Abstract
Objective:
Determine whether adjuvant chemotherapy is associated with a survival benefit in high risk T2–4a, pathologically node-negative distal esophageal adenocarcinoma.
Summary Background Data:
There is minimal literature to substantiate the National Comprehensive Cancer Network (NCCN) guidelines recommending adjuvant therapy for patients with distal esophageal adenocarcinoma and no pathologic evidence of nodal disease.
Methods:
The National Cancer Database was used to identify adult patients with pT2–4aN0M0 esophageal adenocarcinoma who underwent definitive surgery (2004–2015) and had characteristics considered high risk by the NCCN. Patients were stratified by receipt of adjuvant chemotherapy with or without radiation. The primary outcome was overall survival, which was evaluated using Kaplan-Meier and multivariable Cox Proportional Hazards models. A 1:1 propensity score-matched analysis was also performed to compare survival between the groups.
Results:
403 patients met study criteria: 313 (78%) without adjuvant therapy and 90 who received adjuvant chemotherapy with or without radiation (22%). In both unadjusted and multivariable analysis, adjuvant chemotherapy with or without radiation was not associated with a significant survival benefit compared to no adjuvant therapy. In a subgroup analysis of 335 patients without high risk features by NCCN criteria, adjuvant chemotherapy was not independently associated with a survival benefit.
Conclusion:
In this analysis, adjuvant chemotherapy with or without radiation was not associated with a significant survival benefit in completely resected, pathologically node-negative distal esophageal adenocarcinoma, independent of presence of high risk characteristics. The risks and benefits of adjuvant therapy should be weighed before offering it to patients with completely resected pT2–4aN0M0 esophageal adenocarcinoma.
Keywords: esophageal adenocarcinoma, esophageal cancer, adjuvant therapy, chemoradiation
MINI ABSTRACT
To our knowledge, no previous studies have substantiated the National Comprehensive Cancer Network recommendations for adjuvant chemoradiation for patients with high risk T2–4aN0M0 esophageal adenocarcinoma. In an analysis of the National Cancer Database, adjuvant chemotherapy was not found to improve overall survival in these patients.
INTRODUCTION
The National Comprehensive Cancer Network (NCCN) recommends consideration of adjuvant chemotherapy and radiation for ‘high risk’ patients with completely resected T2–4aN0M0 distal esophageal adenocarcinoma who did not receive neoadjuvant therapy. The NCCN defines high risk features as age less than 50 years, high grade tumors, lymphovascular invasion (LVI), and perineural invasion. Several clinical trials have demonstrated improved rates of complete (R0) resection and overall survival for esophageal cancer treated with preoperative chemoradiation (CRT) in addition to surgery.1,2 Other studies have also demonstrated the efficacy of pre- and postoperative chemotherapy in improving survival for patients with operable esophageal adenocarcinoma.3,4 However, the current NCCN recommendations for adjuvant therapy in esophageal adenocarcinoma are largely based on the SWOG9008/INT-0116 trial. In this study, patients with stage IB to IV gastric or esophagogastric junction (EGJ) adenocarcinoma were randomized to either observation or CRT following an R0 resection. Patients in the postoperative CRT group had improved overall and disease-free survival compared to those that received surgery only.5 While there are retrospective studies that suggest adjuvant therapy is beneficial for patients with node positive esophageal cancer6,7, there are no prospective studies, and limited retrospective data, in patients with pathologically node-negative disease.
While much of the current literature regarding paradigms for delivery of chemo- and radiation therapy in esophageal cancer has been focused on the neoadjuvant setting, recent analyses suggest that upward of 12–15% of patients for whom induction therapy is indicated do not receive therapy as recommended.8 It is critical to better understand which patients truly benefit from postoperative therapy given its inherent risks and the variability in guideline concordant use of perioperative therapy. We performed a retrospective analysis of a large, national database to examine the effect of adjuvant chemotherapy on outcomes in patients with node-negative, T2–4a distal esophageal adenocarcinoma who did not undergo induction therapy. We hypothesized that adjuvant chemotherapy would be associated with a survival benefit in high risk patients with T2–4aN0M0 esophageal adenocarcinoma.
METHODS
Data Source
This study was deemed exempt by our Institutional Review Board. The National Cancer Database (NCDB) was the data source for this study: it is a collaborative effort of the American Cancer Society and the American College of Surgeons, and catalogues information about approximately 80% of cancers diagnosed across the United States every year.9 Data are collected prospectively by certified, independent tumor registrars in about 1500 centers.
Study Design
In the first part of the study, the aim was to examine the impact of adjuvant therapy on outcomes in patients with high risk, T2 or more deeply invasive but node-negative distal esophageal adenocarcinoma. The NCDB was used to identify high risk patients with pT2–4aN0M0 distal esophageal adenocarcinoma undergoing esophagectomy between 2004 and 2015 (Figure 1a). Patients who underwent induction therapy of any kind, who suffered a postoperative mortality within 90 days, who were deemed medically unfit for adjuvant chemotherapy, who had missing survival or treatment information, or who had positive resection margins were excluded. While the NCDB catalogues data about patient age, tumor grade, and LVI, we were limited by missing data about LVI for a significant proportion of patients (74%). As a result, we included patients as high risk if they were known to have LVI, but did not use this variable in our multivariable Cox models. The high fraction of missing data also precluded imputation.
Figure 1.
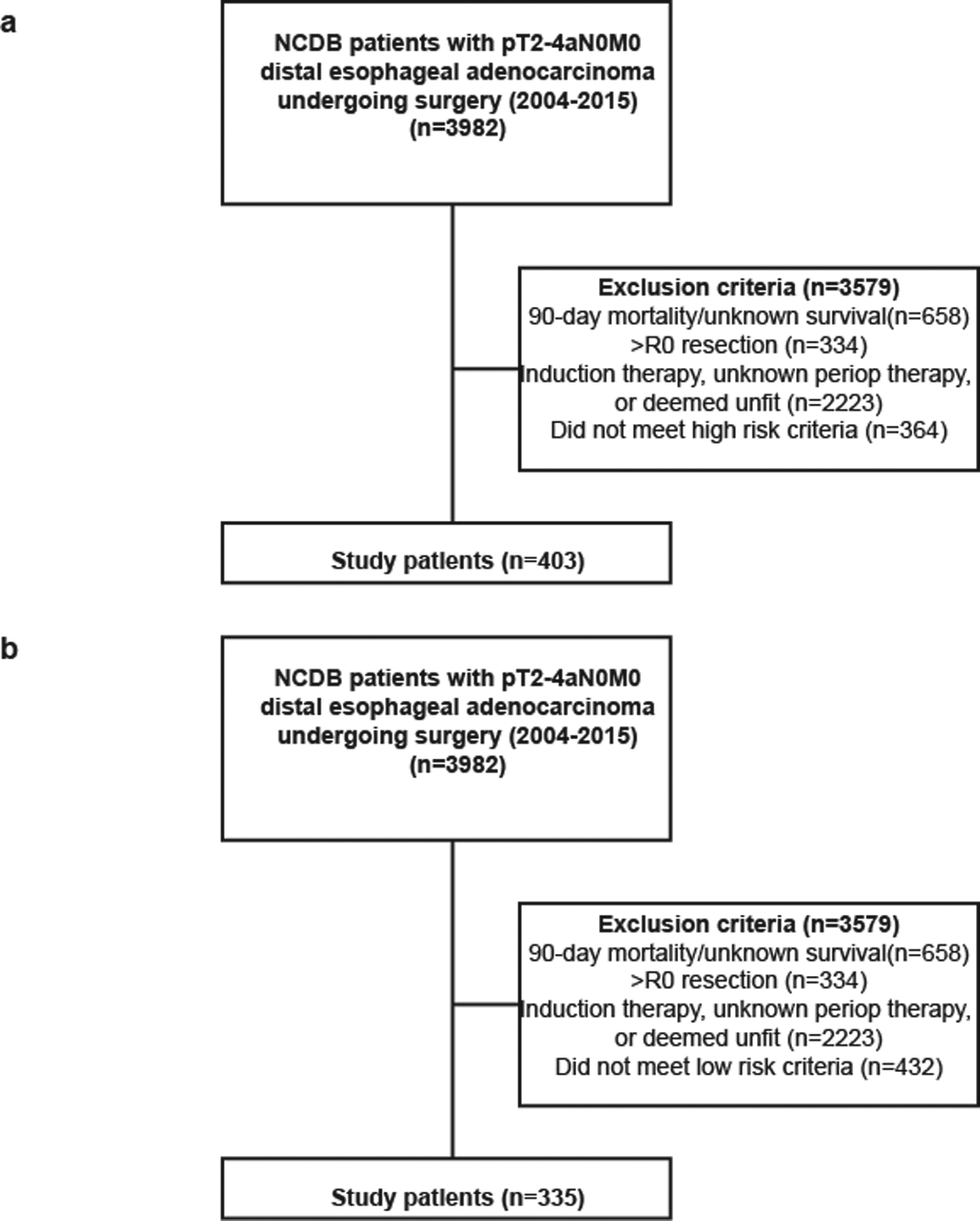
STROBE diagram of (a) high risk and (b) low risk patients analyzed
We initially developed a multivariable Cox proportional hazards model to test interactions between high risk features and adjuvant chemotherapy with or without radiation. This was utilized to verify the validity of high risk characteristics as defined by the NCCN and define criterion for our high risk cohort in our subsequent analyses. We performed this analysis on the overall cohort of 738 patients, regardless of presence of high risk features, with completely resected, distal pT2–4aN0M0 esophageal adenocarcinoma. The interaction terms for grade (ANOVA p=0.001) and age (ANOVA p<0.001 and Supplemental Figure 1) were significant, suggesting that these variables mediated the effect of adjuvant chemotherapy on survival. However, the interaction term for LVI was not significant (ANOVA p=0.51). Because only a subset of patients, based on grade and age, were analyzed thereafter in the study, these interaction terms were not included in the following regression models. The remainder of our analyses were performed in only our high risk cohort or low risk cohort, independently.
We stratified patients with high risk features by receipt of adjuvant chemotherapy with or without radiation. Baseline characteristics between the groups were compared using the Wilcoxon rank sum and Pearson’s chi-squared tests for continuous and categorical variables, respectively. The primary outcome was overall survival, which was computed from diagnosis to any cause mortality. Survival was modeled using Kaplan-Meier and multivariable Cox proportional hazards methods. Variables included in the Cox model were selected based on their modification of treatment effect on overall survival and included age, sex, race, year of diagnosis, Charlson-Deyo comorbidity (CDCC) index, insurance status, treatment at an academic program, pathologic T stage, tumor size, tumor grade, and receipt of adjuvant radiation. An additional analysis was performed stratifying patients by number of high risk factors to evaluate if the presence of >1 risk factor is associated with worse survival or an interaction with adjuvant chemotherapy.
Due to imbalances in baseline characteristics between the two groups, a sensitivity analysis was performed using 1:1 propensity score-matched patient pairs. Using receipt of adjuvant therapy as the exposure, a greedy nearest neighbor algorithm was used that matches patients by propensity for treatment allocation based on a logistic regression model that included variables associated with the outcome, outcome and exposure, and exposure. A caliber width of 0.1 of the standard deviation of the logit of the propensity score was used because a traditional caliper of 0.2 did not achieve covariate balance based on standardized mean differences.10 Variables included in the regression model were age, sex, CDCC score, tumor grade, presence of LVI, facility type, pathologic T stage, and tumor size. There was no replacement of matched controls during matching. The matching process created a group of patients with overlapping propensity for treatment scores, which both eliminated outliers and patients for whom either treatment would be rare. Covariate balance was checked using standardized differences and plots of propensity scores (Supplemental Figure 3). A multivariable Cox model was performed to evaluate the association of adjuvant chemotherapy with survival. For all Cox models, the proportional hazards assumption was checked using Schoenfeld residuals and a ≥10:1 event to degrees of freedom ratio was maintained.
In the second part of the study, patients without high risk features were selected to evaluate the question of whether these patients experience a survival benefit with adjuvant chemotherapy (Figure 1b). Overall survival was evaluated as above. However, due to the small number of patients who received adjuvant therapy in this cohort, a propensity score-matched subgroup analysis was not performed.
Missing data were handled with complete case analysis given the completeness of the NCDB, with exceptions noted above. A two-sided p value equal to or less than 0.05 was considered statistically significant. All statistical analyses were performed using R version 3.5.1 (Vienna, Austria).
RESULTS
High Risk Patients
For the first portion of the study examining our high risk cohort, a total of 403 patients met study criteria: 313 who did not receive adjuvant therapy (78%) and 90 who received adjuvant chemotherapy with or without radiation (22%). Compared to patients who did not receive adjuvant therapy, patients who received adjuvant chemotherapy were more likely to be younger, be privately insured, and have more advanced pathologic T stage (Table 1). The five-year survival for patients not receiving adjuvant therapy and those receiving adjuvant therapy was 44% (95%CI 39–50) and 44% (95%CI 34–56), respectively (Figure 2a). In a multivariable Cox regression, the receipt of adjuvant chemotherapy was not associated with improved survival compared to no adjuvant therapy (Table 2). In a sensitivity analysis of 71 propensity score-matched patient pairs (Supplemental Table 1), five-year survival for patients not receiving adjuvant therapy and those receiving adjuvant chemotherapy was 46% (95%CI 35–60) and 46% (95%CI 35–60), respectively (Figure 2b). Adjuvant chemotherapy was not associated with improved survival compared to no adjuvant therapy in a multivariable regression (Table 3).
Table 1.
Demographic characteristics of study patients
| No adjuvant therapy n=313(%) | Adjuvant chemo with or without radiation n=90 (%) | p value | |
|---|---|---|---|
| Age (years, median) (IQR) | 68 (60–74) | 60 (49–68) | <0.001 |
| Age <50 years | 27(9) | 23(26) | <0.001 |
| Sex (female) | 44(14) | 16(18) | 0.48 |
| Race | 0.85 | ||
| White | 299(97) | 85(96) | |
| Black | 5(2) | 2(2) | |
| Other | 5(2) | 2(2) | |
| Year of diagnosis, median (IQR) | 2008(2006–2010) | 2008 (2006–2009) | 0.46 |
| CDCC Score | 0.74 | ||
| 0 | 214(68) | 63(70) | |
| 1 | 74(24) | 22(24) | |
| 2+ | 25(8) | 5(6) | |
| Insuranee status | 0.05 | ||
| Government | 179(59) | 40(44) | |
| Private | 120(40) | 48(53) | |
| None | 5(1) | 2(2) | |
| Facility location | 0.74 | ||
| Metro | 233(78) | 67(78) | |
| Urban | 55(18) | 17(20) | |
| Rural | 12(4) | 2(2) | |
| Facility type | 0.56 | ||
| Community cancer program | 15(5) | 6(7) | |
| Comprehensive community cancer program | 105(34) | 35(40) | |
| Integrated network cancer program | 22(7) | 6(7) | |
| Academic/research program | 170(55) | 41(47) | |
| Pathologic T stage | <0.001 | ||
| 2 | 186(59) | 28(31) | |
| 3 | 127(41) | 61(68) | |
| 4a | 1(0) | 1(1) | |
| Tumor size (median mm) (IQR) | 31 (22–45) | 32 (22–48) | 0.37 |
| Grade | 0.04 | ||
| Moderately differentiated | 19(6) | 12(14) | |
| Well differentiated | 5(2) | 3(3) | |
| Poorly differentiated | 287(92) | 74(83) | |
| Lymphovascular invasion | 35(39) | 13(62) | 0.09 |
| Adjuvant radiation | 0(0) | 57(63) | <0.001 |
| Median survival (months) (IQR) | 41 (34–61) | 46 (35–65) | 0.80 |
| Mortality events | 189(60) | 59(66) | 0.44 |
| Median follow-up (months) (IQR) | 33(17–68) | 38(20–65) | 0.42 |
IQR indicates Interquartile Range, CDCC, Charlson-Deyo Comorbidity Index
Figure 2.
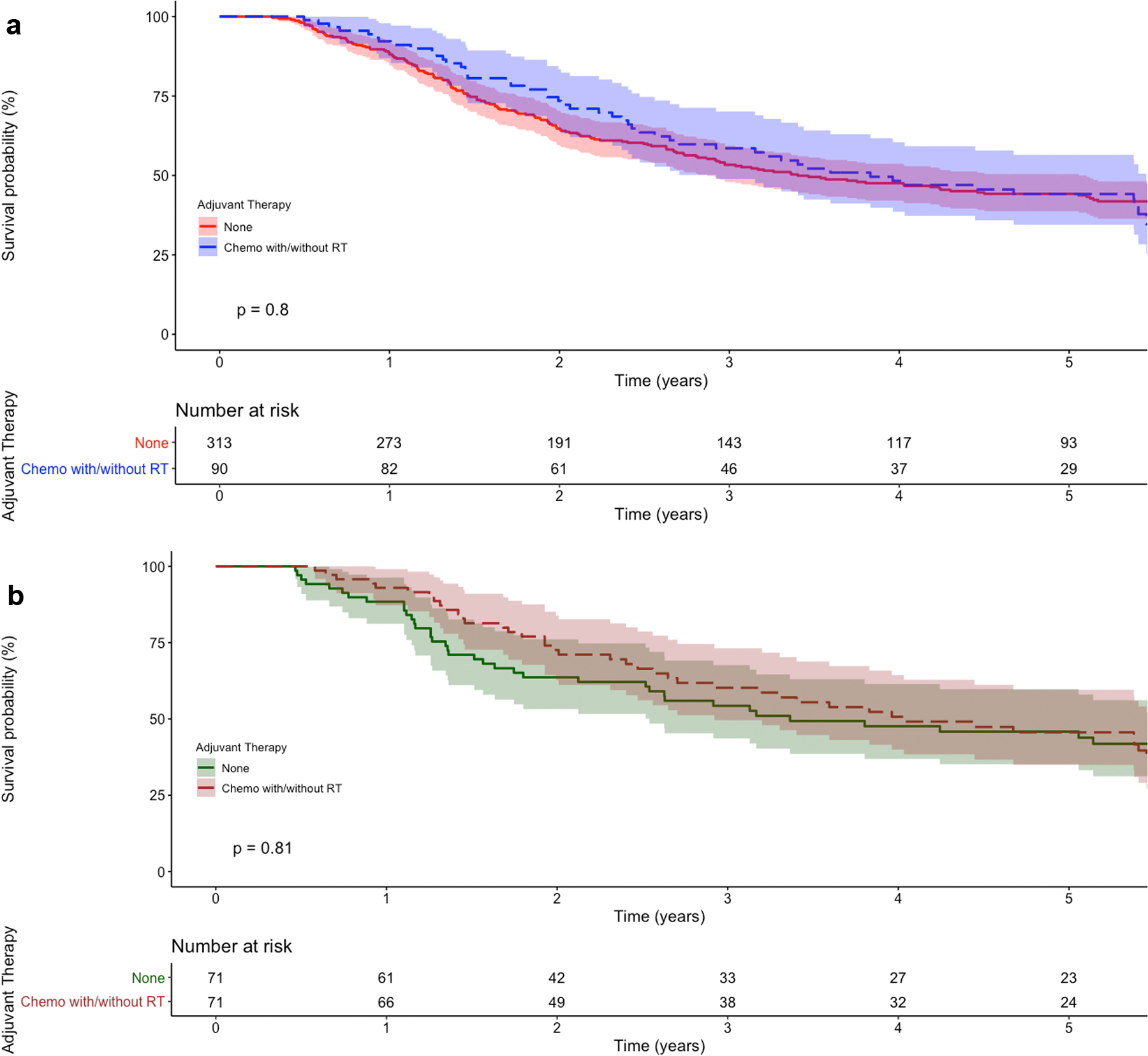
Kaplan-Meier survival curves for (a) unmatched and (b) propensity score-matched high risk patients with pT2–4N0M0 distal esophageal adenocarcinoma, stratified by type of adjuvant therapy. The p value refers to the log-rank test. Shaded regions represent the 95% confidence interval. Numbers at risk are provided beneath the graph
Table 2.
Cox multivariable regression of variables independently associated with survival.
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.04 | 1.02 | 1.05 | 0.003 |
| Sex (female) | 0.63 | 0.42 | 0.94 | 0.02 |
| Race (reference: White) | ||||
| Black | 2.30 | 0.82 | 6.47 | 0.12 |
| Other | 2.54 | 1.11 | 5.82 | 0.03 |
| Year of diagnosis (per year) | 0.97 | 0.92 | 1.02 | 0.26 |
| CDCC score (reference: 0) | ||||
| 1 | 0.87 | 0.63 | 1.21 | 0.42 |
| 2+ | 0.85 | 0.51 | 1.43 | 0.54 |
| Insurance status (reference: government) | ||||
| Private | 0.93 | 0.67 | 1.30 | 0.68 |
| None | 0.82 | 0.19 | 3.53 | 0.78 |
| Facility type (reference: non-academic) | ||||
| Academic/Research Program | 0.80 | 0.60 | 1.05 | 0.11 |
| Pathologic T stage (reference: T2) | ||||
| T3 or T4a | 1.49 | 1.12 | 1.98 | 0.006 |
| Tumor size (per mm) | 1.00 | 0.99 | 1.00 | 0.17 |
| Grade (reference: poorly differentiated) | ||||
| Well differentiated | 0.86 | 0.48 | 1.54 | 0.61 |
| Moderately differentiated | 0.30 | 0.04 | 2.21 | 0.24 |
| Adjuvant radiation (reference: none) | 1.83 | 0.96 | 3.48 | 0.07 |
| Type of adjuvant therapy (reference: none) | ||||
| Chemotherapy with or without radiation | 0.77 | 0.43 | 1.38 | 0.39 |
CDCC indicates Charlson-Deyo Comorbidity Index
Table 3.
Cox multivariable regression of variables independently associated with survival in propensity score-matched patients.
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.03 | 1.01 | 1.06 | 0.01 |
| Sex (female) | 0.49 | 0.26 | 0.93 | 0.03 |
| Facility type (reference: non-academic) | ||||
| Academic/Research Program | 0.85 | 0.55 | 1.31 | 0.46 |
| Pathologic T stage (reference: T2) | ||||
| T3 or T4a | 1.17 | 0.67 | 2.03 | 0.58 |
| Tumor size (per mm) | 1.01 | 0.99 | 1.02 | 0.24 |
| Grade (reference: poorly differentiated) | ||||
| Well/moderately differentiated | 0.41 | 0.18 | 0.98 | 0.05 |
| Adjuvant radiation (reference: none) | 1.73 | 0.89 | 3.34 | 0.10 |
| Type of adjuvant therapy (reference: none) | ||||
| Chemotherapy with or without radiation | 0.86 | 0.45 | 1.63 | 0.64 |
An additional analysis was performed examining patients based on the number of risk factors they had. A total of 346 patients had one risk factor and 57 patients had two or more risk factor (Supplemental Table 2). The five-year survival for patients with one or at least two high risk factors was 44% (95% 39–50) and 43% (95%CI 30–60), respectively (Supplemental Figure 2). In a multivariable Cox model, the presence of at least two risk factors was associated with similar survival compared to a single risk factor (HR 1.32; 95%CI 0.83–2.10; p=0.25). An interaction term of number of risk factors and adjuvant chemotherapy was nonsignificant (ANOVA p=0.46), suggesting the number of risk factors was not associated with the relationship between adjuvant chemotherapy and survival.
Low Risk Patients
A total of 335 patients without high risk features were identified, of whom 291 (87%) did not receive adjuvant therapy and 44 (13%) received adjuvant chemotherapy with or without radiation. Compared to patients who did not receive adjuvant therapy, those who did were more likely to be younger, be diagnosed at an earlier year, be privately insured, have a more advanced pathologic T stage, and have a larger tumor (Table 4). Unadjusted five-year survival for patients not receiving adjuvant therapy and those receiving adjuvant therapy was 50% (95%CI 44–56) and 44% (95%CI 31–61), respectively (Supplemental Figure 4). In a multivariable regression, the receipt of adjuvant chemotherapy was not associated with improved survival compared to no adjuvant therapy (Table 5).
Table 4.
Demographic characteristics of low risk study patients
| No adjuvant therapy n=291 (%) | Adjuvant chemo with or without radiation n=44 (%) | p value | |
|---|---|---|---|
| Age (years, median) (IQR) | 69 (63–76) | 61(58–67) | <0.001 |
| Sex (female) | 51(18) | 8(18) | 1.00 |
| Race | 0.50 | ||
| White | 281(97) | 43(100) | |
| Black | 4(1) | 0(0) | |
| Other | 5(2) | 0(0) | |
| Year of diagnosis, median (IQR) | 2008 (2006–2010) | 2007 (2005–2008) | 0.008 |
| CDCC Score | 0.33 | ||
| 0 | 184(63) | 28(64) | |
| 1 | 83(29) | 15(34) | |
| 2+ | 24(8) | 1(2) | |
| Insurance status | 0.002 | ||
| Government | 195(69) | 19(43) | |
| Private | 86(30) | 25(57) | |
| None | 3(1) | 0(0) | |
| Facility location | 0.62 | ||
| Metro | 224(80) | 32(80) | |
| Urban | 50(18) | 6(15) | |
| Rural | 7(3) | 2(5) | |
| Facility type | 0.85 | ||
| Community cancer program | 13(5) | 1(2) | |
| Comprehensive community cancer program | 105(36) | 18(41) | |
| Integrated network cancer program | 24(8) | 4(9) | |
| Academic/research program | 149(51) | 21(48) | |
| Pathologic T stage | <0.001 | ||
| 2 | 190(65) | 12(27) | |
| 3 | 101(35) | 32(73) | |
| Tumor size (median mm) (IQR) | 30(20–45) | 43(30–61) | <0.001 |
| Grade | 0.50 | ||
| Moderately differentiated | 250(86) | 40(91) | |
| Well differentiated | 41(14) | 4(9) | |
| Adjuvant radiation | 0(0) | 33(75) | N/A |
| Median survival (months) (IQR) | 59(49–74) | 54(33–95) | 0.71 |
| Mortality events | 167(57) | 30(68) | 0.23 |
| Median follow-up (months) (IQR) | 45(24–78) | 48(22–86) | 0.63 |
IQR indicates Interquartile Range, CDCC, Charlson-Deyo Comorbidity Index
Table 5.
Cox multivariable regression of variables independently associated with survival.
| 95% Confidence Interval | ||||
|---|---|---|---|---|
| Predictor | Hazard Ratio | Lower | Upper | p-value |
| Age (per year) | 1.02 | 1.00 | 1.05 | 0.02 |
| Sex (female) | 0.79 | 0.52 | 1.20 | 0.27 |
| Race (reference: White) | ||||
| Black | 3.17 | 0.93 | 10.8 | 0.07 |
| Other | 0.84 | 0.20 | 3.53 | 0.81 |
| Year of diagnosis (per year) | 0.98 | 0.92 | 1.05 | 0.60 |
| CDCC score (reference: 0) | ||||
| 1 | 1.09 | 0.78 | 1.52 | 0.63 |
| 2+ | 1.07 | 0.63 | 1.82 | 0.81 |
| Insurance status (reference: government) | ||||
| Private | 0.67 | 0.45 | 1.01 | 0.06 |
| None | 1.60 | 0.37 | 6.89 | 0.53 |
| Facility type (reference: non-academic) | ||||
| Academic/Research Program | 0.91 | 0.67 | 1.24 | 0.54 |
| Pathologic T stage (reference: T2) | ||||
| T3 or T4a | 1.56 | 1.11 | 2.21 | 0.01 |
| Tumor size (per mm) | 1.00 | 0.99 | 1.01 | 0.90 |
| Grade (reference: moderately differentiated) | ||||
| Well differentiated | 0.46 | 0.26 | 0.82 | 0.009 |
| Adjuvant radiation (reference: none) | 0.89 | 0.29 | 2.71 | 0.84 |
| Type of adjuvant therapy (reference: none) | ||||
| Chemotherapy with or without radiation | 1.03 | 0.36 | 2.92 | 0.95 |
CDCC indicates Charlson-Deyo Comorbidity Index
DISCUSSION
In this analysis of the NCDB, we found that despite NCCN recommendations the majority of patients with T2–4a, node-negative distal esophageal adenocarcinoma failed to receive adjuvant therapy of any kind. Amongst patients with high risk characteristics in our cohort, there was no difference in survival based on the receipt of adjuvant therapy. Similarly, in a separate analysis of patients with T2–4aN0M0 esophageal adenocarcinoma without high risk characteristics, we found that receipt of adjuvant therapy was not associated with improved survival. In aggregate, our findings suggest that adjuvant therapy is not associated with improved overall survival for completely resected, pT2–4aN0M0 distal esophageal adenocarcinoma regardless of the presence of high risk features. Therefore, the risks and benefits of adjuvant therapy should be weighed carefully before being offered to patients receiving upfront surgery for T2 or more deeply invasive but pathologically node-negative distal esophageal adenocarcinoma.
To our knowledge this is the first study exploring the utility of adjuvant therapy in patients with T2–4a distal esophageal adenocarcinoma without pathological evidence of nodal metastatic disease. Currently, for those that did not receive preoperative therapy, the NCCN recommends adjuvant chemoradiation for patients with high risk T2–4a tumors in addition to those that have positive lymph nodes. These recommendations are largely based on the Intergroup trial, INT-0116, which concluded that patients with Stage IB to IV gastric or EGJ adenocarcinoma who receive postoperative CRT have improved overall and relapse-free survival compared to those who receive surgery alone. However, only 20% of the study population had EGJ primary tumors and 80% of patients had positive lymph nodes.5 Therefore, the merit of extrapolating data from this trial to support use of adjuvant therapy in patients with T2–4a, lymph node-negative esophageal adenocarcinoma is unclear. While no previous studies have examined use of adjuvant therapy for node-negative esophageal adenocarcinoma, several retrospective analyses have concluded neoadjuvant therapy offers no survival benefit for these patients.11,12
Several recent, single institution studies have identified characteristics that portend higher risk for recurrence and mortality following resection in esophageal adenocarcinoma which include high tumor grade, presence of lymphovascular invasion, and presence of perineural invasion.13–15 While these characteristics correlate with high risk factors as defined by the NCCN, no previous studies have validated that adjuvant therapy improves survival for patients with completely resected, pT2–4aN0M0 esophageal adenocarcinoma who have tumors with high risk characteristics. In spite of the NCCN recommendations, our findings suggest there is no benefit to adjuvant therapy in these patients. Further, in our low risk cohort, there was no significant difference in 5-year survival between patients who received adjuvant therapy and those who did not. Taken together, these findings suggest that adjuvant therapy may have a limited role for patients with T2 or more deeply invasive, node-negative esophageal adenocarcinoma, and may even place low risk patients at risk for undue harm from treatment toxicity.
This study has several limitations. While we included a propensity score-matched analysis, there is a possibility that unmeasured confounders exist in our data that have not been properly addressed as is inherent with any retrospective study utilizing a large, national database. With respect to the NCDB specifically, our analysis was limited by the granularity of the data available. For one, the NCDB does not catalogue the presence of perineural invasion, which is a recognized high risk feature in patients with esophageal cancer. Further, there is no information on recurrence, so we have no ability to comment on differences in time to recurrence, location of recurrence, or disease-free survival based on adjuvant strategy. Further, we have no insight into specific chemotherapy regimens patients received and the reasons patients were assigned to a certain adjuvant treatment arm. While we assume that any discrepancies in reasons for these therapies would be random between the two groups, we cannot definitively evaluate that with our data source, which introduces the possibility of selection bias. Despite these limitations the large scale of the NCDB allows an analysis beyond the capabilities of single-institution studies, especially in addressing specialized patient populations like in this study.
In conclusion, we found that adjuvant chemotherapy was not associated with a significant survival benefit compared to no adjuvant therapy in completely resected pathologically node-negative distal esophageal adenocarcinoma in this analysis, including patients considered high risk by NCCN. The risks and benefits of adjuvant therapy must therefore be weighed before offering it to patients in this population. Further studies are needed to better delineate the subpopulations of patients with esophageal adenocarcinoma who may best benefit from adjuvant therapy.
Supplementary Material
Supplemental Figure 1. Plot of the interaction term of patient age and receipt of adjuvant chemotherapy. The Y-axis represents an adjusted hazard ratio of mortality and the X-axis increasing patient age in years. The survival curves of patients receiving or not receiving adjuvant therapy are plotted with shaded areas representing the 95% confidence interval
Supplemental Figure 2. Kaplan-Meier survival curves for high risk patients with pT2–4N0M0 distal esophageal adenocarcinoma, stratified by number of high risk factors. The p value refers to the log-rank test. Shaded regions represent the 95% confidence interval. Numbers at risk are provided beneath the graph
Supplemental Figure 3. Balance diagnostics for propensity score-matched high risk patients. (a) Love plot demonstrating the spread of standardized mean differences in matched and unmatched patients and (b) mirror histograms demonstrating the spread of propensity scores between the two treatment groups before and after matching
Supplemental Figure 4. Kaplan-Meier survival curves for low risk patients with pT2–4N0M0 distal esophageal adenocarcinoma, stratified by number of high risk factors. The p value refers to the log-rank test. Shaded regions represent the 95% confidence interval. Numbers at risk are provided beneath the graph
ACKNOWLEDGEMENTS
The American College of Surgeons is in a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals. The data used in the study are derived from a de-identified National Cancer Database file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology used or the conclusions drawn from these data by the investigators.
Funding Sources:
This work received no direct funding.
Drs. Raman and Voigt were supported by a National Institutes of Health T-32 grant 5T32CA093245 in surgical oncology. Dr. Jawitz was supported by a National Institutes of Health T-32 grant 5T32HL069749 in clinical research.
Footnotes
Conflict of Interest:
None to disclose
REFERENCES
- 1.van Hagen P, Hulsholf M, van Lanscot J, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med. 2012;366(22):2074–2084. [DOI] [PubMed] [Google Scholar]
- 2.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham D, Allum W, Stenning S, et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med. 2006;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 4.Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–1721. [DOI] [PubMed] [Google Scholar]
- 5.Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice TW, Adelstein DJ, Chidel MA, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. The Journal of Thoracic and Cardiovascular Surgery. 2003;126(5):1590–1596. [DOI] [PubMed] [Google Scholar]
- 7.Bédard E, Inculet R, Malthaner R, Brecevic E, Vincent M, Dar R. The Role of Surgery and Postoperative Chemradiation Therapy in Patients with Lymph Node Positive Esophageal Carcinoma. Cancer. 2001;91(12):2423–2430. [PubMed] [Google Scholar]
- 8.Samson P, Puri V, Broderick S, Patterson GA, Meyers B, Crabtree T. Adhering to Quality Measures in Esophagectomy Is Associated With Improved Survival in All Stages of Esophageal Cancer. Ann Thorac Surg. 2017;103(4):1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho D, Imai K, King G, Stuart E. MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software. 2011;42(8):1–28. [Google Scholar]
- 11.Gabriel E, Attwood K, Du W, et al. Association Between Clinically Staged Node-Negative Esophageal Adenocarcinoma and Overall Survival Benefit From Neoadjuvant Chemoradiation. JAMA Surg. 2016;151(3):234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantziari S, Gronnier C, Renaud F, et al. Survival Benefit of Neoadjuvant Treatment in Clinical T3N0M0 Esophageal Cancer: Results From a Retrospective Multicenter European Study. Ann Surg. 2017;266(5):805–813. [DOI] [PubMed] [Google Scholar]
- 13.Nobel TB, Livschitz J, Xing XX, et al. Surveillance Implications of Recurrence Patterns in Early Node-Negative Esophageal Adenocarcinoma. Ann Thorac Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackham AU, SM HN, Schell MJ, et al. Recurrence patterns and associated factors of locoregional failure following neoadjuvant chemoradiation and surgery for esophageal cancer. J Surg Oncol. 2018;117(2):150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goense L, van Rossum PSN, Xi M, et al. Preoperative Nomogram to Risk Stratify Patients for the Benefit of Trimodality Therapy in Esophageal Adenocarcinoma. Ann Surg Oncol. 2018;25(6):1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Plot of the interaction term of patient age and receipt of adjuvant chemotherapy. The Y-axis represents an adjusted hazard ratio of mortality and the X-axis increasing patient age in years. The survival curves of patients receiving or not receiving adjuvant therapy are plotted with shaded areas representing the 95% confidence interval
Supplemental Figure 2. Kaplan-Meier survival curves for high risk patients with pT2–4N0M0 distal esophageal adenocarcinoma, stratified by number of high risk factors. The p value refers to the log-rank test. Shaded regions represent the 95% confidence interval. Numbers at risk are provided beneath the graph
Supplemental Figure 3. Balance diagnostics for propensity score-matched high risk patients. (a) Love plot demonstrating the spread of standardized mean differences in matched and unmatched patients and (b) mirror histograms demonstrating the spread of propensity scores between the two treatment groups before and after matching
Supplemental Figure 4. Kaplan-Meier survival curves for low risk patients with pT2–4N0M0 distal esophageal adenocarcinoma, stratified by number of high risk factors. The p value refers to the log-rank test. Shaded regions represent the 95% confidence interval. Numbers at risk are provided beneath the graph


