Abstract
Methamphetamine (METH) use disorder (MUD) is often accompanied by psychotic symptoms, cognitive deficits, and pathological changes in the brains of users. Animals that experimenters injected with drugs also show neurodegenerative changes in their brains. Recently, we have been investigating METH-induced molecular and biochemical consequences in animals that had infused themselves with METH using the drug self-administration (SA) paradigm. In that model, footshocks administered contingently help to separate rats that had already escalated their METH intake into resilient-to-drug (shock-sensitive, SS) or compulsive (shock-resistant, SR) METH takers. Herein, we used that model to test the idea that compulsive METH takers might show evidence of drug-induced autophagic changes in their brains. There were significant increases in mRNA levels of autophagy-related genes including Atg2a, Atg5, Atg14, and Atg16L1 in the rat dorsal striatum. Levels of two autophagy biomarkers, autophagy activating kinase (ULK1) and phospho-Beclin1, were also increased. In addition, we found increased p53 but decreased Bcl-2 protein levels. Moreover, the expression of cleaved initiator caspase-9 and effector caspase-6 was higher in compulsive METH takers in comparison to shock-sensitive rats. When taken together, these results suggest that the striata of rats that had escalated and continue to take METH compulsively the presence of adverse consequences exhibit some pathological changes similar to those reported in post-mortem human striatal tissues. These results provide supporting evidence that compulsive METH taking is neurotoxic. Our observations also support the notion of developing neuro-regenerative agents to add to the therapeutic armamentarium against METH addiction.
Keywords: substance use disorder, punishment, self-administration, addiction, transcription factors
Introduction
Methamphetamine (METH) is the third most commonly abused illicit substance in the world (UNODC 2018). Repeated use of METH can result in METH use disorder (MUD) (Courtney and Ray 2014) that is accompanied by various psychiatric and neurological signs and symptoms (Cadet and Bisagno 2015; DSM5 2013). The manifestations of MUD depend on interactions of brain regions that subsume reward, memory, and habit formation (Balleine and O’Doherty 2010; Knowlton and Patterson 2018). Among these brain areas, the dorsal striatum is involved in habit forming (Cadet et al. 2015; Everitt and Robbins 2016; Hu et al. 2019) and appears to regulate some of the behavioral manifestations of incubation of METH seeking after long intervals of forced drug withdrawal (Caprioli et al. 2017; Rubio et al. 2015; Torres et al. 2017).
In addition to causing addiction, the use of METH is associated with neurodegenerative consequences in animals (Krasnova and Cadet 2009) and humans (Cadet et al. 2014). In fact, rats that are given moderate to large doses of METH suffer from striatal dopamine (DA) depletion and loss of DA transporters (DAT) and serotonin (5-HT) transporters (SERT) (Armstrong and Noguchi 2004; Bittner et al. 1981; Cappon et al. 2000; Fukumura et al. 1998; Guilarte et al. 2003). SERT (Sekine et al. 2006) and DAT (Volkow et al. 2001) deficits are also observed in the brains of human METH users. Similarly, self-administration of METH for 15 hours per day is also associated with depletion of striatal DA, DAT, 5HT, and tyrosine hydroxylase (TH) (Krasnova et al. 2010). Together, these observations support the view that METH is neurotoxic (Cadet et al. 2007).
In addition to the toxic effects of monoaminergic terminals, moderate to large doses of METH can also cause cell death (Cadet et al. 2003) by activating apoptotic pathways in the mammalian brain (Jayanthi et al. 2001; Jayanthi et al. 2004). There is also some evidence that METH might cause autophagic changes in the brain (Pasquali et al. 2008; Roohbakhsh et al. 2016; Xu et al. 2018; Yang et al. 2019). The observations that METH can cause apoptotic and autophagic consequences are consistent with the fact that there exist substantial cross talks between these two processes that are regulated by some common factors (Levine et al. 2005; Maiuri et al. 2007). Autophagy is a conserved and highly regulated process that cells use to degrade cytoplasmic contents (Levine and Kroemer 2008). Impaired regulation of autophagy is associated with protein aggregations observed in neurodegenerative disorders (Nixon and Yang 2011).
METH-induced autophagic changes were first reported by Larsen et al. (2002) who demonstrated that exposure to METH was accompanied by the formation of autophagic granules. Later, Castino et al. (2008) reported that low doses of METH also cause autophagosomes in a cell culture system. Interestingly, pharmacological and genetic inhibition of autophagy in rat dopaminergic cells led to METH-induced apoptosis via caspase-3 activation (Lin et al. 2012), suggesting that autophagic responses might hinder METH-induced apoptosis in their system. This conclusion is consistent, in part, with the demonstration that overexpression of LC3-II (microtubule-associated light chain 3), an autophagy regulatory protein, could protect against METH-induced cell death (Lin et al. 2012). However, there are also reports that inhibition of autophagy via mTOR (negative-regulator of autophagy) might actually reduce METH-induced cell death (Kongsuphol et al. 2009; Li et al. 2012). The latter inferences were supported by a paper that reported that autophagy was an early response in the steps that lead to METH-induced apoptosis (Xu et al. 2018). Because the data on METH-induced autophagic changes were obtained mostly in cell culture systems, this left unanswered the question of whether similar occurrences might be evident in animal models that better mimic human METH use disorder.
Therefore, to further test the possibility that METH can also cause activation of autophagic markers in vivo, we used a model of METH self-administration and measured the expression of autophagy-related genes (Atg), two autophagy protein markers-Beclin and ULK1, and some markers of neuronal damage in the dorsal striatum of rats that had been taking METH compulsively. Herein we report that METH does indeed induce activation of several markers of autophagy. Our results support the notion of using neuroprotective agents as parts of the therapeutic armamentarium against METH addiction in humans (Mohammad Ahmadi Soleimani et al. 2016).
Materials and Methods
Animals and drug treatment
We used male Sprague-Dawley rats (Charles River Labs, Raleigh, NC, USA), weighing 350–400 g that were housed in a humidity and temperature-controlled (22.2± 0.2 °C) room with free access to food and water. Our procedures followed the Guide for the Care and Use of Laboratory Animals (ISBN 0–309–05377–3) and were approved by the National Institute of Drug Abuse Animal Care and Use Committee.
Intravenous surgery
We anesthetized rats with ketamine and xylazine (50 and 5mg/kg, i.p., respectively) and inserted silastic catheters into the jugular veins as described previously (Cadet et al. 2017). We injected buprenorphine (0.1mg/kg, s.c.) one time after surgery to relieve pain and allowed the rats to recover for 5–10 days before METH self-administration training. During the recovery period, the rats were monitored for health, handled daily, and the catheters were flushed every 24–48h with gentamicin (Butler Schein; 5mg/ml) and sterile saline.
Training and punishment phases
We performed the training procedure for METH self-administration as previously described (Cadet et al. 2017; Krasnova et al. 2014). Briefly, we brought rats to the self-administration room on the first day of testing and chronically housed them in self-administration chambers. Animals had free access to food and water that were available in water bottles and feeders hanging on the walls of all self-administration chambers. We trained rats to self-administer dl-METH HCl (0.1 mg/kg/infusion over 3.5 s; 0.1ml/infusion). during three 3-h sessions/day (the sessions were separated by 30min off intervals) for 21 days using a fixed-ratio-1 schedule with 20-s timeouts. At the end of each 3-h session, the house light was turned off and the active lever was retracted. Active lever presses were accompanied by a 5-s compound tone-light cue. Presses on inactive (stationary) lever had no programmed consequences. We connected the catheters of rats to a modified cannula (Plastics One, Minneapolis, MN) attached to a liquid swivel (Instech Laboratories, Inc., Plymouth Meeting, PA, USA) via polyethylene-50 tubing that was protected by a metal spring. We trained rats in sets of 5 days of METH self-administration with 2 days off in order to minimize weight loss. During off days, rats remained housed in self-administration chambers but were disconnected from intravenous self-administration lines. Control rats self-administered saline under the same conditions.
During the punishment phase, rats continued METH self-administration under identical conditions describe above. In addition, 50% of the reinforced lever-presses for METH resulted in the concurrent delivery of a 0.5-s footshock through the grid floor. We set the footshock current at 0.18mA for punishment Day 1, 0.24mA for Day 2, 0.30mA for Day 3 to Day 5, and 0.36 mA for Day 6 to Day 8. The application of this range of shock intensity has previously helped to separate rats into shock-resistant and shock-sensitive animals (Cadet et al. 2017; Torres et al. 2017). Importantly, some animals that self-administered saline received a non-contingent footshock each time the paired animals in the METH SA group received a contingent shock. These animals served as controls for the effects of only shocks on molecular and biochemical markers in the dorsal striatum. Thus, by the end of the behavioral experiments, there were separate groups of rats that were yoked to the corresponding shock-sensitive and -resistant rats, namely yoked SS (YSS) and yoked SR (YSR), respectively. YSR rats received different number of shocks than YSS rats by the end of the shock phase of the experiment.
RNA preparation
Two hours after the last day of self-administration and footshocks, we euthanized the rats by decapitation with guillotine and isolated dorsal striatum samples from the brains. Total RNA was extracted from individual dorsal striatum samples using Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, USA). We assessed RNA integrity using an Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA, USA); RNA samples showed no degradation.
Quantitative RT-PCR analysis of mRNA levels
We reversed-transcribed individual total RNA from Control group (n=10), SR group (n=8), SS group (n=7), YSR group (n=8), and YSS (n=7) into cDNA using Advantage RT-for-PCR kit (Clontech, Mountain View, CA, USA). We generated PCR primers using LightCycler probe design software v. 2.0 (Roche Biosystems, Indianapolis, IN, USA) and purchased gene-specific primers from the Synthesis and Sequencing Facility of Johns Hopkins University (Baltimore, MD, USA). Five hundred nanograms (500 ng) of RNA were reverse-transcribed with oligo dT primers using Advantage RT-for-PCR kit (Clontech). RT-qPCR was performed with Roche LightCycler 480 II using iQ SYBR Green Supermix (Bio-Rad). We normalized the relative amounts of mRNA in each sample to means of OAZ1 and Beta-2 microglobulin (B2M) mRNA. The list of primers used are given in Table S1.
Western Blot
Tissues were homogenized using 10mM Tris HCl, 150 mM NaCl, pH 7.5 in the presence of 1% Nonidet P-40 (NP-40) protein, and phosphatase inhibitor cocktails (Sigma, St. Louis, MO). Total protein concentrations were quantified using BCA assay (Thermofisher Scientific, Waltham, MA). Twenty μg of soluble protein lysate were prepared in solutions that contained Laemmli buffer and 5% β-Mercaptoethanol. Samples were then boiled and resolved using NuPage 10% Bis-Tris Protein Gels (ThermoFisher Scientific, Waltham, MA). Protein were electrophoretically transferred onto PVDF membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 5% BSA in TBST and incubated overnight with primary antibodies at dilutions described by the manufacturer. Primary rabbit polyclonal antibodies including anti-Bcl2 (1:1000; # 2876); anti-ULK1 (1:10000; # 8054); anti-Beclin1 (1:1000; # 3495); anti-phosphoBeclin1 (1:1000; # 14717); anti-cleaved caspase 9 (1:1000; # 9507); anti-cleaved caspase 6 (1:1000; # 9762) and anti-cleaved caspase 3 (1:1000; # 9661) were purchased from Cell Signaling Technologies. Anti-p53 (1:1000; # AHO0132) was purchased from Biosource International. α-Tubulin mouse monoclonal antibody was purchased from Sigma-Aldrich (1:10000, T6074). The antibodies revealed bands at the expected molecular weights for all proteins. Anti-rabbit HRP (1:5000 #7074) and anti-mouse HRP (1:5000 #7076) secondary antibodies were purchased from Cell Signaling Technologies. Anti-Goat HRP (1:5000 sc-2020) was purchased from Santa-Cruz. After secondary antibody incubation, ECL Clarity (Bio-Rad, Hercules, CA) was used to detect bands on ChemiDoc Touch Imaging System (Bio-Rad. Hercules, CA), and intensities were measured with Image Lab 6.0 version (Bio-Rad, Hercules, CA) software.
Statistical Analysis
We analyzed the behavioral data with the statistical program GraphPad Prism and followed significant effects (p< 0.05) with post-hoc contrasts with the repeated measures ANOVA. For the training and shock phases, the dependent variables were the number of METH or saline infusions during 21 training days and 8 foot-shock days. Student’s t-test were used to examine the total METH intake between the two groups compared. We analyzed RT-PCR data and western blot intensities by one-way ANOVA followed by Fischer’s protected least-significant difference test (PLSD) using StatView (version 4.02, SAS Institute, Cary, NC, USA). The null hypothesis was rejected at p<0.05.
Results
Footshock punishment dichotomizes METH self-administering rats into two distinct phenotypes
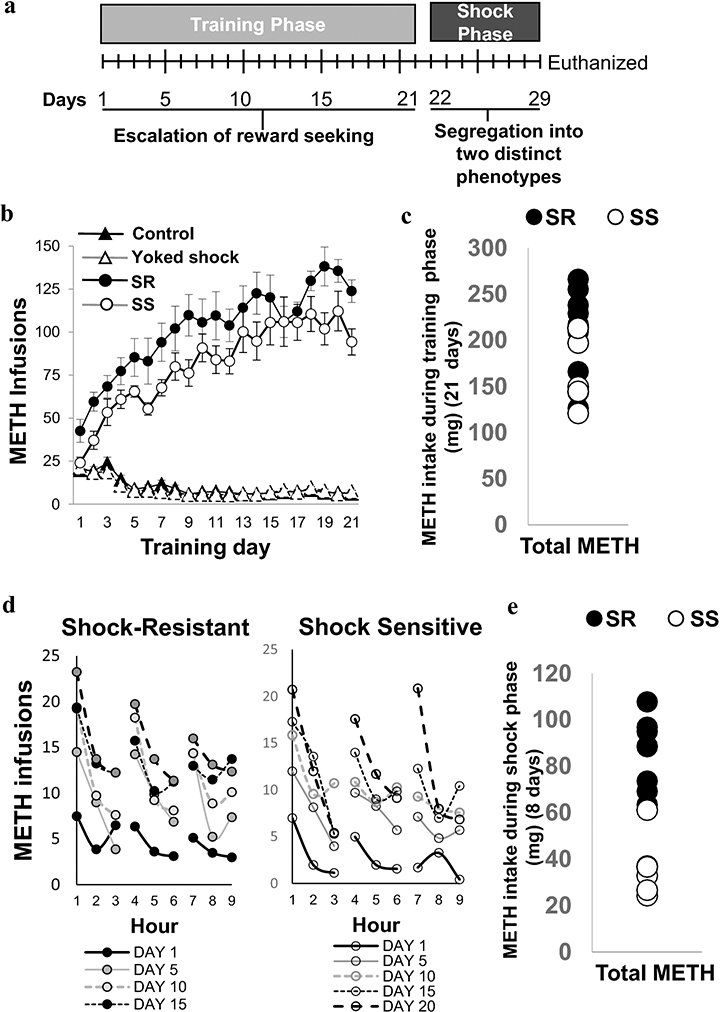
Fig. 1a illustrates the timeline of the present study. Fig. 1b shows the number of METH infusions taken by the rats that self-administered METH (0.1 mg/kg/infusion) for 21 days. Animals in the control (CT, n = 10) and yoked (n = 15) groups self-administered saline. As we previously reported (Cadet et al. 2017; Torres et al. 2017), METH SA rats (n = 15) escalated their METH intake during the training phase (Fig. 1b). Two-way repeated measures ANOVA comparing METH rats to control rats showed significant effects of groups [F (1, 36) = 311.2, p<0.0001], training days [ F (20, 720) = 31.39, p<0.0001], and group x training days interactions [F (20, 720) = 60.54, p<0.0001]. During the 8 days of footshocks or punishment phase, some rats significantly decreased their METH intake (resilient or shock-sensitive, SS) whereas others continued to compulsively press the active lever for METH (compulsive or shock-resistant, SR). Animals that fell post facto into SR (n = 8) and SS (n = 7) groups showed no significant differences in METH intake during the 21 days of METH SA training before footshocks [F (1, 13) = 1. 173, p = 0.08] as per our previous results (Cadet et al., 2017) (Fig. 1c). Fig. 1d illustrates that SR and SS rats took most of the METH infusions within the first hour of each 3-hr interval during SA sessions, thus suggesting that all rats took METH in a binge pattern. These observations are similar to reports that humans self-administer METH in binges (Cheng et al. 2010; Cho and Melega 2002). Fig. 1e illustrates the fact that SR rats took significant more METH than the SS group [F (1, 13) = 6.821, p < 0.0001] during the shock phase. The total METH intake after shock was 85.41 ± 5.38 mg for the SR group and was 36.51 ± 4.54 mg for the SS group. Two-way repeated measures for METH earned included the between-subject factors (treatment groups, SR vs SS) and within-subject factor (shock days), and their interactions. We found significant effects of treatment groups [F(1,13) = 46.53, p<0.0001], shock days [F(7,91) = 6.654, p<0.0001], and the interaction of the two [F(7,91) = 9.348, p<0.0001].
Figure 1. Behavioral effects of extended access to METH SA training and contingent footshock punishment.
(A) Experimental timeline for METH SA included a training phase of 21 days and a shock phase for 8 days. (B) Rats with long access to METH escalate drug intake and increased their METH intake steeply during the first 10 days. METH intake plateaued thereafter. (C) Total METH intake of the shock-resistant (SR, n = 8, denoted in black circles) was similar to that of shock-sensitive (SS, n = 7, denoted in white circles) rats during the 21-day training phase. (D) The patterns of METH infusions during the 3-hr sessions revealed that rats in both SR and SS phenotypes took more METH during the first hour of each session. (E) During the punishment phase, total METH intake of the SR was significantly greater (p< 0.0001) than that of SS rats.
SR and YSR rats received more footshocks (61.64±1.17 shocks) than SS and YSS (25.54±4.92 shocks) rats. It was important to attempt to identify molecular and biochemical consequences of footshocks because footshocks have been shown to influence the striatal expression of some genes including FMO2 (flavin-containing monooxygenase) and PDK4 (pyruvate dehydrogenase kinase 4) (Krasnova et al. 2017). We therefore conducted all the biochemical studies in all the 5 groups that included control (CT), YSR, YSS, SR, and SS rats.
Autophagy-related gene (Atg) mRNA expression and autophagy protein biomarkers are altered in compulsive METH-taking rats
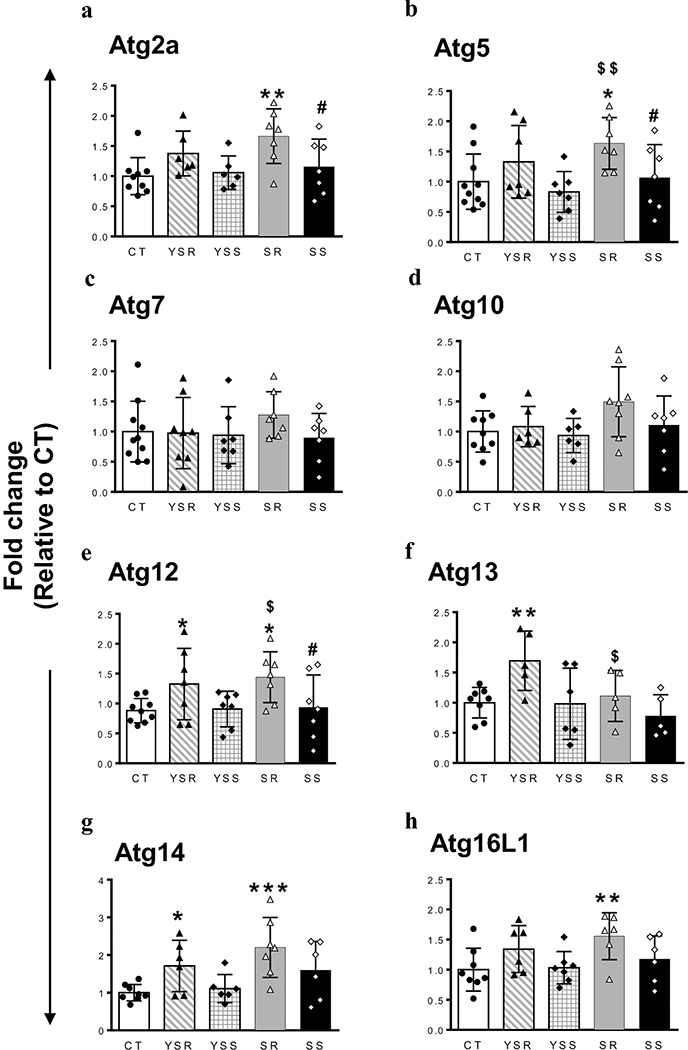
As discussed above, several groups had reported that METH can impact autophagic processes in either cell cultures or animals that experimenters injected with METH (Larsen et al. 2002; Li et al. 2017; Pasquali et al. 2008; Roohbakhsh et al. 2016; Xu et al. 2018; Yang et al. 2019). In our study, we used, for the first time, a self-administration model that is more akin to human conditions because rats self-administer the drug. In addition, we compared compulsive METH takers (SR) to rats that had decreased their METH intake in response to adverse consequences (SS rats). We used the model to measure the expression of several Atg mRNAs in the dorsal striatum because METH negatively impacts that brain region in both rodents and humans (Krasnova and Cadet 2009; Volkow et al. 2001). One-way ANOVA showed that exposure to METH SA was associated with significant changes in Atg2a [F(4,27) = 6.971, P = 0.0005; Fig. 2a] and Atg5 [F(4,33) = 3.072, P = 0.0295; Fig. 2b] mRNA levels, with SR rats showing increased expression in comparison to SS and CT rats. There were also significant changes in Atg12 mRNA [F(4,32) = 2.794, P = 0.0426; Fig. 2c]. These changes were due to increased expression in the SR group in comparison to the SS and CT groups. Atg14 [F(4,28) = 4.633, P = 0.0054; Fig. 2g] and Atg16L1 [F(4,28), P = 0.0488; Fig. 2h]] mRNA levels were also significantly altered. Interestingly, rats that were yoked to the SR group (YSR) showed significant increases in Atg12, Atg13, and Atg14 mRNA levels (Figs. 2e-g), suggesting that large number of footshocks might have activated the expression of these genes. It is also to be noted that YSR rats showed a trend but no significant increases in Atg2a (P = 0.07) and Atg5 (P = 0.17) mRNA levels in comparison to controls (Figs. 2a-b). The fact that Atg2a and Atg5 mRNA levels showed an increasing trend in the YSR rats suggests that footshocks might have contributed to the significant increases in their expression observed in SR rats. No significant changes were observed in the expression of Atg7 and Atg10 transcripts in any group (see Figs. 2c and 2d).
Figure 2. Differential changes in striatal mRNA expression of autophagy-related genes after METH SA and footshocks.
METH SA and footshocks are associated with increased Atg2a (A), Atg5 (B) and Atg12 (E) mRNA levels in the dorsal striatum of SR rats in comparison to control and SS rats. (C and D) Striatal Atg7 and Atg10 mRNA levels show no significant changes in any of the group. (F) Striatal Atg13 mRNA expression was increased only in YSR rats. (G) Striatal Atg14 mRNA expression showed increases in both YSR and SR rats. (H) Atg16L mRNA levels were increased only in SR rats. The values in bar graphs represent means ± SEM (n = 7–10 animals per group). Key to statistics: *, **, *** = p < 0.05, 0.01, 0.001, respectively, in comparison to control rats; #, ##p < 0.05, 0.01, respectively, in comparison to SR rats; $, $ $ p < 0.05, 0.01, respectively, in comparison to YSR.
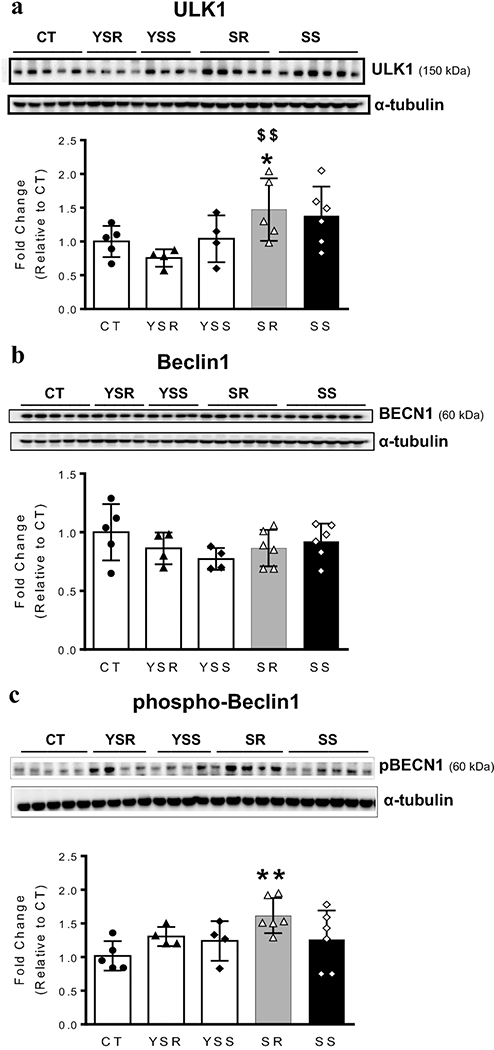
To test if proteins known to interact with ATGs were also affected, we performed Western blot analyses using antibodies specific for Beclin1 (BECN1), phospho-Beclin1 (pBECN1), and its upstream protein regulator, unc51-like autophagy activating kinase1 (ULK1). Significant increases in ULK1 protein levels [F(4,18) =3.133, P = 0.0388; Fig. 3a] were observed in SR animals. There were, however, no significant changes in total BECN1 protein expression [F(4,20) =1.165, P = 0.3558; Fig. 3b]. Importantly, the abundance of phosphorylated BECN1 (pBECN1) in SR rats was increased [F (4,20) =2.866, P = 0.05; Fig. 3c] compared to control rats. These two markers were not significantly impacted in any other group.
Figure 3. Compulsive METH takers exhibit increased expression of autophagy activating kinase (ULK1) and abundance of phosphorylated Beclin1 (pBECN1) protein levels in the rat dorsal striatum.
(A, B, C) Quantitative measures and representative images show respective results of western blot analyses for ULK1, BECN1, and pBECN1 proteins in the rat dorsal striatum. Compulsive METH taking rats (SR) show increased ULK1 (A) and pBECN1 (C) protein abundance. (B) There were no significant changes in BECN1 protein levels in any group. For quantification, Western blotting for ULK1, BECN1, and pBECN1 proteins were normalized to α-tubulin and then analyzed. The values represent means ± SEM (n=4 −6 rats per group). Key to statistics: *, **, *** = p < 0.05, 0.01, 0.001, respectively, in comparison to control rats; #, ##p < 0.05, 0.01, respectively, in comparison to SR rats; $, $ $ p < 0.05, 0.01, respectively, in comparison to YSR.
Upregulation of p53 and downregulation of Bcl2 in the striatum of compulsive METH-taking rats
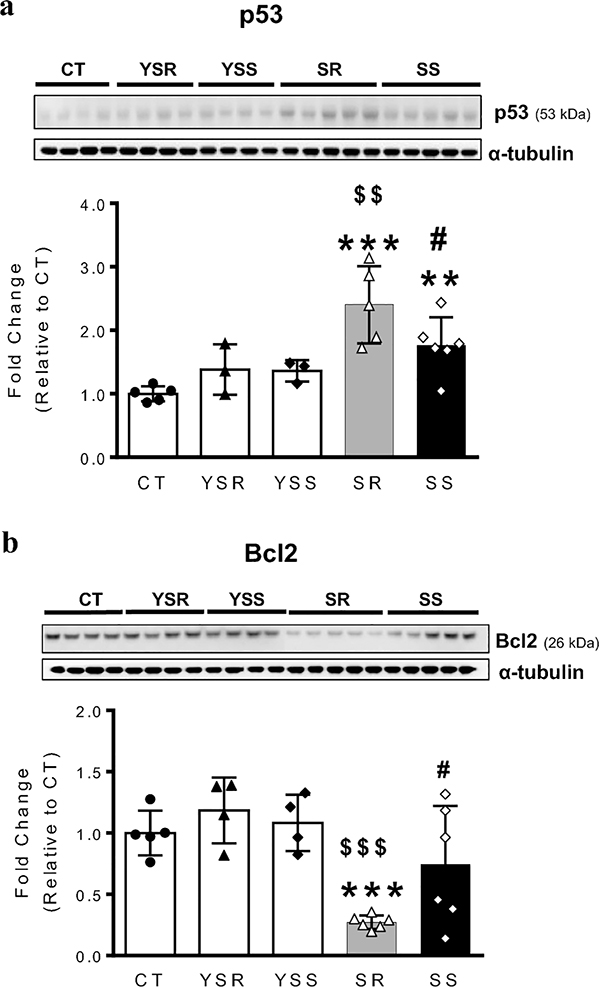
Previous studies have reported that exposure to METH can result in autophagic and apoptotic changes (Cadet et al. 2003; Pasquali et al. 2008). Studies from this lab and others have also reported that METH injections can cause increases in p53 but decreases in Bcl2 protein levels in rodents (Imam et al. 2001; Jayanthi et al. 2001). Interestingly, p53-DNA binding activity in the brain is also increased after METH exposure (Asanuma et al. 2002), suggesting increased p53-dependent transcriptional activity. Moreover, the levels of BECN1 and BCL2 have also been reported to be key determinants of whether cells undergo apoptosis or autophagy (Marquez and Xu 2012) and there is convincing evidence that p53 can regulate autophagic processes (Cordani et al. 2017; White 2016). We thus decided to measure p53 and Bcl2 expression in the METH SA rats. As shown in Fig. 4a, there were significant increases [F(4,17) = 0.8040, P = 0.008] in p53 protein levels in the SR and SS groups in comparison to the control group (Fig. 4a). The p53 levels in SR were also significantly higher than those in the METH SS group (p <0.01; Fig. 4A), indicating that larger amounts of METH caused greater increases in striatal p53 protein expression. In contrast, BCL2 protein levels were significantly decreased [F(4,20) = 8.363, P = 0.0004] only in the SR group (Fig. 4b). Neither p53 nor Bcl2 protein levels were affected in the yoked animals that received only footshocks (Fig. 4).
Figure 4. Compulsive METH takers exhibit increased p53 but decreased Bcl2 protein levels in the dorsal striatum.
(A) Differential expression of p53 protein levels in the SR and SS groups. (B) Bcl2 protein levels are decreased only in compulsive METH takers. α-tubulin was used for normalization. Key to statistics: *** P<0.001 vs. CT; $ $ P<0.01 vs. YSR; # P<0.05 vs. SR.
Regulation of caspase proteins in METH SA rats
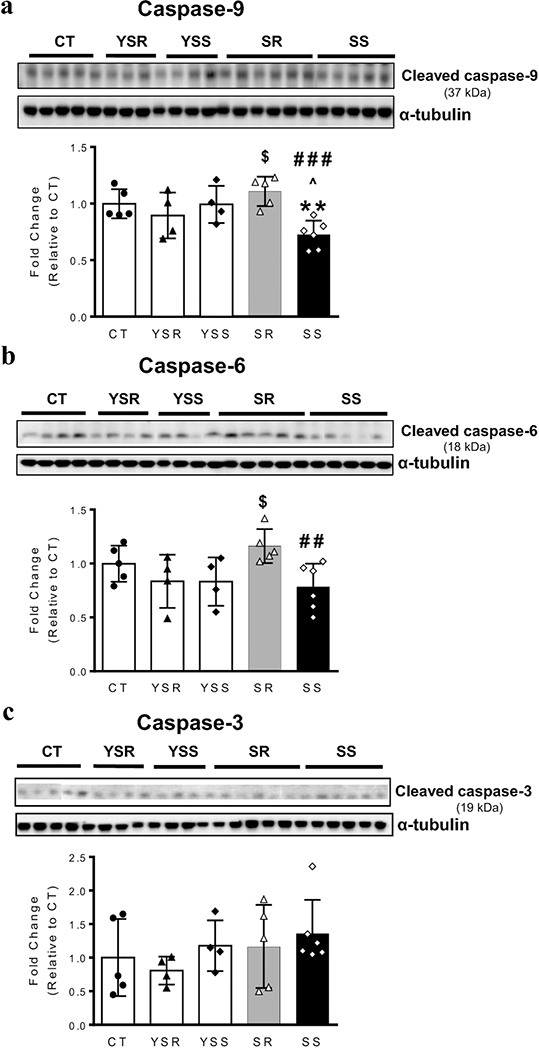
Progression to apoptosis or autophagy involves interactions of proteins that include ATG proteins and caspases (Booth et al. 2019; Shalini et al. 2015; Wu et al. 2014). We have shown previously that METH neurotoxicity is associated with increased activation of caspase-9, −6 and −3 (Cadet et al. 2005; Deng et al. 2002; Jayanthi et al. 2005; Jayanthi et al. 2004). We thus opted to test the idea that METH SA might also be associated with activation of these caspases (Fig. 5). One-way ANOVA revealed significant effects [F(4,19) = 5.157, P = 0.0055] on the cleavage of the initiator caspase 9 (Fig. 5a), with there being lower levels of cleaved caspase-9 in the SS group in comparison to the SR group. We also measured cleaved caspase 6, an effector caspase, and found significant [F(4,19) = 3.074, P = 0.0413] differences between the SS and SR (Fig. 5b). Unexpectedly, METH SA had not effects on caspase-3 cleavage (Fig. 5c).
Figure 5. Effects of METH self-administration on cleavage of caspases-9 and −6 in the dorsal striatum.
Representative western blot bands and corresponding quantitative analysis of the initiator caspase, cleaved caspase-9 (A) and effector, caspase-6 (B). SS rats showed significant decreases in cleaved caspase −9 (p < 0.001) and caspase-6 (p < 0.01) in comparison to SR rats. (C) There were no changes in caspase-3 cleavage in any of the group. α-tubulin was used for normalization. Key to statistics: ** p<0.01 vs. CT; $, $ $ p<0.05, p<0.01 vs. YSR, respectively; ##, ### p<0.01, p<0.001 vs. SR, respectively; ^ p<0.05 vs. YSS.
Discussion
The present study documents, for the first time, significant changes in some markers that are involved in both autophagic and apoptotic processes in animals that self-administered larger quantities of METH in comparison to others that become abstinent after footshocks. Thus, the dorsal striata of compulsive METH takers showed (i) increased Atg2a, Atg5 and Atg12 mRNA levels; (ii) increased ULK1 and pBECN1 abundance; and (iii) increased p53 but decreased Bcl-2 protein expression. These data are discussed in term of their demonstration that METH, taken by self-administration, can also activate pathways that might lead to autophagy and/or neuronal apoptosis.
Our findings are consistent with previous studies that had reported the presence of autophagic changes after exposure to METH (Larsen et al. 2002; Pasquali et al. 2008). At low concentrations, METH may act as a pro-survival factor in dopaminergic neuron by impacting autophagic processes (Ma et al. 2014). Pitaksalee et al. (2015) reported, however, that inhibition of autophagy by caffeine exacerbated METH-induced cell death. Importantly, high doses of METH can cell death by activating multiple death pathways that lead to neuronal apoptosis (Deng et al. 2002; Deng et al. 1999; Hirata and Cadet 1997; Jayanthi et al. 2001; Jayanthi et al. 2004). These pathways include increased expression of p53, decreased expression of Bcl2, and caspase activation, among others (Hirata and Cadet 1997; Jayanthi et al. 2001; Jayanthi et al. 2004). Activation of these pathways is secondary to METH-induced oxidative stress (Cadet and Brannock 1998; Cadet et al. 2003; Cadet et al. 2005)
In the present study, we have found increased ULK1 protein levels in rats that continued to take METH compulsively for 29 days. We thus propose that METH-induced increased ULK1 might have served to promote autophagic changes in the striatum of these rats because activation of the ULK1 complex is involved in the early stages of autophagy (Petherick et al. 2015). This idea is consistent with our observation that these rats exhibit increased phosphorylation of Beclin1 and increased expression of some of its binding partners that include several ATGs (Cao et al. 2017; Matsushita et al. 2007; Otomo et al. 2013). Beclin1 phosphorylation by phosphoglycerate kinase-1 has indeed been shown to play an integral part of the autophagic process (Qian et al. 2017). We also detected increased levels of Atg2a, Atg5, Atg12 and Atg16L mRNAs in the SR group in comparison to both control and SS groups. ATG2a transports lipids from the ER to the growing phagophore at mitochondrial-ER contact sites (Gomez-Sanchez et al. 2018; Osawa et al. 2019). Moreover, the ATG12 conjugation system (ATG12-ATG5-ATG16L complex) is important in autophagosome formation, phagophore elongation, and cargo recognition (Walczak and Martens 2013; Wesselborg and Stork 2015). Thus, when taken together, our novel findings promise to open a new line of inquiry that will help to decipher not only the molecular neurobiology of the neurological and psychiatric deficits but also that of neuropathological abnormalities reported in humans who suffer from METH use disorders (Cadet et al. 2014).
A growing body of evidence suggests that binding of Beclin1 to Bcl2 negatively regulates autophagosome formation by promoting the dissociation between Beclin1 and its binding proteins (Liang et al. 1998; Pattingre et al. 2008). Evidence suggests that the relative amounts of Beclin1 and Bcl-2 family proteins may govern the threshold for transition from cell survival to cell death (Pattingre et al. 2005; Xu et al. 2013). In the present study, we found significant decreases in BCL-2 protein levels in compulsive METH taking rats. Taken together with our observations that p53 protein levels are also increased in these rats, these data suggest that METH SA may induce processes that lead to both autophagic and neuronal apoptosis in the dorsal striatum. This conclusion is consistent with previous data on the neurotoxic effects of METH (Cadet et al., 2003; 2005). Our proposal also agrees with those of Xu et al. (2013) who reported that inhibition of Bcl2 during nutrient deprivation-induced autophagic activity is accompanied by the promotion of cell death.
In summary, we have shown, for the first time, that rats that took large quantities of METH during implementation of a drug self-administration paradigm for several days show significant alterations in markers of autophagy and neuronal apoptosis in their dorsal striatum. These data are summarized in the schema in figure 6 that suggests that METH SA can activate these pathways in the dorsal striatum as a result of METH-induced oxidative stress (Cadet and Brannock 1998). Our observations point to the need to further investigate autophagy- and/or apoptosis-mediated events in various organ systems of humans who abuse METH. Elucidation of these pathways might be of therapeutic benefits.
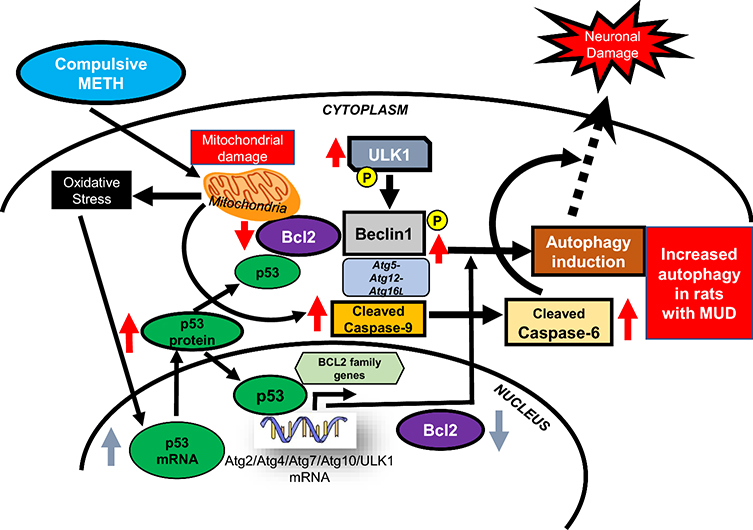
Figure 6. METH-taking activates a p53-Bcl2-ULK1-dependent autophagic cascade in the dorsal striatum.
Schematic representation of activation of autophagic events and eventual neuronal damage in the dorsal striatum of compulsive METH seeking rats. Autophagic events include- initiation of phagophore formation (increased protein expression of ULK1), elongation and completion of autophagosome (upregulation of Atg5-Atg12-Atg16L and activation of pBECN1). Compulsive METH SA by generating oxidative stress may lead to mitochondrial dysfunctions, increased p53 expression, and consequent decreased Bcl2 protein levels. Together these biochemical changes may act to regulate autophagic and apoptotic changes in the dorsal striatum of rats exposed to large amounts of METH.
Supplementary Material
Acknowledgments
This research was supported by funds of the Intramural Research Program of the DHHS/NIH/NIDA.
Abbreviations
- Atg
autophagy related gene
- B2M
beta-2 microglobulin
- BCL-2
B-cell lymphoma 2
- BECN1
Beclin-1
- CT
control group
- DA
dopamine
- DAT
dopamine transporter
- DSM-V
Diagnostic and Statistical Manual of Mental Disorders V
- LC3-II
microtubule-associated light chain 3
- METH
methamphetamine
- mTOR
mammalian target of rapamycin
- MUD
methamphetamine use disorder
- NP-40
Nonidet P-40
- OAZ1
ornithine decarboxylase antizyme
- p53
Tumor protein p53
- SA
self-administration
- SERT
serotonin transporter
- SS
shock-sensitive group
- SR
shock-resistant group
- TH
Tyrosine hydroxylase
- ULK1
unc51-like autophagy activating kinase 1
- 5-HT
serotonin
Footnotes
Disclosure / Conflict of Interest
All the authors declare no competing financial interests or conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Armstrong BD, Noguchi KK (2004) The neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine on serotonin, dopamine, and GABA-ergic terminals: an in-vitro autoradiographic study in rats. Neurotoxicology 25(6):905–14 doi: 10.1016/j.neuro.2004.06.003 [DOI] [PubMed] [Google Scholar]
- Asanuma M, Miyazaki I, Higashi Y, Cadet JL, Ogawa N (2002) Methamphetamine-induced increase in striatal p53 DNA-binding activity is attenuated in Cu,Zn-superoxide dismutase transgenic mice. Neurosci Lett 325(3):191–4 doi: 10.1016/s03043-940(02)00291-4 [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP (2010) Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35(1):48–69 doi: 10.1038/npp.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner SE, Wagner GC, Aigner TG, Seiden LS (1981) Effects of a high-dose treatment of methamphetamine on caudate dopamine and anorexia in rats. Pharmacol Biochem Behav 14(4):481–6 doi: 10.1016/0091-3057(81)90306-3 [DOI] [PubMed] [Google Scholar]
- Booth LA, Roberts JL, Dent P (2019) The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib. Semin Cancer Biol doi: 10.1016/j.semcancer.2019.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V (2015) Neuropsychological Consequences of Chronic Drug Use: Relevance to Treatment Approaches. Front Psychiatry 6:189 doi: 10.3389/fpsyt.2015.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM (2014) Neuropathology of substance use disorders. Acta Neuropathol 127(1):91–107 doi: 10.1007/s00401-013-1221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochemistry international 32(2):117–31 doi: 10.1016/s0197-0186(97)00031-4 [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Jayanthi S, Krasnova IN (2015) Transcriptional and epigenetic substrates of methamphetamine addiction and withdrawal: evidence from a long-access self-administration model in the rat. Mol Neurobiol 51(2):696–717 doi: 10.1007/s12035-0148776-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Brannock C, Krasnova IN, Jayanthi S, Ladenheim B, McCoy MT, Walther D, Godino A, Pirooznia M, Lee RS (2017) Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol Psychiatry 22(8):1196–1204 doi: 10.1038/mp.2016.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X (2003) Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. Faseb J 17(13):1775–88 doi: 10.1096/fj.03-0073rev [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X (2005) Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotox Res 8(3–4):199–206 [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J (2007) Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res 11(3–4):183–202 [DOI] [PubMed] [Google Scholar]
- Cao L, Glazyrin A, Kumar S, Kumar A (2017) Role of Autophagy in HIV Pathogenesis and Drug Abuse. Mol Neurobiol 54(8):5855–5867 doi: 10.1007/s12035-016-0118-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV (2000) Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res 863(1–2):106–11 doi: 10.1016/s0006-8993(00)02107-7 [DOI] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, Shaham Y (2017) Role of Dorsomedial Striatum Neuronal Ensembles in Incubation of Methamphetamine Craving after Voluntary Abstinence. J Neurosci 37(4):1014–1027 doi: 10.1523/JNEUROSCI.3091-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castino R, Lazzeri G, Lenzi P, Bellio N, Follo C, Ferrucci M, Fornai F, Isidoro C (2008) Suppression of autophagy precipitates neuronal cell death following low doses of methamphetamine. J Neurochem 106(3):1426–39 doi: 10.1111/j.1471-4159.2008.05488.x [DOI] [PubMed] [Google Scholar]
- Cheng WS, Garfein RS, Semple SJ, Strathdee SA, Zians JK, Patterson TL (2010) Binge use and sex and drug use behaviors among HIV(−), heterosexual methamphetamine users in San Diego. Subst Use Misuse 45(1–2):116–33 doi: 10.3109/10826080902869620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AK, Melega WP (2002) Patterns of methamphetamine abuse and their consequences. J Addict Dis 21(1):21–34 doi: 10.1300/j069v21n01_03 [DOI] [PubMed] [Google Scholar]
- Cordani M, Butera G, Pacchiana R, Donadelli M (2017) Molecular interplay between mutant p53 proteins and autophagy in cancer cells. Biochim Biophys Acta Rev Cancer 1867(1):19–28 doi: 10.1016/j.bbcan.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ray LA (2014) Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend 143:11–21 doi: 10.1016/j.drugalcdep.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL (2002) Methamphetamine induces apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacol 42(6):837–45 doi: 10.1016/s0028-3908(02)00034-5 [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Tsao LI, Cadet JL (1999) Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J Neurosci 19(22):10107–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM5 (ed)2013) Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association [Google Scholar]
- Everitt BJ, Robbins TW (2016) Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol 67:23–50 doi: 10.1146/annurev-psych-122414-033457 [DOI] [PubMed] [Google Scholar]
- Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV (1998) A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res 806(1):1–7 doi: 10.1016/s0006-8993(98)00656-8 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez R, Rose J, Guimaraes R, Mari M, Papinski D, Rieter E, Geerts WJ, Hardenberg R, Kraft C, Ungermann C, Reggiori F (2018) Atg9 establishes Atg2-dependent contact sites between the endoplasmic reticulum and phagophores. J Cell Biol 217(8):2743–2763 doi: 10.1083/jcb.201710116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS (2003) Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neurosci 122(2):499–513 doi: 10.1016/s0306-4522(03)00476-7 [DOI] [PubMed] [Google Scholar]
- Hirata H, Cadet JL (1997) p53-knockout mice are protected against the long-term effects of methamphetamine on dopaminergic terminals and cell bodies. J Neurochem 69(2):780–90 doi: 10.1046/j.1471-4159.1997.69020780.x [DOI] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Krasnova IN, Gu H, Lu H, Bonci A, Cadet JL, Stein EA, Yang Y (2019) Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals. Proc Natl Acad Sci U S A 116(18):9066–9071 doi: 10.1073/pnas.1819978116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SZ, Itzhak Y, Cadet JL, Islam F, Slikker W Jr., Ali SF (2001) Methamphetamine-induced alteration in striatal p53 and bcl-2 expressions in mice. Brain Res Mol Brain Res 91(1–2):174–8 doi: 10.1016/s0169-328x(01)00139-5 [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL (2001) Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. Faseb J 15(10):1745–52 [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL (2005) Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci U S A 102(3):868–73 doi: 10.1073/pnas.0404990102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL (2004) Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. Faseb J 18(2):238–51 doi: 10.1096/fj.03-0295com [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Patterson TK (2018) Habit Formation and the Striatum. Curr Top Behav Neurosci 37:275–295 doi: 10.1007/7854_2016_451 [DOI] [PubMed] [Google Scholar]
- Kongsuphol P, Mukda S, Nopparat C, Villarroel A, Govitrapong P (2009) Melatonin attenuates methamphetamine-induced deactivation of the mammalian target of rapamycin signaling to induce autophagy in SK-N-SH cells. J Pineal Res 46(2):199–206 doi: 10.1111/j.1600-079X.2008.00648.x [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL (2009) Methamphetamine toxicity and messengers of death. Brain Res Rev 60(2):379–407 doi: 10.1016/j.brainresrev.2009.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Gerra MC, Walther D, Jayanthi S, Ladenheim B, Mccoy MT, Brannock C, Cadet JL (2017) Compulsive methamphetamine taking in the presence of punishment is associated with increased oxytocin expression in the nucleus accumbens of rats. Sci Rep-Uk 7:8331doi: 10.1038/s41598-017-08898-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL (2010) Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS ONE 5(1):e8790 doi: 10.1371/journal.pone.0008790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, Shaham Y, Cadet JL (2014) Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology 39(8):2008–16 doi: 10.1038/npp.2014.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D (2002) Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci 22(20):8951–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH (2005) CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. PNAS 102(52):19186–91 doi: 10.1073/pnas.0509735102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42 doi: 10.1016/j.cell.2007.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Carreria MB, Witonsky KR, Zeric T, Lofaro OM, Bossert JM, Zhang J, Surjono F, Richie CT, Harvey BK, Son H, Cowan CW, Nestler EJ, Shaham Y (2017) Role of Dorsal Striatum Histone Deacetylase 5 in Incubation of Methamphetamine Craving. Biol Psychiatry doi: 10.1016/j.biopsych.2017.12.008. [Epub ahead of print] doi:10.1016/j.biopsych.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu Z, Chen B, Bu Q, Lu W, Deng Y, Zhu R, Shao X, Hou J, Zhao J, Li H, Zhang B, Huang Y, Lv L, Zhao Y, Cen X (2012) Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicol Lett 215(1):1–7 doi: 10.1016/j.toxlet.2012.09.019 [DOI] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B (1998) Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol 72(11):8586–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chandramani-Shivalingappa P, Jin H, Ghosh A, Anantharam V, Ali S, Kanthasamy AG, Kanthasamy A (2012) Methamphetamine-induced neurotoxicity linked to ubiquitin-proteasome system dysfunction and autophagy-related changes that can be modulated by protein kinase C delta in dopaminergic neuronal cells. Neurosci 210:308–32 doi: 10.1016/j.neuroscience.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wan J, Meng J, Banerjee S, Ramakrishnan S, Roy S (2014) Methamphetamine induces autophagy as a pro-survival response against apoptotic endothelial cell death through the Kappa opioid receptor. Cell Death Dis 5:e1099 doi: 10.1038/cddis.2014.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–52 doi: 10.1038/nrm2239 [DOI] [PubMed] [Google Scholar]
- Marquez RT, Xu L (2012) Bcl-2:Beclin 1 complex: multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am J Cancer Res 2(2):214–21 [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Suzuki NN, Obara K, Fujioka Y, Ohsumi Y, Inagaki F (2007) Structure of Atg5.Atg16, a complex essential for autophagy. J Biol Chem 282(9):6763–72 doi: 10.1074/jbc.M609876200 [DOI] [PubMed] [Google Scholar]
- Mohammad Ahmadi Soleimani S, Ekhtiari H, Cadet JL (2016) Drug-induced neurotoxicity in addiction medicine: From prevention to harm reduction. Progress in brain research 223:19–41 doi: 10.1016/bs.pbr.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Nixon RA, Yang DS (2011) Autophagy failure in Alzheimer’s disease--locating the primary defect. Neurobiol Dis 43(1):38–45 doi: 10.1016/j.nbd.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa T, Kotani T, Kawaoka T, Hirata E, Suzuki K, Nakatogawa H, Ohsumi Y, Noda NN (2019) Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat Struct Mol Biol 26(4):281–288 doi: 10.1038/s41594-019-0203-4 [DOI] [PubMed] [Google Scholar]
- Otomo C, Metlagel Z, Takaesu G, Otomo T (2013) Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat Struct Mol Biol 20(1):59–66 doi: 10.1038/nsmb.2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali L, Lazzeri G, Isidoro C, Ruggieri S, Paparelli A, Fornai F (2008) Role of autophagy during methamphetamine neurotoxicity. Ann N Y Acad Sci 1139:191–6 doi: 10.1196/annals.1432.016 [DOI] [PubMed] [Google Scholar]
- Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P (2008) Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie 90(2):313–23 doi: 10.1016/j.biochi.2007.08.014 [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122(6):927–39 doi: 10.1016/j.cell.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Petherick KJ, Conway OJ, Mpamhanga C, Osborne SA, Kamal A, Saxty B, Ganley IG (2015) Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J Biol Chem 290(18):11376–83 doi: 10.1074/jbc.C114.627778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitaksalee R, Sanvarinda Y, Sinchai T, Sanvarinda P, Thampithak A, Jantaratnotai N, Jariyawat S, Tuchinda P, Govitrapong P, Sanvarinda P (2015) Autophagy inhibition by caffeine increases toxicity of methamphetamine in SH-SY5Y neuroblastoma cell line. Neurotox Res 27(4):421–9 doi: 10.1007/s12640-014-9513-9 [DOI] [PubMed] [Google Scholar]
- Qian X, Li X, Cai Q, Zhang C, Yu Q, Jiang Y, Lee JH, Hawke D, Wang Y, Xia Y, Zheng Y, Jiang BH, Liu DX, Jiang T, Lu Z (2017) Phosphoglycerate Kinase 1 Phosphorylates Beclin1 to Induce Autophagy. Mol Cell 65(5):917–931 e6 doi: 10.1016/j.molcel.2017.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohbakhsh A, Shirani K, Karimi G (2016) Methamphetamine-induced toxicity: The role of autophagy? Chem Biol Interact 260:163–167 doi: 10.1016/j.cbi.2016.10.012 [DOI] [PubMed] [Google Scholar]
- Rubio FJ, Liu QR, Li X, Cruz FC, Leao RM, Warren BL, Kambhampati S, Babin KR, McPherson KB, Cimbro R, Bossert JM, Shaham Y, Hope BT (2015) Context-induced reinstatement of methamphetamine seeking is associated with unique molecular alterations in Fos-expressing dorsolateral striatum neurons. J Neurosci 35(14):5625–39 doi: 10.1523/JNEUROSCI.4997-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N (2006) Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 63(1):90–100 doi: 10.1001/archpsyc.63.1.90 [DOI] [PubMed] [Google Scholar]
- Shalini S, Dorstyn L, Dawar S, Kumar S (2015) Old, new and emerging functions of caspases. Cell Death Differ 22(4):526–39 doi: 10.1038/cdd.2014.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Liston C, McEwen BS (2019) Parsing the Hippocampus in Depression: Chronic Stress, Hippocampal Volume, and Major Depressive Disorder. Biol Psychiatry 85(6):436–438 doi: 10.1016/j.biopsych.2019.01.011 [DOI] [PubMed] [Google Scholar]
- Torres OV, Jayanthi S, Ladenheim B, McCoy MT, Krasnova IN, Cadet JL (2017) Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav Brain Res 326:265–271 doi: 10.1016/j.bbr.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC (2018) Global Smart Update: Methamphetamine continues to dominate synthetic drug markets. United Nations Publication; 20(UNIS/CP/648 ) [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J (2001) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21(23):9414–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak M, Martens S (2013) Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy 9(3):424–5 doi: 10.4161/auto.22931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselborg S, Stork B (2015) Autophagy signal transduction by ATG proteins: from hierarchies to networks. Cell Mol Life Sci 72(24):4721–57 doi: 10.1007/s00018-015-2034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E (2016) Autophagy and p53. Cold Spring Harb Perspect Med 6(4):a026120 doi: 10.1101/cshperspect.a026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Che X, Zheng Q, Wu A, Pan K, Shao A, Wu Q, Zhang J, Hong Y (2014) Caspases: a molecular switch node in the crosstalk between autophagy and apoptosis. Int J Biol Sci 10(9):1072–83 doi: 10.7150/ijbs.9719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HD, Wu D, Gu JH, Ge JB, Wu JC, Han R, Liang ZQ, Qin ZH (2013) The pro-survival role of autophagy depends on Bcl-2 under nutrition stress conditions. PLoS ONE 8(5):e63232 doi: 10.1371/journal.pone.0063232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Huang E, Luo B, Cai D, Zhao X, Luo Q, Jin Y, Chen L, Wang Q, Liu C, Lin Z, Xie WB, Wang H (2018) Methamphetamine exposure triggers apoptosis and autophagy in neuronal cells by activating the C/EBPbeta-related signaling pathway. Faseb J:fj201701460RRR doi: 10.1096/fj.201701460RRR [DOI] [PubMed] [Google Scholar]
- Yang G, Zeng X, Li J, Leung CK, Zhang D, Hong S, He Y, Huang J, Li L, Li Z (2019) Protective effect of gastrodin against methamphetamine-induced autophagy in human dopaminergic neuroblastoma SH-SY5Y cells via the AKT/mTOR signaling pathway. Neurosci Lett 707:134287 doi: 10.1016/j.neulet.2019.134287 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.