Abstract
Background
Past clinical trials of docosahexaenoic Acid (DHA) supplements for the prevention of Alzheimer's disease (AD) dementia have used lower doses and have been largely negative. We hypothesized that larger doses of DHA are needed for adequate brain bioavailability and that APOE4 is associated with reduced delivery of DHA and eicosapentaenoic acid (EPA) to the brain before the onset of cognitive impairment.
Methods
33 individuals were provided with a vitamin B complex (1 mg vitamin B12, 100 mg of vitamin B6 and 800 mcg of folic acid per day) and randomized to 2,152 mg of DHA per day or placebo over 6 months. 26 individuals completed both lumbar punctures and MRIs, and 29 completed cognitive assessments at baseline and 6 months. The primary outcome was the change in CSF DHA. Secondary outcomes included changes in CSF EPA levels, MRI hippocampal volume and entorhinal thickness; exploratory outcomes were measures of cognition.
Findings
A 28% increase in CSF DHA and 43% increase in CSF EPA were observed in the DHA treatment arm compared to placebo (mean difference for DHA (95% CI): 0.08 µg/mL (0.05, 0.10), p<0.0001; mean difference for EPA: 0.008 µg/mL (0.004, 0.011), p<0.0001). The increase in CSF EPA in non-APOE4 carriers after supplementation was three times greater than APOE4 carriers. The change in brain volumes and cognitive scores did not differ between groups.
Interpretation
Dementia prevention trials using omega-3 supplementation doses equal or lower to 1 g per day may have reduced brain effects, particularly in APOE4 carriers. Trial Registration: NCT02541929.
Funding
HNY was supported by R01AG055770, R01AG054434, R01AG067063 from the National Institute of Aging and NIRG-15-361854 from the Alzheimer's Association, and MGH by the L. K. Whittier Foundation. This work was also supported by P50AG05142 (HCC) from the National Institutes of Health. Funders had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Keywords: DHA, Omega-3, RCT, Alzheimer's disease, Dementia, APOE
Research In Context.
Evidence before this study
Animal models and epidemiological studies support an association between docosahexaenoic acid and eicosapentaenoic acid (DHA and EPA) intake or blood levels with lower incidence of Alzheimer's disease (AD) dementia. Yet, systematic reviews of randomized clinical trials using lower doses of omega-3 supplements for dementia prevention have been negative. DHA supplementation dosing needed for DHA brain delivery is not clear.
Added value of this study
In this placebo-controlled trial, cognitively unimpaired adults were provided with a vitamin B complex and randomized to 2 gs per day of DHA supplementation or placebo over 6 months. A modest increase in cerebrospinal fluid (CSF) DHA levels was observed following supplementation, with APOE4 carriers having a lower increase than non-carriers.
Implications of all the available evidence
Dementia prevention trials that use omega-3 doses of equal or less than 1 g per day may have reduced brain effects, and especially for APOE4 carriers.
Alt-text: Unlabelled box
1. Introduction
A meta-analysis of 21 epidemiological studies with 181,580 participants identified that greater intake and blood levels of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) omega-3 fatty acids (n-3 FA) were associated with lower Alzheimer's disease (AD) risk [1]. Lower levels of blood DHA have also been associated with lower cognitive function, smaller hippocampal volumes, and the accumulation of amyloid plaques in the brain imaged by PET [2]. Both DHA and EPA promote synaptic plasticity, and are associated with enhanced anti-oxidation processes and inhibition of synaptic loss [3,4]. Long-term use of large dose DHA supplementation have anti-amyloidogenic properties in AD animal models, reducing β-amyloid (Aβ42) levels and diminishing neuronal loss [5]. Although DHA and EPA can be synthesized de novo from precursor lipids containing alpha-linolenic acid (ALA), this mechanism is not very efficient and these n-3 fatty acids have to be obtained largely from seafood consumption [6]. EPA can also be produced endogenously through retro-conversion of dietary DHA by β-oxidation. The current Western diet, however, contains considerably lower amounts of DHA and EPA compared to the n-6 fatty acid, arachidonic acid (AA) [7]. Lower ratio of n-3 to n-6 fatty acids (DHA/AA, EPA/AA) in the circulation is associated with higher measures of neuroinflammation [8,9] and increased cardiovascular disease risk [10].
Despite these observations, DHA supplementation in clinical trials was not effective in preventing symptomatic progression in participants already diagnosed with AD [11]. In addition, a systematic review of trials with mostly lower doses (less than 1 g per day) of DHA supplementation for the primary prevention of AD showed no benefit on cognitive function in cognitively healthy older people [12]. DHA brain bioavailability after supplementation in prevention trials is not clear.
Challenges to dementia prevention studies include identifying a population at risk of progressing to dementia before the onset of irreversible neurodegeneration. Aging, APOE4, positive family history of dementia [13], and sedentary lifestyles [14] increase the risk of developing dementia. Carbon-13 tracer studies suggest that both aging and carrying the APOE4 allele accelerate DHA oxidation and loss [15,16]. Younger and cognitively healthy APOE4 carriers show an increase in DHA brain uptake by PET compared to non-carriers suggesting compensation for a brain DHA deficiency state [17]. Data on brain DHA penetrance after supplementation is mostly limited to patients with cognitive impairment. In OmegAD, 2.3 gs of n-3 FA supplementation per day over 6 months led to a modest increase in CSF n-3 FA levels [18]. In the ADCS sponsored DHA trial, plasma and CSF DHA levels increased after 2 gs per day over 18 months with a greater increase in APOE4 non-carriers compared to carriers [11,16]. The effect of APOE4 on brain DHA and EPA delivery in cognitively unimpaired individuals is not known. To determine the delivery of DHA and EPA to the brain before the onset of cognitive impairment, we conducted a randomized controlled clinical trial stratified by APOE4 status among persons with dementia risk factors using high-dose (2 gs per day) DHA supplementation. Since adequate vitamin B levels are required to incorporate n-3 FA into circulating phospholipids [19], all participants were provided with a vitamin B supplement.
2. Methods
Participants: Participants were recruited from the Los Angeles area between 2016 and 2018. Inclusion criteria included: English and Spanish speaking cognitively unimpaired men and women ages 55 and older with a first-degree family history of dementia. Exclusion criteria included: current smokers (or a recent history of smoking within less than 5 years), history of cardiovascular disease defined by a prior heart attack, coronary artery revascularization, renal failure or blindness, a diagnosis of cancer in the past 6 months, uncontrolled hyper- or hypothyroidism, taking anti-coagulants such as warfarin, consumption of n-3 polyunsaturated fatty acids (PUFA) capsules for the last 3 months, regular exercise (>150 min of aerobic exercise per week), and heavy drinking (>30 units of alcohol per week). All participants completed the National Alzheimer Coordinating Center Uniform data set (UDS 3.0) neuropsychology battery. Participants meeting mild cognitive impairment core criteria [20] or clinical diagnosis of dementia were excluded. The study was approved by the USC IRB (HS-14-00864). All participants provided signed informed consent. The trial was registered with clinical trials.gov (NCT02541929).
Intervention: In a single centered double-blind trial, participants were randomized to 2152 mg per day of DHA or placebo in identically appearing capsules and treated over 6 months. Participants were required to take four 1000 mg soft-gel capsules per day which contained either 538 mg DHA (treatment arm) or identically-appearing capsules containing corn/soy oil (placebo arm) manufactured and provided by DSM, Columbia, MD. The EPA content of the capsules was <0.1% of the total fatty acid composition. The full content of the DHA capsules is provided in Supplementary Table 1. Participants were provided written instructions to limit their intake of PUFA intake during the trial. All participants were provided and instructed to take two vitamin B complex supplements per day each containing 500 mcg of vitamin B12, 50 mg of vitamin B6 and 400 mcg of folic acid (Homocysteine Modulators, Solgar, NY). The full supplement content is provided in Supplementary Table 2. A randomization sequence was developed by the biostatistics team, with randomization fidelity monitored by the trial data manager/analyst. Only the trial statistician and one designated research coordinator had access to the randomization list. Randomization was (1:1) between DHA and placebo and was stratified by APOE4 carrier status, with a blocking factor that was not revealed to investigators. Participants came for three visits: screening, baseline and 6 months visits. CSF and plasma were done fasting overnight, and CSF was collected in polypropylene tubes. Compliance was assessed by pill counting.
Outcomes: The primary outcome was the change in CSF DHA levels at 6 months. Secondary outcomes included changes in CSF EPA levels, hippocampal volume and entorhinal cortex thickness on magnetic resonance imaging (MRI); and exploratory outcomes were the Montreal Cognitive Assessment (MoCA) to assess global cognition, Craft Stories and California Verbal Learning Test 2 (CVLT2) for verbal memory, and Trail making A and B tests for speed and executive functions as described [2].
Fatty acids measurements: For CSF, a 0.25 mL aliquot was transferred into a reaction vial containing an internal standard (C23:0 triglyceride) and dried in a Speedvac for 40 min at 60°C. For plasma, a 25 uL aliquot was combined with the internal standard in a reaction vial. Thereafter the methylating reagent [boron trifluoride in methanol (14%), toluene, and methanol (35/30/35 v/v); 0.5 mL] was added, the vial vortexed and heated at 100°C for 45 min. After cooling, 0.5 mL of distilled water and hexane (0.25 mL for CSF and 0.5 mL for plasma) were added. Fatty acid analysis was performed by GC MS using a Shimadzu GC2010 Plus with a 100 m SP2560 capillary column as described previously [21].
Other measurements: APOE genotyping was assessed using qPCR with isoform-specific primers as described [22]. Blood biomarkers were completed at Huntington Hospital clinical laboratory (Pasadena, CA). CSF levels of Aβ42 were measured using the MSD multiplex assay [23].
MRI methods: We collected structural MRI scans (MPRAGE) at baseline and follow-up on 28 participants (Siemens Magnetom Prisma 3T; TR 2300 ms; TE 2.95 ms; 1.1 × 1.1 × 1.2 mm). Hippocampal segmentations were performed using FreeSurfer. Each segmentation was passed or failed by a trained reviewer following a visual quality control assessment using a protocol based on the recommendations of the European Alzheimer's Disease Consortium and the Alzheimer's Disease Neuroimaging Initiative (EADC–ADNI) harmonized hippocampal protocol [22]. The mean of right and left hippocampal volumes divided by intracranial volume estimated by FreeSurfer was used in the statistical analyses. One right hippocampal segmentation at baseline and one left hippocampal segmentation at follow-up failed segmentation based on our protocol. In these cases, only the measure of the remaining acceptable segmentation was used in the analyses rather than a right-left mean. Two subjects also had bilaterally failed hippocampal segmentations at follow-up, leaving a total of 26 participants who had usable hippocampal segmentations at both baseline and follow-up. For entorhinal cortex thickness, FreeSurfer adequately segmented gray versus white matter in the entorhinal cortex, using an in-house quality control protocol. The mean of right and left entorhinal cortex thickness was included in the statistical analyses.
2.1. Data sharing
Coded individual participant data and a data dictionary defining each field in the set will be made available to others upon request to the corresponding author, on January 1, 2022. Additional documents (study protocol, statistical analysis plan, informed consent form) can be made available by request to corresponding author on Jan 1, 2021. Data will be shared after approval of a proposal, with a signed data access agreement.
2.2. Statistical analysis
Sample size determination: In a prior study in patients with mild AD [24], 44 participants had measures of DHA in CSF at baseline and 18 months after receiving 2 g per day of DHA treatment (DHA arm, n = 29, and placebo arm, n = 15). The difference in CSF DHA levels after 18 months was 1.0024% (in weight percentage units) in the DHA-treated arm and −0.257% in the placebo-treated arm with a standard deviation of 1.026. Based on these estimates, a sample size of 26 (13 per group) would have 80% power to detect a 1.22 difference in CSF DHA after treatment. Given a possible 20% dropout rate, a total of 32 participants were required to be randomized to detect this difference.
Data analysis: Baseline variables were compared using Fisher's exact test and Wilcoxon two sample rank sum test. Changes in fatty acids (post-pre) as a function of treatment and genotype were modeled using an ANCOVA model within a general linear model framework. Residuals were evaluated for normality and homoscedasticity. One cognitive variable (CVLT Trial 5) with a non-normal distribution was ranked and the regression model was run on both the untransformed and ranked variables. Interaction of treatment and APOE genotype was modeled as a function of fatty acid levels. A p-value below 0.05 (two-sided) was considered statistically significant for the primary outcome-change in CSF DHA. The analysis was done on a modified intention-to-treat basis (limited to trial completers with available follow-up outcome measures) and all subjects were included regardless of adherence. For analysis of the imaging and cognitive scores, multiple comparisons were not controlled for any other variables and, therefore, p-values for results should be considered as nominal. All statistical analyses used R (http://www.R-project.org/) and SAS software version 9.4 (Cary, NC).
3. Results
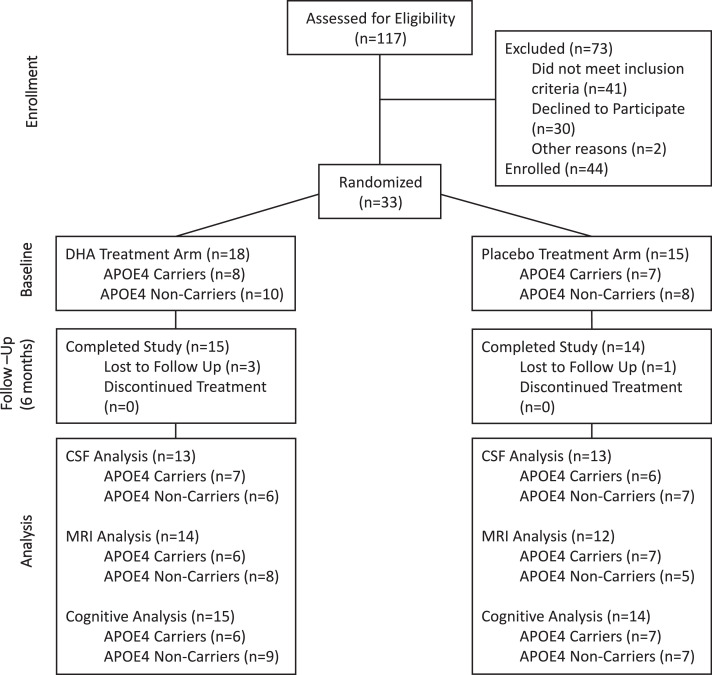
One hundred and seventeen individuals were screened for trial eligibility. Thirty-three participants were randomized into placebo (n = 15; APOE4 carriers=7; non-carriers=8) and DHA treatment arms (n = 18, APOE4 carriers=8; non-carriers=10), as shown in Fig. 1. All but one participant had a Clinical Dementia Rating (CDR) score of 0. Twenty-six individuals completed lumbar punctures and MRI imaging, and 29 completed cognitive assessments at baseline and 6 months. The majority of participants were women, and there were more males in the placebo group than in the DHA group. A summary of the demographic and clinical characteristics of the cohort based on treatment is provided in Table 1. The information is further grouped by APOE4 status and presented in Supplementary Table 3. Blood and imaging biomarkers, as well as baseline neuropsychological measures, are provided in Supplementary Table 4. For the analysis results of primary, secondary, and exploratory outcomes, the mean differences in change between treatment groups with 95% confidence interval are presented. Overall, the intervention was well tolerated, the majority of adverse events were minor (mostly gastrointestinal) and did not differ by treatment arms (Supplementary Table 5). Mean adherence by pill count in the overall sample was 80.4% (placebo mean (SD)=79.8 (21.8); DHA mean (SD)=80.9 (17.3)).
Fig. 1.
Consort diagram. From 117 participants screened for eligibility, 33 were randomized into placebo (n = 15, APOE4=7, non-APOE4=8) and DHA (n = 18, APOE4=8, non-APOE4=10) treatment arms.
Table 1.
Demographic and baseline clinical characteristics by treatment arm.
| Placebo (N = 15) | DHA (N = 18) | P-value | |
|---|---|---|---|
| Age in years, median (min, max) | 69 (58, 79) | 68.5 (58, 90) | 0.46 |
| Males, n (%) | 4 (27%) | 2 (11%) | 0.37 |
| Race, n (%) | 0.39 | ||
| White (Non-Hispanic) | 7 (47%) | 11 (61%) | |
| Hispanic | 5 (33%) | 6 (33%) | |
| Black | 0 | 1 (6%) | |
| Asian | 2 (13%) | 0 | |
| Other | 1 (7%) | 0 | |
| APOE genotype, n (%)a | 0.99 | ||
| E2/E3 | 2 (13%) | 3 (17%) | |
| E3/E3 | 6 (40%) | 6 (33%) | |
| E3/E4 | 6 (40%) | 6 (33%) | |
| E4/E4 | 1 (7%) | 2 (11%) | |
| BMI (kg/m2), median (IQR) | 32.0 (7.3) | 27.8 (5.8) | 0.13 |
| HgbA1c (%), median (IQR) | 5.7 (0.2) | 5.8 (0.6) | 0.66 |
| MoCA, median (min, max)b | 27 (22, 30) | 28 (19, 30) | 0.62 |
The exact genotype of one of the non-APOE4 carriers in the DHA arm was not determined.
Sample size for HgbA1c, MoCA: placebo (N = 15), DHA (N = 17).
3.1. Change in CSF and plasma fatty acids after the intervention
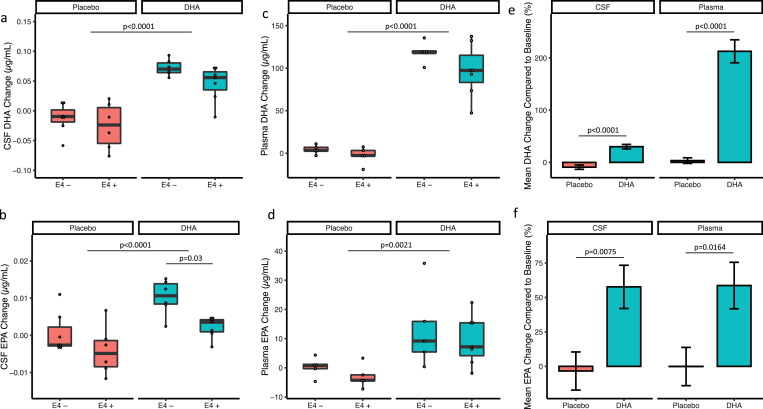
At baseline, higher CSF DHA levels were significantly correlated with higher CSF Aβ42 (r = 0.54; p = 0.0033). Fatty acid levels measured at baseline in CSF and plasma are presented in Supplementary Tables 6 and 7. After six months of DHA supplementation, there was a significant increase in CSF DHA in the DHA supplement arm (n = 13) compared with placebo (n = 13) (mean difference (95% CI): 0.08 (0.05, 0.10) µg/ml; p<0.0001; Fig. 2a). There was a trend for a greater increase in CSF DHA in APOE4 non-carriers compared to carriers (0.02 (−0.003, 0.04), p = 0.08, Cohen's effect size d = 0.41) but the interaction between the treatment group and APOE group on the change of CSF DHA was not significant (p = 0.61; Fig. 2a). CSF EPA levels were increased after DHA supplementation (0.008 (0.004, 0.01) µg/ml; p <0.0001; Fig. 2b). Change in CSF EPA levels in both groups was significantly greater in APOE4 non-carriers compared to carriers (0.006 (0.003, 0.009) µg/ml; p = 0.001). Interaction of APOE genotype and treatment on the change in CSF EPA level was not significant (p = 0.54). The changes in all CSF fatty acids by treatment group are presented in Supplementary Table 8.
Fig. 2.
Comparison of post-intervention difference in CSF DHA and EPA levels at baseline and 6-months based on treatment arm and APOE4 status. Comparison of a) change in CSF DHA between DHA and placebo treatments arms separated by APOE4 status, b) change in CSF EPA between treatment arms separated by APOE4 status, c) change in plasma DHA between treatments arms separated by APOE4 status, and d) change in plasma EPA between treatment arms separated by APOE4 status, e) mean DHA% change compared to baseline in CSF and plasma, f) mean% change in EPA compared to baseline in CSF and plasma. The p values were derived from a linear model ANCOVA.
Plasma DHA levels after DHA supplementation was increased compared with placebo (106.4 (87.8, 125.1) µg/ml, p<0.0001; Fig. 2c). EPA plasma levels were also increased with DHA supplementation (13.8 (5.8, 21.7) µg/ml, p = 0.002; Fig. 2d). DHA and EPA plasma levels did not significantly differ between APOE4 carriers and non-carriers (DHA: 14.7 (−4.2, 33.7) µg/ml, p = 0.12; EPA: 4.4 (−3.4, 12.3) µg/ml, p = 0.25; Fig. 2B and D). When comparing the percentage changes in plasma to CSF, participants in the DHA treatment arm exhibited a 200% increase in plasma DHA, but only a 28% increase in CSF DHA (Fig. 2e). By comparison, the percentage increase in CSF and plasma EPA levels were similar after supplementation (Fig. 2f). The change in all plasma fatty acids by treatment group is presented in Supplementary Table 9. Increasing plasma DHA was associated with an increase in CSF DHA (R = 0.91, p<0.001; Supp Figure 1a). APOE genotype did not modify this association. In contrast to DHA, no correlation was observed between the changes in plasma and CSF EPA (Supp Figure 1b).
Levels of CSF AA did not differ after DHA treatment (−0.02 (−0.07, 0.02) µg/ml, p = 0.35) and there was no interaction between treatment and APOE genotype on levels of CSF AA (p = 0.32). Plasma AA levels were significantly lower after DHA supplementation (−45.5 (−75.4, −15.6) µg/ml, p = 0.005). This difference in change was significant among APOE4 non-carriers (−57.7 (−115.1, −0.4) µg/ml, p<0.05) but not among APOE4 carriers. The interaction between APOE genotype and treatment arm on plasma AA was not significant (p = 0.40).
The change in the ratio of DHA/AA and EPA/AA in CSF and plasma was similar to total DHA and EPA concentrations. CSF DHA/AA was increased among those taking DHA supplements compared with those taking placebo (0.27 (0.20, 0.34), p<0.0001), but without a significant difference between APOE4 groups. There was an increase in CSF EPA/AA among those taking DHA supplements versus placebo (0.03 (0.02, 0.04), p<0.0001); and was greater among non-carriers compared to APOE4 carriers (0.01 (0.002, 0.03), p = 0.02), but without a significant interaction between treatment and genotype (p = 0.26). After treatment, change in plasma DHA/AA ratio (0.63 (0.48, 0.78), p<0.0001), and plasma EPA/AA ratio (0.09 (0.03, 0.15), p = 0.004) differed by treatment group but did not differ significantly between APOE4 carriers and non-carriers.
3.2. Secondary and exploratory outcomes
There were no significant differences in the change of hippocampal volume (−0.002 (−0.009, 0.005), p = 0.56) or entorhinal cortex thickness (−0.11 (−0.25, 0.03), p = 0.13) between DHA and placebo treatment arms. Cohen's effect size d of the hippocampal and entorhinal volume changes after treatment were 0.24 and 0.54 respectively. Changes in individual cognitive scores are shown in Table 2. With the exception of a nominally significant difference in change of scores in CVLT Trial 5 of list learning (DHA treated arm learned one additional word than the placebo treated arm), all other measures of cognition did not significantly differ between treatment arms.
Table 2.
Changes in cognitive scores by treatment arm.
| Cognitive Test | Placebo (N = 14) Mean (SD) |
DHA (N = 15) Mean (SD) |
Difference between treatment arms Mean (95% CI)a |
P-value |
|---|---|---|---|---|
| MoCA | 0.3 (1.6) | −0.2 (1.6) | −0.5 (−1.7, 0.7) | 0.40 |
| Craft immediate recall | 1.7 (3.1) | 0.3 (3.2) | −1.4 (−3.9, 1.0) | 0.23 |
| Craft delayed recall | 0.4 (2.8) | 1.1 (2.8) | 0.7 (−1.5, 2.8) | 0.52 |
| CVLT Trial 5 | −0.8 (2.1) | 1.1 (2.2) | 1.9 (0.2, 3.5) | 0.03 |
| CVLT Trial 5 (ranked) | 9.9 (7.1) | 14.6 (7.2) | 4.7 (−0.8, 10.2) | 0.09 |
| CVLT delayed recall | 1.3 (2.6) | 1.9 (2.6) | 0.6 (−1.4, 2.6) | 0.53 |
| Trails A | −2.8 (10.2) | −3.8 (10.3) | −1.0 (−8.8, 6.9) | 0.80 |
| Trails Bb | −1.5 (40.0) | 3.5 (40.2) | 5.0 (−26.3, 36.3) | 0.74 |
Mean difference (DHA minus placebo) in change score.
Sample size for Trails B: placebo (N = 14), DHA (N = 14).
4. Discussion
In this trial, we sought to determine the levels of DHA and EPA in CSF following DHA supplementation in cognitively unimpaired individuals at risk of dementia, and whether the changes in CSF DHA and EPA levels are related to APOE4 carrier status. We observed a modest (28%) increase in CSF DHA levels with 2152 mg per day of TG-based DHA supplementation. This finding has implications for past clinical trials that used lower doses (e.g. 1 g daily of TG-based DHA supplements per day or less) and were overwhelmingly negative [11]. Using lower doses of n-3 FA supplements may have resulted in limited n-3 FA brain delivery.
Another aspect affecting the response to DHA supplementation is APOE4 status. APOE4 non-carriers showed a trend toward greater CSF DHA levels and significantly greater EPA levels compared with APOE4 carriers. Lower CSF DHA and EPA levels in APOE4 following DHA supplementation could result from lower brain uptake or greater brain consumption.
Although EPA levels are disproportionately low in the brain when compared with other n-3 FAs such as DHA and AA (up to 250 times lower) [25], this does not equate to lesser biological significance. EPA plays fundamental roles in neuroinflammation and neural proliferation processes [[25],[26]]. EPA can work to attenuate the effects of inflammatory mediators such as interleukin-1β (IL-1β) via various cell signaling pathways [27]. EPA also serves as a source of eicosanoids and resolvins involved in mitigating inflammation and excitotoxicity in the brain [[28],[29]]. This finding contributes to our understanding as to why APOE4 carriers with limited omega-3 intake might be at a greater risk of neuroinflammation and AD progression [30].
We define high dose supplementation as greater than 2 g of TG-based DHA consumption per day. We acknowledge this definition is limited as our study only tested one DHA dose. In addition, different DHA formulations or populations may require distinct dosing strategies that are determined by several factors (discussed below). Nevertheless, two important variables determining the delivery of DHA to tissues are the dose and duration of supplementation. From a pharmacokinetic standpoint, DHA levels in plasma phospholipids are saturated after 6 weeks of supplementation with 2 g per day of TG-based DHA [31]. However, a brain DHA tracer study reveals a much slower brain DHA turnover than plasma with an estimated brain DHA half-life of around 2.5 years [32]. This suggests a much longer time is needed to remodel brain DHA and corroborated by the slower increase in CSF DHA after supplementation over time compared to plasma. For example, in OmegAD, 1720 mg of DHA [in ethyl ester form] per day over 6 months was associated with only 11% increase in CSF DHA levels, as opposed to a 200% increase in plasma DHA levels [18]. The ADCS-sponsored DHA trial in mild AD used slightly larger doses than OmegAD [2 g of TG derived DHA daily] but over 18 months and observed a 38% increase in CSF DHA levels as opposed to a 207% increase in plasma DHA levels [33]. In the current study, 2.1 g of TG-DHA per day over 6 months in cognitively unimpaired individuals was associated with a 28% increase in CSF DHA and a 200% increase in plasma DHA levels. Together these data suggest that short term (for example less than 1 year) of TG-based DHA with doses less than 1 g per day will be less likely to lead to meaningful remodeling of tissue DHA levels, particularly in the brain.
In addition to dosing and duration of n-3 FA supplementation, vitamin B levels, baseline n-3 FA levels, age, sex, BMI, and physical activity affect the levels of n-3 FA in the circulation following supplementation. In the current study, all participants were provided a high dose vitamin B complex supplement. B vitamins are necessary for the incorporation of DHA into circulating phospholipids [19]. Emerging evidence suggests that vitamin B influences DHA's supplementation efficacy on cognitive outcomes. In VITACOG, brain atrophy and possibly some cognitive benefits of vitamin B supplementation were dependent on the parallel presence of high levels of DHA and EPA [34]. An analysis of OmegAD indicated that the effect of n-3 FA supplementation on cognitive outcomes was influenced by baseline homocysteine levels [35]. Lower baseline n-3 FA levels, older age, female sex, and higher physical activity also appear to enhance the circulating levels of n-3 FA in response to supplementation [36].
Another consideration for determining an effective n-3 FA dose is a correlation between tissue levels and improved clinical outcome data. There is some evidence to support that n-3 FA doses greater than 2 g per day provide a dose “outcome” response. In cardiovascular disease prevention, REDUCE-IT using 4 g of ethyl-ester EPA per day [37] was associated with lower rate of ischemic events including cardiovascular death. In contrast, trials using lower doses were inconsistent or largely negative (reviewed in [38]). More recently, MAPT [39], ORIGIN [40], VITAL [41] and Do-HEALTH [42] used n-3 FA supplements with doses less or equal to 1 g per day. In trials with cognitive outcomes, higher DHA doses or plasma levels were generally associated with improvements in cognitive outcomes, whereas trials using lower doses were not [11,43,44]. For example, in OmegAD, improvements in cognitive outcomes were associated with the greatest plasma levels of n-3 FA achieved after 2.3 g per day supplementation [45]. In lipiDIDiet, 1.2 g of DHA + 300 mg of EPA had positive (but inconsistent) effects on some of the cognitive outcomes [46]. In the ADCS sponsored clinical trial, treatment with 2 g of TG-DHA per day was not associated with cognitive benefit, but the response differed by APOE4 status with non-APOE4 carriers showing signs of cognitive improvement. These findings are corroborated in animal AD and APOE4 models showing neuroprotective effects of TG-DHA supplementation by long term supplementation with human doses equivalent to 3 g per day or more [47]. Whether long term high-dose DHA supplementation in persons at increased risk of AD but before the onset of cognitive impairment, such as in APOE4 carriers, can delay the symptomatic onset of cognitive decline remains an important unanswered question.
N-3 FA supplement formulation does not appear to impact its plasma bioavailability. N-3 FA can be produced by microalgae (TG-based), refined from fish oils (ethyl esters or resterified TG-based), or krill oils (phospholipid “PL”-based) [48]. Direct comparisons of the different formulations do not support an advantage for TG or PL-based formulations on plasma levels [48], although animal models indicate that PL-DHA may increase in the brain more efficiently than other formulations [49]. Unesterified fatty acids and specific phospholipids such as lysophosphatidylcholine (LPC DHA) are the preferred brain DHA substrates [50]. Following absorption, dietary TG-DHA is hydrolyzed by lipases to produce free or unesterified DHA and LPC DHA. A second phase PL-DHA is produced from hepatic metabolism of TG-DHA. When in free fatty acid form, DHA detaches from albumin in the plasma and is transported via passive diffusion across the outer membrane of the blood-brain barrier (BBB) [41]. LPC DHA is transported along the inner membrane of the BBB via the major facilitator superfamily domain-containing 2α (MFSD2α) receptor [51]. Accordingly, LPC DHA formulations may produce faster enrichment of brain DHA concentrations than TG-based DHA formulations [52], but this has not yet been demonstrated in human studies.
In the current study, we observed an increase in EPA levels after DHA supplementation which is likely secondary to retroconversion from DHA. Although acute carbon tracer studies suggest minimal retroconversion of DHA to EPA in tissues [53], longer-term DHA supplementation leads to an increase in circulatory EPA by up to 10% [54,55]. This process involves peroxisomal oxidation of DHA and occurs in both astrocytes and hepatocytes [56]. Furthermore, the EPA content of the n-3 FA capsules provided in this trial was very small (<0.1%), making it a less plausible explanation for the observed increase in plasma or CSF EPA levels following supplementation. The lower rate of retroconversion of DHA to EPA in APOE4 carriers may signify peroxisomal dysfunction. Peroxisomes play a key role in the production of reactive oxygen species and contribute to the oxidation of long-chain PUFAs [57]. In AD mouse models, decreased efficiency of peroxisomal β-oxidation was observed in the hippocampus and was associated with the accumulation of toxic very long chain fatty acids [58]. It is plausible that older APOE4 carriers with evidence of oxidation markers in tissues may not benefit from n-3 FA supplementation, underscoring the need for early intervention.
We also found a positive correlation between higher CSF DHA levels and higher CSF Aβ42 (suggestive of lower amyloid deposition) at baseline. Using PiB PET scanning, we previously found a correlation between lower serum DHA levels and increased amyloid deposition in patients without dementia but with vascular risk factors [59]. In the ADCS-sponsored DHA clinical trial, we identified a similar association between CSF DHA and CSF Aβ42 in patients with mild AD [24]. Together, these findings indicate an association between greater brain amyloid accumulation and lower brain DHA levels that appear before the onset of cognitive impairment and is independent of APOE4 status. However, changes in CSF DHA levels were not associated with changes in CSF Aβ42. It is possible longer exposure to larger concentrations of DHA in the brain are needed to affect Aβ42 metabolism [60,61] and slow brain amyloid accumulation.
The strength of this study is in the selection of participants with a positive family history of dementia, stratifying recruitment by APOE groups, low baseline omega-3 consumption and a sedentary lifestyle, but who were not cognitively impaired. The present trial pilot, unlike prior studies, investigated levels of DHA in CSF of cognitively normal adults before any indication of cognitive impairment. The APOE genotype differences in CSF DHA and EPA levels that may arise before cognitive deterioration or development of AD suggest potential preventive measures. Although this was a randomized trial, there were significantly fewer males and lower baseline fasting triglyceride levels in the DHA treatment arm. This is a limitation of the smaller sample size in this trial. We acknowledge that changes in CSF DHA and EPA levels do not simply reflect brain uptake, but represent a complex dynamic relationship occurring within the brain involving both the uptake of these n-3 FA from the circulation and their recycling/consumption from brain cells. As a result, we could not determine from this study whether lower DHA and EPA in CSF of APOE4 carriers are due to lower uptake across the BBB or greater utilization within brain cells. Future investigations should seek a better understanding of the APOE4-related mechanisms that determine DHA and EPA brain bioavailability, as well as the development of alternative formulations to enhance brain DHA delivery in this population. The current study was not planned to detect an interaction effect on CSF fatty acids by treatment and APOE groups nor to detect DHA treatment effects on brain imaging or clinical cognitive outcomes. We are currently testing the effect of high dose DHA supplementation on CSF fatty acid levels, imaging, and cognitive outcomes in a larger ongoing trial, PreventE4 (NCT03613844).
In summary, our study suggests that higher doses of n-3 FA (2 g per day or more of TG-DHA) are needed to ensure adequate brain delivery, particularly in APOE4 carriers. Although it is possible that n-3 FA supplementation may not slow cognitive decline, past low dose (1 g per day or less) n-3 FA supplementation trials in dementia prevention may not have provided adequate brain levels to fully evaluate the efficacy of n-3 FA supplementation on cognitive outcomes.
Declaration of Competing Interest
The authors have no conflict of interest.
Acknowledgments
HNY and WJM had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We are grateful for the generous donation of DHA and placebo supplements by DSM Nutritional Products and Vitamin B supplements from Solgar. We thank Bill Harris from OmegaQuant for his assistance in measuring n-3 FA levels in plasma and CSF.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102883.
Appendix. Supplementary materials
References
- 1.Zhang Y., Chen J., Qiu J., Li Y., Wang J., Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2016;103(2):330–340. doi: 10.3945/ajcn.115.124081. [DOI] [PubMed] [Google Scholar]
- 2.Yassine H.N., Feng Q., Azizkhanian I., Rawat V., Castor K., Fonteh A.N. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 2016;73(10):1208–1216. doi: 10.1001/jamaneurol.2016.1924. [DOI] [PubMed] [Google Scholar]
- 3.Calon F.L., GP, Yang F., Morihara T., Teter B., Ubeda O., Rostaing P., Triller A., Salem N., Jr., Ashe K.H., Frautschy S.A., Cole G.M. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43(5):633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouwens MvdR O., Dellschaft N., Bromhaar M.G., de Groot L.C., Geleijnse J.M., Muller M., Afman L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells. Am J Clin Nutr. 2009;90:415–424. doi: 10.3945/ajcn.2009.27680. [DOI] [PubMed] [Google Scholar]
- 5.Hooijmans C., Rutters F., Dederen P., Gambarota G., Veltien A., Van Groen T. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD) Neurobiol Dis. 2007;28(1):16–29. doi: 10.1016/j.nbd.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 6.de Magalhães J., Muller M., Rainger G.Ed., Steegenga W. Fish oil supplements, longevity, and aging. Aging. 2016;8(8):1578–1582. doi: 10.18632/aging.101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson D., R. Block, Mousa S.A. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr. 2012;3(1):1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funk C. Prostaglandins and leukotrienes advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 9.Thomas J., Thomas C., Radcliffe J., Itsiopoulos C. Omega-3 fatty acids in early prevention of inflammatory neurodegenerative disease: a focus on Alzheimer's disease. Biomed Res Int. 2015;2015:172801. doi: 10.1155/2015/172801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itakura H., Yokoyama M., Matsuzaki M., Saito Y., Origasa H., Ishikawa Y. Relationships between plasma fatty acid composition and coronary artery disease. J Atheroscler Thromb. 2011;18(2):99–107. doi: 10.5551/jat.5876. [DOI] [PubMed] [Google Scholar]
- 11.Yassine H.N., Braskie M.N., Mack W.J., Castor K.J., Fonteh A.N., Schneider L.S. Association of docosahexaenoic acid supplementation with alzheimer disease stage in apolipoprotein E epsilon4 carriers: a review. JAMA Neurol. 2017 doi: 10.1001/jamaneurol.2016.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sydenham E., Dangour A.D., Lim W.S. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD005379.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Yi D., Lee Y., Byun M.S., Lee J.H., Ko K., Sohn B.K. Synergistic interaction between APOE and family history of Alzheimer's disease on cerebral amyloid deposition and glucose metabolism. Alzheimers Res Ther. 2018;10(1):84. doi: 10.1186/s13195-018-0411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Head D., Bugg J.M., Goate A.M., Fagan A.M., Mintun M.A., Benzinger T. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69(5):636–643. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chouinard-Watkins R., Rioux-Perreault C., Fortier M., Tremblay-Mercier J., Zhang Y., Lawrence P. Disturbance in uniformly 13C-labelled DHA metabolism in elderly human subjects carrying the apoE epsilon4 allele. Br J Nutr. 2013;110(10):1751–1759. doi: 10.1017/S0007114513001268. [DOI] [PubMed] [Google Scholar]
- 16.Chouinard-Watkins R., Plourde M. Fatty acid metabolism in carriers of apolipoprotein E epsilon 4 allele: is it contributing to higher risk of cognitive decline and coronary heart disease? Nutrients. 2014;6(10):4452–4471. doi: 10.3390/nu6104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yassine H.N., Croteau E., Rawat V., Hibbeln J.R., Rapoport S.I., Cunnane S.C. DHA brain uptake and APOE4 status: a PET study with [1-11 C]-DHA. Alzheimers Res Ther. 2017;9(1):23. doi: 10.1186/s13195-017-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund Levi Y., Vedin I., Cederholm T., Basun H., Faxen Irving G., Eriksdotter M. Transfer of omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich omega-3 fatty acid preparation in patients with Alzheimer's disease: the OmegAD study. J Intern Med. 2014;275(4):428–436. doi: 10.1111/joim.12166. [DOI] [PubMed] [Google Scholar]
- 19.Khot V., Kale A., Joshi A., Chavan-Gautam P., Joshi S. Expression of genes encoding enzymes involved in the one carbon cycle in rat placenta is determined by maternal micronutrients (folic acid, vitamin B12) and omega-3 fatty acids. Biomed Res Int. 2014;2014 doi: 10.1155/2014/613078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dement: J Alzheimer's Assoc. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimenez E.Y., Mangani C., Ashorn P., Harris W.S., Maleta K., Dewey K.G. Breast milk from women living near Lake Malawi is high in docosahexaenoic acid and arachidonic acid. Prostaglandins Leukot Essent Fatty Acids. 2015;95:71–78. doi: 10.1016/j.plefa.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Rawat V., Wang S., Sima J., Bar R., Liraz O., Gundimeda U. ApoE4 alters ABCA1 membrane trafficking in astrocytes. J Neurosci. 2019;39(48):9611–9622. doi: 10.1523/JNEUROSCI.1400-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nation D.A., Sweeney M.D., Montagne A., Sagare A.P., D'Orazio L.M., Pachicano M. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. doi: 10.1038/s41591-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yassine H.N., Rawat V., Mack W.J., Quinn J.F., Yurko-Mauro K., Bailey-Hall E. The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer's disease. Alzheimers Res Ther. 2016;8(1):25. doi: 10.1186/s13195-016-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazinet R.P., Metherel A.H., Chen C.T., Shaikh S.R., Nadjar A., Joffre C. Brain eicosapentaenoic acid metabolism as a lead for novel therapeutics in major depression. Brain Behav Immun. 2020;85:21–28. doi: 10.1016/j.bbi.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Dyall S.C. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Y., Pu K., Duan W., Chen H., Chen L., Wang Y. Involvement of Akt/CREB signaling pathways in the protective effect of EPA against interleukin-1β-induced cytotoxicity and BDNF down-regulation in cultured rat hippocampal neurons. BMC Neurosci. 2018;19(1):52. doi: 10.1186/s12868-018-0455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohli P., Levy B.D. Resolvins and protectins: mediating solutions to inflammation. Br J Pharmacol. 2009;158(4):960–971. doi: 10.1111/j.1476-5381.2009.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tassoni D., Kaur G., Weisinger R.S., Sinclair A.J. The role of eicosanoids in the brain. Asia Pac J Clin Nutr. 2008;17(Suppl 1):220–228. [PubMed] [Google Scholar]
- 30.Tao Q., Ang T.F.A., DeCarli C., Auerbach S.H., Devine S., Stein T.D. Association of chronic low-grade inflammation with risk of Alzheimer Disease in ApoE4 carriers. JAMA Netw Open. 2018;1(6) doi: 10.1001/jamanetworkopen.2018.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arterburn L., Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006;83(6 Suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 32.Umhau J.C., Zhou W., Carson R.E., Rapoport S.I., Polozova A., Demar J. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res. 2009;50(7):1259–1268. doi: 10.1194/jlr.M800530-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yassine H.N., Rawat V, Mack W.J., Quinn J.F., Yurko-Mauro K., Bailey-Hall E., Aisen P.S., Chui H.C., Schneider L.S. The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer's disease. Alzheimer's Rea Therapy. 2016;8:25. doi: 10.1186/s13195-016-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jernerén F., Elshorbagy A.K., Oulhaj A., Smith S.M., Refsum H., Smith A.D. Brain atrophy in cognitively impaired elderly: the importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 2015;102(1):215–221. doi: 10.3945/ajcn.114.103283. [DOI] [PubMed] [Google Scholar]
- 35.Jernerén F., Cederholm T., Refsum H., Smith A.D., Turner C., Palmblad J. Homocysteine status modifies the treatment effect of omega-3 fatty acids on cognition in a randomized clinical trial in mild to moderate Alzheimer's disease: the OmegAD study. J Alzheimers Dis. 2019;69(1):189–197. doi: 10.3233/JAD-181148. [DOI] [PubMed] [Google Scholar]
- 36.Flock M.R., Skulas-Ray A.C., Harris W.S., Etherton T.D., Fleming J.A., Kris-Etherton P.M. Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc. 2013;2(6) doi: 10.1161/JAHA.113.000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 38.Bhatt D.L., Steg P.G., Brinton E.A., Jacobson T.A., Miller M., Tardif J.C. Rationale and design of REDUCE-IT: reduction of cardiovascular events with icosapent ethyl-intervention trial. Clin Cardiol. 2017;40(3):138–148. doi: 10.1002/clc.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrieu S., Guyonnet S., Coley N., Cantet C., Bonnefoy M., Bordes S. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 40.Cukierman-Yaffe T., Bosch J., Diaz R., Dyal L., Hancu N., Hildebrandt P., et al. Effects of basal insulin glargine and omega-3 fatty acid on cognitive decline and probable cognitive impairment in people with dysglycaemia: a substudy of the ORIGIN trial. Lancet Diabetes Endocrinol 2014;2(7):562–72. [DOI] [PubMed]
- 41.Manson J.E., Cook N.R., Lee I.-.M., Christen W., Bassuk S.S., Mora S. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2018;380(1):23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chocano-Bedoya P.O., Bischoff-Ferrari H.A. DO-HEALTH: vitamin D3-omega-3-home exercise-healthy aging and longevity trial—dietary patterns in five European countries. In: Weaver CM, Bischoff-Ferrari H, Daly RM, Wong M-S, editors. Proceedings of the 10th international symposium on nutritional influences on bone health. Springer International Publishing; Cham: 2019. pp. 3–10. [Google Scholar]
- 43.Sydenham E., Dangour A.D., Lim W.-.S. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Sao Paulo Med J. 2012;130(6):419. doi: 10.1590/S1516-31802012000600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yurko-Mauro K., Alexander D.D., Van Elswyk M.E. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eriksdotter M., Vedin I., Falahati F., Freund-Levi Y., Hjorth E., Faxen-Irving G. Plasma fatty acid profiles in relation to cognition and gender in Alzheimer's disease patients during oral omega-3 fatty acid supplementation: the OmegAD study. J Alzheimers Dis. 2015;48(3):805–812. doi: 10.3233/JAD-150102. [DOI] [PubMed] [Google Scholar]
- 46.Soininen H., Solomon A., Visser P.J., Hendrix S.B., Blennow K., Kivipelto M., et al. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer's disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol 2017;16(12):965–75. [DOI] [PMC free article] [PubMed]
- 47.Hooijmans C.R., Pasker-de Jong P., de Vries R., Ritskes-Hoitinga M. The effects of long-term omega-3 fatty acid supplementation on cognition and Alzheimer's pathology in animal models of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimer's Dis. 2012;28(1):191–209. doi: 10.3233/JAD-2011-111217. [DOI] [PubMed] [Google Scholar]
- 48.Schuchardt J.P., Hahn A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2013;89(1):1–8. doi: 10.1016/j.plefa.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 49.Liu L., Bartke N., Van Daele H., Lawrence P., Qin X., Park H.G. Higher efficacy of dietary DHA provided as a phospholipid than as a triglyceride for brain DHA accretion in neonatal piglets. J Lipid Res. 2014;55(3):531–539. doi: 10.1194/jlr.M045930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J.J., Green P., John Mann J., Rapoport S.I., Sublette M.E. Pathways of polyunsaturated fatty acid utilization: implications for brain function in neuropsychiatric health and disease. Brain Res. 2015;1597:220–246. doi: 10.1016/j.brainres.2014.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen L.N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509(7501):503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 52.Patrick R.P. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer's disease. Faseb J. 2019;33(2):1554–1564. doi: 10.1096/fj.201801412R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Metherel A.H., Chouinard-Watkins R., Trépanier M.-.O., Lacombe R.J.S., Bazinet R.P. Retroconversion is a minor contributor to increases in eicosapentaenoic acid following docosahexaenoic acid feeding as determined by compound specific isotope analysis in rat liver. Nutr Metab. 2017;14:75. doi: 10.1186/s12986-017-0230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conquer J.A., Holub B.J. Dietary docosahexaenoic acid as a source of eicosapentaenoic acid in vegetarians and omnivores. Lipids. 1997;32(3):341–345. doi: 10.1007/s11745-997-0043-y. [DOI] [PubMed] [Google Scholar]
- 55.Grønn M., Christensen E., Hagve T.-.A., Christophersen B.O. Peroxisomal retroconversion of docosahexaenoic acid (22:6(n−3)) to eicosapentaenoic acid (20:5(n−3)) studied in isolated rat liver cells. Biochim Biophys Acta (BBA) – Lipids Lipid Metab. 1991;1081(1):85–91. doi: 10.1016/0005-2760(91)90254-f. [DOI] [PubMed] [Google Scholar]
- 56.Champeil-Potokar G., Hennebelle M., Latour A., Vancassel S., Denis I. Docosahexaenoic acid (DHA) prevents corticosterone-induced changes in astrocyte morphology and function. J Neurochem. 2016;136(6):1155–1167. doi: 10.1111/jnc.13510. [DOI] [PubMed] [Google Scholar]
- 57.Chouinard-Watkins R., Plourde M. Fatty acid metabolism in carriers of apolipoprotein E epsilon 4 allele: is it contributing to higher risk of cognitive decline and coronary heart disease? Nutrients. 2014;6(10):4452–4471. doi: 10.3390/nu6104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fanelli F., Sepe S., D'Amelio M., Bernardi C., Cristiano L., Cimini A. Age-dependent roles of peroxisomes in the hippocampus of a transgenic mouse model of Alzheimer's disease. Mol Neurodegener. 2013;8(1):8. doi: 10.1186/1750-1326-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yassine H.N., FQ, Azizkhanian I., Rawat V., Castor K., Fonteh A.N., Harrington M.G., Zheng L., Reed B.R., DeCarli C., Jagust W.J., Chui H.C. Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol. 2016;73(10):1208–1216. doi: 10.1001/jamaneurol.2016.1924. [DOI] [PubMed] [Google Scholar]
- 60.Eckert G.P., Chang S., Eckmann J., Copanaki E., Hagl S., Hener U. Liposome-incorporated DHA increases neuronal survival by enhancing non-amyloidogenic APP processing. Biochim Biophys Acta. 2011;1808(1):236–243. doi: 10.1016/j.bbamem.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Grimm M.O., Kuchenbecker J., Grösgen S., Burg V.K., Hundsdörfer B., Rothhaar T.L. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J Biol Chem. 2011;286(16):14028–14039. doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.