Abstract
Uncontrolled decomposition of agro-industrial waste leads to extensive contamination of water, land, and air. There is a tremendous amount of waste from various sources which causes serious environmental problems. The concern in the disposal problems has stimulated research interest in the valorization of waste streams. Valorization of the wastes not only reduces the volume of waste but also reduces the contamination to the environment. Waste from food industries has great potential as primary or secondary feedstocks for biopolymer production by extraction or fermentation with pre-treatment or without pre-treatment by solid-state fermentation to obtain fermentable sugars. Various types of waste can be used as substrates for the production of biomaterials but recently more focus has been observed on the agro-industrial wastes which have a high rate of production worldwide. This review collates in detail the different food wastes used for biopolymer, technologies for the production and characterization of the biopolymers, and their economic/technical viability.
Keywords: Materials science, Food technology, Decomposition, Agro-industrial waste, Waste valorization
Materials science, Food technology, Decomposition, Agro-industrial waste, Valorization biomaterials.
1. Introduction
The rising global population has resulted in increased food processing demand and a concomitant accentuation in food processing wastes. According to the recent report of the Food and Agriculture Organization (FAO), approximately 1.3 billion tons of food is wasted each year (Blakeney, 2019; CNN, 2020). Food waste sources include food industries and post-harvesting agro-processing which poses a significant threat to the environment (Hansen and Cheong, 2007; Malik and Grohmann, 2011) at both pre-market and post-market sites. These wastes are commonly disposed at landfill sites or employed in preparing compost. However, value-added products developed from these food wastes by recycling them into commercially viable goods would not only promote the recovery of the waste but also earn better returns (Theagarajan et al., 2019a).
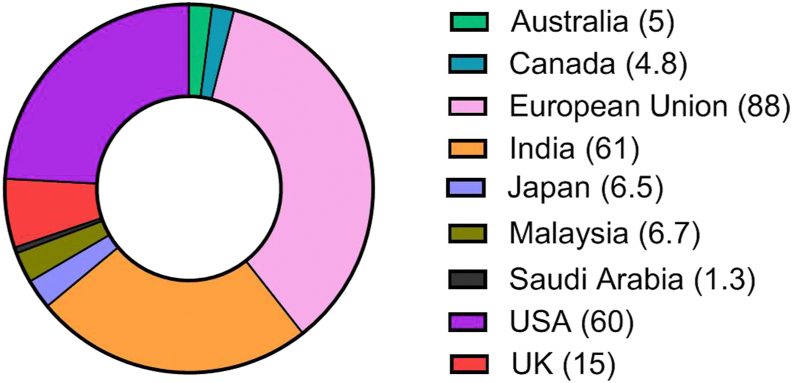
It has been observed that the generation of food waste varies globally depending on geographical features. Figure 1 summarizes the estimated food waste in a few countries. The European Union (EU) generates the maximum waste followed by India and the USA. An intriguing aspect is that besides developing India, developed nations of the EU and the USA are also contributing to waste creation. This entails responsibility on the part of these countries to better utilize the disposed materials to reduce the existing bioburden. These two entities can involve practice and policies that would reduce the said load of wastes. Perhaps the following disquisition would be of some use.
Figure 1.
Food waste around the world in the year 2018 (million tonnes).
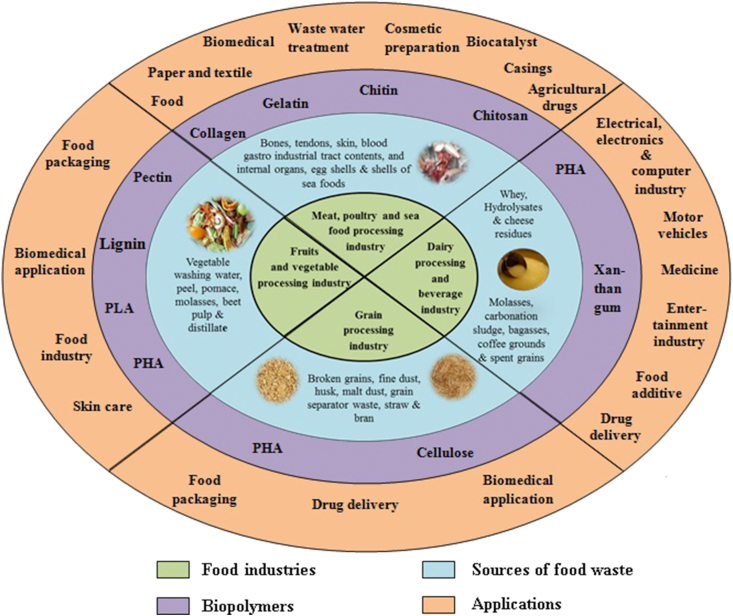
Among many products generated from food wastes, biopolymers are gaining more interest owing to their biodegradability/compostability, biocompatibility, and their bio-based nature. Biopolymer production from food wastes could be accomplished through extraction or fermentation, either with pre-treatment or, without pre-treatment by solid-state fermentation to acquire fermentable sugars. Biopolymers generated from the food wastes have biodegradability, bio-functionality, biostability, biocompatibility, and also offer a wide spectrum of chemical and mechanical properties that can be used for several applications (Bayón et al., 2018; Theagarajan et al., 2019b). In fact, researchers opine that useful products could be designed with such wastes even with low impact technologies. The recycled products would find applications in medicine, food industries, biosensors, industrial plastics, clothing fabrics, water treatment chemicals, cosmetics, pharmaceuticals, and even as data storage elements (Sanchez-Vazquez et al., 2013). Figure 2 shows the application of biopolymers produced from different waste streams. The plethora of avenues depicted in the said figure does indicate possibilities that could be explored. Few of them indeed have been worked upon, and they have been debated at length in the present review. But, many more that are shown in the figure, could be possible domains of exploration of application. While biopolymers are inherently biocompatible and biodegradable, they have limited industrial application at large scale industries because of its poor mechanical, thermal and barrier properties (Yoha et al., 2019). Interestingly, given the multitude of areas of application, it is rather strange that process engineers/scientists are yet to fully engage the wasted materials as useful bioresources. More attention to the shown areas of research is surely warranted.
Figure 2.
Applications of biopolymers produced from different waste streams.
Polylactic acid (PLA) and polyhydroxyalkanoates (PHA) are the key biopolymers in the market for biodegradable plastics. The demand for PLA is predicted to be double by 2023 (European Bioplastics, 2017). In 2017, worldwide production of PHA from commercial manufacturers was up to 2.05 million tons (Tsang et al., 2019). The global market size of PHA is estimated to grow from USD 57 million in 2019 to USD 98 million by 2024 (ReportLinker, 2019). This increases the demand for renewable, eco-friendly, and bio-based materials such as bagasse, zein, casein, plant starch, etc. Moreover, the bioplastic industries are predicted to be a serious player in the economy in the future. Notably, effluents from food oil and food waste industries contain an ample amount of carbon and nutrients that can be potential raw materials for bacterial fermentation to produce PHA. The bioplastic market will double in value in the next few years, rising at 17% per year from 2017 to an estimated market value of $7.2 billion in 2022. Market of biopolymer coatings are expected to exceed $1.3 billion by 2024 (Global Market Insights, 2017). The industry of bio-based polymer coatings used in packaging, derives its raw material from starch, chitosan, soy protein, PLA and whey protein. With the thrust of eco-friendly resources, market growth is promising. In this context, it is mentionable that commercialized biopolymer coatings have been implemented by many companies such as Meridian Holding Group Inc., NatureWorks, NovamontS.p.A, Cargill and EcoSynthetix (Global Market Insights, 2017).
2. Different biopolymers and their conventional sources
Reportedly, biopolymers are formed by enzyme-catalyzed polymerase chain reactions, during complex cellular metabolic processes, under natural conditions. Various types of biodegradable polymers are being utilized in various fields, chiefly in medical applications. Natural biodegradable polymers include polysaccharides (cellulose derivatives, chitosan, starch), and protein-based polymers (collagen, gelatin, and albumin); whereas, aliphatic polyesters, poly-anhydrides, poly (alkylcyanoacrylates), polyaminoacids, phosphorous based polymers, and acrylic polymers are synthetic biodegradable polymers. Biodegradable polymers derived from petroleum resources are polyglycolide (PGA), PLA, poly (lactide-co-glycolide) (PLGA), polycaprolactone (PCL), poly (butylene succinate) (PBS), poly (p-dioxanone) (PPDO), polycarbonate, polyamides, and poly (esteramide)s, polyurethanes, polyanhydrides, and vinyl polymers. PHA and poly(hydroxybutyrate-co-hydroxyvalerate) [P(3HB-co-3HV)] are biodegradable polymers produced by microbial fermentation.
Biopolymers like cellulose, starch, pectin, chitin/chitosan, and derivatives are generally derived from agro-waste industries. Table 1 summarizes product-specific wastes from food industries. Polysaccharides are the most abundant natural biopolymer found in plants, animals, and microorganisms. On the other hand, collagen is an abundant protein-based biopolymer derived from mammals. Skins from cattle and pigs as well as their bones, mostly from abattoirs are the main sources of collagen. Fish wastes (scales, bones), eggshells, and chicken processing waste are used for the production of collagen (Kaya et al., 2015). They are used in the industries of food, pharmaceutical, cosmetics, and leather. Type I collagen, fibrillar type collagen responsible for the tensile strength of extracellular matrix in bone, accounts for about 90% of the overall collagen and is found mainly in the animal's skin, tendon, and ligament as well as the organic portion of the calcified tissue of bone and teeth (Arunmozhivarman et al., 2017). It has a wide range of applications in the pharmaceutical, biomedical, and food industries.
Table 1.
Product specific waste from industries.
| Types of waste | Waste products | Food industries | Biopolymers | References |
|---|---|---|---|---|
| Abattoir waste | Bones, tendons, skin, contents of gastro industrial tract, blood and internal organs, eggshells, shells of seafood | Meat, poultry and seafood processing industry | Collagen, gelatine, chitin/chitosan, PHA | Tarafdar and Biswas (2013), Munasinghe et al. (2015), Arunmozhivarman et al. (2017), Ponkham et al. (2011), Prameela et al. (2017), (Banerjee and Mahapatra (2012)),(Palareti et al. (2016),(Palareti et al., 2016), (Povolo et al. (2012),(Koller and Braunegg, 2015, Koller and Rodr, 2015) |
| Waste from the rice mill, grist mill, malt house | Broken grains, fine dust, husk, malt dust, grain separator waste, straw, bran | Grain processing industry | PHA | Gasser et al. (2014), Cesário et al. (2014), Mostafa and Tayeb (2015), Aslan et al. (2016) |
| Waste from the milk production unit, cheese production | Whey, hydrolysate and cheese residue | Dairy processing industry | PHA (Carbon and nitrogen sources), xanthan gum | Niknezhad et al. (2015), Pais et al. (2015), Colombo et al. (2019) |
| Waste from preparation and processing of fruit, juice industries, oil mill | Vegetable washing water, peel, pomace, molasses, beet pulp, distillate | Fruit and vegetables processing industries, | PHA (carbon source), pectin, lignin, cellulose, PLA and Xanthan gum | Liang et al. (2014), Bilanovic et al. (2010), Martinez et al. (2014), Liang et al. (2014), Jonglertjunya et al. (2014), Szymańska-Chargot et al. (2017), Di Donatoa et al. (2014), Elain et al. (2016), Tapia-Blácido et al. (2017), Follonier et al. (2014) |
| Waste from the production of both non-alcoholic and alcoholic beverages | Molasses, carbonation sludge, bagasse, spent grains, spent coffee grounds | Waste from brewery and distillery industries manufactures of coffee and tea | PHA | Karmee (2018), Obruca et al. (2014), Cruz et al., 2015a, Cruz et al., 2015b |
Gelatine is another widely used biopolymer in the food, pharmaceuticals, and photographic fields (Killekar et al., 2012). About 95% of gelatine is derived commercially from porcine and bovines. Gelatin is abundantly found in bones, tendons, skin, and cartilage (derived from partial hydrolysis of fibrous collagen) of animals (Sockalingam and Abdullah, 2015). The second most abundant biopolymer on earth is chitin which is N-acetyl- + -glucosamine units linked via β (1 → 4) linkage (Cheba et al., 2011). It is regarded as the major structural component of the exoskeleton of marine zooplankton, including corals, jellyfish, shrimp, crab, prawn, and lobsters (Kandile et al., 2018); besides, the cell wall of fungi and cuticles of insects. The alkaline deacetylation of chitin produces chitosan that is used widely in the food, medicinal, pharmaceutical, textile, agriculture, cosmetics, and water treatment industries (Al-Manhel et al., 2018).
Lignocellulosic biomass is composed of cellulose (35–50%), hemicelluloses (15–35%), lignin (15–25%), and several inorganic materials. The composition of agro-industrial residues, including sugarcane bagasse, corn cobs, wheat straw, banana, soybean hull, oil palm mesocarp, rice straw, and soybean straw and hulls are the sources of the lignocellulosic material (Tapia-Blácido et al., 2017). Food wastes such as peanut husk, citrus peel, straw, and corn stover have high cellulose concentrations as well (Sanchez-Vazquez et al., 2013). The formation of a hydrogen bond network between hydroxyl groups of cellulose plays a major role in governing the physical properties of cellulosic materials. These networks are relatively resistant to oxidizing agents and strong alkali. Considering their resilience, cellulose fibers are nowadays being used to provide stability to thermoplastics or thermosetting polymer matrices that are replacing glass fibers (Sanchez-Vazquez et al., 2013).
Hemicellulose is the most abundant renewable plant material after cellulose. They are interconnected by hydrogen bonds with cellulose moieties and are bound by covalent bonds to lignin (Peng et al., 2009). Lignin is another polymer that is comprised of three primary lignin monomers viz. p-hydroxyphenyl alcohol (H), coniferyl alcohol (G), and synapyl alcohol (S). Lignin is a thermoplastic polymer consisting of G-units in soft-wood, G-S-H units in herbaceous plants, and G-S units in hard-wood. This biopolymer provides mechanical rigidity to the cell wall, protection against microbial attack (Huang et al., 2019).
Xanthan gum is another important biopolymer which is produced about 30,000 tons per year worldwide (Stredansky and Conti, 1999). It is a hetero-polysaccharide produced by the genus Xanthomonas. It is commonly used in food, pharmaceutical, and petrochemical industries. Generally in industries, Xanthomonas campestris is used for the production of xanthan gum (Mesomo et al., 2009). Food wastes, such as cheese whey, spent malt grains, citrus wastes are also good sources of xanthan gum.
PHA is another class of biodegradable polyesters which is produced by various microorganisms as intracellular carbon and energy storage materials, also some domains of archea can synthesize PHA. It is produced using agro-industrial by-products comprising of carbon and nitrogen sources. PHA is synthesized and accumulated as inclusions by microorganisms when the available supply of nitrogen source is reduced, but the source of carbon is excessive (Salgaonkar and Bragança, 2017). PHA accumulation occurs in a broad range in Gram-positive and Gram-negative bacterial species, and archea (Koller et al., 2017).
Various microorganisms can produce differently composed PHA co- and tetra polymers. Such polyesters have a wide range of applications in the medical, agriculture, and marine industries. The capability of PHA biosynthesis substantially enhances the survival of bacteria under numerous stress factors such as heat shock, UV radiation, acidic pH, and osmotic pressure (Obruca et al., 2018). poly-3-hydroxybutyrate (P3HB), poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHx), poly(3-hydroxybutyrate-co-3-hydroxyoctanoate) (PHBO) and P(3HB-co-3HV) are the polymeric esters under the group of PHAs. For the synthesis of PHAs by bacterial fermentation, microbes such as Alcaligenes, Azotobacter, Agrobacterium, Azospirilum, Aeromonas, recombinant E. coli, Pseudomonas sp., Cupriavius, Rhizobium, Rhodobacter, Bacillus, and Sphaerotilus are used (Sanchez-Vazquez et al., 2013; Obruca et al., 2018).
3. Production of biopolymers from different food wastes
3.1. Chitin production
A substantial amount of seafood production worldwide is discarded as processing waste, including trimmings, fins, heads, skin, shells, and viscera. Chitin isolation from crustacean shells (exoskeleton) is both time and energy-consuming. Conventionally chemical treatments have been practiced for the extraction of chitin and chitosan from crustacean by-products. The two basic steps in chemical methods are first, deproteinization by alkali treatment followed by demineralization by acidic treatments at high temperature, with subsequent bleaching with reagents to attain colorless products. Mostly NaOH and HCl are preferred for these two processes. The advantages of the chemical method in chitin purification are energy-effectiveness, shorter processing time, and relatively less processing (Kaya et al., 2015).
In the biological process, two methods are used for extraction of chitin: enzymatic and fermentation. In the enzymatic process, enzymes such as proteases are used for deproteinization of crustacean shell, instead of alkali treatments. These proteolytic enzymes are generally obtained from Lactobacillus spp., Pseudomonas aeruginosa K-187, Serratia marcescens FS-3, and Bacillus subtiltis or, from the gut bacteria in the shrimp intestine or, by using the proteases present in the biowaste (Gadgey et al., 2017).
3.1.1. Shrimp shell
The optimal conditions for the chitin and chitosan production from shrimp shell waste have been reported using 3% HCl and 4% NaOH, resulting in the recovery of 15.40% chitosan and 14.02% chitin (Hossain et al., 2014). Another group of researchers concluded that the yield of chitosan was low (at only 12.93%) when dried shrimp shells were demineralized with 1 N HCl at ambient temperature for 6 h. The shells were further treated with 3.5% NaOH at 65 °C for 2 h for deproteinization (Al-Manhel et al., 2018). But in another find, the yield of chitosan was higher (19%) when shrimp shells were demineralized with 5% HCl for 24 h, with deproteinization obtained by 5% NaOH at 60 °C and shell exposure time of 48 h (Arafat et al., 2015).
In one investigation, lactic acid bacteria (LAB), Lactobacillus plantarum was used as a starter culture in microbial fermentation of shrimp waste for obtaining chitin and carotenoids (Prameela et al., 2017). A recent study states that the pre-treatment of crustacean shell waste by atmospheric pressure dielectric barrier discharge plasma resulted in intensified protein removal. The plasma was generated with N2. The yield of chitin was found to be higher (37.6%) for 6 min treatment of plasma compared to 3 and 1.5 min (25 and 33.2%, respectively) treatment (Borić et al., 2018). It is reported to be an effective and sustainable form of pre-treatment for removing invaluable compounds.
3.1.2. Prawn shell
Report on the extraction of chitin and chitosan from prawn shells is very scarce. A study on the same concluded that NaOH (5%) and HCl (0.5%) for deproteinization and demineralization could be effective in achieving a yield of 35% chitin and 25% chitosan (Mohammed et al., 2013). In another study, the yield of chitosan extracted from prawn shells was found to be 22.08 ± 0.13% when 5% NaOH was used for deproteinization of shells followed by 4% HCl for demineralization and finally 50% NaOH was applied for deacetylation (Muley et al., 2018).
3.1.3. Crab shell
Chitin has been synthesized from crab shells also, using 7% HCl for demineralization and 5% NaOH for deproteinization, producing a yield of 10.60–12.73% (Pandharipande and Bhagat, 2016). Scientists have conducted experiments to extract chitin from crab shells by changing the concentration of NaOH (1 N and 5%) in the deproteinization step, and HCl (at 0.5 N, 1 N and 7%) in the demineralization step, along with the sample to solvent ratio at 1:10 and 1:15. From the two experiments, it was evident that 7% HCl, 1:10 sample to solvent ratio and 5% NaOH produced the highest yield of chitin (6%) (Aung et al., 2018). In another approach, chitin from crab, crayfish, and shrimp shells was reportedly isolated with NaClO for 10 min, ahead of demineralization and deproteinization procedures. This treatment brings the yield of chitin at about 13.4% for crab, 15.3% for crayfish, and 14.8% for shrimp shells (Kaya et al., 2015). Hajji and team employed 6 protease producing Bacillus strains: B. subtilis A26, B. mojavencis A21, B. pumilus A1, B. Amyloliquefaciens An6, B. licheniformis NH1 and B. cereus BG1 to ferment crab shell wastes. They observed high proteolytic activity in B. pumilus A1 followed by B. licheniformis NH1. Notably, all strains achieved high deproteinization rates for the crab shells (Hajji et al., 2015). Chakravarty and his team used protease producing bacteria (Bacillus megaterium NH21 or Serratia marcescens Db11) and organic acid-producing bacterium Lactobacillus plantarum to ferment lobster shell wastes. A combination of Serratia marcescens Db11 and Lactobacillus plantarum (Lactobacillus was added in the media after 3 days of culture) resulted in a yield of chitin of about 82.56% from lobster biomass with high efficiency of total deproteinization (87.19%) and demineralization (89.59%) (Chakravarty et al., 2018). Generally, the fermentation process of chitin extraction is costly compared to chemical and enzymatic methods. However, the extraction cost of chitin can be reduced using agro-wastes as the carbon source for microbial fermentation (Gadgey et al., 2017).
3.2. Production of collagen
Collagen is extracted from discarded parts of chicken, egg, and fish. Acid and enzymatic hydrolyses are mostly used for this extraction. NaCl, acetic acid, and pepsin are used to hydrolyze non-collagenous protein at cross-linking sites. The yield of collagen mostly depends on the acetic acid concentration, pepsin content, and duration of hydrolysis (Araújo et al., 2018). Nagai and Suzuki (2000) reported that acetic acid (0.5 M) could be used to extract collagen from skin and fins of fishes; whereas bone collagen could be decalcified with 0.5 M EDTA (ethylenediaminetetraacetic acid). The yield of collagen from these fish waste was found to be 36–54%.
To explore the effect of salts on the extraction of collagen from poultry wastes, Zhou and co-workers employed 0.45 M NaCl for the extraction of salt soluble collagen, 0.5 M acetic acid for extraction of acid-soluble collagen and 0.1% pepsin with a ratio of 1:80 (w/v) for extraction of pepsin soluble collagen from the skin of chicken feet. Interestingly, pepsin soluble collagen showed the highest yield of 49.10% followed by acid-soluble collagen (14.49%) and salt soluble collagen (1.13%) (Zhou et al., 2016). In a separate study, removal of non-collagenous proteins was obtained by mixing the sample with 0.1 N NaOH, which was further defatted with butyl alcohol, and then the organic matter was removed by soaking the defatted sample in 0.1 N HCl. These pre-treatments reportedly increased the yield of collagen by 38.7% (Munasinghe et al., 2015).
Collagen has also been extracted from chicken skin by treating it with 0.5 M acetic acid, 1% pepsin; the product contained fat and non-collagenous protein which were removed by 20% ethanol and 0.1 N NaOH. The yield of type I collagen in this extraction process was about 10–12% (Arunmozhivarman et al., 2017). An optimization study was carried out for the extraction process of collagen from chicken feet with variables such as acetic acid concentration, pepsin content, and hydrolysis time, in response to yield. The highest yield of collagen (72.98%) was found at 0.3 mol/L of acetic acid, 0.2% of pepsin, and 12 h of hydrolysis (Araújo et al., 2018). Other process parameters could be worked out to further improve upon the process efficiency, such as unit operations, particle size, the effect of sample moisture, and likewise.
3.3. Recovery of lignin and hemicelluloses
The major source of lignocelluloses is the sugarcane bagasse, a cheap by-product of the sugarcane industry. It mainly contains cellulose, hemicelluloses, lignin, and ash (Peng et al., 2009). Lignin is a derivative of lignocellulose which is more resistant to most types of biological stresses compared to cellulose and hemicellulose. Lignin is reportedly extracted with aqueous solution and ethanol solution. The yield of lignin has been reported to be higher with 40% NaOH in distilled water (20.4%) than that of 40% NaOH in 50% ethanol (9%) (Jonglertjunya et al., 2014).
For sugarcane bagasse, the presence of fibrous residues makes its microbial degradation slower and more difficult. To overcome this complexity, pre-treatment of bagasse has been carried out to improve the substrate availability and fermentation process. Hemicelluloses are extracted from cereal straw and bagasse by alkali treatment. The addition of alkali at higher concentrations cleaves the α-ether linkage between lignin and hemicelluloses in bagasse (Peng et al., 2009). Alkaline treatment of bagasse with 1% and 3% NaOH resulted in the yield of 25.1% hemicelluloses (i.e. 74.90% of original hemicellulose present in bagasse). The hemicellulosic fraction obtained by sequential extraction has been successfully sub-fractioned by graded precipitation with ethanol (Peng et al., 2009). Alkali treatment had been proved to be an effective method for fractionating alkali-soluble lignin and hemicelluloses from bagasse and straw. Hydrogen peroxide (H2O2) is also used for both lignifications and solubilization of hemicelluloses (Brienzo et al., 2009). According to researchers, the yield of hemicelluloses from sugarcane bagasse reached 94.5% under 4% alkaline H2O2 treatment at 40 °C, for 10 h reaction time. But a minimal degradation of hemicelluloses was achieved at the optimal conditions of 6% H2O2 for 4 h at 20 °C; the resultant hemicelluloses showed 4.6–14.1% of associated lignin content (Brienzo et al., 2009).
3.4. Cellulose production
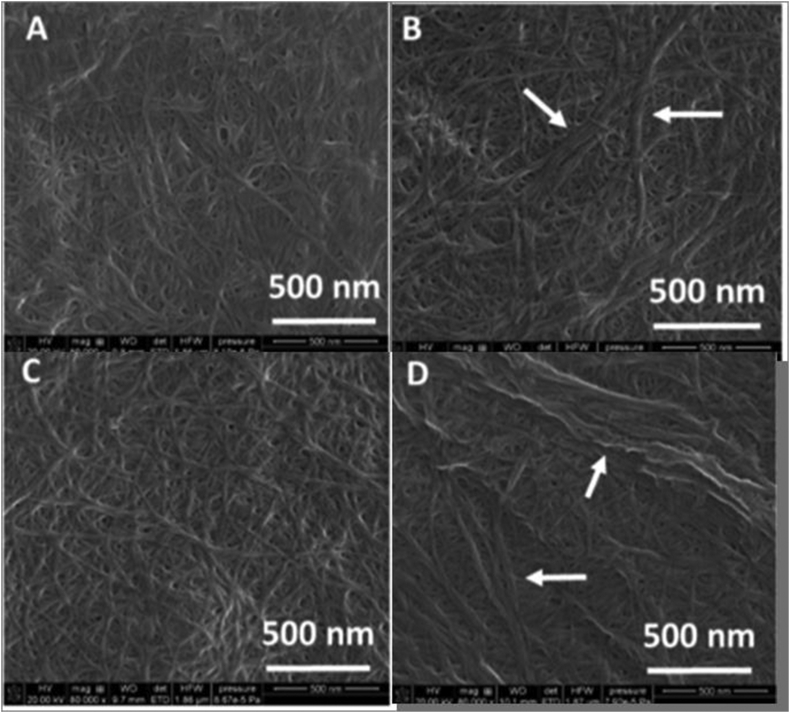
For cellulose production, a mild acid-alkali condition is used for fractioning of fruit and vegetable pomace. Fruit pomace contains higher amounts of cellulose, hemicelluloses, pectin, and starch and, therefore, it can be used as carbon sources for the fermentation. Cellulose content is reportedly high in cucumber (16.13%) and low for tomato and apple (8.60% and 8.81%, respectively) pomace which is evident from the SEM image of their microstructure (Figure 3). This illustration depicts most density of microfibrils in cucumber and least in carrot. This information can decide the fate of the constituents that would be derived from these vegetables and fruits. Depending on cellulose content, suitable application of these biomaterials could be opined.
Figure 3.
SEM image of cellulose isolated from the pomace of the a) apple b) cucumber c) tomato d) carrot (Szymańska-Chargot et al., 2017).
Initially, the substrates are boiled for 10 min to remove phenolic compounds, sugars, and parts of water-soluble polysaccharides and then treated with HCl to remove pectin polysaccharides. After that, the residues are subjected to alkali treatment for the removal of hemicelluloses followed by bleaching with sodium hypochlorite solution; cellulose is recovered as the resulting precipitate (Szymańska-Chargot et al., 2017). Similarly, cellulose was isolated from rice husk by chemical treatment with sodium hydroxide and neutralized with HCl (Shukla, 2013). This approach for the isolation of components from pomace did not require lengthy processing time or use of toxic chemicals and therefore reduces the cost and environmental hazards.
3.5. Production of xanthan gum
Xanthan gum is largely produced in different compositions, viscosity, and yields from different strains of X. campestris using cheese whey and citrus waste. Apple pomace is considered as a suitable substrate for the production of xanthan since it contains a high concentration of soluble sugars and also other substances such as pectin and organic acids, required for sustaining X. campestris. Grape pomace has also been used as the substrate but the yield obtained is very low due to its low absorption capacity and low sugar content (Stredansky and Conti, 1999). The same bacteria have also been used for the production of xanthan gum from jackfruit seed powder combined with peptone, citric acid, K2HPO4, and KH2PO4 where 51.62 g L−1 yield of the product was obtained (Katherine et al., 2017).
In a potato-chips production plant, starch is an everyday waste, mainly due to slicing and washing processes. Production of biopolymers from potato processing has been carried out using enzymatic hydrolysis. Potato starch waste and malt in the ratio of 90:10 yield highest glucose concentration by converting about 96% of starch in the waste. Potato waste has also been used as an alternate substrate for X. campestris NRRL B-1003 bacterial strain for xanthan gum production by solid-state fermentation, submerged fermentation, and semi-solid state fermentation. Experiments reveal that yields of xanthan in solid and semi-solid fermentation are higher than submerged state fermentation (Bilanovic et al., 2010).
Kitchen waste hydrolysate has also been used by the bacteria to produce 11.73 g L−1 of xanthan by batch fermentation (Li et al., 2016). Even to enhance the yield of the product, chicken feather peptone has been utilized as an enhancer supplement in the production media which increased the production of xanthan by 1.73 fold (Ozdal and Kurbanoglu, 2018). Coconut shell and cocoa husks have also been studied for the production of xanthan gum by X. campestris with minimal supplementation of urea and potassium (da Silva et al., 2018).
3.6. PHA and PHB production
The commonly used microbial strains for the production of PHB and PHA are Bacillus sp., Comamonass p., Cupriavidus sp., Pseudomonas sp., Hydrogenophaga sp., Azotobacter sp., Burkholderia sp., Acinetobacter sp., Haloferax sp., and Azohydromonas sp. A pre-treatment is required to convert food waste into PHA. This treatment is either through chemical or biological processing, for digestion of the sample matrix. Subsequently, PHA producing microorganisms utilize the carbon source made available to produce PHA (Nielsen et al., 2017; Rodriguez-Perez et al., 2015). It is noteworthy that waste substrates utilized for the production of PHA contain different concentrations of nitrogen and phosphorus. For the production of PHA, an appropriate ratio of carbon: nitrogen: phosphorous is needed, an excess concentration of any of these nutrients may cause inhibition (Rodriguez-Perez et al., 2015). The properties of PHA such as hardness and brittleness depend on their structure, and depending on the bacteria and the substrate used, the structure of monomers varies. The most widely recognized microbial PHA is poly 3- hydroxybutyric acid (PHB), a homopolymer of 3-hydroxybutyrate. This homopolymer is brittle and biodegradable and thermoplastic; however, only limited industrial use owing to high production cost of PHB is reported till date (Reis et al., 2008; Johnston et al., 2018).
3.6.1. Dairy waste
Wastes from the dairy industry contain soluble organics, suspended solids, and trace organics (fats, oils, and grease, minerals, and phosphates), and necessary ingredients for the biopolymer production by microbial fermentation (Sarkar et al., 2006; Bosco and Chiampo, 2010). Table 2 enlists various microbial strains used for the production of biopolymers from dairy wastes. PHB is the major biopolymer synthesized from the microorganisms isolated from dairy waste. During fermentation, buttermilk acts as a carbon source at pH 7, 37 °C, at a shaker speed of 150 rpm that results in maximum production of PHB (Mehta et al., 2017). Moreover, buttermilk is the cheapest source to produce microbial polymer from the microorganisms isolated from dairy wastes.
Table 2.
Various microbial strains used for the production of biopolymers from dairy wastes.
| Source | Microbial strains | Biopolymers | Yield | References |
|---|---|---|---|---|
| Fermented cheese whey | Activated sludge | PHA | 28.2 g L−1 | Colombo et al. (2019) |
| Cheese whey lactose | Xanthomonas campestris and Xanthomonas pelargonii | Xanthan gum | 0.42g of xanthan g−1 of lactose; 0.27 g of xanthan g−1 of lactose |
Niknezhad et al. (2015) |
| Cheese whey | Haloferax mediterranei | P(3HB-co-3HV) | 9.6 g L−1 | Pais et al. (2015) |
| Whey | Dairy wastewater as innoculum | PHA | 0.284 g L−1 | Bosco and Chiampo (2010) |
| Whey supernatant | Thermus thermophilus HB8 | PHA | 0.57 g L−1 | Pantazaki et al. (2009) |
| Cheese whey | Xanthomonas campestris pv. Mangiferaeindicae IBSF 1230 | Xanthan gum | 46.8 g L−1 of gum | Mesomo et al. (2009) |
| Whey permeate | Pseudomonas hydrogenovora | PHA | 1.27 g L−1 | Koller et al. (2017) |
| Milk whey | X. campestris pv. campestris – 2149 | Xanthan gum | 21.91 g L−1 | Nery et al. (2008) |
| Whey lactose |
Haloferax mediterranei Pseudomonas hydrogenovora Hydrogenophaga pseudoflava |
PHA | 5.5 g L−1 1.3 g L−1 2.7 g L−1 |
Koller et al. (2017) |
| Whey and corn steep liquor | Recombinant Escherichia coli | PHB | 6.12 g L−1 | Nikel et al. (2005) |
| Lactose and whey permeate | Sinorhizobium 41 meliloti, Hydrogenophage pseudoflava DSM 1034 | PHA |
Sinorhizobium 41 meliloti: 0.483 g L−1 Hydrogenophage pseudoflava: 0.375 g L−1 |
Povolo and Casella (2003) |
| Whey | Ralstonia eutropha | PHB | 0.17 g L−1 | Marangoni et al. (2002) |
3.6.2. Waste from potato processing
Alcaligenes eutrophus uses fermentable substrate from potato waste and barley malt for the production of about 5 g L−1 PHB (Rusendi and Sheppard, 1995). Ralstonia eutropha NCIMB 11599 can be used along with saccharified waste potato for PHB production at about 1.47g (L. h)−1(Haas et al., 2008).
3.6.3. Waste from the sugarcane industry
Utilization of sugarcane bagasse as a carbon substrate for the production of PHA by four halophilic archaeal isolates namely Halococcus salifodinae strain BK6 (AB588757), Haloferax volcanii strain BBK2 (AB588756), Haloarcula japonica strain BS2 (HQ455798) and Hgm. borinquense strain E3 (AB904833) has shown significant results. In a study, among the four strains, Hgm. borinquense strain E3 (AB904833) was chosen for further study as its growth increases to 30% (v/v) of sugarcane bagasse hydrolysate during screening. Thus, the resulting production was about 45.7–50.4% PHA (Salgaonkar and Bragança, 2017).
3.6.4. Fish waste
Halophilic bacterium, Salinivibrio sp. M318 isolated from fermenting shrimp paste uses combined sources of carbon and nitrogen from fish sauce, mixtures of waste fish oil, and glycerol for the production of PHB. Highest PHB production (42%, w/w) was obtained in this study with glycerol and waste fish oil as carbon source and 51.7% (w/w) when the fish sauce was used as a nitrogen source (Van Thuoc et al., 2019). In a different study, fish solid waste was used as a substrate for the production of PHB using Bacillus subtiltis (KP172548). The production of PHB was about 1.62 g L−1. These are all inexpensive substrates for the production of biopolymer and simultaneously reduce environmental problems (Mohapatra et al., 2017).
3.6.5. Poultry waste
Interestingly, chicken feather hydrolysate as nitrogen source and waste frying oil as a carbon source are used by Cupriavidus necator H16 for the production of PHA. These inexpensive waste products increased the yield of PHA by 50% compared to that of control cultivation, especially with the addition of 10% (v/v) chicken feather hydrolysate in the media, produced by microwave-assisted alkaline hydrolysis (Palareti et al., 2016). Iva Pernicova and team employed Pseudomonas putida KT2440 to ferment chicken feather waste for the production of mcl-PHA. The highest PHA yield of 1.42 g L−1 were observed in the metabolically active bacterial biomass when it was transferred from the biodegraded feather into nitrogen-limited mineral media (Pernicova et al., 2019). Cynobacterium, Nostoc muscorum Agardh with 10 g L−1 poultry litter supplementation along with 10% CO2 supply resulted in 144.2 mg L−1 yield of PHB along with P(3HB-co-3HV) copolymer (0.77 g L−1) (Bhati and Mallick, 2015).
3.6.6. Industrial fat-containing waste
Fatty acids-containing waste such as olive oil distillate (OOD), used cooking oil (UCO), and biodiesel fatty acids by-product have been used as the cheapest carbon source for the PHA production. Fats from refinery waste, sludge, and margarine, waste frying oil, waste water from oil mills are useful feedstock for the PHA production (Hassan et al., 2013; Cruz et al., 2015a; Rodriguez-Perez et al., 2015). Microbes such as Cupriavidus necator, Pseudomonas sp., Comamonas testosteroni, and Azotobacter vinelandii grow efficiently using fat from industrial wastes and have the capability of converting these fatty acids containing substrates into PHA (Cruz et al., 2015a). The yield of PHA is higher (5.50 g L−1) in OOD for C. necator compared to UCO (4.60 g L−1) (Cruz et al., 2015a). C. necator is a suitable candidate for the production of high amounts of polymers. Fatty acid substrates such as glycerol, methyl esters derived from different fatty acids, oleic acids, and fatty wastes have been used for the PHA production. Bacterial fermentation is widely used in the industrial process for the production of PHAs. . The online monitoring process is also developed for PHA production by utilizing UCO as a carbon source with Cupriavidus necator. The yield of PHA in UCO is found to be lower (7.40 g L−1) (Cruz et al., 2015b), compared to used cooking oil from palm (9.50 g L−1) (Kamilah et al., 2014). Therefore, sugar and/or fat-containing wastes can be the best candidate for PHA production.
Two cultures, C. necator DSM545 and its mutant strain phaZ1 do not differ in terms of growth and polymer content. For the wild type C. necator DSM545, there was a reduction in the intracellular PHA to 30% of the initial content under carbon starvation, but the strain C. necator sp-1 (phaZ1) maintained its PHA content at 85% even after 96 h incubation (Povolo et al., 2015). Favaro et al. (2019) employed this wild type C. necator DSM545 and Pseudomonas (P.) oleovorans DSM 1045, for the production of PHA from lipid residues such as crude glycerol, glycerol from biodiesel and a slaughterhouse, and biodiesel obtained from fatty acid residues. Different combinations of substrates were tested as the source of carbon for bacterial growth and its PHA accumulation. Results showed that both of them were able to grow in all the substrates; especially C. necator DSM 545 could produce copolymers P(3HB-co-3HV) and it could be used for the production of both scl- and mcl-PHAs.
Lipid-rich surplus streams from industrial sectors undergo chemical transformation into crude glycerol phase and biodiesel, and are used for the production of different types of PHA. In fermentation I, Cupriavidus necator DSM 545 utilized carbon source from animal-based crude glycerol phase for the production of homopolyester PHB which results in a yield of 0.29 g g−1 cell dry mass (CDM). Fermentation II was accomplished using saturated biodiesel fraction (SFAE) as the sole carbon source by Cupriavidus necator DSM 545 which results in a yield of about 0.6 g g−1 CDM of P(3HB-co-3HV), which is higher than the yield when glycerol was used as carbon source. Fermentation III and IV have been carried out by the bacterial strain Ps. Citronellolis DSM 50332 and Ps. Chlororaphis DSM 50083 using SFAE as a sole carbon source. The yield for SFAE conversion biomass amounted to 0.59 g g−1 using Ps. Citronellolis DSM 50332 and 0.62 g g−1 using Ps. Chlororaphis DSM 50083. The biopolyesters obtained were 3-hydroxyoctanoate (3-HO) and 3-hydroxydecanoate (3-HD) and, to a minor extent, 3-hydroxydodecanoate (3-DD), 3-hydroxynonanoate (3-HN), 3-hydroxyhexanoate (3-HHx) and 3-hydroxyheptanoate (3-HHp) monomers (Koller and Braunegg, 2015).
3.6.7. Waste cooking oil
The safe disposal of used cooking oil is one of the problems faced by the food processing industries. Interestingly, waste frying oil from chips and chicken frying industries is found to be a suitable substrate for PHA production. In one of the studies, used rapeseed oil produced maximum PHB at 0.9 g L−1 from 20 g L−1 of oil (Verlinden et al., 2011). In another study, chemical mutagen ethyl methane sulphonate was used to mutate the wild type strain of Cupriavidus necator H16 (CCM 3726) to increase the yield of PHA production from waste frying rapeseed oil. In comparison with wild type strain, the yield of PHA was found to be higher (16.0 g L−1) in the mutant strain, possibly due to the improved activities of enzymes involved in oxidative stress response. Importantly, the regulation of aeration or redox balance and mild oxidative stress conditions can enhance the production of PHA in mutant and PHA accumulating bacteria (Obruca and Snajdar, 2013).
Verlinden et al. (2011) describe that the concentration of PHB is higher when fermentation is carried out with Cupriavidus necator with waste frying oil compared to pure vegetable oil, due to various nitrogen sources suited for the production of PHB. When Cupriavidus necator is grown on oils, obtained PHB are found to be free from residual oil traces. Besides, the presence of high free fatty acids increases the uptake of fatty acid by C. necator into the β-fatty acid oxidation cycle, which contributes to increased production of PHB (Kamilah et al., 2014). Bacterial strains such as Acinetobacter sp. (BT1), Pseudomonas stutzeri (BT2), Pseudomonas stutzeri (BT3), Pseudomonas sp. (SP-13), Pseudomonas sp. (SP-20), Pseudomonas sp. (AA4), Bacillus altitudinis (FF1), Bacillus pumilus (FS1) were isolated from soil and slaughterhouse waste. It followed a procedure specifically altered for selecting strains able to grow on waste cooking oil, commercial lard and tallow as carbon source. The strains SP-13, SP-20 and BTI showed better results in terms of % of polymer production per cell dry mass (Povolo et al., 2012).
3.6.8. Palm oil mill waste
Effluent from palm oil mills is one of the most important sources of pollutants in the palm oil industries. It contains a large levels of total solids, oil, and grease and has very high chemical oxygen demand (COD) and biochemical oxygen demand (BOD). This effluent is a potential source of carbon and nitrogen for microbial growth. PHA is reportedly produced from the mixed organic acids obtained from anaerobically treated residual oil, and lignocellulosic materials in the effluent, which make a renewable and cheap carbon source (Hassan et al., 2013). Comamonas sp. EB172 obtained from palm oil mill effluent accumulates PHA of about 59%. This bacterial strain is used for the biosynthesis of homopolymer and copolymer of P(3HB) and P(3HB-co-3HV) from oil mill effluent (Zakaria et al., 2010a, Zakaria et al., 2010b).
Production of PHB has also been investigated with native strains of Bacillus sp., Bacillus megaterium, and Lactobacillus lactis using residual glycerol by-product from palm oil, waste frying oil, castor oil, jatropha oil, and whey as carbon sources (substrate). Using the said concept, Bacillus megaterium grown in combined substrate system using glycerol as carbon source has resulted in a promising yield of PHB (2.81 g L−1) (Gómez Cardozo et al., 2016).
3.6.9. Olive oil mill waste
Waste from olive oil mill is another pollutant to potable water sources and responsible for changes in the microbial population of soil. However, this waste can well be used for the production of PHA. C. necator results in the highest polymer yield of 7.7 g L−1 when cultivated on olive oil distillate; in fact, it is one of the best scl-PHA (short-chain-length polyhydroxyalkanoates) producers (Cruz et al., 2015a). The halophilic organism, Haloferax mediterranei has been used to produce PHA from the oil mill wastewater (OMW) in research activities. A study revealed that PHA yield was lower (0.2 g L−1) when recovered from OMW compared with cheese whey (7.92 g L−1), whey sugars (12.2 g L−1), glycerol (16.24 g L−1), rice bran and starch (77.8 g L−1). The researchers involved in the said study also witnessed the production of copolymer PHBHV even without the addition of extra carbon source (Alsafadi and Al-mashaqbeh, 2016). Sunflower meal (SFM) hydrolysates, crude glycerol, and levulinic acid have been used as sole feedstock for the production of PHB and P(3HB-co-3HV) by fed-batch fermentation using the strain Cupriavidus necator DSM 7237. The yield was about 27 g L−1 PHB and continuous supplementation of levulinic acid led to the production of 23.4 g L−1 P(3HB-co-3HV) (Kachrimanidou et al., 2014). In another study, Kachrimanidou et al. (2015) employed the same bacterial strain using SFM and crude glycerol as carbon source which resulted in higher yield of (57 g L−1) PHB without any commercial nutrient supplement (Kachrimanidou et al., 2015).
3.6.10. Fruit and vegetable wastes
Agro-industrial wastes from the food industries include tomato and lemon processing wastes, residues from fruit juice industries, and crop residues. To reduce environmental pollution, proper disposal of such biomass is crucial. Interestingly, PHB has been reportedly produced from vegetable wastes such as tomato, carrot, and fennel (Di Donatoa et al., 2014).
Strains from thermophilic bacteria Bacillus thermantarcticus and Geobacillus thermoleovorans subsp. stromboliensis, (type strain Pizzo; DSM15392), halophilic bacteria Halobacillus alkaliphilus (type strain FP5; DSM18525), and halophilic archaeon Haloterrigena hispanica (type strain FP1; DSM18328) use carbon source from agro-industrial vegetable wastes such as tomato, lemon, and carrot for PHB production. Carrot waste as a sole carbon source produces a comparable amount of PHB (1.25 mg g−1 dry cell) in comparison with complex media (1.35 mg g−1 dry cell) (Di Donatoa et al., 2014). As a result, vegetable waste can be used as fermentation media for the production of bacterial biomass and biopolymers. Wine lees and crude glycerol are used as nutrient and carbon sources in batch and fed-batch fermentation for the production of PHB using the strain Cupriavidus necator DSM 7237. The yield was about 30.1 g L−1 (Dimou et al., 2015). Therefore, the biorefining of food waste could lead to the development of a sustainable process for production of biopolymers in cost-effective manner.
Pomaces from apricot, grapes, and cherries can be used as substrates for producing mcl-PHA with Pseudomonas strains (P. putida KT2440 and P. resinovorans). Using wasted frying oil and the said microorganism, mcl-PHA has been produced. The yield of mcl-PHA was 1.4 g (L of pomace)−1with apricot as substrate and 21.3 g (L of pomace)−1for solaris grape. The study reported that the use of hydrolyzed pomace for the initial growth stage of P. resinovorans followed by the addition of waste frying oil results in higher yield (21.3 g L−1) of PHA (Follonier et al., 2014). Interestingly, enzyme pre-treated grape pomace from winery waste has also been used as a carbon source by C. necator for the production of PHA (Martinez et al., 2016). In another investigation, to obtain volatile fatty acid-rich effluent, grape pomace was anaerobically digested under batch acidogenic conditions. The yield of PHA at 20% acidic effluent was found to be lower (49%) compared to 40% acidic effluent (63%) (Martinez et al., 2016).
Food wastes are divergent which renders them a suitable and interesting candidate for the production of biopolymers. The pomace of white grapes is a promising growth substrate for P. putida KT2440 for the biosynthesis of mcl-PHA. Grape pomace was supplemented with fatty acids (50 mol% of octanoic acid and 50 mol% of 10-undecenoic acid) for PHA accumulation in a study. The 2-step fermentation strategy finally achieved a biopolymer concentration of 5.8 g L−1mcl-PHA. Another important result in this study was, it produced 583 g of biopolymer from 40 kg of Gewürztraminer white grape pomace (Follonier et al., 2015). Grandfils and Reis (2019) employed Pseudomonas citronellolis NRRL B-2504 for the production of mcl-PHA using soluble fractions from apple pulp waste as a substrate. The yield was about 1.2 g L−1 of mcl-PHA. Volatile fatty acids derived from hydrolysis of pea shells (PS), potato peels (PP), onion peels (OP), and apple pomaces (AP) have been used by Bacillus spp. for the production of PHA. Defined mixed culture of Bacillus spp. was used for efficient hydrolysis of biowaste. The yield of PHA was 100 mg L−1 when combinations of hydrolysates PS and PP were employed along with 1% of glucose supplementation. The PHB yield was about130 mg L−1 for PS and AP in the ratio of 2:1 post addition of 1% of glucose, and for the PS: OP combination in the ratio of 2:1, the yield of PHB was 550 mg L−1. Another combination of PS:OP and PS:AP in the ratio of 2:1 was effective in controlling the biowastes consumed individually, and enhanced the yield of the copolymer PHA and homopolymer (Kumar et al., 2015). Therefore, volatile fatty acid-rich effluents can be employed as an inexpensive substrate for the production of PHA.
Agro-food industrial by-products can also be used as the cheapest source to produce biopolymers. Leguminous processing water (LPW) rich in saccharose and stachyose, fruit processing water (FPW) rich in glucose, and fructose were used to PHA production using marine bacterial species Halomonas i4786. The yield of PHA thus found was 1.6 g L−1 in LPW and 1.8 g L−1 in FPW, respectively, under the batch mode of cultivation in a 5 L fermenter (Elain et al., 2016).
3.6.11. Spent coffee grounds
The liquid waste stream from the coffee industry is named as spent coffee grounds (SCG). Worldwide, around 6 million tonnes of spent coffee grounds are generated annually (Tokimoto et al., 2005). SCG can also be collected from cafeterias, restaurants, fast food chains, etc. Cupriavidus necator H16 uses oil derived from SCG as a substrate for the PHB production, and it has been observed that the yield of PHB is higher for coffee oil (10 g L−1) compared to other waste frying oils such as rapeseed oil (7.3 g L−1), palm oil (6.9 g L−1) and sunflower oil (5.7 g L−1) (Obruca et al., 2014). This enhanced yield is reported by the said authors to be due to higher FFA (7.1) content in the coffee seed oil that stimulates growth and metabolic activity of Cupriavidus necator. In another study, oil extracted from SCG by supercritical carbon dioxide extraction was used as the carbon source for PHA production by Cupriavidus necator DSM 428 strain. The volumetric polymer productivity of PHA in this work was found to be 4.7 g L−1day−1(Cruz et al., 2014).
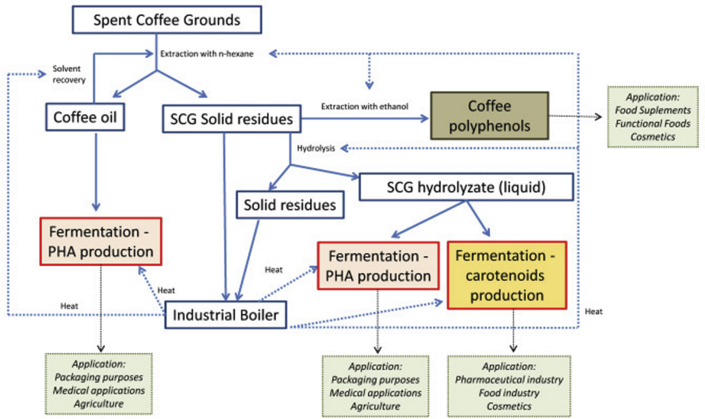
Two bacterial strains namely Bacillus megaterium and Burholderia cepacia have been investigated to be capable of producing PHA from spent coffee ground hydrolysate (SCGH). Obruca and team observed that detoxification of SCG by 30% ethanol before hydrolysis enhanced the yield of PHA by 25%. Combination of both these methods produced approx. 20% conversion of SCG to PHA. They recovered copolymer P(3HB-co-3HV) from Bacillus megaterium and PHB homopolymer by the bacteria Burholderia cepacia (Obruca et al., 2015). Halomonas halophila has the potential to produce PHA from the fermentable sugars derived from SCG. Detoxified spent coffee ground hydrolysates have been used as carbon substrates. Three methods were employed for the production of SCG hydrolysates to receive high content of released saccharides. The first method was SCGs (25% (w/v)) with 4 vol.% H2SO4, the second method was hydrolysis of defatted SCGs (25% (w/v)) with 4 vol.% H2SO4 and the third method was hydrolysis of defatted SCGs with the reduced content of phenolics (25% (w/v)) with 4 vol.% H2SO4 detoxified with polymeric non-ionic exchanger (Amberlite XAD4). Out of these three methods, the second method was found to have the highest yield of PHB of about 0.95 g L−1 (Grandfils and Reis, 2019). Thus underutilized waste from the coffee industry has been used as a substrate for the production of value-added products (Figure 4). The representative image describes the hierarchical events that occur for the effective utilization of coffee waste. These could be effective intermediates to confectionaries wherein low cost substitutes could be identified in them. Besides, use in medical, cosmetics, food packaging industries emphasizes the potential of coffee wastes as seed material for consumer goods, other than food.
Figure 4.
Diagram of the potential process of SCG conversion into PHAs and carotenoids (Obruca et al., 2015).
3.6.12. Rice mill, grist mill, malt house dissipate
Agricultural wastes such as bran and straw from rice and wheat are emerging sources of raw materials for the preparation of composite materials. Because of the cost-effectiveness and vast availability in industries, they have gained a huge interest in the past decades. Wheat straw is used as a substrate for the PHB production by Burkholderia sacchari DSM17165 (Cesário et al., 2014). The polymer yield was about 0.22 g P (3HB) g−1 of total sugar. Molasses, wheat, and rice bran and plant saps have been reportedly used as major raw materials for the PHA production at low cost. During this process, first, VFA (volatile fatty acids) in activated sludge are formed by the conversion of carbon sources from wastewater, followed by PHAs accumulation in mixed cell culture. Use of rice grain distillery wastewater as the carbon source provided 67% yield of PHA and extruded rice and wheat bran by using Haloferox mediterranei resulted in 56% of PHA (Huang et al., 2006). Wheat straw hydrolysate can accumulate about 72% of PHA using Burkholderia sacchari DSM17165 (Cesário et al., 2014; Aslan et al., 2016).
In another endeavor, Akaraonye et al. (2012) engaged sugarcane molasses as the main carbon source for PHB production by fermentation using Bacillus cereus SPV. The maximum yield of PHB was around 4.05 g L−1in a 2 L fermenter, and 3.45 g L−1 in shake flask. Mostafa and team obtained cellulose acetate from flax fibers by bleaching the sulfuric acid-catalyzed acetylation process. The yield of cellulose acetate from flax fibers was found to be higher i.e. 81%. Flax fibers are recommended for the commercial production of cellulose acetate due to its higher production yield. The authors opine that cellulose acetate produced from flax fibers is a suitable material for manufacture of plastic tools, packages, and salt containers (Mostafa and Tayeb, 2015).
3.7. Production of other biopolymers
P(3HB-co-3HV), is a biopolymer that was synthesized by Cupriavidus necator DSM545 in the presence of excess carbon and limited nutrients, using whey and invert sugar as sources of carbon and energy. Propionic acid is necessary for the production of the copolymer. Also, the fraction of 3-hydroxyvalerate in the final product can be controlled by altering the levels of propionic acid (Marangoni et al., 2002; Vandamme et al., 2004).
The production of lactic acid (LA) from potato waste has been studied using microbes such as Lactobacillus var. and Rhizopus. The mixed culture fermentation process has been used for the production of LA from potato waste and other waste streams such as sewage solids under optimal temperature without pre-treatment of enzymatic hydrolysis. Compared to traditional pure culture fermentation, converting food waste using mixed microbial culture in sequencing bioreactor (SBR) can reduce the operating cost for PLA production (Liang et al., 2014). Lactic acid production by anaerobic fermentation was performed by potato peel waste (PPW) with mixed microbial culture collected from local municipal wastewater (Moscow, Idaho) in a SBR is yet another avenue. In a corresponding trial, the yield of LA was considerably high (0.25 gg−1PPW) when the resource was fermented with gelatinized PPW and compared with un-gelatinized PPW (Liang et al., 2014). This draws curious attention to the effect of pre-treatments, pre-considering on the process effectiveness. In another study, an enzymatic hydrolysate obtained from the potato residues were used as a substrate in fermentation with Lactobacillus casei along with yeast supplementation for improved production of lactic acid (Smerilli et al., 2016). Potato residues from the food processing industries have also been used as a starchy substrate for the production of LA under non-sterile conditions by thermophilic bacteria Geobacillus stearothermophilus (Smerilli et al., 2015).
Gelatin is a biopolymer derived from the partial acid or alkaline hydrolysis of fibrous collagen found in tendons, bones, skins, and cartilage of animals. Gelatin is reportedly extracted from fish scales that contain a high quantity of protein. The scales of blue tilapia fish were soaked in 3% HCl (1:6 w/v) for 16 h that produced11.88% of gelatin (Sockalingam and Abdullah, 2015). This indicates that fish scales are a good source of gelatin.
4. Characterization of biopolymers for effective use of recovered moieties
Although recovery of biopolymers from wasted bioproducts is very important for best bioresource utilization, the assessment of the recovered biopolymer is equally essential. High quality of recovered products would help in achieving the best prices and manufacture better-finished products with them. Hence, besides developing biopolymers from waste sources, their characterization is also important to gauge their best utility. This is rather critical since these valorized bioresources could be employed in different processes and products, depending upon their quality.
To study the morphological and crystalline characteristics of biopolymer, X-ray diffraction (XRD) and scanning electron microscope (SEM) have been used in few studies. Numbers of techniques have been developed to trace PHA producing organisms in natural habitat, to quantify PHA in microbial biomass. Quantitative and structural analysis of isolated PHA is of enhancing significance for kinetic analysis of PHA formation process and to control the large scale PHA production process. Several PHA building blocks can be estimated with about 150–200 HAs which requires high-tech methodologies such as Nuclear Magnetic Resonance (NMR) or Mass Spectrometry (MS). Development of genetic methods to identify the PHA producing organisms are very rapid and clarification of enzymatic background of PHA biosynthesis resulted in employing techniques such as Southern Blot Hybridization (SBH), Fluorescence In Situ Hybridization (FISH) or Polymerase Chain Reaction (PCR). Structural characterization and information on functional groups of polyesters and their interactions are determined by NMR, Infrared (IR), and Raman spectroscopy. Optical microscopy, fluorescence staining, and fluorescence microscopy are used for rapid and direct screening test for imaging PHA granules (Koller and Rodr, 2015).
Characterization of PHA has been done using XRD analysis, UV-visible spectrophotometry, Differential Scanning calorimeter (DSC) analysis, Proton nuclear magnetic resonance (H-NMR) and Fourier transform infrared spectroscopy (FTIR) (Salgaonkar and Bragança, 2017). The FT-IR spectra for PHA, collagen, and lignin were obtained in the range of 400–4000 cm−1 (Jonglertjunya et al., 2014; Alsafadi and Al-mashaqbeh, 2016; Zhou et al., 2016). According to few researchers, the degree of deacetylation (DDA) in chitin and chitosan can be estimated in FT-IR using band ratio method and NMR using integrals of the peak of the proton, while, hemicelluloses can be analyzed by FT-IR and spectrum normalized to band nearest 900 cm−1(Brienzo et al., 2009). Others suggest use of GC-MS (Gas chromatography-mass spectrometry) to analyze the monomer composition of PHA. The technique could also be used for the quantification of the amount of PHA produced at different time points. The number average molecular weight (Mn) of PHA is determined by Gel permeation chromatography (GPC) coupled with refractive index detector (Obruca et al., 2014; Gasser et al., 2014). The molecular weights of hemicellulosic fractions were determined by GPC with Knauer differential refractometer. Size exclusion chromatography was used to analyze the molecular weight distribution of polymers and polydispersity index (PDI) (Kamilah et al., 2014). The thermal property of PHA has been analyzed by DSC with a temperature profile of over -30 °C to -200 °C, at a heating rate of 10 °C/min. Cellulose nitrate filters membrane of 0.45μm was used to measure the cell dry weight (CDW) (Zakaria et al., 2010a, Zakaria et al., 2010b). High-pressure liquid chromatography (HPLC) is used for performing carbohydrate analysis (Liang et al., 2014).
Besides, the soluble lignin concentration can be determined by UV-visible spectrophotometry at 290 nm (Jonglertjunya et al., 2014). High-performance anion-exchange chromatography (HPAEC) equipped with an amperometric detector could be used to determine the neutral sugar in the hemicellulosic subfractions. Thermal behavior of hemicelluloses and lignin can be analyzed using differential thermal analysis (DTA) and thermogravimetric analysis (TGA) with a heating rate of 10 °C/min in temperature between 30 °C and 500 °C. In NMR analysis for hemicelluloses, the H NMR spectrum is recorded at 300 MHZ whereas for the C NMR spectrum was recorded at 74.5 MHz (Peng et al., 2009). Characterization of chitin and chitosan is carried out using FT-IR, NMR, TGA, XRD, and elemental analysis. Molecular mass distribution of chitosan was determined by GPC equipped with multi-angle laser light scattering (MALLS) and refractive index detectors (Mohammed et al., 2013).
5. Conclusion
Managing the waste derived from food processing industries is a serious environmental concern. Although the waste from the food processing industry is not an alarming bioburden, its uncontrolled accumulation is disadvantageous. Especially, when these wastes could be used for developing other valuable products as biopolymers, their valorization makes a good sense. The exposition has highlighted various sources for resourcing these wastes and multifarious techniques that could be employed as a remedial measure for waste valorization. The waste products from food are also much in industrial demand, which otherwise has to be synthetically synthesized. Plastic bag bans and global warming initiatives are driving increased market opportunities for bio-based plastics. There has been a tremendous effort to incorporate low-value nanomaterials into the biopolymers to reduce the cost of the final product. And also, the production of different biopolymers from a biorefineries will reduce greenhouse gas emissions significantly. The products from food wastes also compensate for the production cost of these biopolymers, owing to fractional raw material costs incurred compared with fresh raw material. These biopolymers have major applications in industries such as biomedical fields, food industries, electrical and electronic products, agricultural products, automation products, cosmetic preparation, wastewater treatments, biocatalysts casings, and the entertainment industry. Therefore, the production of biopolymers from food wastes could act as a key measure to minimize food waste disposal at land-fills and river streams. Future researchers are advised to explore more in the scalability of these lab-scale processes to pilot plant and industrial scales, investigate process modeling aspects, and develop experiments employing green techniques and green solvents, thus minimizing use of non-GRAS solvent. This would aid not only waste valorization, but also environmental sustenance.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Akaraonye E., Moreno C., Knowles J.C., Keshavarz T., Roy I. Poly(3-hydroxybutyrate) production by Bacillus cereus SPV using sugarcane molasses as the main carbon source. Biotechnol. J. 2012;7(2):293–303. doi: 10.1002/biot.201100122. [DOI] [PubMed] [Google Scholar]
- Al-Manhel A.J., Al-Hilphy A.R.S., Niamah A.K. Extraction of chitosan, characterisation and its use for water purification. J. Saudi Soc. Agric. Sci. 2018;17:186–190. [Google Scholar]
- Alsafadi D., Al-mashaqbeh O. A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. N. Biotechnol. 2016;34:47–53. doi: 10.1016/j.nbt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Arafat A., Samad S.A., Masum S., Moniruzzaman M. Preparation and characterization of chitosan from shrimp shell waste. Int. J. Sci. Eng. Res. 2015;6:538–541. [Google Scholar]
- Araújo Í.B.D.S., Bezerra T.K.A., Nascimento E.S.D., Gadelha C.A.D.A., Santi-Gadelha T., Madruga M.S. Optimal conditions for obtaining collagen from chicken feet and its characterization. Food Sci. Technol. 2018;38:167–173. [Google Scholar]
- Arunmozhivarman K., Abraham R.J.J., Appa V., Parthiban M. Extraction and molecular characterization of collagen from poultry meat processing by-product ( chicken skin ) Int. J. Pure App. Biosci. 2017;5:1085–1091. [Google Scholar]
- Aslan A.N., Ali M.M., Morad N.A., Tamunaidu P. Vol. 36. IOP Publishing; 2016. Polyhydroxyalkanoates production from waste biomass; p. 12040. (IOP Conference Series: Earth and Environmental Science). 1. [Google Scholar]
- Aung K.P., Win D.S.Z., Thu D.S.L. Study on chitin extraction from crab shells waste. Int. J. Sci. Eng. Appl. 2018;7:437–441. [Google Scholar]
- Banerjee D., Mahapatra S. Fungal tannase: a journey from strain isolation to enzyme applications. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2012;6(2):49–60. [Google Scholar]
- Bayón B., Berti I.R., Gagneten A.M., Castro G.R. Waste to Wealth. Springer; Singapore: 2018. Biopolymers from wastes to high-value products in biomedicine; pp. 1–44. [Google Scholar]
- Bhati R., Mallick N. Carbon dioxide and poultry waste utilization for production of polyhydroxyalkanoate biopolymers by Nostoc muscorum Agardh : a sustainable approach. J. Appl. Phycol. 2015;28(1):161–168. [Google Scholar]
- Bilanovic, D. D., Malloy, S. H., & Remeta, P., 2010. U.S. Patent No. 7,727,747. Washington, DC: U.S. Patent and Trademark Office.
- Blakeney M. Food Loss and Food Waste. Edward Elgar Publishing; 2019. Drivers of food waste. [Google Scholar]
- Borić M., Puliyalil H., Novak U., Likozar B. An intensified atmospheric plasma-based process for the isolation of the chitin biopolymer from waste crustacean biomass. Green Chem. 2018;20(6):1199–1204. [Google Scholar]
- Bosco F., Chiampo F. Production of polyhydroxyalcanoates (PHAs) using milk whey and dairy wastewater activated sludge. Production of bioplastics using dairy residues. J. Biosci. Bioeng. 2010;109:418–421. doi: 10.1016/j.jbiosc.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Brienzo M., Siqueira A.F., Milagres A.M.F. Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochem. Eng. J. 2009;46(2):199–204. [Google Scholar]
- Cesário M.T., Raposo R.S., de Almeida M.C.M., van Keulen F., Ferreira B.S., da Fonseca M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014;31(1):104–113. doi: 10.1016/j.nbt.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Chakravarty J., Yang C.L., Palmer J., Brigham C.J. Chitin extraction from lobster shell waste using microbial culture-based methods. Appl. Food Biotechnol. 2018;5:141–154. [Google Scholar]
- Cheba B.A. Chitin and chitosan: marine biopolymers with unique properties and versatile applications. Glob. J. Biotechnol. Biochem. 2011;6:149–153. [Google Scholar]
- CNN Global food waste twice as high as previously estimated, study says. REPORT CNN. 2020. https://edition.cnn.com/2020/02/20/health/global-food-waste-higher/index.html Available online:
- Colombo B., Pepè T., Reis M., Scaglia B., Adani F. Bioresource Technology Polyhydroxyalkanoates ( PHAs ) production from fermented cheese whey by using a mixed microbial culture. Bioresour. Technol. 2019;218:692–699. doi: 10.1016/j.biortech.2016.07.024. [DOI] [PubMed] [Google Scholar]
- Cruz M.V., Paiva A., Lisboa P., Freitas F., Simões P., Barreiros S., Reis M.A.M. Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour. Technol. 2014;157:360–363. doi: 10.1016/j.biortech.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Cruz M.V., Freitas F., Paiva A., Mano F., Ramos A.M., Reis M.A.M., Dionı M. Valorization of fatty acids-containing wastes and byproducts into short- and medium-chain length polyhydroxyalkanoates. New Biotechnol. 2015;33(1):206–215. doi: 10.1016/j.nbt.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Cruz M.V., Sarraguc M.C., Reis M.A.M. Online monitoring of P ( 3HB ) produced from used cooking oil with near-infrared spectroscopy. J. Biotechnol. 2015;194:1–9. doi: 10.1016/j.jbiotec.2014.11.022. 194. [DOI] [PubMed] [Google Scholar]
- da Silva J.A., Cardoso L.G., de Jesus Assis D., Gomes G.V.P., Oliveira M.B.P.P., de Souza C.O., Druzian J.I. Xanthan gum production by Xanthomonas campestris pv. campestris IBSBF 1866 and 1867 from lignocellulosic agroindustrial wastes. Appl. Biochem. Biotechnol. 2018;186:750–763. doi: 10.1007/s12010-018-2765-8. [DOI] [PubMed] [Google Scholar]
- Di Donatoa P., Finorea I., Anzelmoa G., Lamaa L., Nicolausa B., Poli A. Biomass and biopolymer production using vegetable wastes as cheap substrates for extremophiles. Chem. Eng. 2014;38 [Google Scholar]
- Dimou C., Kopsahelis N., Papadaki A., Papanikolaou S., Kookos I.K., Mandala I., Koutinas A.A. Wine lees valorization: biorefinery development including production of a generic fermentation feedstock employed for poly(3-hydroxybutyrate) synthesis. Food Res. Int. 2015;73:81–87. [Google Scholar]
- Elain A., Grand L., Corre Y., Fellic M. Le, Tilly L., Loulergue P., Audic J., Elain A. Valorisation of local agro-industrial processing waters as growth media for polyhydroxyalkanoates (PHA) production. Ind. Crop. Prod. 2016;80:1–5. [Google Scholar]
- European Bioplastics e.V. REPORT European Bioplastics; 2017. Global Production Capacities of Bioplastics 2018-2023.www.european-bioplastics.org/market/ Available online: [Google Scholar]
- Favaro L., Basaglia M., Rodriguez J.E.G., Morelli A., Ibraheem O., Pizzocchero V., Casella S. Bacterial production of PHAs from lipid-rich by-products. Appl. Food Biotechnol. 2019;6:45–52. [Google Scholar]
- Follonier S., Goyder M.S., Silvestri A., Crelier S., Kalman F., Riesen R., Zinn M. Fruit pomace and waste frying oil as sustainable resources for the bioproduction of medium-chain-length polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014;71:42–52. doi: 10.1016/j.ijbiomac.2014.05.061. [DOI] [PubMed] [Google Scholar]
- Follonier S., Riesen R., Zinn M. Pilot-scale production of functionalized mcl-PHA from grape pomace supplemented with fatty acids. Chem. Biochem. Eng. Q. 2015;29(2):113–121. [Google Scholar]
- Gadgey K.K. Studies on extraction methods of chitin from crab shell and investigation of its mechanical properties. Int. J. Mech. Eng. Technol. 2017;8:220–231. [Google Scholar]
- Gasser E., Ballmann P., Dröge S., Bohn J., König H. Microbial production of biopolymers from the renewable resource wheat straw. J. Appl. Microbiol. 2014;117:1035–1044. doi: 10.1111/jam.12581. [DOI] [PubMed] [Google Scholar]
- Global Market Insights . 2017. Biopolymer Coatings Market.https://www.gminsights.com/industry-analysis/biopolymer-coatings-market Accessed from: [Google Scholar]
- Gómez Cardozo J.R., Mora Martínez A.L., Yepes Pérez M., Correa Londoño G.A. Production and characterization of polyhydroxyalkanoates and native microorganisms synthesized from fatty waste. Int. J. Pol. Sci. 2016;2016 [Google Scholar]
- Grandfils C., Reis M.A.M. Production of medium-chain length polyhydroxyalkanoates by Pseudomonas citronellolis grown in apple pulp waste. Appl. Food Biotechnol. 2019;6(1):71–82. [Google Scholar]
- Haas R., Jin B., Zepf F.T. Production of poly(3-hydroxybutyrate) from waste potato starch. Biosci. Biotechnol. Biochem. 2008;72(1):253–256. doi: 10.1271/bbb.70503. [DOI] [PubMed] [Google Scholar]
- Hajji S., Ghorbel-Bellaaj O., Younes I., Jellouli K., Nasri M. Chitin extraction from crab shells by Bacillus bacteria. Biological activities of fermented crab supernatants. Int. J. Biol. Macromol. 2015;79:167–173. doi: 10.1016/j.ijbiomac.2015.04.027. [DOI] [PubMed] [Google Scholar]
- Hansen C.L., Cheong D.Y. Elsevier Inc; 2007. Agricultural Waste Management in Food Processing, Handbook of Farm Dairy and Food Machinery. [Google Scholar]
- Hassan M.A., Yee L.N., Yee P.L., Ariffin H., Raha A.R., Shirai Y., Sudesh K. Sustainable production of polyhydroxyalkanoates from renewable oil-palm biomass. Biomass Bioenergy. 2013;50:1–9. [Google Scholar]
- Hossain M.S., Iqbal A. Production and characterization of chitosan from shrimp waste. J. Bangladesh Agric. Univ. 2014;12:153–160. [Google Scholar]
- Huang T.Y., Duan K.J., Huang S.Y., Chen C.W. Production of polyhydroxyalkanoates from inexpensive extruded rice bran and starch by Haloferax mediterranei. J. Ind. Microbiol. Biotechnol. 2006;33:701–706. doi: 10.1007/s10295-006-0098-z. [DOI] [PubMed] [Google Scholar]
- Huang J., Fu S., Gan L., editors. Elsevier; 2019. pp. 25–50. (Lignin Chemistry and Applications). [Google Scholar]
- Johnston B., Radecka I., Hill D., Chiellini E., Ilieva V.I. The microbial production of polyhydroxyalkanoates from waste polystyrene fragments attained using oxidative degradation. Polymers. 2018;10:957. doi: 10.3390/polym10090957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonglertjunya W., Juntong T., Pakkang N., Srimarut N., Sakdaronnarong C. Properties of lignin extracted from sugarcane bagasse and its efficacy in maintaining postharvest quality of limes during storage. LWT - Food Sci. Technol. 2014;57:116–125. [Google Scholar]
- Kachrimanidou V., Kopsahelis N., Papanikolaou S., Kookos I.K., De Bruyn M., Clark J.H., Koutinas A.A. Sunflower-based biorefinery: poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from crude glycerol, sunflower meal and levulinic acid. Bioresour. Technol. 2014;172:121–130. doi: 10.1016/j.biortech.2014.08.044. [DOI] [PubMed] [Google Scholar]
- Kachrimanidou V., Kopsahelis N., Alexandri M., Strati A., Gardeli C., Papanikolaou S., Komaitis M., Kookos I.K., Koutinas A.A. Integrated sunflower-based biorefinery for the production of antioxidants, protein isolate and poly(3-hydroxybutyrate) Ind. Crop. Prod. 2015;71:106–113. [Google Scholar]
- Kamilah H., Sudesh K., Yang T.A., Division F.T. Characteristics of used palm olein and its bioconversion into polyhydroxybutyrate by Cupriavidus necator H16. Malays. J. Microbiol. 2014;10:139–148. [Google Scholar]
- Kandile N.G., Zaky H.T., Mohamed M.I., Nasr A.S., Ali Y.G. Extraction and characterization of chitosan from shrimp shells. Open J. Org. Polym. Mater. 2018:33–42. [Google Scholar]
- Karmee S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018;72:240–254. doi: 10.1016/j.wasman.2017.10.042. [DOI] [PubMed] [Google Scholar]
- Katherine R.F., Muthukumaran C., Sharmila G., Kumar N.M., Tamilarasan K., Jaiganesh R. Xanthan gum production using jackfruit-seed-powder-based medium: optimization and characterization. 3 Biotech. 2017;7(4):248. doi: 10.1007/s13205-017-0876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya M., Baran T., Karaarslan M. A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Nat. Prod. Res. 2015;29:1477–1480. doi: 10.1080/14786419.2015.1026341. [DOI] [PubMed] [Google Scholar]
- Killekar V.C., Koli J.M., Sharangdhar S.T., Metar S.Y. Functional properties of gelatin extracted from skin of black kingfish ( Ranchycentron Canadus ) Indian J. Fundam. Appl. Life Sci. 2012;2:106–116. [Google Scholar]
- Koller M., Braunegg G. Biomediated production of structurally diverse poly ( hydroxyalkanoates ) from surplus streams of the animal processing industry. Polimery. 2015;60 [Google Scholar]
- Koller M., Rodr A. Techniques for tracing PHA-producing organisms and for qualitative and quantitative analysis of intra- and extracellular PHA. Eng. Life Sci. 2015;15(6):558–581. [Google Scholar]
- Koller M., Maršálek L., de Sousa Dias M.M., Braunegg G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. N. Biotechnol. 2017;37:24–38. doi: 10.1016/j.nbt.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Kumar P., Ray S., Kalia V.C. Microbial biotechnology and genomics, CSIR -institute of genomics and integrative biology. Bioresour. Technol. 2015 [Google Scholar]
- Li P., Li T., Zeng Y., Li X., Jiang X., Wang Y., Xie T., Zhang Y. Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr. Polym. 2016;151:684–691. doi: 10.1016/j.carbpol.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Liang S., McDonald A.G., Coats E.R. Lactic acid production from potato peel waste by anaerobic sequencing batch fermentation using undefined mixed culture. Waste Manag. 2014;45:51–56. doi: 10.1016/j.wasman.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Malik A., Grohmann E., editors. Environmental protection Strategies for Sustainable Development. Springer Science & Business Media; 2011. pp. 65–109. 3. [Google Scholar]
- Marangoni C., Furigo A., De Aragão G.M.F. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Ralstonia eutropha in whey and inverted sugar with propionic acid feeding. Process Biochem. 2002;38:137–141. [Google Scholar]
- Martinez G.A., Domingos J.B., Rebecchi S., Fava F., Bologna U., Porto C. Da, Natolino A., Decorti D., Udine U. 2014. An Agro-Industrial Waste Valorization : Biopolymer Production from Dephenolized and Fermented Grape Pomace 1–6. [Google Scholar]
- Martinez G.A., Rebecchi S., Decorti D., Domingos J.M., Natolino A., Del Rio D.…Fava F. Towards multi-purpose biorefinery platforms for the valorisation of red grape pomace: production of polyphenols, volatile fatty acids, polyhydroxyalkanoates and biogas. Green Chem. 2016;18:261–270. [Google Scholar]
- Mehta V., Patel E., Vaghela K., Marjadi D., Dharaiya N. Production of biopolymer from dairy waste : an approach to alternate synthetic plastic. Int. J. Res. Biosci. 2017;6(4):1–8. 6. [Google Scholar]
- Mesomo M., Silva M.F., Boni G., Padilha F.F., Mazutti M., Mossi A., de Oliveira D., Cansian R.L., di Luccio M., Treichel H. Xanthan gum produced by Xanthomonas campestris from cheese whey: production optimisation and rheological characterisation. J. Sci. Food Agric. 2009;89:2440–2445. [Google Scholar]
- Mohammed M.H., Williams P.A., Tverezovskaya O. Extraction of chitin from prawn shells and conversion to low molecular mass chitosan. Food Hydrocolloids. 2013;31:166–171. [Google Scholar]
- Mohapatra S., Sarkar B., Samantaray D.P., Daware A., Maity S., Pattnaik S., Bhattacharjee S. Bioconversion of fish solid waste into PHB using Bacillus subtilis based submerged fermentation process. Environ. Technol. 2017;38:3201–3208. doi: 10.1080/09593330.2017.1291759. [DOI] [PubMed] [Google Scholar]
- Mostafa N.A., Tayeb A.M. Production of biodegradable plastic from agricultural wastes. Arab. J. Chem. 2015:4–11. [Google Scholar]
- Muley A.B., Chaudhari S.A., Mulchandani K.H., Singhal R.S. Extraction and characterization of chitosan from prawn shell waste and its conjugation with cutinase for enhanced thermo-stability. Int. J. Biol. Macromol. 2018;111:1047–1058. doi: 10.1016/j.ijbiomac.2018.01.115. [DOI] [PubMed] [Google Scholar]
- Munasinghe K.A., Schwarz J.G., Whittiker M. Utilization of chicken by-products to form collagen films. J. Food Process. 2015;2015:1–6. [Google Scholar]
- Nagai T., Suzuki N. Isolation of collagen from fish waste material - skin, bone and fins. Food Chem. 2000;68:277–281. [Google Scholar]
- Nery T.B.R., Brandão L.V., Esperidião M.C.A., Druzian J.I. Biossíntese de goma xantana a partir da fermentação de soro de leite: Rendimento e viscosidade. Quim. Nova. 2008;31:1937–1941. [Google Scholar]
- Nielsen C., Rahman A., Rehman A.U., Walsh M.K., Miller C.D. Food waste conversion to microbial polyhydroxyalkanoates. Microb. Biotechnol. 2017;10:1338–1352. doi: 10.1111/1751-7915.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikel P.I., Pettinari M.J., Méndez B.S., Galvagno M.A. Statistical optimization of a culture medium for biomass and poly(3-hydroxybutyrate) production by a recombinant Escherichia coli strain using agroindustrial byproducts. Int. Microbiol. 2005;8:243–250. [PubMed] [Google Scholar]
- Niknezhad S.V., Asadollahi M.A., Zamani A., Biria D., Doostmohammadi M. Optimization of xanthan gum production using cheese whey and response surface methodology. Food Sci. Biotechnol. 2015;24:453–460. [Google Scholar]
- Obruca S., Snajdar O. Application of random mutagenesis to enhance the production of polyhydroxyalkanoates by Cupriavidus necator H16 on waste frying oil. World J. Microbiol. Biotechnol. 2013;29(12):2417–2428. doi: 10.1007/s11274-013-1410-5. [DOI] [PubMed] [Google Scholar]
- Obruca S., Petrik S., Benesova P. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014;98(13):5883–5890. doi: 10.1007/s00253-014-5653-3. [DOI] [PubMed] [Google Scholar]
- Obruca S., Benesova P., Kucera D., Petrik S., Marova I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol. 2015;32(6):569–574. doi: 10.1016/j.nbt.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Obruca S., Sedlacek P., Koller M., Kucera D., Pernicova I. Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: biotechnological consequences and applications. Biotechnol. Adv. 2018;36:856–870. doi: 10.1016/j.biotechadv.2017.12.006. [DOI] [PubMed] [Google Scholar]
- Ozdal M., Kurbanoglu E.B. Valorisation of chicken feathers for xanthan gum production using Xanthomonas campestris MO-03. J. Genet. Eng. Biotechnol. 2018;16:259–263. doi: 10.1016/j.jgeb.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais J., Serafim L.S., Freitas F., Reis M.A.M. Conversion of cheese whey into poly ( 3-hydroxybutyrate- co -3- hydroxyvalerate ) by Haloferax mediterranei. N. Biotechnol. 2015;33(1):224–230. doi: 10.1016/j.nbt.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Palareti G., Legnani C., Cosmi B., Antonucci E., Erba N., Poli D., Testa S., Tosetto A. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016;38:42–49. doi: 10.1111/ijlh.12426. [DOI] [PubMed] [Google Scholar]
- Pandharipande S.L., Bhagat P.H. Synthesis of chitin from crab shells and its utilization in preparation of nanostructured film. Int. J. Sci. Eng. Technol. Res. 2016;5:2278–7798. [Google Scholar]
- Pantazaki A.A., Papaneophytou C.P., Pritsa A.G., Liakopoulou-Kyriakides M., Kyriakidis D.A. Production of polyhydroxyalkanoates from whey by Thermus thermophilus HB8. Process Biochem. 2009;44:847–853. [Google Scholar]
- Peng F., Ren J.L., Xu F., Bian J., Peng P., Sun R.C. Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J. Agric. Food Chem. 2009;57(14):6305–6317. doi: 10.1021/jf900986b. [DOI] [PubMed] [Google Scholar]
- Pernicova I., Enev V., Marova I., Obruca S. Interconnection of waste chicken feather biodegradation and keratinase and mcl -PHA production employing Pseudomonas putidaKT2440. Appl. Food Biotechnol. 2019;6(1):83–90. [Google Scholar]
- Ponkham W., Limroongreungrat K., Sangnark A. Extraction of collagen from hen eggshell membrane by using organic acids. Thai J. Agric. Sci. 2011;44:354–360. [Google Scholar]
- Povolo S., Casella S. Bacterial production of PHA from lactose and cheese whey permeate. Macromol. Symp. 2003;197:1–9. [Google Scholar]
- Povolo S., Romanelli M.G., Fontana F., Basaglia M., Casella S. Production of polyhydroxyalkanoates from fatty wastes. J. Polym. Environ. 2012;20:944–949. [Google Scholar]
- Povolo S., Basaglia M., Fontana F., Morelli A., Casella S. Poly(hydroxyalkanoate) production by Cupriavidus necator from fatty waste can be enhanced by phaZ1 inactivation. Chem. Biochem. Eng. Q. 2015;29:67–74. [Google Scholar]
- Prameela K., Venkatesh K., Vani K.D., Sudesh Kumar E., Mohan C.H.M. Eco-friendly extraction of biopolymer chitin and carotenoids from shrimp waste. IOP Conf. Ser. Mater. Sci. Eng. 2017;225 [Google Scholar]
- Reis K.C., Pereira J., Smith A.C., Carvalho C.W.P., Wellner N., Yakimets I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008;89:361–369. [Google Scholar]
- 2019. https://www.reportlinker.com/p04660850/Polyhydroxyalkanoate-Market-by-Type-Manufacturing-Technology-Application-Global-Forecast-to.html?utm_source=GNW
- Rodriguez-Perez Santiago, Antonio Serrano, Pantión Alba A., Alonso-Fariñas Bernabé. Challenges of scaling-up PHA production from waste streams. A review. J. Environ. Manag. 2015;205:215–230. doi: 10.1016/j.jenvman.2017.09.083. [DOI] [PubMed] [Google Scholar]
- Rusendi D., Sheppard J.D. Hydrolysis of potato processing waste for the production of poly-β-hydroxybutyrate. Bioresour. Technol. 1995;54:191–196. [Google Scholar]
- Salgaonkar B.B., Bragança J.M. Utilization of sugarcane bagasse by Halogeometricum borinquense strain E3 for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Bioengineering. 2017;4:50. doi: 10.3390/bioengineering4020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vazquez S.A., Hailes H.C., Evans J.R.G. Hydrophobic polymers from food waste: resources and synthesis. Polym. Rev. 2013;53:627–694. [Google Scholar]
- Sarkar B., Chakrabarti P.P., Vijaykumar A., Kale V. Wastewater treatment in dairy industries — possibility of reuse. Desalination. 2006;195:141–152. [Google Scholar]
- Shukla S.K. Preparation and characterization of cellulose derived from rice husk for drug delivery. Adv. Mater. Lett. 2013;4(9):714–719. [Google Scholar]
- Smerilli M., Neureiter M., Wurz S., Haas C., Frühauf S., Fuchs W. Direct fermentation of potato starch and potato residues to lactic acid by Geobacillus stearothermophilus under non-sterile conditions. J. Chem. Technol. Biotechnol. 2015;90:648–657. doi: 10.1002/jctb.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerilli M., Neureiter M., Haas C., Frühauf S., Fuchs W. Valorization of potato-processing residues for the production of lactic acid. Chem. Biochem. Eng. Q. 2016;30:255–263. [Google Scholar]
- Sockalingam K., Abdullah H.Z. Extraction and characterization of gelatin biopolymer from black Tilapia ( Oreochromis mossambicus ) scales 020053. AIP Conf. Proc. 2015;1669(1) [Google Scholar]
- Stredansky M., Conti E. Xanthan production by solid state fermentation. Process Biochem. 1999;34:581–587. [Google Scholar]
- Szymańska-Chargot M., Chylińska M., Gdula K., Kozioł A., Zdunek A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers. 2017;9(10):495. doi: 10.3390/polym9100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Blácido D.R., Maniglia B.C., Martelli-Tosi M. Biopolymers from sugarcane and soybean lignocellulosic biomass. Sustain. Polym. from Biomass. 2017:227–253. from Biomass. [Google Scholar]
- Tarafdar A., Biswas G. Extraction of chitosan from prawn shell wastes and examination of its viable commercial applications. Int. J. Theor. Appl. Res. Mech. Eng. 2013:17–24. [Google Scholar]
- Theagarajan T., Malur Narayanaswamy L., Dutta S., Moses J.A., Chinnaswamy A. Valorisation of grape pomace (cv. Muscat) for development of functional cookies. Int. J. Food Sci. Technol. 2019;54(4):1299–1305. [Google Scholar]
- Theagarajan R., Dutta S., Moses J.A., Anandharamakrishnan C. Alginates for food packaging applications. Alginates. 2019:205–232. [Google Scholar]
- Tokimoto T., Kawasaki N., Nakamura T., Akutagawa J., Tanada S. Removal of lead ions in drinking water by coffee grounds as vegetable biomass. J. Colloid Interface Sci. 2005;281:56–61. doi: 10.1016/j.jcis.2004.08.083. [DOI] [PubMed] [Google Scholar]
- Tsang Y.F., Kumar V., Samadar P., Yang Y., Lee J., Song H., Kim K., Kwon E.E., Jae Y. Production of bioplastic through food waste valorization. Environ. Int. 2019;127:625–644. doi: 10.1016/j.envint.2019.03.076. [DOI] [PubMed] [Google Scholar]
- Van Thuoc D., My D.N., Loan T.T., Sudesh K. Utilization of waste fish oil and glycerol as carbon sources for polyhydroxyalkanoate production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019;141:885–892. doi: 10.1016/j.ijbiomac.2019.09.063. [DOI] [PubMed] [Google Scholar]
- Vandamme P., Coenye T. Taxonomy of the genus Cupriavidus: a tale of lost and found. Int. J. Syst. Evol. Microbiol. 2004;54:2285–2289. doi: 10.1099/ijs.0.63247-0. [DOI] [PubMed] [Google Scholar]
- Verlinden R.A.J., Hill D.J., Kenward M.A., Williams C.D., Piotrowska-seget Z. Production of polyhydroxyalkanoates from waste frying oil by Cupriavidus necator. Amb. Express. 2011;1:11. doi: 10.1186/2191-0855-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoha K.S., Leena M.M., Moses J.A., Anandharamakrishnan C. Properties of food packaging biocomposites and its impact on environment. Compos. Environ. Eng. 2019:347–381. [Google Scholar]
- Zakaria M.R., Ariffin H., Johar N.A.M., Abd-Aziz S., Nishida H., Shirai Y., Hassan M.A. Biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer from wild-type Comamonas sp. EB172. Polym. Degrad. Stabil. 2010;95(8):1382–1386. [Google Scholar]
- Zakaria M.R., Tabatabaei M., Ghazali F.M., Abd-Aziz S., Shirai Y., Hassan M.A. Polyhydroxyalkanoate production from anaerobically treated palm oil mill effluent by new bacterial strain Comamonas sp. EB172. World J. Microbiol. Biotechnol. 2010;26(5):767–774. [Google Scholar]
- Zhou C., Li Y., Yu X., Yang H., Ma H., Yagoub A.E.G.A., Cheng Y., Hu J., Otu P.N.Y. Extraction and characterization of chicken feet soluble collagen. LWT - Food Sci. Technol. 2016;74:145–153. [Google Scholar]