Abstract
To arrive at a coherent understanding of the relation between glucocorticoids and the human brain, we systematically reviewed the literature for studies examining the associations between endogenous or exogenous cortisol and human brain function. Higher levels of endogenous cortisol during psychological stress were related to increased activity in the middle temporal gyrus and perigenual anterior cingulate cortex (ACC), decreased activity in the ventromedial prefrontal cortex, and altered function (i.e., mixed findings, increased or decreased) in the amygdala, hippocampus and inferior frontal gyrus. Moreover, endogenous cortisol response to psychological stress was related to increased activity in the inferior temporal gyrus and altered function in the amygdala during emotional tasks that followed psychological stress. Exogenous cortisol administration was related to increased activity in the postcentral gyrus, superior frontal gyrus and ACC, and altered function in the amygdala and hippocampus during conditioning, emotional and reward-processing tasks after cortisol administration. These findings were in line with those from animal studies on amygdala activity during and after stress.
Keywords: cortisol, endogenous, exogenous, fMRI, psychological stress
1. Introduction
While considerable research has examined the long-term adverse effects of glucocorticoids on the brain (Andela et al., 2015; Judd et al., 2014; Lupien et al., 2009), glucocorticoids also affect brain function acutely following endogenous release or exogenous administration (Dedovic et al., 2009a). Acute changes in glucocorticoids are thought to influence a range of processes including general memory, arousal and emotional processing (Joels, 2018; Ulrich-Lai and Herman, 2009). Yet, a coherent account of how such acute changes in endogenous and exogenous cortisol influence human brain function as measured using functional magnetic resonance imaging (fMRI) is still lacking. This is due partly to the challenge of co-evaluating data from studies that have employed a variety of experimental methodologies, imaging protocols and analytical approaches, as well as differences in dosing, timing and frequency of cortisol measurements. In this review, we aim to identify commonalties and differences across studies, search for replicated findings, and summarize how acute cortisol is associated with human brain function.
Glucocorticoids, specifically corticosterone in rodents and cortisol in humans, are the end-product of the hypothalamic-pituitary-adrenal (HPA) axis, which plays an important role in the stress response (de Kloet et al., 2005; Reul and De Kloet, 1986). Cortisol crosses the blood-brain barrier and binds to both glucocorticoid and mineralocorticoid receptors in the brain (de Kloet et al., 2005; Reul and De Kloet, 1986). Cortisol has both acute non-genomic and slow gene-mediated effects on the brain. The acute effects seem to be mostly related to activation of specific circuits, whereas slow effects seem to be mostly related to normalization of earlier enhanced activity (Joels, 2018). In the current review we focus on the acute effects of cortisol. Animal studies have the ability to directly manipulate the HPA axis to address predictable hypotheses and have shown acute effects of glucocorticoids on activity of the amygdala, hippocampus, paraventricular nucleus and prefrontal cortex (Joels et al., 2012; Ulrich-Lai and Herman, 2009). These effects might facilitate a quick and adaptive response to stressors (Joels, 2018; Ulrich-Lai and Herman, 2009). It is important to bridge animal and human research on the acute effects of glucocorticoids on brain function, as individual differences in these effects could be related to individual differences in coping with stress (de Kloet et al., 2005).
Recent human studies have investigated associations between acute increases in endogenous and exogenous cortisol levels and the brain under controlled conditions. Studies of endogenous cortisol usually induce elevations in cortisol by employing short, acute psychological stressors, such as tasks involving uncontrollability and/or social-evaluative threat (Dickerson and Kemeny, 2004). A meta-analysis has shown that cortisol peaks 0–20 minutes after such stressors (Dickerson and Kemeny, 2004). Studies of exogenous cortisol use oral or intravenous administration, usually in a placebo-controlled design. Each type of study has advantages and disadvantages (Table 1). In short, studies of endogenous cortisol are most similar to stressful events in daily life and induce a natural physiological response. However, the endogenous cortisol response might be related to subjective experience of the stressor which is variable. For example, one of the more frequently employed stressors, the Trier Social Stress Test (Kirschbaum et al., 1993), elicits a two-to-three fold increase in cortisol in about 70–80% of participants 10–20 minutes after the stressor (Kudielka et al., 2007). Therefore, it is difficult to differentiate a failed stress induction from a dysregulated stress response system, especially if only one system is investigated (Andrews et al., 2013). On the other hand, exogenous administration of cortisol provides tight experimental control and induces higher levels of cortisol, which might improve signal-to-noise ratio in terms of cortisol elevation, and the opportunity to test for causal effects. However, administration of exogenous cortisol does not represent a natural physiological stress reaction, as the stress response is a combination of three interacting systems (sympathetic nervous system, HPA-axis and subjective emotional experience (Andrews et al., 2013). As endogenous and exogenous cortisol might reflect different processes, it is important to integrate the findings both within each domain (endogenous versus exogenous) as well as across the two domains. This will provide a coherent understanding of associations between glucocorticoids and human brain function, as well as to identify important areas for future research.
Table 1.
Overview of advantages and disadvantages of studies using endogenous and exogenous cortisol measures.
| Endogenous cortisol | Exogenous cortisol | |
|---|---|---|
| Advantages | HPA-axis is centrally activated, with coordinated and controlled release of CRH, ACTH and finally cortisol. | Control over dosage |
| Stress response is combination of three interacting systems: SNS, HPA-axis, subjective emotional experience (Andrews et al., 2013). These systems interact naturally, in a coordinated fashion and with the expected magnitude and speed. | Control over timing | |
| Immune system changes unfold naturally as a consequence of first ANS, and then HPA-axis changes. | Control with placebo is possible | |
| Blinding is possible | ||
| Repeated exposure under exact dosage control is possible | ||
| Increases cortisol levels in all participants Specificity - only cortisol is changed | ||
| Disadvantages | The stressor might not induce changes in cortisol. | Same dosage of cortisol may have different effects in different subjects, due to individual variation in number of glucocorticoid receptors, weight, or sex (Abercrombie et al., 2011). |
| Response magnitude might be small and cannot be controlled | Cortisol increase independent of ACTH and CRH increase, resulting in an atypical CNS state (no CRH stimulation, but glucocorticoid feedback). | |
| Between-subject differences in stress reactivity | Cortisol increase independent of SNS and subjective emotional experience. | |
| Double-blind experimental designs are not possible | Low ecological validity | |
| Habituation complicates longitudinal or repeat-exposure experiments | ||
| Effect of stressor might change across development (Gunnar et al., 2009) | ||
Note: HPA = hypothalamic-pituitary-adrenal; CRH = corticotropin-releasing hormone; ACTH = adrenocorticotropic hormone; SNS = sympathetic nervous system; ANS = autonomic nervous system; CNS = central nervous system
We systematically reviewed the literature examining the relation between endogenous and exogenous cortisol and human brain function. We reviewed fMRI studies that measured brain activity during a psychological stressor or cortisol administration, and during tasks performed shortly after a psychological stressor or cortisol administration. In this way, we focused on the acute effects of cortisol. The first research question is how cortisol relates to brain activity as measured with fMRI during (a) psychological stress, or (b) cortisol administration. The second research question is how cortisol relates to brain activity during fMRI tasks after (a) psychological stress, or (b) cortisol administration. Based on findings from animal research (Joels et al., 2012; Ulrich-Lai and Herman, 2009), we hypothesize that endogenous and exogenous cortisol are related to activity in the amygdala, hippocampus, paraventricular nucleus and prefrontal cortex.
Regarding the latter hypothesis, the literature has focused on brain activity during a variety of tasks: conditioning, emotional stimuli, reward processing, decision-making, and non-emotional tasks (mostly working memory, detection and memory retrieval tasks). Conditioning tasks usually present one stimulus paired with an aversive stimulus and one stimulus without an aversive stimulus, to measure how participants learn to distinguish between stimuli (Lonsdorf et al., 2017). Emotional tasks usually present participants with pictures of emotional faces (Vuilleumier and Pourtois, 2007) or pictures from the International Affective Picture System (IAPS) (Lang et al., 2001). Reward processing tasks are used to measure how participants respond to anticipating and receiving rewards (Wang et al., 2016). Decisionmaking tasks measure how participants make (risky) decisions (Schonberg et al., 2011). Non-emotional tasks measure working memory or memory retrieval of neutral stimuli such as neutral images or spatial locations (Roozendaal et al., 2009).
2. Methods
2.1. Search strategy
Inclusion criteria and search terms were defined separately for studies on endogenous and exogenous cortisol. For studies on endogenous cortisol, the following inclusion criteria were used: (a) acute psychological laboratory stressors, defined as tasks that lasted less than one hour and did not serve a function outside the laboratory setting (Dickerson and Kemeny, 2004), including physical-psychological stressor combinations; (b) fMRI during or shortly after the psychological stressor or control condition (maximum of 30 minutes between stressor and fMRI1); (c) salivary or plasma cortisol assessed before and during/after the psychological stressor; (d) healthy adult humans; (e) in English. A literature search was performed on PubMed and Web of Knowledge using the following search terms: fMRI (or functional MRI or functional magnetic resonance or neuroimaging) and cortisol (or HPA or hypothalamic-pituitary-adrenal axis or neuroendocrine or hydrocortisone or psychoneuroimmunology or psychoimmunology or psychoneuroendocrinology), excluding rat.
For studies on exogenous cortisol, the following inclusion criteria were used: (a) cortisol administration; (b) placebo-controlled; (c) fMRI during or after cortisol administration (on the same day); (d) healthy adult humans; (e) in English. A literature search was performed on PubMed and Web of Knowledge using the following search terms: fMRI (or functional MRI or functional magnetic resonance or neuroimaging) and cortisol and administration (or exogenous or oral or IM or IV or cortisone or corticosterone or corticosteroid or corticoid or glucocorticoid or prednisone or prednisolone or dexamethasone or hydrocortisone or glucocorticoid or methylprednisolone or steroid or cosyntropin or corticorelin), excluding rat. Both literature searches were performed before September 12th, 2019. No limit was set for year of publication or country/region of study. To ensure literature saturation, we scanned the reference lists of included studies and relevant reviews and meta-analyses identified through the search. After duplicates were removed at least two review authors independently screened the titles and abstracts yielded by the search. We obtained full text for all titles that appeared to meet the inclusion criteria or where there was any uncertainty. At least two review authors screened the full text reports independently and decided whether these meet the inclusion criteria, disagreements were resolved through discussion. We recorded the reasons for excluding studies after full-text screening (see flow diagram in Supplementary Figure 1). If correlations between cortisol and brain activity were not reported in the manuscript, we contacted the authors to gather that information.
3. Results
3.1. Literature overview
The literature search for endogenous cortisol resulted in 1083 manuscripts, of which 65 studies met the inclusion criteria (see flow diagram in Supplementary Figure 1). The correlation between cortisol and brain activity during psychological stress was reported in 22 of these studies, but there was high variability in reported statistics: five studies reported Montreal Neurological Institute (MNI) coordinates (Akdeniz et al., 2014; Boehringer et al., 2015; Kukolja et al., 2008; Radke et al., 2018; Root et al., 2009), six studies reported correlation coefficients for different regions of interest (ROIs) (Admon et al., 2015; Dahm et al., 2017; Lederbogen et al., 2011; Mareckova et al., 2017; Ming et al., 2017; Pruessner et al., 2008), four studies reported no statistics but rather reported that no effects arose (Dedovic et al., 2014; Kogler et al., 2015; Kogler et al., 2017; Orem et al., 2019), five studies only compared cortisol responders and non-responders (Dedovic et al., 2009b; Khalili-Mahani et al., 2010; Streit et al., 2014; van Stegeren et al., 2007; Wheelock et al., 2016), and two studies focused on perfusion fMRI data (Wang et al., 2007; Wang et al., 2005). The correlation between cortisol and brain activity during tasks right after psychological stress was reported in 20 studies. Eighteen studies measured both cortisol and brain activity during or after psychological stress but did not report the correlation between the two, and we did not get the information after we contacted the authors. In addition, five studies focused on connectivity analyses, one study focused on changes in cortisol elicited by exercise treatment, and one study did not include the cortisol data in the manuscript.
The literature search for exogenous cortisol resulted in 328 manuscripts, of which 30 studies met the inclusion criteria (see flow diagram in Supplementary Figure 2). We included different cortisol compounds in the search, but almost all studies used hydrocortisone, except two studies using prednisolone (Buades-Rotger et al., 2016; Serfling et al., 2019). The correlation between cortisol and brain activity during cortisol administration was only examined and reported in one study (Lovallo et al., 2010). The correlation between cortisol and brain activity during tasks after cortisol administration was reported in 29 studies.
The inconsistency in statistical procedures, paradigms, and definitions of cortisol response precluded quantitative review of cross-study associations between endogenous or exogenous cortisol and human brain function. Therefore, we used a qualitative, systematic approach to answer the two research questions. Only effects that were replicated were interpreted and described in the text (Figure 1–2); however, all findings are reported in Tables 2, 3, and 4. We focused on the associations between acute cortisol and human brain function, as the direction of the effect is unclear as cortisol might not have had the time to impact the brain. In addition, the cortisol response was measured differently across studies (e.g. area under the curve, peak minus baseline).
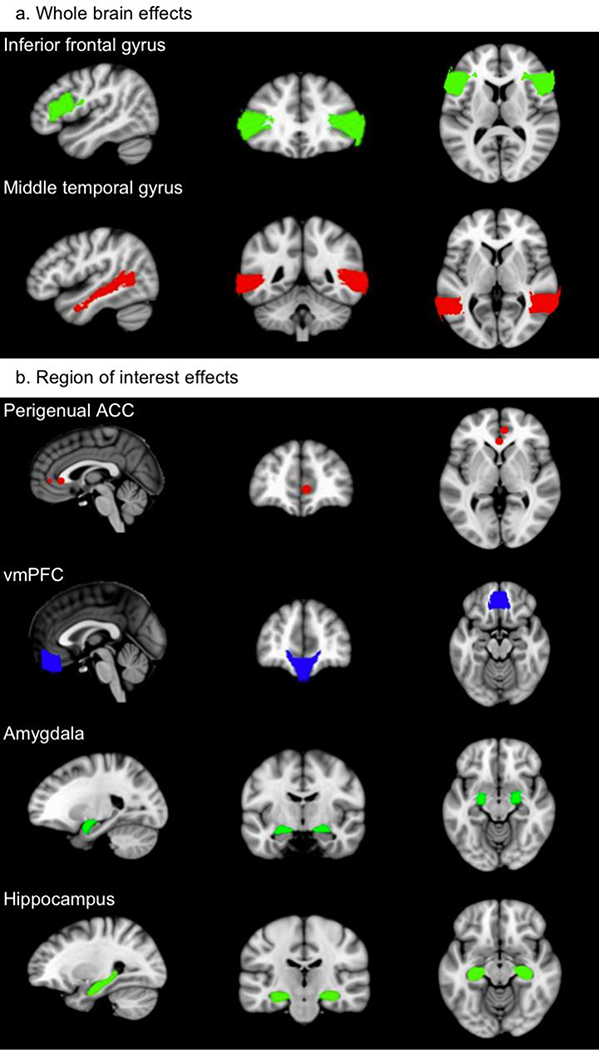
Figure 1.
Illustration of the brain regions that were related to endogenous cortisol levels in at least two studies at either whole-brain (a) or region of interest level (b) during psychological stress in the MRI scanner (regions from Harvard-Oxford atlas, perigenual ACC shown as 5 mm spheres around the peak coordinates from Boehringer et al. (2015) and Akdeniz et al. (2014)).
Note: red represents a positive correlation with cortisol, blue a negative correlation, and green altered function (positive in some studies, negative in others).
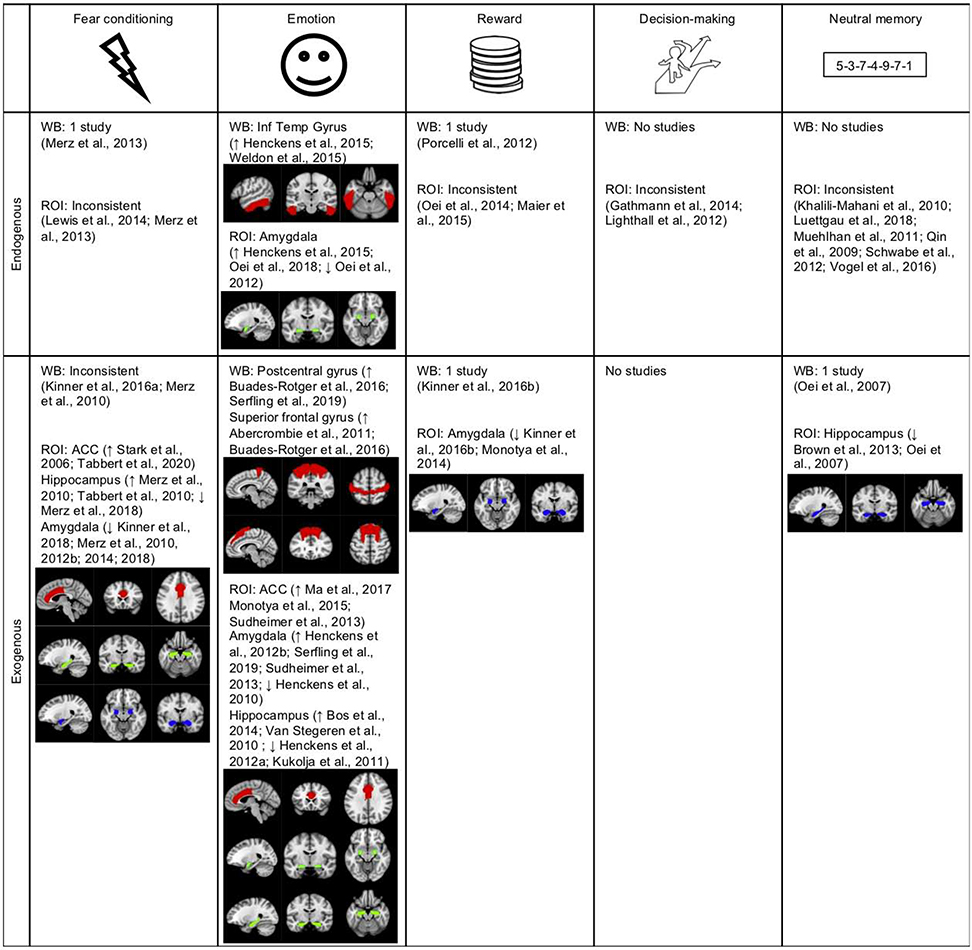
Figure 2.
Summary of replicated findings on the associations between brain activity and endogenous and exogenous cortisol after respectively psychological stress and cortisol administration (regions from Harvard-Oxford atlas).
Note: red represents a positive correlation with cortisol, blue a negative correlation, and green altered function (positive in some studies, negative in others). WB = whole-brain; ROI = region of interest; ACC = anterior cingulate cortex; Inf Temp Gyrus = inferior temporal gyrus; sup fr gyrus = superior frontal gyrus.
Table 2.
Overview of studies on the associations between endogenous cortisol and brain activity during psychological stress.
| Reference | Participants (mean age, percentage females) | Stressor | Analysis | Results for HVs For ↑ cortisol - reported as either correlation with cortisol, or as a group difference |
|---|---|---|---|---|
| Whole brain | ||||
| Kukolja et al., 2008 | 20 HV young (Mage=23.8, 45%) 12 HV old (Mage=58.5, 33.33%) |
Encoding and recognition task | WB | WB encoding: interaction cortisol and age in L middle frontal gyrus (pos relation in young subjects) WB retrieval: interaction cortisol and age in L middle frontal gyrus, R inferior frontal gyrus, R hippocampal formation (neg relation older subjects) |
| Radke et al., 2018 | 80 HV (Mage=24.54, 50%) | Cyberball task (social exclusion) | WB | ↑ inferior frontal gyrus, ↑ middle temporal gyrus |
| Dedovic et al., 2014 | 26 HV (Mage=21.9, 53.8%) 23 sub depr (Mage=21.9, 47.8%) |
MIST | WB ROI |
WB: No significant associations ROI: No significant associations with hippocampus, medial OFC, sgACC |
| Orem et al., 2019 | 239 HV (Mage=19.44, 47.3%) | MIST | WB ROI |
WB: No significant associations ROI: No significant associations with amygdala, hippocampus |
| Root et al., 2009 | 20 HV (Mage=31, 45%) | Fear evoking images | WB ROI |
WB: ↑ L middle temporal gyrus and ↑ frontal regions (including R middle frontal gyrus and R middle orbital frontal gyrus), ↓ L&R insula, ↓ frontal regions (including R superior and inferior frontal gyri), ↓ L&R lateral orbital frontal gyrus ROI: ↑ amygdala, ↑ hippocampus, ↓ vmPFC |
| ROI | ||||
| Pruessner et al., 2008 | 40 HV (Mage=23.1, 50%) | MIST | ROI Resp vs non-resp |
ROI: ↓ hippocampus, no association with ACC Resp < non-resp: L ACC, R orbitofrontal/inferior frontal gyrus, L dlPFC |
| Kogler et al., 2015 | 40 HV (Mage=24.5, 57.5%) | MIST | ROI | No significant associations with amygdala, hippocampus, putamen |
| Admon et al., 2015 | 29 HV (Mage=45.7, 45.7%) 31 rMDD (Mage=47.4, 51.5%) |
International Affective Picture System (IAPS) | ROI | No significant associations with bilateral caudate, NAcc, putamen rMDD: ↑ caudate |
| Akdeniz et al., 2014 | 23 HV German (Mage=23.35, 60.9%) 23 HV Migrant (Mage=22.61, 52.2%) |
ScanSTRESS (arithmetic task) | ROI | ↑ pACC |
| Boehringer et al., 2015 | 25 HV (Mage=41.88, 56%) | MIST | ROI | ↑ pACC* |
| Lederbogen et al., 2011 | 32 HV (Mage=43.6, 50%) | MIST | ROI | ↓ amygdala, ↓ hippocampus |
| Mareckova et al., 2017 | 41 HV (0.46%) 31 psychosis (0.48%) 27 MDD (0.52%) Mage males=45.02, Mage females=44.33 |
IAPS | ROI | ↓ L OFC, ↓ L mPFC** |
| Kogler et al., 2017 | 77 HV (Mage=24.56, 51.9%) | MIST | ROI | No significant associations with R insula, R STG |
| Dahm et al., 2017 | 83 HV (Mage=27, 59%) | ScanSTRESS (mental spatial rotation and arithmetic subtraction) | ROI (mediation analysis) | No significant associations with amygdala, dACC, insula Mediation analysis: conscientiousness mediates the relationship between insula & amygdala activation and cortisol |
| Ming et al., 2017 | 36 HV (Mage=22.19, 50%) 36 MDE (Mage=22.81, 52.78%) 33 rem MDE (Mage = 21.67, 51.52%) |
MIST | ROI | ↓ vmPFC |
| van Stegeren et al., 2007 | 14 HV responders (50%) 14 HV non-responders (50%) Mage=20.93 |
emotional images | ROI Resp vs non-resp |
ROI: ↑ amygdala Resp > non-resp: amygdala |
| Responders vs non-responders | ||||
| Dedovic et al., 2009 | 7 HV responders 13 HV non-responders |
eventMIST | Resp v non-resp | Direct comparison not significant (resp > non-resp: dmPFC L&R, dlPFC R, temporal lobe L) |
| Khalili-Mahani et al., 2010 | 9 HV responders (0%) 10 HV non-responders (0%) |
MIST | Resp vs non-resp | ROI: ↓ L hippocampus Resp > non-resp: middle temporal gyrus, R lateral PFC, R rostral ACC Resp < non-resp: hippocampus |
| Streit et al., 2014 | 42 HV (Mage=28, 47%) | ScanSTRESS (arithmetic task) | Resp vs non-resp | Resp > non-resp: amygdala |
| Wheelock et al., 2016 | 53 HV (Mage=18.68, 43.4%) | MIST | Resp vs non-resp | ROI: ↓ vmPFC Resp < non-resp: vmPFC, PCC, insula, superior temporal gyrus |
| Cerebral blood flow | ||||
| Wang et al., 2005 | 23 HV stress (Mage=24.1, 47%) 7 HV control (Mage=23, 60%) |
arithmetic task | CBF | ROI: ↑ ventral R PFC, ↑ ACC, ↑ precuneus, ↑ L&R angular gyri/inferior parietal cortex, ↑ antromedial prefrontal cortex |
| Wang et al., 2007 | 32 HV (Mage=23.55, 50%) | arithmetic task | CBF | Males: ↑ R PFC, ↓ L OFC/ inferior FC Females: ↑ L thalamus, ↑ dACC, ↓ L IFC |
Note: HV = healthy volunteer; WB = whole brain; L = left; R = right; pos = positive; neg = negative; MIST = Montreal Imaging Stress Task; ROI = region of interest; OFC = orbitofrontal cortex; sgACC = subgenual anterior cingulate cortex; vmPFC = ventromedial prefrontal cortex; Resp = responders; non-resp = non-responders; ACC = anterior cingulate cortex; d1PFC = dorsolateral prefronal cortex; rMDD = major depressive disorder in remission; NAcc = nucleus accumbens; pACC = perigenual anterior cingulate cortex; mPFC = medial prefrontal cortex; STG = superior temporal gyrus; dACC = dorsal ACC; dmPFC = dorsomedial prefrontal cortex; IFC = inferior frontal cortex
Does not survive correction for multiple comparisons
only in females
Table 3.
Overview of studies on the associations between endogenous cortisol and brain activity on tasks after psychological stress.
| Reference | Participants (mean age, percentage females) | Stressor | Task | Time between end of stressor and task | Results for HVs For ↑ cortisol - reported as either correlation with cortisol, or as a group difference (stress vs control) |
|---|---|---|---|---|---|
| Conditioning tasks | |||||
| Merz et al., 2013 | 48 HV stress (Mage=23.22, 50%) 48 HV control (Mage=23.22, 50%) |
TSST outside scanner | Fear conditioning task | 25 min | WB: no significant associations ROI: ↓ L NAcc* no associations with ACC, L amygdala |
| Lewis et al., 2014 | 7 HV stress responder (Mage=25.43, 57.1%) 9 HV stress non-responder (Mage=21.56, 55.6%) 13 HV control (Mage=23.41, 50%) |
Cold pressor in scanner | Conditioning task | 0 min | Resp > non-resp: R ventral putamen No significant associations with medial PFC, hippocampus, paracentral lobule |
| Emotional tasks | |||||
| Weldon et al., 2015 | 24 HV (Mage=37.2, 38%) | fMRI scan itself | Face processing task | 0 min | WB: Fear-neutral: ↑ precuneus, ↑ lingual gyrus Anger-neutral: ↑ inferior temporal gyrus, ↑ superior parietal lobule, ↑ precuneus, ↑ rectal gyrus, ↓ cerebellar tonsil, ↓ inferior semi-lunal lobule Sad-neutral: ↑ bilateral motor cortex, ↑ posterior cingulate, ↑ R precuneus Happy-neutral: ↑ R putamen, ↑ rostral cingulate, ↑ L posterior middle temporal gyrus and precentral gyrus, ↑ bilateral precuneus, ↑ posterior cingulate, ↑ middle cingulate, ↑ cerebellum, ↑ inferior parietal lobule Faces-animals: ↑ L superior frontal gyrus |
| Henckens et al., 2015 | 118 HV (0%) | Movie clip in scanner | Face processing task | 0 min | WB: Emotional faces: ↑ bilateral amygdala, ↑ hippocampus, ↑ parahippocampal gyrus, ↑ inferior temporal gyrus, Fearful vs happy: ↓ cluster in midbrain, covering the locus coeruleus, hypothalamus and amygdala↑ ROI: ↑ amygdala |
| Shermohammed et al., 2017 | 29 HV stress (Mage=21.13, 51.72%) 25 HV control (Mage=20.89, 48%) |
speech stress induction task (adapted from TSST) outside scanner math stress induction task (adapted from MIST) in scanner |
Cognitive Reapprasal task | Time of going into scanner for speech stressor 0 min for math stressor | WB: no significant associations |
| Li et al., 2019 | 27 HV (Mage=24.25, 0%) [25 included in cortisol analysis] | TSST outside scanner | Emotional face recognition | Time of going into scanner | ROI: ↑ L hippocampus in ll 5-HTTLPR carriers but not in ss/sl 5-HTTLPR carriers |
| Oei et al., 2012 | 17 HV control (Mage=24, 0%) 17 HV stress (Mage=24.5, 0%) |
TSST outside scanner | Working memory task | Time of going into scanner | ROI: ↓ ventral system, ↓ amygdala, ↓ IFG No significant associations with dorsal system (dlPFC and LPC) |
| Oei et al., 2018 | 61 offspring (Mage=66.23, 50.8%) 43 partners (Mage=65.86, 51.2%) |
TSST outside scanner | Emotional Sternberg task | 15 min | ROI: ↑ L amygdala No significant associations with right amygdala, R IFG, cingulate |
| Van Leeuwen et al., 2018 | 39 HV (Mage=33.73, 0%) 29 siblings of patients with SZ (Mage=33.17, 0%) |
TSST outside scanner | IAPS pictures | 33 min after start stressor (stressor around 13 min) | ROI: no significant associations in HV with precuneus, mPFC, L precentral gyrus, L STG, L&R vlPFC, superior frontal gyrus, middle cingulate, anterior insula, cerebellum |
| Reward tasks | |||||
| Porcelli et al., 2012 | 16 HV control (Mage=23.4, 50%) 16 HV stress (Mage=23.4, 50%) |
Cold pressor in scanner | Card guessing task | 0 min | WB: No significant associations |
| Oei et al., 2014 | 20 HV control (Mage=21.5, 0%) 17 HV stress (Mage=22.4, 0%) |
TSST outside scanner | Backwards masked task | 10 min | ROI: L&R NAcc |
| Maier et al., 2015 | 22 HV control (Mage=21, 0%) 29 HV stress (Mage=21, 0%) |
SPECT outside scanner | Food choice task | 12–17 min | ROI: No significant associations with amygdala, ventral striatum |
| Decision-making tasks | |||||
| Gathmann et al., 2014 | 16 HV stress (Mage=23.69, 43.75%) 17 HV control (Mage=24.06, 52.9%) |
TSST outside scanner | N back tast, game of dice task | around 30 min | ROI: ↓ L middle frontal gyrus-dorsolateral prefrontal area (BA 9) |
| Lighthall et al., 2012 | 23 HV stress (Mage=22.4, 48%) 24 HV control (Mage=22.4, 50%) |
Cold pressor outside scanner | Balloon Analogue Risk Task | 24 min after start stressor (stressor around 3 min) | ROI: ↑ dorsal striatum* No significant associations with insula |
| Non-emotional tasks | |||||
| Qin et al., 2009 | 13 HV control (Mage=20, 100%) 14 HV stress (Mage =21, 100%) |
Movie clip in scanner | N-back task | 0 min | ROI: ↑ MPFC extending to ACC |
| Schwabe & Wolf, 2012 | 29 HV control (Mage=23.8, 50%) 30 HV stress (Mage=23.8, 50%) |
SPECT outside scanner | Probabilistic classification learning task | 25 min | ROI: ↑ caudate (cortisol baseline, not cortisol increase) |
| Vogel et al., 2016 | 98 HV (Mage=21.9, 0%)** Stress/placebo Control placebo Stress/MR-blocker Control/MR-blocker |
SPECT outside scanner | Spatial memory task | 17 (+/− 4) min | ROI: ↑ amygdala (in placebo condition) |
| Khalili-Mahani et al., 2010 | 9 HV responders (0%) 10 HV non-responders (0%) |
MIST in scanner | Image recognition task | 0 min | Resp > non-resp: hippocampus during encoding; R rostral ACC, L lateral PFC during recognition |
| Luettgau et al., 2018 | 29 HV (Mage=23.48, 0%) | TSST outside scanner | n-back task | around 20 min | ROI: No significant associations withdlPFC |
| Muehlhan et al., 2011 | 29 HV (Mage=23.8, 12.8%) | fMRI scan itself | Visual detection task | 0 min | ROI: No significant associations with thalamus |
Note: HV = healthy volunteer; TSST = Trier Social Stress Task; SPECT = cold pressor with social element; WB = whole brain; ROI = region of interest; L = left; R = right; NAcc = nucleus accumbens; ACC = anterior cingulate cortex; Resp = responder; non-resp = non-responder; PFC = prefrontal cortex; MIST = Montreal Imaging Stress Task; IFG = inferior frontal gyrus; dlPFC = dorsolateral prefrontal cortex; LPC = lateral parietal cortex; BA = Brodman area; MPFC = medial prefrontal cortex
Only in males
Only total participants was reported, not the numbers per group.
Table 4.
Overview of studies on the associations between exogenous cortisol and brain activity on tasks after cortisol administration.
| Reference | Participants (mean age, percentage females) | Drug (dosage) | Task | Time between administration and task | Results for HVs For cortisol versus placebo |
|---|---|---|---|---|---|
| Conditioning tasks | |||||
| Merz et al., 2012b | 62 cort (72.5%) 60 placebo (75%) |
Hydrocortisone (30 mg) | Acquisition (implicit) | 45 min | ROI: ↓ L amygdala, ↓ L anterior parahippocampal gyrus (treatment*sex hormone status) |
| Merz et al., 2010 | 20 cort (50%) 19 placebo (47.4%) |
Hydrocortisone (30 mg) | Acquisition | 45 min | WB: no significant difference ROI: ↓ amygdala; treatment*sex: insula, hippocampus*, thalamus* |
| Stark et al., 2006 | 17 cort (47.1%) 17 placebo (52.9%) Mage= 24.2 |
Hydrocortisone (30 mg) | Acquisition | 15 min | ROI UCS: ↑ R ACC and PCC Treatment*time: dlPFC Treatment*sex: anterior cingulate, lateral OFC, mPFC |
| Tabbert et al., 2010 | 10 cort (100%) 10 placebo (100%) Mage=23.2 |
Hydrocortisone (30 mg) | Acquisition Extinction | 45 min before start scan | Acquisition: ACC, hippocampus (CS+>CS−) Extinction: hippocampus, thalamus (CS−>CS+) No significant difference in insula, amygdala |
| Merz et al., 2012a | 49 cort (61%) 49 placebo (59%) |
Hydrocortisone (30 mg) | Acquisition Extinction | 45 min | Acquisition: no significant differences Extinction: no significant differences |
| Merz et al., 2018 | 20 cort (Mage=24.1, 0%) 20 placebo (Mage=24.8, 0%) |
Hydrocortisone (30 mg) | Extinction | 50 min | ROI: ↓ amygdala, ↓ R anterior parahippocampal gyrus, ↓ R hippocampus during early extinction |
| Merz et al., 2014 | 16 cort (Mage=24.3, 0%) 16 placebo (Mage=24.9, 0%) |
Hydrocortisone (30 mg) | Extinction | 45 min | ROI: ↓ amygdala, ↓ medial frontal gyrus, ↓ NAcc |
| Kinner et al., 2016a | 30 cort (50%) 30 placebo (50%) |
Hydrocortisone (30 mg) | Extinction recall | 40 min | WB: no significant differences ROI: vmPFC (treatment*sex interaction: ↓ men ↑ women in cortisol group) |
| Kinner et al., 2018 | 32 cort (50%) 32 placebo (50%) Mage=23.6 |
Hydrocortisone (30 mg) | Renewal and reinstatement test | 40 min | ROI: ↑ R amgydala (men), ↓ R amgydala (women) No significant differences in vmPFC, OFC, dACC, insula, NAcc, hippocampus |
| Emotional tasks | |||||
| Buades-Rotger et al., 2016 | 20 HV (Mage=24.35, 0%) | Prednisolone (250 mg) | Negative and neutral human figures | 240 min | WB: ↑ primary somatosensory cortex/postcentral gyrus, ↑ middle frontal gyrus, ↑ superior frontal gyrus, ↑ inferior frontal gyrus, ↓ anterior cerebellum ROI: ↑ R inferior frontal gyrus, ↑ supplementary motor area/medial frontal gyrus, ↑ L caudate, ↑ L amgydala |
| Monotya et al., 2015 | 19 HV (Mage=22.6, 0%) | Hydrocortisone (40 mg) and primogel (320 mg) | Fear-and-Escape Task | 80 min | WB: ↓ midbrain (main effect drug) ROI: AIC (inescapable threat anticipation), ↑ AIC & dACC (escapable threat) |
| Ma et al., 2017 | 40 HV (Mage=22.8, 50%) | Hydrocortisone (100 mg) | Shifted-Attention Emotion Appraisal Task (SEAT) | 120 min before start scan | WB: dmPFC (for treatment × sex: ↓ females ↑ males) ROI: vmPFC, sgACC (for treatment × sex: ↑ females) |
| Abercrombie et al., 2018 | 46 minimal EA (Mage=26.1, 100%) 14 moderate EA (Mage=31.4, 100%) 15 severe EA (Mage=28.6, 100%) |
Hydrocortisone (20 mg) | Memory encoding with IAPS pictures | 90 min | WB: L BA6-whole cluster, bilateral thalamus, R BA6/BA40 Moderating effects of childhood EA severity |
| Abercrombie et al., 2011 | 41 HV (26.7, 56.1%) 19 depressed (27.1, 52.6%) |
Hydrocortisone (15 mg) | Emotional sentence processing task | 60 min | WB: ↑ L superior frontal gyrus (only men) |
| Henckens et al., 2012a | 18 HVs (median age=23, 0%) | Hydrocortisone (20 mg) | Picture encoding task | 30 min (fast) 180 min (slow) |
Fast effects of cortisol WB: no significant differences ROI: no significant difference in hippocampus Slow effects of cortisol WB: ↓ middle frontal gyrus ROI: ↓ hippocampus |
| Bos et al., 2014 | 21 HV (Mage=22.8, 0%) | Hydrocortisone (40 mg) and primogel (320 mg) | Cry task | 60 min | WB: no significant differences ROI: ↑ hippocampus, ↑ thalamus |
| Henckens et al., 2012b | 22 fast cort (0%) 22 slow cort (0%) 21 placebo (0%) |
Hydrocortisone (10 mg) | Emotional interference task | 60 min (fast) 270 min (slow) |
Fast effects of cortisol WB: no significant differences ROI: ↑ amygdala* Slow effects of cortisol WB: ↓ cuneus ROI: no significant difference in amygdala |
| Serfling et al., 2019 | 20 HV (Mage=24.6, 0%) | Prednisolone (250 mg) | Go/Nogo with food stimuli | 240 min | WB: ↑ postcentral gyrus (NoGo relative to Go trials) ROI: ↑ L amygdala, ↓ R putamen (main effects), ↑ insula, ↑ amygdala (food-go>object-go) No significant differences in ACC, hippocampus |
| Kukolja et al., 2011 | 13 cort (Mage=24.1, 46.2%) 16 placebo (Mage=25.2, 50%) 13 RBX (Mage=24.1, 53.8%) 16 RBX-cort (Mage=24.9, 50%) |
Hydrocortisone (30 mg) | Episodic encoding task with IAPS pictures | 145 min | ROI: ↓ L hippocampus [for cort only] |
| Sudheimer et al., 2013 | 21 cort (Mage=21.5, 55%) 20 extended dose cort (Mage=21.7, 50%) 20 placebo (Mage=21.8, 50%) |
Hydrocortisone (100 mg) | Emotional face processing task | 120 min | ROI: ↓ sgACC (sadness), ↑ sgACC & amygdala (happy faces) No differnces in vmPFC, amygdala |
| Van Stegeren et al., 2010 | 12 placebo/cort (0%) 12 placebo/placebo (0%) 12 placebo/YOH (0%) 12 YOH/cort(0%) |
Hydrocortisone (20 mg) and yohimbine (20 mg) | Emotional picture processing task | 45 min before start scan (yohimbine 60 min before start scan) | ROI: ↑ R hippocampus, ↑ L frontal gyrus [for placebo/cort] |
| Henckens et al., 2010 | 22 fast cort (Mage=21, 0%) 23 slow cort (Mage=21, 0%) 23 placebo (Mage=21, 0%) |
Hydrocortisone (10 mg) | Emotional picture processing task | 75 min 285 min |
Fast effects of cortisol ROI: ↓ amygdala Slow effects of cortisol ROI: ↓ amygdala |
| Kukolja et al., 2008 | 15 cort (Mage=23.9, 46.7%) 16 placebo (Mage=25.2, 50%) 15 RBX (Mage=24.1, 46.78%) 15 RBX-cort (Mage=25.1, 46.7%) |
Hydrocortisone (30 mg) | Emotional faces | 105 min | Do not report cortisol only vs placebo |
| Reward tasks | |||||
| Kinner et al., 2016b | 30 cort (50%) 30 placebo (50%) |
Hydrocortisone (30 mg) | MID | 60 min | WB: no significant differences ROI: ↓ L pallidum, ↓ R anterior parahippocampal gyrus Treatment*sex interaction: L precuneus, anterior cingulate, L anterior parahippocampal gyrus (verbal reward), R amygdala (monetary reward)*, L hippocampus (verbal vs monetary reward) (↓ men, ↑ women) |
| Monotya et al., 2014 | 20 HV (Mage=23, 0%) | Hydrocortisone (40 mg) and primogel (320 mg) | MID | 50 min | ROI: ↓ NAcc, ↓ BLA |
| Non-emotional tasks | |||||
| Oei et al., 2007 | 20 HV (Mage=22.8, 0%) | Hydrocortisone (20 mg) | Word recognition task | 60 min | WB: ↓ R superior frontal gyrus, ↓ L putamen, ↓ R precuneus (only neutral words) ROI: ↓ R hippocampus (pooled words), ↓ hippocampus (only neutral words) |
| Brown et al., 2013 | 15 HV (Mage=25.3, 60%) | Hydrocortisone (80 mg, 5 times before scan, last dose morning of scan) | Novelty detection task (indoor & outdoor scenes) | 240 min | ROI: ↓ hippocampus |
| Fleischer et al., 2019 | 33 HV (Mage=28.3, 100%) | Hydrocortisone (10 mg) | Autobiographical memory test | 45 min | ROI: ↓ R amPFC |
| Henckens et al., 2011 | 22 fast cort (Mage=21, 0%) 23 slow cort (Mage=21, 0%) 23 placebo (Mage=21, 0%) |
Hydrocortisone (10 mg) | n-back task | 30 min (fast) 240 min (slow) |
Fast effects of cortisol ROI: No significant difference in dlPFC Slow effects of cortisol ROI: ↑ dlPFC |
Note: WB = whole brain; ROI = region of interest; L = left; R = right; cort = cortisol; H&B = Hormones and Behavior; UCS = unconditioned stimulus; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex; mPFC = medial prefrontal cortex; SCAN = Social Cognitive Affective Neuroscience; NAcc = nucleus accumbens; vmPFC = ventromedial prefrontal cortex; dACC = dorsal ACC; HV = healthy volunteer; AIC = anterior insula; dmPFC = dorsomedial prefrontal cortex; sgACC = subgenual cingulate; EA = emotional abuse; BA = Brodman area; RBX = reboxetine; IAPS = International Affective Picture System; YOH = yohimbine; MID = monetary incentive delay task; BLA = basolateral amygdala; amPFC = anterior medial PFC
p-value between 0.05 and 0.1
3.2. Is cortisol related to brain activity during psychological stress or cortisol administration?
3.2.1. Endogenous cortisol
In these studies, the stressor occurred while the participants were in the MRI scanner, so cortisol probably peaks right after the MRI scan. It should be noted that the relation between cortisol and brain activity is correlational, and that these associations might also be influenced by other variables. At the whole-brain level, higher levels of endogenous cortisol during psychological stress in the MRI scanner were related to increased activity in the middle temporal gyrus and altered function (increased activity in some and decreased activity in other studies) in the inferior frontal gyrus (Table 2, Figure 1). Specifically, for the middle temporal gyrus, higher cortisol levels were related to more activity in response to fear evoking images (Root et al., 2009) and during social exclusion in the Cyberball task (Radke et al., 2018). For the inferior frontal gyrus, higher cortisol levels were related to less activity in response to fear evoking images (Root et al., 2009) and during memory retrieval in older subjects (Kukolja et al., 2008). However, higher cortisol levels were related to more activity in this same region during social exclusion in the Cyberball task (Radke et al., 2018). Two studies did not find associations between cortisol and brain activity on a whole-brain level during the Montreal Imaging Stress Task (MIST) (Dedovic et al., 2014; Orem et al., 2019), although they did not find increased levels of cortisol in response to this task either.
ROI analyses of endogenous cortisol have focused on different ROIs based on the literature or on whole-brain findings. These studies revealed that higher levels of endogenous cortisol were related to increased activity in the perigenual anterior cingulate cortex (ACC), decreased activity in the ventromedial prefrontal cortex (vmPFC) and altered function in the amygdala and hippocampus (Figure 1). First, higher endogenous cortisol levels were related to increased activity in the perigenual ACC during the MIST (Boehringer et al., 2015) and during ScanSTRESS (an arithmetic task) (Akdeniz et al., 2014). Of note, other regions of the ACC were not related to endogenous cortisol during the MIST (Dedovic et al., 2014; Pruessner et al., 2008) nor during ScanSTRESS (Dahm et al., 2017). Second, higher endogenous cortisol levels were related to reduced activity in the vmPFC during the MIST (Ming et al., 2017; Wheelock et al., 2016) and while watching fear evoking images (Root et al., 2009). Third, an increased endogenous cortisol response was related to increased activity in the amygdala while watching fear evoking images (Root et al., 2009; van Stegeren et al., 2007), and to decreased activity in the amygdala during the MIST (Lederbogen et al., 2011). In contrast, three other studies found no relation between cortisol and amygdala activity during psychological stress (Dahm et al., 2017; Kogler et al., 2015; Orem et al., 2019). Fourth, higher levels of endogenous cortisol were related to decreased activity in the hippocampus during the MIST (Khalili-Mahani et al., 2010; Lederbogen et al., 2011; Pruessner et al., 2008), and to increased activity in the hippocampus while watching fear evoking images (Root et al., 2009). Three studies using the MIST showed no relation between endogenous cortisol and activity in the hippocampus (Dedovic et al., 2014; Kogler et al., 2015; Orem et al., 2019), but the MIST did not elicit a significant increase in cortisol in these studies.
Since psychological stressors elicit a cortisol response in only a fraction of participants (Dickerson and Kemeny, 2004; Kudielka et al., 2007), six studies focused on the comparison between those participants who demonstrated an increase in cortisol (‘responders’) and those who did not (‘non-responders’). Two of these studies found that responders showed more activity in the amygdala than non-responders during ScanSTRESS (Streit et al., 2014) and while watching fear-evoking images (van Stegeren et al., 2007).
3.2.2. Exogenous cortisol
Only one study investigated the relation between exogenous cortisol and brain activity during hydrocortisone administration and found decreased activity in the hippocampus and amygdala (Lovallo et al., 2010).
3.3. Is cortisol related to brain activity during tasks performed after psychological stress or cortisol administration?
3.3.1. Endogenous cortisol
In these studies, the stressor occurred before the participants performed a task in the MRI scanner, so the peak in cortisol is around the same time as the task. It should be noted that the relation between cortisol and brain activity is correlational, and that these associations might also be influenced by other variables. Only two studies investigated associations between endogenous cortisol and brain function during a conditioning task and reported that higher levels of cortisol were related to less activity in the left nucleus accumbens (NAcc) in men but not women (Merz et al., 2013), and to more activity in the right ventral putamen (Lewis et al., 2014). Endogenous cortisol during psychological stress was related to increased activity in the inferior temporal gyrus and altered function in the amygdala during subsequent emotional tasks (Figure 2 and Table 3).
At the whole-brain level, increased endogenous cortisol was related to increased activity in the inferior temporal gyrus while processing angry compared to neutral faces (Weldon et al., 2015) and emotional faces compared to neutral faces (Henckens et al., 2015). However, there were no whole-brain findings in a cognitive reappraisal task (Shermohammed et al., 2017). On the ROI level, two studies revealed that higher cortisol during psychological stress was related to more activity in the amygdala while processing emotional faces (Henckens et al., 2015; Oei et al., 2018), whereas one study found less activity in the amygdala during distracting IAPS pictures (Oei et al., 2012).
Two studies that focused on reward tasks did not report significant correlations with cortisol (Maier et al., 2015; Porcelli et al., 2012), and one reported a positive correlation between cortisol and activity in the NAcc (Oei et al., 2014). Only two studies focused on decision-making tasks and examined distinct ROIs: striatum (Lighthall et al., 2012) and middle frontal gyrus-dorsolateral prefrontal area (Gathmann et al., 2014) – hence no findings could be replicated. Similarly, six studies administered a non-emotional task in the MRI scanner after psychological stress (Khalili-Mahani et al., 2010; Luettgau et al., 2018; Muehlhan et al., 2011; Qin et al., 2009; Schwabe and Wolf, 2012; Vogel et al., 2016), but these studies examined different ROIs so there were no replicated findings.
3.3.2. Exogenous cortisol
In these studies, participants performed tasks in the MRI scanner after cortisol administration. Here, the associations between cortisol and brain activity are most likely to be related to cortisol, because of the tight experimental control with a placebo. Exogenous cortisol was related to increased activity in the ACC and hippocampus, and decreased activity in the amygdala during conditioning. First, during fear acquisition, exogenous cortisol was related to increased activity in the ACC to the unconditioned stimulus (Stark et al., 2006) and to the threat stimulus compared to the safe stimulus (Tabbert et al., 2010). Second, exogenous cortisol was related to decreased amygdala activity in response to the threat stimulus during fear acquisition (Merz et al., 2010, 2012b) and during fear extinction (Merz et al., 2018; Merz et al., 2014). Third, exogenous cortisol was related to increased activity in the hippocampus in response to a threat stimulus compared to the safe stimulus (Tabbert et al., 2010). However, this effect was at trend-level and only found in women (men showed the opposite effect) in Merz et al. (2010). During fear extinction, exogenous cortisol was related to increased activity in the hippocampus in response to the safe stimulus compared to the threat stimulus (Tabbert et al., 2010), and decreased activity in the right hippocampus in response to the extinguished stimulus during early extinction (Merz et al., 2018). However, another study found no significant relation between cortisol and brain activity during acquisition and extinction (Merz et al., 2012a).
Exogenous cortisol was also related to increased activity in the postcentral gyrus, superior frontal gyrus and ACC, and altered function in the amygdala and hippocampus during emotional tasks after cortisol administration (see Figure 2 and Table 4). On the whole brain level, exogenous cortisol was related to increased activity in the postcentral gyrus while watching negative and neutral human features (Buades-Rotger et al., 2016) and during a Go/NoGo task with food stimuli, which was administered only in men (Serfling et al., 2019). Furthermore, exogenous cortisol was related to increased activity in the superior frontal gyrus while watching negative and neutral human features (Buades-Rotger et al., 2016) and during an emotional sentence processing task (Abercrombie et al., 2011). On the ROI level, higher cortisol levels were related to increased activity in the ACC during escapable threat in a Fear-and-Escape task (Montoya et al., 2015), during the viewing of happy faces in an emotional face processing task (in contrast to decreased ACC activity in response to sad faces) (Sudheimer et al., 2013), and only in women, not men, during a shifted-attention emotion appraisal task (Ma et al., 2017). No association between exogenous cortisol and ACC activity was found in one study that included only men using a Go/NoGo task with food (Serfling et al., 2019). Furthermore, higher cortisol levels were related to more activity in the amygdala during the viewing of aversive words (Henckens et al., 2012b) and happy faces (Sudheimer et al., 2013) in emotion interference tasks and during a Go/NoGo task with food in a study with only men (Serfling et al., 2019). Higher cortisol levels were also related to less activity in the amygdala in men when processing emotional pictures (Henckens et al., 2010). Finally, higher cortisol levels were related to more activity in the hippocampus during a cry task (Bos et al., 2014) and an emotional picture processing task (van Stegeren et al., 2010), and to less activity in the hippocampus during an episodic encoding task with emotional and neutral IAPS pictures (Henckens et al., 2012a; Kukolja et al., 2011). No relation between exogenous cortisol and hippocampus activity during a Go/NoGo task with food were found in a study that included only men (Serfling et al., 2019).
Only two studies measured brain activity during reward tasks after cortisol administration, both found that exogenous cortisol was related to reduced activity in the amygdala (Kinner et al., 2016b; Montoya et al., 2014). However, Kinner et al. (2016b) found this effect only in men, whereas women showed the opposite effect, though this interaction with sex was at trend-level. In non-emotional tasks, exogenous cortisol was related to reduced activity in the hippocampus in a word recognition task (Oei et al., 2007) and in a novelty detection task with indoor and outdoor scenes (Brown et al., 2013), but not in an autobiographical memory test (Fleischer et al., 2019).
4. Discussion
The goal of the current study was to systematically review the literature on the associations between endogenous and exogenous cortisol and human brain function. We reviewed results from fMRI studies that measured brain activity during a psychological stressor or cortisol administration, and during tasks after a psychological stressor or cortisol administration. These studies revealed many inconsistent findings, due to heterogeneity in methodology, paradigms, cortisol sampling, and analyses. However, there were three sets of replicated findings: First, higher levels of endogenous cortisol were related to increased activity in the middle temporal gyrus and perigenual ACC, decreased activity in the vmPFC, and altered function (increased activity in some studies and decreased in others) in the amygdala, hippocampus and inferior frontal gyrus during psychological stress. Second, endogenous cortisol during psychological stress was related to increased activity in the inferior temporal gyrus and altered function in the amygdala during emotional tasks that followed psychological stress. Third, exogenous cortisol (hydrocortisone in all except two studies) was also related to increased activity in the postcentral gyrus, superior frontal gyrus and ACC, and altered function in the amygdala and hippocampus during conditioning, emotional and reward-processing tasks after cortisol administration.
The inconsistent associations between endogenous and exogenous cortisol and brain activity did not seem to be related to type of stressor, method of assessing cortisol (e.g., area under the curve, peak minus baseline), or task after the stressor. Associations with activity in the amygdala were most consistently found across all studies; cortisol was related to amygdala activity during and after psychological stress. Similar findings emerged in the studies on exogenous cortisol. Studies of endogenous and exogenous cortisol both have their unique advantages, with studies of endogenous cortisol being more ecologically valid and studies of exogenous cortisol offering tighter experimental control. That both endogenous and exogenous cortisol were associated with activity in the amygdala might suggest a specific effect of cortisol independent of other effects of stress. These amygdala findings are in line with a previous review (Dedovic et al., 2009a). However, the effects were generally small, in different directions, had different peak coordinates, and were not statistically significant in all studies.
The relation between cortisol and amygdala activity is consistent with animal studies, demonstrating that the limbic system plays an important role in the stress response (Ulrich-Lai and Herman, 2009). Furthermore, in rhesus monkeys, the central nucleus of the amygdala is a core component of the neural circuit underlying anxious temperament, which is related to an increased cortisol response in a stressful situation (Kalin, 2017). The amygdala interacts with the hippocampus and medial prefrontal cortex in regulating effects of glucocorticoids on spatial memory retrieval (Roozendaal et al., 2003) and working memory (Roozendaal et al., 2004). Indeed, activity in the hippocampus and vmPFC was also related to cortisol in the human studies reviewed here. Furthermore, some studies showed that differential amygdala connectivity is related to endogenous (Mareckova et al., 2017; Vaisvaser et al., 2013; Vogel et al., 2016) or exogenous cortisol (Buades-Rotger et al., 2016; Henckens et al., 2012b). However, these effects were found in different brain regions (right dorsomedial prefrontal cortex, hippocampus, hypothalamus, middle frontal gyrus, precentral and postcentral gyrus, striatum, and thalamus) and some studies did not find significant associations between cortisol and amygdala connectivity (de Voogd et al., 2017; Henckens et al., 2010). Future research should focus on the connectivity between the amygdala, hippocampus, and vmPFC, during or shortly after stress, to gain insight into the mechanisms through which the amygdala influences memory in response to stress.
Cortisol has both acute non-genomic and slow gene-mediated effects on brain function (Joels, 2018). Here we focused on the acute effects of cortisol, with neuroimaging during or shortly after psychological stress or cortisol administration. In studies of endogenous cortisol, cortisol would peak right after or during neuroimaging, and thus it is unknown if cortisol has had the time to impact neural activity. Therefore, the relation between endogenous cortisol and brain function is solely correlational. In studies of exogenous cortisol, the associations are more likely to be related to cortisol, due to tight experimental control with a placebo. However, the time between cortisol administration and subsequent neuroimaging varied, which makes it unclear if cortisol has had the time to impact neural activity. It is important to take the time between the stressor or cortisol administration and the subsequent fMRI task into account, as this could reflect different underlying processes. A few studies compared the relation between brain function and the acute versus slow effects of cortisol (Henckens et al., 2012a; Henckens et al., 2010, 2011, 2012b) and most of these showed differences between the acute and slow effects (Henckens et al., 2012a; Henckens et al., 2011, 2012b). Therefore, future research should compare the acute and slow effects of cortisol to enhance understanding of the modulatory effects of timing.
While the negative effects of cortisol on human brain functioning are usually emphasized, some studies have shown positive effects of cortisol. For example, patients with depression were better at retaining a relatively positive memory bias after cortisol administration (Abercrombie et al., 2011), and patients with social anxiety disorder showed a reduced early bias for implicit social threat after cortisol administration (van Peer et al., 2010). The positive effects of cortisol administration are putatively achieved by inhibiting automatic processing of goal-irrelevant threatening information and by increasing early approach-avoidance responses (Putman and Roelofs, 2011). Moreover, some studies have examined whether cortisol can be used as a treatment for internalizing disorders. For instance, dexamethasone was more effective than placebo in the treatment of depression (Arana et al., 1995), and glucocorticoids reduced symptoms in post-traumatic stress disorder and specific phobias (de Quervain and Margraf, 2008). These studies on the positive effects of cortisol highlight the complexity of the effects of glucocorticoids on the brain.
Several limitations should be taken into account. First, we were not able to do a meta-analysis due to considerable heterogeneity in methodology: different stress tasks, fMRI paradigms, cortisol dosage, frequency and time of the day, the time between stress and the following fMRI-task, cortisol response calculations (such as area under the curve, difference scores, etc.), and analytical approaches employed. Second, not all stress tasks elicited a significant increase in cortisol. One reason might be that essential components of stress tasks, such as uncontrollability and social evaluation (Dickerson and Kemeny, 2004), were not present in these tasks. Also, the increase in cortisol is highly variant within individuals and across populations (e.g. clinical versus non-clinical), reducing the possibility of generalization. Third, null findings might not have been reported. Fourth, most studies were conducted by a few research groups, some studies possibly tested overlapping participants, underlining the need for independent replication. Fifth, we focused on the associations between acute increases in cortisol level and human brain function as measured with fMRI, but these associations can be studied at different levels using many techniques (e.g. cell level, EEG). Sixth, the pitfalls of studies of endogenous and exogenous cortisol should be taken into account. Even though studies of endogenous cortisol are more ecologically valid, the increase in cortisol level is small and the response to the stressor might be dependent on subjective experience of the stressor, which varies between people. Exogenous cortisol administration provides tight experimental control, but does not reflect a natural physiological stress reaction.
We have several recommendations for future studies on the associations between cortisol and human brain function. First, we recommend a more standardized approach to reporting results. For whole-brain analysis, results should be reported from all contrasts that were tested. For ROI analysis, anatomically defined ROIs should be used and findings from all ROIs should be reported. All results should be reported, even if associations were not statistically significant, as this would allow for future meta-analysis of the associations between brain function and cortisol. Second, we recommend within-subjects designs, to compare associations between cortisol and brain activity within individuals, whenever possible. Third, future research should investigate the role of cortisol on brain activity in psychopathology. For example, cortisol might suppress hippocampus activation, increase activity in the amygdala, and reshape dendrites in the hippocampus, amygdala and ventral PFC in patients with mood disorders (Erickson et al., 2003). These findings should be replicated and the effects of cortisol on the development and maintenance of psychopathology should be studied further. Fourth, previous research has shown that the long-term adverse effects of cortisol change over development (Lupien et al., 2009), and that adolescents are particularly sensitive to effects of stress (McCormick et al., 2010). Therefore, we recommend investigating the associations between acute increases in cortisol levels and the brain during adolescence, when most internalizing disorders develop (Kessler et al., 2005). Fifth, many studies have found interactions between cortisol and sex (Kinner et al., 2016a; Merz et al., 2010, 2012b; Stark et al., 2006), whereas other studies have only included men (Henckens et al., 2012a; Serfling et al., 2019). Thus, future research should recruit large samples with the same number of men and women to further investigate sex differences in the associations between cortisol and the brain. In addition, in women, sex hormone levels over the course of the menstrual cycle and the use of hormonal contraceptives might also influence the relation between cortisol and the brain (Herrera et al., 2019; Herrera et al., 2016). However, this has not been studied extensively. Sixth, the increase in cortisol level in studies of exogenous cortisol is larger than the increase in cortisol level in studies of endogenous cortisol. Future studies with a lower dose of exogenous cortisol should supplement the findings from studies of endogenous cortisol. Seventh, many different factors influence the cortisol response, such as time of day, diurnal cortisol slopes and dosage. For instance, animal research has shown that glucocorticoids act in an ultradian, pulsatile pattern (Lightman and Conway-Campbell, 2010). In healthy humans, the same dose of cortisol administered in different patterns influenced brain function and behavioral responses in several emotional and cognitive tasks differently (Kalafatakis et al., 2018). We recommend studying whether individual differences in this pulsatile pattern are related to individual differences in the associations between cortisol and brain function. Eight, future studies should use a multimodal assessment of the stress response, by combining effects of cortisol, other physiological measures, subjective anxiety, and neural measures.
In summary, our systematic review on the associations between endogenous and exogenous cortisol and human brain function shows that cortisol is related to amygdala activity during and after psychological stress, a finding confirmed by cortisol administration studies. These findings support results from prior animal studies demonstrating amygdala activity during and after stress. Future studies should focus on connectivity between the amygdala and other brain regions during and after stress, therapeutic effects of cortisol, and the modulating effects of development, sex and psychopathology on the relation between cortisol and human brain function.
Supplementary Material
Highlights.
We reviewed relations between endogenous/exogenous cortisol and human brain function.
Studies reported inconsistent findings, due to heterogeneity in methods and analyses.
Associations between cortisol and amygdala activity were most consistently found.
Acknowledgments
The authors would like to thank Dr. Peter Schmidt and Prof. Ned H. Kalin for their feedback on previous versions of the manuscript. This work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (ZIA-MH-002782 for D.S. Pine and ZIA-MH-002786 for A. Stringaris).
Footnotes
Declarations of interest: none.
Cortisol peaks 0–20 minutes after a stressor (Dickerson and Kemeny, 2004), so cortisol would most likely peak during the fMRI task.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie HC, Jahn AL, Davidson RJ, Kern S, Kirschbaum C, Halverson J, 2011. Cortisol’s effects on hippocampal activation in depressed patients are related to alterations in memory formation. Journal of Psychiatric Research 45, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Holsen LM, Aizley H, Remington A, Whitfield-Gabrieli S, Goldstein JM, Pizzagalli DA, 2015. Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol Psychiatry 78, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdeniz C, Tost H, Streit F, Haddad L, Wust S, Schafer A, Schneider M, Rietschel M, Kirsch P, Meyer-Lindenberg A, 2014. Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA psychiatry 71, 672–680. [DOI] [PubMed] [Google Scholar]
- Andela CD, van Haalen FM, Ragnarsson O, Papakokkinou E, Johannsson G, Santos A, Webb SM, Biermasz NR, van der Wee NJA, Pereira AM, 2015. Cushing’s syndrome causes irreversible effects on the human brain: a systematic review of structural and functional magnetic resonance imaging studies. Eur. J. Endocrinol 173, R1–R14. [DOI] [PubMed] [Google Scholar]
- Andrews J, Ali N, Pruessner JC, 2013. Reflections on the interaction of psychogenic stress systems in humans: The stress coherence/compensation model. Psychoneuroendocrinology 38, 947–961. [DOI] [PubMed] [Google Scholar]
- Arana GW, Santos AB, Laraia MT, McLeodbryant S, Beale MD, Rames LJ, Roberts JM, Dias JK, Molloy M, 1995. Dexamethasone for the treatment of depression - A randomized, placebo-controlled, double-blind trial. American Journal of Psychiatry 152, 265–267. [DOI] [PubMed] [Google Scholar]
- Boehringer A, Tost H, Haddad L, Lederbogen F, Wüst S, Schwarz E, Meyer-Lindenberg A, 2015. Neural correlates of the cortisol awakening response in humans. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos PA, Montoya ER, Terburg D, van Honk J, 2014. Cortisol administration increases hippocampal activation to infant crying in males depending on childhood neglect. Human brain mapping 35, 5116–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Lu H, Denniston D, Uh J, Thomas BP, Carmody TJ, Auchus RJ, Diaz-Arrastia R, Tamminga C, 2013. A randomized, placebo-controlled proof-of-concept, crossover trial of phenytoin for hydrocortisone-induced declarative memory changes. J Affect Disord 150, 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buades-Rotger M, Serfling G, Harbeck B, Brabant G, Kramer UM, 2016. Prednisolone increases neural reactivity to negative socio-emotional stimuli in healthy young men. European Neuropsychopharmacology 26, 1176–1189. [DOI] [PubMed] [Google Scholar]
- Dahm AS, Schmierer P, Veer IM, Streit F, Gorgen A, Kruschwitz J, Wust S, Kirsch P, Walter H, Erk S, 2017. The burden of conscientiousness? Examining brain activation and cortisol response during social evaluative stress. Psychoneuroendocrinology 78, 48–56. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F, 2005. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci 6, 463–475. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Margraf J, 2008. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: A novel therapeutic approach. European Journal of Pharmacology 583, 365–371. [DOI] [PubMed] [Google Scholar]
- de Voogd LD, Klumpers F, Fernandez G, Hermans EJ, 2017. Intrinsic functional connectivity between amygdala and hippocampus during rest predicts enhanced memory under stress. Psychoneuroendocrinology 75, 192–202. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC, 2009a. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage 47, 864–871. [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Engert V, Lue SD, Andrews J, Efanov SI, Beaudry T, Pruessner JC, 2014. Psychological, endocrine and neural responses to social evaluation in subclinical depression. Soc Cogn Affect Neurosci 9, 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Rexroth M, Wolff E, Duchesne A, Scherling C, Beaudry T, Lue SD, Lord C, Engert V, Pruessner JC, 2009b. Neural correlates of processing stressful information: An event-related fMRI study. Brain Res 1293, 49–60. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, 2004. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin 130, 355–391. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, Schulkin J, 2003. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and Biobehavioral Reviews 27, 233–246. [DOI] [PubMed] [Google Scholar]
- Fleischer J, Metz S, Dusenberg M, Grimm S, Golde S, Roepke S, Renneberg B, Wolf OT, Otte C, Wingenfeld K, 2019. Neural correlates of glucocorticoids effects on autobiographical memory retrieval in healthy women. Behavioural brain research 359, 895–902. [DOI] [PubMed] [Google Scholar]
- Gathmann B, Schulte FP, Maderwald S, Pawlikowski M, Starcke K, Schafer LC, Scholer T, Wolf OT, Brand M, 2014. Stress and decision making: neural correlates of the interaction between stress, executive functions, and decision making under risk. Experimental Brain Research 232, 957–973. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A, 2009. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology 34, 953–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, Klumpers F, Everaerd D, Kooijman SC, van Wingen GA, Fernandez G, 2015. Inter-individual differences in stress sensitivity: Basal and stress-induced cortisol levels differentially predict neural vigilance processing under stress. Social Cognitive Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, Pu ZW, Hermans EJ, van Wingen GA, Joels M, Fernandez G, 2012a. Dynamically changing effects of corticosteroids on human hippocampal and prefrontal processing. Human brain mapping 33, 2885–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joels M, Fernandez G, 2010. Time-dependent effects of corticosteroids on human amygdala processing. Journal of Neuroscience 30, 12725–12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joels M, Fernandez G, 2011. Time-dependent corticosteroid modulation of prefrontal working memory processing. Proceedings of the National Academy of Sciences of the United States of America 108, 5801–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joels M, Fernandez G, 2012b. Time-dependent effects of cortisol on selective attention and emotional interference: a functional MRI study. Frontiers in integrative neuroscience 6, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AY, Faude S, Nielsen SE, Locke M, Mather M, 2019. Effects of hormonal contraceptive phase and progestin generation on stress-induced cortisol and progesterone release. Neurobiol. Stress 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera AY, Nielsen SE, Mather M, 2016. Stress-induced increases in progesterone and cortisol in naturally cycling women. Neurobiol. Stress 3, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M, 2018. Corticosteroids and the brain. J. Endocrinol. 238, R121–R130. [DOI] [PubMed] [Google Scholar]
- Joels M, Sarabdjitsingh RA, Karst H, 2012. Unraveling the time domains of corticosteroid hormone influences on brain activity: Rapid, slow, and chronic modes. Pharmacol. Rev. 64, 901–938. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Brown ES, Wolkowitz OM, Sternberg EM, Bender BG, Bulloch K, Cidlowski JA, de Kloet ER, Fardet L, Joels M, Leung DYM, McEwen BS, Roozendaal B, Van Rossum EFC, Ahn J, Brown DW, Plitt A, Singh G, 2014. Adverse consequences of glucocorticoid medication: Psychological, cognitive, and behavioral effects. American Journal of Psychiatry 171, 1045–1051. [DOI] [PubMed] [Google Scholar]
- Kalafatakis K, Russell GM, Harmer CJ, Munafo MR, Marchant N, Wilson A, Brooks JC, Durant C, Thakrar J, Murphy P, Thai NJ, Lightman SL, 2018. Ultradian rhythmicity of plasma cortisol is necessary for normal emotional and cognitive responses in man. Proceedings of the National Academy of Sciences of the United States of America 115, E4091–E4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, 2017. Mechanisms underlying the early risk to develop anxiety and depression: A translational approach. European Neuropsychopharmacology 27, 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions’ of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Khalili-Mahani N, Dedovic K, Engert V, Pruessner M, Pruessner JC, 2010. Hippocampal activation during a cognitive task is associated with subsequent neuroendocrine and cognitive responses to psychological stress. Hippocampus 20, 323–334. [DOI] [PubMed] [Google Scholar]
- Kinner VL, Merz CJ, Lissek S, Wolf OT, 2016a. Cortisol disrupts the neural correlates of extinction recall. Neuroimage 133, 233–243. [DOI] [PubMed] [Google Scholar]
- Kinner VL, Wolf OT, Merz CJ, 2016b. Cortisol alters reward processing in the human brain. Hormones and Behavior 84, 75–83. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH, 1993. The Trier Social Stress Test - A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 28, 76–81. [DOI] [PubMed] [Google Scholar]
- Kogler L, Gur RC, Derntl B, 2015. Sex differences in cognitive regulation of psychosocial achievement stress: brain and behavior. Human brain mapping 36, 1028–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Seidel EM, Metzler H, Thaler H, Boubela RN, Pruessner JC, Kryspin-Exner I, Gur RC, Windischberger C, Moser E, Habel U, Derntl B, 2017. Impact of self-esteem and sex on stress reactions. Scientific Reports 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C, 2007. Ten years of research with the Trier Social Stress Test (TSST) – revisited, in: Harmon-Jones E, Winkielman P (Eds.), Social Neuroscience. Guilford Press, New York. [Google Scholar]
- Kukolja J, Klingmuller D, Maier W, Fink GR, Hurlemann R, 2011. Noradrenergic-glucocorticoid modulation of emotional memory encoding in the human hippocampus. Psychological Medicine 41, 2167–2176. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel C, Wolf O, Fink G, 2008. Increased cortisol levels in cognitively challenging situations are beneficial in young but not older subjects. Psychopharmacology (Berl) 201, 293–304. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2001. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings Technical report A-5. The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Lederbogen F, Kirsch P, Haddad L, Streit F, Tost H, Schuch P, Wüst S, Pruessner JC, Rietschel M, Deuschle M, 2011. City living and urban upbringing affect neural social stress processing in humans. Nature 474, 498–501. [DOI] [PubMed] [Google Scholar]
- Lewis AH, Porcelli AJ, Delgado MR, 2014. The effects of acute stress exposure on striatal activity during Pavlovian conditioning with monetary gains and losses. Frontiers in behavioral neuroscience 8, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighthall NR, Sakaki M, Vasunilashorn S, Nga L, Somayajula S, Chen EY, Samii N, Mather M, 2012. Gender differences in reward-related decision processing under stress. Soc Cogn Affect Neurosci 7, 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman SL, Conway-Campbell BL, 2010. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 11, 710–718. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, Heitland I, Hermann A, Kuhn M, Kruse O, Drexler SM, Meulders A, Nees F, Pittig A, Richter J, Romer S, Shiban Y, Schmitz A, Straube B, Vervliet B, Wendt J, Baas JMP, Merz CJ, 2017. Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience and Biobehavioral Reviews 77, 247–285. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Robinson JL, Glahn DC, Fox PT, 2010. Acute effects of hydrocortisone on the human brain: an fMRI study. Psychoneuroendocrinology 35, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luettgau L, Schlagenhauf F, Sjoerds Z, 2018. Acute and past subjective stress influence working memory and related neural substrates. Psychoneuroendocrinology 96, 25–34. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Ma ST, Abelson JL, Okada G, Taylor SF, Liberzon I, 2017. Neural circuitry of emotion regulation: Effects of appraisal, attention, and cortisol administration. Cognitive Affective & Behavioral Neuroscience 17, 437–451. [DOI] [PubMed] [Google Scholar]
- Maier SU, Makwana AB, Hare TA, 2015. Acute stress impairs self-control in goal-directed choice by altering multiple functional connections within the brain’s decision circuits. Neuron 87, 621–631. [DOI] [PubMed] [Google Scholar]
- Mareckova K, Holsen L, Admon R, Whitfield-Gabrieli S, Seidman LJ, Buka SL, Klibanski A, Goldstein JM, 2017. Neural - hormonal responses to negative affective stimuli: Impact of dysphoric mood and sex. Journal of Affective Disorders 222, 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, Thomas C, Waters P, 2010. Investigations of HPA function and the enduring consequences of stressors in adolescence in animal models. Brain and Cognition 72, 73–85. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Hamacher-Dang TC, Stark R, Wolf OT, Hermann A, 2018. Neural underpinnings of cortisol effects on fear extinction. Neuropsychopharmacology 43, 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Hermann A, Stark R, Wolf OT, 2014. Cortisol modifies extinction learning of recently acquired fear in men. Social cognitive and affective neuroscience 9, 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT, 2010. Investigating the impact of sex and cortisol on implicit fear conditioning with fMRI. Psychoneuroendocrinology 35, 33–46. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT, 2012a. Neuronal correlates of extinction learning are modulated by sex hormones. Social cognitive and affective neuroscience 7, 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT, 2012b. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Hormones and Behavior 62, 531–538. [DOI] [PubMed] [Google Scholar]
- Merz CJ, Wolf OT, Schweckendiek J, Klucken T, Vaitl D, Stark R, 2013. Stress differentially affects fear conditioning in men and women. Psychoneuroendocrinology 38, 2529–2541. [DOI] [PubMed] [Google Scholar]
- Ming QS, Zhong X, Zhang XC, Pu WD, Dong DF, Jiang YL, Gao YD, Wang X, Detre JA, Yao SQ, Rao HY, 2017. State-independent and dependent neural responses to psychosocial stress in current and remitted depression. American Journal of Psychiatry 174, 971–979. [DOI] [PubMed] [Google Scholar]
- Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J, 2014. Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology 47, 31–42. [DOI] [PubMed] [Google Scholar]
- Montoya ER, van Honk J, Bos PA, Terburg D, 2015. Dissociated neural effects of cortisol depending on threat escapability. Human brain mapping 36, 4304–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlhan M, Lueken U, Wittchen HU, Kirschbaum C, 2011. The scanner as a stressor: evidence from subjective and neuroendocrine stress parameters in the time course of a functional magnetic resonance imaging session. International journal of psychophysiology : official journal of the International Organization of Psychophysiology 79, 118–126. [DOI] [PubMed] [Google Scholar]
- Oei NYL, Both S, van Heemst D, van der Grond J, 2014. Acute stress-induced cortisol elevations mediate reward system activity during subconscious processing of sexual stimuli. Psychoneuroendocrinology 39, 111–120. [DOI] [PubMed] [Google Scholar]
- Oei NYL, Elzinga BM, Wolf OT, de Ruiter MB, Damoiseaux JS, Kuijer JPA, Veltman DJ, Scheltens P, Rombouts S, 2007. Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain imaging and behavior 1, 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NYL, Jansen SW, Veer IM, Slagboom PE, van de Grond J, van Heemst D, 2018. Stress evokes stronger medial posterior cingulate deactivations during emotional distraction in slower paced aging. Biological psychology 135, 84–92. [DOI] [PubMed] [Google Scholar]
- Oei NYL, Veer IM, Wolf OT, Spinhoven P, Rombouts S, Elzinga BM, 2012. Stress shifts brain activation towards ventral ‘affective’ areas during emotional distraction. Soc Cogn Affect Neurosci 7, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orem TR, Wheelock MD, Goodman AM, Harnett NG, Wood KH, Gossett EW, Granger DA, Mrug S, Knight DC, 2019. Amygdala and prefrontal cortex activity varies with individual differences in the emotional response to psychosocial stress. Behav. Neurosci. 133, 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli AJ, Lewis AH, Delgado MR, 2012. Acute stress influences neural circuits of reward processing. Frontiers in neuroscience 6, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S, 2008. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance Imaging studies. Biol Psychiatry 63, 234–240. [DOI] [PubMed] [Google Scholar]
- Putman P, Roelofs K, 2011. Effects of single cortisol administrations on human affect reviewed: Coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology 36, 439–448. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G, 2009. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry 66, 25–32. [DOI] [PubMed] [Google Scholar]
- Radke S, Seidel EM, Boubela RN, Thaler H, Metzler H, Kryspin-Exner I, Moser E, Habel U, Derntl B, 2018. Immediate and delayed neuroendocrine responses to social exclusion in males and females. Psychoneuroendocrinology 93, 56–64. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, De Kloet ER, 1986. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. Journal of Steroid Biochemistry 24, 269–272. [DOI] [PubMed] [Google Scholar]
- Root JC, Tuescher O, Cunningham-Bussel A, Pan H, Epstein J, Altemus M, Cloitre M, Goldstein M, Silverman M, Furman D, Ledoux J, McEwen B, Stern E, Silbersweig D, 2009. Frontolimbic function and cortisol reactivity in response to emotional stimuli. Neuroreport 20, 429–434. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Griffith QK, Buranday J, de Quervain DJF, McGaugh JL, 2003. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: Dependence on the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America 100, 1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S, 2009. Stress, memory and the amygdala. Nat. Rev. Neurosci 10, 423–433. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL, 2004. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. Journal of Neuroscience 24, 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Poldrack RA, 2011. Mind the gap: bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn. Sci. 15, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT, 2012. Stress modulates the engagement of multiple memory systems in classification learning. Journal of Neuroscience 32, 11042–11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfling G, Buades-Rotger M, Harbeck B, Kramer UM, Brabant G, 2019. The corticosteroid prednisolone increases amygdala and insula reactivity to food approach signals in healthy young men. Psychoneuroendocrinology 99, 154–165. [DOI] [PubMed] [Google Scholar]
- Shermohammed M, Mehta PH, Zhang J, Brandes CM, Chang LJ, Somerville LH, 2017. Does psychosocial stress impact cognitive reappraisal? Behavioral and neural evidence. Journal of Cognitive Neuroscience 29, 1803–1816. [DOI] [PubMed] [Google Scholar]
- Stark R, Wolf OT, Tabbert K, Kagerer S, Zimmermann M, Kirsch P, Schienle A, Vaitl D, 2006. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. Neuroimage 32, 1290–1298. [DOI] [PubMed] [Google Scholar]
- Streit F, Haddad L, Paul T, Frank J, Schafer A, Nikitopoulos J, Akdeniz C, Lederbogen F, Treutlein J, Witt S, Meyer-Lindenberg A, Rietschel M, Kirsch P, Wust S, 2014. A functional variant in the neuropeptide S receptor 1 gene moderates the influence of urban upbringing on stress processing in the amygdala. Stress (Amsterdam, Netherlands) 17, 352–361. [DOI] [PubMed] [Google Scholar]
- Sudheimer KD, Abelson JL, Taylor SF, Martis B, Welsh RC, Warner C, Samet M, Manduzzi A, Liberzon I, 2013. Exogenous glucocorticoids decrease subgenual cingulate activity evoked by sadness. Neuropsychopharmacology 38, 826–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, Stark R, 2010. Cortisol enhances neural differentiation during fear acquisition and extinction in contingency aware young women. Neurobiology of Learning and Memory 94, 392–401. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP, 2009. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, Fruchter E, Wald I, Pine DS, Tarrasch R, Bar-Haim Y, Hendler T, 2013. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Frontiers in human neuroscience 7, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Peer JM, Spinhoven P, Roelofs K, 2010. Psychophysiological evidence for cortisol-induced reduction in early bias for implicit social threat in social phobia. Psychoneuroendocrinology 35, 21–32. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Roozendaal B, Kindt M, Wolf OT, Joels M, 2010. Interacting noradrenergic and corticosteroid systems shift human brain activation patterns during encoding. Neurobiology of Learning and Memory 93, 56–65. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts S, 2007. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiology of Learning and Memory 87, 57–66. [DOI] [PubMed] [Google Scholar]
- Vogel S, Klumpers F, Schroder TN, Oplaat KT, Krugers HJ, Oitzl MS, Joels M, Doeller CF, Fernandez G, 2016. Stress induces a shift towards striatum-dependent stimulus-response learning via the mineralocorticoid receptor. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G, 2007. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia 45, 174–194. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA, 2007. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci 2, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA, 2005. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proceedings of the National Academy of Sciences of the United States of America 102, 17804–17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KS, Smith DV, Delgado MR, 2016. Using fMRI to study reward processing in humans: past, present, and future. J. Neurophysiol 115, 1664–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon AL, Hagan M, Van Meter A, Jacobs RH, Kassel MT, Hazlett KE, Haase BD, Vederman AC, Avery E, Briceno EM, Welsh RC, Zubieta JK, Weisenbach SL, Langenecker SA, 2015. Stress response to the functional magnetic resonance imaging environment in healthy adults relates to the degree of limbic reactivity during emotion processing. Neuropsychobiology 71, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MD, Harnett NG, Wood KH, Orem TR, Granger DA, Mrug S, Knight DC, 2016. Prefrontal cortex activity is associated with biobehavioral components of the stress response. Frontiers in human neuroscience 10, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.