Abstract
Prior fMRI studies have reported relationships between memory-related activity in the hippocampus and in-scanner memory performance, but whether such activity is predictive of longitudinal memory change remains unclear. Here, we administered a neuropsychological test battery to a sample of cognitively healthy older adults on three occasions, the second and third sessions occurring one month and three years after the first session. Structural and functional MRI data were acquired between the first two sessions. The fMRI data were derived from an associative recognition procedure and allowed estimation of hippocampal effects associated with both successful associative encoding and successful associative recognition (recollection). Baseline memory performance and memory change were evaluated using memory component scores derived from a principal components analysis of the neuropsychological test scores. Across participants, right hippocampal encoding effects correlated significantly with baseline memory performance after controlling for chronological age. Additionally, both left and right hippocampal associative recognition effects correlated negatively with longitudinal memory decline after controlling for age, and the relationship with the left hippocampal effect remained after also controlling for left hippocampal volume. Thus, in cognitively healthy older adults, the magnitude of hippocampal recollection effects appears to be a robust predictor of future memory change.
Keywords: aging, encoding, episodic memory, hippocampus, retrieval
1. Introduction
As they age, healthy adults typically demonstrate reduced performance in multiple cognitive domains. One of these domains is episodic memory (Nyberg and Pudas, 2019) which, following Tulving (1983), we define here as memory for unique events. Numerous functional magnetic resonance imaging (fMRI) studies have investigated the neural correlates of episodic memory processing in older adults (for review, see Rajah et al., 2015; Tromp et al., 2015), but whether any of these correlates are predictive of performance on standardized memory tests, or changes in test performance over time, remains largely unknown. In the present study, we focused on the possible predictive roles of encoding- and retrieval-related neural activity in the hippocampus, a structure that is both necessary for episodic memory (Eichenbaum, 2017; Moscovitch et al., 2017) and has repeatedly been implicated in age-related memory decline (e.g. Persson et al., 2006; Rodrigue et al., 2013; for review, see Leal and Yassa, 2015).
As we discuss below, fMRI studies have employed two classes of experimental contrasts to examine the neural correlates of episodic memory at encoding and retrieval, respectively. The ‘subsequent recollection procedure’ entails contrasts between the neural activity elicited by study items according to whether the items were successfully recollected on a subsequent memory test or were judged as studied merely on the basis of an acontextual sense of familiarity. Resulting differences in neural activity will be referred to below as encoding effects. Similarly, the neural correlates of successful recollection – hereafter recollection effects – are identified by contrasting the neural activity elicited by memory test items according to whether the items were successfully recollected or judged as studied on the basis of familiarity alone. As is described in more detail in the Materials and Methods, in the present study these contrasts were performed in the context of an associative recognition test in which participants were required to discriminate between pairs of test words presented in the same pairing as at study (‘intact’ pairs), and test pairs comprising words that had been studied on two different study trials (‘rearranged’ pairs). The neural correlates of recollection were operationalized as the contrast between neural activity elicited by intact items according to whether the items were correctly judged intact, or wrongly judged as rearranged (see de Chastelaine et al., 2016a, 2016b, for a detailed rationale for this contrast).
Numerous cross-sectional studies have examined the effects of age on fMRI correlates of episodic memory encoding (e.g. de Chastelaine et al., 2011, 2016a; Kim and Giovanello, 2011; Miller et al., 2008; for review, see Maillet and Rajah, 2014) and retrieval (e.g. Dennis et al., 2008; de Chastelaine et al., 2016b; Duarte et al., 2008; Wang et al., 2016; Wang and Giovanello, 2016; for review, see Giovanello and Dew, 2015). In the case of the hippocampus, findings from both encoding and retrieval studies are mixed; whereas some studies reported null effects of age on encoding- or retrieval-related hippocampal effects (e.g. Angel et al., 2016; Dulas and Duarte, 2016; Miller et al., 2008), others have reported that the effects were either enhanced (e.g. Dulas and Duarte, 2011; Duverne et al., 2008) or attenuated in older adults (e.g. Daselaar et al., 2006; Dennis et al., 2008, Dulas and Duarte, 2014). However, it should be noted that, in these prior studies, memory performance was not always matched or statistically controlled across age groups. For example, whereas simple recognition memory performance was equated between young and older adults in Daselaar et al. (2006), estimates of recollection were higher in the young group. Similarly, Dennis et al. (2008) and Dulas and Duarte (2014) reported significantly lower memory performance in their older samples. As discussed previously (e.g. de Chastelaine et al., 2016b; Rugg and Morcom, 2005), the interpretation of age-related reductions in encoding- and retrieval-related functional activity is problematic when the reductions are accompanied by age differences in memory performance. In such cases, it is difficult to determine whether functional differences between age groups should be attributed to age or to performance.
As was just noted, numerous studies have investigated the effects of age on hippocampal functional correlates of encoding and retrieval. However, a substantially smaller number of studies have examined whether such correlates are associated with performance on the experimental memory task. Moreover, only one study has described relationships between these correlates and performance on standardized memory tests, and only two studies have examined whether hippocampal functional correlates might be predictive of longitudinal memory change. Turning first to associations between hippocampal effects and experimental memory performance, the findings are mixed. Four studies reported age-invariant, positive relationships between the magnitude of hippocampal encoding (de Chastelaine et al., 2016a) or retrieval (Daselaar et al., 2006; de Chastelaine et al., 2016b; Wang et al., 2016) effects and memory performance. By contrast, other studies examining such relationships in samples of older adults have reported negative relationships for encoding- (Miller at al., 2008) or retrieval-related effects (Carr et al., 2017), or a null relationship (Dulas and Duarte, 2011, 2016). In a similar vein, Daselaar et al. (2015) reported a negative relationship between hippocampal recollection effects and a composite index of memory function derived from a neuropsychological test battery. A possible reason for these inconsistent findings might lie in the heterogeneity of the samples of older participants employed in different studies; we return to this issue in the Discussion.
In comparison to studies that examined relationships between encoding- and recollection-related hippocampal effects and memory performance, markedly fewer studies have examined whether these effects are predictive of longitudinal memory change. Indeed, with the exception of Hantke et al. (2013) and Leal et al. (2017), studies examining relationships between hippocampal functional activity and longitudinal memory change have employed measures of hippocampal activity estimated either relative to an implicit baseline, or through non-mnemonic contrasts (O’Brien et al., 2010; Persson et al., 2012; Pudas et al., 2013, 2014; Woodard et al., 2010). Thus, it is not possible to classify these studies according to whether hippocampal activity was encoding- or retrieval-related. One study (Woodard et al., 2010) employed a prospective design and reported that a baseline measure of ‘hippocampal’ activity (an amalgam of activity in the hippocampus and parahippocampal cortex) elicited by a contrast between famous and non-famous names contributed significantly to regression models that predicted whether participants’ memory scores would remain stable or decline over the following 18 months. Hantke et al. (2013) described additional analysis of the same data set and reported that, unlike in the fame judgment task, neural activity differentiating between ‘old’ and ‘new’ recognition memory judgments did not contribute to prediction of future memory decline.
In another two studies employing longitudinal designs, Persson et al. (2012) reported that decline in left hippocampal activity over 6 years was positively associated with longitudinal memory change over a 20 year period. Similarly, O’Brien et al. (2010) reported that longitudinal decline in right hippocampal activity was positively related to memory decline over 2 years. In two final studies (Pudas et al., 2013, 2014), the relationship between hippocampal activity and retrospective memory change was investigated. Pudas et al. (2013) reported that older adults whose memory performance had remained stable over the preceding 15–20 years demonstrated higher levels of hippocampal activity than did adults in whom memory tended to decline over the same period. In a further analysis of the same data set, Pudas et al. (2014) reported that older adults’ hippocampal activity was positively related to their midlife memory performance in addition to performance at the time of scanning.
Together, the above-mentioned longitudinal findings indicate that hippocampal activity can be predictive of individual differences in longitudinal memory change in healthy older adults. As was previously noted, however, the measures of hippocampal activity employed in these studies cannot easily be understood in terms of neural activity directly related to episodic encoding or retrieval operations. One study relevant to this issue is that of Leal et al. (2017), who contrasted hippocampal activity elicited by visual scenes according to whether the scenes were confidently recognized or forgotten on a subsequent memory test. The resulting subsequent memory effect in the right hippocampus was unrelated to change in CVLT Long-Delay Free Recall performance over an average follow-up period of 2.7 years. It seems possible, however, that this null finding is a reflection of the marked divergence between the nature of the experimental items (visual scenes) and standardized test materials (words).
Here, we employed the verbal associative recognition procedure described previously to obtain measures of encoding- and recollection-related hippocampal effects in healthy older adults, and examined the relationships between these measures, baseline verbal memory performance and, most saliently, longitudinal (three year) memory change. In addition, we examined whether any such relationships were mediated by hippocampal volume, given that age-related volume reductions in the hippocampus are well documented (e.g. Fraser et al., 2015; Fjell et al., 2009; Raz et al., 2005), and hippocampal volume has sometimes been reported to be predictive of memory performance and memory change in older adults (e.g. Gorbach et al., 2017; Rosen et al., 2003; but see Charlton et al., 2010; Carmichael et al., 2012 for examples of null results).
Motivated by prior findings that encoding and recollection-related hippocampal effects are positively correlated with experimental memory performance (e.g. de Chastelaine et al., 2016a; Wang et al., 2016), we hypothesized that these effects would also be predictive of memory metrics derived from standardized neuropsychological tests. Findings from longitudinal studies linking hippocampal activity to memory change (Persson et al. 2012; O’Brien et al. 2010; Pudas et al. 2013) lead to the further prediction that any relationship detected between hippocampal encoding or recollection effects and longitudinal memory decline should be negative; that is, larger hippocampal effects at baseline should be associated with lower decline over the follow-up period.
2. Materials and Methods
In the present report, we describe neuropsychological test data obtained in three test sessions, separated by one month and three years respectively. The data from session 1 have been described previously (de Chastelaine et al., 2015, 2016a, 2016b, 2017, 2019; King et al., 2018), but the data from the succeeding two sessions have not been previously reported. The data pertaining to the relationships between the test scores and structural and functional hippocampal measures have also not been reported previously.
2.1. Participants
Participants were 67 heathy older adults recruited from the greater Dallas community. They comprised a sub-set of 69 older adults who received the same neuropsychological test battery (see below) on two sessions spaced over a one-month period and who were eligible for structural and functional neuroimaging. The two additional participants were excluded from all analyses (including the PCA conducted on the session 1 neuropsychological test scores, see below), because of abnormal anatomical scans. Intracranial and hippocampal volumetric data from one participant and intracranial volumetric data from one additional participant were excluded because of low quality structural images. Functional data from 3 participants were excluded because of near-chance performance on the in-scanner memory task (2 participants) or insufficient ‘associative miss’ trials (1 participant; see below for details about the functional MRI session).
A subsample of 55 participants were re-administered the neuropsychological test battery approximately 3 years after test sessions 1 and 2 [12 older adults did not participate in session 3 due to death (N =1), relocation from the Dallas area (N = 5), loss of contact (N = 5) or failure to attend (N = 1)]. Out of these 55 participants, volumetric data from 1 participant, and functional data from 2 other participants were excluded for one of the reasons mentioned above.
All participants were right-handed, fluent in English by age 5, had no history of neurological or psychiatric disease and had normal or corrected to normal vision. They each gave informed consent according to procedures approved by the UT Dallas and University of Texas Southwestern Institutional Review Boards. They were compensated at the rate of $30 per hour for their participation.
2.2. Neuropsychological test battery
The neuropsychological test battery comprised the California Verbal Learning Test-II (CVLT; immediate and delayed cued recall, immediate and delayed free recall, and delayed recognition, Delis et al., 2000), the Logical Memory test of the Wechsler Memory Scale (WMS-IV, Logical Memory tests I and II; Wechsler, 2009), the Digit Span test (Forward and Backward) of the Wechsler Adult IQ Scale Revised (WAIS-R, Wechsler, 2001), the Digit/Symbol Coding test of the WAIS-R (SDMT, written version), Trail Making Tests A and B (Reitan and Wolfson, 1985), letter and category fluency tests (FAS; Spreen and Benton, 1977), the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) and Raven’s Progressive Matrices (short version; Raven et al., 2000). We separately scored hits and false alarms in the CVLT delayed recognition test. Forward and Backward scores were summed to yield a single Digit Span score. Because the scores on the different CVLT recall tests were so highly correlated (rs > .79, ps <.001), we generated a single, composite CVLT recall score by averaging the scores across 4 different tests (i.e. across immediate and delayed free and cued recall). For the same reason, a composite Logical Memory score was computed by averaging the scores of the immediate- and delayed tests (the scores correlated at r = .84, p < .001). These composite memory scores, together with the scores on each of the other neuropsychological tests, were used for all further analysis (see Table 2).
Table 2.
Performance and performance change across sessions for each of the neuropsychological tests (N = 67 for full group, N = 55 for longitudinal subgroup, standard deviations in parentheses).
| Task | Session | ||||
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 3 | |
| Full group | Longitudinal subgroup | ||||
| FASa, b | 45.21 (12.53) | 49.09 (12.74) | 45.04 (12.63) | 48.40 (11.58) | 47.56 (13.26) |
| Logical Memory Compositea, b, c | 27.59 (5.39) | 31.73 (5.45) | 27.51 (5.57) | 31.93 (5.39) | 28.39 (5.57) |
| SDMTa, b, c | 49.46 (8.50) | 51.91 (8.20) | 49.45 (9.18) | 51.80 (8.73) | 49.09 (8.46) |
| Trail A (ms) | 32.69 (11.24) | 30.25 (11.10) | 31.96 (9.40) | 30.75 (11.71) | 31.89 (10.18) |
| Trail B (ms)a, b, c | 75.01 (45.70) | 59.82 (18.51) | 71.44 (30.60) | 59.44 (18.32) | 69.69 (30.54) |
| Digit Span | 18.27 (4.36) | 17.87 (4.24) | 18.25 (4.30) | 17.71 (4.09) | 18.15 (4.26) |
| Category Fluency (Animals)a, b | 22.45 (5.56) | 23.96 (5.39) | 22.35 (5.50) | 23.69 (5.44) | 22.93 (5.80) |
| WTAR (Full-Scale IQ)c | 112.64 (5.43) | 113.00 (5.16) | 112.95 (5.21) | 113.04 (5.15) | 112.11 (4.63) |
| Raven’s | 9.57 (2.13) | 9.91 (1.87) | 9.49 (2.25) | 9.85 (1.87) | 9.56 (2.63) |
| CVLT Hitsa, b | 14.82 (1.31) | 15.42 (.96) | 14.84 (1.33) | 15.36 (1.01) | 15.25 (1.13) |
| CVLT False Alarms | 1.97 (2.18) | 1.84 (2.53) | 1.93 (2.13) | 1.93 (2.71) | 2.09 (2.52) |
| CVLT recall Compositea, b, c | 12.00 (2.47) | 13.63 (2.02) | 11.95 (2.57) | 13.54 (2.17) | 12.80 (2.55) |
Note.
session 1≠session 2, p < .05 for full group;
session 1 ≠ session2, for longitudinal subgroup, p < .05;
session 2 ≠ session 3, p < .05.
Following the initial administration of the test battery, potential participants were excluded from the MRI session if they had 1) scores > 1.5 SDs below the age-appropriate norm on any long-term memory sub-test (CVLT or Logical memory) or on any two other tests; 2) an estimated full-scale IQ < 100 as indexed by performance on the WTAR, or 3) a score on the Mini-Mental State Examination (MMSE) < 27.
Participants who met the inclusion criteria were re-administered the test battery approximately one month later (session 2, range = 14–64 days, mean = 32 days). The majority of the participants were also re-tested after approximately 3 years (session 3, range = 2.9–3.2 years, mean = 3.0 years). Session 2 was included in an effort to attenuate possible re-test effects at session 3, which would lead to an underestimation of cognitive change. This approach was based on evidence that re-test effects tend to be greater for an initial re-test session than for subsequent sessions (Salthouse and Tucker-Drob, 2008) and, in addition, are evident across inter-test delays of several years (Salthouse, 2009). In the present case, we re-administered the neuropsychological test battery after a period (1 month) too short for the scores to be affected by age-related cognitive change. As is detailed below, the mean of the scores obtained in the two sessions served as the baseline for the assessment of change at session 3. Averaging scores across sessions 1 and 2 not only had the benefit of attenuating session 3 re-test effects, but also of providing more reliable estimates of baseline performance than those provided from a single test session (note however that the results reported below for the relationships between fMRI measures and memory performance and change were essentially identical when session 2 scores were employed as the baseline, see Supplementary Material). Missing values from one participant for SDMT, Trail A and Trail B tests at session 3 were replaced by the mean performance of the remaining participants for that session.
A principal component analysis (PCA) was used to reduce the raw scores from the neuropsychological test battery to scores on latent cognitive constructs (component scores). PCA was conducted on the session 1 test data of the 67 eligible participants who provided scores for that session (see the section of Participants above). The raw scores were standardized prior to being subjected to PCA. The four principal components with eigenvalues > 1 were retained and subjected to Varimax rotation (Kaiser, 1958). The resulting component loadings are given in Supplemental Table 1, where it can be seen that the components can be broadly characterized as representing constructs associated with memory, fluency, speed, and crystallized IQ. To maintain comparability of component scores across sessions, for each test in the full group, we combined the test scores from session 1 and session 2 into the same dataset and standardized them together. The component loadings were then applied to the standardized test scores from each session to obtain the component scores for that session. A similar procedure was used to calculate the standardized component scores for the longitudinal subgroup, with the exception that for each test, the scores from all three sessions were combined into a single dataset and then standardized. In both cases, memory component scores averaged across sessions1 and 2 comprised the baseline scores.
2.3. In-scanner associative memory task
A single MRI scanning session, during which both functional and structural data were acquired, occurred between the initial two administrations of the neuropsychological test battery (average of 22 days after Session 1). The fMRI procedure has been described in detail previously (de Chastelaine et al., 2016a, 2016b) and is described only briefly here. Before entering the scanner, participants were instructed on and practiced both the encoding and retrieval phases of the experimental associative recognition test; thus, encoding was not incidental. During an initial functional scan, participants encoded a series of 240 trial-unique word pairs in the context of a relational task (which of the denoted objects would ‘fit’ into which) presented in two consecutive study blocks. After the encoding phase, participants exited the scanner and rested. They re-entered the scanner 15 min later for a scanned associative recognition test that was administrated in three consecutive blocks. The test items comprised 160 ‘intact’ word pairs (pairs re-presented from study), 80 ‘rearranged’ pairs (comprising studied words that were re-paired between study and test), and 80 ‘new’ pairs (pairs of unstudied words). Instructions were to discriminate between the three classes of word pair, signaling the judgment on each trial by pressing one of three buttons. For each of the study and test blocks, there were two buffer pairs at the start and two buffer pairs in the middle, which followed a halfway 30-s break. The study pairs were pseudo-randomly intermixed with 80 null trials and the test pairs were pseudo-randomly intermixed with 106 null trials (de Chastelaine et al., 2016a, 2016b). For both study and test, neither pairs belonging to the same category nor null trials occurred more than three times successively. A fixation cross was continuously present during each null trial.
2.4. MRI acquisition
Functional and structural images were acquired with a Philips Achieva 3T MR scanner (Philips Medical System, Andover, MA USA) equipped with a 32-channel head coil. Functional scans were acquired during both the study and test phases. The functional data were obtained using a T2*-weighted EPI sequence incorporating the following parameters: TR = 2 s, TE = 30 ms, flip angle = 70°, FOV = 240 × 240, matrix size = 80 × 78. Each EPI volume included 33 × 3 mm thick slices with a 1 mm inter-slice gap and an in-plane resolution of 3×3 mm. Slices were acquired oriented parallel to the AC-PC line in ascending order and positioned for full coverage of the cerebrum and most of the cerebellum. Following the second functional scanning session, diffusion tensor images (DTI) and high-resolution T1-weighted images were acquired. The T1-weighted images were acquired with an MP-RAGE pulse sequence (TR = 8.1 ms, TE = 3.7 ms, FOV = 256 × 224, voxel size = 1×1×1 mm, 160 slices, sagittal acquisition).
2.5. Data preprocessing and analysis
MRI data were preprocessed in SPM8 (Wellcome Department of Cognitive Neurology, London, UK). The functional images were motion and slice-time corrected, realigned and spatially normalized using a sample-specific template generated across young, middle-aged and older adults. The template was created by first normalizing the mean volume of each participant’s functional time series (separately for study and test) with reference to a standard EPI template based on the MNI reference brain (Cocosco et al., 1997; see also de Chastelaine et al., 2015, 2016a, 2016b, 2017; King et al., 2018). The normalized mean images were averaged within each group and the resulting 3 mean images were then averaged to generate a template that was equally weighted with respect to the 3 age groups. Images were resampled into 3 mm isotropic voxels and smoothed with an isotropic 8 mm full-width half-maximum Gaussian kernel. For the purposes of template formation and anatomical localization of functional effects, the T1 images were normalized with a procedure analogous to that applied to the functional image but using as an initial template the standard T1-weighed MNI reference brain.
Given our previous findings indicating that both encoding- and recollection-related hippocampal effects were localized primarily to the anterior hippocampus (de Chastelaine et al., 2016a, 2016b), we elected to quantify these functional effects using the anatomically defined anterior hippocampus as the region of interest (ROI). This approach ensured that we sampled encoding- and retrieval- related activity in an unbiased manner from the same hippocampal voxel sets. Following Poppenk et al. (2013), the hippocampal ROIs were defined as the portions of left and right hippocampus anterior to y= −21 in MNI space. The ROIs were manually traced on the across-group average T1 anatomical template following the hippocampal segmentation protocol used by Arnold et al. (2015) (see below).
There were two events of interest for the analysis of hippocampal encoding effects: intact study pairs that were later endorsed as intact (subsequent associative hits) and intact pairs that were later incorrectly identified as rearranged (subsequent associative misses). Intact pairs later incorrectly identified as new were separately modeled, along with all other study pairs and buffer pairs modeled as events of no interest. Analysis of the fMRI recollection effects adopted a similar approach, but with the events of interest comprising correctly endorsed intact pairs (associative hits) and intact pairs incorrectly identified as rearranged (associative misses). Pairs correctly endorsed as rearranged, new pairs correctly endorsed as new, and intact pairs incorrectly endorsed as new were also separately modeled. As for the encoding data, all other test and buffer pairs were modeled as events of no interest. For both the encoding and retrieval data, the rest breaks were also modeled, along with 6 regressors representing motion-related variance and constants representing means across each scan session. Null trials and inter-stimulus intervals were implicitly modeled as the baseline.
For each participant, parameter estimates extracted from voxels falling within the anatomically defined hippocampal ROIs were averaged for each event of interest. Encoding effects were operationalized as greater BOLD activity for items that went on to be classed as associative hits than for items that became associative misses. Recollection effects were operationalized as greater BOLD activity for associative hits than for associative misses. The rationale for these contrasts is detailed in de Chastelaine et al. (2016b). In brief, the contrasts are assumed to isolate neural activity related to the successful recollection of inter-item associations while holding constant the familiarity strength of the individual test items.
2.6. Manual tracing of the hippocampus and estimation of hippocampal volume
Manual tracing of the whole hippocampus was performed using 3DSlicer/v.4.4.0 (https://www.slicer.org) on each participant’s T1-weighted images. Following the hippocampal segmentation protocol by Arnold et al. (2015), hippocampal boundaries were defined laterally and medially by the lateral ventricle, anteriorly by the hippocampal-amygdala transitional zone, posteriorly by the crus of the fornix, inferiorly by the subiculum, and superiorly by the alveus. The volume of interest (VOI) included CA1, CA2/3, dentate gyrus/CA4, alveus and fimbria, avoiding subiculum, the entorhinal cortex and the hippocampus-amygdala transitional zone. Hippocampal volume was estimated by summing the number of voxels within the traced regions. Prior to analysis, hippocampal volume estimates were residualized against intracranial volume (ICV), which was traced from every 12th slice of the transverse plane and estimated using Analyze 11 (https://analyzedirect.com/). Left and right volumes of the anterior hippocampus were estimated by curtailing measurement at the first slice after which the uncal notch was no longer visible. These latter estimates were obtained at the request of a reviewer to give an approximate correspondence with the hippocampal ROIs employed for the functional analyses. The findings reported below were unchanged in all but two minor respects when we repeated the analyses using the anterior rather than the whole hippocampal volume estimates (see Supplemental Material).
2.7. Statistical analyses
We were interested in examining the extent to which the encoding effects and hippocampal recollection effects predicted, 1) in-scanner recollection performance, and 2) baseline memory and longitudinal memory change, as indexed by the memory component scores derived from the neuropsychological test data.
Recollection performance (pR) was indexed by performance on the in-scanner associative recognition task and was estimated as the difference between the proportion of correctly endorsed intact pairs (associative hits) and the proportion of intact pairs incorrectly identified as rearranged (associative misses) (de Chastelaine et al., 2016a, 2016b). In the case of the neuropsychological test data, the average of session 1 and session 2 standardized memory component scores (see the section of Neuropsychological test battery above) provided the baseline against which the scores for session 3 were compared.
To examine whether the hippocampal encoding effects were reliable at the group level, and to examine their lateralization, we conducted a 2 (condition: subsequent associative hit vs. subsequent associative miss) × 2 (hemisphere) ANOVA on parameter estimates extracted from the hippocampal ROIs. An analogous 2 (condition: associative hit vs. associative miss) × 2 (hemisphere) ANOVA was conducted on the parameter estimates extracted from the hippocampal ROIs at retrieval.
We used partial correlation analyses to examine whether encoding- and retrieval-related hippocampal effects, the predictors of primary interest, were related either to in-scanner recollection performance measured by pR, or the baseline memory component scores derived from the test battery (see Neuropsychological test battery above). In addition, we constructed a series of linear mixed effects models to examine whether the effects were predictive of mean memory performance (averaged across baseline and session 3) or longitudinal memory change. Each model included a random intercept term to accommodate individual differences in baseline memory scores. We included chronological age as a predictor in all these analyses because preliminary analyses indicated that this variable was correlated with both hippocampal functional effects and memory performance with small-to-medium effect sizes (absolute rs ranging from .10 to .32). The linear mixed models took the following general form:
where memoryij refers to individual i’s memory performance at session j. Age is participant’s (uncentered) age at baseline, and session is test session (baseline coded as 0, session 3 coded as 1). Hippo_effect refers to either the hippocampal encoding or recollection effect at baseline, and hippo_effect × session refers to the interaction between the hippocampal effect and test session. B denotes fixed-effects estimates, b0 denotes estimates for participant-specific random-effects (i.e. baseline memory scores), and e is the residual error. For those models in which functional activity was found to be a significant predictor, the model was expanded to include hippocampal volume. We constructed additional models to directly examine whether hippocampal volume was predictive of memory performance or memory change.
The ANOVA and correlational analyses were conducted using SPSS v.25 (IBM Corp., Armonk, NY). Linear mixed effects models were estimated in R software (R core Team 2018) using the lmer function from the lme4 package (Bates et al., 2015).
3. Results
3.1. Participant characteristics
Demographic information, and summary measures of hippocampal volume and intracranial volume (ICV) for both the full group the longitudinal subgroup are given in Table 1. Consistent with the impression given by the table, the volume of the left hippocampus was significantly smaller than that of the right hippocampus; for the full group, t(65) = 6.06, p < .001, for the longitudinal subgroup, t(53) = 6.62, p < .001.
Table 1.
Demographic information, summary measures of hippocampal volume and intracranial volume for the study participants (standard deviations in parentheses).
| Variable | |
|---|---|
| Full Group | |
| N | 67 |
| Age at Session 1 (yrs) | |
| M | 68.2 (3.6) |
| Range | 63 – 76 |
| Gender | 37 F, 30 M |
| Education (yrs) | 17.2 (2.3) |
| Left hippocampcal volume (cc) | 3.15 (.43) |
| Right hippocampal volume (cc) | 3.33 (.42) |
| ICV (cc) | 1469.98 (122.22) |
| Longitudinal subgroup | |
| N | 55 |
| Age at Session 1 (yrs) | |
| M | 68.3 (3.7) |
| Range | 63 – 76 |
| Gender | 28 F, 27 M |
| Education (yrs) | 17.3 (2.4) |
| Left hippocampcal volume(cc) | 3.18 (.41) |
| Right hippocampal volume (cc) | 3.39 (.39) |
| ICV (cc) | 1479.05 (127.32) |
3.2. Neuropsychological test performance
Mean neuropsychological test performance is given in Table 2 for each of the test sessions. As is evident from the table, for most tests, performance on the first two sessions was well matched between the full group and the longitudinal subgroup. In both groups, there was an overall improvement across tests from session 1 to session 2. In the longitudinal group, mean performance generally showed modest evidence of change between sessions 2 and 3.
Memory component scores for each test session, and the baseline score averaged across sessions 1 and 2 are shown in Table 3. Performance on session 2 was significantly higher than that on session 1 for both the full group, t(66) = 9.69, p < .001 and the longitudinal subgroup, t(54) = 8.36, p < .001. pR (associative recognition performance) for the full group (M = .30, SD = .15) was closely similar to that for the longitudinal subgroup (M = .28, SD = .14). Together with the findings for the neuropsychological test battery (see Table 2), this finding provides reassurance that attrition of the sample between sessions 2 and 3 was non-selective in respect of baseline cognitive performance. Baseline memory component scores were significantly correlated with memory scores at session 3, r = .87, p < .001, indicating high test-retest reliability between baseline and session 3. Finally, pR was moderately correlated with baseline memory component scores in both groups (for full group, r = .40, p = .001; for longitudinal subgroup, r = .48, p < .001).
Table 3.
Standardized memory component score for each session and change score over three years (standard deviations in parentheses).
| Session | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | baseline (1&2) | change (1&2 – 3) | |
| Full group | −.85 (2.64) | .85 (2.23) | N/A | N/A | N/A |
| Longitudinal subgroup | −.73 (2.69) | .86 (2.30) | −.12 (2.73) | .06 (2.41) | .19 (1.35) |
Note. For the longitudinal subgroup, memory scores were significantly higher for session 2 than session 3, t(54) = 5.37, p < .001.
The difference score between baseline and session 3 is also shown in Table 3. A t test comparing these scores revealed no evidence for longitudinal memory change at the level of the whole sample [t(54) = 1.03, p = .308]. Individual memory changes from baseline to session 3 are illustrated in Figure 1. As is evident from the figure, most participants demonstrated relatively small changes in memory over the three year follow-up interval.
Figure 1.
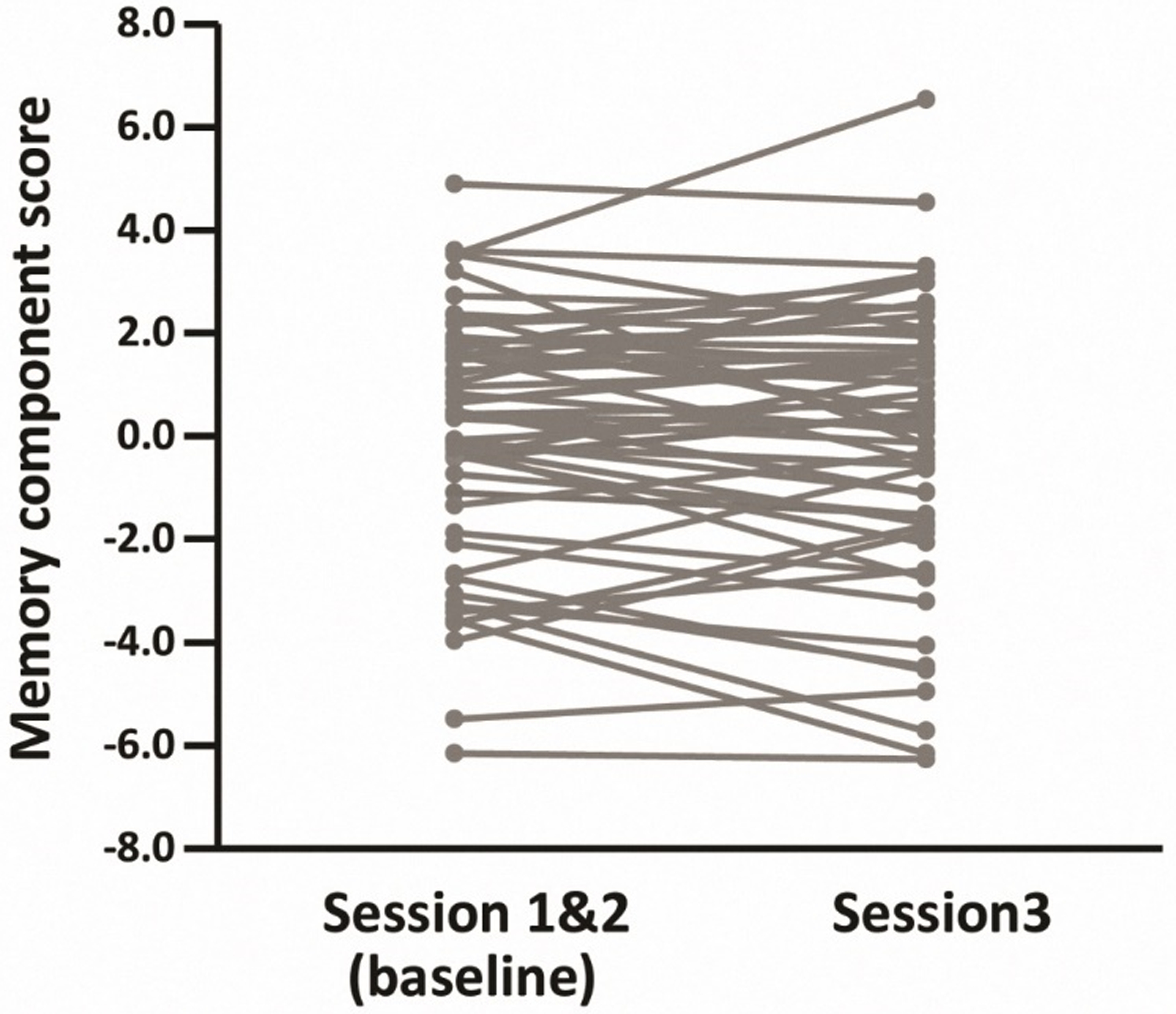
Individual memory component scores at baseline and session 3 for the longitudinal subgroup (N = 55). Each line represents memory change for an individual participant.
3.3. Functional hippocampal effects
Note that for all analyses of baseline data the findings for the full group and the longitudinal subgroup were equivalent. Therefore, we only report the findings from the full group here. Findings for the longitudinal subgroup can be found in the Supplementary Material.
We first examined whether the functional effects were reliable at the group level, and whether there was any evidence of lateralization in the effects (see Materials and Methods). For the encoding data, neither the main effect of condition nor the condition × hemisphere interaction was significant, ps > .075, partial η2s < .05. An analogous ANOVA conducted on the parameter estimates for associative hits and misses at retrieval revealed a significant effect of condition, F(1, 63) = 32.76, p < .001, partial η2 = .34, indicative of greater hippocampal activity for associative hits (M = .10) than for associative misses (M = −.39). This effect interacted significantly with hemisphere, F(1, 63) = 6.38, p = .014, partial η2 = .09, reflecting larger recollection effects in the left hemisphere (M = .59) compared to the right hemisphere (M = .39). Simple effects analyses indicated that recollection effects were reliable in both hemispheres (ps < .001).
3.4. Correlations between functional and structural measures and memory performance
Partial correlations (controlling for age) between hippocampal encoding effects, hippocampal recollection effects and in-scanner recollection performance (pR) are given in the left panel of Table 4, while correlations with the baseline memory scores are shown in the right panel of the table. As is evident from the table, with only one exception, the correlations with pR were significant (see also Figure 2). In contrast to the findings for pR, only the right hippocampal encoding effect was significantly correlated with baseline memory scores (see also Figure 3).
Table 4.
Correlations between hippocampal encoding and recollection effects, associative recognition performance (pR) and baseline memory score, after controlling for age (N = 64).
| pR | baseline memory score | |||
|---|---|---|---|---|
| r | p | r | p | |
| Encoding effect | ||||
| Left hippocampus | .40 | .001 | .13 | .324 |
| Right hippocampus | .38 | .002 | .29 | .024 |
| Recollection effect | ||||
| Left hippocampus | .24 | .057 | −.09 | .464 |
| Right hippocampus | .36 | .004 | −.05 | .682 |
Figure 2.
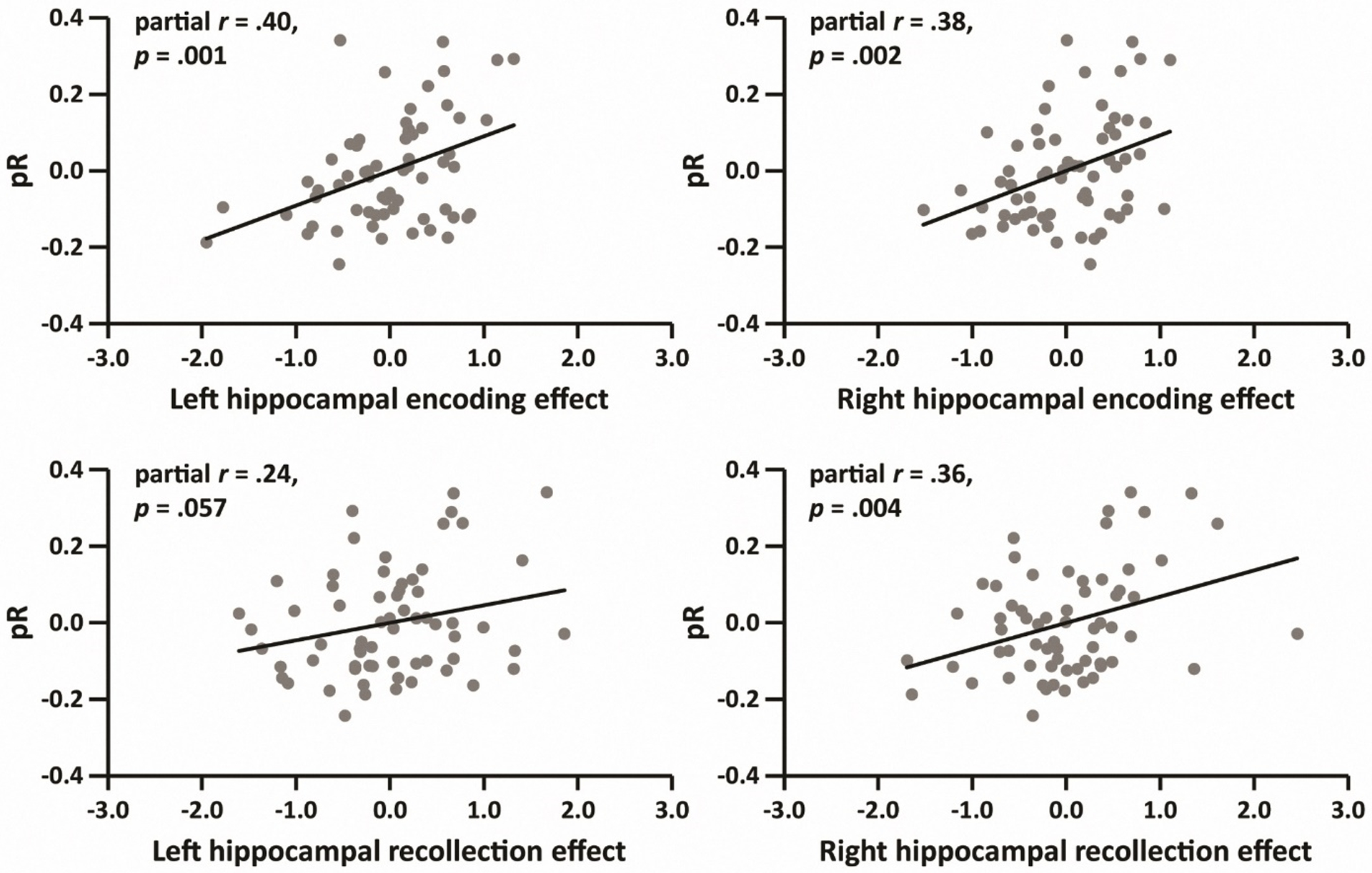
Partial correlations (controlling for age) between pR and functional hippocampal effects (N = 64).
Figure 3.
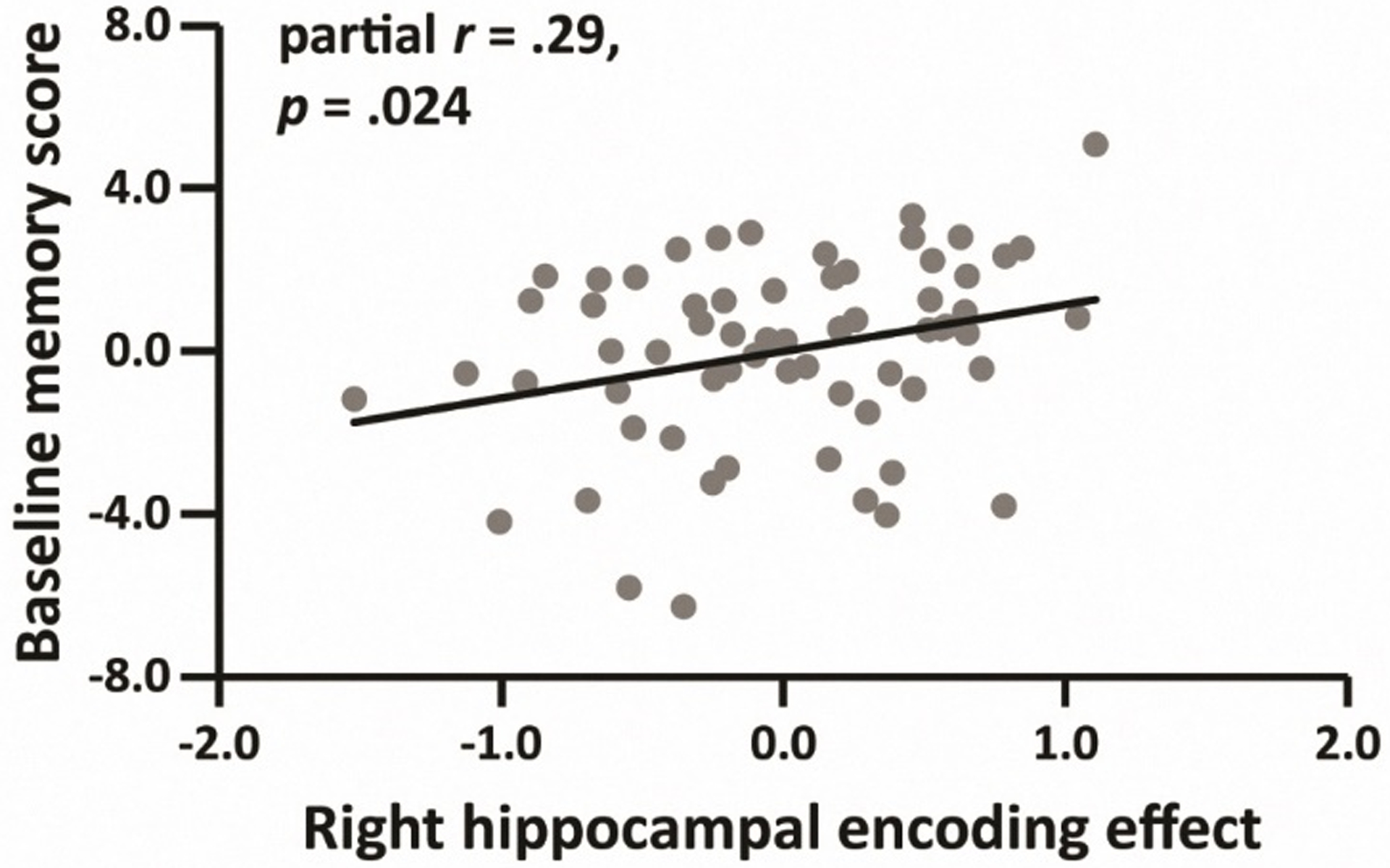
Relationship between the right hippocampal encoding effect and baseline memory score, after controlling for age (N = 64).
To investigate whether any of the relationships between the hippocampal effects and memory performance were mediated by hippocampal volume, we repeated the foregoing analyses with hippocampal volume as an additional covariate. All of the correlations with pR listed in Table 4 remained significant (partial rs > .36, ps < .005). However, the relationship between right hippocampal encoding effect and baseline memory scores was not significant after controlling for right hippocampal volume (partial r = .24, p = .059).
Finally, we examined the direct association between hippocampal volume and pR or baseline memory scores. In contrast to the findings for the functional effects, hippocampal volume was not significantly correlated with pR (for left hippocampus, r = .11, p = .401; for right hippocampus, r = .01, p = .922) or baseline memory scores (for left hippocampus, r = .02, p = .852; for right hippocampus, r = −.10, p = .418).
3.5. Predictors of longitudinal memory change
Based on the general model described in the Materials and Methods (see ‘statistical analyses’), four linear mixed effects models (Models 1–4) – one for each of the hippocampal effects (i.e. left hippocampal encoding effect, right hippocampal encoding effect, left hippocampal recollection effect, and right hippocampal recollection effect) – were constructed. For each model, we were interested in: 1) the contribution of the hippocampal effect, which reflects the strength of the relationship between the effect and mean memory performance, and 2) the hippocampal effect × session interaction, which indexes the relationship between the hippocampal effect and memory change.
Results for each model are shown in Table 5. As is evident from the table, the right hippocampal encoding effect (Model 2) was a significant predictor of overall memory performance in the absence of a significant interaction between the effect and session. This finding is consistent with the results reported in Table 4 and Figure 3.
Table 5.
Linear mixed effects regression results for the encoding- and recollection-related hippocampal effects predicting memory performance and memory change.
| Parameter | B (SE) | df | t | p |
|---|---|---|---|---|
| Model 1 | ||||
| Intercept | 12.07 (6.16) | 50 | 1.96 | .056 |
| Age | −.18 (.09) | 50 | −1.96 | .056 |
| Left_hippo_Enc | .42 (.50) | 58 | .84 | .405 |
| Session | −.15 (.19) | 51 | −.79 | .433 |
| Left_hippo_Enc × Session | −.00 (.27) | 51 | −.01 | .993 |
| Model 2 | ||||
| Intercept | 8.96 (6.07) | 50 | 1.48 | .147 |
| Age | −.13 (.09) | 50 | −1.46 | .150 |
| Right_hippo_Enc | 1.18 (.54) | 58 | 2.18 | .033 |
| Session | −.15 (.18) | 51 | −.82 | .417 |
| Right_hippo_Enc × Session | .05 (.30) | 51 | .18 | .855 |
| Model 3 | ||||
| Intercept | 12.76 (6.33) | 50 | 2.02 | .049 |
| Age | −.18 (.09) | 50 | −1.99 | .052 |
| Left_hippo_Re | −.32 (.45) | 56 | −.71 | .479 |
| Session | −.52 (.20) | 51 | −2.55 | .014 |
| Left_hippo_Re × Session | .71 (.22) | 51 | 3.23 | .002 |
| Model 4 | ||||
| Intercept | 12.48 (6.21) | 50 | 2.01 | .050 |
| Age | −.18 (.09) | 50 | −2.00 | .051 |
| Right_hippo_Re | −.09 (.47) | 57 | −.19 | .852 |
| Session | −.33 (.20) | 51 | −1.67 | .102 |
| Right_hippo_Re × Session | .52 (.25) | 51 | 2.12 | .039 |
Note: Left_hippo_Enc: Left hippocampal encoding effect; Right_hippo_Enc: Right hippocampal encoding effect; Left_hippo_Re: Left hippocampal recollection effect; Right_hippo_Re: Right hippocampal recollection effect.
As is also evident from Table 5, in contrast with Model 2, for Models 3 and 4 the hippocampal recollection effects significantly interacted with test session. Since we used baseline scores (the mean of Sessions 1 and 2) as the reference session, these results indicate that the magnitude of hippocampal recollection effects, especially those in the left hippocampus, was inversely related to longitudinal memory decline. That is, those participants with the largest effects tended to demonstrate the least decline in memory performance over the follow-up period. To visualize these effects, we plotted the interactions using simple slopes based on model-derived parameters (Figure 4A) and, in addition, we computed and plotted the partial correlations between the age-residualized hippocampal recollection effects and residualized memory change (Figure 4B).
Figure 4.
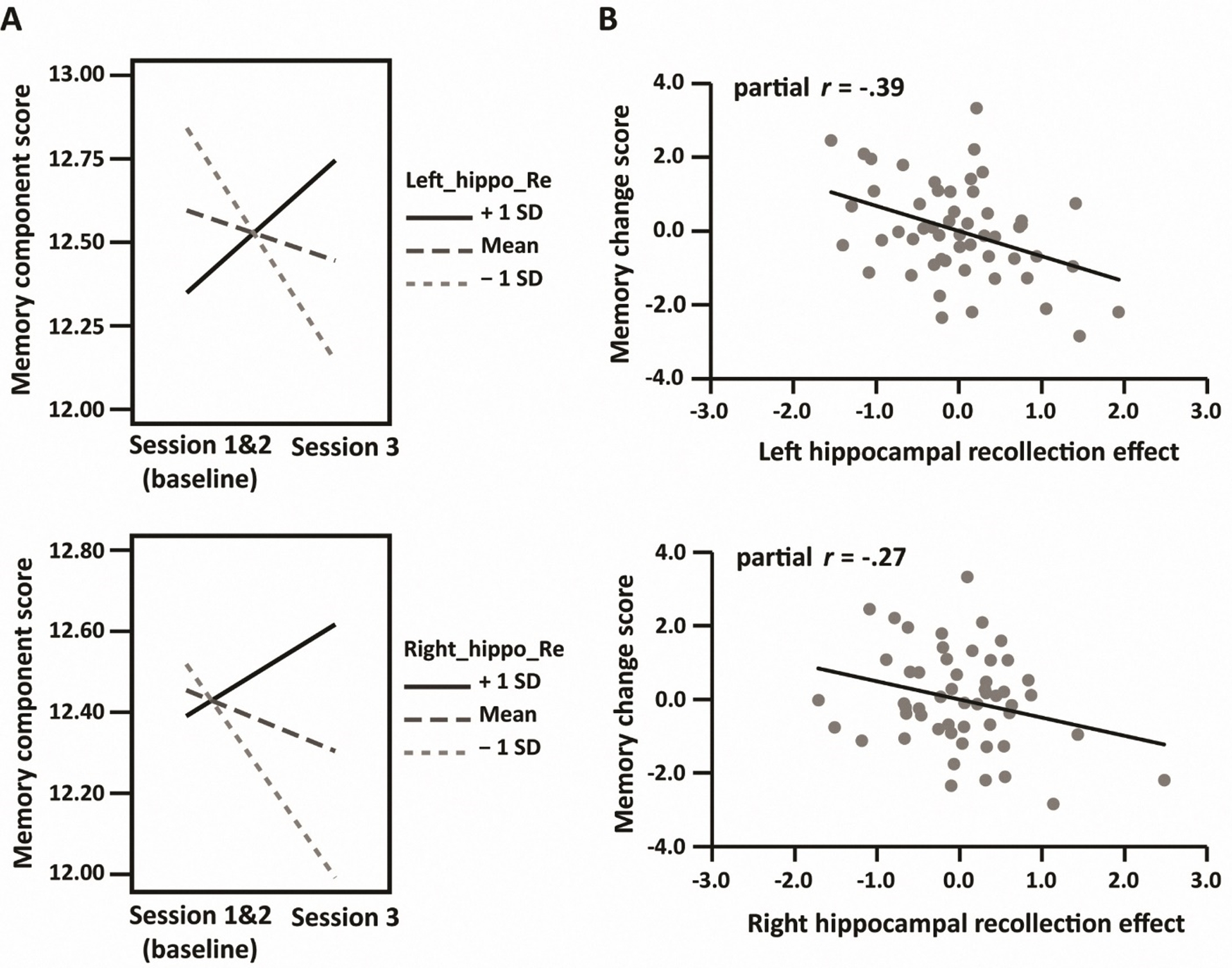
A: Left (upper) and right (lower) hippocampal recollection effect × session interactions visualized with simple slopes (mean ±1SD). B: scatter plots depicting the relationships between memory change scores (baseline minus session 3) and hippocampal recollection effects, controlling for age and baseline scores.
To examine the possible role of hippocampal volume in mediating these relationships, we constructed follow-up regression models in which either left or right hippocampal volume and the hippocampal volume × session interaction were entered in Models 2, 3 and 4 as additional predictor variables. In the presence of these additional variables, neither the relationship between the right hippocampal encoding effect and memory performance, nor the interaction between the right hippocampal recollection effect and session, were significant [respectively: B = .91, t(56) = 1.57, p = .123, B = .44, t(49) = 1.54, p = .130]. However, the interaction between the left hippocampal recollection effect and session remained significant [B = .66, t(49) = 2.79, p = .007].
We also performed two linear mixed effects analyses to directly examine the relationship between hippocampal volume and memory performance or change. The two models included either left or right hippocampal volume, session and hippocampal volume × session as independent variables of interest, and longitudinal memory performance as the dependent variable. We did not identify a significant effect in either model (ps > .222).
3.6. Correlations among hippocampal functional effects and hippocampal volume
Simple correlations between hippocampal encoding effects, hippocampal recollection effects and hippocampal volume are given in Table 6. As is evident from the table, the encoding effect did not significantly correlate with the ipsilateral recollection effect in either hemisphere. Furthermore, neither the left nor the right hemisphere functional effects correlated with their respective hippocampal volumes.
Table 6.
Simple correlations among hippocampal encoding effects, hippocampal recollection effects and hippocampal volumes in the full group (N = 65 for hippocampal volume, N = 64 for hippocampal effects).
| Variable | By variable | r | p |
|---|---|---|---|
| Ipsilateral correlations | |||
| Left_hippo_Enc | Left_hippo_Re | −.01 | .966 |
| Left_hippo_vol | Left_hippo_Enc | −.13 | .304 |
| Left_hippo_vol | Left_hippo_Re | −.06 | .656 |
| Right_hippo_Enc | Right_hippo_Re | −.00 | .974 |
| Right_hippo_vol | Right_hippo_Enc | −.15 | .241 |
| Right_hippo_vol | Right_hippo_Re | −.22 | .080 |
| Contralateral correlations | |||
| Left_hippo_Enc | Right_hippo_Enc | .59 | < .001 |
| Left_hippo_Re | Right_hippo_Re | .65 | < .001 |
| Left_hippo_vol | Right_hippo_vol | .78 | < .001 |
Note. Left_hippo_Enc: Left hippocampal encoding effect; Left_hippo_Re: Left hippocampal recollection effect; Left_hippo_vol: Left hippocampal volume; Right_hippo_Enc: Right hippocampal encoding effect; Right_hippo_Re: Right hippocampal recollection effect; Right_hippo_vol: Right hippocampal volume. Volume measures were residualized against ICV.
As is also evident from Table 6, in contrast to the ipsilateral correlations, there were robust positive across-hemisphere correlations for both classes of functional effect. Similarly, left and right hippocampal volumes were positively correlated.
3.7. Specificity of the relationships between hippocampal functional effects and memory
We took two steps to examine whether the hippocampal functional effects were selectively predictive of memory performance and memory change. First, we examined the relationships between these hippocampal effects and the component scores for the three other cognitive domains. Second, we tested whether the hippocampal encoding and recollection effects remained as significant predictors of memory performance or memory change after controlling for the variance shared with other cognitive domains. Specifically, we calculated the mean scores across the other three domains at both baseline and session 3 (hereafter, the mean of these scores is termed ‘MOTH-COG’). To ascertain whether functional brain measures explained variance unique to memory measures, these scores were included as additional covariates in the relevant statistical models.
Complete results of these analyses can be found in Section 10 of the Supplemental Material. Here we briefly describe the most important findings. In the case of baseline cognitive performance, after controlling for age there were no significant correlations between hippocampal encoding- or recollection effects and fluency, speed or crystallized IQ component scores (absolute partial rs < .25, ps > .056). Furthermore, after controlling for both age and variance shared with other cognitive domains, hippocampal encoding and recollection effects continued to correlate significantly with pR (partial rs > .28, ps < .024). However, the previously identified relationship between the right hippocampal encoding effect and baseline memory component scores was no longer significant (partial r = .18, p = .153).
In the case of longitudinal cognitive change, we constructed a series of linear mixed effects models to examine whether hippocampal functional effects were predictive of longitudinal change in the fluency, speed or crystalized IQ component scores. The only significant finding was that the right hippocampal encoding effect predicted mean fluency performance [B = .95, t(59) = 2.04, p = .046]. There was no evidence that the hippocampal effects were predictive of longitudinal change in any of the three domains.
To examine whether the relationships identified in Models 2–4 (see Table 5) reflected brain-behavior associations unique to memory performance and memory change, we constructed additional linear mixed models in which MOTH-COG and the MOTH-COG × Session interaction were included as additional predictors. With these additional predictors included, the right hippocampal encoding effect no longer predicted mean memory performance [B = .52, t(62) = 1.13, p = .263]. In contrast, both left and right hippocampal recollection effects continued to predict memory change [respectively: B = .55, t(50) = 2.79, p =.007, B = .54, t(50) = 2.58, p = .013].
4. Discussion
The primary aim of the present study was to investigate the relationships between encoding- and recollection-related hippocampal effects, memory performance and three-year longitudinal memory change in a sample of healthy older adults. We found that while right hippocampal encoding effects were correlated with baseline memory performance, both left and right hippocampal recollection effects were predictive of memory change, such that larger effects were associated with less decline in performance over the three-year follow-up period. To our knowledge, this is the first evidence to link hippocampal recollection effects obtained at baseline to longitudinal memory change in older adults.
Before discussing these findings, we note that re-test effects can pose significant obstacles for the interpretation of longitudinal data. Such effects can persist over several years and lead to the underestimation of cognitive change (Nyberg et al., 2016; Salthouse, 2009). In light of prior findings that re-test effects for verbal memory tend to diminish after the first re-test session (Salthouse and Tucker-Drob, 2008), we adopted a burst measurement design (Salthouse and Nesselroade, 2010) and re-administered the neuropsychological test battery shortly after the first test session. For the reasons outlined in the Materials and Methods, we elected to employ test scores averaged over these initial two sessions to estimate baseline performance. When assessed against this baseline, memory performance did not demonstrate a reliable decline over the follow-up period at the group level. However, this finding should not necessarily be taken as evidence that the memory performance of our sample remained stable over this period. Notably, if it is assumed that session 2 performance provides the best correction for session 3 re-test effects, then memory scores declined significantly and robustly at the group level (see Table 3). Given that there is no basis for preferring one of these measures of baseline performance over the other (or any other weighting of session 1 and session 2 performance), we elected to employ arguably the most stable measure, and to interpret the ensuing metric of memory change in relative rather than absolute terms. That being said, as is reported in the Supplementary Material, our main findings were unaffected when session 2 scores alone were employed as the baseline measure.
We note that an alternative to the burst measurement procedure adopted here would be to employ parallel versions of each test, eliminating the component of re-test effects that results from prior exposure to the same test items. Obviously, this approach is possible only when a sufficient number of versions of a test are available to allow separate versions to be employed at each test session. This was not the case in the present study. Furthermore, it is worth noting that the employment of parallel tests tends to reduce, rather than eliminate, re-test effects, and does so to varying degrees depending on the specific test (Salthouse and Tucker-Drob, 2008).
As was noted in the Introduction, prior fMRI studies examining across-participants relationships between encoding- and recollection-related hippocampal effects and memory performance focused on measures of performance derived from the memory task employed to estimate the fMRI effects (e.g. Daselaar et al., 2006; de Chastelaine et al., 2016a, 2016b; Wang et al., 2016). The novel aspect of the present study is the extension of these analyses to ‘offline’ neuropsychological measures of baseline memory performance and its change over time. However, it should be noted that the positive age-invariant correlations between hippocampal encoding and recollection effects with in-scanner memory performance described in our prior reports (de Chastelaine et al., 2016a, 2016b) were also evident in the present sample, which comprised a subgroup of the 136 participants described in those reports. The present findings lend credence to the proposal that these fMRI effects index the efficacy of functionally significant mnemonic processes in older adults. Indeed, a multiple regression model predicting the in-scanner associative recognition performance of our baseline sample of 64 older participants from their four hippocampal functional effects (i.e. left and right encoding and recollection effects) accounted for more than 30% of the variability in performance (adjusted R2 = .318, p < .001).
As already noted, the principal focus of the present study was not on the relationship between fMRI effects and in-scanner memory performance, but rather, the relationship between these effects and memory metrics derived from standardized neuropsychological test scores. In the case of baseline performance, we identified a significant positive relationship between baseline scores and the right hippocampal encoding effect, and obtained a convergent result from the linear mixed effects model (Model 2) that employed this fMRI effect as a predictor of memory performance in the longitudinal subgroup (although, obviously, these two findings should not be viewed as independent). Turning to the longitudinal component of the study, we found that both hippocampal recollection effects were predictive of memory change, although the relationship in the left hippocampus was the more robust.
Whereas the sizeable correlations between in-scanner memory performance and hippocampal encoding and recollection effects point to the functional significance of both classes of effect, this does not mean that they reflect common, or even closely related, cognitive operations. Moreover, the finding that the across-subject correlations between two classes of effect were essentially zero (Table 6) indicates that the effects do not both reflect individual differences in some ‘trait-like’ factor such as hippocampal functional integrity or efficacy. Arguably, these findings are understandable given the differing roles proposed for the hippocampus during encoding and retrieval (e.g. Rugg et al., 2015). At encoding, the hippocampus is held to be responsible for ‘binding’ patterns of cortical activity elicited by an event into a sparse, content- addressable memory representation. As was discussed in de Chastelaine et al. (2016a), in light of this proposed role, the relationship between hippocampal encoding effects and memory performance could be an indirect rather than a direct one. That is, the relationship might reflect individual differences, not in the functional efficacy of the hippocampus, but in the amount or the quality of the information about a study event that it receives. For example, there is evidence that both subsequent memory performance and hippocampal encoding effects are sensitive to the amount of attentional resources that are directed toward a study event or a subset of its features (e.g. Aly and Turk-Browne, 2016; Uncapher and Rugg, 2009). Therefore, the present findings for associative memory performance might be a reflection of individual differences in the processing resources or attentional strategies engaged by participants while they performed the study task. The finding that (right) hippocampal encoding effects also predicted baseline memory performance suggests that these individual differences also contributed to across participant variability in baseline memory scores. This proposal receives further support from the additional finding that performance on the experimental memory test (indexed by the pR metric) and baseline memory performance were robustly correlated, a finding reminiscent of prior reports that performance on experimental tests of memory correlates with performance on standardized neuropsychological memory tests (e.g. Anderson et al., 2008; Davidson and Glisky, 2002).
The contribution of the hippocampus to successful episodic retrieval is distinct from that at encoding. Recollection is held to occur when a retrieval cue activates a hippocampal memory representation sufficiently to give rise to ‘pattern completion’, which restores the representation to an active state. In turn, this leads to reinstatement of the encoded pattern of cortical activity, providing access to mnemonic content (see Rugg et al., 2015, for review). From this perspective, therefore, the determinants of the magnitude of hippocampal recollection effects are distinct from those moderating encoding effects, and will include such factors as the efficacy of cue processing and the amount and specificity of the information retrieved in response to the cue (Mayes et al., 2019; Rugg et al., 2012). Since these factors would be expected to contribute to memory performance, it is perhaps unsurprising that hippocampal recollection effects correlated significantly with associative recognition performance in the present study, while at the same time correlating negligibly with hippocampal encoding effects.
Why was memory change correlated with hippocampal recollection effects, but not with encoding effects? We conjecture that this dissociation reflects a combination of two factors. First, that age-related memory change largely reflects a decline in the ability to recollect qualitative information about past events, rather than in memory processes that do not depend heavily on the hippocampus, such as familiarity (see Koen and Yonelinas, 2014, for review of the extensive cross-sectional literature supporting this contention). Second, that hippocampal recollection effects provide a ‘purer’ or more direct index of the structure’s contribution to recollection than do encoding effects which, as noted previously, are likely sensitive to a multiplicity of processes that depend on extra-hippocampal regions. The relationship between hippocampal recollection effects and memory change can then be explained if it is assumed that the effects are indicative of both the current functional integrity of the structure, and its resilience to future age-related functional degradation. This resilience may result either from a relatively low rate of functional decline or, as a reviewer suggested, from a ‘raised functional baseline’, such that a greater level of ‘hippocampal reserve’ is available to support memory function in the face of neural decline. Arbitrating between these and other possible accounts seems a worthy goal for future research.
In the present study, all correlations between functional hippocampal effects and memory performance were positive: larger effects predicted higher performance on the in-scanner memory task, as well as higher baseline memory scores and less decline in these scores over three years. These findings stand in contrast to those from other studies where either a null (Dulas and Duarte, 2011, 2016) or a negative relationship (Carr et al., 2017; Daselaar et al., 2015; Miller at al., 2008) between hippocampal memory effects and memory performance was reported. Whereas null findings can plausibly be attributed to any number of factors that might have obscured a ‘true’ relationship, notably, lack of statistical power arising from small sample sizes, findings of a negative relationship clearly conflict with the present results (and those of, for example, Daselaar et al., 2006; de Chastelaine et al., 2016a, 2016b; Wang et al., 2016). One possibility is that these disparate findings reflect variation in the cognitive status of the older adult samples employed in the different studies. Notably, it has been reported that older adults with mild cognitive impairment (MCI) likely attributable to prodromal Alzheimer’s Disease (AD) demonstrate hippocampal ‘hyperactivity’ – an elevation of task-related hippocampal responses relative to age-matched, low-risk controls (e.g. Bakker et al., 2015; Dickerson et al., 2005; Putcha et al., 2011). Moreover, negative correlations between task-related hippocampal activity and memory performance have been reported in MCI samples (Bakker et al., 2012; Yassa et al., 2010). Thus, if samples of older adults include a sufficient number of individuals at high risk for AD, a negative relationship between hippocampal memory effects and performance might be anticipated. In the present study, participants were screened to exclude individuals with cognitive profiles or medical histories indicative of elevated risk for prodromal AD (see Materials and Methods). Of importance, all but two of the participants in our longitudinal subgroup continued to meet these inclusion criteria when tested at follow-up. [For one of the two participants who failed to meet the criteria, CVLT hit rate and the score on the Raven’s progressive matrices test both fell below criterion; performance on all other tests was well above criterion, however. The other participant had an estimated FSIQ that fell below 100, but, again, otherwise demonstrated scores well above criterion]. Thus, we assume our initial screening procedure was reasonably effective. A similar approach was adopted in Miller et al. (2008), but it is perhaps noteworthy that the participants included in the study of Carr et al. (2017) comprised a mixture of healthy and cognitively impaired older adults. No information about the cognitive profiles of the participants employed in Daselaar et al. (2015) was provided in that report.
A last noteworthy feature of the findings in respect of the hippocampal memory effects is the specificity with which they predicted memory performance, rather than cognitive performance more generally (see supplementary material). Not only did the effects show little evidence of correlating with non-mnemonic component scores, both right and left hippocampal recollection effects continued to predict memory change when regression models were expanded to include the additional predictor variables of the mean performance across the three other cognitive domains, and its interaction with session. These findings are especially salient given that the memory component score shared a significant fraction of its variance with the other component scores (rs = .387, .519 and .588 for crystallized IQ, fluency and speed respectively, all p < .002). They suggest that, at least in cognitively healthy older adults, individual differences in hippocampal functional effects are poor predictors of cognitive ability outside of the relatively narrow domain of memory.
In contrast to the functional effects, we were unable to identify significant relationships between hippocampal volume and either baseline memory performance or memory change. Perhaps unsurprisingly in light of these null findings, hippocampal volume also had little or no mediating influence on the relationship between the functional effects and these behavioral measures. The present null findings in respect of hippocampal volume are not without precedent. The numerous prior studies examining the relationship between hippocampal volume and memory performance in healthy older adults have yielded an inconsistent pattern. Whereas some studies reported a positive correlation (e.g. Ezzati et al., 2016; O’Shea et al., 2016; Rosen et al., 2003), others have failed to find such evidence (e.g. Charlton et al., 2010; Walhovd et al., 2010; for reviews, see Kaup et al., 2011; Van Petten, 2004). Similar inconsistencies also exist for longitudinal studies examining relationships between hippocampal volume and memory decline (see Gorbach et al., 2017; Mungas et al., 2005 for examples of positive findings; see Cardenas et al., 2011; Carmichael et al., 2012 for examples of null results; for review, see Oschwald et al., 2019). As in the case of the inconsistent findings regarding the relationship between hippocampal functional effects and memory performance discussed previously, these inconsistent findings in respect of hippocampal volume may also reflect variation across studies in the proportion of participants with incipient neuropathology. Notably, hippocampal volume has consistently been reported to predict memory performance and longitudinal memory change in participants with MCI (Fellgiebel and Yakushev., 2011; Grundman et al., 2003; Nathan et al., 2017; Mungas et al., 2005; Stoub et al., 2010; Leong et al., 2017). Thus, inclusion in an experimental sample of older adults at high risk for, or progressing toward MCI, would likely exaggerate the relationships between hippocampal volume and memory performance. The present findings raise the possibility that, in cognitively and, arguably, neurologically healthy older adults, measures of hippocampal function might be more sensitive predictors of memory performance and change than measures of hippocampal structure.
In the present sample of older adults, hippocampal volume demonstrated a robust asymmetry in favor of the right hemisphere. Similar findings have been reported previously not only older adults, but in young and middle-aged samples also (e.g. Woolard and Heckers, 2012; Wellington et al., 2013; for reviews, see Pedraza et al., 2004; Shi et al., 2009), indicating that the asymmetry is unlikely to be a consequence of aging. Indeed, similarly sized hippocampal asymmetries were evident in the groups of young and middle-aged adults who, along with the present older sample, contributed the fMRI data described in prior reports (e.g. de Chastelaine et al., 2016a, 2016b, see Supplemental Material). Although positive correlations between degree of the asymmetry and measures of verbal memory and fluency were reported in one study (Woolard and Heckers, 2012), its functional significance remains obscure.
Finally, we note a number of limitations of the present study. First, the sample size was modest, limiting statistical power and constraining the size of the effects that could be detected. Second, since we only assessed memory performance on what was, effectively, two occasions, we were unable to characterize the trajectory of memory change in our participants. This limitation is compounded by the relatively short follow-up period of three years. Third, the associations with hippocampal effects identified here could conceivably reflect individual differences not only in neural activity but in one or more vascular factors, such as cerebrovascular reactivity (CVR) – an important non-neural determinant of BOLD signal magnitude (e.g. Liu et al., 2013; Lu et al., 2011; Tsvetanov et al., 2015). Since we did not control for CVR, we cannot rule out some influence of this variable. Finally, our test battery did not include measures of visual or spatial long-term memory. Thus, we cannot ascertain whether the null effects we observed for the relationship between hippocampal volume and verbal memory performance extend to non-verbal memory. Clearly, future research would benefit from the employment of larger samples subjected to multiple test sessions over a longer overall follow-up period and a more extensive test battery, along with functional methods that correct for or which are insensitive to individual differences in neurovascular coupling.
These limitations notwithstanding, consistent with prior findings reviewed in the Introduction, the present results suggest that hippocampal functional activity is predictive of both individual differences in memory performance and longitudinal memory change in cognitively unimpaired older adults. Going beyond prior reports, the results further suggest that experimental contrasts that isolate the role of the hippocampus in recollection-based memory judgments might hold promise as predictors of future memory performance in this population.
Supplementary Material
Acknowledgements
We gratefully acknowledge the contributions of Hannah Stanton and Kay Moolenijzer for their assistance with participant recruitment and neuropsychological data collection. We also gratefully acknowledge the contribution of Amber Kidwai to the measurement of anterior hippocampal volumes. We thank the staff of the UTSW Advanced Imaging Center for their assistance with MRI data collection.
Funding
This work was supported by the National Institute on Aging (grant number 1RF1AG039103).
References
- Aly M, Turk-Browne NB. 2016. Attention promotes episodic encoding by stabilizing hippocampal representations. Proc Natl Acad Sci. 113:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. 2008. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 22:177–187. [DOI] [PubMed] [Google Scholar]
- Angel L, Bastin C, Genon S, Salmon E, Fay S, Balteau E, Maquet P, Luxen A, Isingrini M, Collette F. 2016. Neural correlates of successful memory retrieval in aging: Do executive functioning and task difficulty matter?. Brain Res. 1631:53–71. [DOI] [PubMed] [Google Scholar]
- Arnold SJ, Ivleva EI, Gopal TA, Reddy AP, Jeon-Slaughter H, Sacco CB, Francis AN, Tandon N, Bidesi AS, Witte B, Poudyal G, Pearlson GD, Sweeney JA, Clementz BA, Keshavan MS, Tamminga CA. 2015. Hippocampal volume is reduced in schizophrenia and schizoaffective disorder but not in psychotic bipolar I disorder demonstrated by both manual tracing and automated parcellation (FreeSurfer). Schizophr Bull. 41:233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Albert MS, Krauss G, Speck CL, Gallagher M. 2015. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage: Clinical. 7: 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. 2012. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 74:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. lme4: Linear mixed-effects models using Eigen and S4 (R package version 1.1–7). URL http://CRAN.R-project.org/package=lme4.
- Cardenas VA, Chao LL, Studholme C, Yaffe K, Miller BL, Madison C, Buckley ST, Mungas D, Schuff N, Weiner MW. 2011. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging. 32:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Mungas D, Beckett L, Harvey D, Farias ST, Reed B, Olichney J, Miller J, DeCarli C. 2012. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging. 33:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr VA, Bernstein JD, Favila SE, Rutt BK., Kerchner GA, Wagner AD. 2017. Individual differences in associative memory among older adults explained by hippocampal subfield structure and function. Proc Natl Acad Sci. 114:12075–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton RA., Barrick TR, Markus HS, Morris RG. 2010. The relationship between episodic long-term memory and white matter integrity in normal aging. Neuropsychologia. 48:114–122. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RKS, Pike GB, Evans AC. 1997. Brainweb: Online interface to a 3D MRI simulated brain database. Neuroimage. 5:425. [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. 2006. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 16:1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Iyengar V, Davis SW, Eklund K, Hayes SM, Cabeza RE. 2015. Less wiring, more firing: low-performing older adults compensate for impaired white matter with greater neural activity. Cereb Cortex. 25:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. 2002. Neuropsychological correlates of recollection and familiarity in normal aging. Cogn Affect Behav Neurosci 2:174–186. [DOI] [PubMed] [Google Scholar]
- de Chastelaine M, Donley BE, Kennedy KM, Rugg MD. 2019. Age moderates the relationship between cortical thickness and cognitive performance. Neuropsychologia, 132:107136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. 2015. Sensitivity of negative subsequent memory and task-negative effects to age and associative memory performance. Brain Res. 1612:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. 2016a. The relationships between age, associative memory performance, and the neural correlates of successful associative memory encoding. Neurobiol Aging. 42:163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. 2016b. The neural correlates of recollection and retrieval monitoring: Relationships with age and recollection performance. Neuroimage. 138:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Mattson JT, Wang TH, Donley BE, Rugg MD. 2017. Independent contributions of fMRI familiarity and novelty effects to recognition memory and their stability across the adult lifespan. Neuroimage. 156:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. 2011. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb Cortex. 21:2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. 2000. California Verbal Learning Test, 2nd edn. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. 2008. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 34:791–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. 2005. Increased hippocampal activation in mildcognitive impairment compared to normal aging and AD. Neurology. 65:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. 2008. The effects of aging on the neural correlates of subjective and objective recollection. Cereb Cortex. 18:2169–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. 2011. The effects of aging on material-independent and material-dependent neural correlates of contextual binding. Neuroimage. 57:1192–1204. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. 2014. Aging affects the interaction between attentional control and source memory: an fMRI study. J Cogn Neurosci. 26:2653–2669. [DOI] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. 2016. Age-related changes in overcoming proactive interference in associative memory: The role of PFC-mediated executive control processes at retrieval. Neuroimage. 132:116–128. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. 2008. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol Aging. 29:1902–1916. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H 2017. Prefrontal–hippocampal interactions in episodic memory. Nat Rev Neurosci. 18:547–558. [DOI] [PubMed] [Google Scholar]
- Ezzati A, Katz MJ, Lipton ML, Zimmerman ME, Lipton RB. 2016. Hippocampal volume and cingulum bundle fractional anisotropy are independently associated with verbal memory in older adults. Brain Imaging Behav. 10:652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellgiebel A, Yakushev I. 2011. Diffusion tensor imaging of the hippocampus in MCI and early Alzheimer’s disease. J Alzheimers Dis. 26:257–262. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. 2009. One-year brain atrophy evident in healthy aging. J Neurosci. 29: 15223–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser MA, Shaw ME, Cherbuin N. 2015. A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. Neuroimage. 112:364–374 [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Dew ITZ. 2015. Relational memory and its relevance to aging In Addis DR, Barense M, Duarte A, editors. The Wiley handbook on the cognitive neuroscience of memory. Oxford: Wiley-Blackwell; p 371–392 [Google Scholar]
- Gorbach T, Pudas S, Lundquist A, Orädd G, Josefsson M, Salami A, de Luna X, Nyberg L. 2017. Longitudinal association between hippocampus atrophy and episodic-memory decline. Neurobiol Aging. 51:167–176. [DOI] [PubMed] [Google Scholar]
- Grundman M, Jack CR Jr, Petersen RC, Kim HT, Taylor C, Datvian M, Weiner MF, DeCarli C, DeKosky ST, van Dyck C, et al. 2003. Hippocampal volume is associated with memory but not nonmemory cognitive performance in patients with mild cognitive impairment. J Mol Neurosci. 20:241–248. [DOI] [PubMed] [Google Scholar]
- Hantke N, Nielson KA, Woodard JL, Breting LMG, Butts A, Seidenberg M, Smith JC, Durgerian S, Lancaster M, Matthews M, Sugarman MA, Rao SM. 2013. Comparison of semantic and episodic memory BOLD fMRI activation in predicting cognitive decline in older adults. JINS. 19:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser HF. 1958. The varimax criterion for analytic rotation in factor analysis. Psychometrika, 23:187–200. [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. 2011. A review of the brain structure correlates of successful cognitive aging. J Neuropsychiatry Clin Neurosci. 23:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Giovanello KS. 2011. The effects of attention on agerelated relational memory deficits: fMRI evidence from a novel attentional manipulation. J Cogn Neurosci. 23: 3637–3656. [DOI] [PubMed] [Google Scholar]
- King DR, de Chastelaine M, Rugg MD. 2018. Recollection-related increases in functional connectivity across the healthy adult lifespan. Neurobiol Aging. 62:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koen JD, Yonelinas AP. 2014. The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer’s disease on recollection and familiarity: A meta-analytic review. Neuropsychol Rev. 24:332–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Landau SM, Bell RK, Jagust WJ. 2017. Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. Elife, 6:e22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Yassa MA. 2015. Neurocognitive aging and the hippocampus across species. Trends Neurosci. 38:800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong RLF, Lo JC, Sim SKY, Zheng H, Tandi J, Zhou J, Chee MWL. 2017. Longitudinal brain structure and cognitive changes over 8 years in an East Asian cohort. Neuroimage. 147: 852–860. [DOI] [PubMed] [Google Scholar]
- Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Park DC, Lu H. 2013. Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. Neuroimage. 78:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J, Park DC. 2011. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 21:1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D, Rajah MN. 2014. Age-related differences in brain activity in the subsequent memory paradigm: a meta-analysis. Neurosci Biobehav Rev. 45:246–257. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Montaldi D, Roper A, Migo EM, Gholipour T, Kafkas A. 2019. Amount, not strength of recollection, drives hippocampal activity: A problem for apparent word familiarity‐related hippocampal activation. Hippocampus. 29:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamäki M, Sperling RA. 2008. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci. 105:2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, Nadel L. 2016. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu Rev Psychol. 67:105–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. 2005. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 65:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PJ, Lim YY, Abbott R, Galluzzi S, Marizzoni M, Babiloni C, et al. 2017. Association between CSF biomarkers,hippocampal volume and cognitive function in patients withamnestic mild cognitive impairment (MCI). Neurobiol Aging. 53:1–10 [DOI] [PubMed] [Google Scholar]
- Nyberg L, Pudas S, Lundquist A. 2016. Structural and functional imaging of aging: longitudinal studies In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. New York (NY): Oxford University Press; p 155–182 [Google Scholar]
- Nyberg L, Pudas S. 2019. Successful memory aging. Annu Rev Psychol. 70:219–243. [DOI] [PubMed] [Google Scholar]
- O’Brien JL, O’keefe KM, LaViolette PS, DeLuca AN, Blacker D, Dickerson BC, Sperling RA. 2010. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 74:1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oschwald J, Guye S, Liem F, Rast P, Willis S, Röcke C, Lutz Jäncke, Martin M, Mérillat S. 2019. Brain structure and cognitive ability in healthy aging: a review on longitudinal correlated change. Rev Neuroscience. 31:1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea A, Cohen R, Porges EC, Nissim NR, Woods A.J. 2016. Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. 8:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza O, Bowers D, Gilmore R. 2004. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. JINS. 10:664–678. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. 2006. Structure–function correlates of cognitive decline in aging. Cereb Cortex. 16:907–915. [DOI] [PubMed] [Google Scholar]
- Persson J, Pudas S, Lind J, Kauppi K, Nilsson LG, Nyberg L. 2012. Longitudinal structure–function correlates in elderly reveal MTL dysfunction with cognitive decline. Cereb Cortex. 22(10):2297–2304. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. 2013. Long-axis specialization of the human hippocampus. Trends Cog Sci. 17:230–240. [DOI] [PubMed] [Google Scholar]
- Pudas S, Persson J, Josefsson M, de Luna X, Nilsson LG, Nyberg L. 2013. Brain characteristics of individuals resisting age-related cognitive decline over two decades. J Neurosci. 33:8668–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudas S, Persson J, Nilsson LG, Nyberg L. 2014. Midlife memory ability accounts for brain activity differences in healthy aging. Neurobiol Aging. 35:2495–2503. [DOI] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O’Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. 2011. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J Neurosci. 31:17680–17688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, Maillet D, Grady CL, 2015. Episodic memory in healthy older adults In Addis DR, Barense M, Duarte A, editors. The Wiley handbook on the cognitive neuroscience of memory. Oxford: Wiley-Blackwell; p 347–370. [Google Scholar]
- Raven J, Raven JC, Courth JH. 2000. Manual for Raven’s progressive matrices and vocabulary scales. Section 4: the advanced progressive matrices. [Google Scholar]
- Raven J, Raven JC, Courth JH. 2000. Manual for Raven’s progressive matrices and vocabulary scales Section 4: the advanced progressive matrices. San Antonio, TX: Harcourt Assessment [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Daugherty AM, Haacke EM, Raz N. 2013. The role of hippocampal iron concentration and hippocampal volume in age-related differences in memory. Cereb Cortex. 23:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, Gabrieli JD, Stoub T, O’Hara R, Friedman L, Yesavage JA, deToledo-Morrell L. 2003. Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behav Neurosci. 117:1150–1160. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Uncapher MR. 2015. Encoding and retrieval in episodic memory: Insights from fMRI In Addis DR, Barense M, Duarte A, editors. The Wiley handbook on the cognitive neuroscience of memory. Oxford: Wiley-Blackwell; p 84–107 [Google Scholar]
- Rugg MD, Morcom AM. 2005. The relationship between brain activity, cognitive performance, and aging: the case of memory In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. New York (NY): Oxford University Press; p 132–154 [Google Scholar]
- Rugg MD, Vilberg KL, Mattson JT, Sarah SY, Johnson JD, Suzuki M. 2012. Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia. 50:3070–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Nesselroade JR. 2010. Dealing with short-term fluctuation in longitudinal research. J Gerontol B Psychol Sci Soc Sci. 65:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Tucker-Drob EM. 2008. Implications of short-term retest effects for the interpretation of longitudinal change. Neuropsychology. 22:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. 2009. When does age-related cognitive decline begin?. Neurobiol Aging. 30:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T. 2009. Hippocampal volume and asymmetry inmild cognitive impairment and Alzheimer’s disease: meta-analyses of MRI studies. Hippocampus. 19:1055–1064. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. 1977. Neurosensory center comprehensive examination for aphasia. Victoria, BC, Canada: Neuropsychology Laboratory. [Google Scholar]
- Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, deToledo-Morrell L. 2010. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: relation to memory function. Neurobiol Aging. 31:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp D, Dufour A, Lithfous S, Pebayle T, Després O. 2015. Episodic memory in normal aging and Alzheimer disease: insights from imaging and behavioral studies. Ageing Res Rev. 24:232–262. [DOI] [PubMed] [Google Scholar]
- Tsvetanov KA, Henson RN, Tyler LK, Davis SW, Shafto MA, Taylor JR, Williams N, Cam C, Rowe JB. 2015. The effect of ageing on f MRI: Correction for the confounding effects of vascular reactivity evaluated by joint f MRI and MEG in 335 adults. Hum Brain Mapp. 36:2248–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E 1983. Elements of Episodic Memory. Oxford: Oxford University Press. [Google Scholar]
- Uncapher MR, Rugg MD. 2009. Selecting for memory? The influence of selective attention on the mnemonic binding of contextual information. J Neurosci. 29:8270–8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petten C 2004. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 42:1394–1413. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, McEvoy LK, Brewer J, Karow DS, Salmon DP, Fennema-Notestine C. 2010. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiol Aging. 31:1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, Johnson JD, de Chastelaine M, Donley BE, Rugg MD. 2016. The effects of age on the neural correlates of recollection success, recollection-related cortical reinstatement, and post-retrieval monitoring. Cereb Cortex. 26:1698–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Giovanello KS. 2016. The role of medial temporal lobe regions in incidental and intentional retrieval of item and relational information in aging. Hippocampus. 26:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 2001. Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D 2009. Wechsler Memory Scale–Fourth Edition. San Antonio, TX: Pearson. [Google Scholar]
- Wellington RL, Bilder RM, Napolitano B, Szeszko PR. 2013. Effects of age on prefron-tal subregions and hippocampal volumes in young and middle-aged healthy humans. Hum. Brain Mapp. 34:2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Smith JC, Antuono P, Durgerian S, Guidotti L, Zhang Q, Butts A, Hantke N, Lancaster M, Rao SM. 2010. Prediction of cognitive decline in healthy older adults using fMRI. J Alzheimers Dis. 21:871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolard AA, Heckers S. 2012. Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res. 201:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. 2010. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage. 51:1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


