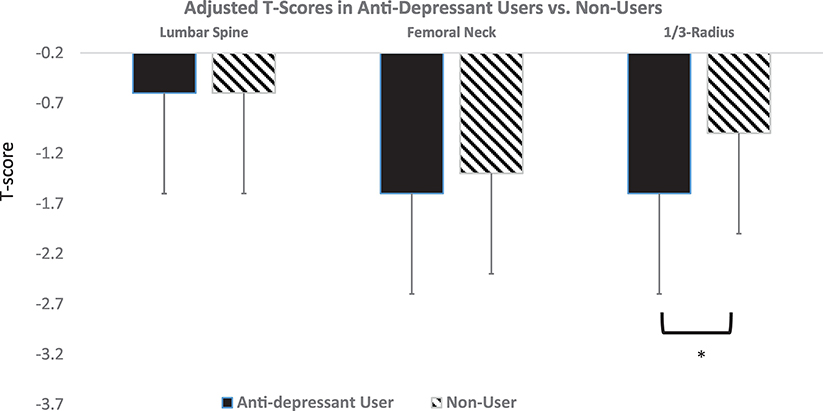
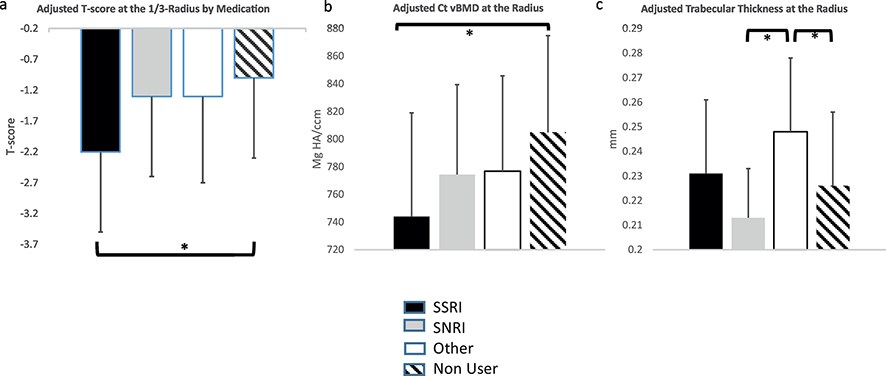
As shown in Figure 1, average T-scores at the hip site and forearm were in the osteopenic range, while mean T-score was normal at the lumbar spine. Age- and weight-adjusted T-score by DXA was lower in current users at the 1/3-radius (−1.6±1.1 vs. −1.0±1.4, p=0.04), but not at other sites (Figure 1a). When anti-depressant users were compared separately by category (SSRI vs. SNRI vs. other) to each other and non-users, only SSRI users had lower adjusted T-scores at the radius compared to non-users (Figure 2a). Mean TBS was in the degraded range in both groups but the between-group difference in age- and weight-adjusted TBS did not reach statistical significance (Table 2). As shown in Table 2, there was no difference in the frequency of vertebral fractures by VFA in anti-depressant users compared to non-users (p=0.56). Similarly, there was no difference in DXA-measured subtotal (excluding head) fat or lean mass by DXA (Table 2). Age- and weight adjusted physical function, as measured by grip strength, however, was 13.3% lower in anti-depressant users vs. non-users (p=0.04). There were no differences in the prevalence of vertebral compression fractures, TBS, body composition or grip strength by antidepressant category (data not shown).
Figure 1:
Age- and weighted-adjusted T-score by DXA at the lumbar spine, femoral neck, and 1/3-radius in anti-depressant users (black) and non-users (hatched). *p<0.05
Figure 2:
Comparison of age- and weight adjusted skeletal parameters stratified by anti-depressant category: a) T-score at the 1/3 radius by DXA; b) cortical volumetric BMD by HR-pQCT at the 4% radius and c) trabecular thickness at the 4% radius by HRpQCT. Different categories of medication use are represented by different colors: black indicates to SSRI users, gray SNRI users, white “other” antidepressants, and hatched no SSRI use.
Table 2.
Trabecular Bone Score, Body Composition, Vertebral Fracture Prevalence, and Physical Function by Anti-Depressant Use
| DXA | ||||
|---|---|---|---|---|
| Non-Users | Current Users | P-value | Adjusted P-value* | |
| Trabecular Bone Score | 1.185±1.205 | 1.145±1.196 | 0.14 | 0.11 |
| Lean Mass (%) | 60.7±6.3 | 61.2±5.1 | 0.72 | 0.80 |
| Fat Mass (%) | 39.3±6.3 | 38.8±5.1 | 0.72 | 0.80 |
| Percent with Vertebral Fracture by VFA | 14.7% | 19.2% | 0.56 | 0.42 |
| Other Skeletal Parameters | ||||
| Grip Strength (kgs force) | 16.6±5.3 | 14.4±4.8 | 0.07 | 0.04 |
Values represent mean ± SD or percentages; VFA=vertebral fracture assessment
adjusted for age and weight
As shown in Table 3, by HR-pQCT, age- and weight-adjusted cortical (Ct) volumetric BMD (vBMD) was 4.8% lower in users vs. non-users at the 4% radius (p=0.007), but there was no difference in cortical thickness or porosity. Unadjusted skeletal stiffness was lower in anti-depressant users but differences in skeletal stiffness were attenuated and did not differ after adjusting for age and weight (Table 3). Differences in trabecular vBMD and trabecular microstructure at the radius were not significant. When anti-depressant users were compared separately by category (SSRI vs. SNRI vs. other) to each other and non-users, Ct. vBMD was lower in SSRI users vs. non-users only (Figure 2b) after adjusting for covariates, while none of the other groups differed from each other. On the other hand, adjusted trabecular thickness was higher in individuals using other anti-depressants compared to non-SSRI users and SNRI users (Figure 2c). There were no differences at the relative tibia site before or after adjustment for age and weight and no effect of anti-depressant class (data not shown).
Table 3.
Skeletal Microstructure and Stiffness by HR-pQCT in Current Anti-Depressant Users and Non-Users
| 4% Radius | ||||
|---|---|---|---|---|
| Non-Users | Current Users | P-value | Adjusted P-value* | |
| Tb. vBMD (mgHA/ccm) | 127.2±37.6 | 136.8±39.0 | 0.24 | 0.20 |
| Ct. vBMD (mgHA/ccm) | 804.7±65.6 | 766.6±78.8 | 0.01 | 0.007 |
| Tb. Number (1/mm) | 1.247±0.241 | 1.337±0.219 | 0.08 | 0.06 |
| Tb. Thickness (mm) | 0.226±0.02 | 0.234±0.05 | 0.38 | 0.07 |
| Tb. Spacing (mm) | 0.809±0.241 | 0.737±0.16 | 0.06 | 0.13 |
| Ct. Thickness (mm) | 0.783±0.176 | 0.735±0.152 | 0.20 | 0.22 |
| Ct. Porosity (%) | 1.1±0.8 | 1.2±0.8 | 0.52 | 0.51 |
| Stiffness (kN/mm) | 47.1±14.2 | 40.0±9.8 | 0.03 | 0.22 |
| 30% tibia | ||||
| Ct. vBMD (mgHA/ccm) | 1014.8±39.7 | 997.9±52.0 | 0.06 | 0.04 |
| Ct. Thickness (mm) | 4.2±0.9 | 4.2±0.7 | 0.99 | 0.93 |
| Ct. Porosity (%) | 1.3±1.1 | 1.8±1.2 | 0.04 | 0.02 |
| Stiffness (kN/mm) | 220.6±42.0 | 210.2±22.6 | 0.11 | 0.17 |
Values represent mean ± SD or percentages
adjusted for age and weight
Tb=trabecular; Ct=cortical; vBMD=volumetric bone mineral density; mgHA=milligrams of hydroxyapatite; ccm=cubic centimeters; mm=millimeters; kN=kilo Newton
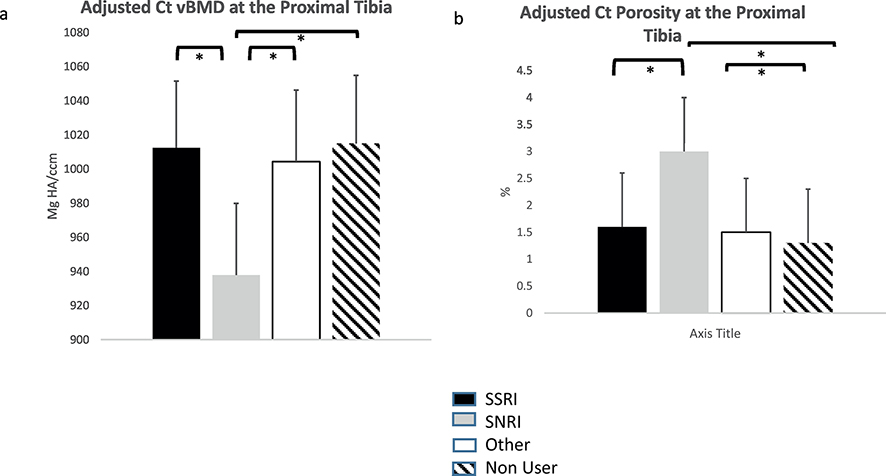
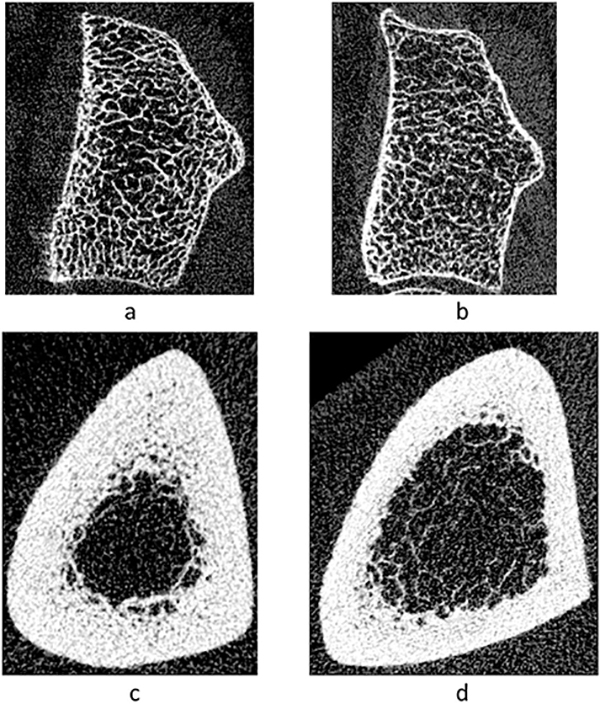
At the predominantly cortical proximal tibia, however, age- and weight-adjusted cortical vBMD was lower in anti-depressant users (p=0.02). Age- and weight-adjusted cortical porosity was 39.7% higher at this site as well in users vs. non-users (p=0.02). As shown in Figure 3, when anti-depressant users were compared by category, SNRI-users had significantly lower adjusted Ct vBMD and higher porosity compared to all other groups at the proximal tibia. The other groups did not differ from each other. Figure 4 shows representative images from the radius and proximal tibia sites in anti-depressant users and non-users.
Figure 3:
Comparison of age- and weight adjusted cortical indices at the proximal 30% tibia stratified by anti-depressant category: a) cortical volumetric BMD by HR-pQCT; b) cortical porosity by HR-pQCT. Different categories of medication use are represented by different colors: black indicates SSRI users, gray SNRI users, white “other” anti-depressants, and hatched no SSRI use.
Figure 4:
Gray scale images of a cross-sectional slice from HR-pQCT scans showing bone microstructure in an anti-depressant user and non-user. Distal radius (4%) scan from (a) an anti-depressant user and (b) non-user; proximal tibia scan from (c) an anti-depressant user and (d) non-user
Table 4 indicates step-wise multiple regression models assessing age, weight, race, anti-depressant use, use of osteoporosis medications, tobacco and alcohol use, physical activity, vitamin D intake and self-rated health as potential predictors of cortical vBMD at the radius by HR-pQCT and 1/3-radius T-score by DXA. In the final model, age and anti-depressant use were significant predictors of cortical vBMD by HR-pQCT (Table 4). The parameter estimate is interpreted to mean that antidepressant use was associated with 39.9 mgHA/ccm lower cortical vBMD adjusted for age. For 1/3-radius T-score, age, weight and current anti-depressant use were independent predictors of BMD. Antidepressant use was associated with 0.6 SD lower T-score adjusted for age and weight. The association between current anti-depressant use and grip strength was of borderline statistical significance (p=0.06) after adjusting for age, weight, race, self-rated health and alcohol use all of which were significant predictors (data not shown; all p<0.05).
Table 4.
Multiple Regression* Models of Skeletal Outcomes
| Parameter Estimate (β) | Standard Error | P-value | Model R2 | |
|---|---|---|---|---|
| Cortical vBMD at the Radius (HR-pQCT) | ||||
| Age (per 10 years) | −26.7 | 8.2 | 0.001 | R2=0.094 P=0.0002 |
| Current Use of Anti-Depressant | −39.9 | 14.2 | 0.006 | |
| T-score 1/3-Radius (DXA) | ||||
| Age (per 10 years) | −3.8 | 1.6 | 0.02 | R2=0.184 P<0.0001 |
| Weight (per 10 pounds) | 0.1 | 0.03 | <0.0001 | |
| Current Use of Anti-Depressant | −0.6 | 0.3 | 0.04 | |
Table shows significant predictors in final stepwise model
R2indicates the percent of the variance accounted for by predictors in the model.
Because there was a higher percentage of white women in the anti-depressant user vs. non-user group and cortical microstructure has been observed to be deteriorated in white women compared to Black and Caribbean Hispanic women, we performed the analysis excluding white women to ensure these results were not driven by between-group differences in race/ethnicity. Among non-Caucasian women (n=153), between-group differences in demographics were similar to that of the whole cohort. Anti-depressant users (n=16) were shorter (59.8±2.5 vs. 62.1±2.7 inches, p=0.002). Additionally, non-Caucasian anti-depressant users consumed less vitamin D compared to non-users (668±483 vs. 1542±1853 IU daily, p<0.0001). Otherwise, there were no differences in age, weight, BMI, physical activity, smoking, alcohol use, or other demographic/lifestyle factors. In this subgroup, anti-depressant users had lower age- and weight-adjusted cortical vBMD (808.9±67.8. vs. 758.4±66.0 mgHA/ccm, p=0.005) and tended to have lower cortical thickness (0.804±0.17 vs. 0.722±0.16 mm, p=0.07) at the radius. However, users also tended to have higher age- and weight-adjusted trabecular number (1.360±0.238 vs. 1.240±0.230 1/mm, p=0.05) and thickness (0.241±0.03 vs. 0.227±0.03 mm, p=0.05). There were no differences in stiffness at the radius and microstructure at the 7.3% tibia site or BMD by DXA after adjusting for age and weight. At the proximal tibial site, age and weight-adjusted cortical porosity also tended to be higher in non-white users vs. non-users (1.2±1.0 vs. 1.8±1.2 %, p=0.05).
Discussion
In this analysis, we have shown that current anti-depressant use is associated with cortical, but not trabecular, skeletal deterioration. Further, the results suggest that both SSRIs and SNRIs, but not “other” anti-depressants, are associated with these findings. To our knowledge, this is the first study to show that skeletal microstructure, as assessed by HR-pQCT, is worse in those taking anti-depressants. It is also the only HRpQCT study to assess multiple categories of anti-depressants. We believe the results are important because they may provide insight into the potential mechanism by which anti-depressants increase the risk of fracture in older women. The subgroup analysis by anti-depressant category implies there are likely class-specific effects. Multiple studies suggest that patients taking anti-depressants have an increased risk of fractures at skeletal sites that are predominantly cortical (hip and non-vertebral), which is congruent with the pattern of cortical bone loss identified in our study. Further, the results indicating effects were limited to those taking SSRIs and SNRIs provide indirect evidence supporting the potential importance of serotonin in human skeletal health.
Serotonin is an endogenous regulator of bone health in animal models, but it has opposing effects depending on its origin. Peripheral (gut-derived) serotonin is a negative regulator, while brain-derived serotonin increases bone formation [14, 34]. SSRIs inhibit serotonin re-uptake by the 5hydroxytryptamine transporter (5HTT) expressed in serotonergic neurons, leading to an increase in serotonin in the synaptic space. Animal studies suggest long-term use of SSRIs results in bone loss due to desensitization of the serotonin (Htr2c) receptor, leading to a central decrease in serotonin signaling that causes an increase in sympathetic activity [14]. Ultimately, the increase in sympathetic activity reduces bone formation and increases bone resorption [14, 35]. It is unclear whether SNRIs act on bone in a similar manner, but their mechanism of action to block reuptake of synaptic serotonin and norepinephrine makes it plausible. “Other” anti-depressants were not associated with microstructural deterioration, but rather, this group had better trabecular thickness compared to some other groups. This finding however, must be interpreted with caution as the “other” group included women taking medications in different classes acting via different mechanisms, only some of which affect serotonin levels. Further work is needed to determine if detrimental skeletal effects from particular medications within the “other” antidepressant category may have been masked by the heterogeneity within this group. Further, it will be important to determine if an increase in serotonin by any drug mechanism is associated with skeletal deterioration or if this is specific to SSRIs and SNRIs. Additionally, whether anti-depressants that only increase adrenergic tone, but not serotonin, or act via other mechanisms have harmful skeletal effects will also be important to consider.
Only one other study utilized HR-pQCT to assess skeletal microstructure in anti-depressant users. In contrast to our study, that investigation in older Swedish women, did not show any association between anti-depressant use and skeletal microstructure [16]. The reasons for the differences are not clear. The study by Larsson et al. was larger than our study and included only those taking SSRIs. In contrast to our study, which utilized a second generation HR-pQCT instrument, the prior study by Larsson utilized the lower resolution first generation instrument. This may have limited the ability to detect differences. Both studies enrolled women of a similar age, but the racial composition of the cohorts differed. Our cohort was composed predominantly of non-Caucasian women, while the participants in the Swedish study were presumably mostly white. It is unclear whether race modifies the effect of anti-depressants on bone health.
Khosla et al. reported a relationship between serum serotonin levels and microstructure by HRpQCT in the general population, rather than those on anti-depressants [36]. In this study serum (peripheral) serotonin levels were inversely associated with bone volume fraction, trabecular number, and trabecular thickness at the radius consistent with the negative regulation of bone by peripheral serotonin. Cortical parameters, however, were not reported. One recent study reported an association between the trabecular bone score and SSRI use, but the relationship was not independent of other covariates, such as age, BMI, diabetes and alcohol use [23].
The reason for the preferential loss of cortical bone is not clear. Our results suggest skeletal site specific effects that may, in part, be explained by the compartmental pattern of deterioration. We found worse microstructure at the 1/3 radius by DXA and the proximal tibia, sites that are predominantly composed of cortical bone, as well as the distal radius by HRpQCT. In contrast, we did not find differences at the distal tibia by HRpQCT. The incongruence between the two tibial sites may be explained by the fact that the cortex is much thicker at the proximal versus the distal tibia [37]. Some, but not all, studies indicate associations between anti-depressant use and BMD by DXA at the hip and spine. Most studies have not assessed the cortical 1/3-radius by DXA. Two studies that evaluated the forearm, did indeed show reduced BMD in anti-depressant users [19, 38]. Given the pattern of increased risk of fracture at skeletal sites predominantly composed of cortical bone (hip and non-vertebral) in antidepressant users, one might have anticipated antidepressants to cause preferential loss of cortical BMD. While HR-pQCT is not available clinically, our data imply that DXA measurement of BMD at the 1/3 radius could be useful in identifying those at risk.
We found that anti-depressant users tended to have reduced physical function in the absence of reduced physical activity or muscle mass. The influence was, however, was only of borderline statistical significance, after adjusting for covariates. It is unclear if this is due to serotonin specifically or other mechanisms, as there was no class-effect. A recent study limited to those on SSRIs, however, found similar results [16]. Performance on tests evaluating grip strength, walking speed and rising from standing were lower among SSRI users, but muscle mass was not affected [16]. The mechanisms by which serotonin affect muscle function have only begun to be explored. A recent study found that when serotonin levels are increased via ingestion of the SSRI, paroxetine, arm/upper extremity muscle function was increased if the muscle was “unfatigued”, but decreased if the muscle was fatigued (i.e. subjected to repeated sustained contraction) as measured by reduced time- to- task failure and reduced force [39]. This mechanism could explain the grip strength findings and also predispose to falls, particularly during sustained exertion, if other muscle groups such as the quadriceps, are affected similarly. Falls may explain fractures at cortical sites (hip and non-vertebral) reported in other studies [2, 3]. Notwithstanding, sample size limitations, we did observe that SSRI and SNRI use was associated with pelvic fractures, which generally occur after a fall. On the other hand, we did not find anti-depressant use was associated with falls, which has been reported in a number of studies [12, 24, 25, 40, 41].
We found that white non-Hispanic white women were more likely than non-Caucasian or Hispanic women to be taking anti-depressants. This finding is consistent with several prior studies that have assessed racial and ethnic disparities in depression treatment [8, 42]. The reasons for this are likely multifactorial and include cultural differences in seeking or wanting treatment, provider bias in prescribing anti-depressants, and access to care and or coverage of prescriptions among other factors [43, 44]. There are also racial/ethnic differences in skeletal health. Caucasians have been shown to have worse cortical parameters by HRpQCT compared to Black and Caribbean Hispanic individuals [45–47]. We are, however, reassured that our findings are not simply to due race/ethnic differences between groups as the sub-group analysis of non-Caucasian women indicated similar findings to the full cohort.
Our study has several limitations. First, our study is cross-sectional. While our regression models assessed the influence of many confounders and our analysis comparing anti-depressant users to other anti-depressant users by category may have mitigated the effect of some confounders, we cannot exclude the possibility there may be residual confounding due to depression itself or unmeasured lifestyle factors associated with depression or its treatment. A randomized clinical trial assessing the effect of antidepressant use on musculoskeletal endpoints is a stronger design and would be optimal to demonstrate causality. Second, with a larger sample size, we may have been able to detect effects of individual medications and smaller effects on other skeletal or muscle outcomes, particularly stiffness as this value was available only in a subset. Future larger studies will be helpful to confirm class/medication specific effects and mechanisms. We were also unable to study the association in men due to the small number of men taking anti-depressants. Lastly, we do not have information regarding duration of anti-depressant use, which would have been helpful in delineating if length of therapy is associated with the extent of skeletal deterioration.
Our study also has several strengths. Participants were enrolled from a racially diverse population-based cohort, which limits selection bias and increases the generalizability of these findings. Further, our analysis was conducted in those most at risk for fracture, elderly women, in whom anti-depressant treatment is common. We had comprehensive information on skeletal covariates and thoroughly assessed musculoskeletal health with multiple modalities by measuring areal BMD, body composition, and TBS from DXA, skeletal microstructure and estimated bone strength by HR-pQCT, physical function and falls, and vertebral fracture. Finally, we assessed HR-pQCT using a relative offset to ensure same region of interest was assessed across the cohort to account for differences in height and limb length. Additionally, we also scanned participants at a proximal tibial site, providing further insight into effects on cortical bone.
In summary, anti-depressant use was associated with cortical, but not trabecular, deterioration in elderly women. The results suggest class specific effects and may provide insight into the mechanism by which anti-depressants increase the risk of hip and non-vertebral fractures and also provide indirect support for the importance of serotonin as a regulator of bone health in humans. DXA screening that includes assessment of BMD at the forearm may be useful in identifying older female anti-depressant users at risk for fracture.
Highlights.
Areal bone mineral density (BMD) and cortical volumetric BMD were lower at the 1/3-radius by DXA and 4% radius as well as the 30% tibia by HR-pQCT, respectively, in current antidepressant users compared to non-users
Effects on BMD were only present in users of certain classes of anti-depressants, namely selective serotonin reuptake inhibitor and serotonin-norepinephrine reuptake inhibitor
These findings provide insight into the mechanism by which anti-depressants may contribute to the increased fracture risk in older women
Acknowledgements
We acknowledge the WHICAP study participants and the WHICAP investigators, research and support staff for their contributions to this study.
Funding: Funding for this work was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases R01 AR071986; the National Institute on Aging grants PO1AG07232, R01AG037212, and RF1AG054023. Data collection and sharing for this project was supported by the Washington Heights-Hamilton Heights Inwood Columbia Aging Project (WHICAP).
Funding: R01AR071986, PO1AG07232, R01AG037212, RF1AG054023
Footnotes
The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yadav VK, et al. , A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell, 2009. 138(5): p. 976–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Q, et al. , Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case-control studies. Osteoporos Int, 2012. 23(1): p. 365–75. [DOI] [PubMed] [Google Scholar]
- 3.Vestergaard P, Rejnmark L, and Mosekilde L, Selective serotonin reuptake inhibitors and other antidepressants and risk of fracture. Calcif Tissue Int, 2008. 82(2): p. 92–101. [DOI] [PubMed] [Google Scholar]
- 4.Eom CS, et al. , Use of selective serotonin reuptake inhibitors and risk of fracture: a systematic review and meta-analysis. J Bone Miner Res, 2012. 27(5): p. 1186–95. [DOI] [PubMed] [Google Scholar]
- 5.Power C, et al. , Bones of Contention: A Comprehensive Literature Review of Non-SSRI Antidepressant Use and Bone Health. J Geriatr Psychiatry Neurol, 2019: p. 891988719882091. [DOI] [PubMed] [Google Scholar]
- 6.Moura C, et al. , Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int, 2014. 25(5): p. 1473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CY, et al. , Serotonergic antidepressant use and the risk of fracture: a population-based nested case-control study. Osteoporos Int, 2016. 27(1): p. 57–63. [DOI] [PubMed] [Google Scholar]
- 8.Pratt LA Antidepressant Use Among Persons Aged 12 and Over: United States, 2011–2014. 2017. [cited 2019; Available from: https://www.cdc.gov/nchs/products/databriefs/db283.htm. [PubMed]
- 9.Wadhwa R, et al. , Serotonin reuptake inhibitors and bone health: A review of clinical studies and plausible mechanisms. Osteoporos Sarcopenia, 2017. 3(2): p. 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray WA, et al. , Psychotropic drug use and the risk of hip fracture. N Engl J Med, 1987. 316(7): p. 363–9. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, et al. , Use of selective serotonin-reuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet, 1998. 351(9112): p. 1303–7. [DOI] [PubMed] [Google Scholar]
- 12.Collaboration FT, Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet, 2019. 393(10168): p. 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavoie B, Lian JB, and Mawe GM, Regulation of Bone Metabolism by Serotonin. Adv Exp Med Biol, 2017. 1033: p. 35–46. [DOI] [PubMed] [Google Scholar]
- 14.Ortuno MJ, et al. , Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat Med, 2016. 22(10): p. 1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou C, et al. , Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int, 2018. 29(6): p. 1243–1251. [DOI] [PubMed] [Google Scholar]
- 16.Larsson B, et al. , Normal Bone Microstructure and Density But Worse Physical Function in Older Women Treated with Selective Serotonin Reuptake Inhibitors, a Cross-Sectional PopulationBased Study. Calcif Tissue Int, 2018. 103(3): p. 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haney EM, et al. , Association of low bone mineral density with selective serotonin reuptake inhibitor use by older men. Arch Intern Med, 2007. 167(12): p. 1246–51. [DOI] [PubMed] [Google Scholar]
- 18.Spangler L, et al. , Depressive symptoms, bone loss, and fractures in postmenopausal women. J Gen Intern Med, 2008. 23(5): p. 567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauma PH, et al. , The association between major depressive disorder, use of antidepressants and bone mineral density (BMD) in men. J Musculoskelet Neuronal Interact, 2015. 15(2): p. 17785. [PMC free article] [PubMed] [Google Scholar]
- 20.Rauma PH, et al. , Effects of antidepressants on postmenopausal bone loss - A 5-year longitudinal study from the OSTPRE cohort. Bone, 2016. 89: p. 25–31. [DOI] [PubMed] [Google Scholar]
- 21.Diem SJ, et al. , Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab, 2013. 98(11): p. 4355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saraykar S, et al. , Association of Selective Serotonin Reuptake Inhibitors and Bone Mineral Density in Elderly Women. J Clin Densitom, 2018. 21(2): p. 193–199. [DOI] [PubMed] [Google Scholar]
- 23.Goldman AL, et al. , VITamin D and OmegA-3 TriaL (VITAL) bone health ancillary study: clinical factors associated with trabecular bone score in women and men. Osteoporos Int, 2018. 29(11): p. 2505–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcum ZA, et al. , Antidepressant Use and Recurrent Falls in Community-Dwelling Older Adults: Findings From the Health ABC Study. Ann Pharmacother, 2016. 50(7): p. 525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macri JC, et al. , Association between Antidepressants and Fall-Related Injuries among LongTerm Care Residents. Am J Geriatr Psychiatry, 2017. 25(12): p. 1326–1336. [DOI] [PubMed] [Google Scholar]
- 26.Tang MX, et al. , Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology, 2001. 56(1): p. 49–56. [DOI] [PubMed] [Google Scholar]
- 27.Bonnick SL, et al. , Importance of precision in bone density measurements. J Clin Densitom, 2001. 4(2): p. 105–10. [DOI] [PubMed] [Google Scholar]
- 28.Hans D, et al. , Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom, 2011. 14(3): p. 302–12. [DOI] [PubMed] [Google Scholar]
- 29.Genant HK, et al. , Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res, 1993. 8(9): p. 1137–48. [DOI] [PubMed] [Google Scholar]
- 30.Bouxsein ML, et al. , Guidelines for assessment of bone microstructure in rodents using microcomputed tomography. J Bone Miner Res, 2010. 25(7): p. 1468–86. [DOI] [PubMed] [Google Scholar]
- 31.Walker MD, et al. , Application of high-resolution skeletal imaging to measurements of volumetric BMD and skeletal microarchitecture in Chinese-American and white women: explanation of a paradox. J Bone Miner Res, 2009. 24(12): p. 1953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Washburn RA, et al. , The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol, 1993. 46(2): p. 153–62. [DOI] [PubMed] [Google Scholar]
- 33.Rickels K, et al. , Buspirone in depressed outpatients: a controlled study. Psychopharmacol Bull, 1990. 26(2): p. 163–7. [PubMed] [Google Scholar]
- 34.Yadav V, Serotonin: The Central Link between Bone Mass and Energy Metabolism, in Translational Endocrinology of Bone. 2013, Elsevier; p. 51–62. [Google Scholar]
- 35.Elefteriou F, et al. , Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature, 2005. 434(7032): p. 514–20. [DOI] [PubMed] [Google Scholar]
- 36.Modder UI, et al. , Relation of serum serotonin levels to bone density and structural parameters in women. J Bone Miner Res, 2010. 25(2): p. 415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fink HA, et al. , Association of High-resolution Peripheral Quantitative Computed Tomography (HR-pQCT) bone microarchitectural parameters with previous clinical fracture in older men: The Osteoporotic Fractures in Men (MrOS) study. Bone, 2018. 113: p. 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams LJ, et al. , Selective serotonin reuptake inhibitor use and bone mineral density in women with a history of depression. Int Clin Psychopharmacol, 2008. 23(2): p. 84–7. [DOI] [PubMed] [Google Scholar]
- 39.Kavanagh JJ, McFarland AJ, and Taylor JL, Enhanced availability of serotonin increases activation of unfatigued muscle but exacerbates central fatigue during prolonged sustained contractions. J Physiol, 2019. 597(1): p. 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ensrud KE, et al. , Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc, 2002. 50(10): p. 1629–37. [DOI] [PubMed] [Google Scholar]
- 41.Thapa PB, et al. , Antidepressants and the risk of falls among nursing home residents. N Engl J Med, 1998. 339(13): p. 875–82. [DOI] [PubMed] [Google Scholar]
- 42.McGregor B, et al. , Racial and Ethnic Disparities in Treatment and Treatment Type for Depression in a National Sample of Medicaid Recipients. Psychiatr Serv, 2020: p. appips201900407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong EC, et al. , Racial and Ethnic Differences in Mental Illness Stigma and Discrimination Among Californians Experiencing Mental Health Challenges. Rand Health Q, 2017. 6(2): p. 6. [PMC free article] [PubMed] [Google Scholar]
- 44.Jimenez DE, et al. , Cultural beliefs and mental health treatment preferences of ethnically diverse older adult consumers in primary care. Am J Geriatr Psychiatry, 2012. 20(6): p. 533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Putman MS, et al. , Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res, 2013. 28(10): p. 2177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker MD, et al. , Cortical microstructure compensates for smaller bone size in young Caribbean Hispanic versus non-Hispanic white men. Osteoporos Int, 2017. 28(7): p. 2147–2154. [DOI] [PubMed] [Google Scholar]
- 47.Zhou B, et al. , Bone density, microarchitecture and stiffness in Caucasian and Caribbean Hispanic postmenopausal American women. Bone Res, 2014. 2: p. 14016. [DOI] [PMC free article] [PubMed] [Google Scholar]