Abstract
A surge to increase the production via usage of chemicals at both industrial and agricultural arena has forced humans to be routinely and imprudently exposed to a wide variety of endocrine disrupting chemicals. The overall aim of the study was to evaluate possible relation that might exist between bisphenol-A (BPA) and the adipose tissue hormones, and further impact on adiposopathy. In the present study, the role of BPA, an “endocrine disruptor” with respect to adiposopathy was evaluated in type 2 diabetes mellitus patients. For the study, 150 healthy control subjects and 150 newly diagnosed diabetes patients were recruited. Fasting venous blood samples was analyzed for several biochemical parameters such as serum glucose, lipid profile, insulin, adiponectin, leptin, TNF-α, IL-6, IL-1, free fatty acid. Concentrations of BPA were also measured both in control and diabetic subjects. Serum BPA concentration was found to be significantly higher in diabetic subjects in comparison to the control subjects. Levels of BPA were found to be positively correlated with BMI and WC in diabetic subjects. Also, it was found to be positively correlated with leptin and negatively correlated with adiponectin in diabetic subjects. Therefore, the current study suggested more deleterious effect of BPA on diabetes and its pathophysiology.
Keywords: Bisphenol-A, Adiposopathy, Type 2 diabetes mellitus, Endocrine disruptors, Adipose tissues
Introduction
With changing lifestyle, obesity and diabetes are emerging as huge public health crisis and threatening the economies of all nations, particularly developing countries. According to International Diabetes Federation (IDF), in 2015 about 415 million people had diabetes mellitus worldwide. This figure is projected to be 642 million by the year 2040. South Asians have a disproportionately higher incidences of type-2 diabetes mellitus (T2DM) and it occurs at 50% higher rates in them as compared to other racial groups [1]. Diabetes develops 5–10 years earlier in them and is one of the principle causes of premature heart attack and death. According to IDF, there were 69.2 million diabetic adults in India in 2015, the second highest number for any country, only after China. Asian Indians also show a “lean fat” phenotype characterized by relatively lower BMI, but higher body fat content, particularly visceral fat. The high prevalence and early age of onset of T2DM in this population is attributable to this excess fat. Clustering of diabetes and other components of metabolic syndrome has currently been defined as a formal disease of adipose tissue, so called adiposopathy (or sick fat); the later defined as pathogenic adipose tissue dysfunction and is promoted and exacerbated by fat accumulation (adiposity) [2].
Many genetic and environmental risk factors such as diet, physical inactivity, and sleep disturbances contribute to the increased prevalence of diabetes in this ethnic group. However, preventive strategies based on management of traditional risk factors have been more or less unsuccessful in controlling the current epidemic of diabetes. The scientific community has shown considerable interest in understanding the contribution of non-traditional risk factors to the diabetes epidemic, especially environmental chemicals and particularly endocrine-disrupting chemicals (EDCs). Researchers addressing the role of environmental chemicals in the development of metabolic disorders, like obesity and T2DM, have rapidly expanded. Epidemiological and experimental evidence suggested an association between exposure to EDCs and T2DM, especially since the exposure to chemicals increased massively in the last decade [3, 4].
Several EDCs that are responsible for the increasing prevalence of human diseases including obesity, and diabetes have been found to alter the hormonal and homeostatic systems [5]. One such endocrine disruptor is bisphenol-A (BPA). It is used as a constituent monomer in polycarbonate plastics, which are used extensively in water bottles and food packaging and in the production of oxidant used in the lining of canned goods [6]. BPA is wield its effects through endocrine disruption, epigenetic modification, cytokine release and oxidative stress. BPA can be urinary eliminated with half life of 4–6 h but being partially lipophilic, it also accumulates in human fat, can therefore potentially cause adipose tissue dysfunction [7]. It antagonizes the action of PPARγ, resulting in decreased expression of adiponectin. Not only this, BPA also increases secretion of interleukin-6 (IL-6) [8]. IL-6 is mostly regarded as a pro-inflammatory cytokine that promotes inflammation under various pathological conditions. Its anti-inflammatory and regenerative properties have also been increasingly recognized [9].BPA also increased the secretion of another pro-inflammatory cytokine, tumor necrosis factor-α (TNFα). Therefore, the diabetogenic mechanism of BPA action could be due to enhanced release of pro-inflammatory adipokine such as IL-6 and TNF-α from human adipose tissue [10]. In vitro study had shown that BPA can influence adipogenesis, increase expression of adipogenic genes, and promote inflammation. Hugo et al. studied the effect of low dose of BPA on secretion of adiponectin from adipose explants and mature adipocytes. They found that low nanomolar doses (1 and 10 nM) inhibited adiponectin secretion from adipose tissue and mature adiposities [11].
The studies related to the role of BPA in the T2DM associated adipose tissue dysfunction (adiposopathy) in Asian Indians are few. Therefore, to have a conclusive remark, more statistically significant studies are the need of the hour. In the present study, we have evaluated the role of BPA in T2DM associated adipose tissue dysfunction in Asian Indians. Specifically, the role of adipocytokines like adiponectin, leptin, TNF-α, IL-6, IL-1α, and free fatty acid in T2DM and their association with BPA was studied. The primary focus of the present study was to evaluate the effect of BPA on adipose tissue hormones, adiposity, insulin resistance, and insulin secretion in Asian Indians T2DM patients.
Materials and Methods
Subjects
The study was conducted on 150 T2DM patients diagnosed as per American Diabetes Association criteria (age 44.12 ± 8.5 year, M:F ratio 57.33 vs. 42.67). The patients with secondary diabetes mellitus (DM), other endocrinopathy, on drugs such as steroids, Pioglitazone, Metformin and with conditions that could influence insulin resistance, IHD, infections were excluded from the study. Also, T2DM patients who were directly exposed to chemicals or having cancer, renal failure or HIV were excluded. 150 healthy normal glucose tolerant controls (age 44.1 ± 10.4 years, M:F ratio 55.33 vs. 44.67) were studied as controls. The institutional ethics committee of SMS Medical College and Hospital, Jaipur, Rajasthan, India, approved the study protocol with reference number 110/EC/2012. Informed written consent was obtained from all the subjects.
Anthropometric Measurements
Anthropometric measurements were taken with height and weight measured to the nearest 0.1 cm and 0.5 kg, respectively. Waist circumference (WC) was measured at the horizontal circumference between the lowest rib margin and the iliac crest. Hip circumference was measured at the maximum circumference over the buttocks. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Waist to hip ratio (WHR) was calculated as waist circumference divided by hip circumference (HC).
Collection and Analysis of Blood Samples
All the subjects were asked to fast overnight, before collection of blood. The collected blood samples were allowed to clot. Serum was collected after centrifugation of clotted blood at 1300–1800 rpm for 10 min. Serum glucose and lipids were measured as per manufacturers’ instructions on fully automated analyzer (AU400/Kopran). Total cholesterol (Anamol), Triglycerides (Beacon), Phospholipids (Beacon), HDL (LoGotech), Glucose (Recombigen), FFA (Randox) were analyzed. VLDL was calculated using formula: VLDL (conc.) = TG/5. Serum insulin was estimated by chemiluminescent immunometric assay using Immulite 2000 machine. Adipokines, such as adiponectin (BioVendor); leptin (Diagnostics Biochem, Canada Inc); TNF-α, IL-6 and IL-1 (AviBion, Orgenium Laboratories, Finland), C-peptide (Dia Metra) and endocrine disruptor, BPA (Cusabio Biotech) were measured by ELISA (Mindray MR-96 A) methods as per manufacturers’ instructions.
Insulin resistance index was assessed for homeostasis model assessment (HOMA-IR) using the formula [12, 13]:
The following table includes blood analysis and estimations done in terms of method, principle, results and permissible limits with remarks about offbeat concentrations.
Results and Discussion
The roles of genetic predisposition and life-style related factors as cause of obesity and metabolic disorders like diabetes is well known, however, the role of environmental factors in its pathogenesis cannot be ignored. Due to intense industrialization and increased usage of chemicals in agriculture, humans have become more exposed to endocrine disrupting chemicals. One such EDC is bisphenol-A, omnivorously present in plastic packaging products of everyday use. Therefore, the current study was focused to assess the role of BPA as an endocrine disruptor in T2DM associated adipose tissue dysfunction in Asian Indians.
Anthropometric Parameters of Adiposity
The anthropometry parameters of adiposity were measured in all 300 subjects as summarized in Table 1. In diabetics, all these parameters i.e. body mass index (BMI), waist circumference (WC), hip circumference (HC), and W:H ratio (0.96 ± 0.085 vs. 0.93 ± 0.096) were found to be significantly higher in comparison with the controls.
Table 1.
Anthropometric measurements of diabetic versus control group
| Character | Mean ± SD | p value | |
|---|---|---|---|
| Diabetic | Control | ||
| Number | 150 | 150 | |
| Age (years) | 44.12 ± 8.5 | 44.1 ± 10.4 | 0.98 |
| BMI (kg/m2) | 26.52 ± 4.39 | 23.45 ± 2.4 | 0.00 |
| WC (cm) | 94.37 ± 11.08 | 82.28 ± 12.67 | 0.00 |
| HC (cm) | 98.13 ± 11.7 | 87.96 ± 12.79 | 0.00 |
| W:H ratio | 0.96 ± .0.085 | 0.93 ± 0.096 | 0.00 |
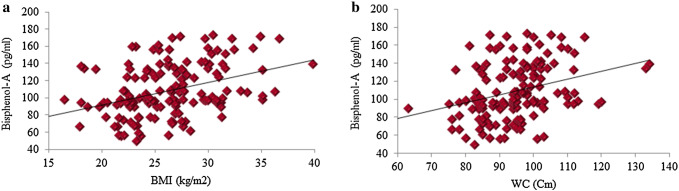
Diabetic group had significantly higher BPA concentration as compared to control people (109.04 ± 30.32 vs. 102.04 ± 20.67). On serum analysis of all 300 subjects the levels of BPA, a known endocrine disrupter was found to be higher in diabetic people as compared with healthy control. A remarkable positive correlation was observed between anthropometric parameters in BPA exposed diabetic patients, as shown in Fig. 1.
Fig. 1.
Bisphenol-A was shown to be positively correlated with BMI (a) and WC (b) in diabetic group
Biochemical Parameters
Serum glucose, lipid profile, insulin, HOMA-IR, HOMA-β levels were measured in all the subjects and the results are summarized in Table 2. In diabetics, plasma glucose, insulin, HOMA-R were significantly higher, whereas HOMA-β were significantly lower when compared with the controls. Diabetic were also found to have higher concentrations of total lipid, phospholipid, triglyceride, and VLDL and LDL cholesterol. Whereas HDL cholesterol concentration was significantly lower in diabetics vs control subjects.
Table 2.
Summation of various biochemical parameters and their comparison
| Character | Mean ± SD | p value | |
|---|---|---|---|
| Diabetic | Control | ||
| Number | 150 | 150 | |
| Glucose (mg/dl) | 170.03 ± 56.12 | 90.9 ± 9.79 | 0.00 |
| Serum insulin (µU/l) | 6.50 ± 5.48 | 4.66 ± 3.66 | 0.00 |
| C-peptide (ng/ml) | 1.72 ± 0.80 | 3.13 ± 1.02 | 0.00 |
| HOMA-IR | 2.65 ± 2.1 | 1.05 ± 0.84 | 0.00 |
| HOMA-β | 29.4 ± 33.39 | 70.49 ± 25.19 | 0.00 |
| Total lipids (mg/dl) | 605.68 ± 127.15 | 555.04 ± 113.94 | 0.00 |
| Phospholipids (mg/dl) | 205.21 ± 37.01 | 190.68 ± 36.16 | 0.00 |
| Triglycerides (mg/dl) | 156.18 ± 73.51 | 119.39 ± 50.01 | 0.00 |
| Total cholesterol (mg/dl) | 191.57 ± 41.60 | 180.47 ± 39.86 | 0.01 |
| HDL-C (mg/dl) | 43.74 ± 5.39 | 47.47 ± 7.15 | 0.00 |
| LDL-C (mg/dl) | 116.56 ± 37.9 | 109.40 ± 34.58 | 0.00 |
| VLDL-C (mg/dl) | 31.22 ± 14.74 | 23.84 ± 10.11 | 0.00 |
| FFA (mmol/l) | 0.99 ± 0.10 | 0.52 ± 0.21 | 0.00 |
All the studies were done in triplicates to analyze the biochemical parameters in control and diabetics
The whole study was conducted keeping in view the effect of endocrine disruptor, BPA on diabetic patients. When levels of BPA in serum of all the subjects was measured, it was present considerably at elevated levels in diabetic patients. But no correlation was found for BPA with TG, HDL and HOMA-IR in both control and diabetic as shown in Table 3.
Table 3.
Correlation of BPA with Biochemical parameter in diabetics and controls
| Variables | Control | Diabetic | ||
|---|---|---|---|---|
| r | p | r | p | |
| TG | 0.082 | 0.31 | − 0.015 | 0.85 |
| HDL | 0.035 | 0.66 | − 0.013 | 0.85 |
| Insulin | 0.044 | 0.58 | 0.140 | 0.07 |
| HOMA-IR | 0.038 | 0.63 | 0.083 | 0.312 |
| FFA | − 0.020 | 0.80 | − 0.024 | 0.77 |
Analysis of Presence of Adipokines
Adipokines are significant factor either in development or progression of diabetes. Adiponectin concentrations (inversely), leptin and IL-6 levels (positively) were associated with increased risk of T2DM even after adjustment for anthropometric measurements. Lower circulating adiponectin levels and elevated inflammatory cytokines are strongly associated with increased risks of obesity-related diseases including diabetes. Serum BPA can suppress adiponectin and increased IL-6 and TNF-α release even at nanomolar concentrations, proving that BPA could be the bona fide EDC that adversely affects metabolic homeostasis [10].
The relation of BPA with anthropometric parameters of adiposity, insulin resistance and adipocytokines like adiponectin, leptin, TNF-α, IL-6, IL-1α, free fatty acid was studied in diabetics and control subjects summarized in Table 4.
Table 4.
Concentrations of various adipokines in studied groups
| Character | Mean ± SD | p value | |
|---|---|---|---|
| Diabetic | Control | ||
| Adiponectin (µg/ml) | 4.12 ± 1.39 | 6.86 ± 2.79 | 0.00 |
| Leptin (ng/ml) | 11.54 ± 5.47 | 14.44 ± 3.44 | 0.00 |
| TNF-α (pg/ml) | 87.88 ± 26.77 | 82.12 ± 27.45 | 0.07 |
| IL-6 (pg/ml) | 103.89 ± 16.83 | 101.76 ± 13.37 | 0.22 |
| IL-1α (pg/ml) | 62.42 ± 10.53 | 60.15 ± 7.73 | 0.03 |
All the studies were done in triplicates to analyze the influence and impact of BPA on concentrations of various cytokines in control and diabetics.
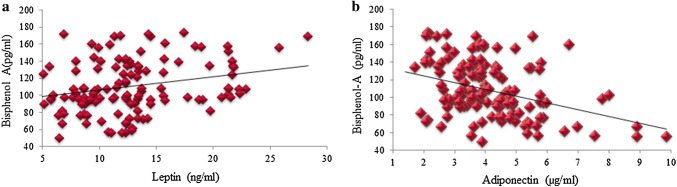
The current study has clearly shown that circulatory levels of TNF-α and IL1-α were elevated in diabetics. But there was no association between circulatory BPA level and these cytokines in either group as shown in Table 5. Though, some of previous studies had shown that BPA increases secretion of pro-inflammatory cytokines like TNF-α and IL-6 [14]. No association between BPA and these cytokines was seen in our study, it could be speculated that BPA gets accumulated in adipose tissue and its circulatory levels in a cross sectional study may not reflect true tissue level action of this endocrine disruptor. As BPA is lipophilic and gets accumulated in adipose tissue and the experimental evidences suggested that it could alter adipogenesis and adipocytokine secretion. Moreover, adipocytokines are known to influence insulin resistance and beta cell dysfunction and diabetes [15]. Therefore, altered adipocytokine secretion from adipose tissue could be one of mechanism of diabetogenic action of BPA. In this connection, an interesting finding of this study is that in diabetic subjects, serum BPA levels were positively correlated with BMI, WC, and leptin levels and negatively correlated with adiponectin levels. Significant positive correlations of leptin (r = 0.261, p = 0.00) were observed with BPA in diabetes (Table 5, Fig. 2a). Whereas, a negative correlation with adiponectin (r = −0.398, p = 0.00) was found with BPA in diabetic patients (Fig. 2b). Such correlation was not seen in control group. Therefore, these findings supported the above-mentioned hypothesis that relationship between BPA and the adipose tissue hormone secretion could be one of the mechanisms of adipose tissue dysfunction (adiposopathy) in diabetics.
Table 5.
Correlation between serum levels of BPA and adipokines in control vs diabetic groups
| Variables | Control | Diabetic | ||
|---|---|---|---|---|
| r | p | r | p | |
| Leptin | 0.099 | 0.22 | 0.261 | 0.00 |
| Adiponectin | − 0.115 | 0.16 | − 0.398 | 0.00 |
| TNF-α | − 0.066 | 0.94 | − 0.047 | 0.56 |
| IL-6 | − 0.108 | 0.18 | − 0.036 | 0.66 |
| IL-1α | − 0.054 | 0.51 | 0.035 | 0.66 |
| FFA | − 0.020 | 0.80 | − 0.024 | 0.77 |
Significant positive correlation of leptin (r = 0.261, p = 0.00) and negative correlation with adiponectin (r = − 0.398, p = 0.00) was found with Bisphenol-A in diabetic patients in comparison to normal, healthy, non-obese controls
Fig. 2.
Correlation of bisphenol-A with adiponectin and leptin in diabetic group. BPA was found to be significantly positively correlated with leptin (a) and observed to possess negative impact on adiponection (b)
Inflammatory cytokines play a major role as regulator of the metabolism of adipose tissues. Several studies had reported an increase in the plasma concentrations of these cytokines in obese subjects. In particular, these pro-inflammatory cytokines have been investigated extensively for their potential role in the development of obesity-induced complications [16]. The findings of this study, which included high BPA levels and its association with obesity, insulin level and adipokines levels, suggested its role in diabetes associated adipose tissue dysfunction in this population also. Therefore, its presence in patients is warning for further deterioration of diabetic condition. BPA is known to cause hyper secretion of insulin. In our case, BPA was found to be negatively correlated with adiponectin, a key regulator of insulin sensitivity and tissue inflammation. To the best of our knowledge, this is the first study from India that showed possible positive correlation of BPA with diabetes, obesity and associated adipose tissue dysfunction. Our study is in consistent with a few in vivo studies conducted earlier which suggested that BPA exposure might have a role in weight gain and obesity development through several mechanisms, including its actions on pre-adipocytes [17, 18].
Though, epidemiological evidences suggested relationship between BPA and diabetes, but limited information is available for the mechanism of BPA activity that might cause adipose tissue dysfunction [19]. A recent study had shown deleterious effect of chronic exposure of BPA on human primary adipocytes undergoing differentiation [19]. In the current study, levels of adiponectin were not only much reduced, but also showed a reciprocal relationship with BPA levels in diabetics. Therefore, it supports the hypothesis that BPA could contribute to pathogenesis of T2DM via altered adipose tissue adiponectin secretion. Several research studies had observed both positive and negative correlation between BPA and leptin levels [20]. The findings of present study are in consistent with the previously reported work. Moreover, these findings pointed towards interrelationship between BPA, adiposity, adipose tissue hormones and diabetes.
Though our data suggested possible correlation of BPA with a few obesity related hormones such as leptin, adiponectin and cytokines, yet a detailed longitudinal investigation is required to determine the mechanistic approach of BPA activity in adiposopathy.
Conclusion
Bisphenol A, a major component in polycarbonates and is being found in a myriad of products including plastic food and beverage containers. The main mechanism by which the population is exposed to BPA is through leaching from plastic products. BPA is thought to wield its effects through endocrine disruption, epigenetic modification, cytokine release and oxidative stress. Insulin resistance is a key etiological factor for T2DM. It is known worldwide that BPA exposure, particularly early in life, may alter adipose energy homeostasis in a way that is promoting fat storage and obesity that could either lead to onset of diabetes or have harmful effects on already existing diabetic condition by means of dysregulation of adipokines and cytokines. The detailed study done so far had driven to following conclusions where a positive relation was deducted between levels of BPA and existing diabetic condition. A clear change in levels of adipokines is an indicator that BPA, a known EDC must be used very wisely for it can lead to increased pathology of T2DM. More detailed study is the need of the hour to investigate possible mechanism and impact of BPA exposure on incidences of diabetes.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Ethical Standards
Ethics Committee approval for conduct of above mentioned study. Reference no. 110/EC/2012 by Office of the Ethics committee, SMS Medical college & attached Hospital, Jaipur
Research Involving Human Participants and/or Animals
Ethics Committee approval has been obtained.
Informed Consent
Duly signed, written informed consent from all the participants was obtained.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jyoti Jain and Nidhi Gupta contributed equally as first authors.
References
- 1.International Diabetes Federation . IDF diabetes atlas. 8. Brussels: International Diabetes Federation; 2017. [PubMed] [Google Scholar]
- 2.Punjabi P, Mathur P, Gupta RC, Mathur I, Thanvi J, Gupta D, Mathur SK. Autonomic dysfunction in Asian Indian T2DM patients is related to body fat content instead of insulin resistance: a DEXA study. J Basic Appl Sci. 2014;10:212–219. doi: 10.6000/1927-5129.2014.10.29. [DOI] [Google Scholar]
- 3.Duan Y, Yao Y, Wang B, Han L, Wang L, Sun H, Chen L. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: a case-control study. Environ Pollut. 2018;243(Pt B):1719–1726. doi: 10.1016/j.envpol.2018.09.093. [DOI] [PubMed] [Google Scholar]
- 4.Ogo FM, de Lion Siervo GEM, Staurengo-Ferrari L, de Oliveira Mendes L, Luchetta NR, Vieira HR, Fattori V, Verri WA, Jr, Scarano WR, Fernandes GSA. Bisphenol A exposure impairs epididymal development during the peripubertal period of rats: inflammatory profile and tissue changes. Basic Clin Pharmacol Toxicol. 2018;122(2):262–270. doi: 10.1111/bcpt.12894. [DOI] [PubMed] [Google Scholar]
- 5.Coster SD, Larebeke NV. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health. 2012;2012:1–52. doi: 10.1155/2012/713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez MF, Arrebola JP, Taoufiki J, Navalon A, Ballesteros O, Pulgar R, et al. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol. 2007;207(2):215–221. doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Wang B, Tang Q, Liu J, Yang X. Bisphenol A triggers proliferation and migration of laryngeal squamous cell carcinoma via GPER mediated upregulation of IL-6. Cell Biochem Funct. 2017;35(4):209–216. doi: 10.1002/cbf.3265. [DOI] [PubMed] [Google Scholar]
- 9.LaPensee CR, Hugo ER, Ben-Jonathan N. Insulin stimulates interleukin-6 expression and release in LS14 human adipocytes through multiple signaling pathways. Endocrinology. 2008;149:5415–5422. doi: 10.1210/en.2008-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol-A on adipokine release from human adipose tissue: implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304:49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol-A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabet Care. 2004;27(6):1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 14.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 15.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147:50–55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 16.Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol-A accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84:319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- 17.Phrakonkham P, Viengchareun S, Belloir C, Lombe’s M, Artur Y, Canivenc-Lavier MC. Dietary xenoestrogens differentially impair 3T3-L1 preadipocyte differentiation and persistently affect leptin synthesis. J Steroid Biochem Mol Biol. 2008;110:95–103. doi: 10.1016/j.jsbmb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Dunmore SJ, Brown EPJ. The role of adipokines in β-cell failure of type 2 diabetes. J Endocrinol. 2013;216:T37–T45. doi: 10.1530/JOE-12-0278. [DOI] [PubMed] [Google Scholar]
- 19.Verbanck M, Canouil M, Leloire A, Dhennin V, Coumoul X, Yengo L, et al. Low- dose exposure to bisphenols A, F and S of human primary adipocyte impacts coding and non-coding RNA profiles. PLoS ONE. 2017;12(6):e0179583. doi: 10.1371/journal.pone.0179583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rönn M, Lind L, Örberg J, Kullberg J, Söderberg S, Larsson A, et al. Bisphenol-A is related to circulating levels of adiponectin, leptin and ghrelin, but not to fat mass or fat distribution in humans. Chemosphere. 2014;112:42–48. doi: 10.1016/j.chemosphere.2014.03.042. [DOI] [PubMed] [Google Scholar]