ABSTRACT
Tubulin enters the cilium by diffusion and motor-based intraflagellar transport (IFT). However, the respective contribution of each route in providing tubulin for axonemal assembly remains unknown. Using Chlamydomonas, we attenuated IFT-based tubulin transport of GFP–β-tubulin by altering the IFT74N–IFT81N tubulin-binding module and the C-terminal E-hook of tubulin. E-hook-deficient GFP–β-tubulin was incorporated into the axonemal microtubules, but its transport frequency by IFT was reduced by ∼90% in control cells and essentially abolished when the tubulin-binding site of IFT81 was incapacitated. Despite the strong reduction in IFT, the proportion of E-hook-deficient GFP–β-tubulin in the axoneme was only moderately reduced. In vivo imaging showed more GFP–β-tubulin particles entering cilia by diffusion than by IFT. Extrapolated to endogenous tubulin, the data indicate that diffusion provides most of the tubulin required for axonemal assembly. We propose that IFT of tubulin is nevertheless needed for ciliogenesis, because it augments the tubulin pool supplied to the ciliary tip by diffusion, thus ensuring that free tubulin there is maintained at the critical concentration for plus-end microtubule assembly during rapid ciliary growth.
KEY WORDS: Flagella, Cilia, Microtubule, Axoneme, Calponin homology domain, IFT81, IFT74, Diffusion
Highlighted Article: Using GFP-tagged β-tubulin, we show that most of the tubulin required for axonemal assembly enters Chlamydomonas cilia by diffusion rather than by intraflagellar transport.
INTRODUCTION
Tubulin is the main protein of the axoneme, the structural core of cilia and flagella (Borisy and Taylor, 1967). The microtubules of the axoneme provide the scaffold to anchor dynein arms and other protein complexes and serve as tracks for intraflagellar transport (IFT). During ciliary assembly, vast amounts of tubulin move from the cell body, the place of tubulin synthesis, into the organelle. For example, a 12-µm long axoneme, as it is typical for Chlamydomonas, which we used in this study, contains ∼350,000 tubulin dimers (Bhogaraju et al., 2014). Studies in several species have revealed that tubulin enters cilia by both IFT and diffusion (Craft et al., 2015; Hao et al., 2011; Luo et al., 2017).
During IFT, arrays of IFT particles (IFT trains) are moved distally and proximally along the axonemal microtubules by means of the motor proteins kinesin and IFT dynein, respectively. These IFT trains carry ciliary building blocks, such as tubulin and axonemal dyneins, into the growing organelle (Cole et al., 1998; Kozminski et al., 1993). IFT speed and frequency are similar in growing and non-growing cilia, but the frequency of tubulin transport is upregulated while cilia elongate and attenuated in non-growing cilia, indicating tight control of delivery of tubulin to the cilium by IFT (Craft et al., 2015).
Much less is known about diffusion of tubulin into the cilium. Diffusion into the cilium of proteins and complexes larger than ∼50 kDa is severely restricted by the ciliary gate formed by the transition zone, and it is thought that association with IFT enables such proteins and complexes to pass through the ciliary gate (Awata et al., 2014; Breslow et al., 2013; Kee et al., 2012; Lin et al., 2013). Indeed, the absence of IFT cargo adapters and mutation of the cargo binding sites on IFT particles largely diminishes the presence of the respective cargoes from cilia, indicating that they critically depend on IFT for ciliary entry and accumulation (Ahmed et al., 2008; Badgandi et al., 2017; Berbari et al., 2008; Dai et al., 2018; Hou and Witman, 2017; Hunter et al., 2018). However, tubulin, presumably in a form no smaller than the 110-kDa α-tubulin–β-tubulin dimer, diffuses into cilia, and this diffusion occurs in both growing and fully formed cilia (Craft et al., 2015; Harris et al., 2016; Kee and Verhey, 2013). Tubulin entry into cilia by diffusion continues when IFT is switched off using the temperature-sensitive kinesin-2 mutant fla10-1 (Craft et al., 2015). Spatial mapping showed that ∼30% of all GFP–tubulin enters full-length primary cilia by diffusion through the lumen of the axonemal cylinder (Luo et al., 2017). To determine the respective contributions of IFT and diffusion in supplying tubulin for axonemal assembly, we manipulated IFT–tubulin interactions to attenuate tubulin supplied by the IFT route.
In vitro interaction studies revealed that the N-terminal domains of the IFT particle proteins IFT74 and IFT81 form a bipartite tubulin-binding module (Bhogaraju et al., 2013). In detail, the calponin homology (CH) domain of IFT81 binds to the globular cores of the α-tubulin–β-tubulin dimer, providing specificity. The basic N terminus of IFT74 likely binds the acidic C-terminal E-hook of β-tubulin, stabilizing the IFT–tubulin interaction. To test the role of the IFT74N–IFT81N tubulin-binding module in vivo, Kubo et al. (2016) expressed IFT74Δ130, which lacks the tubulin-interacting N-terminal 130 residues of IFT74, and IFT81-CH5E, in which five basic residues in the IFT81 CH domain were replaced with glutamate to abolish tubulin–IFT interaction, in corresponding ift74-2 and ift81-1 null mutants (Bhogaraju et al., 2013; Brown et al., 2015). However, Chlamydomonas expressing IFT81-CH5E or IFT74Δ130 still assemble near full-length cilia, albeit at reduced rates (Kubo et al., 2016). In both mutants, transport of GFP–α-tubulin by IFT is reduced, potentially explaining the delay in ciliary growth. A double mutant (ift74-2 IFT74Δ130 ift81-1 IFT81-CH5E) assembles severely truncated cilia with an otherwise normal ultrastructure, supporting the notion that the IFT74N–IFT81N module promotes ciliogenesis by facilitating IFT transport of tubulin (Kubo et al., 2016). Although great care was taken to ensure that the above-described manipulations of IFT81 and IFT74 were specifically affecting tubulin transport, defects in IFT itself cannot be excluded. The strain expressing the N-terminally truncated IFT74, for example, displays anomalies in IFT speed and frequency, and the abundance of various IFT proteins in the cell body is altered (Brown et al., 2015). Further, phospholipase D is enriched in the cilia, indicative of defects in retrograde IFT (Brown et al., 2015; Lechtreck et al., 2013). Due to the short length of the cilia, IFT could not be assessed directly in the double mutant. It is therefore of interest to analyze how changes in tubulin itself affect its transport by IFT. Here, we focused on the E-hook of β-tubulin, which, as noted above, has been predicted to interact with the N-terminal domain of IFT74.
In the ciliate Tetrahymena thermophila, genomic replacement of wild-type β-tubulin with versions lacking a functional E-hook severely impairs cell viability and ciliary assembly (Duan and Gorovsky, 2002; Xia et al., 2000). Thus, approaches altering the entire tubulin pool of a cell are unsuited to determine the specific effect of such manipulations on tubulin transport by IFT. Here, we expressed GFP-tagged α- and β-tubulins in Chlamydomonas to levels of ∼10% of the total tubulin and analyzed how the altered E-hooks affect IFT transport without perturbing either IFT itself, IFT of the endogenous tubulin, or ciliary assembly. This approach has the potential to shed light on the relative contributions of IFT and diffusion in ciliary tubulin supply by revealing how a reduction in IFT of a specific assembly-competent GFP-tagged tubulin derivative affects its share in the axoneme.
We show that deletion of the E-hook of β-tubulin severely reduces its ability to bind to IFT trains. Nevertheless, E-hook-deficient β-tubulin remained abundant in the axoneme, suggesting a smaller than anticipated role of IFT in providing tubulin for axonemal assembly. Although our results reveal that most of the tubulin for axonemal assembly is supplied by diffusion, we postulate that tubulin transport by IFT ensures that enough soluble tubulin is available near the ciliary tip to promote axonemal elongation and ciliary growth.
RESULTS
The E-hook of β-tubulin promotes tubulin–IFT interaction in vivo
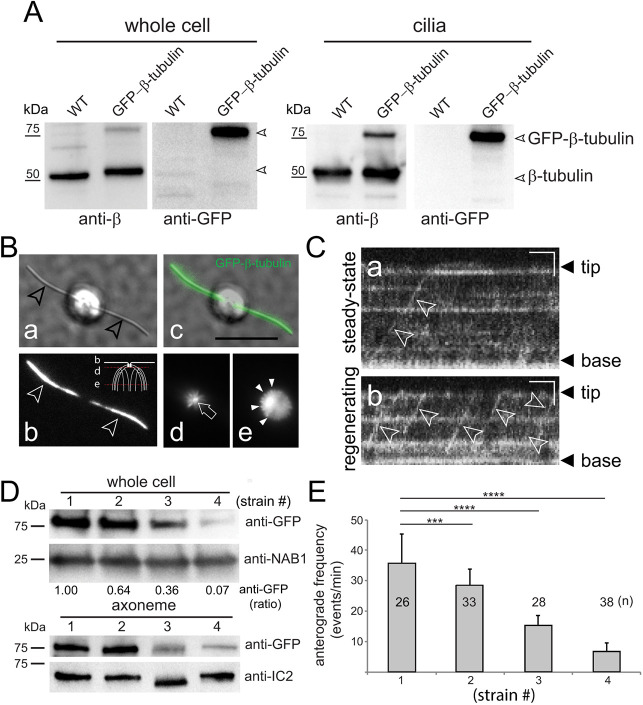
Superfolder GFP (GFP) was fused to the N terminus of β-tubulin and expressed in wild-type Chlamydomonas (Fig. 1). Western blotting using anti-GFP and anti-β-tubulin confirmed expression and the presence of GFP–β-tubulin in cilia, and we observed a similar ratio between tagged and untagged tubulin in the whole-cell and cilia samples (Fig. 1A). Live-cell imaging showed that GFP–β-tubulin is incorporated into both cytoplasmic and axonemal microtubules (Fig. 1B). As previously reported for GFP–α-tubulin, IFT of GFP–β-tubulin was rarely observed in full-length cilia but occurred with high frequency in growing cilia obtained by first deciliating the cells using a pH shock (Fig. 1C) (Craft et al., 2015). Therefore, the transport frequencies for all tubulin constructs used in this study were obtained using regenerating cilia with a length of ∼4–9 µm. A comparison of strains expressing different amounts of tagged β-tubulin revealed an approximately linear correlation between the total amount of GFP–β-tubulin expressed, the frequency of its IFT transport in regenerating cilia, and its share in the axoneme (Fig. 1D,E).
Fig. 1.
GFP–β-tubulin is transported by IFT and incorporated into microtubules. (A) Western blot analysis of whole cells and isolated cilia from a wild-type strain expressing GFP–β-tubulin and an untransformed control strain (WT). Western blots were probed with a polyclonal antibody to β-tubulin and with anti-GFP; the position of endogenous β-tubulin, the GFP-tagged version, and marker proteins are indicated. (B) Live-cell imaging of a cell expressing GFP–β-tubulin in DIC (a) and low-angle illumination (b, d, e); an overlay image of a and b is shown in c. The still images present a focal series showing the level of the cilia (marked by arrowheads in a and b), the basal bodies (arrow in d) surrounded by the root microtubules, and the cortical microtubules of the cell body (arrowheads in e). The diagram in b indicates the different focal planes. Scale bar: 10 µm. (C) Kymograms showing IFT transport (arrowheads) of GFP–β-tubulin in full-length (a) and regenerating (b) cilia. The tip and the base of the cilia are indicated. Scale bars: 2 s (horizontal) and 2 µm (vertical). (D) Western blots of whole-cell and axoneme samples obtained from four wild-type strains (numbers 1–4) expressing different levels of GFP–β-tubulin. Blots were stained with anti-GFP; antibodies to the cell body protein nucleic acid binding protein 1 (NAB1) and the axonemal dynein intermediate chain 2 (IC2) were used as loading controls for the cell body and axonemes, respectively. For quantitation of the whole cell:axoneme anti-GFP ratio, the intensity of the GFP–tubulin band in the highest expressing strain was set to 1.00; all values were corrected for the anti-NAB1 and anti-IC2 signals. (E) Frequency of GFP–β-tubulin anterograde transport during ciliary regeneration in the strains shown in Fig. 1D. Data are mean±s.d. The significance based on a two-tailed t-test is indicated (***P<0.001; ****P<0.0001).
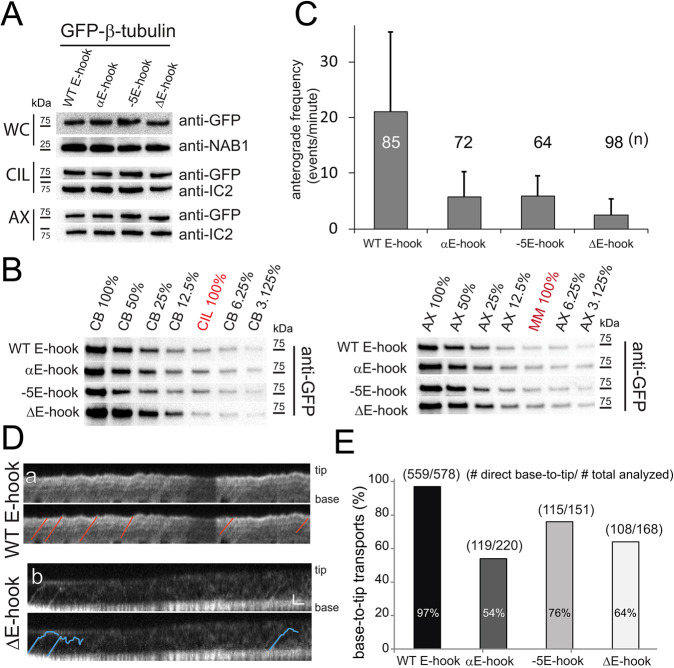
To investigate the role of the E-hook of β-tubulin (βE-hook) in IFT of tubulin, GFP-tagged β-tubulin was modified by replacing the βE-hook with the E-hook of α-tubulin (αE-hook), by replacing five glutamic acid residues in the E-hook with alanine to undermine charged-based interactions (−5E-hook), and by deleting of the C-terminal 13 residues (ΔE-hook; Fig. 2, Table S1). Cells expressing the transgenic GFP–tubulins grew normally, showed wild-type motility, assembled full-length cilia at normal rates, and IFT of tubulin progressed at standard velocities (Table S1, data not shown). To determine whether the E-hook modifications affected the frequency of GFP–β-tubulin transport, we selected strains expressing similar amounts of the transgenes, as determined by western blotting of whole-cell samples (Fig. 2A). For all four constructs, ∼8% of the total GFP-tagged protein was in the cilia and ∼90% of the ciliary GFP–tubulin was in the axonemal fraction, indicating that the fusion proteins entered cilia and were assembly competent (Fig. 2B). All three mutations in the βE-hook resulted in significant reductions in the frequency of anterograde tubulin IFT, with the ΔE-hook construct being the most affected with a ∼90% reduction in anterograde IFT (2.4 events/min, s.d. 2.9 events/min, n=98 cilia analysed compared to 21 events/min, s.d. 14.3 events/min, n=85 for wild-type β-tubulin; Fig. 2C).
Fig. 2.
The E-hook of β-tubulin promotes transport by IFT. (A) Western blot analyses of whole cells (WC), isolated cilia (CIL), and axonemes (AX) from strains expressing GFP–β-tubulin with wild-type and modified E-hooks. Anti-GFP was used to visualize the tagged β-tubulins; antibodies to NAB1 and IC2 were used to visualize loading controls for WC and CIL/AX, respectively. (B) Left and right panels show western blots of dilution series comparing the amount of GFP–β-tubulin in the deciliated cell bodies (CB) to that in cilia (CIL), and the amount of GFP–β-tubulin in the axonemes (AX) to that in the detergent-soluble membrane and matrix fraction (MM), respectively. A value of 100% indicates that equivalents of the two fractions were loaded (i.e. one cell body per two cilia). (C) The average frequency of anterograde IFT events observed for the GFP-tagged β-tubulins. The number of regenerating cilia analyzed for each strain (n) and the s.d. are indicated. (D) Kymograms showing transport of full-length (WT E-hook) and E-hook-deficient (ΔE-hook) β-tubulin in regenerating cilia. Selected tracks are marked on the lower duplicate panel for each strain. Note the reduced frequency and reduced run length of transport events involving the truncated GFP–β-tubulin (blue lines). Scale bars: 1 s (horizontal) 1 µm (vertical). (E) The percentage of IFT events that proceeded non-stop from ciliary base to tip for wild-type (WT E-hook) and modified GFP–β-tubulins. The number of processive base-to-tip events and the total number of events analyzed are indicated.
Loss of the βE-hook weakens the interaction of tubulin with IFT in vitro (Bhogaraju et al., 2013), so we investigated how loss of the βE-hook affects the processivity of tubulin transport in cilia. IFT transport of GFP–α-tubulin mostly (∼98%) proceeds in one run to the tip, indicative of a stable interaction with IFT trains (Craft et al., 2015). Similarly, 97% (n=559 IFT events) of transport events involving wild-type GFP–β-tubulin proceeded nonstop to the tip of the elongating cilia. In contrast, such processive IFT events were reduced by 25–45% for the constructs with modified E-hooks (Fig. 2D,E). In those strains, GFP–β-tubulin was observed converting from IFT transport to diffusion, indicative of a dissociation from IFT (Fig. 2D).
Considering the apparent importance of the βE-hook for tubulin binding to IFT, we transplanted the E-hook and neighboring regions of β-tubulin onto GFP or mNeonGreen (mNG; Fig. S1A). Similar to GFP alone, the GFP– and mNG–βE-hook fusion proteins readily entered cilia by diffusion. Whereas IFT transport of GFP alone was not observed, GFP– and mNG–βE-hook fusions underwent anterograde IFT, albeit at extremely low frequencies (<0.05 events/min) indicating a rather weak binding to IFT trains (Fig. S1B). In summary, the βE-hook is neither sufficient nor necessary for IFT transport; its loss, however, reduces the frequency and processivity of tubulin transport, supporting the view that the βE-hook stabilizes IFT–tubulin interactions.
High frequency transport of tubulin by IFT requires the E-hook of β-tubulin
Bhogaraju et al. (2013) proposed that tubulin binding by IFT trains involves the CH domain of IFT81 and an interaction between the E-hook of β-tubulin and the N-terminal domain of IFT74 (Fig. 3A). To further investigate the validity of this model, we analyzed tubulin transport in ift81-1 IFT81-CH5E, an ift81 null mutant expressing a variant IFT81 in which five basic residues (K73, R75, R85, K112 and R113) in the CH domain critical for tubulin binding were replaced with glutamates, and in ift74-2 IFT74Δ130, an ift74 null mutant rescued with a version of IFT74 lacking the proposed N-terminal tubulin-binding domain (Fig. 3B–F) (Bhogaraju et al., 2013; Brown et al., 2015; Kubo et al., 2016).
Fig. 3.
Loss of the E-hook attenuated IFT of GFP–β-tubulin. (A) Schematic presentation of tubulin binding by the IFT74N–IFT81N module (as proposed by Bhogaraju et al., 2013). (B) The frequency of anterograde IFT events observed for full-length (WT) and truncated (ΔE-hook) GFP-tagged β-tubulins in cilia of the ift81-1 IFT81 and the ift81-1 IFT81-CH5E strains. The number of regenerating cilia analyzed for each strain (n) is indicated. Data are mean+s.d. The corresponding western blot comparing the amounts of wild-type and ΔE-hook GFP–β-tubulin in whole-cell samples of the strains is shown below the graph. Tagged tubulin was detected with anti-GFP, anti-NAB1 staining was used to verify equal loading. (C,D) Anterograde transport of full-length and E-hook-deficient GFP–β-tubulin (C) and GFP–α-tubulin (D) in the ift74-2 IFT74Δ130 strain and wild-type (WT) controls. Data are mean+s.d., and n for each strain is indicated. Western blots of whole-cell samples documenting similar expression of the transgenes are below. (E) Frequency of anterograde IFT for full-length and E-hook-deficient GFP–α-tubulin in the ift81-1 IFT81 and the ift81-1 IFT81-CH5E strains. Data are mean+s.d., and n for each strain is indicated. The corresponding western blots of the whole-cell samples of these strains are shown below the graph. (F) Anterograde transport of αE-hook–GFP–β-tubulin in the ift81-1 IFT81 and the ift81-1 IFT81-CH5E strains. Data are mean+s.d., and n for each strain is indicated. The corresponding western blots of the whole cell samples are shown. Diagrams in B–F depict the mutations in tubulin, IFT74 and IFT81 in each strain.
The E-hook-deficient β-tubulin is predicted to be unable to interact with the N-terminal domain of IFT74 and, thus, its binding to IFT trains should depend on the CH domain of IFT81. In the ift81-1 IFT81-CH5E background, strongly reduced transport frequencies were observed for full-length β-tubulin, whereas anterograde IFT of ΔE-hook β-tubulin was essentially abolished (∼1% compared to full-length β-tubulin in strains with wild-type IFT81; Fig. 3B). Sporadic transport of E-hook-deficient β-tubulin in ift81-1 IFT81-CH5E could be due to residual binding by IFT81-CH5E or low capacity binding elsewhere on the IFT train. The ift74-2 IFT74Δ130 strain lacks the proposed βE-hook-binding site of IFT74. The anterograde IFT frequencies of full-length and E-hook-deficient β-tubulin were similarly low in this strain, suggesting that both bind with similar strength to IFT trains presumably via the remaining IFT81-CH site (Fig. 3C). Thus, the lack of the N-terminal region of IFT74 renders IFT unable to discriminate between full-length and E-hook-deficient β-tubulin.
We wondered whether IFT transport specifically requires the E-hook of β-tubulin or whether the E-hook of α-tubulin also contributes to IFT. In wild-type IFT cells, transport of E-hook-deficient α-tubulin was somewhat reduced but still occurred at a high frequency when compared to the transport of E-hook-deficient β-tubulin (Fig. 3D,E). Also, strains expressing ift74-2 IFT74Δ130 or ift81-1 IFT81-CH5E transported full-length and E-hook-deficient α-tubulin with similar, albeit low frequencies (Fig. 3D,E). Thus, the IFT system is largely insensitive to the loss of the αE-hook. Furthermore, substitution of the E-hook of GFP–β-tubulin with that of α-tubulin did not support normal transport frequencies in wild type or the ift81-1 IFT81-CH5E background (Figs 2C and 3F). The data support the model proposed by Bhogaraju et al. (2013) in which the E-hook of β-tubulin interacts with the N-terminal domain of IFT74.
Near abrogation of IFT of GFP–β-tubulin only moderately reduces its axonemal presence
Tubulin enters cilia by diffusion as well as IFT (Craft et al., 2015; Luo et al., 2017). The expression of a tagged assembly-competent tubulin with strongly decreased binding to IFT allowed us to assess the respective contributions of IFT and diffusion in supplying tubulin for axonemal assembly. If, for example, ∼90% of the ciliary tubulin were delivered by IFT, one would expect that a strong reduction in IFT of a specific tubulin species would be reflected by a similarly strong reduction of its share in the axoneme. Towards this end, we analyzed the correlation between the frequency of IFT and the axonemal share for full-length and E-hook-deficient GFP–β-tubulin (Fig. 4). Loss of the βE-hook reduced the frequency of IFT by ∼90% in wild type (Figs 3C and 4A), the ift81-1 IFT81 strain (expressing a wild-type IFT81 transgene; Fig. 3B), and the ift81-1 IFT81-CH5E strain (Fig. 3B), each in comparison to full-length GFP–β-tubulin in the same background. Western blot analyses of axonemes, however, documented only a moderate reduction of E-hook-deficient GFP–β-tubulin compared to the full-length version (Figs 2A and 4B; Fig. S2A,B). Quantitative analysis of western blots from nine independent cilia isolates (four, three and two in the wild-type (Fig. 4C), ift81 IFT81 (Fig. S2C), and ift81-1 IFT81-CH5E (Fig. S2C) backgrounds, respectively) showed an average reduction of ∼17% (s.d. 35%) for truncated versus full-length GFP–β-tubulin. Western blotting of isolated axonemes using anti-β-tubulin confirmed that full-length and truncated GFP–β-tubulin were present in similar ratios to the endogenous β-tubulin (Fig. 4D); for this experiment we used a polyclonal anti-Chlamydomonas-β-tubulin because most commercial antibodies to β-tubulin react with the E-hook (Silflow and Rosenbaum, 1981). Even in the near absence of transport by IFT, as observed for E-hook-deficient GFP–β-tubulin in the ift81-1 IFT81-CH5E strain (∼99% reduction in frequency in comparison to GFP–β-tubulin in wild type; Fig. 3B), the truncated GFP-tagged tubulin was still well represented in the cilia (Fig. S2D; n=2 independent isolates). In live-cell imaging, the cilia of cells expressing full-length GFP–β-tubulin (in the ift20 IFT20–mCherry background to facilitate identification) and of cells expressing E-hook-deficient GFP–β-tubulin were of similar brightness (Fig. 4E). In summary, the strong reduction in the frequency of IFT of E-hook-deficient β-tubulin is not matched by a proportional reduction of its presence in the axoneme.
Fig. 4.
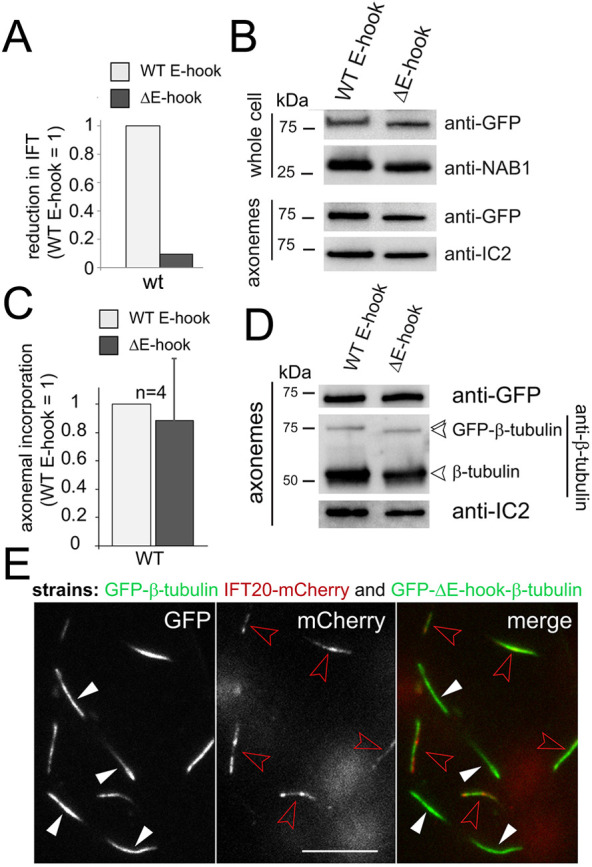
Abated IFT of E-hook-deficient tubulin does not cause a proportional reduction of its presence in the axoneme. (A) The reduction in IFT transport of truncated (ΔE-hook) versus full-length (WT E-hook) β-tubulin in control cells. The frequency observed for the full-length GFP–β-tubulin was set to 1. See Fig. 3C for the original data, s.d. and n values. (B) Western blot of control cells, comparing whole-cell and axoneme samples of the indicated strains using anti-GFP and antibodies to NAB1 and IC2, as loading controls. (C) Bar chart showing the change in the axonemal amounts of E-hook-deficient (ΔE-hook) versus full-length β-tubulin in control cells, as deduced from western blots probed with anti-GFP. The band intensity of the full-length GFP–β-tubulin was set to 1. n, number of independent cilia isolates. Data are mean+s.d. (D) Western blot comparing isolated axonemes from a wild-type strain expressing full-length or truncated β-tubulin stained with anti-GFP, anti-β-tubulin, and anti-IC2. (E) TIRF images showing the GFP and mCherry signals in cilia of live cells. Cells expressing E-hook-deficient GFP–β-tubulin (white arrowheads) and cells expressing full-length GFP–β-tubulin in the ift20-1 IFT20–mCherry background (red arrowheads) were mixed to allow for a comparison of the GFP-signal strength with the same microscope settings. Scale bar: 10 µm.
Our data suggest a limited quantitative contribution of IFT in providing tubulin for axonemal assembly. However, cells with defects in the IFT74N–IFT81N tubulin-binding module largely fail to assemble cilia. We therefore considered possible alternative mechanisms that could potentially explain the surprisingly limited effect that the reduction in IFT frequency of the truncated β-tubulin had on its presence in the axoneme.
First, if retrograde IFT had a higher affinity for endogenous full-length β-tubulin than for truncated GFP–β-tubulin, the latter could become enriched in the cilium. The frequencies of retrograde tubulin traffic, however, were negligibly low compared to anterograde traffic and were similar in range for full-length GFP–β-tubulin and its derivatives (Fig. S2E,F).
Second, the total amount of GFP–β-tubulin tolerated in axonemal microtubules could be limited, and even low IFT frequencies as observed for truncated GFP–β-tubulin could still be sufficient to saturate GFP–tubulin incorporation. In this case, there might not be a difference in the amount of full-length GFP–β-tubulin versus E-hook-deficient GFP–β-tubulin incorporated into the axoneme. We consider this situation unlikely, because the IFT frequency and axonemal quantity of full-length GFP–β-tubulin correlated well over a wide range of expression levels (Fig. 1D,E).
Third, different ciliary growth rates could be accompanied by distinct contributions of IFT and diffusion to axonemal assembly. Indeed, reduced transport of GFP–tubulin correlates with slow ciliary elongation (Kubo et al., 2016). In our experiments, we used regenerating cilia to determine IFT frequency of GFP–tubulin, whereas cilia assembled postmitotically in the course of the normal cell cycle were used for our biochemical analyses. Regenerating cilia require ∼90 min to grow 12 µm, whereas postmitotic assembly is considerably slower, taking ∼4 h to build an ∼8-µm long cilium (Madey and Melkonian, 1990; Rosenbaum et al., 1969). Therefore, postmitotic ciliary assembly could involve less IFT and more diffusion of precursors, potentially explaining the high share of ΔE-hook GFP–β-tubulin in cilia despite its low frequency of IFT. Western blot analysis, however, did not reveal a significant difference in the share of full-length and E-hook-deficient β-tubulin in postmitotic versus regenerated axonemes (Fig. S2G).
Fourth, E-hook-deficient β-tubulin could incorporate into the axoneme at a significantly higher rate than the full-length protein. In this case, one would expect the E-hook deficient version to be more abundant in the axoneme than full-length GFP–β-tubulin under conditions when both proteins show the same frequency of IFT, as is the case in the ift74-2 IFT74Δ130 background, when tubulin transport relies solely on the CH domain of IFT81. However, analysis of ift74-2 IFT74Δ130 axonemes revealed similar amounts of full-length and truncated GFP–β-tubulin (Fig. S2H). In conclusion, our observations are explained best by assuming that IFT transports only a fraction of the tubulin present in the axoneme and that most tubulin enters cilia by diffusion.
Diffusional entry of GFP–tubulin particles into cilia outnumbers entry via IFT
If entry by diffusion provides more tubulin for axonemal assembly than IFT, the number of GFP–tubulin particles entering cilia by diffusion should exceed those entering via IFT. To estimate tubulin influx, we bleached and imaged full-length and growing cilia of a wild-type strain expressing GFP–β-tubulin at high laser intensities until individual GFP–tubulin particles became visible (Fig. 5A). Because diffusing GFP–β-tubulin moves swiftly, identifying single particles required movies to be recorded at 20–40 frames per second (f.p.s.; compared to 10 f.p.s. for standard recordings); high laser intensities prevented unbleached soluble GFP–tubulin from re-accumulating in the ciliary matrix, which would obscure individual particles (Fig. 5B). In addition to the random back-and-forth motion typical of free diffusion, some GFP–β-tubulin particles moved unidirectionally for extended distances toward the tip or base of the cilia with velocities exceeding those of IFT (≥10 µm/s; Fig. 5B, red arrows). A similar behavior was previously described for EB1–mNG movements in cilia and could represent non-random diffusion (Harris et al., 2016). In the most proximal region of the cilium, high-frequency entry of GFP–tubulin mostly by diffusion generated a dense signal, often preventing a reliable particle count; for scoring, we used a more distal region, in which individual tracks were typically resolved (Fig. 5C; Fig. S3A, Movie 1).
Fig. 5.
GFP–β-tubulin rapidly enters cilia by diffusion. (A) Still images (a, b) of a wild-type cell expressing GFP–β-tubulin with full-length cilia prior (a) and during bleaching (b). The corresponding kymogram (c) shows how diffusion (arrows) and IFT (arrowhead) of GFP–β-tubulin become apparent as axonemal GFP–β-tubulin is bleached. Scale bars: 2 s (horizontal) and 2 µm (vertical). The movies were recorded at 40 f.p.s. (B) Gallery of kymograms showing diffusion (white arrows) and IFT (arrowheads) of GFP–β-tubulin in bleached full-length (a–d) and regenerating (e–g) cilia. A subset of GFP–β-tubulin diffusion occurs rapidly and almost unidirectional along the cilia (red arrows). At the ciliary tip, diffusing GFP–tubulin becomes transiently immobilized (indicated by white brackets); more stable association is visible in panels e and g (indicated by red brackets). Scale bars: 2 s (horizontal) 2 µm (vertical). (C) Kymograms of bleached full-length (a) and regenerating (b) cilia showing the entry of GFP–β-tubulin into cilia by diffusion (arrows) and IFT (arrowheads). Note accumulation of GFP–tubulin at the tip of growing cilia. Scale bars: 2 s (horizontal) 2 µm (vertical). (D) Frequency of GFP–β-tubulin entry by IFT and diffusion in regenerating (reg) and full-length (FL) cilia. The standard deviation, number of cilia analyzed and probability value (P) based on a two-tailed t-test are indicated. (E) Kymograms of GFP–β-tubulin moving by IFT in a growing cilium. GFP photobleaching events are marked by green arrowheads; bleaching appears to occur in one (a,b) or two steps (c,d). Loading (filled arrowhead in f) and unloading (open arrowheads in e and f) of GFP–β-tubulin from IFT is indicated. Based on movies recorded at 20 f.p.s. Scale bars: 2 s (horizontal) 2 µm (vertical). In B,C,E: T, position of cilia tip; B, position of cilia base.
GFP–tubulin entry by IFT occurred at frequencies of 1.7 particles/min and 24.2 particles/min into full-length and regenerating cilia, respectively, whereas entry by diffusion into full-length and regenerating cilia was observed at an average of 108 particles/min and 101 particles/min, respectively (Fig. 5D; Fig. S3A). In addition to the frequency of IFT, determining the number of GFP–tubulin cargoes per train is critical for assessing GFP–tubulin influx via IFT. IFT trains encompass on average ∼50 IFT-B complexes and each IFT train provides multiple binding sites for tubulin (Jordan et al., 2018). However, most GFP–β-tubulin particles moving by IFT bleached in one or two steps, indicating the presence of just one or two GFPs per train (Fig. 5E panels a–d). Furthermore, the brightness of GFP–tubulin moving by IFT was typically in a range similar to that of the faster-moving diffusing particles, which is particularly apparent when GFP–β-tubulin particles switch from IFT to diffusion and vice versa (Fig. 5E panels e and f). We conclude that each IFT train carries only one or a few GFP–β-tubulin particles. GFP–β-tubulin is expressed to ∼10% of the total β-tubulin level (Figs 1A and 4D). Therefore, based on the above observations, we estimate that ∼17 tubulin dimers/s enter cilia by diffusion compared to ∼4–8 dimers/s by IFT at peak entry rates. Of note, the IFT frequency of GFP–tubulin declines progressively as cilia get longer, whereas its entry by diffusion continues at high frequency in full-length cilia (Fig. 5C,D; Craft et al., 2015). Although the dense traffic of GFP–tubulin in cilia allowed only an approximation of tubulin influx, the imaging data strongly support the hypothesis that more tubulin enters cilia by diffusion than by IFT.
Tubulin entering the cilium by diffusion rapidly reaches the tip and is incorporated into the axoneme
IFT moves tubulin processively to the ciliary tip and releases it near the growing end of the axoneme, which could increase the chance of incorporation into the axoneme when compared to tubulin entering cilia by diffusion, which likely moves freely through the transition zone and therefore is expected to exit the cilium at a similar rate as it enters if it is not captured by a microtubule end. To analyze the behavior of tubulin near the ciliary tip, we bleached only the tip region using a focused laser beam and imaged GFP–β-tubulin re-entering the bleached region (Fig. S3B–D; Movie 2).
In full-length cilia, diffusing GFP–β-tubulin particles often became stationary at the ciliary tip for∼0.3–3.3 s (average 0.77 s, s.d. 0.8 s, n=16) likely indicating transient assembly into the axoneme (Fig. 5B). Interestingly, in a subset of cilia, two GFP–tubulin dots spaced ∼600 nm apart recovered near the ciliary tip, likely indicating limited turnover at the ends of both the A-tubule and the slightly shorter B-tubule (Fig. S3B) (Ringo, 1967). Overall, the incorporation of GFP–β-tubulin into the tip of full-length cilia was limited, as previously reported for mCherry–α-tubulin (Fig. 5C panel a; Fig. S3C panels a and b) (Harris et al., 2016).
A more pronounced recovery of the tip signal was observed in growing cilia reflecting axonemal growth (Fig. 5C panel b; Fig. S3C panels c and d). Recovery of the signal at the tip of growing cilia was also observed in cells expressing E-hook-deficient β-tubulin (Fig. S3B panel d). In growing cilia, one or more diffusing GFP–β-tubulin particles typically entered the bleached tip region prior to the arrival the first GFP–tubulin particle via IFT (Fig. 5B panels e–g; Fig. S3D). GFP–β-tubulin particles approaching the tip of growing cilia by IFT or diffusion remained mobile, dwelled transiently or remained stationary until the signal was bleached (Fig. 5B); the latter putatively indicates incorporation into the axoneme (Fig. 5B panels e and g; Fig. S3C,D). Thus, GFP–β-tubulin moving by both IFT and diffusion appeared to be added to the ciliary tip, indicating that both routes contribute to axonemal assembly.
DISCUSSION
Tubulin enters cilia by diffusion and as a cargo of IFT. The latter is regulated in a ciliary-length-dependent manner and it is likely that this regulation of tubulin influx contributes to ciliary length control (Craft et al., 2015; Hao et al., 2011; Kubo et al., 2016). To explore the respective contributions of each route in providing tubulin for axonemal assembly, we manipulated tubulin IFT by taking advantage of the detailed knowledge of IFT–tubulin interactions. Previously, in vitro studies showed that the E-hook of β-tubulin confers stable binding of tubulin dimers to the IFT81N–IFT74N module by interacting with IFT74 (Bhogaraju et al., 2013). In agreement with this model, removal of the E-hook of GFP-tagged β-tubulin reduced the frequency of its transport by IFT by ∼90%. However, the strong reduction in IFT was not mirrored by a comparative reduction of the share of the tagged E-hook-deficient β-tubulin present in cilia. We propose that the predominant role of IFT with regard to tubulin is to elevate intraciliary tubulin concentration, especially near the tip of the growing cilium, rather than to provide the bulk of tubulin needed for ciliary assembly.
IFT74 specifically interacts with the β-tubulin E-hook to promote tubulin IFT
Bhogaraju and colleagues (2013) showed that tubulin-binding by the IFT74N–IFT81N module was significantly reduced when the E-hook of β-tubulin was removed by a brief subtilisin treatment. Subtilisin treatment only mildly affected tubulin binding by IFT81N alone, suggesting that the positively charged IFT74N interacts with the negatively charged E-hook of β-tubulin. Our in vivo observations support this model and provide the following additional insights: (1) The E-hook of β-tubulin is not essential for binding to IFT trains or incorporation into the axonemal microtubules; it is also not sufficient to support IFT of fusion cargoes. (2) IFT74N’s interaction with tubulin is specific for the β-tubulin tail, whereas the negatively charged E-hook of α-tubulin is expendable. (3) GFP–tubulin transport was essentially abolished when the deletion of the βE-hook was combined with mutations in IFT81N, an arrangement thought to disrupt both interactions of the IFT74N–IFT81N module with tubulin. This suggests that the IFT74N–IFT81N module is the major tubulin-binding site on anterograde IFT trains and argues against the presence of additional tubulin-binding sites independent of the IFT74N–IFT81N module (Bhogaraju et al., 2014). This statement is supported by the recent finding from Kubo et al. (2016) that double mutants in IFT74N and IFT81N are essentially unable to assemble cilia.
The bulk of axonemal tubulin is supplied by diffusion
Complete replacement of endogenous β-tubulin with versions lacking the E-hook or possessing mutated E-hooks has been shown to interfere with cell division and axonemal assembly (Nielsen et al., 2001; Thazhath et al., 2002). Here, we circumvented problems resulting from the loss of endogenous β-tubulin by expressing small amounts of GFP-tagged tubulin derivatives in cells possessing endogenous wild-type tubulin. This approach allowed us to analyze IFT transport of tagged modified tubulins independently of ciliary assembly. Surprisingly, a strong reduction in IFT of E-hook-deficient GFP–β-tubulin was not matched by a proportional reduction of its share in the axoneme, suggesting that a large portion of GFP–β-tubulin enters the cilium by diffusion. However, because the axonemes of our transgenic strains are composed mostly of endogenous tubulin, it raises the question of whether results for the tagged tubulins are representative of tubulin in general. Direct editing of the endogenous tubulin genes has not yet been achieved in Chlamydomonas, preventing us from testing whether the tagged tubulins are fully functional, i.e. would support cell viability and axonemal assembly in the absence of wild-type tubulin. In budding yeast, α-tubulin with a C-terminal GFP tag is not able to replace endogenous tubulin, although co-expression is tolerated (Carminati and Stearns, 1997; Huh et al., 2003). Because of such issues, complementation assays for tagged tubulins are rarely executed. Although we cannot exclude a functional restriction, we note that N-terminally GFP-tagged α- and β-tubulin are incorporated into multiple types of microtubules in Chlamydomonas, that their expression apparently does not affect microtubule-based processes or cell viability, and that GFP–α-tubulin undergoes acetylation and polyglutamylation in cilia (Craft et al., 2015). Given that GFP–β-tubulin appears to function like endogenous β-tubulin in these ways, it is reasonable to assume that it serves as a good proxy for the endogenous tubulin in axonemal assembly. We conclude that, in Chlamydomonas, most of the tubulin used for axonemal assembly enters the cilium by diffusion. Similarly, Luo and colleges (2017) reported that ∼30% of EGFP–α-tubulin enters full-length primary cilia through the center of the axoneme by diffusion, with additional diffusion events probably occurring outside of the axonemal cylinder, where IFT trains move (Luo et al., 2017).
Estimates of the tubulin transport capacity of IFT support this notion. Although several other IFT proteins possess CH domains, only those of IFT81 and IFT54 bind tubulin, and the CH-domain of IFT54 is expendable for ciliary assembly (Bhogaraju et al., 2014; Taschner et al., 2016; Zhu et al., 2017). Thus, it is likely that the IFT74N–IFT81N module forms, or is part of, the only high-capacity tubulin-binding site of IFT. An average-length anterograde IFT train (∼300 nm) contains ∼50 IFT-B complexes, and ∼60 IFT trains enter a regenerating cilium each minute (Dentler, 2005; Jordan et al., 2018). Although not shown experimentally, the low stability of small tubulin oligomers suggests that the transported form of tubulin is an α–β dimer. Under these assumptions, and assuming that every train is completely loaded, IFT could transport ∼60% of the tubulin required to assemble a 10-µm long axoneme in 60 min and about a third of the ∼9000 tubulin dimers required each minute during rapid ciliary growth (∼350 nm/min; Bhogaraju et al., 2014). Apparently, the IFT pathway simply lacks the capacity to be the major provider of axonemal tubulin during rapid ciliary assembly. Also, the photobleaching kinetics and brightness of GFP–β-tubulin while moving by IFT indicate that the trains are only partially loaded with tubulin. Our fluorescence recovery after photobleaching (FRAP) analysis revealed that this shortfall is made up for by a continuous high-frequency influx of GFP–tubulin by diffusion, which occurs in both growing and full-length cilia [this study and Craft et al. (2015)].
In full-length cilia, IFT transport of GFP–tubulin was rarely observed, and tubulin particles appear to associate only transiently to the axonemal plus end (Harris et al., 2016). Thus, IFT appears to have a limited role as a tubulin carrier during the maintenance of full-length Chlamydomonas cilia. However, significant tubulin exchange at the tip has been observed in zygotic cilia of Chlamydomonas, indicating that these are more dynamic than those of vegetative cells (Marshall and Rosenbaum, 2001), and in primary cilia of Caenorhabditis elegans (Hao et al., 2011). In the latter, FRAP analysis revealed fast incorporation of YFP-tagged tubulin β-4 at the ends of the middle and distal segments, suggesting that both the A- and the B-tubules undergo dynamic length changes largely driven by IFT-based tubulin transport (Hao et al., 2011).
A capture-and-carry model for tubulin transport by IFT
IFT is known to be essential for cilia assembly. It transports tubulin into cilia, and defects in the IFT74N–IFT81N tubulin-binding module reduce the ciliary growth rate or impair ciliogenesis, indicating that tubulin transport by IFT is critical for the assembly of Chlamydomonas cilia (Bhogaraju et al., 2013; Kubo et al., 2016). How can these observations be reconciled with the data presented here suggesting that most tubulin enters cilia by diffusion? Similar to the assembly of sperm flagella in Drosophila, apicomplexan parasites such as Plasmodium rapidly assemble numerous axonemes within the cell body cytoplasm of the mother cell without IFT, because IFT genes are absent in Plasmodium (Billker et al., 2002; Briggs et al., 2004; van Dam et al., 2013). Apparently, diffusion is sufficient to provide the tubulin necessary for axonemal assembly in these situations, as is generally assumed to be the case for other large-scale microtubule-based structures such as the mitotic spindle. Thus, axonemal assembly per se does not require IFT of tubulin. It seems likely that the geometry of projecting cilia, especially the narrowness of their connection to the cell body cytoplasm and their small volume, could explain IFT's importance in ciliogenesis. In Chlamydomonas, the opening into the cilium corresponds to just ∼0.01% of the surface of the plasma membrane and the cross-sectional area available for diffusion through the transition zone is likely to be substantially less due to the doublet microtubules and numerous other structures present in the transition zone (Ringo, 1967). As a result, the transition zone will function as a passive physical barrier, delaying equilibration between the cell body and ciliary pools of soluble tubulin. Because of this delay, we hypothesize that diffusion alone is insufficient to supply new tubulin to the cilium fast enough to support axonemal assembly during phases of rapid ciliary growth. If correct, a quickly polymerizing axoneme embedded in a small volume of ciliary matrix could deplete soluble tubulin near the tip, causing tubulin to drop below the critical concentration for microtubule assembly, stalling elongation of the axoneme and potentially triggering depolymerization. IFT proteins are present on cytoplasmic microtubules radiating from the ciliary base (Brown et al., 2015; Richey and Qin, 2012; Wingfield et al., 2017) and IFT trains are assembled over an extended area around the basal bodies, likely allowing them to capture tubulin from this larger area, carry it through the transition zone and unload it near the ciliary tip (Brown et al., 2015; Wingfield et al., 2017). Thus, IFT will likely overcome the limitations of tubulin entry by diffusion originating from the narrowness of the transition zone (Schuss et al., 2007). Even with the bulk of axonemal tubulin entering cilia by diffusion, motor-based IFT could maintain the concentration of tubulin near the tip above a threshold critical for efficient axonemal assembly and cilia elongation, thereby ensuring rapid uninterrupted axonemal growth. Further experimentation should be able to test this hypothesis.
MATERIALS AND METHODS
Strains and culture conditions
Chlamydomonas reinhardtii was maintained in modified M medium at room temperature, aeration with 0.5% CO2, and a light/dark cycle of 14:10 h (Witman, 1986). Strain CC-620 was used as wild type for most experiments; other strains used included ift72-2 IFT74Δ130 and ift81-1 IFT81-CH5E (Kubo et al., 2016) (http://www.chlamycollection.org/).
Generation of transgenic strains
The TUB2 gene encoding β-tubulin was synthesized omitting the second intron and introducing flanking BamH1 and EcoR1 sites (Genewiz). The TUA1 gene in the previously described vector pBR25-sfGFP-α-tubulin (Craft et al., 2015; Rasala et al., 2013) was replaced with the modified TUB2 using digestion with BamH1 and EcoR1 and ligation. Changes in the sequence encoding the E-hook of β-tubulin were prepared as follows: gene segments encoding the modified C termini (E-hooks) of β-tubulin were synthesized (Genewiz), excised with EcoRI and EcoRV, and ligated into the pBR25-sfGFP vector digested with the same enzymes, taking advantage of a unique EcoRV site near the 3′ end of the TUB2 gene. Plasmids were restricted with KpnI and XbaI, and a fragment encompassing the functional Ble::GFP-β-tubulin cassette was gel purified and transformed into the various C. reinhardtii strains by electroporation. TAP plates (https://www.chlamycollection.org/methods/media-recipes/tap-and-tris-minimal/) with 10 μg/ml zeocin were used to select transformants. Transformant colonies were transferred to liquid media in 96-well plates and screened by total internal reflection fluorescence (TIRF) microscopy for expression of GFP. Expression of GFP–α-tubulin has been described previously (Craft et al., 2015). The ift20-1 IFT20–mCherry strain was transformed with the construct encoding sfGFP–β-tubulin to obtain a double-tagged strain (Lechtreck et al., 2009).
Isolation and fractionation of cilia
To isolate cilia for biochemical analysis we followed the protocol by Witman (1986). Cells were concentrated by centrifugation (1150 g, 3 min, room temperature) and washed with 10 mM HEPES, pH 7.4. Cells were resuspended in HMS (10 mM HEPES, 5 mM MgSO4 and 4% sucrose) and deciliated by the addition dibucaine (final concentration of 4.17 mM; Sigma-Aldrich) and vigorous pipetting. The cell bodies were removed by centrifugation and cilia were sedimented (17,000 g, 4°C, 20 min). Isolated cilia were resuspended in a microtubule-stabilizing buffer, HMEK (30 mM HEPES, 5 mM MgSO4, 0.5 mM EGTA and 25 mM KCl) plus protease inhibitor (P9599; 1:100; Sigma-Aldrich), and demembranated by addition of NP-40 Alternative (1% final concentration; EMD Millipore) on ice for 20 min. Axonemes were separated from the membrane and matrix fraction by centrifugation (30,000 g, 4°C, 20 min) and fractions were analyzed using SDS–PAGE and western blotting.
Western blotting
Ciliary proteins were separated using SDS–PAGE and transferred to PVDF membrane (Immobilon; Millipore). The following primary antibodies were used: rabbit anti-Chlamydomonas-β-tubulin (1:2000; a kind gift from Joel Rosenbaum, Yale University, New Haven, CT; Silflow and Rosenbaum, 1981), mouse monoclonal anti-IC2 (1:50; King and Witman, 1990), rabbit anti-GFP (1:500; Invitrogen), and rabbit anti-NAB1 (1:5000; Agrisera). Western blots were developed using anti–mouse or anti–rabbit secondary antibodies conjugated to horseradish peroxidase (Molecular Probes) and chemiluminescence substrate (SuperSignal West Dura; Thermo Fisher Scientific). A ChemiDoc MP imaging system was used for imaging, and Image Lab (both Bio-Rad Laboratories) was used for signal quantification via densitometry.
Ciliary regeneration
Cells were washed and resuspended in M medium, deciliated by a pH shock (pH ∼4.2 for 45 s), transferred to fresh M medium, and allowed to regrow cilia under constant light with agitation (Lefebvre, 1995). To delay the onset of regeneration, cells were kept on ice until needed. To initiate regeneration, cells were diluted into ambient temperature M medium.
In vivo microscopy
For in vivo imaging of GFP–tubulin by through-the-objective TIRF illumination, we used a Nikon Eclipse Ti-U equipped with a 60× NA1.49 objective and 40-mW 488-nm and 75-mW 561-nm diode lasers (Spectraphysics; Lechtreck, 2013, 2016). The excitation lasers were cleaned up with a Nikon GFP/mCherry TIRF filter and the emission was separated using an image splitting device (Photometrics DualView2 with filter cube 11-EM). Specimens were prepared as follows. Cells (∼10 µl) were placed inside a ring of vacuum grease onto a 24×60 mm No. 1.5 cover glass and allowed to settle (∼1–3 min). The observation chamber was closed by inverting a 22×22 mm No. 1.5 cover glass with ∼10 μl of 5 mM HEPES pH 7.3, 5 mM EGTA onto the larger cover glass. Images were recorded at 10–40 f.p.s. using an iXON3 camera (Andor) and the NIS-Elements Advanced Research software (Nikon). To image structures located deeper in the cell body, such as cytoplasmic microtubules (Fig. 1B panels d and e), we used a smaller incident angle, allowing the light to penetrate deeper into the specimen. ImageJ (National Institutes of Health) with the LOCI plugin (University of Wisconsin, Madison WI) and multiple kymogram plugin (European Molecular Biology Laboratory) were used to generate kymograms as previously described (Lechtreck, 2013). To photobleach the cilia, the intensity of the 488-nm laser was increased to ∼10% for 4–12 s; alternatively, a ciliary segment was bleached using a focused laser beam moved by a motorized steering mirror. To image diffusing GFP–tubulin at high frame rates, laser intensity was increased to 10–50%.
Video analysis
IFT transport events were identified manually in ImageJ. The angle tool was used to determine the velocity of the transport events. Data were transferred to Microsoft Excel for further analysis. If not stated differently in the figure legend, the n values state the number of cilia analyzed. Individual frames and kymograms were saved in ImageJ, Adobe Photoshop was used to adjust brightness and contrast, and figures were mounted in Illustrator.
Supplementary Material
Acknowledgements
We thank Joel Rosenbaum (Yale University) for the kind gift of anti-Chlamydomonas-β-tubulin).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.F.L.; Methodology: J.C.V.D.W., J.A.H., T.K., G.B.W., K.F.L.; Formal analysis: J.C.V.D.W., J.A.H., T.K.; Investigation: J.C.V.D.W., J.A.H., T.K., K.F.L.; Resources: T.K.; Writing - original draft: J.C.V.D.W., J.A.H., T.K., G.B.W., K.F.L.; Writing - review & editing: G.B.W., K.F.L.; Visualization: J.C.V.D.W., J.A.H.; Supervision: G.B.W., K.F.L.; Funding acquisition: G.B.W., K.F.L.
Funding
This study was supported by a Uehara Memorial Foundation Research Fellowship for Research Abroad (to T.K.), the Robert W. Booth Endowment at the University of Massachusetts Medical School (to G.B.W.), and grants by the National Institutes of Health (R37GM030626 and R35GM122574 to G.B.W. and R01GM110413 to K.F.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at https://jcs.biologists.org/lookup/doi/10.1242/jcs.249805.supplemental
References
- Ahmed N. T., Gao C., Lucker B. F., Cole D. G. and Mitchell D. R. (2008). ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J. Cell Biol. 183, 313-322. 10.1083/jcb.200802025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awata J., Takada S., Standley C., Lechtreck K. F., Bellve K. D., Pazour G. J., Fogarty K. E. and Witman G. B. (2014). NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. J. Cell Sci. 127, 4714-4727. 10.1242/jcs.155275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgandi H. B., Hwang S. H., Shimada I. S., Loriot E. and Mukhopadhyay S. (2017). Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J. Cell Biol. 216, 743-760. 10.1083/jcb.201607095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari N. F., Lewis J. S., Bishop G. A., Askwith C. C. and Mykytyn K. (2008). Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. USA 105, 4242-4246. 10.1073/pnas.0711027105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogaraju S., Cajanek L., Fort C., Blisnick T., Weber K., Taschner M., Mizuno N., Lamla S., Bastin P., Nigg E. A. et al. (2013). Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341, 1009-1012. 10.1126/science.1240985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogaraju S., Weber K., Engel B. D., Lechtreck K. F. and Lorentzen E. (2014). Getting tubulin to the tip of the cilium: one IFT train, many different tubulin cargo-binding sites? BioEssays 36, 463-467. 10.1002/bies.201400007 [DOI] [PubMed] [Google Scholar]
- Billker O., Shaw M. K., Jones I. W., Ley S. V., Mordue A. J. and Sinden R. E. (2002). Azadirachtin disrupts formation of organised microtubule arrays during microgametogenesis of Plasmodium berghei. J. Eukaryot. Microbiol. 49, 489-497. 10.1111/j.1550-7408.2002.tb00234.x [DOI] [PubMed] [Google Scholar]
- Borisy G. G. and Taylor E. W. (1967). The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J. Cell Biol. 34, 525-533. 10.1083/jcb.34.2.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow D. K., Koslover E. F., Seydel F., Spakowitz A. J. and Nachury M. V. (2013). An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J. Cell Biol. 203, 129-147. 10.1083/jcb.201212024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs L. J., Davidge J. A., Wickstead B., Ginger M. L. and Gull K. (2004). More than one way to build a flagellum: comparative genomics of parasitic protozoa. Curr. Biol. 14, R611-R612. 10.1016/j.cub.2004.07.041 [DOI] [PubMed] [Google Scholar]
- Brown J. M., Cochran D. A., Craige B., Kubo T. and Witman G. B. (2015). Assembly of IFT trains at the ciliary base depends on IFT74. Curr. Biol. 25, 1583-1593. 10.1016/j.cub.2015.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati J. L. and Stearns T. (1997). Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 138, 629-641. 10.1083/jcb.138.3.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D. G., Diener D. R., Himelblau A. L., Beech P. L., Fuster J. C. and Rosenbaum J. L. (1998). Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993-1008. 10.1083/jcb.141.4.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J. M., Harris J. A., Hyman S., Kner P. and Lechtreck K. F. (2015). Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J. Cell Biol. 208, 223-237. 10.1083/jcb.201409036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Barbieri F., Mitchell D. R. and Lechtreck K. F. (2018). In vivo analysis of outer arm dynein transport reveals cargo-specific intraflagellar transport properties. Mol. Biol. Cell 29, 2553-2565. 10.1091/mbc.E18-05-0291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. (2005). Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J. Cell Biol. 170, 649-659. 10.1083/jcb.200412021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J. and Gorovsky M. A. (2002). Both carboxy-terminal tails of α- and β-tubulin are essential, but either one will suffice. Curr. Biol. 12, 313-316. 10.1016/S0960-9822(02)00651-6 [DOI] [PubMed] [Google Scholar]
- Hao L., Thein M., Brust-Mascher I., Civelekoglu-Scholey G., Lu Y., Acar S., Prevo B., Shaham S. and Scholey J. M. (2011). Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat. Cell Biol. 13, 790-798. 10.1038/ncb2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. A., Liu Y., Yang P., Kner P. and Lechtreck K. F. (2016). Single-particle imaging reveals intraflagellar transport-independent transport and accumulation of EB1 in Chlamydomonas flagella. Mol. Biol. Cell 27, 295-307. 10.1091/mbc.e15-08-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y. and Witman G. B. (2017). The N-terminus of IFT46 mediates intraflagellar transport of outer arm dynein and its cargo-adaptor ODA16. Mol. Biol. Cell 28, 2420-2433. 10.1091/mbc.e17-03-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S. and O'Shea E. K. (2003). Global analysis of protein localization in budding yeast. Nature 425, 686-691. 10.1038/nature02026 [DOI] [PubMed] [Google Scholar]
- Hunter E. L., Lechtreck K., Fu G., Hwang J., Lin H., Gokhale A., Alford L. M., Lewis B., Yamamoto R., Kamiya R. et al. (2018). The IDA3 adapter, required for IFT transport of I1 dynein, is regulated by ciliary length. Mol. Biol. Cell 29, 886-896. 10.1091/mbc.E17-12-0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. A., Diener D. R., Stepanek L. and Pigino G. (2018). The cryo-EM structure of intraflagellar transport trains reveals how dynein is inactivated to ensure unidirectional anterograde movement in cilia. Nat. Cell Biol. 20, 1250-1255. 10.1038/s41556-018-0213-1 [DOI] [PubMed] [Google Scholar]
- Kee H. L., Dishinger J. F., Blasius T. L., Liu C. J., Margolis B. and Verhey K. J. (2012). A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nat. Cell Biol. 14, 431-437. 10.1038/ncb2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee H. L. and Verhey K. J. (2013). Molecular connections between nuclear and ciliary import processes. Cilia 2, 11 10.1186/2046-2530-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M. and Witman G. B. (1990). Localization of an intermediate chain of outer arm dynein by immunoelectron microscopy. J. Biol. Chem. 265, 19807-19811. [PubMed] [Google Scholar]
- Kozminski K. G., Johnson K. A., Forscher P. and Rosenbaum J. L. (1993). A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA 90, 5519-23. 10.1073/pnas.90.12.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Brown J. M., Bellve K., Craige B., Craft J. M., Fogarty K., Lechtreck K. F. and Witman G. B. (2016). The IFT81 and IFT74 N-termini together form the major module for intraflagellar transport of tubulin. J. Cell Sci. 129, 2106-2119. 10.1242/jcs.187120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck K. F. (2013). In vivo imaging of IFT in Chlamydomonas flagella. Methods Enzymol. 524, 265-284. 10.1016/B978-0-12-397945-2.00015-9 [DOI] [PubMed] [Google Scholar]
- Lechtreck K. F. (2016). Methods for studying movement of molecules within cilia. Methods Mol. Biol. 1454, 83-96. 10.1007/978-1-4939-3789-9_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck K. F., Brown J. M., Sampaio J. L., Craft J. M., Shevchenko A., Evans J. E. and Witman G. B. (2013). Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J. Cell Biol. 201, 249-261. 10.1083/jcb.201207139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck K. F., Johnson E. C., Sakai T., Cochran D., Ballif B. A., Rush J., Pazour G. J., Ikebe M. and Witman G. B. (2009). The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 187, 1117-1132. 10.1083/jcb.200909183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. A. (1995). Flagellar amputation and regeneration in Chlamydomonas. Methods Cell Biol. 47, 3-7. 10.1016/S0091-679X(08)60782-7 [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Niewiadomski P., Lin B., Nakamura H., Phua S. C., Jiao J., Levchenko A., Inoue T. and Rohatgi R. (2013). Chemically inducible diffusion trap at cilia reveals molecular sieve-like barrier Nat. Chem. Biol. 9, 437-443. 10.1038/nchembio.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Ruba A., Takao D., Zweifel L. P., Lim R. Y. H., Verhey K. J. and Yang W. (2017). Axonemal lumen dominates cytosolic protein diffusion inside the primary cilium. Sci. Rep. 7, 15793 10.1038/s41598-017-16103-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madey P. and Melkonian M. (1990). Flagellar development during the cell cycle in Chlamydomonas reinhardtii. Botanica Acta 103, 97-102. 10.1111/j.1438-8677.1990.tb00133.x [DOI] [Google Scholar]
- Marshall W. F. and Rosenbaum J. L. (2001). Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 155, 405-414. 10.1083/jcb.200106141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M. G., Turner F. R., Hutchens J. A. and Raff E. C. (2001). Axoneme-specific β-tubulin specialization: a conserved C-terminal motif specifies the central pair. Curr. Biol. 11, 529-533. 10.1016/S0960-9822(01)00150-6 [DOI] [PubMed] [Google Scholar]
- Rasala B. A., Barrera D. J., Ng J., Plucinak T. M., Rosenberg J. N., Weeks D. P., Oyler G. A., Peterson T. C., Haerizadeh F. and Mayfield S. P. (2013). Expanding the spectral palette of fluorescent proteins for the green microalga Chlamydomonas reinhardtii. Plant J. 75, 545-556. 10.1111/tpj.12165 [DOI] [PubMed] [Google Scholar]
- Richey E. A. and Qin H. (2012). Dissecting the sequential assembly and localization of intraflagellar transport particle complex B in Chlamydomonas. PLoS ONE 7, e43118 10.1371/journal.pone.0043118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo D. L. (1967). Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J. Cell Biol. 33, 543-571. 10.1083/jcb.33.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. L., Moulder J. E. and Ringo D. L. (1969). Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J. Cell Biol. 41, 600-619. 10.1083/jcb.41.2.600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuss Z., Singer A. and Holcman D. (2007). The narrow escape problem for diffusion in cellular microdomains. Proc. Natl. Acad. Sci. USA 104, 16098-16103. 10.1073/pnas.0706599104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow C. D. and Rosenbaum J. L. (1981). Multiple alpha- and beta-tubulin genes in Chlamydomonas and regulation of tubulin mRNA levels after deflagellation. Cell 24, 81-88. 10.1016/0092-8674(81)90503-1 [DOI] [PubMed] [Google Scholar]
- Taschner M., Weber K., Mourao A., Vetter M., Awasthi M., Stiegler M., Bhogaraju S. and Lorentzen E. (2016). Intraflagellar transport proteins 172, 80, 57, 54, 38, and 20 form a stable tubulin-binding IFT-B2 complex. EMBO J. 35, 773-790. 10.15252/embj.201593164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazhath R., Liu C. and Gaertig J. (2002). Polyglycylation domain of beta-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat. Cell Biol. 4, 256-259. 10.1038/ncb764 [DOI] [PubMed] [Google Scholar]
- van Dam T. J. P., Townsend M. J., Turk M., Schlessinger A., Sali A., Field M. C. and Huynen M. A. (2013). Evolution of modular intraflagellar transport from a coatomer-like progenitor. Proc. Natl. Acad. Sci. USA 110, 6943-6948. 10.1073/pnas.1221011110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J. L., Mengoni I., Bomberger H., Jiang Y.-Y., Walsh J. D., Brown J. M., Picariello T., Cochran D. A., Zhu B., Pan J. et al. (2017). IFT trains in different stages of assembly queue at the ciliary base for consecutive release into the cilium. Elife 6, e26609 10.7554/eLife.26609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B. (1986). Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 134, 280-290. 10.1016/0076-6879(86)34096-5 [DOI] [PubMed] [Google Scholar]
- Xia L., Hai B., Gao Y., Burnette D., Thazhath R., Duan J., Bre M. H., Levilliers N., Gorovsky M. A. and Gaertig J. (2000). Polyglycylation of tubulin is essential and affects cell motility and division in Tetrahymena thermophila. J. Cell Biol. 149, 1097-1106. 10.1083/jcb.149.5.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Liang Y., Gao F. and Pan J. (2017). IFT54 regulates IFT20 stability but is not essential for tubulin transport during ciliogenesis. Cell. Mol. Life Sci. 74, 3425-3437. 10.1007/s00018-017-2525-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.