Abstract
Hyperhomocysteinemia known to be associated with increased thrombotic tendency has been considered as a risk factor for coronary artery disease, atherosclerosis, venous thrombosis, and stroke. There are three main genes MTHFR, cystathionine beta-synthase (CBS) and methionine synthase (MS) and it's genetic variant that are known to influence the homocysteine metabolism leading to hyperhomocysteinemia. There is scarcity of Indian data on hyperhomocysteinemia and genetics variants in patients with thrombosis. Hence the objective of present study was to determine MTHFR, CBS, and MS genetic variants in thrombosis patients from Indian population. Genetic variant analysis was performed on thrombosis patients to detect MTHFR C677T (rs1801133), MTHFR A1298C (rs1801131), MS A2756G (rs1805087) and CBS T833C (rs5742905) mutations. The mutant allele frequencies of MTHFR 677T, MTHFR 1298C, MS2756G and CBS 833C were observed to be 16.1%, 37.5%, 34.1% and 5.8% respectively. MTHFR 677TT genotype was observed to be significantly associated with elevated homocysteine (Hcy) levels (64.65 μmol/L) alleles as compared to CC alleles (32.43 μmol/L) and CT alleles (30.54 μmol/L). MTHFR A1298C, MS A2756G and CBS T833C genotypes did not showed significant association with higher Hcy levels. Thus, in Indian patients with thrombosis only MTHFR T677T genotype was observed to be significantly associated with hyperhomocysteinemia.
Electronic supplementary material
The online version of this article (10.1007/s12291-019-00846-9) contains supplementary material, which is available to authorized users.
Keywords: Hyperhomocysteinemia, MTHFR mutants, Thrombosis, Indians
Introduction
Hyperhomocysteinemia known to be associated with increased thrombotic tendency has been considered as a risk factor for coronary artery disease (CAD) and atherosclerosis. However, few studies have reported no association between acute myocardial infarction (MI) and hyperhomocysteinemia. Mild to moderate hyperhomocysteinemia is attributed to genetic factors such as mutation in methylene tetrahydrofolate reductase (MTHFR) genes or due to environmental factors such as deficiency of vitamin B12 or folic acid. Mutations in the MTHFR gene reduces enzyme activity thus increasing plasma homocysteine (Hcy) [1], and this effect can be compensated by supplementation of folate, which is an important co-factor in the conversion of homocysteine to methionine [2]. The three main genes MTHFR, cystathionine beta-synthase (CBS) and methionine synthase (MS) and there genetic variants influences homocysteine metabolism causing hyperhomocysteinemia.
MS plays an important role in the homocysteine metabolism as a key enzyme in the remethylation pathway. The MS gene has several polymorphisms of which A2756G SNP (rs1805087) is the most prevalent polymorphism which leads to the substitution of aspartic acid by glycine. The condensation of serine and homocysteine is catalyzed by CBS enzyme to form cystathionine. Abnormal CBS activity leads to the manifestation of two major clinical conditions, such as hyperhomocysteinemia and homocystinuria. Of the large number of mutations identified in human CBS gene [3], a transition mutation, T833C (rs5742905) is the common mutation.
The two most common MTHFR gene single nucleotide polymorphisms (SNPs) C677T (rs1801133) and A1298C (rs1801131) have been reported to affect the enzyme activity. The homozygotes (T677T) and heterogzygotes (C677T) genotypes reduces enzyme activity by about 70% and 40% respectively and thereby causing elevation of plasma homocysteine levels [4] and reduction in DNA methylation [5]. Whereas the A1298C SNP results in the reduction of MTHFR activity to a lesser extent than in the case of C677T [4].
Many studies have reported the association of MTHFR C677T and A1298C mutations with cardiovascular diseases, neural-tube defects, various types of cancer, male infertility, pregnancy complications, and other complex diseases where the role of folate is largely documented. Increased homocysteinemia levels affects the vascular structure. Therefore, hyperhomocysteinemia has been related with a wide range of disorders such as cardiovascular diseases, stroke, and venous thrombosis. Several studies have reported that C677T and A1298C polymorphisms might be associated with DVT due to hyperhomocysteinemia.
High heterogeneity have been reported in frequency distribution of both C677T and A1298C mutations among different ethnic groups [6]. There is scarcity of data on MTHFR gene variants in thrombotic patients with hyperhomocysteinemia from Western India. Hence the present study was planned to determine association of genetic variants with homocysteine levels in patients with thrombosis amongst Western Indian populations.
Materials and Methods
Study Subjects
This study involves retrospective data analysis of patients with thrombosis and prescribed for MTHFR mutation analysis by the consulting physician between January 2012 till December 2016. The study design and protocols were approved by the Institutional Review Board (IRB). Written informed consent was obtained from all the patients, and the study was conducted following the tenets of the Helsinki Declaration. MTHFR mutation analysis was performed on patients with thrombosis affecting different sites such deep venous thrombosis (DVT), pulmonary embolism (PE), portal venous thrombosis (PVT), superior sagittal thrombosis (SST), cerebral venous thrombosis (CVT), etc. Patients with other diseases or illness such as malignancies, delayed growth defects, and gynaecological problems were excluded. History regarding the presence of traditional risk factors like hypertension, diabetes mellitus, family history of bleeding disorders and hyperhomocysteinemia were also documented.
Genotyping
DNA was extracted by modified Miller et al. [7] protocol. ARMS PCR was standardized for detection of C677T, and A1298C, A2756G and T833C mutations using primers and cycling conditions described in Online Resource 1. Representative samples were sent for DNA sequencing for validation and confirmation.
Statistical Analysis
Allele frequencies and genotype frequencies were calculated by counting method and Chi square analysis was performed for deviation from Hardy-Weinberg equilibrium using online calculator (www.husdyr.kvl.dk/htm/kc/popgen/genetik/applets/kitest.htm). T test and Chi-square test were performed for comparison of homocysteine levels and MTHFR mutants using Graphpad Prism software (version 7.02). p values < 0.05 were considered significant.
Results
Of the 155 thrombotic patients screened, 124 patients were included in the study whose samples were sent for both MTHFR mutation analysis and Hcy estimation. The mean age of patients was observed to be 35 (± 17) years and included 91 males and 33 females. The clinical presentation of these 124 patients showed that 32% had DVT, 29% had brain thrombosis and 12% had CVST (additional data are given in Online Resource 2).
The genotype and allele frequencies of C677T, A1298C, A2756G and T833C alleles are demonstrated in Table 1. The heterozygous and mutant alleles exhibited highest frequencies for A1298C allele than C677T. Though the MTHFR 1298C allele frequency (37.5%) and MS 2756G allele frequency (34.1%) were higher than the MTHFR 677T frequency (16.1%), no significant association was observed between mutant allele frequency and Hcy levels. In contrast to this the 677T allele frequency exhibited increasing trend with Hcy levels and was significantly highest in Hcy level of > 30 μmol/L.
Table 1.
MTHFR Genotype and Allele frequnecies and homocysteine levels
| C667T Mutation (N = 124) | A1298C Mutation (N = 124) | MS A2756G Mutation (N = 69) | CBS T833C Mutation (N = 69) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype frequencies % (n) | ‘T’ allele frequency % | Genotype frequencies % (n) | ‘C’ allele frequency % | Genotype frequencies % (n) | ‘G’ allele frequency % | Genotype frequencies % (n) | ‘C’ allele frequency % | |||||||||
| CC | CT | TT | AA | AC | CC | AA | AG | GG | TT | TC | CC | |||||
| Hcys levels between 0 – 10 μmol/L | 81.5 (22) | 18.5 (5) | 0 | 9.3 | 37 (10) | 33.3 (9) | 29.6 (8) | 46.3 | 53.3 (8) | 20 (3) | 26.7 (4) | 36.7 | 86.7 (13) | 13.3 (2) | 0 | 6.7 |
| Hcys levels between 10 – 15 μmol/L | 78.3 (18) | 21.7 (5) | 0 | 10.9 | 26.1 (6) | 60.9 (14) | 13 (3) | 43.5 | 66.7 (8) | 33.3 (4) | 0 | 16.7 | 83.3 (10) | 16.7 (2) | 0 | 8.3 |
| Hcys levels between 15 – 30 μmol/L | 71.9 (23) | 21.9 (7) | 6.2 (2) | 17.2 | 53.1 (17) | 40.6 (13) | 6.25 (2) | 26.56 | 40.9 (9) | 54.6 (12) | 4.5 (1) | 31.8 | 86.4 (19) | 13.6 (3) | 0 | 6.8 |
| Hcys levels between > 30 μmol/L | 69.1 (29) | 16.7 (7) | 14.3 (6) | 22.6 (p = 0.00069)* | 40.5 (17) | 45.2 (19) | 14.3 (6) | 36.9 | 30 (6) | 50 (10) | 20 (4) | 45 (p < 0.002)* | 95 (19) | 5 (1) | 0 | 2.5 (p = 0.0131)* |
| Total (n = 124) | 74.2 (92) | 19.4 (24) | 6.5 (8) | 16.1 (p = 0.0015)* | 40.3 (50) | 44.4 (55) | 15.3 (19) | 37.5 | 45 (31) | 42 (29) | 13 (9) | 34.1 | 88.4 (61) | 11.6 (8) | 0 | 5.8 |
*Hardy–Weinberg equilibrium showed significance
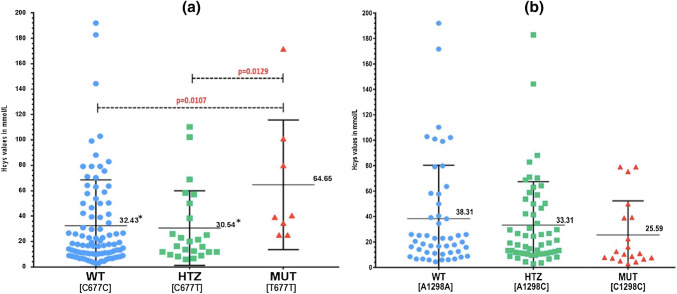
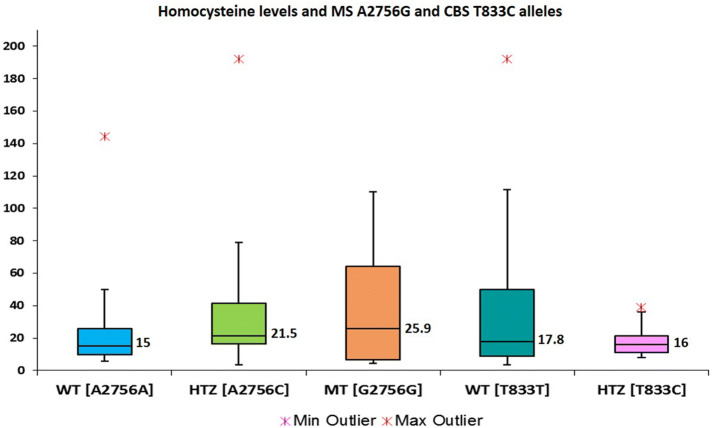
Hcy levels were observed to be significantly higher in MTHFR T677T alleles as compared with MTHFR C677C (p = 0.0107) and MTHFR C677T (p = 0.0129) alleles (Fig. 1a). In case of A1298C mutations the Hcy levels did not showed significant differences between MTHFR C1298C mutant alleles as compared to MTHFR A1298A and MTHFR A1298C alleles (Fig. 1b). Similar trend was also observed in patient groups with DVT and brain thrombosis (Fig. 2a, b). Since 17 patients (38%) with elevated Hcy levels had wild type genotype for both C677T and A1298C alleles, sub-group analysis was performed for MS A2756G and T833C alleles. Sub-group analysis on 69 patients did not showed significant association of MS A2756G and CBS T833C alleles with hyperhomocysteinemia (Fig. 3).
Fig. 1.
Allelic distribution and homocysteine levels. a C677T alleles and b A1298C alleles
Fig. 2.
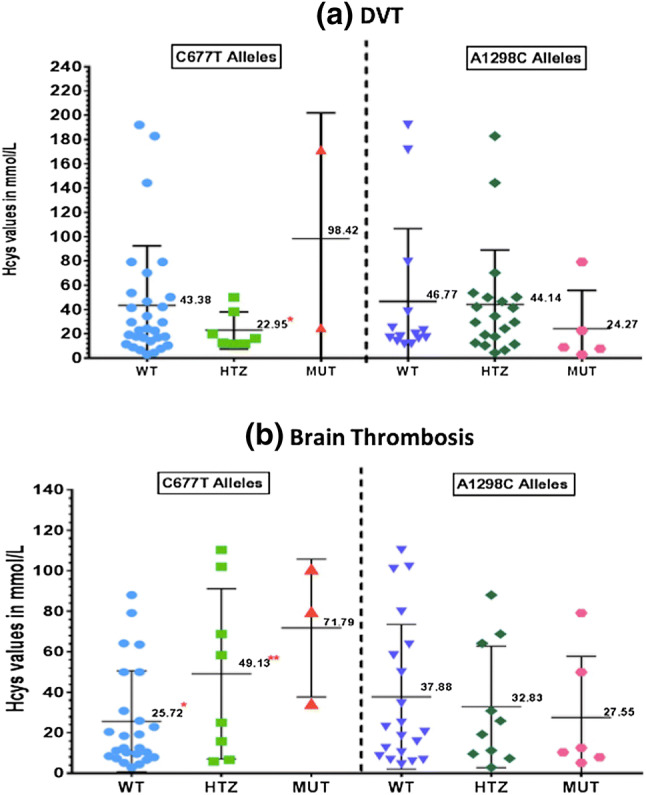
Allelic distribution and homocysteine levels in a patients with DVT and b patients with brain thrombosis
Fig. 3.
Homocysteine levels and MSA2756G alleles and CBS T833C alleles
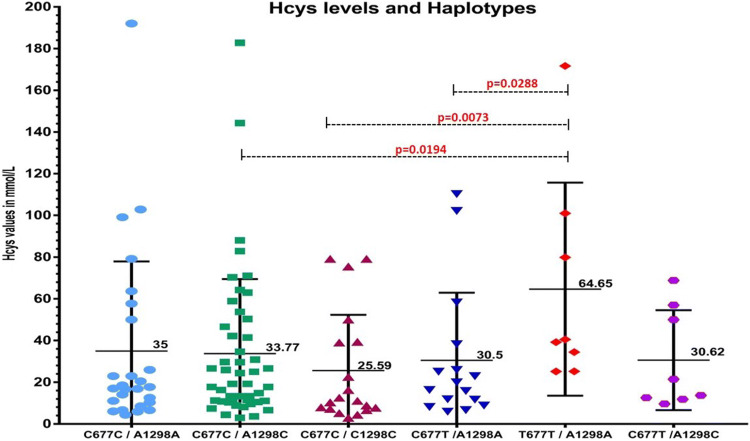
The distribution of MTHFR haplotypes with Hcy levels is illustrated in Fig. 4. The haplotype analysis was done for MTHFR C677T and MTHFR A1298C variants, which showed significantly higher levels of Hcy in T677T/A1298A as compared C677C/A1298C, C677C/C1298C and C677T/A1298A haplotypes. The Hcy levels did not showed significant differences in compound heterozygous C677T/A1298C haplotypes. The MTHFR haplotypes C677C/A1298C, C677C/C1298C, C677T/A1298A and compound heterozygous C677T/A1298C were detected in patients with varying range of Hcy levels. However the detrimental haplotype T677T/A1298A has been detected only in patients with hyper homocysteinemia (Fig. 5).
Fig. 4.
MTHFR haplotypes and homocysteine levels
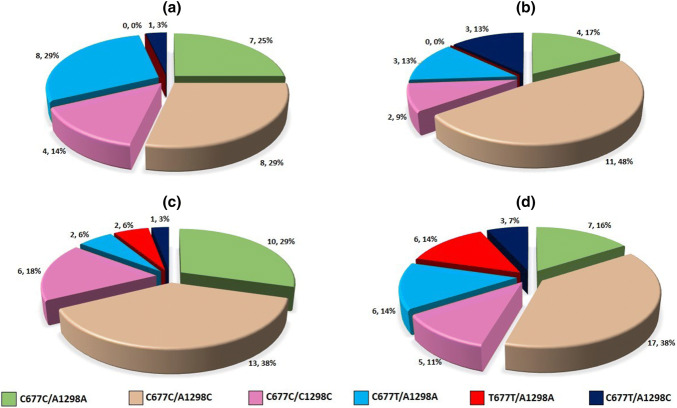
Fig. 5.
Distribution of MTHFR haplotypes in patients with different homocysteine levels. a Hcy levels 0–10 μmol/L (n = 28); b Hcy levels 10–15 μmol/L (n = 23); c Hcy levels 15–30 μmol/L (n = 34); d Hcy levels >30 μmol/L (n = 44)
A sub-group analysis for MS A2756G and CBS T833C mutation detection was performed on 69 patients. The allele frequency of MS 2756G allele and CBS 833C allele was observed to be 34.1% and 5.8% respectively.
Discussion
Hyperhomocysteinemia has been associated with various complex disorders. The levels of plasma homocysteine can potentially be elevated either due to environmental (including diet) and/or genetic factors. Among the dietary factors, folate and vitamin B12 play a critical role in the metabolism of homocysteine. Since most of the Indian population is deficient in vitamin B12, presumably due to their adherence to a strict vegetarian diet, it has been hypothesized that genetic polymorphisms responsible for the metabolism of homocysteine might have greater impact on hyperhomocysteinemia. In the present study hyperhomocysteinemia was observed in 78% of thrombotic patients and hypertension was observed in 28% patients.
The MTHFR 677T allele frequency in the present study (16.1%) was similar to the average frequency (14%) reported by Indian Genome Variation Consortium. The ‘T’ allele frequency amongst Indian populations has been reported [8] to show high variability with 16.7% in North India, followed by 9.9% in North East India, 7.8% in Western India, 7.6% in South India and 1.1% in East India. Linguistically, the average ‘T’allele frequency has been reported to be 12.8% in the Indo-European group, followed by 9.9% amongst the Tibeto-Burman, 6.5% in Dravadian, and absent in Austro-Asiatic population. Worldwide, 677T allele frequency has been reported to be highest in Europe ranging from 24.1 to 64.3%, followed by North America (6–64.3%), East Asia (2–55%), South America (2–48.7%), Asia (2.5–45%), Africa (0–35.5%), Siberia (8–31.5%) and Oceania (2.9–28.6%) [8]. Allele frequency was observed to be 14.9% amongst Sri Lankan population [9]. The presence of TT genotype among all non-tribal populations and a high frequency of 677T allele from north Indian population, which are linguistically Indo-European compared to tribal populations, is indicative of the gene flow from Eurasian populations into north Indian populations [8].
Preponderance of MTHFR 1298C alleles was observed to be more than the 677T allele in the present study. The MTHFR 1298 C allele frequency amongst thrombotic patient in the present study was 37.5%. Saraswathy et al. [8] reported high incidence of 1298C amongst the east Indian populations (34.6%), followed by west (27%), south (24.3%), north (16.8%) and northeast Indian populations (13.5%). The MTHFR 1298C allele frequency have been reported to show wide variability, ranging from 43% in New Delhi, 10% in Chandigarh in the Northern India to 40.4% in Tamil Nadu. Linguistically, the allele was most frequent among the Dravidian populations (28.7%), followed by Austro-Asiatic (22.8%), Indo-European (21.3%) and Tibeto-Burman populations (13.5%). Worldwide, high allele frequency has been reported in East Asia ranging from 18 to 70%, followed by Asia (17–44.6%), Europe (24–40%), Siberia (38%), Africa (13–32.3%), South America (0–15%) and North America (14.7%) [8]. The present data showed a higher frequency of 1298C (37.5%) than that of Americas (0–15%). Perera et al. [9] reported allele frequencies of ‘C’ allele frequency to be 31.3% in SriLankan populations.
Elevated homocysteine levels has toxic effects on the vascular structure [10] and has been associated with disorders such as cardiovascular diseases, stroke, and venous thrombosis. DVT is a multi-factorial disorder and genetic factors has been accountable for approximately 60% DVT cases [11]. MTHFR C677T polymorphism is most commonly studied hereditary defect responsible for hyperhomocysteinemia. Ghaznavi et al. [12] have reported ‘T’allele frequency to be 24% amongst DVT patients in Iranian population. Soltanpour et al. [13] have reported the ‘T’ allele frequency to be 21% and 27.2% in controls and venous thrombosis patients respectively. Heijer et al. [14] demonstrated in a meta-analysis that T677T genotype was associated with a 20% increased risk of venous thrombosis. Literature have reported controversial findings regarding association of C677T polymorphism, hyperhomocysteinemia and DVT [15, 16]. Significant association of MTHFR C677T genotypes with elevated homocysteine in DVT patients has been reported in Macedonian population. Another study from Macedonian patients with DVT also reported association between MTHFR C677T and A1298C genotypes with homocysteine levels [17]. Similarly in the present study T677T homozygous mutant was observed to be significantly associated with hyperhomocysteinemia in DVT patients. In the present study, the C1298C homozygous mutant did not showed significant association with hyperhomocysteinemia in DVT patients. Iranian population did not showed significant risk between MTHFR A1298C mutant and venous thrombosis [18]. Soltanpour et al. [18] have reported ‘C’ allele frequency of MTHFR A1298C to be 36.5% and 38.5% respectively amongst controls and patients with venous thrombosis respectively. Hossini et al. [19] have reported that genetic variants of FV Leiden, MTHFR C677T, MTHFR A1298C and PAi-1 4G/5G are significantly associated with an increased risk of DVT.
Indian studies have reported contradictory findings regarding association of MTHFR C677T gene mutations and risk of Ischemic stroke. Kumar et al. [20] have reported significant association whereas Kalita et al. [21] did not reported significant association inspite of reporting high frequency of MTHFR T677T mutants. Das et al. [22] have reported MTHFR C677T to be an important risk factor for Ischemic stroke and hemorrhagic stroke amongst South Indian populations. The 677T allele frequency was reported to be 14%, 17% and 12% amongst Ischemic stroke, hemorrhagi stroke and controls respectively. Bharatkumar et al. [23] have reported that Hcy as a risk factor for Indian patients with CVT and MTHFR T677T genotype to be responsible for increased Hcy levels. Kumar et al. [20] reported an association between MTHFR C677T polymorphism and ischemic stroke. Li et al. [24] have reported association between C677T mutations and Hcy levels in SVT patients. Numerous studies have reported inconsistent results on associations between MTHFR T677T genotypes and ischemic stroke. This variant have reported to be show more pronounced association with ischemic stroke amongst Asian [25] patients than Caucasians [26]. In the present study sub-group analysis in brain thrombosis showed significantly elevated of Hcy levels in patients with MTHFR T677T genotypes, whereas no significance was observed with MTHFR C1298C genotypes. There is limited literature on A1298C mutation from Indian population in brain thrombosis. A study for Tunisia reported definite correlation between A1298C and CVT [27].
These two functional and well characterized SNPs in the MTHFR gene, C677T and A1298C, have been associated with decreased enzyme activity and increased levels of plasma homocysteine. The homozygotes (T677T) and heterogzygotes (C677T) genotypes reduces enzyme activity by about 70% and 40% respectively and thereby causing elevation of plasma homocysteine levels, whereas the A1298C SNP results in the reduction of MTHFR activity to a lesser extent than in the case of C677T [4]. Several studies in different populations have reported that MTHFR T677T genotype is associated with high levels of homocysteine. Our results corroborate with previous findings which reported significant association between T677T mutant and hyperhomocysteinemia than the C1298C mutant. Additionally, the haplotype T677T/A1298A was associated with significantly higher levels of Hcy. Increased homocysteine levels damages blood vessels and has been associated with disorders such as cardiovascular diseases, stroke, and venous thrombosis. Though the compound heterozygous C677T/A1298C variants has reduce the enzyme activity, these did not showed any significance with Hcy levels. Ghaznavi et al. [12], reported that higher plasma homocysteine levels in patients with MTHFR T677T genotype than those with C677C and C677T genotypes. These findings are in concordance with the ACMG guidelines which states that the variants C677T, A1298C and C677T/A1298C compound heterozygous are unlikely to be of clinical significance [28]. Although MTHFR A1298C polymorphism has been associated with hyperhomocysteinemia, there is scarcity of data on its prevalence in patients with thrombosis in Indian population. Markan et al. [29] and Kumar et al. [30] reported significantly higher Hcy levels in subjects with A1298C polymorphism in patients with hypertension and CAD respectively. In contrast to these findings, hyperhomocysteinemia was not significantly associated with A1298C in the thrombotic patients in the present study.
The wild type haplotype C677C/A1298A has been detected in 17 patients with elevated Hcy levels (> 15 μmol). Hence a sub-group analysis was performed on 69 patients to detect whether MS and CBS genetic variants are responsible for hyperhomocysteinemia. MS plays an important role in the Hcy metabolism as a key enzyme in the remethylation pathway. The most prevalent A2756G SNP have been reported to be commonly and heterogeneously distributed amongst the worldwide populations. The M2756G allele frequency has been reported to be 8.5% in Chinese population [31] followed by 11.8% in Thais [32], 13.1% in Korean [33], 17.3% in Japanese [34], 18.8% in Hispanics [35], 19.9% in Caucasians [35] and 23.8% in African Americans [35]. A study from Northern India have reported frequency to be 24% [36], whereas in the present study the frequency was highest (34.1%).
Zhang et al. [37] reported frequency of CBS 833C allele to be 31.1% and 34.3% in hypertension and control groups respectively. In a meta analysis, Ding et al. [38] have reported that CBS T833C polymorphisms to be associated with an increased risk of stroke and that ‘C’ allele is likely to be an important risk factor for stroke. Kumar et al. [39] have reported no association of CBS T833C polymorphisms in CAD patients amongst Indian population. There is lack of data on CBS gene mutation amongst patients with thrombosis in Indian population. In the present study no significant association was observed between MS and CBS gene mutations and hyperhomocysteinemia.
Homocysteine level has been found to be significantly affected by many non-genetic factors, such as gender, age, and plasma/serum levels of folate and vitamin B12 amongst Asian populations. Additionally, environmental factors such as vegetarian diet, which is known to be associated with increased risk of Vitamin B12 deficiency, could also lead to hyperhomocysteinemia. Geneitc variants such as genes involved in the transport and/or absorption of folate, vitamin B6 or vitamin B12 could be highly prevalent in our population. Kumar et al. [40] reported significant association between choline dehydrogenase (CHDH A119C) and plasma homocysteine in Indian population. A genome-wide approach using linkage and association studies in large population could be a powerful tool in the detection of unknown polymorphisms associated with hyperhomocysteinemia in the Indian population.
The study may pose limitation due to small size and genetic analysis is limited to known mutations. However, from perspective of Indian population there is lack of data on genetic variants, Hcy levels and thrombosis from Indian population. Despite of the limitations, the present study provides significant association of MTHFR 677TT mutants with hyperhomocysteinemia in thrombotic patients from Western Indian populations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1: ARMS PCR conditions used for detection of MTHFR C677T and A1298C mutations. (DOC 51 kb)
Online Resource 2: Clinical distribution of thrombosis patients. BCS: Budd Chairy Syndrome; CVST:; DVT: Deep Vein Thrombosis; HPVT: Hepatic portal vein thrombosis; PE: Pulmonary Embolism; PVT: Portal vein thrombosis (JPEG 71 kb)
Acknowledgements
We would like to acknowledge the National Health Education Society of P. D. Hinduja Hospital & MRC for their support throughout the study.
Author contributions
All the authors have accepted responsibility for the entire content of this submitted manuscript and approve submission.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional review board (IRB) and with the 1964 Helsinki declaration and its later amendments.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Minal Umesh Paradkar, Email: minal.deshmukh@hindujahospital.com.
Balkrishna Padate, Email: dr_cbalakrishnan@hindujahospital.com.
Swarup A. V. Shah, Email: dr_swarup.shah@hindujahospital.com
Hiral Vora, Email: hiralvora9513@gmail.com.
Tester F. Ashavaid, Email: dr_tashavaid@hindujahospital.com
References
- 1.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Mattews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 2.Ueland PM, Hustad S, Schneede J, Refsum H, Vollset SE. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci. 2001;22:195–201. doi: 10.1016/S0165-6147(00)01675-8. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Weiberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetehydrofolate reductase (MTHFR) association with decreased enzyme activity. Mol Genet Metab. 1998;64:169–172. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 5.Friso S, Choi S, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider JA, Rees DC, Liu YT, Clegg JB. Worldwide distribution of a common methylenetetrahydrofolate reductase mutation. Am J Hum Genet. 1997;62:1258–1260. doi: 10.1086/301836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraswathy KN, Asghar M, Samtani R, Murry B, Mondal PR, Ghosh PK, et al. Spectrum of MTHFR gene SNPs C677T and A1298C: a study among 23 population groups of India. Mol Biol Rep. 2012;39:5025–5031. doi: 10.1007/s11033-011-1299-8. [DOI] [PubMed] [Google Scholar]
- 9.Perera R, Chandrasena LG, Indrakumar J, Peiris H. MTHFR C677T and A1298C gene polymorphisms and Ischemic Heart Disease (IHD) in a Sri Lankan population—a preliminary study. Indian J Mednodent Allied Sci. 2017;5:1–8. doi: 10.5958/2347-6206.2017.00001.2. [DOI] [Google Scholar]
- 10.Kluijtmans LA, van den Heuvel LP, Boers GH, Frosst P, Stevens EM, van Oost BA, et al. Moleuclar genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylenetetrahydrofolate reductase gene is a genetic risk factor for cardiovascular disease. Am J Hum Genet. 1996;58:35–41. [PMC free article] [PubMed] [Google Scholar]
- 11.Souto JC, Almasy L, Borrell M, Blanco-Vaca F, Mateo J, Soria JM, et al. Genetic susceptibility to thrombosis and its relationship to physiological risk factors: the GAIT study. Am J Hum Genet. 2000;67:1452–1459. doi: 10.1086/316903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghaznavi H, Soheili Z, Samiei S, Soltanpour S. Association of methylenetetrahydrofolate reductase C677T polymorphism with hyperhomocysteinemia and deep vein thrombosis in the Iranian population. Vasc Spec Int. 2015;31:109–114. doi: 10.5758/vsi.2015.31.4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soltanpour MS, Soheili Z, Pourfathollah AA, Samiei S, Meshkani R, Deyhim MR, et al. The association between common C677T mutation in Methylenetetrahydrofolate reductase gene and the risk of venous thrombosis in an Iranian population. Lab Med. 2008;39:97–100. doi: 10.1309/BHB986MDHMGQP9VG. [DOI] [Google Scholar]
- 14.Heijer MD, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3:292–299. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 15.Brezovska-Kavrakova J, Krstevska M, Bosilkova G, Alabakovska S, Panov S, Orovchanec N. Hyperhomocysteinemia and of methylenetetrahydrofolate reductase (C677T) genetic polymorphism in patients with deep vein thrombosis. Mater Sociomed. 2013;25:170–174. doi: 10.5455/msm.2013.25.170-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elhassan HO, Abdalla M. Methylenetertahydrofolate reductase (MTHFR C677T) polymorphism in Sudanese patients with deep vein thrombosis. Int J Biomed Res. 2015;6:323–326. doi: 10.7439/ijbr.v6i5.2023. [DOI] [Google Scholar]
- 17.Spiroski I, Kedev S, Antov S, Arsov T, Krstevska M, Dzhekova-Stojkova S, et al. Methylenehydrofolate reductase (MTHFR-677 and MTHFR-1298) genotypes and haplotypes and plasma homocysteine levels in patients with occlusive artery disease and deep venous thrombosis. Acta Biochim Pol. 2008;55:587–594. doi: 10.18388/abp.2008_3065. [DOI] [PubMed] [Google Scholar]
- 18.Soltanpour MS, Soheili Z, Pourfathollah AA, Samiei S, Meshkani R, Kiani AA, et al. The A1298C mutation in methylenetetrahydrofolate reductase gene and its association with idiopathic venous thrombosis in an Iranian population. Lab Med. 2011;42:213–216. doi: 10.1309/LM5LWXCHVZY9RFOM. [DOI] [Google Scholar]
- 19.Hosseini S, Kalantar E, Hosseini MS, Tabibian S, Shamsizadeh M, Dorgalaleh A. Genetic risk factors in patients with deep venous thrombosis, a retrospective case control study on Iranian population. Thromb J. 2017;13:35. doi: 10.1186/s12959-015-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Misra S, Hazarika A, Kumar P, Sagar R, Pathak A, et al. Association between methylenetetrahydrofolate reductase (MTHFR) C677T gene polymorphism and risk of ischemic stroke in North Indian population: a hospital based case-control study. Egypt J Med Hum Genet. 2016;17:359–365. doi: 10.1016/j.ejmhg.2016.01.001. [DOI] [Google Scholar]
- 21.Kalita J, Srivastava R, Bansal V, Agarwal S, Misra UK. Methylenetetrahydrofolate reductase gene polymorphism in Indian stroke patients. Neurol India. 2006;54:260–263. doi: 10.4103/0028-3886.27148. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Roy S, Kaul S, Jyothy A, Munshi A. MTHFR gene (C677T) polymorphism in Ischemic stroke, its subtypes and hemorrhagic stroke in a South Indian populations. Acta Med Int. 2018;2:28–33. [Google Scholar]
- 23.Bharatkumar VP, Nagaraja D, Christopher R. Hyperhomocysteinemia and methylenetetrahydrofolate reductase C677T polymorphism in cerebral venous-sinus thrombosis. Clin Appl Thromb Hemost. 2014;20:78–83. doi: 10.1177/1076029612466285. [DOI] [PubMed] [Google Scholar]
- 24.Li SL, Yue XY, Wang H, Zhang L, Lu ZJ. MTHFR C677T, homocysteine and risk of splanchnic vein thrombosis: a pooled analysis of published epidemiological studies. J Genet Mutat Disord. 2015;1:101. [Google Scholar]
- 25.Soriente L, Coppola A, Madonna P, Cerbone AM, Di Minno G, Orefice G, et al. Homozygous C677T mutation of the 5, 10 methyltetrahydrofolate reductase gene and hyperhomocysteinemia in Italian patients with a history of early-onset ischemic stroke. Stroke. 1998;29:869–871. doi: 10.1161/01.STR.29.4.869. [DOI] [PubMed] [Google Scholar]
- 26.Markus HS, Ali N, Swaminathan R, Sankaralingam A, Molloy J, Powell J. A common polymorphism in the methylenetetrahydrofolate reductase gene, homocsyeteine and ischemic cerebrovascular disease. Stroke. 1997;28:1739–1743. doi: 10.1161/01.STR.28.9.1739. [DOI] [PubMed] [Google Scholar]
- 27.Fekih-Mrissa N, Klai S, Mrad M, Zaouali J, Sayeh A, Nsiri B, et al. Role of methylenetetrahydrofolate reductase A1298C polymorphism in cerebral venous thrombosis. Blood Coagul Fibrinolysis. 2013;24:118–119. doi: 10.1097/MBC.0b013e32835707cd. [DOI] [PubMed] [Google Scholar]
- 28.Hickey SE, Curry CJ, Toriello HV. ACMG practice guideline: lack of evidence for MTHFR polymorphism testing. Genet Med. 2013;15:153–156. doi: 10.1038/gim.2012.165. [DOI] [PubMed] [Google Scholar]
- 29.Markan S, Sachdeva M, Sehrawat B, Kumari S, Jain S, Khullar M. MTHFR 677 CT/MTHFR 1298 CC genotypes are associated with increased risk of hypertension in Indians. Mol Cell Biochem. 2007;302:125–131. doi: 10.1007/s11010-007-9434-5. [DOI] [PubMed] [Google Scholar]
- 30.Kumar J, Das SK, Sharma P, Karthikeyan G, Ramakrishnan L, Sengupta S. Homocysteine levels are associated with MTHFR A1298C polymorphism in Indian population. J Hum Genet. 2005;50:655–663. doi: 10.1007/s10038-005-0313-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhao HI, Li XQ, Zhang ZX, Bi XH, Wang B, Zhang JW. Association analysis of methionine synthase gene 2756 A > G polymorphism and Alzheimer disease in a Chinese population. Brain Res. 2008;1204:118–122. doi: 10.1016/j.brainres.2008.01.064. [DOI] [PubMed] [Google Scholar]
- 32.Sirachainan N, Wongruangsri S, Kajanachumpol S, Pakakasama S, Visudtibhan A, Nuchprayoon I, et al. Folate pathway genetic polymorphisms and susceptibility of central nervous system tumors in Thai children. Cancer Detect Prev. 2008;32:72–78. doi: 10.1016/j.cdp.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Kim Y, Lee I, Lee J, Yang D, Park K, et al. Polymorphisms involved in the folate metabolizing pathway and risk of multiple myeloma. Am J Hematol. 2007;82:798–801. doi: 10.1002/ajh.20967. [DOI] [PubMed] [Google Scholar]
- 34.Morita H, Kurihara H, Sugiyama T, Hamada C, Kurihara Y, Shindo T, et al. Polymorphism of the methionine synthase gene. Association with homocysteine metabolism and late-onset vascular diseases in the Japanese population. Artheroscler Thromb Vasc Biol. 1999;19:298–302. doi: 10.1161/01.ATV.19.2.298. [DOI] [PubMed] [Google Scholar]
- 35.Conroy JM, Trivedi G, Sovd T, Caggana M. The allele frequency of mutations in four genes that confer enhanced susceptibility to venous thromboembolism in and unselected group of New York state newborns. Thromb Res. 2000;99:317–324. doi: 10.1016/S0049-3848(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 36.Shekari M, Sobti R, Kordi Tamandani D, Suri V. Impact of methylenetetrahydrofolate reductase (MTHFR) codon (677) and methionine synthase (MS) codon (2756) on risk of cervical carcinogenesis in North Indian population. Arch Gynecol Obstet. 2008;278:517–524. doi: 10.1007/s00404-008-0623-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Wang H, Sun HW, Chen YL, Ouyang JY, Wang YU, et al. Correlation between cystathionine bsynthase T833C genetic polymorphism and primary hypertension. Exp Ther Med. 2014;8:713–718. doi: 10.3892/etm.2014.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding R, Lin S, Chen D. The association of cystathionine β synthase (CBS) T833C polymorphism and the risk of stroke: a meta-analysis. J Neurol Sci. 2012;312:26–30. doi: 10.1016/j.jns.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 39.Kumar J, Garg G, Ganesan K, Sengupta S. Cystathionine β-synthase 844ins68 polymorphism is not associated with level of homocysteine and cysteine in an Indian population. Biomarkers. 2010;15:283–287. doi: 10.3109/13547501003658106. [DOI] [PubMed] [Google Scholar]
- 40.Kumar J, Garg G, Kumar A, Sundaramoorthy E, Sanapala KR, Ghosh S, et al. Single nucleotide polymorphisms in homocysteine metabolism pathway genes: association of CHDH A119C and MTHFR C677T with hyperhomocysteinemia. Circ Cardiovasc Genet. 2009;2:599–606. doi: 10.1161/CIRCGENETICS.108.841411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1: ARMS PCR conditions used for detection of MTHFR C677T and A1298C mutations. (DOC 51 kb)
Online Resource 2: Clinical distribution of thrombosis patients. BCS: Budd Chairy Syndrome; CVST:; DVT: Deep Vein Thrombosis; HPVT: Hepatic portal vein thrombosis; PE: Pulmonary Embolism; PVT: Portal vein thrombosis (JPEG 71 kb)