Abstract
Acute kidney injury (AKI) is characterized by fast decline in renal function within a short period of time. Renal ischemic–reperfusion (I–R) injury is the main cause of AKI. This study aims to investigate the possible nephroprotective effect of lycopene on renal ischemic–reperfusion injury in mice model. Forty Swiss Albino adult male mice were randomly allocated onto one of the four study groups: sham group: mice had median laparotomy under anesthesia with no procedures performed, renal tissues and blood samples were collected. ischemic–reperfusion group (I–R-control): mice underwent median laparotomy under anesthesia, followed by 30 min bilateral renal ischemia. Renal tissues and blood samples were collected after 2 h from reperfusion. Vehicle-treated group: mice were pretreated with intra 1% dimethyl sulfoxide 30 min before inducing ischemia. Lycopene-treated group: mice were pretreated with 10 mg/kg intraperitoneal injection of lycopene 30 min before inducing renal ischemia. Renal tissues, and blood samples were collected after 2 h from reperfusion. Blood and tissue samples were collected to look for evidence of inflammation and necrosis. Blood urea nitrogen, serum creatinine as well as plasma NGAL levels were significantly increased in the active control group (P ≤ 0.05), when compared to the sham group. Similarly, renal levels of Notch2/Hes 1, TLR 2, IL-6, Bax, and F2-isoprostane were significantly increased in the active control group as compared to the sham group (P ≤ 0.05). Moreover, lycopene treatment was found to be significantly effective in reducing the increased levels of these markers after I–R injury (P ≤ 0.05).
Keywords: Renal ischemia–reperfusion injury, Lycopene, Notch2/hes1 protein, IL-6, NF-κB
Introduction
Acute kidney injury (AKI) is becoming a common pathological disorder, characterized by rapid deterioration of renal function with a relatively short period. AKI can be classified according to the etiology into the major categories: (pre-renal, renal, and post-renal). The prerenal causes of AKI account for 25%, the intrarenal causes account for 35–70%, while the postrenal causes generally account for < 5% [1]. Ischemic–reperfusion (I–R) injury is the main cause of AKI. Acute ischemia can be defined as a sudden drop in blood flow which causes inadequate oxygen and nutrients supply to the kidney tissue. This cause contributes to 80–90% of the renal etiologies of AKI [2].
Studies have found that AKI is associated with increased inflammatory rate. This is manifested by an increased expression of human Toll-like receptor 2 (TLR2), due to the high inflammatory response associated with kidney damage [3–5]. In addition, AKI may increase the activation of “Notch” signaling pathway due to the acute loss of blood supply to the renal tissue [6]. Notch2 protein is necessary to control local cellular proliferation. This step is necessary to recover from the acute kidney injury [6, 7]. Therefore, inhibition of the resulting high ‘Notch2/hes-1’ signaling is a necessary step to resume normal renal tissue re-perfusion and healing [6].
Lycopene is a lipophilic pigment, responsible for the red color of various fruits, such as tomato, watermelon, guava or grapefruit. It belongs to the carotenoid family, with an open-polyene chain structure similar to β-carotene but lacking a β-ionone ring [8–10]. Lycopene can ameliorate the levels of several antioxidant enzymes [11]. In addition, it acts as a scavenger substance to get rid of the harmful oxidative stress products [12]. Studies also found that lycopene supplementation may help reducing the level of inflammation as manifested by significant reduction in the levels of cytokines and interleukins (such as TNFα, IL-1β, IL-6) [13].
This study aims to investigate the possible nephroprotective effect of lycopene on renal ischemic–reperfusion injury in mice model, in terms of inhibiting the expected inflammatory response resulting from rapid (and acute) decline in blood supply to the normal renal tissue.
Materials and Methods
A total of 40 adult males ‘Swiss Albino’ mice (17–18 weeks of age), were included in this study. The animals were kept at the Animal House, University of Kufa, with a controlled temperature of (25 ± 2 °C) and a humidity (60–65%), with alternating 12 h light:12 h dark cycle. The mice were fed a standard chow diet with water. This study was approved by the Institutional Animal Care and Use Committee (IACUC—2017/19), University of Kufa, Al-Najaf, Iraq.
Study Design
After 1 week of acclimatization, the mice were randomly allocated into four study groups (10 mice in each):
Sham group Mice had median laparotomy under anesthesia with no procedures performed, renal tissues and blood samples were collected.
Ischemic–reperfusion group (IR-control) Mice underwent median laparotomy under anesthesia, followed by 30 min bilateral renal ischemia. Renal tissues and blood samples were collected after 2 h from reperfusion.
Vehicle-treated group Mice were pretreated with intra 1% dimethyl sulfoxide 30 min before inducing ischemia.
Lycopene treated group Mice were pretreated with 10 mg/kg intraperitoneal injection of lycopene 30 min before inducing renal ischemia. Renal tissues, and blood samples were collected after 2 h from reperfusion.
Experimental Procedure
Anesthesia was induced by an intraperitoneal injection of ketamine (100 mg/kg body weight, Kepro, Holland) and xylazine (10 mg/kg, Kepro, Holland) [14]. While sedated, mice were placed on the surgery board, a midline laparotomy incision was made to expose the abdomen and the intestines were retracted to expose the renal pedicles. Then the microvascular clamps were positioned around the right and left renal pedicles for 30 min. During the whole procedure, mice were kept well hydrated with warm sterile saline at a constant temperature (37 °C) [15]. After ischemia, the microvascular clamps were removed for reperfusion. The incision line in the abdominal region was closed using 3/0 silk suture. After 2 h, anesthesia was administered to all animals including the control group, and bilateral nephrectomy was performed via laparotomy [16], the animals are euthanized and both blood, as well as tissue samples, were collected for analysis.
Collection and Preparation of Samples
At the end of the surgical experiment, 2 milliliters of blood samples were collected from the kidneys and were placed in two separate tubes. The first sample, blood was placed in a plain tube to coagulate at 37 °C then centrifuged to measure the levels of urea and creatinine using enzymatic colorimetric methods [17]. The second sample, blood was placed in an EDTA as an anticoagulant after it is mixed at 3000 rpm for 15 min. ELISA technique was used to detect the levels of plasma NGAL (Mouse Lipocalin-2 ELISA Kit (NGAL) (ab199083)), Tissue IL-6 (Human IL-6 ELISA Kit (ab178013)), TLR-2 (Human TLR2 ELISA Kit (ab227897)), and F2-isoprostane (Anti-8 iso Prostaglandin F2 alpha antibody (ab2280)) in blood and tissue samples. In addition, high-throughput flow cytometry technology and a systems immunology approach was applied to identify potential predictive biomarkers of Notch 2/Hes1, using an automated sample loading system multiple samples are aspirated from multiwell plates and delivered directly into the flow cell of a flow cytometer. The method involves the sequential extraction of samples from the wells into a single length of transfer tubing, where samples are separated from one from another by air gaps. The stream of samples from the entire plate is delivered directly into the flow cell of the cytometer [18].
Renal Function and Histology
Blood urea nitrogen (BUN) and serum creatinine were measured to assess the kidney function. Histologically, renal tissues (renal cortex and pelvis), were fixed immediately in 10% formaldehyde and were processed in paraffin tissue blocks. Histopathological examination was performed to look for any abnormalities like loss of brush border and tubular dilation. A scoring system was implemented based on the percentage of tissue damage: 0: no damage; 1: < 25%; 2: 25–50%; 3: 50–75%; 4: > 75% [19].
Statistical Analysis
Statistical analyses were performed using SPSS (version 22, IBM, USA). Data were expressed using mean ± SEM. Analysis of Variance (ANOVA) was used for multiple comparisons and LSD post hoc test. Kruskal–Wallis test was used for the non-parametric data. P value was considered significant at ≤ 0.05.
Results
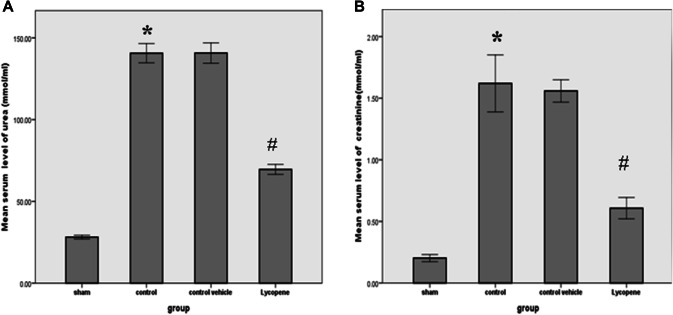
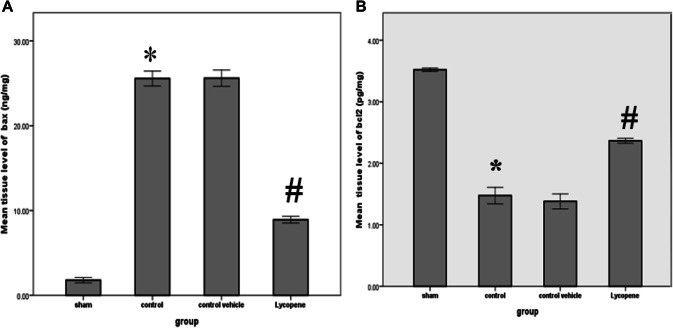
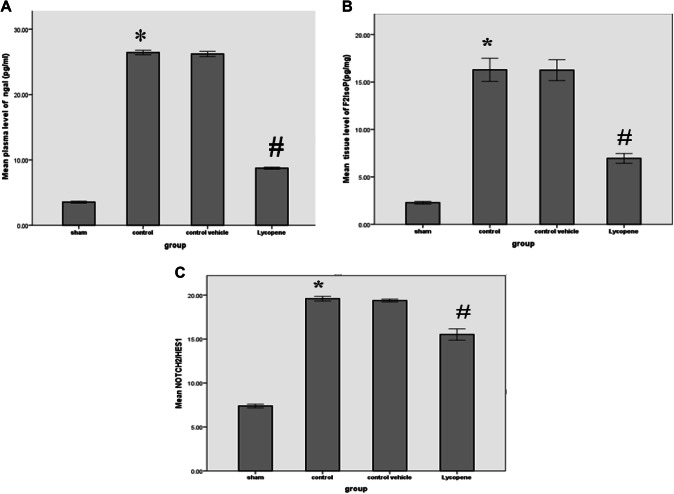
The levels of blood urea and serum creatinine were significantly lower in the lycopene treated group as compared to the control vehicle group (P ≤ 0.05) (Fig. 1). In addition, the levels of these markers were significantly increased in the control group, when compared with the sham group (P ≤ 0.05) (Fig. 1). There was a significant increase in the tissue levels of Bax among the control group when compared to the sham group (P ≤ 0.05). However, the level of this marker appears to be significantly reduced in the lycopene treated group (P ≤ 0.05). This is associated with a significant rise in the Bcl-2 level in the lycopene treated group when compared to the control group (P ≤ 0.05) (Fig. 2). Similarly, there was a significant reduction in the NGAL tissue level in the lycopene treated group when compared to the control group (P ≤ 0.05) (Fig. 3a).
Fig. 1.
The mean levels of a blood urea and b serum creatinine both in mmol/ml among the four study groups. Data were expressed as Mean ± SEM, significant P < 0.05. *Control versus sham; #Lycopene versus control vehicle
Fig. 2.
The mean tissue levels of a Bax (ng/mg) and b Bcl-2 (pg/mg) among the four study groups. Data were expressed as Mean ± SEM, significant P < 0.05. *Control versus sham; #Lycopene versus control vehicle
Fig. 3.
The mean tissue levels of a NGAL (pg/mg); b F2IsoP (pg/mg); c Notch2/Hes1, among the four study groups. Data were expressed as Mean ± SEM, significant P < 0.05. *Control versus sham; #Lycopene versus control vehicle
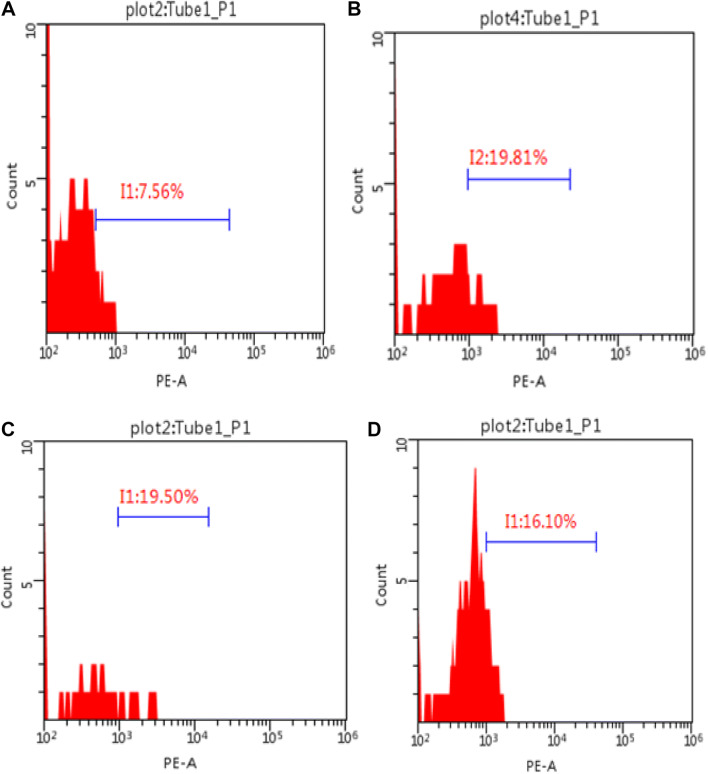
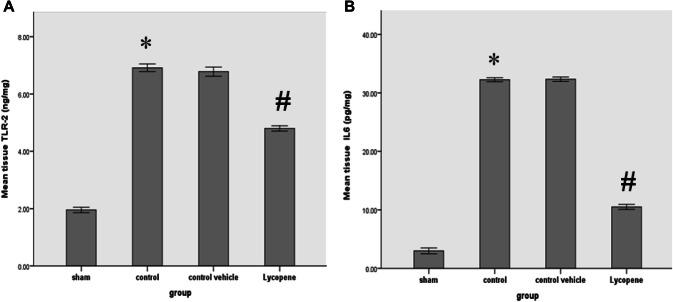
Our study also indicated a significant reduction in the F2IsoP tissue level among the lycopene treated group when compared to the control group (P ≤ 0.05) (Fig. 3b). Similarly, the renal level Noch2/Hes1 was significantly lower in the lycopene treated group, as compared to the control group (P ≤ 0.05) (Fig. 3c). Figure 4 shows the percentage of the renal Notch2/Hes1 among the four study groups (Fig. 4). The renal level TLR-2 was also significantly lower in the lycopene treated group, as compared to the control group (P ≤ 0.05) (Fig. 5). The renal level IL-6 was also significantly lower in the lycopene treated group, as compared to the control group (P ≤ 0.05) (Fig. 5).
Fig. 4.
Flow cytometry graphs showing the percentage of the Notch/Hes-1 among the four study groups. a sham; b control; c control vehicle, d lycopene treated group
Fig. 5.
a The mean tissue levels of TLR-2 (ng/mg) and b IL-6 (pg/mg), among the four study groups among the four study groups. Data were expressed as Mean ± SEM, significant P < 0.05. *Control versus sham; #Lycopene versus control vehicle
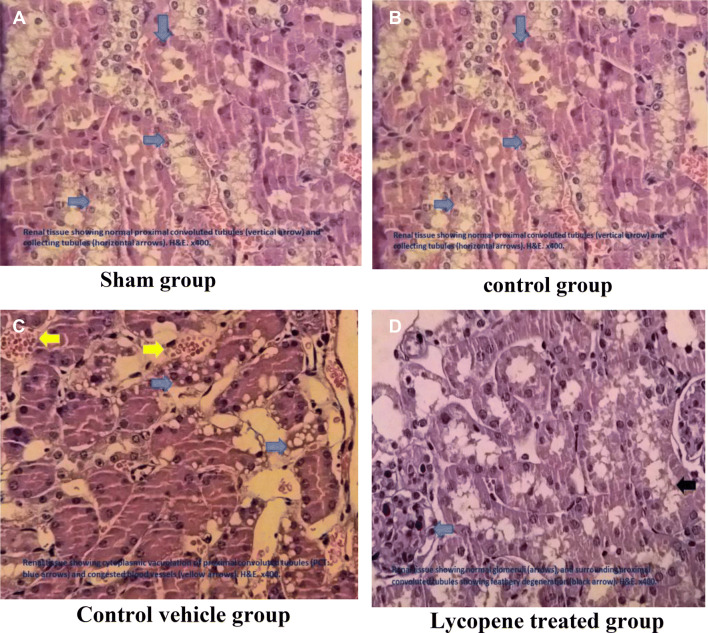
Acute kidney injury was assessed in the renal mice for the four study groups. There were normal renal structures in the sham group. The score of the control group (and and the control vehicle group) was significant different from the sham group (P ≤ 0.05), 70% (60%) had severed renal injury, 30% (40%) had a moderate renal injury. Moreover, the lycopene treated group had an overall improvement in the total renal tissue scores when compared to the control group score with only 20% slight damage and 60% moderate renal damage (Fig. 6).
Fig. 6.
Histopathological examination of the mice renal tissues among the four study groups. Blue arrows refer to the cytoplasmic vacuolation of proximal convoluted tubule PCT, Yellow arrows refer to the congested blood vessel
Discussions
Ischemic–reperfusion injury possess acute stress on normal tissue. This is due to the lack of sufficient oxygen and nutrient supply, which is associated with a series of inflammatory reactions that may cause serious local (and general) tissue damage [20]. In AKI there is an activation of wide range of inflammatory responses, mainly driven by the local oxidative stress and lipid peroxidation [20]. Previous studies showed that lycopene possess anti-carcinogenic, anti-inflammatory and antioxidant effects, therefore, this study investigated its beneficial supplementation in AKI cases in mice model [21]. Although blood urea nitrogen and serum creatinine were significantly increased in the control study group as compared to the sham group, lycopene supplementation appears to have a protective effect against further renal tissue damage. Studies showed that I–R injury causes significant rise in the levels of serum creatinine and alanine aminotransferase just within 30–45 min after the induction of the acute ischemic injury in animal models [22], this may also be associated with a significant rise in the inflammatory responses [23]. Lycopene supplementation was significantly effective in reducing the damaging effects of the I–R injury [21].
Both tissue levels IL-6 and TLR-2 were significantly elevated in the control group as compared to the sham group. However, lycopene supplementation appeared to be effective in reducing the inflammatory response as represented by the significant decrease in the levels of these two parameters in the lycopene treated group when compared to the control group. Previous studies showed that the serum levels of IL-6 and malondialdehyde (a marker of lipid peroxidation) were significantly increased in response to renal I–R injury in rat model [24]. This is associated with an increases in the mRNA expression of interleukin-6 [25]. Other studies concluded that the signal activation of the TLR-2 may contribute to the pathogenesis of the I–R injury [26]. Lycopene suppresses the synthesis of prostaglandin, prostacyclin, thromboxane, and leukotriene by regulating cyclooxygenase and lipoxygenase via ERK, p38MAPK, and NF-κB signaling pathway, and thereby it prevents the reactions that cause inflammation, making it an excellent protective supplement against a wide variety of diseases [21]. Moreover, the renal Bax levels appears to significantly increase in the control group when compared to the sham group. However, lycopene supplementation appeared to protect the renal tissue from this harmful rise. Moreover, Bcl2 levels appeared to have an opposite change in its level as it significantly increased in response to lycopene supplementation before inducing I–R injury, probably due to the increased expression of caspase-3 enzyme [27]. Plasma levels of NGAL significantly increased in response to AKI, however, this increase appears to be ameliorated with lycopene pretreatment [28], making NGAL a ‘promising’ screening marker to determine tissue damage in response to acute ischemia [25].
There is still no sufficient data available to explain the lycopene effects on the expression of the plasma NGAL levels.
Renal levels of F2 isoprostane were significantly increased after AKI, however, lycopene supplementation appeared to have a protective effect against the I–R injury. F2-isoprostanes is a product of non-cyclooxygenase free radical-induced peroxidation of arachidonic acid. Studies showed that this marker represent a reliable index of total lipid peroxidation [29]. Lycopene supplementation produces significant reduction in the degree of lipid peroxidation, perhaps due to its strong ‘antioxidant’ effect [21]. Similarly, the tissue Notch2/Hes-1 levels were significantly increased in response to I–R injury, suggesting an important role of the Notch signaling pathway as part of the pathogenesis of AKI. Previous studies showed that the activation of the Notch signaling pathway in myeloid cells aggravates the hepatic ischemia–reperfusion injury by enhancing the inflammation response through down-regulation of NF-κB [6]. An effect which was ameliorated by lycopene supplementation.
The histopathological findings from this study are consistent with the biochemical findings and proved a wide variety of tissue damage (including loss of brush borders, dilation of renal tubules, cytoplasmic vacuolation, and glomerular changes. This agrees with the findings from other studies [30]. Lycopene supplementation was significantly helpful in reducing the overall tissue damage resulted from the I–R injury [11, 21].
Conclusions
Based on the findings of our study, we conclude that lycopene supplementation plays a protective role against renal ischemia–reperfusion injury in the mice model. This is due to its anti-oxidant and anti-inflammatory as manifested by the significant reduction in the levels of IL-6, NGAL and F2 isoprostane in experimental mice with acute kidney injury, with lycopene supplementation when compared to the control group.
Acknowledgements
We acknowledge the support of technical staff at Department of Pharmacology and Therapeutics, Faculty of Medicine, University of Kufa.
Funding
This work received no external funds.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11(5):264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Luodelete M, Dong C, Li B, Zhang W, Nie P, et al. Pathological spectrum of glomerular disease in patients with renal insufficiency: a single-center study in Northeastern China. Ren Fail. 2019;41(1):473–480. doi: 10.1080/0886022X.2019.1620774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrar CA, Keogh B, McCormack W, O’Shaughnessy A, Parker A, Reilly M, et al. Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. FASEB J. 2012;26(2):799–807. doi: 10.1096/fj.11-195396. [DOI] [PubMed] [Google Scholar]
- 4.Zhao H, Perez JS, Lu K, George AJ, Ma D. Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306(8):F801–F811. doi: 10.1152/ajprenal.00469.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta VK, Siddiqi NJ, Ojha AK, Sharma B. Hepatoprotective effect of Aloe vera against cartap- and malathion-induced toxicity in Wistar rats. J Cell Physiol. 2019;234(10):18329–18343. doi: 10.1002/jcp.28466. [DOI] [PubMed] [Google Scholar]
- 6.Asanuma K, Oliva Trejo JA, Tanaka E. The role of Notch signaling in kidney podocytes. Clin Exp Nephrol. 2017;21(1):1–6. doi: 10.1007/s10157-016-1247-y. [DOI] [PubMed] [Google Scholar]
- 7.Gupta VK, Singh R, Sharma B. Phytochemicals mediated signalling pathways and their implications in cancer chemotherapy: challenges and opportunities in phytochemicals based drug development: A review. Biochem Compd. 2017;5(1):2. doi: 10.7243/2052-9341-5-2. [DOI] [Google Scholar]
- 8.Richelle M, Lambelet P, Rytz A, Tavazzi I, Mermoud AF, Juhel C, et al. The proportion of lycopene isomers in human plasma is modulated by lycopene isomer profile in the meal but not by lycopene preparation. Br J Nutr. 2012;107(10):1482–1488. doi: 10.1017/S0007114511004569. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, Sharma B. Natural products and their antioxidant potential. Nat Prod. 2012;8(2):72–87. [Google Scholar]
- 10.Singh R, Sharma B. Certain traditional indian plants and their therapeutic applications: a review. VRI Phytomed. 2013;1(1):1–11. [Google Scholar]
- 11.Pereira BLB, Reis PP, Severino FE, Felix TF, Braz MG, Nogueira FR, et al. Tomato (Lycopersicon esculentum) or lycopene supplementation attenuates ventricular remodeling after myocardial infarction through different mechanistic pathways. J Nutrition Biochem. 2017;46:117–124. doi: 10.1016/j.jnutbio.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Gleize B, Zhang L, Caris-Veyrat C, Renard Cmgc. A D-optimal mixture design of tomato-based sauce formulations: effects of onion and EVOO on lycopene isomerization and bioaccessibility. Food Funct. 2019;10(6):3589–602. [DOI] [PubMed]
- 14.Dodelet-Devillers A, Zullian C, Vachon P, Beaudry F. Assessment of stability of ketamine-xylazine preparations with or without acepromazine using high performance liquid chromatography-mass spectrometry. Can J Vet Res. 2016;80(1):86–9. [PMC free article] [PubMed]
- 15.Wang L, Liu X, Chen H, Chen Z, Weng X, Qiu T, et al. Effect of picroside II on apoptosis induced by renal ischemia/reperfusion injury in rats. Exp Therap Med. 2015;9(3):817–822. doi: 10.3892/etm.2015.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skrypnyk NI, Harris RC, de Caestecker MP. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Visual Exp JoVE. 2013;9(78):50495. [DOI] [PMC free article] [PubMed]
- 17.Salazar JH. Overview of Urea and Creatinine. Lab Med. 2014;45(1):e19–e20. doi: 10.1309/LM920SBNZPJRJGUT. [DOI] [Google Scholar]
- 18.Black CB, Duensing TD, Trinkle LS, Dunlay RT. Cell-based screening using high-throughput flow cytometry. Assay Drug Dev Technol. 2011;9(1):13–20. doi: 10.1089/adt.2010.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riser BL, Barreto FC, Rezg R, Valaitis PW, Cook CS, White JA, et al. Daily peritoneal administration of sodium pyrophosphate in a dialysis solution prevents the development of vascular calcification in a mouse model of uraemia. Nephrol Dial Transpl. 2011;26(10):3349–3357. doi: 10.1093/ndt/gfr039. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Yin X, Li J, Zhang L. Overexpression of microRNA-204-5p alleviates renal ischemia-reperfusion injury in mice through blockage of Fas/FasL pathway. Exp Cell Res. 2019;381(2):208–214. doi: 10.1016/j.yexcr.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Kaya C, Karabulut R, Turkyilmaz Z, Sonmez K, Kulduk G, Gulbahar O, et al. Lycopene has reduced renal damage histopathologically and biochemically in experimental renal ischemia-reperfusion injury. Ren Fail. 2015;37(8):1390–1395. doi: 10.3109/0886022X.2015.1064742. [DOI] [PubMed] [Google Scholar]
- 22.Farag MM, Ahmed GO, Shehata RR, Kazem AH. Thymoquinone improves the kidney and liver changes induced by chronic cyclosporine A treatment and acute renal ischaemia/reperfusion in rats. J Pharm Pharmacol. 2015;67(5):731–739. doi: 10.1111/jphp.12363. [DOI] [PubMed] [Google Scholar]
- 23.Jia Y, Zhao J, Liu M, Li B, Song Y, Li Y, et al. Brazilin exerts protective effects against renal ischemia-reperfusion injury by inhibiting the NF-κB signaling pathway. Int J Mol Med. 2016;38(1):210–216. doi: 10.3892/ijmm.2016.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su M, Ren S, Zhong W, Han X. Impact of propofol on renal ischemia/reperfusion endoplasmic reticulum stress. Acta Cirurgica Brasileira. 2017;32(7):533–539. doi: 10.1590/s0102-865020170070000004. [DOI] [PubMed] [Google Scholar]
- 25.Sueud T, Hadi NR, Abdulameer R, Jamil DA, Al-Aubaidy HA. Assessing urinary levels of IL-18, NGAL and albumin creatinine ratio in patients with diabetic nephropathy. Diab Metabol Syndr Clin Res Rev. 2019;13(1):564–568. doi: 10.1016/j.dsx.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Liu L, Pang XL, Shang WJ, Xie HC, Wang JX, Feng GW. Over-expressed microRNA-181a reduces glomerular sclerosis and renal tubular epithelial injury in rats with chronic kidney disease via down-regulation of the TLR/NF-kappaB pathway by binding to CRY1. Mol Med. 2018;24(1):49. doi: 10.1186/s10020-018-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou JQ, Qiu T, Zhang L, Chen ZB, Wang ZS, Ma XX, et al. Allopurinol preconditioning attenuates renal ischemia/reperfusion injury by inhibiting HMGB1 expression in a rat model. Acta Cirurgica Brasileira. 2016;31(3):176–182. doi: 10.1590/S0102-865020160030000005. [DOI] [PubMed] [Google Scholar]
- 28.Fang DY, Lu B, Hayward S, de Kretser DM, Cowan PJ, Dwyer KM. The role of activin A and B and the benefit of follistatin treatment in renal ischemia-reperfusion injury in mice. Transpl Direct. 2016;2(7):e87. doi: 10.1097/TXD.0000000000000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maschirow L, Khalaf K, Al-Aubaidy HA, Jelinek HF. Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes Biomarkers as a possible tool for early disease detection for rural screening. Clin Biochem. 2015;48(9):581–585. doi: 10.1016/j.clinbiochem.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Liu M, Wang H, Zhang J, Yang X, Li B, Wu C, et al. NF-kappaB signaling pathway-enhanced complement activation mediates renal injury in trichloroethylene-sensitized mice. J Immunotoxicol. 2018;15(1):63–72. doi: 10.1080/1547691X.2017.1420712. [DOI] [PMC free article] [PubMed] [Google Scholar]