Abstract
Background & Aims:
Smooth muscles of the lower esophageal sphincter (LES) and skeletal muscle of the crural diaphragm (esophagus hiatus) provide the sphincter mechanisms at the esophagogastric junction (EGJ). We investigated differences in the 3-dimensional (3D) pressure profile of the LES and hiatal contraction between normal subjects and patients with achalasia esophagus.
Methods:
We performed a prospective study of 10 healthy subjects (controls; 7 male; mean age, 60±15 years; mean body mass index, 25 ± 2) and 12 patients with a diagnosis of achalasia (7 male; mean age, 63 ±13 years; mean body mass index, 26 ± 1), enrolled at a gastroenterology clinic. Participants underwent 3D high-resolution manometry (3DHRM) with a catheter equipped with 96 transducers (for the EGJ pressure recording). A 0.5mm metal ball was taped close to the transducer number 1 of the 3DHRM catheter. EGJ pressure was recorded at end-expiration (LES pressure) and at the peak of forced inspiration (hiatal contraction). Computed tomography (CT) scans were performed to localize the circumferential location of the metal ball on the catheter. Esophagus, LES, stomach, right, and left crus of the diaphragm and spine were segmented in each CT scan slice images to construct the 3D morphology of the region.
Results:
The metal ball was located at the 7 o’clock position in all controls. The circumferential orientation of metal ball was displaced 45–90 degrees in patients with achalasia compared with controls. The 3D-pressure profile of the EGJ at end-expiration and forced inspiration revealed marked differences between the groups. The LES turns to the left as it entered from the chest into the abdomen, forming an angle between the spine and LES. The spine–LES angle was smaller in patients with achalasia (1040) compared with controls (1240). Five of the 10 subjects with achalasia had physical breaks in the left crus of the diaphragm
Conclusions:
Besides LES, the 3D pressure profile of the EGJ can indicate anatomical and functional abnormalities of the crural diaphragm muscle in patients with achalasia esophagus. Further studies are needed to define the nature of hiatal and crural diaphragm dysfunction in patients with achalasia of the esophagus.
Keywords: diagnostic, dysmotility, esophageal contraction, esophageal disorder
INTRODUCTION
The sphincter mechanism at the esophagogastric junction (EGJ), often referred to as the lower esophageal sphincter (LES) is complex. Franz Ingelfinger, the editor of New England Journal of Medicine, called it “the sphincter that is a sphinx”1. With improvements in the pressure recording techniques over years, it has become clear that the sphincter mechanism at the esophagogastric junction (EGJ) has two components, 1) smooth muscle lower esophageal sphincter (LES) and, 2) skeletal muscle crural diaphragm (CD)2, 3. The right and left crus muscles form the esophageal hiatus through which the esophagus enters from the thorax into abdomen. The LES and hiatus are anatomically superimposed on each other, the hiatus surrounds the cranial half of the LES. The EGJ pressure at end-expiration is generally thought to be due to the LES and increase in pressure with inspiration is due to the CD or hiatal contraction4. The increase in pressure with inspiration is directly proportional to the depth of inspiration or the force of diaphragmatic contraction5. The CD contributes to the end-expiratory EGJ pressure during increases in intra-abdominal pressure such as, coughing, sniffing, abdominal compression and straight leg raise6.
The functional characteristics of the LES and CD (hiatus), i.e., contraction pressure profile and hiatal anatomy were recently described using the 3D-high resolution manometry catheter and CT scan imaging in normal healthy subjects7. We found that the anatomical length of LES is greater on the lesser curvature as compared to the greater curvature of the stomach. The hiatus is located at the cranial half of the LES and it has a horizontal pressure profile on the manometry recording. The LES and hiatal contraction related pressures are greater towards the left side, i.e., at the angle of HIS. The 3D reconstruction of the EGJ anatomy from the CT scan images allowed us to locate the precise orientation of LES and CD pressure profile in the EGJ anatomy. Furthermore, CT images allowed us to determine the formation of hiatus from the right and left crus of the diaphragm and the anatomical relationship between the LES and hiatus.
The goal of our study was to extend our observations made in controls to patients with achalasia esophagus using 3D-EGJ pressure recordings and CT imaging. We compared differences in the 3D pressure profile of the LES and CD (hiatal contraction), and hiatal anatomy between controls and patients with achalasia esophagus. Our study suggests dysfunctional esophageal hiatus (crural diaphragm) in patients with achalasia esophagus.
METHODS
Studies were performed in 10 controls (7 males, 60±15 years, body mass index 25 ± 2 and 12 patients (7 males, 63 ±13 years, body mass index 26 ± 1 selected from the GI clinic at UCSD with a diagnosis of achalasia 2 esophagus (per Chicago classification definition8). Achalasia esophagus patients were being investigated as a part of the work up of dysphagia symptom. None of these subjects had any prior medical or surgical treatment for achalasia esophagus. The protocol for these studies was approved by the Institutional Review Board for the Protection of Human Subjects of the University of California San Diego, and all subjects signed an approved consent form prior to their participation in the study.
A 0.5mm diameter metal ball (BB) was flattened by placing in a large vice and taped to the 3DHRM esophageal catheter (Medtronic Inc. MN, USA), just proximal to the transducer #1 of the first ring of 96 transducers designated for the EGJ pressure recording. Subject’s nose and throat were anesthetized using 2% lidocaine gel and spray, and the manometry catheter was passed through nose and positioned in such a fashion that the EGJ (LES) high pressure zone was located on the segment of the catheter equipped with 96 pressure transducers. All studies were performed with the subject in the supine position. Following 10 swallows with 5ml water to ensure normal esophageal motility in controls and achalasia 2 pattern in the patients, baseline recordings were obtained for 10 minutes. Subjects were then asked to take 5 deep breaths (forced inspiration, FI) and the catheter position was adjusted, if necessary, to ensure that the EGJ was located on the part of the catheter equipped with the 96 pressure transducers. The catheter was firmly taped to the nose after manometry recordings and a CT scan was performed, from the mid thorax to mid-abdomen in end expiration, with subjects in the supine position. A non-contrast CT scans was performed using GE, HD 750, 64-slice CT scanner. Images were acquired at 100–120 KV and 200–300 milliamps depending on patient’s BMI. The 2.3 mm, coronal DICOM images (30–40 images) from the CT scan were uploaded into an advanced 3D visualization software program, AMIRA (Thermo Fisher Scientific, Waltham, Mass. USA).
Image Analysis:
Images from the CT scan were reviewed to localize the axial and circumferential orientation of the metal ball on the catheter. Additionally, the DICOM images were imported into the AMIRA computer software program and following structures were outlined in each image: 1) right and left crus of diaphragm, as they originate from the lumbar spine and go around the esophagus to form the esophageal hiatus; 2) thoracic and abdominal esophagus along with the proximal part of the stomach; 3) aorta; and 4) lower thoracic and upper lumbar vertebral bodies, and intervertebral discs. Each structure was assigned different color in the reconstructed 3D anatomy of the region. The angle between the spine and part of the esophagus below the diaphragmatic hiatus (LES) was measured from the reconstructed 3D anatomy of the region of interest, as shown in figure 2.
Figure 2:
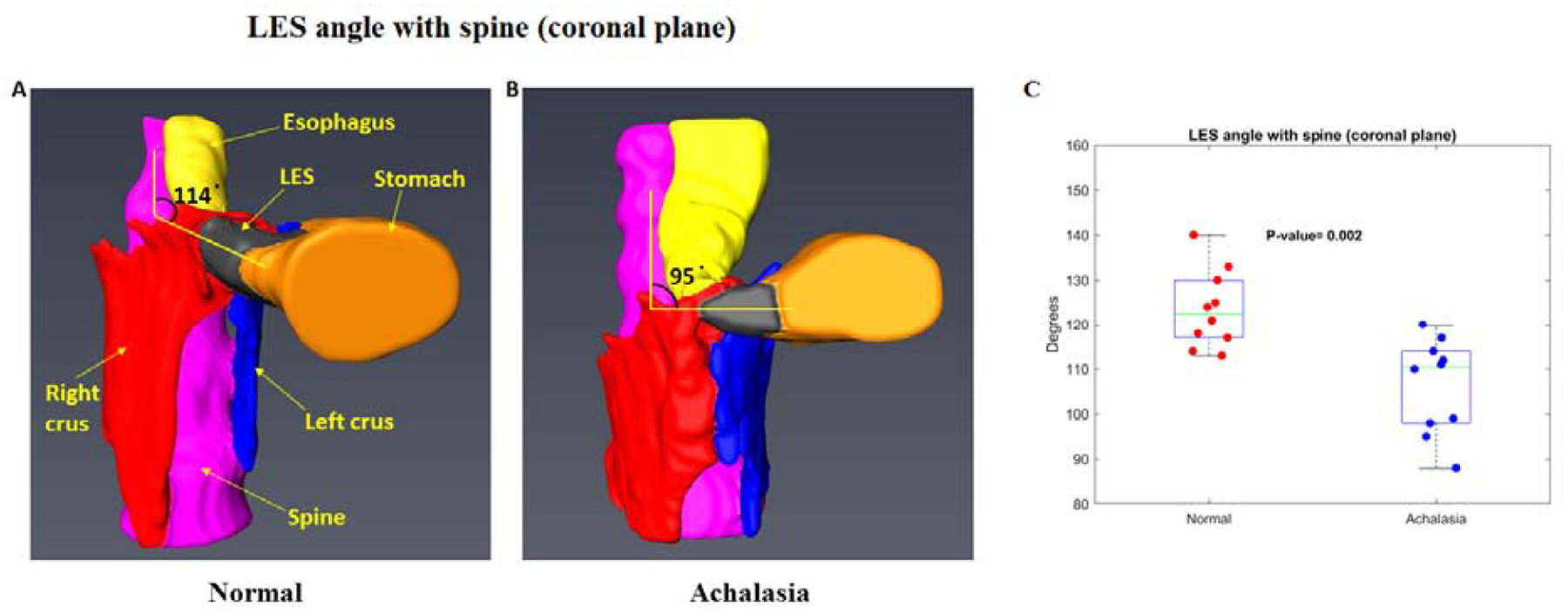
Spine-LES angle in a control (A) and patient with achalasia esophagus (B). Note that the angle is less (acute) in achalasia esophagus compared to controls. C, show the dot plot of the spine LES angle in all subjects, (see results for details)
3D Pressure Topography Morphometric Analysis
The EGJ pressure was measured at five different time points each, at end-expiration (EE) and forced inspiration (FI). The data were exported into an excel sheet and Matlab (Mathworks, Inc., Natick, MA) for further analysis. The unfolded cylindrical pressure profile (2D-HDRM surface plot) was treated as a surface and following surface topographical parameters were extracted: 1) Altitude: peak pressure in the pressure profile, 2) Slope: maximum rate of change in the EGJ pressure elevation, 3) Curvature: the rate of the change of the slope (the second derivative of the elevation surface), which itself divides into profile and planform curvatures. Profile, being the curvature of the pressure surface along the steepest downhill direction with negative values indicating convex and positive values indicating concave pressure surface. Planform denotes the horizontal component of the curvature. It is perpendicular to the direction of the maximum slope. A value of zero indicates the surface is linear. Profile curvature relates to the convergence and divergence of flow across a surface. 4) Rugosity (roughness or topographic complexity) which represents the degree of flatness of the 3D pressure surface.
Statistical Analysis:
Data are reported as median and interquartile range (IQR), if not otherwise stated and analyzed with appropriate parametric or non-parametric tests. The assumption of normality was verified using the Shapiro-Wilk W test. P-values < .05 were considered statistically significant.
RESULTS
Studies were successful in all 10 controls and 10 of the 12 achalasia patients. Two achalasia subjects were excluded because one showed normal peristalsis on the day of study even though clinical HRM study performed earlier had suggested type 2 achalasia. In the second patient, technical issue with the manometry recording prevented adequate analysis. Figure 1 shows the axial CT images from controls and patients with achalasia esophagus. From the axial CT images, we could localize the circumferential orientation of transducer #1 of 96 pressure transducers designated for the EGJ pressure recordings. In controls, the circumferential location of the metal ball (BB) was at 7 0 clock position in all subjects, even though no effort had been made to place the catheter in any specific orientation. On the other hand, in patients with achalasia esophagus, variability in the circumferential location of the metal BB was observed, one at 12 0 clock, one at 2 O clock, two at 6 O clock, three at 7 O clock and three at 8 Oclock (Figure 1).
Figure 1:
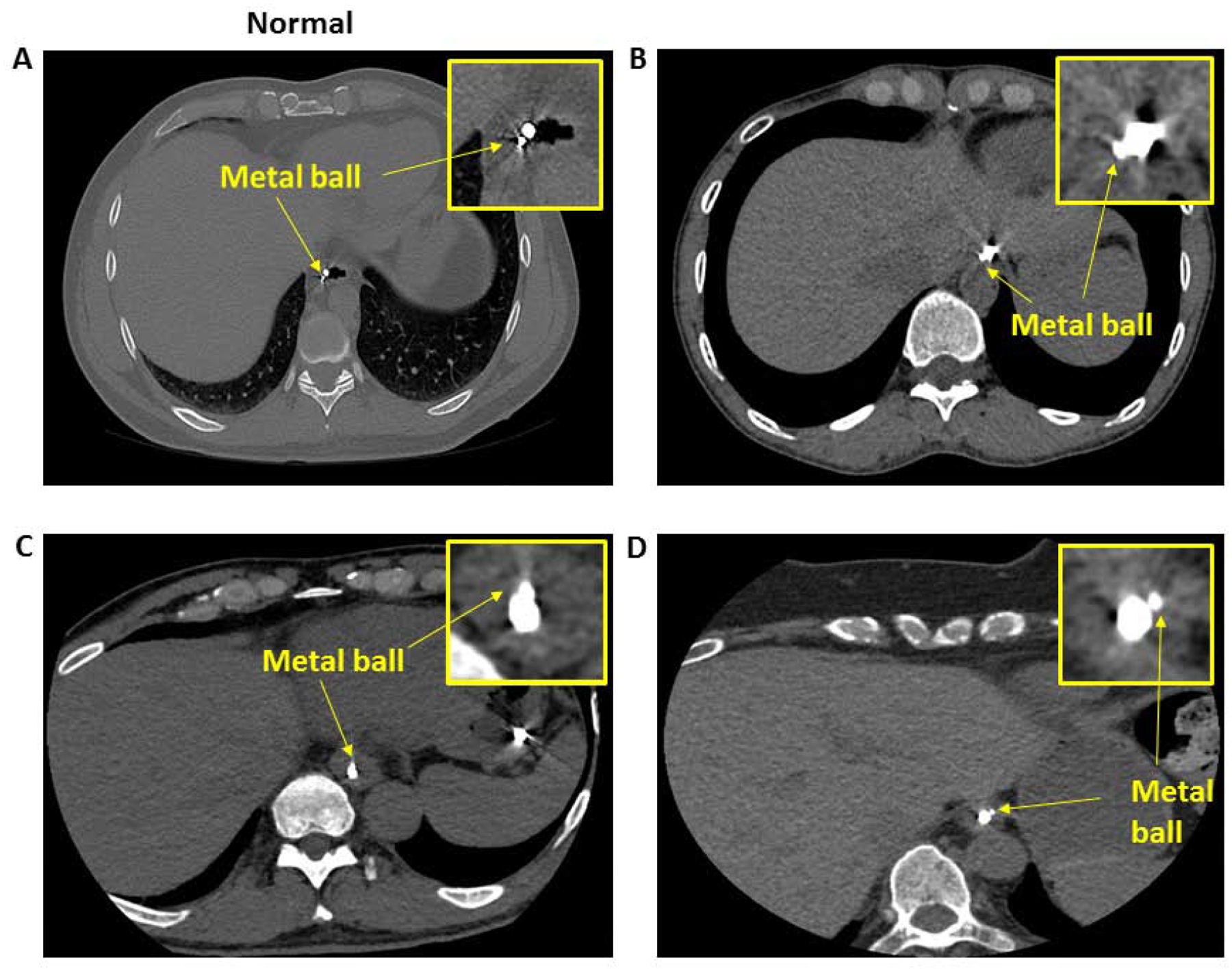
Axial CT scan of the normal and achalasia patients: A (control), B, C & D (3 patients with achalasia esophagus. Note the circumferential location of the metal ball (BB) is at 7 O’ clock position in a control, and varies in different patients with achalasia esophagus.
The 3D reconstructed image of the esophagus, LES, stomach, right crus, left crus and spine revealed that the esophagus bends to the left and anterior as it enters from the chest into the abdomen. The proximal margin of the hiatus coincided with the upper edge of the LES. We initially determined the angle that the esophagus makes with the LES as it enters into the abdomen, however, the thoracic part of the esophagus was not always in the coronal plan in all patients because several patients with achalasia esophagus had tortuous and dilated esophagus. Therefore, instead of the esophagus-LES angle, we measured the spine LES angle in coronal and sagittal planes. The coronal and sagittal spine-LES angle in controls were 124±80 and 48±70 respectively. The comparable spine-LES angles in achalasia patients were 106±100 and 43±70. The difference in the coronal spine-LES angle was significantly different (p <0.05) and there was trend in the sagittal spine-LES angle (p=.08), between the 2 groups (Figure 2).
Morphology of Esophageal Hiatus
The right crus divided into right and left branches, and surrounds the esophagus to form the hiatus in all controls and achalasia esophagus patients. The hiatus was larger in patients because of the larger diameter LES located in the hiatus. None of the controls revealed any breaks in the right or left crus muscles of the diaphragm (Figure 3). On the other hand, 5 of the 10 achalasia patients revealed breaks in the left crus of the diaphragm, which ranged from 7 mm to 28 mm in cranio-caudal length. Nine of the 10 achalasia subjects also revealed degenerative changes in the distal thoracic and upper lumbar spine (T11 to L2) in the form of large osteophytes, loss of disc height and disc herniation.
Figure 3:
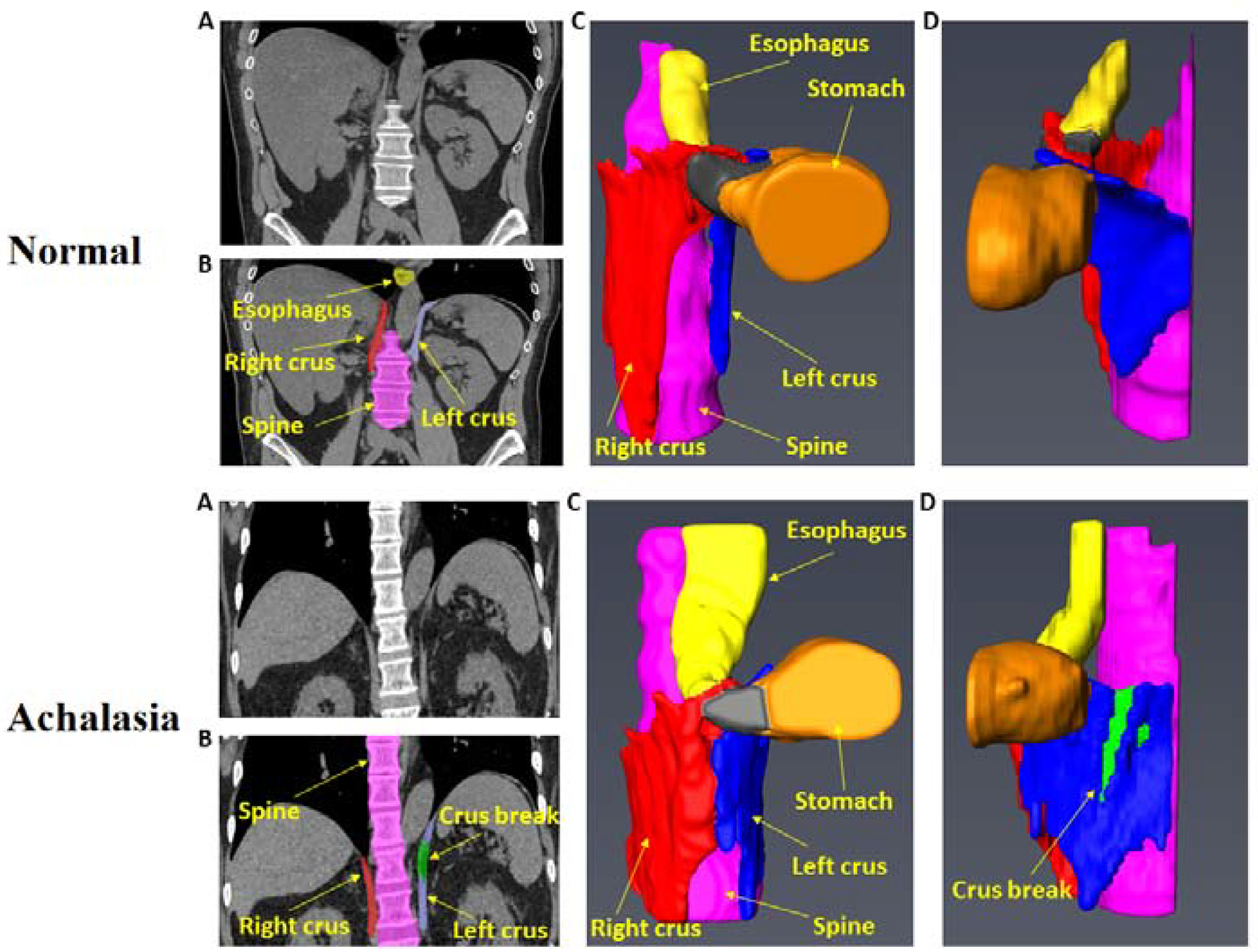
3D reconstruction of the esophageal hiatus, left and right crus of the diaphragm from the CT scan images. Note a large break in the left crus of the diaphragm in achalasia patient.
3D-High Resolution Manometry
In normal subjects, at end expiration, the unfolded 2D image of the HPZ of cylindrical EGJ subjects resembled the shape of a “V” with the longer arm located between 9–12 O clock (lesser curvature and anterior surface of the stomach), and the shorter arm towards the angle of HIS (greater curvature of the stomach, between 3 – 6 O clock). In normal subjects, the shape and orientation of the EGJ-HPZ stayed the same at end-expiration and FI, however the pressures increased significantly with forced inspiration (FI) or in other words diaphragmatic contraction. The FI resulted in an increase in the EGJ pressure that was present all around the circumference and the profile appeared as a horizontal pressure band just below the upper border of the EGJ-HPZ. The increase in pressure with FI was circumferentially asymmetric with greater pressure between 3 to 6 O clock position as compared to the other regions, (Figure 4A).
Figure 4:
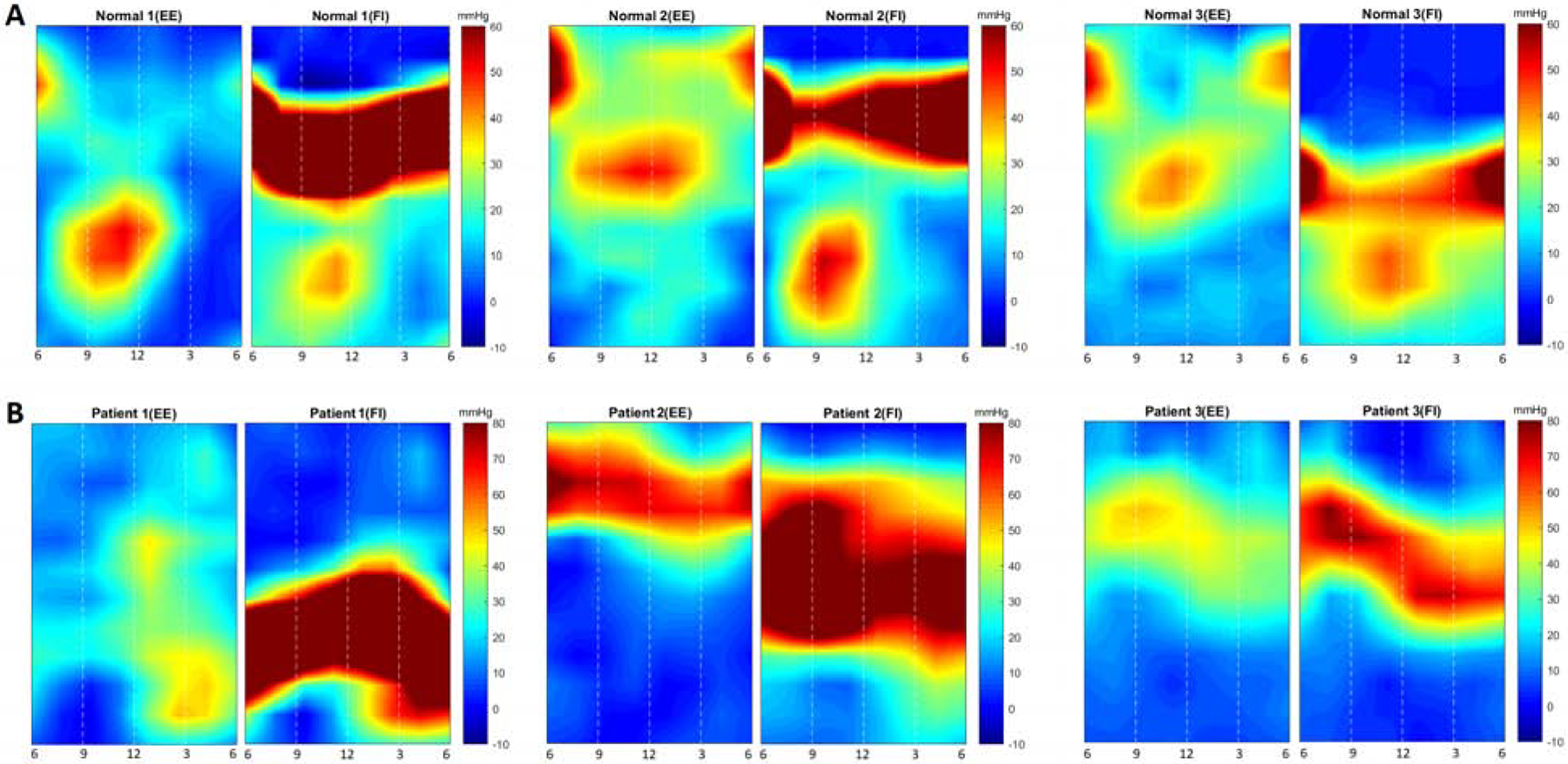
2D pressure profile of the esophago-gastric junction (EGJ) from of controls and 3 achalasia patients at end-expiration (EE) and forced inspiration (FI). Note the shape of the pressure profile is relatively uniform with the longer side located along the lesser curvature of the stomach and shorter side towards the greater curvature. With FI, the pressure increase is located at the proximal (cranial) part of the high pressure zone. In patients with achalasia esophagus the pressure profile is deformed at both, end-expiration and forced inspiration.
In patients with achalasia esophagus, on the other hand, the EGJ-HPZ was markedly different in visual appearance (Figure 4B). There were differences in the shape, circumferential orientation of the long arm of the HPZ, pressure distribution within the HPZ at end-expiration as well as with the FI. 1) Shape: Unlike the “V” shape of the EGJ profile in normal subjects, it was of varying shapes in achalasia subjects; no consistent shape was observed at either EE or FI. The EGJ appeared rotated with the longer arm of the HPZ at different circumferential orientations as compared to normal subjects. 2) Pressure distribution within the EGJ pressure profile was different in achalasia subjects with marked variability, 3) The location of diaphragmatic contraction with in the EGJ-HPZ was markedly different as compared to normal subjects and it was different among different achalasia patients (Figure 4B).
Quantitative analysis of the EGJ pressure profile (topographical analysis) revealed that at end-expiration, the surface pressure range was lower in the normal group (median 12 mmHg, (IQR 4.7)), as compared to achalasia patients (19 mmHg (IQR 6.6), p<0.01. At FI, Min and Max pressure surface profile curvatures were lower and higher respectively in the normal subjects as compared to achalasia patients (Figures, 5 & 6) suggesting less variability in the pressure surface curvature distribution. The range of slope and max slope values at FI were significantly different between the two groups (p<0.05). Finally, median rugosity at FI (median 1.02(0.22)) was lower in normal compared to achalasia patients (median 1.14(0.36)), indicating rougher or a more irregular pressure distribution in achalasia patients. No statistically significant differences were observed between the planform curvatures (Figure 6).
Figure 5:
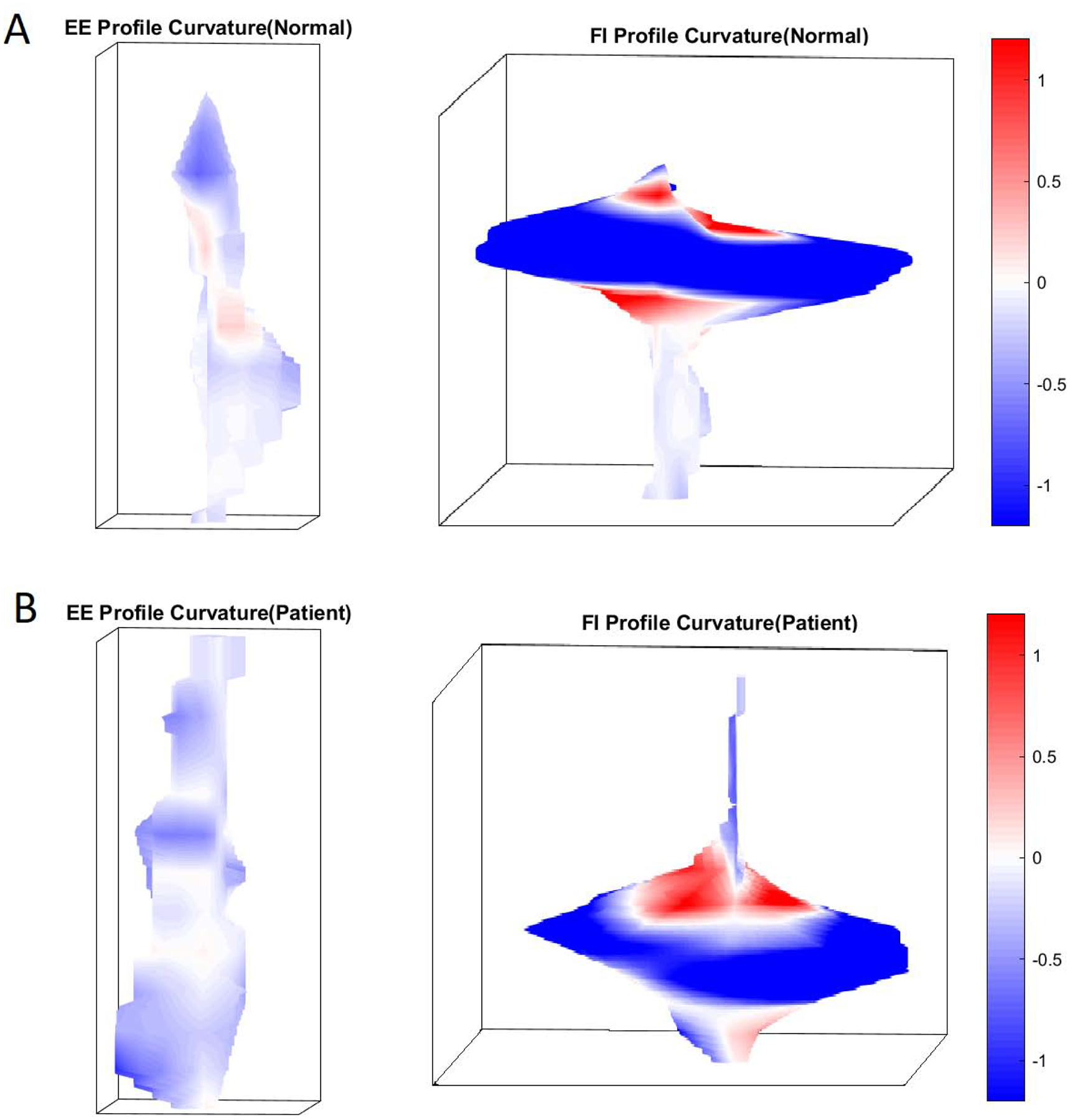
D pressure profile of the esophagogastric junction (EGJ) at end-expiration (EE) and forced inspiration (FI) in a control and a patient with achalasia esophagus. Note the difference in the shape of the pressure profile.
Figure 6:
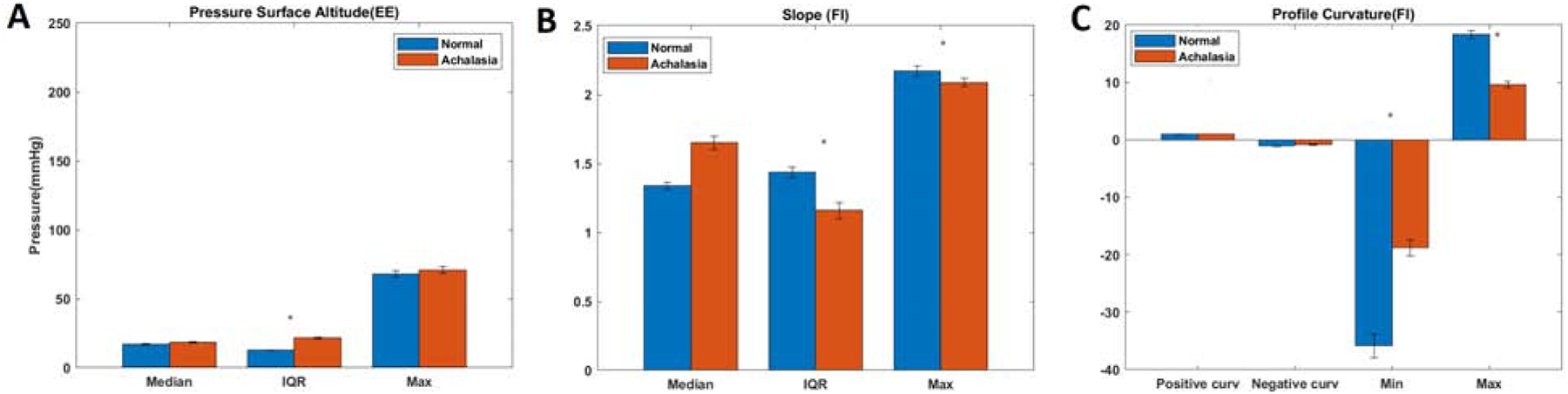
Quantitative analysis of the topography of the EGJ pressure profile: comparison between normal and achalasia esophagus patients. (A) pressure surface altitude at end-expiration (EE), (B) Slope at forced inspiration (FI), and (C) Profile curvature at forced inspiration (FI).
DISCUSSION
In summary, our data reveals the following novel information; 1) the esophagus makes an angle with the LES as it enters from the thorax into abdomen, at the level of esophageal hiatus. The spine-LES angle is smaller (acute) in patients with achalasia esophagus as compared to normal subjects, 2) we observed physical breaks in the left crus of the diaphragm in 5 of the 10 achalasia esophagus subjects that were not observed in normal subjects. 3) 3D pressure profile of the EGJ in patients with achalasia esophagus revealed much more variability as compared to normal subjects.
The esophagus makes a bend to the left and anteriorly as it enters into the abdomen, which has been described before, even though the reason for the bending is not known. Several studies reveal that the smooth muscle LES is physically located within the hiatus, i.e., hiatus and LES are anatomically superimposed on each other. The latter is suggested by the fact that the increase in LES pressure with inspiration related to the hiatal or crural diaphragm contraction is superimposed on the LES pressure recording in normal subjects. Simultaneous manometry and CT imaging reveals that in normal subjects the hiatus surrounds the proximal half of the LES. We believe that the bending of the esophagus/LES to the left is related to the pull of the hiatus on the esophagus by the CD. Esophageal hiatus is formed by the right crus of the diaphragm in majority of subjects. Right crus muscle originates from the right side of the lumbar vertebra (L1 to L3) and it divides into two bundles to encircle the esophagus/LES. In fact, the fibers of the two bundles of right crus cross each other in a “scissor like” fashion before forming the esophageal hiatus9. Left crus originating from the left side of lumbar vertebrae and joins the left branch of right crus in strengthening the esophageal hiatus. Right and left crus are not midline structures and it is likely that “pillar like” crus muscles pull the esophagus/LES in one direction or the other. We recently reported that the intra-sphincteric injection of botox paralyzes the esophageal hiatus10 and we observed that it also obliterates the esophago-LES angle (supplementary figure 1). Based on the above reasoning, we believe that the difference in the esophago-LES angle between normal and achalasia patients is likely related to the dysfunction of the esophageal hiatus. We observed large breaks in the left crus of diaphragm in achalasia subjects, an observation that has never been reported, which we believe may have an effect on the esophagus hiatus. The esophago-LES angle that we describe in this study is different from the angle between the esophagus and fundus of stomach, also known as angle of HIS. The latter is likely related to the clasp and sling fibers of the LES and is likely responsible for the formation of the flap valve at the EGJ. It is considered to be an important component of the antireflux barrier by many investigators. Sophisticated MRI and HRM studies prove that the flap value angle is greater in patients with reflux disease as compared to normal subjects11, 12.
The circumferential pressure asymmetry of the LES is well known and has been observed by many investigators13, 14. Using infusion manometry and eight sides located at the same axial level, Richardson et all observed that the pressures are greater in the left posterior direction; the reasons for which is believed to be related to the asymmetric push from the diaphragmatic hiatus. We studied the EGJ pressure asymmetry at end-expiration (considered to be due to the smooth muscle LES) as well as at the peak of inspiration7 (LES + hiatal contraction). Based on the concurrently performed 3D pressure profile of the EGJ and CT scan imaging, we provided an alternate explanation for the LES pressure asymmetry in our earlier paper, i.e, unique morphology of the LES7. The axial length of the LES is longer towards the lesser curvature as compared to the greater curvature of the stomach. Physical principle dictates equal forces in the opposite directions (Newton’s first law). Force is measured by the pressure multiplied by the area of contact between the manometry probe and LES. A shorter area of contact on the left side (shorter length of the LES high pressure zone) will result in a higher pressure on the left as compared to the right which is exactly what we found. The hiatus/crural diaphragm contraction, similar to LES is circumferential; it is the difference in area of contact around the circumference between the manometry probe and surrounding around structures (LES and hiatus) that results in circumferential pressure asymmetry. The unique, “noose shaped” morphology of the LES and hiatus is most likely the reason for circumferential pressure asymmetry9. One may argue how can right crus muscle, placed obliquely across the LES15 can result in a horizontal pressure profile on the LES. The manometry catheter, similar to LES, makes a sharp bend to the left at the level of hiatus which results in the hiatus and manometry catheter to be at almost right angle to each other, which is the reason for the horizontal pressure profile of the hiatus on the manometry catheter.
The shape and pressure distribution of the EGJ high pressure zone at expiration and inspiration in normal subjects is quite reproducible, based upon which we constructed an average pressure profile of the EGJ at end-expiration and forced inspiration in normal subjects. On the other hand, in patients with achalasia esophagus the pressure distribution with in the EGJ high pressure zone is quite variable with many peaks and valleys, different from normals, at both end expiration and peak inspiration. We used novel analytical methods to determine differences in the topography or in other words the pressure distribution within the EGJ high pressure zone. The right and left crus of the diaphragm that form the esophageal hiatus are relatively small and inaccessible muscles, however they serve extremely important function in maintaining the integrity of antireflux barrier. The pressure generated by the hiatal contraction is much greater than the LES, e.g., with forced inspiration the EGJ is 100–150mm Hg. On the other hand, the EGJ pressure at end-expiration (LES pressure) is usually 10–30mmHg. This large increase in the EGJ pressure with inspiration is required to counter the large increase in gastroesophageal pressure gradient generated during forced respiration under normal physiological conditions5. We speculate that the explanation for the differences between EGJ pressure topography of achalasia patients and controls is related to the structural/anatomical issues around the LES, i.e., the hiatus formed by the two crural diaphragm muscle. We observed differences in the esophago-LES angle, breaks in the crus of the diaphragm, and markedly different EGJ pressure profile with forced inspiration in achalasia subjects compared to controls. Based on the above reasoning we speculate the possibility of hiatal dysfunction in achalasia esophagus.
Current understanding is that the degeneration of inhibitory neurons of the esophagus and LES is the main cause of neuromuscular dysfunction in achalasia esophagus16. However, several other observations in patients with achalasia esophagus are worthy of consideration. The neuromuscular dysfunction in achalasia esophagus is located only above the hiatus, as suggested by findings of normal gastric emptying and normal small and large bowel motility in achalasia patients17. Furthermore, there is low prevalence of hiatus hernia in patients with achalasia esophagus18. In controls, with each swallow the LES migrates into chest, also known as physiological herniation19. On the other hand, we found lack of physical separation between the LES and hiatus/crural diaphragm with swallows in achalasia patients, or in other words a tight anchoring between the LES and hiatus. The latter explains low prevalence of hiatus hernia in achalasia esophagus20. A number of studies from our laboratory prove that the LES relaxation is mediated through longitudinal muscle contraction of the esophagus that results in esophageal shortening21–24. May be, the hiatus dysfunction (tight anchoring with LES) prevents axial shortening of distal esophagus and contributes to impaired LES relaxation, which in turn leads to neuromuscular changes in the LES and esophagus. A series of studies by Schulze-Deleriu et al in early 1990’s found neuromuscular alterations in the obstructed opossum esophagus that resemble findings in achalasia esophagus, i.e., dilated tortuous esophagus, muscular hypertrophy and loss of inhibitory innervation25–29. Further studies are needed to define the anatomical and functional differences in hiatal function in larger number of patients with achalasia esophagus and possibly even other esophageal motility disorders.
Few limitations of our study are worthy of consideration, 1) One may wonder why high resolution manometry (HRM) studies in achalasia patients have not revealed abnormalities of the EGJ pressure profile described in the current study? The reason is that even though each ring of pressure sensor in the HRM catheter, placed one centimeter apart, is made up of 8 pressure transducer, the system averages those 8 numbers to provide only one value in the color pressure topograph and therefore does not reveal information on the circumferential asymmetry of the EGJ, which is not the case with the 3D HRM catheter that we used for this study. 2) Number of achalasia patients and controls studied are relatively small. We conducted careful studies using innovative, “state of the art” manometry system and CT scan imaging. Furthermore, we used novel analytical methods to study the EGJ functional morphology/topography. We believe that our findings are likely to be reproducible in large number of patients. 3) Our study was not designed to determine the cause and effect relationship between anatomical and functional abnormalities of EGJ in achalasia esophagus. Furthermore, the reason for the anatomical and functional alteration of hiatal function is not clear from our study. We speculate that the abnormality of the crus muscles may be related to the pathology of lumbar vertebrae and intervertebral discs from which the crus muscles arise, as has been observed in other paraspinal muscles30–32. We found evidence of large osteophytes and other degenerative changes in the lumbar spine in some but not all achalasia patients. The two crus muscles and hiatus are relatively small and inaccessible muscles and can’t be fully studied by the CT scan imaging. Future studies with MR imaging may provide better proof of the abnormalities of structure and function of two crus muscles in patients with achalasia esophagus.
Supplementary Material
What you need to know:
Background and Context:
Smooth muscles of the lower esophageal sphincter (LES) and skeletal muscle of the crural diaphragm (esophagus hiatus) provide the sphincter mechanisms at the esophagogastric junction (EGJ).
New Findings:
The 3-dimensional pressure profile of the EGJ can indicate anatomical and functional abnormalities of the crural diaphragm muscle in patients with achalasia esophagus.
Limitations:
Further studies are needed to define the nature of hiatal and crural diaphragm dysfunction in patients with achalasia of the esophagus
Impact:
This information might be used to identify anatomical and functional abnormalities of the crural diaphragm muscle in patients with achalasia esophagus.
Lay Summary:
The authors identified an alteration in the esophagus of patients with achalasia
Financial Support:
This work was supported by a NIH Grant DK060733
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COI: None of the authors have any conflict of interest
Bibliography:
- 1.Ingelfinger FJ. The sphincter that is a sphinx. N Engl J Med 1971;284:1095–6. [DOI] [PubMed] [Google Scholar]
- 2.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med 1997;336:924–32. [DOI] [PubMed] [Google Scholar]
- 3.Brasseur JG, Ulerich R, Dai Q, et al. Pharmacological dissection of the human gastro-oesophageal segment into three sphincteric components. J Physiol 2007;580:961–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle JT, Altschuler SM, Nixon TE, et al. Role of the diaphragm in the genesis of lower esophageal sphincter pressure in the cat. Gastroenterology 1985;88:723–30. [DOI] [PubMed] [Google Scholar]
- 5.Mittal RK, Rochester DF, McCallum RW. Electrical and mechanical activity in the human lower esophageal sphincter during diaphragmatic contraction. J Clin Invest 1988;81:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittal RK, Fisher M, McCallum RW, et al. Human lower esophageal sphincter pressure response to increased intra-abdominal pressure. Am J Physiol 1990;258:G624–30. [DOI] [PubMed] [Google Scholar]
- 7.Mittal RK, Zifan A, Kumar D, et al. Functional morphology of the lower esophageal sphincter and crural diaphragm determined by three-dimensional high-resolution esophago-gastric junction pressure profile and CT imaging. Am J Physiol Gastrointest Liver Physiol 2017;313:G212–G219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zifan A, Kumar D, Cheng LK, et al. Three-Dimensional Myoarchitecture of the Lower Esophageal Sphincter and Esophageal Hiatus Using Optical Sectioning Microscopy. Sci Rep 2017;7:13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar D, Zifan A, Mittal RK. Botox Injection into the Lower Esophageal Sphincter Induces Hiatal Paralysis and Gastroesophageal Reflux. Am J Physiol Gastrointest Liver Physiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curcic J, Fox M, Kaufman E, et al. Gastroesophageal junction: structure and function as assessed by using MR imaging. Radiology 2010;257:115–24. [DOI] [PubMed] [Google Scholar]
- 12.Curcic J, Roy S, Schwizer A, et al. Abnormal structure and function of the esophagogastric junction and proximal stomach in gastroesophageal reflux disease. Am J Gastroenterol 2014;109:658–67. [DOI] [PubMed] [Google Scholar]
- 13.Winans CS. Manometric asymmetry of the lower-esophageal high-pressure zone. Am J Dig Dis 1977;22:348–54. [DOI] [PubMed] [Google Scholar]
- 14.Richardson BJ, Welch RW. Differential effect of atropine on rightward and leftward lower esophageal sphincter pressure. Gastroenterology 1981;81:85–9. [PubMed] [Google Scholar]
- 15.Kumar D, Zifan A, Ghahremani G, et al. Morphology of the Esophageal Hiatus: Is It Different in 3 Types of Hiatus Hernias? J Neurogastroenterol Motil 2020;26:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology 2013;145:954–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckardt VF, Krause J, Bolle D. Gastrointestinal transit and gastric acid secretion in patients with achalasia. Dig Dis Sci 1989;34:665–71. [DOI] [PubMed] [Google Scholar]
- 18.Binder HJ, Clemett AR, Thayer WR, et al. Rarity of Hiatus Hernia in Achalasia. N Engl J Med 1965;272:680–2. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatek MA, Nicodeme F, Pandolfino JE, et al. Pressure morphology of the relaxed lower esophageal sphincter: the formation and collapse of the phrenic ampulla. Am J Physiol Gastrointest Liver Physiol 2012;302:G389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mittal RK, Caplin M, Zifan A. Evidence for the abnormal anchoring between the lower esophageal sphincter and crural diaphragm in patients with achalasia esophagus. Neurogastroenterology and Motility 2019; 31:111 [Google Scholar]
- 21.Mittal RK. Regulation and dysregulation of esophageal peristalsis by the integrated function of circular and longitudinal muscle layers in health and disease. Am J Physiol Gastrointest Liver Physiol 2016;311:G431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, Sandler B, Bhargava V, et al. Antireflux action of Nissen fundoplication and stretch-sensitive mechanism of lower esophageal sphincter relaxation. Gastroenterology 2011;140:442–9. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Bhargava V, Mittal RK. Mechanism of stretch-activated excitatory and inhibitory responses in the lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol 2009;297:G397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dogan I, Bhargava V, Liu J, et al. Axial stretch: A novel mechanism of the lower esophageal sphincter relaxation. Am J Physiol Gastrointest Liver Physiol 2007;292:G329–34. [DOI] [PubMed] [Google Scholar]
- 25.Shirazi S, Schulze-Delrieu K. Role of altered responsiveness of hypertrophic smooth muscle in manometric abnormalities of the obstructed opossum oesophagus. Neurogastroenterol Motil 1996;8:111–9. [DOI] [PubMed] [Google Scholar]
- 26.Lu C, Schulze-Delrieu K, Shirazi S, et al. Dynamic imaging of obstructed opossum esophagus. From altered load to altered contractility. Dig Dis Sci 1994;39:1377–88. [DOI] [PubMed] [Google Scholar]
- 27.Tung HN, Shirazi S, Schulze-Delrieu K, et al. Morphological changes of myenteric neurons in the partially obstructed opossum esophagus. J Submicrosc Cytol Pathol 1993;25:357–63. [PubMed] [Google Scholar]
- 28.Conklin JL, Du CA, Schulze-Delrieu K, et al. Hypertrophic smooth muscle in the partially obstructed opossum esophagus. Excitability and electrophysiological properties. Gastroenterology 1991;101:657–63. [DOI] [PubMed] [Google Scholar]
- 29.Tung HN, Schulze-Delrieu K, Shirazi S, et al. Hypertrophic smooth muscle in the partially obstructed opossum esophagus. The model: histological and ultrastructural observations. Gastroenterology 1991;100:853–64. [DOI] [PubMed] [Google Scholar]
- 30.Hodges P, Holm AK, Hansson T, et al. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976) 2006;31:2926–33. [DOI] [PubMed] [Google Scholar]
- 31.Shahidi B, Hubbard JC, Gibbons MC, et al. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J Orthop Res 2017;35:2700–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahidi B, Parra CL, Berry DB, et al. Contribution of Lumbar Spine Pathology and Age to Paraspinal Muscle Size and Fatty Infiltration. Spine (Phila Pa 1976) 2017;42:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


