Abstract
Background
Low-dose interleukin-2 (ld-IL-2) enhances regulatory T-cell (Treg) function in auto-inflammatory conditions. Neuroinflammation being a pathogenic feature of amyotrophic lateral sclerosis (ALS), we evaluated the pharmacodynamics and safety of ld-IL-2 in ALS subjects.
Methods
We performed a single centre, parallel three-arm, randomised, double-blind, placebo-controlled study. Eligibility criteria included age < 75 years, disease duration < 5 years, riluzole treatment > 3 months, and a slow vital capacity ≥ 70% of normal. Patients were randomised (1:1:1) to aldesleukin 2 MIU, 1 MIU, or placebo once daily for 5 days every 4 weeks for 3 cycles. Primary outcome was change from baseline in Treg percentage of CD4+ T cells (%Tregs) following a first cycle. Secondary laboratory outcomes included: %Treg and Treg number following repeated cycles, and plasma CCL2 and neurofilament light chain protein (NFL) concentrations as surrogate markers of efficacy. Safety outcomes included motor-function (ALSFRS-R), slow vital capacity (SVC), and adverse event reports. This trial is registered with ClinicalTrials.gov, NCT02059759.
Findings
All randomised patients (12 per group), recruited from October 2015 to December 2015, were alive at the end of follow-up and included in the intent-to-treat (ITT) analysis. No drug-related serious adverse event was observed. Non-serious adverse events occurred more frequently with the 1 and 2 MIU IL-2 doses compared to placebo, including injection site reactions and flu-like symptoms. Primary outcome analysis showed a significant increase (p < 0·0001) in %Tregs in the 2 MIU and 1 MIU arms (mean [SD]: 2 MIU: +6·2% [2·2]; 1 MIU: +3·9% [1·2]) as compared to placebo (mean [SD]: -0·49% [1·3]). Effect sizes (ES) were large in treated groups: 2 MIU ES=3·7 (IC95%: 2·3–4·9) and 1 MIU ES=3·5 (IC95%: 2·1–4·6). Secondary outcomes showed a significant increase in %Tregs following repeated cycles (p < 0·0001) as compared to placebo, and a dose-dependent decrease in plasma CCL2 (p = 0·0049). There were no significant differences amongst the three groups on plasma NFL levels.
Interpretation
Ld-IL-2 is well tolerated and immunologically effective in subjects with ALS. These results warrant further investigation into their eventual therapeutic impact on slowing ALS disease progression.
Funding
: The French Health Ministry (PHRC-I-14-056), EU H2020 (grant #633413), and the Association pour la Recherche sur la SLA (ARSLA).
Keywords: Amyotrophic lateral sclerosis, Randomised clinical trial, Low dose interleukin-2, Neuro-inflammation, Biomarkers, Regulatory T cells
Research in context.
Evidence before this study
We searched PubMed up to Feb 1st 2020 with the terms “(interleukin-2 OR IL-2)” AND “(clinical trials)”. We also searched PubMed with the terms “(interleukin-2 OR IL-2)” AND “(ALS OR motor neurone disease OR motor neuron disease)” as well as “(ALS OR motor neurone disease OR motor neuron disease)” AND “(regulatory T cells OR Tregs)”. We also searched PubMed under the terms “(interleukin-2 OR IL-2)” AND “(randomised clinical trials)”. We searched www.clinicaltrials.gov for published randomised placebo-controlled trials using low dose IL-2 (ld-IL-2). We imposed no language restrictions on any of the searches. Searches revealed evidence of a satisfactory profile of safety and tolerability across diverse immunological disorders but no published randomised controlled trials (Class 1 evidence) on ld-IL-2 or agents thought to act in a similar fashion in ALS or other neurological disorders.
Added value of this study
Despite many studies on the potential benefits of ld-IL-2 on auto-immune and neurodegenerative disorders, including ALS, there have been very few randomised controlled trials in the field. This study adds Class 1 evidence to the rapidly developing research area and provides new evidence on Treg responsiveness to repeated cycles of ld-IL-2, while extending the understanding of the pharmacodynamic impact of ld-IL-2 in the context of neuroinflammation in ALS.
Implications of all the available evidence
This study, built on existing evidence that shows that ld-IL-2 expands Treg numbers and Treg frequency (when expressed as a percentage of CD4+ T cells) in healthy volunteers and in autoimmune and inflammatory disorders, provides a sound evidence base for future clinical studies of ld-IL-2 in ALS and other neurological disorders.
Alt-text: Unlabelled box
1. Introduction
Amyotrophic Lateral Sclerosis (ALS) is a fatal neuromuscular disorder characterised by progressive muscle wasting and weakness. Since the introduction of riluzole two decades ago [1], trials have failed to deliver more effective disease-modifying remedies. Recent consensus guidelines for ALS trials emphasise the importance of incorporating biomarkers of target engagement and disease activity at an early stage of therapeutic development [2].
Microglial cell activation is evident in the pathology of ALS at all disease stages [3] and in the transgenic SOD1 ALS mouse in which expression of macrophage-typical cytokines precedes clinical symptoms [4]. Furthermore, biomarkers of neuro-inflammation are elevated in patients with ALS and have been shown to correlate with disease severity and predict disease progression [5,6].
Although evidence of a neuro-inflammatory contribution to ALS pathogenesis is compelling [7], until now all therapeutic attempts to modify the neuro-inflammatory response in the ALS clinical context have failed [8]. However, most of these trials have targeted non-specific suppression of neuro-inflammation. Such approaches have a high risk of harm for people with ALS, where toxicity may outweigh a beneficial drug effect. In this context, reinforcing physiological tolerogenic dominance within the neuroimmuno-inflammatory system may provide a more effective approach to control cytopathic neuroinflammatory states compared to aggressive general immune suppression.
CD4+FOXP3+ regulatory T-cells (Tregs) physiologically regulate immune responses, contributing to the induction and maintenance of tolerance, thus preventing the onset of autoimmune and inflammatory diseases [9]. Previous studies have shown that in ALS patients, decreased levels of Tregs were correlated with increased disease severity and were predictive of disease progression and survival, suggesting that they may be a potential target for therapy [10], [11], [12]. Tregs are exclusively reliant on the cytokine Interleukin 2 (IL-2) for their generation, activation and survival [13]. Low dose IL-2 (ld-IL-2) administration induces the selective expansion of Tregs in mice and humans [14,15]. Several clinical trials exploring the therapeutic potential of ld-IL-2 in auto-immune and inflammatory conditions have now been reported, showing the feasibility and clinical safety of this approach [16]. Building upon our experience with ld-IL-2 in type-1 diabetes [17], we therefore examined the safety and pharmacodynamic effects of ld-IL-2 in ALS through a phase-2a, randomised, double-blind, placebo-controlled trial. Our primary objective was to verify within a small pilot study, whether immune and inflammatory parameters of ALS patients could be modified with low dose IL-2 therapy towards an improved tolerogenic state, with an acceptable safety profile.
2. Methods
2.1. Study design and participants
This three-arm, randomised (1:1:1), double-blind, single-centre study of 2 doses of ld-IL-2 in parallel versus placebo included patients at the Montpellier Amyotrophic Lateral Sclerosis Reference Centre in France. The study protocol was submitted by the Sponsor (Centre Hospitalier Universitaire de Nîmes) and approved by an independent ethics committee (Le Comité de Protection des Personnes Sud Méditerranée III; reference number: 2014.09.01-ter), declared on clinicaltrials.gov (NCT02059759) and was designed for adults less than 75 years old with probable, or laboratory-supported probable or definite ALS as defined by El Escorial Revised ALS diagnostic criteria [18]. The main inclusion criteria consisted of disease duration of less than 5 years, stable on riluzole treatment for over three months, and a vital capacity ≥ 70% of normal. Patients with severe cardiac or pulmonary disease, cancer, other life-threatening diseases, respiratory or feeding assistance, clinical signs of infection, positive serology (IGM) for recent infections (cytomegalovirus, Epstein-Barr virus), or human immunodeficiency virus, auto-immune disorders (except asymptomatic Hashimoto thyroiditis), any clinically significant laboratory abnormality (excepting cholesterol, triglyceride and glucose), or other diseases precluding functional assessments were excluded, as were those who had received a vaccination in the 8 weeks preceding the first experimental dosing. All patients provided signed informed consent before entering the study.
2.2. Randomisation and masking
Allocation was performed and blinding assured via a web-based inclusion and randomisation (with blocking) application. A statistician otherwise not involved in the study prepared randomisation lists. The size of blocks (3) remained undisclosed to all participants until unblinding. Clinical treatment unit (CTU) preparation and labelling were performed by a pharmacist at the Clinical Trial Pharmacy Unit of the University Hospitals of Montpellier, France, who was the only unblinded trial researcher. All laboratory assays were completely blinded. Samples were only identified by barcodes with the correspondence to randomisation number, time in the study (number of sampling time points=7), or treatment group (placebo, 1 MIU IL-2, 2 MIU IL-2), unknown to the laboratory producing the data. Prior to unblinding, accuracy of sample labelling was tested via genotyping of 20 common single nucleotide polymorphisms (LGC Genomics Division, Hoddenson, UK) allowing us to correct one mismatch on two samples over 251 samples collected.
2.3. Procedures
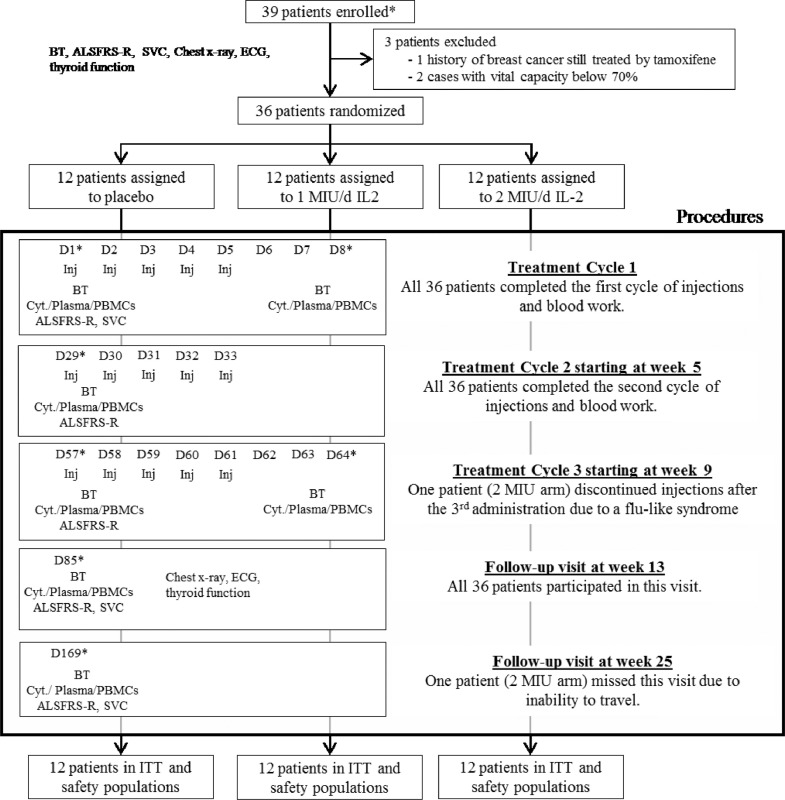
Upon inclusion by an investigating physician, baseline assessments were performed and included: routine blood haematology, biochemistry, and thyroid function tests, slow vital capacity, ALS Functional Rating Scale Revised (ALSFRS-R), chest x-ray, and electrocardiogram (Fig. 1). Following randomisation (≤ 2 weeks before first administration on day 1), patients started a 5-day cycle of once-daily sub-cutaneous injections. 5-day cycles were repeated twice, on weeks 5 and 9, for a total of 3 cycles of treatment per patient (Fig. 1). After the last treatment cycle, all patients were followed up for safety monitoring for a further 3 months.
Fig. 1.
Trial profile. ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Score – Revised; BT: Routine blood tests (see supplementary methods for detail); Cyt: Fresh Blood Cytometry (see supplementary methods for detail); SVC: slow vital capacity; PBMCs: Peripheral Blood Mononuclear Cells; Inj: sub-cutaneous injection; D: day; ECG: electrocardiogram; ITT: intention-to-treat. *Time frames corresponding to at-hospital visits.
Proleukin® (aldesleukin) at 22 MIU vials was purchased from Novartis-Pharma France. Clinical trial unit pharmaceutical preparation consisted of visually identical 1 ml polypropylene syringes containing 0·5 ml of either placebo (glucose for injection preparation D 5% solution), or 1 MIU or 2 MIU of IL-2, according to randomisation.
Assessments performed during the 6-month study period are indicated in Fig. 1. Vital signs, concomitant medications and adverse events were assessed at each visit. Slow vital capacity was assessed according to current recommendations (https://www.encals.eu/outcome-measures).
2.4. Clinical immunophenotyping
Clinical flow cytometry was performed by the Department for Cell and Tissue Engineering of the Montpellier University Hospital, France, within 2 h of phlebotomy. Peripheral blood was drawn into EDTA tubes and stained with two panels of monoclonal antibodies to identify CD3+, CD4+, CD8+ and regulatory T cells (Tregs; CD4+ CD25+ CD127low/- FoxP3+), NK cells, B lymphocytes and monocytes. A representative gating scheme is shown in supplementary Fig. 1. B cells, NK cells and CD3+ T cells are expressed as percentages of total lymphocytes; CD4+ and CD8+ T cells are expressed as percentages of CD3+ cells. Tregs are expressed as percentages of CD4+T cells. Effector T cells (Teffs) are calculated as the difference between total CD4+ T cells and Tregs and expressed as percentages of CD4+ T-cells. Monocytes are expressed as percentages of CD45+ leukocytes.
2.5. Mechanistic immunophenotyping
At each study visit, 20 ml blood was collected into sodium heparin tubes and peripheral blood mononuclear cells (PBMC) isolated and cryopreserved as detailed in the supplementary methods. For analysis of Treg function, cryopreserved PBMC were thawed and stained with a cocktail of monoclonal antibodies (details in supplementary methods). Suppression assays were then performed in V-bottom 96-well plates by co-culturing 500 sorted CD4+CD25−/low CD127+ Teffs in the presence or absence of CD4+CD25high CD127low Tregs at various ratios (Treg:Teff 0:1, 1:2 and 1:1) with 1 × 103 CD19+B cells. Cells were stimulated with PHA (4 μg/ml; Alere) and incubated at 37 °C, 5% CO2, for 6 days. Proliferation was assessed by the addition of 0·5 μCi/well [3H] thymidine (PerkinElmer) for the final 20 h of co-culture. Conditions were run in 6 replicates, and proliferation readings (counts per minute [CPM]) averaged. Any samples with averaged proliferation less than 3000 CPM from the Teff wells alone were excluded. The percentage suppression in each culture was calculated using the following formula: percent suppression = 100 − [(CPM in the presence of Tregs ÷ CPM in the absence of Tregs) × 100]. All time points from an individual were analysed concurrently.
2.6. Plasma chemokine determination
Plasma chemokine analysis was performed on −80 °C frozen plasma samples at Humanitas Clinical and Research Centre, Milan (Italy). CCL2 and CCL17 plasma levels were measured by Multiplex bead assay (Luminex Human HS Cytokine Panel - R&D Systems), and CCL18 by ELISA (Quantikine ELISA kit (DCL180B, R&D Systems).
2.7. Plasma neurofilament light chain protein determination
Plasma concentrations of the neurofilament light chain (NFL) protein were measured using an immunoassay with electrochemiluminescent detection (Meso Scale Discovery) at Queen Mary University of London, London (UK), as previously described in detail [19]. Plasma NFL concentration was also measured using Single Molecule Array (Simoa) technology, at the University of Gothenburg (Sweden), as previously described in detail [20].
Full details of clinical and biochemical phenotyping methods are provided in the supplementary material.
2.8. Outcomes
The primary pharmacodynamic outcome was the change in Tregs as a percentage of CD4+ T-lymphocytes on day 8 measured by clinical flow cytometry. Secondary pharmacodynamics were Treg number and percentage at all time-points, including expression as incremental areas-under-the-curves (iAUC), and plasma levels of CCL2 and NFL as markers of disease activity. Exploratory analyses included measurements of the number and frequency of leucocyte populations by flow cytometry as well as Treg cell functionality tests. Monocyte polarisation in response to treatment was investigated through analysis of their chemokine production profile (CCL17 and CCL18). Safety was assessed through a systematic check for predefined events (injection site reactions, flu-like symptoms, fatigue, gastro-intestinal signs, allergic reaction), abnormal vital signs, ECG results, chest radiographs, laboratory tests, and records of all adverse events reported during the study. As a secondary clinical outcome, changes in clinical function (ALSFRS-R and slow vital capacity (SVC)) with time were assessed throughout the study.
2.9. Sample size
Previous data [17] demonstrated that 6 patients per group achieved 88% power to detect a 60% increase in Tregs at p α=0·05 (Mann & Whitney test). Because impact on disease activity is also of primary interest, we retained 12 patients per group to achieve 80% power at p α(2-tailed)= 0·05 for detecting a 40% decrease in plasma NFL-MSD (Mann- Whitney U test) based on a previous ALS study [19].
Interim safety data were evaluated once by an Independent Data and Safety Monitoring Board (DSMB) after the first 12 patients were included and had completed a first cycle of treatment including day 8 assessments. The interim report remained undisclosed until the end of the study.
2.10. Statistical analyses
Categorical variables are described as absolute and relative frequencies. Quantitative variables are summarized by mean, standard deviation, 95% confidence interval (95% CI), median and range. Effect size with 95% CI was calculated for the primary pharmacodynamics outcome. Flow cytometry parameters were analysed as changes from baseline at D8 (primary criteria) and D64, i.e. absolute differences between each time point and baseline (D1). Overall immune cell changes with time for the first (D1, D8, D29) and third cycles (D57, D64, D85) were summarised as incremental time-normalised areas-under-the-curves (iAUC, using the trapezoidal method), minus D1 or D57 values respectively; iAUCt for trough values were calculated using values measured at D1, D29, D57 and D85 minus D1. Eosinophil counts were analysed in the same way as cytometry parameters. ALSFRS-R measures were summarised by regression slopes from D1 to D85. For SVC and NFL-MSD, absolute differences between D85 and baseline D1 were analysed. For CCL2, CCL17 and CCL18, baseline normalised values at D64 were analysed. For statistical inferences with regards to differences between the three study arms, we used a Fisher-Hayter's two-stage MCP testing strategy approach by first using a Kruskal-Wallis H test (KW-H) checking for differences among all groups, and only when significant at p < 0·05, Mann-Whitney U tests (MW-U) were used for pairwise comparisons of each active arm to placebo, to pinpoint the detected differences without the need for further adjusting the nominal p value threshold [21]. Dose-response relationships on summary measures were analysed using Jonckheere-Terpstra J test (JT-J) assessing whether the change in outcome variable was constantly increasing across levels of doses (linear trend test). For within group comparisons of time-points we used a Wilcoxon match-paired signed rank test (Wx-W test).
The full protocol is available upon request and access to material & data are subjected to preliminary agreement with the Sponsor.
3. Results
Between September 21st and December 4th 2015, thirty-nine patients were screened. Of these, 3 were excluded and 36 randomized (Fig. 1). After 12 inclusions and 1 cycle of treatment, the independent DSMB found no safety concerns, and inclusions continued. With only one exception (see Fig. 1), all randomised patients completed the 3 cycles of treatment over 3 months and the 3-month post treatment follow-up. All 36 randomised patients were included in the intention-to-treat and safety populations (Fig. 1). Of 252 maximum possible assessments for clinical and laboratory measurements for primary/secondary outcomes in the trial, all but one were available for analysis (Fig. 1).
Patient characteristics and disease history are shown in Table 1 and Supplementary Tables 2 and 3. Though between-group baseline differences were not statistically significant, the 2 MIU group had a higher female-to-male ratio, and slightly more severe disease features. Nonetheless, no clear imbalance that would influence the results was identified between groups.
Table 1.
Demographic and clinical baseline characteristics of study participants.
| Placebo (n = 12) | IL2 at 1 MIU/d (n = 12) | IL2 at 2 MIU/d (n = 12) | |
|---|---|---|---|
| Age | |||
| Mean (SD) | 56·45 (9·57) | 54·98 (10·99) | 57·68 (12·91) |
| Median (Range) | 56·20 (42·2 to 69·7) | 54·80 (40·2 to 75·4) | 61·25 (36·5 to 76·6) |
| Sex (female) | 3 (25%) | 5 (41·7%) | 3 (25%) |
| BMI | |||
| Mean (SD) | 26·80 (5·6) | 25·34 (2·53) | 24·39 (1·71) |
| Median (Range) | 25·10 (22·2 to 43·4) | 24·90 (21·90 to 28·7) | 24·35 (21·6 to 26·7) |
| Age at onset | |||
| Mean (SD) | 54·27 (9·85) | 52·43 (11·02) | 55·80 (12·86) |
| Median (Range) | 55·30 (38·1 to 68·0) | 52·20 (37·4 to 72·6) | 58·25 (35·1 to 76·0) |
| Disease duration (years) | |||
| Mean (SD) | 2·2 (1·44) | 2·60 (1·33) | 1·96 (1·44) |
| Median (Range) | 1·75 (0·5 to 5·0) | 2·85 (0·9 to 4·6) | 1·45 (0·6 to 4·6) |
| Duration of riluzole treatment (months) | |||
| Mean (SD) | 16·58 (12·49) | 20·70 (14·90) | 14·18 (11·47) |
| Median (Range) | 12·35 (4·6 to 39·8) | 17·45 (5·0 to 45·1) | 11·10 (3·1 to 34·0) |
| Diagnosis | |||
| Definite | 5 (41·7%) | 6 (50%) | 4 (33·3%) |
| Probable | 5 (41·7%) | 6 (50%) | 3 (25%) |
| Probable – laboratory supported | 2 (16·7%) | 0 (0%) | 5 (41·7%) |
| Familial form | 0 | 2 (16·7%) | 2 (16·7%) |
| Site of onset | |||
| Limb | 11 (92%) | 11 (92%) | 9 (75%) |
| Bulbar | 1 (8%) | 1 (8%) | 3 (25%) |
| Slow vital capacity (percentage predicted) | |||
| Mean (SD) | 94·4 (12·4) | 101·5 (18·1) | 93·6 (16·3) |
| Median (Range) | 96·5 (77·00 to 119·00) | 101·0 (79·00 to 132·00) | 94·5 (72·00 to 118·00) |
| ALSFRS-R score | |||
| Mean (SD) | 38·8 (3·4) | 38·0 (4·8) | 37·8 (5·3) |
| Median (Range) | 38·5 (34·00 to 45·00) | 38·0 (30·00 to 44·00) | 39·5 (26·00 to 44·00) |
| NFL-MSD (pg/ml) | |||
| Mean (SD) | 127.84 (89.90) | 135.55 (76.80) | 178.19 (94.84) |
| Median (Range) | 116.6 (6.7 - 349.2) | 103.4 (46.2 - 245.5) | 144.2 (109.1 - 460.0) |
Categorical data are presented as number (%). ALSFRS = Amyotrophic Lateral Sclerosis Functional Rating Score – Revised. BMI = Body Mass Index. SD = standard deviation. NFL-MSD= neurofilament light chain protein – electroluminescent detection method (Meso Scale Discovery).
Clinical tolerance was satisfactory at both doses of IL-2. During the entire follow-up (D1-D169), no drug-related serious adverse event (SAE) occurred and most non-serious adverse events (NSAEs) were transient and of mild to moderate grades. During the treatment period (D1-D85), frequencies of patients presenting NSAEs during cycles were higher in the IL-2 groups, n = 11 (92%) and n = 12 (100%) at the 1 and 2 MIU/day doses respectively, compared to n = 3 (25%) with placebo (Table 2). Local reactions at injection sites (erythema, pain) were the most common NSAEs of comparable frequency in the 2 active treatment groups (all patients except one experienced injection site reactions) while only one patient reported such an event in the placebo group. Flu-like symptoms (including myalgia, chills, fever, arthralgia), which are characteristic of IL-2 treatment [17], were reported only for the 2 MIU/day dose (25%). One patient in the 2 MIU/day group withdrew from treatment after 2 days of treatment in the third cycle because of severe flu-like symptoms not responding to symptomatic therapy (see Fig. 1).
Table 2.
Safety– number of patients (frequency) presenting non-serious adverse events during treatment cycles.
| Adverse events | Treatment group |
Total (N = 36) | ||
|---|---|---|---|---|
| 2 MIU (N = 12) | 1 MIU (N = 12) | Placebo (N = 12) | ||
| Injection site reactions | 12 (100%) | 11 (91·7%) | 1 (8·3%) | 24 (66·7%) |
| Flu-like symptoms | 3 (25·0%) | 0 | 0 | 3 (8·3%) |
| Fatigue | 2 (16·7%) | 1 (8·3%) | 2 (16·7%)0 | 5 (13·9%) |
| Gastro-intestinal signs | 2 (16·7%) | 1 (8·3%) | 0 | 3 (8·3%) |
| Rhinitis | 2 (16·7%) | 0 | 0 | 2 (5·6%) |
| Nasopharyngitis | 1 (8·3%) | 1 (8·3%) | 0 | 2 (5·6%) |
| Headache/Migraine | 4 (33·3%) | 0 | 0 | 4 (11·1%) |
| Chest pain | 1 (8·3%) | 0 | 0 | 1 (2·8%) |
| Cold sweat | 0 | 1 (8·3%) | 0 | 1 (2·8%) |
| Arthralgia | 1 (8·3%)0 | 0 | 0 | 1 (2·8%) |
| Myalgia | 0 | 0 | 1 (8·3%) | 1 (2·8%) |
| Total | 12 (100%) | 11 (91·7%) | 3 (25·0%) | 26 (72·2%) |
Outside treatment cycles, only one case of nausea/vomiting was imputed to treatment among other AEs. One patient (1 MIU/day group) with a history of prostatic adenoma developed severe urinary retention 10 days after the last administration and required hospitalisation for prostatic surgery. Other events were related to ALS disease or other pre-existing conditions.
No abnormalities were observed among the routine laboratory parameters, except for an elevation of C - reactive protein at day 8 in the one patient developing flu-like symptoms in the 2 MIU/day group, and another at D57 in relation to a viral infection. As for haematology parameters, no significant changes were observed except for eosinophil counts that were significantly increased compared to placebo at day D8 and D64 in the 2 MIU/day group (Supplementary Table 2); changes of a lesser degree were observed at 1 MIU/day and were significant only at D64. In the 2 MIU/day group, 3 patients presented eosinophil increases above 1·5 × 109/l but remained asymptomatic. All counts were close to baseline values at D169 (no significant differences between groups) and all within normal range.
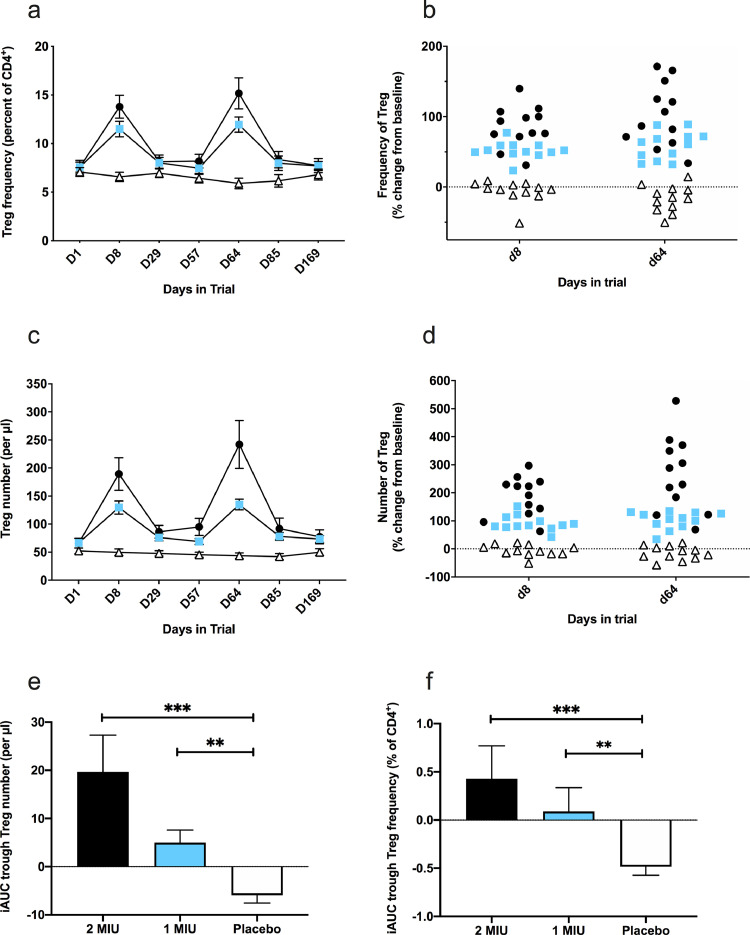
The a priori defined primary pharmacodynamic outcome of an increase in the frequency of Tregs as a percentage of CD4+ T-lymphocytes at D8 was dose-dependent (JT-J test, p < 0.0001) and highly significant for both treated arms (MW-U test, p < 0·0001; 2 MIU: mean [SD]: +6·2% [2·2]; 1 MIU: mean [SD]: +3·9% [1·2]) as compared to placebo (mean [SD]: −0·5% [1·3]) (see Table 3, Fig. 2a and b). Effect sizes were large for both IL-2 groups: 2 MIU ES=3·7 (95% CI: 2·3–4·9); 1 MIU ES=3·5 (95% CI: 2·1–4·6). Furthermore, when examining the range of change from baseline in Treg frequency, a clear distinction was observed in all IL-2 recipients (range of change: +23 to +139%) with no overlap with the placebo group (range of change: −51 to +9%, Fig. 2a). Secondary outcomes for Tregs revealed that the frequency and absolute count significantly and dose-dependently (JT-J test, p < 0.0001 for both frequency and absolute count) increased compared to baseline and placebo during subsequent treatment cycles (Fig. 2a–d, Table 3 and Supplementary Table 2). Furthermore, the peak during cycle 3 tended to be higher than that observed during cycle 1, suggesting that successive treatment cycles have residual effects that might be cumulative. This suggestion is further supported by significantly higher iAUC trough Treg levels (measuring the residual Treg change before beginning a new cycle) in IL-2 arms as compared to placebo (Fig. 2e and f, Table 3 and Supplementary Table 2). Overall, the 2 MIU arm resulted in higher Treg peaks and trough levels than the 1 MIU arm. Ld-IL-2 also resulted in a moderate increase in the frequency and number of NK cells for both IL-2 groups (maximum 1·7 fold increase in number at D64 for the 2 MIU group); an increase in the number of CD8 T cells for both IL-2 groups (maximum 1·4 fold increase in number at D64 for the 2 MIU group); an increase in the number of CD4+ Teffs for both ld-IL-2 groups (maximum 1·6 fold increase in number at D64 for the 2 MIU group) and a decrease in the frequency of monocytes in the 2 MIU group at D64 (all data shown in Table 3 and Supplementary Table 2).
Table 3.
Immune cell parameters at baseline (D1) and response.
| Dose group | Baseline D1 | D8 change from D1 | D64 change from D1 | iAUC trough D29-D57-D85 | D169 change from D1 | |
|---|---|---|---|---|---|---|
| CD8 (% CD3+) | 2 MIU | 25·0 (8·9) | −3·1 (1·7)** | −4·3 (2·6)** | −1·4 (1·1) | −1·4 (1·4) |
| 1 MIU | 24·1 (6·2)) | −2·0 (1·2)* | −2·7 (1·3)** | −1·0 (0·6) | −0·6 (1·4) | |
| Placebo | 26·1 (9·0) | −0·4 (2·3) | −0·3 (2·5) | −0·9 (1·5) | −1·5 (2·4) | |
| Treg (% CD4+) | 2 MIU | 7·6 (2·4) | 6·2 (2·2)**** | 7·6 (3·9)**** | 0·4 (1·2)*** | 0·2 (1·0) |
| 1 MIU | 7·6 (1·8) | 3·9 (1·2)**** | 4·4 (1·5)**** | 0·1 (0·9)** | 0·1 (0·9) | |
| Placebo | 7·1 (1·5) | −0·5 (1·3) | −1·2 (1·2) | −0·5 (0·3) | −0·3 (1·4) | |
| NK (% CD45+) | 2 MIU | 2·4 (1·0) | 1·4 (0·9)*** | 1·2 (0·9)*** | 0·4 (0·5) | 0·2 (0·5) |
| 1 MIU | 3·2 (2·1) | 1·1 (1·0)** | 1·4 (0·8)*** | 0·2 (0·5) | 0·1 (0·9) | |
| Placebo | 3·6 (2·4) | −0·2 (0·8) | −0·1 (0·7) | −0·2 (0·6) | −0·6 (1·4) | |
| CD19 (% CD45+) | 2 MIU | 3·5 (2·0) | −0·3 (0·9) | −0·2 (1·1) | −0·1 (0·8) | 0·3 (1·2) |
| 1 MIU | 3·3 (1·0) | −0·3 (0·4) | −0·2 (0·2) | −0·1 (0·3) | 0·3 (0·5) | |
| Placebo | 3·4 (1·8) | −0·1 (0·9) | −0·2 (0·6) | −0·2 (0·5) | −0·1 (0·4) | |
| Monocytes (% CD45+) | 2 MIU | 6·7 (1·2) | −0·6 (1·4) | −1·4 (1·3)** | −0·7 (1·3) | −0·9 (1·7) |
| 1 MIU | 5·4 (1·2)* | 0·5 (0·5) | −0·04 (0·6) | 0·1 (0·5) | −0·1 (0·5) | |
| Placebo | 7·3 (2·5) | 0·2 (1·0) | 0·1 (1·0) | 0·2 (0·6) | −0·2 (0·8) | |
| Eosinophils (% total WBCa) | 2 MIU | 2·7 (1·8) | 3·3 (1·5)*** | 6·8 (4·1) *** | 1·8 (1·0)*** | 0·4 (1·3) |
| 1 MIU | 2·3 (1·8) | 1·0 (0·8) | 2·5 (2·2)** | 0·6 (0·5) | −0·2 (0·7) | |
| Placebo | 2·3 (0·9) | 0·6 (0·7) | 0·4 (1·2) | 0·2 (0·6) | 0·6 (1·0) |
Results are expressed as relative frequency: mean (SD). a total leukocytes from haematology lab. Comparisons of each dose group to placebo group by Mann-Whitney test: *p< 0·05, ⁎⁎p<0·01, ⁎⁎⁎p<0·001, ⁎⁎⁎⁎p<0·0001.
Fig. 2.
Effect of IL-2 treatment on Treg number and frequency. Panels a to d: change in frequency (a-b) and absolute number (c-d) of Tregs throughout the study for all three arms (open triangles, placebo; blue squares, 1 MIU of IL2; black circles, 2 MIU of IL2). a & c: data points indicate mean values, and error bars their associated SEMs. b & d: change in the number and frequency of Tregs between baseline and the three days after the final injection of one treatment cycle (D8) or 3 treatment cycles (D64). Data points represent the per-patient change in Treg frequency (b) and number (d). Three group comparisons by the Kruskal-Wallis H test at D8 and D64 (p < 0.0001) for panel b and d. Panels e-f: iAUC of trough levels of Tregs during the study. Data points indicate mean values, and error bars their associated SEMs for Treg number (e) and frequency (f). Verum to placebo comparisons by the Mann-Whitney U test: *** p < 0.001, ** p < 0.01.
Exploratory analyses of Treg phenotype and function were performed using cryopreserved PBMC focussing primarily on responses at baseline and following 3 cycles of treatment (D1 and D64). We observed good correlation between the frequency of Tregs defined in blood by clinical cytometry and when sorting Tregs from cryopreserved PBMC (R2=0·91, p < 0·0001). Similar to results in fresh blood, analysis of cryopreserved PBMC revealed a significant increase in the frequency of Tregs following 3 cycles of ld-IL-2 treatment (Supplementary Fig. 3a–c).
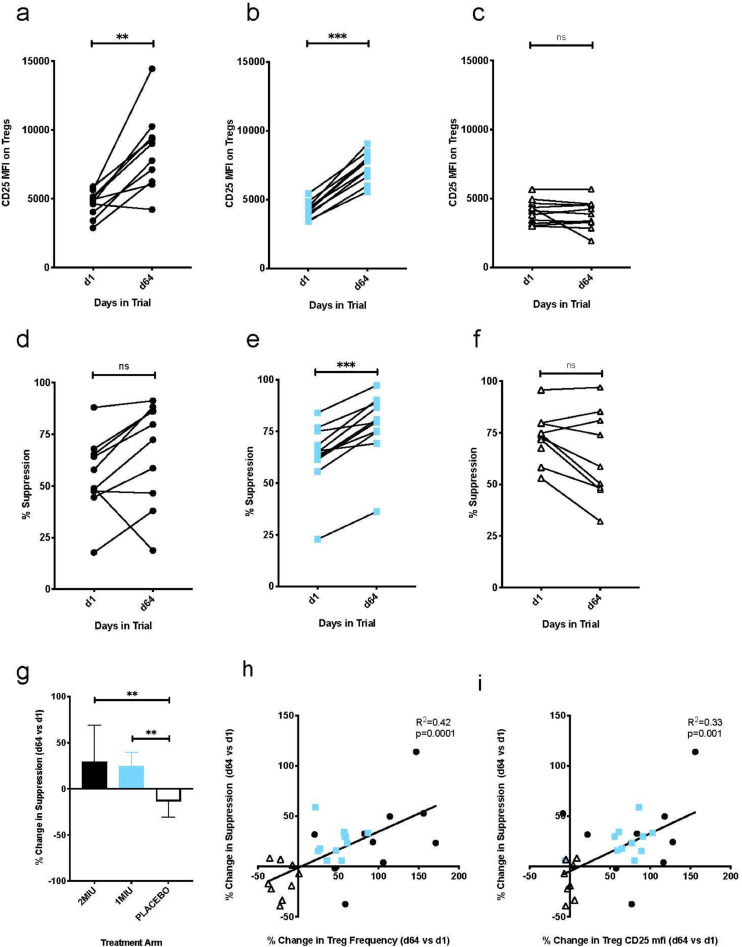
Furthermore, batched analysis on the same day of all time-points from a single individual allowed direct comparison of CD25 expression on Tregs quantified through median fluorescence intensity (mfi) (Fig. 3a–c). Comparisons of percent change from baseline after three cycles (D64) showed a significant dose dependent increase in CD25 expression on Tregs (JT-J test, p < 0.0001). Median increase in Tregs CD25 mfi was 1.94 fold in the 2MIU arm and 1.84 fold in the 1MIU arm (CD25 mfi, median [range], D1 vs D64: 2MIU 4651 [2892–5886] vs 9015 [4220–14446], Wx-W test, p = 0·002; 1 MIU = 4230 [3388–5423] vs 7778 [5564–9037], Wx-W test, p = 0·001) compared to no change in the placebo arm (4105 [2992–5656] vs 3867 [1923–5669], Wx-W test, p = 0·83).
Fig. 3.
Effect of IL-2 treatment on Treg phenotype and suppressive function. Panel a to c: CD25 mfi expression on Tregs at baseline (D1) and 3 days after completion of 3 treatment cycles (D64) in all three study groups: (a) 2 MIU, (b) 1 MIU and (c) placebo. Panels d to f: autologous suppressive function of Tregs measured by in vitro co-culture assay at baseline (D1) and 3 days after completion of 3 treatment cycles (D64) in individuals treated with (d) 2 MIU, (e) 1 MIU and (f) placebo. Panel g: Change in suppressive function of Tregs following 3 cycles of treatment relative to baseline levels in all three groups. Bars represent mean values, and error bars their associated SEMs. Panels h-i: Relationship between the relative change in Treg frequency (h) and Treg CD25 mfi (i) measured by mechanistic immunophenotyping cytometry (x-axis) and Treg suppressive function (Y axis) following 3 cycles of treatment (values at D64 vs D1). Open triangles denote individuals receiving placebo, blue squares 1 MIU and black circles 2 MIU of IL2. **** p < 0.0001, *** p < 0.001, ** p < 0.01, ns: p > 0.05 by the Wilcoxon match paired sign rank test (a to f) and by the Mann-Whitney test (g).
A smaller, but still significant increase in CD25 expression was also observed on Teffs (CD25 mfi, median [range], D1 vs D64: 2 MIU= 426 [116–897] vs 528 [122–948], Wx-W test, p = 0·02; 1 MIU= 336 [132–478] vs 362 [124–577], Wx-W test, p = 0·001; placebo= 251 [195–919] vs 263 [158–881], Wx-W test, p = 0·24; Supplementary Fig. 3d–f).
Treg function was assessed by in vitro co-culture assays using Teffs from the corresponding time point as responder cells. In cultures lacking Tregs, we observed no effect of ld-IL-2 administration on the proliferation of responder T cells (Supplementary Fig. 3g–i). However, we did observe an increase in suppressive function of Tregs following 3 cycles of ld-IL-2 therapy, which reached statistical significance for the 1 MIU dose (percent suppression, median [range], D1 vs D64: 2 MIU = 53% [18-88] vs 76% [19-91], Wx-W test, p = 0·06; 1 MIU = 65% [23-84] vs 80% [36-97], Wx-W test, p = 0·001; Fig. 3d and e). In contrast, we observed a slight decrease in Treg function in the placebo group (percent suppression, median [range], D1 vs D64: placebo= 73% [53–95] vs 59% [32-97]; Wx-W test, p = 0·07; Fig. 3f). When comparing the percent change in Treg suppressive function over the treatment period (relative to suppression at baseline), we observed a significant difference between both groups treated with ld-IL-2 when compared to placebo (KW-H test, p < 0.0021, 2 MIU vs placebo, MW-U test, p = 0·0076; 1 MIU vs placebo, MW-U test, p = 0·001; Fig. 3g). We also assessed the relationship between the change in Treg suppressive function and the change in Treg frequency or change in CD25 expression in response to treatment for each individual. In both cases, we observed a highly significant correlation between these measurements (Treg suppressive function vs Treg frequency: R2=0·42, p = 0·0001, Treg suppressive function vs Treg CD25 mfi: R2=0·33, p = 0·001 Fig. 3h-i) with individuals who received ld-IL-2 clearly clustering away from those who received placebo.
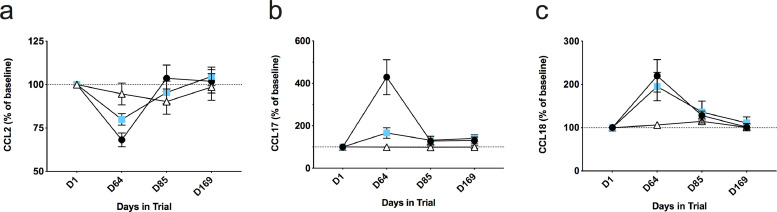
Plasma levels of the CCL2 chemokine were assessed in order to evaluate the potential for a therapeutic impact of ld-IL-2 on individuals with ALS through change in this marker of disease activity. Following the third treatment cycle (D64), we observed a significant difference between the three groups in the plasma levels of CCL2 (KW-H test, p = 0·005, Fig. 4a), both active dose groups showing a dose-dependent change in CCL2 levels (JT-J test, p = 0.0049), which were significantly reduced compared to placebo with the 2 MIU dose (MW-U test, p = 0·005), though not reaching statistical significance with the 1 MIU dose (MW-U test, p = 0·06). Second, we measured plasma levels of chemokines associated with macrophage/microglial polarization (CCL17 and CCL18). We observed a significant difference between treatment groups at D64 in CCL17 and CCL18 (KW-H tests, p = 0·0001 and 0·0028, respectively, Fig. 4b and c), with an increase in both treatment groups compared to placebo (MW-U tests, CCL17: 2 MIU p = 0·0001 and 1 MIU p = 0·0138; CCL18: 2 MIU p = 0·0012 and 1 MIU p = 0·0094). For all three chemokines, in both treated group values returned to baseline by D85 (cycle 3 trough level).
Fig. 4.
Effect of IL-2 treatment on plasma chemokine concentrations. Panels a to c: variation in plasma chemokine levels throughout the study for CCL2 (a), CCL17 (b) and CCL18 (c). Concentrations are expressed as a percentage of baseline value for each individual and points indicate mean values, and error bars their associated SEMs. Open triangles denote individuals receiving placebo, blue squares 1 MIU and black circles 2 MIU of IL2. Three dose comparisons at D64 panels a to c, by the Kruskal-Wallis rank test: (a) p < 0.005; (b) p < 0.0001; (c) p < 0.0028.
These results suggest that ld-IL-2 treatment was associated with a decrease of an inflammatory marker associated with ALS, and a concomitant shift of monocytes towards the M2 phenotype.
With regard to disease progression, we did not observe any significant differences among the three groups with regards to time related changes in the ALSFRS-R score (KW-H test, p = 0·12), slow vital capacity (KW-H test, p = 0·59), or plasma NFL-MSD levels (KW-H test, p = 0·84; supplementary Fig. 4). For NFL, re-analysis of the plasma samples using the Simoa approach provided similar results (KW-H test, p = 0·30). However, in the overall population, none of these parameters showed statistically significant changes over the treatment period – ALSFRS-R (points/month) mean slope [95% CI]= −0·8 [−2·4, +0·8]; SVC (percent predicted) mean change at D85 from D1 [95% CI]= −2·2 [−23·4, +19·0]; NFL-MSD D85 change from D1, (pg/ml) mean change [95% CI] = + 0·63 [−62·5, + 63·7]; NFL-SIMOA D85 change from D1, (pg/ml) mean change [95% CI] = −1·64 [−26·25, +22·97] – demonstrating that these parameters were poorly sensitive to change over the relatively short treatment period.
4. Discussion
First, our results show that IL-2 at two low doses was clinically well tolerated in ALS subjects over three cycles, and no further safety issues were detected following treatment withdrawal. In keeping with previous reports [16], our findings show that ALS patients, who are particularly vulnerable to treatment toxicity, can withstand repeated cycles of treatment with ld-IL-2. The safety of ld-IL-2 is further supported by the lack of significant deterioration in ALSFRS-R or SVC over the treatment period across all groups, and within groups, including placebo. The lack of any detectable functional change may be related to the relative insensitivity to change of these clinical outcome measures over the short treatment period, but is nonetheless reassuring in terms of safety.
Second, we observed a significant, dose dependent increase in both the absolute number and relative frequency of Tregs in both the 2 MIU and 1 MIU groups. Comparing these results to those obtained in a double-blind randomised clinical trial in individuals with Type 1 Diabetes [17], we observed a similar magnitude of Treg response to ld-IL-2, with a ~1·5-fold increase in the Treg percentage of CD4+ cells following 5 days of treatment at 1 MIU.
Of note, all individuals in both groups on active treatment showed an increase in Treg number and frequency. Thus, although it has been suggested that Tregs from ALS patients may have impaired endogenous responsiveness to IL-2 [22], potentially making them unresponsive to ld-IL-2 treatment, in this cohort of ALS patients we observed no evidence of intrinsic impairment in Treg responsiveness to ld-IL-2.
Third, we have shown that the increase in Treg response was sustained over 4 weeks following a 5-day treatment cycle. This is important as optimum clinical efficacy is likely to require a sustained increase in Treg levels, and therefore potentially a continuous life-long treatment with ld-IL-2. We selected our treatment schedule based on the study in type 1 diabetes [17], and the present study confirms a significant expansion in Treg number and frequency at trough levels (i.e. before the start of each treatment cycle) and also that this expansion increases with repeated cycles. However, it remains to be verified whether our treatment schedule is the most effective for controlling neuro-inflammation in ALS, or whether different treatment schedules (e.g., more frequent administration of ld-IL-2) will be more therapeutically useful.
Taken together, our findings suggest that in this ALS cohort there was no loss of sensitivity to ld-IL-2 with repeated administration. Consistent with this and in agreement with other reports [16], we observed that ld-IL-2 leads to a preferential increase in expression of CD25 on Tregs. This may increase sensitivity of Treg cells to both administered and endogenous IL-2, thus potentially enhancing and sustaining any treatment effect.
In order to understand the relevance of the change in Treg number to any treatment effect, we assessed Treg function before and after ld-IL-2 administration. We used an autologous co-culture assay to measure the ability of FACS-isolated Tregs to suppress the proliferation of CD4+ effector T cells. Our results demonstrate that, overall, treatment with ld-IL-2 results in both an increase in Treg frequency and in Treg suppressive function. This improvement in Treg function is highly significant in the 1 MIU arm, with all individuals showing an increase in function, while in the 2 MIU group only a trend toward significance was observed, due to increased variation in response between individuals. This improvement of Treg function following ld-IL-2 treatment is consistent with previous observations in ALS that the suppressive function of Tregs among individuals with ALS could be improved by ex vivo expansion driven by recombinant IL-2 [22]. The dual efficacy of ld-IL-2 is illustrated by the highly significant correlation between increased Treg frequency and function, though we also observed individual variation in treatment response with some treated individuals showing a more significant change in either Treg frequency or Treg function and others showing an increase in both. Conversely, individuals in the placebo group tended to lose either Treg frequency or function during the same timeframe.
With regard to ld-IL-2 effects on blood markers of ALS disease activity, we found a significant and dose-dependent reduction in the plasma concentration of CCL2. CCL2 is a small chemokine belonging to the C—C subfamily which signals through the CC chemokine receptor 2 (CCR2) and drives circulating leucocytes towards sites of neuroinflammation [23]. CCL2 knockout mice have reduced infiltration of circulating leucocytes at sites of neuroinflammation and resistance to disease in models of autoimmunity and inflammation [23], suggesting this pathway plays a role in driving pathogenesis. Elevated CCL2 expression levels have been observed in neural tissue from individuals with ALS and are associated with infiltration and activation of macrophages and microglia. CCL2 levels in biological fluids are also elevated in individuals with ALS and have been shown to correlate with disease score [24] and survival [5], indicating that CCL2 is a useful biomarker of disease activity.
Mechanisms underlying the polarisation of myeloid cell activation states have been proposed to harbour significant potential for intervening in the progression of neurodegenerative diseases, including ALS [25]. In experimental ALS models, an M1/inflammatory microglial phenotype characterizes end-stage disease phases and exerts neurotoxic activity with detrimental outcomes, while a shift to an M2/immunoregulatory microglial phenotype has been shown to protect motor neurons [26]. Monocytes also show polarised activation, with distinct chemokine expression profiles recognized as suitable markers for defining their M1 or M2 profiles [27]. In experimental ALS models, inflammatory monocytes are recruited to the spinal cord and contribute to disease progression by increasing neuronal loss and reducing lifespan [28]. Interestingly, ld-IL-2 treatment was associated with a significant increase of plasma levels of CCL17 and CCL18, in keeping with a change in macrophage/microglial polarisation towards an immunoregulatory/M2-like phenotype [29]. To our knowledge, there is no information on CCL17 in blood in ALS, while CCL18 has been investigated, but no significant association with disease progression was observed [30]. It will be important in future ALS studies to investigate these and other biomarkers of microglial polarisation in CSF. Overall, the changes in inflammatory biomarkers of macrophage activation and polarisation are consistent with a role of ld-IL-2 in controlling cytopathic microglial activation associated with ALS progression.
Tregs are known to influence macrophage activation and polarisation, primarily towards an M2-like phenotype [31], raising the possibility that these changes are a direct result of the increased number or functional capacity of Tregs induced by ld-IL-2. However, cells of the monocyte-macrophage lineage do express functional IL-2 receptors, and their expression of CD25 (IL2RA) is increased under inflammatory conditions [32], in keeping with the notion that macrophage/microglial polarisation may also occur as a direct result of ld-IL-2 acting directly on these cells.
Finally, we did not observe across groups any significant difference in changes in plasma NFL concentrations in response to treatment. Although there was an over 20% increase in the mean of NFL levels in the placebo group consistent with the disease progression over time, this difference was not statistically different from zero due to a large variance in this group. In contrast, no increase was observed in the two ld-IL-2 treated groups. Based on published data [19], we estimated that it should be possible to detect a treatment effect with relatively few patients per group, but in the context of this randomised (and strictly blinded) study, post-hoc power analysis suggests that this is a large underestimate, as supported by recent reports [33]. In the light of these findings it is possible that analyses of neurofilament proteins in the CSF will be more informative [34].
Altogether, considering the dose-dependency of responses over Treg expansion and function and of inflammatory markers CCL2, CCL17 and CCL18, the selection of the 2 MIU IL-2 dose seems more appropriate for further clinical development. However, the 1MIU proved better tolerated though still significantly effective compared to placebo. It is likely that a flexible dose approach in further clinical development should be considered.
The main limitation of this study is that the results were obtained on a small sample of a highly selected population of slowly progressing patients over a short period of treatment. Although this design minimises informative censoring due to death which hampers analysis of repeated measures in ALS studies [35], it does not allow us to generalise our findings to the overall ALS population, nor does it provide the power to detect even large changes in clinical parameters. The demonstration in an ALS population that ld-IL-2 is engaging the Treg target was a necessary step towards the next level demonstrating ld-IL-2′s potential clinical efficacy in slowing down the rate of disease progression. In a recent experiment using the SOD1 mouse ALS model [36], it was shown that in vivo Treg expansion using an IL-2/IL-2 antibody complex was associated with a significant increase in survival. Nonetheless, preclinical ALS models have unfortunately proved inadequate predicting clinical outcomes in drug development, and in the case of ld-Il-2, are unlikely to help defining how much Treg amplification is required to achieve clinical benefit.
In conclusion, this study shows that ld-IL-2 is safe over 3 monthly cycles in people with ALS. In addition, we provide clear evidence for in vivo amplification of Treg numbers, frequency and suppressive function with ld-IL-2. Importantly, in the light of the previous findings of raised CCL2 in plasma and CSF of ALS patients, our observation that plasma CCL2 is decreased in a dose-related fashion to ld-IL-2 treatment supports the notion that this therapeutic approach may translate into an effective therapy. A phase 2b/3 study based on these observations is ongoing (www.mirocals.eu; ClinicalTrials.gov NCT03039673), which may confirm the usefulness of these biomarkers as early surrogate outcomes for clinical efficacy.
Contributors
GBS and PNL conceived the study and hypotheses. GBS, CP, PNL, WC and CS were involved in the clinical trial design. GBS, TT, JLV, JK, AM, SS, AAC, CM & CG were involved in designing the specific laboratory tests of the study. WC, RJM & NP were involved in clinical data collection. JLV, JDV, MM, TT, CG, ML, JK, PS, AM, UA & HZ were involved in specific laboratory data collection. CP, TT, CG & ML were involved in data management and data analysis. GBS, CP, WC, TT, PNL, CG, ML, AM, HZ were involved in data interpretation. GBS, TT, PNL, CP, CS, CG, MM, ML, AM, HZ, WC were involved in drafting the manuscript. All authors critically reviewed the manuscript.
Data safety monitoring board
Pr Bertrand Diquet, Pharmacologie (CHU Angers), Pr Philippe Couratier, Neurologie (CHU Limoges), Pr B. Asselain, Biométrie, Institut Curie (Paris).
Declaration of Competing Interest
Drs. Camu, Mickunas, Payan, Juntas Morales, Pageot, Masseguin, Suehs, De Vos, Saker, Andreasson and Veyrune have nothing to disclose. Dr. Bensimon reports grants from French Health Ministry (PHRC-I), ARSLA and EU HORIZON 2020, during the conduct of the study; in addition, Dr. Bensimon has a patent (WO 2012123381 A1) with royalties paid to Assistance Publique Hopitaux de Paris (APHP), Institut National de la Sante et de la Recherche Medicale INSERM, and Sorbonne Universite. Drs. Bensimon, Tree, Leigh, Locati, Garlanda, Shaw, Kirby, Malaspina have a patent (B75649EPD40021) pending. Dr. Malaspina reports grants from EU HORIZON 2020, grants from MND Association UK, grants and other from Barts and the London Charity, and from UCB Pharma SPRL, during the conduct of the study; and from F. Hoffmann-La Roche outside the submitted work. Dr. Zetterberg reports personal fees from Samumed, Roche Diagnostics, Denali, CogRx and Wave, outside the submitted work. Dr. Kirby reports grants from The Nimes University Hospital Center (CHU Nimes) and grants from EU HORIZON 2020, during the conduct of the study. Dr. Shaw reports grants from EU HORIZON 2020, Sheffield component and MIROCALS (633413), outside the submitted work. Dr. Al-Chalabi reports involvement as Chief Investigator for LEVALS clinical trial and European CI for REFALS clinical trial for OrionPharma, as well as consultancy from Mitsubishi Tanabe Pharma, consultancy and involvement in debating panel for Cytokinetics Inc, consultancy from Chronos Therapeutics, GSK, Lilly, and from Biogen Idec, outside the submitted work.
Acknowledgments
Acknowledgement
We wish to thank Drs Fabienne Bringer & Audrey Castet-Nicolas for the preparation of the Investigational Medicinal Products, Sebastien Alphandery for the monitoring of the Study, Marina Sironi for technical assistance in cytokine and chemokine analysis, and all the individuals with ALS who volunteered to join this study, knowing that it was unlikely to benefit them individually but might aid the development of a new therapy in the future.
Funding
This study was funded by a government call-for-tender for academic/investigator-driven research (French Ministry of Health, PHRC-I, 2014, n° 14-056), the French ALS patient Association ARSLA and EU H2020 Grant no. 633413. The Sponsor (CHU-Nimes, France) of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing
The full protocol is available upon request and access to material and data are subjected to preliminary agreement with the Sponsor (DRC@chu-nimes.fr).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102844.
Contributor Information
Timothy Tree, Email: timothy.tree@kcl.ac.uk.
Gilbert Bensimon, Email: gbensimon.psl@gmail.com.
Appendix. Supplementary materials
References
- 1.Bensimon G., Lacomblez L., Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. ALS/Riluzole Study Group. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg L.H., Sorenson E., Gronseth G., Macklin E.A., Andrews J., Baloh R.H. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology. 2019;92:e1610–e1623. doi: 10.1212/WNL.0000000000007242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troost D., Van den Oord J.J., Vianney de Jong J.M. Immunohistochemical characterization of the inflammatory infiltrate in amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 1990;16:401–410. doi: 10.1111/j.1365-2990.1990.tb01276.x. [DOI] [PubMed] [Google Scholar]
- 4.Alexianu M.E., Kozovska M., Appel S.H. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- 5.Gille B., De Schaepdryver M., Dedeene L., Goossens J., Claeys K.G., Van Den Bosch L. Inflammatory markers in cerebrospinal fluid: independent prognostic biomarkers in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2019;90:1338–1346. doi: 10.1136/jnnp-2018-319586. [DOI] [PubMed] [Google Scholar]
- 6.Thonhoff J.R., Beers D.R., Zhao W., Pleitez M., Simpson E.P., Berry J.D. Expanded autologous regulatory T-lymphocyte infusions in ALS: a phase I, first-in-human study. Neurol Neuroimmunol Neuroinflamm. 2018;5:e465. doi: 10.1212/NXI.0000000000000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans M.C., Couch Y., Sibson N., Turner M.R. Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol Cell Neurosci. 2013;53:34–41. doi: 10.1016/j.mcn.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Khalid S.I., Ampie L., Kelly R., Ladha S.S., Dardis C. Immune modulation in the treatment of amyotrophic lateral sclerosis: a review of clinical trials. Front Neurol. 2017;8:486. doi: 10.3389/fneur.2017.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wing K., Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 10.Rentzos M., Evangelopoulos E., Sereti E., Zouvelou V., Marmara S., Alexakis T. Alterations of T cell subsets in ALS: a systemic immune activation? Acta Neurol Scand. 2012;125:260–264. doi: 10.1111/j.1600-0404.2011.01528.x. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani S., Garbelli S., Pasini A., Alimonti D., Perotti C., Melazzini M. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J Neuroimmunol. 2009;210:73–79. doi: 10.1016/j.jneuroim.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Henkel J.S., Beers D.R., Wen S., Rivera A.L., Toennis K.M., Appel J.E. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med. 2013;5:64–79. doi: 10.1002/emmm.201201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malek T.R., Bayer A.L. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 14.Zorn E., Nelson E.A., Mohseni M., Porcheray F., Kim H., Litsa D. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Q., Adams J.Y., Penaranda C., Melli K., Piaggio E., Sgouroudis E. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahvildari M., Dana R. Low-dose IL-2 therapy in transplantation, autoimmunity, and inflammatory diseases. J Immunol Baltim Md 1950. 2019;203:2749–2755. doi: 10.4049/jimmunol.1900733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartemann A., Bensimon G., Payan C.A., Jacqueminet S., Bourron O., Nicolas N. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2013;1:295–305. doi: 10.1016/S2213-8587(13)70113-X. [DOI] [PubMed] [Google Scholar]
- 18.Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 19.Gaiottino J., Norgren N., Dobson R., Topping J., Nissim A., Malaspina A. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE. 2013;8:e75091. doi: 10.1371/journal.pone.0075091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gisslén M., Price R.W., Andreasson U., Norgren N., Nilsson S., Hagberg L. Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: a Cross-Sectional Study. EBioMedicine. 2016;3:135–140. doi: 10.1016/j.ebiom.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keselman H.J., Cribbie R., Holland B. The pairwise multiple comparison multiplicity problem: an alternative approach to familywise and comparison wise Type I error control. Psychol Methods. 1999;4:58–69. doi: 10.1037/1082-989X.4.1.58. [DOI] [Google Scholar]
- 22.Thonhoff J.R., Simpson E.P., Appel S.H. Neuroinflammatory mechanisms in amyotrophic lateral sclerosis pathogenesis. Curr Opin Neurol. 2018;31:635–639. doi: 10.1097/WCO.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor T., Borsig L., Heikenwalder M. CCL2-CCR2 Signaling in Disease Pathogenesis. Endocr Metab Immune Disord Drug Targets. 2015;15:105–118. doi: 10.2174/1871530315666150316120920. [DOI] [PubMed] [Google Scholar]
- 24.Nagata T., Nagano I., Shiote M., Narai H., Murakami T., Hayashi T. Elevation of MCP-1 and MCP-1/VEGF ratio in cerebrospinal fluid of amyotrophic lateral sclerosis patients. Neurol Res. 2007;29:772–776. doi: 10.1179/016164107X229795. [DOI] [PubMed] [Google Scholar]
- 25.Mammana S., Fagone P., Cavalli E., Basile M., Petralia M., Nicoletti F. The role of macrophages in neuroinflammatory and neurodegenerative pathways of alzheimer's disease, amyotrophic lateral sclerosis, and multiple sclerosis: pathogenetic cellular effectors and potential therapeutic targets. Int J Mol Sci. 2018;19:831. doi: 10.3390/ijms19030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao B., Zhao W., Beers D.R., Henkel J.S., Appel S.H. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol. 2012;237:147–152. doi: 10.1016/j.expneurol.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Butovsky O., Siddiqui S., Gabriely G., Lanser A.J., Dake B., Murugaiyan G. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrivastava R., Shukla N. Attributes of alternatively activated (M2) macrophages. Life Sci. 2019;224:222–231. doi: 10.1016/j.lfs.2019.03.062. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Merino L., Iridoy M., Galbete A., Roldán M., Rivero A., Acha B. Evaluation of chitotriosidase and CC-chemokine ligand 18 as biomarkers of microglia activation in amyotrophic lateral sclerosis. Neurodegener Dis. 2018;18:208–215. doi: 10.1159/000490920. [DOI] [PubMed] [Google Scholar]
- 31.Tiemessen M.M., Jagger A.L., Evans H.G., van Herwijnen M.J.C., John S., Taams L.S. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holter W., Goldman C.K., Casabo L., Nelson D.L., Greene W.C., Waldmann T.A. Expression of functional IL 2 receptors by lipopolysaccharide and interferon-gamma stimulated human monocytes. J Immunol Baltim Md 1950. 1987;138:2917–2922. [PubMed] [Google Scholar]
- 33.Piehl F., Kockum I., Khademi M., Blennow K., Lycke J., Zetterberg H. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler Houndmills Basingstoke Engl. 2018;24:1046–1054. doi: 10.1177/1352458517715132. [DOI] [PubMed] [Google Scholar]
- 34.de Flon P., Laurell K., Sundström P., Blennow K., Söderström L., Zetterberg H. Comparison of plasma and cerebrospinal fluid neurofilament light in a multiple sclerosis trial. Acta Neurol Scand. 2019;139:462–468. doi: 10.1111/ane.13078. [DOI] [PubMed] [Google Scholar]
- 35.Ramchandani R., Schoenfeld D.A., Finkelstein D.M. Global rank tests for multiple, possibly censored, outcomes. Biometrics. 2016;72:926–935. doi: 10.1111/biom.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheean R.K., McKay F.C., Cretney E., Bye C.R., Perera N.D., Tomas D. Association of regulatory T-cell expansion with progression of amyotrophic lateral sclerosis: a study of humans and a transgenic mouse model. JAMA Neurol. 2018;75:681–689. doi: 10.1001/jamaneurol.2018.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.