Significance
Evidence of climate change impacts on biodiversity is accumulating, but a growing literature also reveals highly variable rates and even directions of these responses. We propose that this complexity arises because biological processes do not respond to climate in direct and linear ways and, therefore, that complex biodiversity responses could be simplified by rescaling climate in terms of the underlying biological processes. Applying this “plant’s eye view” approach to a montane grassland climate change experiment, we find that variation in local extinction and colonization rates in response to temperature and precipitation change across landscapes emerge from unexpected and understudied biotic interactions. Thus, biotic rescaling can simultaneously test mechanistic hypotheses and enhance generality in understanding of biodiversity responses to climate change.
Keywords: colonization, extinction, context dependency, temperature, precipitation
Abstract
Generality in understanding biodiversity responses to climate change has been hampered by substantial variation in the rates and even directions of response to a given change in climate. We propose that such context dependencies can be clarified by rescaling climate gradients in terms of the underlying biological processes, with biotic interactions as a particularly important process. We tested this rescaling approach in a replicated field experiment where entire montane grassland communities were transplanted in the direction of expected temperature and/or precipitation change. In line with earlier work, we found considerable variation across sites in community dynamics in response to climate change. However, these complex context dependencies could be substantially reduced or eliminated by rescaling climate drivers in terms of proxies of plant−plant interactions. Specifically, bryophytes limited colonization by new species into local communities, whereas the cover of those colonists, along with bryophytes, were the primary drivers of local extinctions. These specific interactions are relatively understudied, suggesting important directions for future work in similar systems. More generally, the success of our approach in explaining and simplifying landscape-level variation in climate change responses suggests that developing and testing proxies for relevant underlying processes could be a fruitful direction for building more general models of biodiversity response to climate change.
Evidence of climate change impacts on biodiversity is rapidly accumulating (1, 2), both for species-level range shifts (3–7) and for changes in community composition (8–10). However, attempts to synthesize climate change impacts have been stymied by substantial variation in rates and even directions of biodiversity response to a given change in climate (6, 11–13). This dependence of the response to climate change on the ambient climate of the site, referred to as baseline dependency or context dependency, greatly limits both our understanding of observed climate change impacts, as well as our ability to forecast future biodiversity trends (14–16).
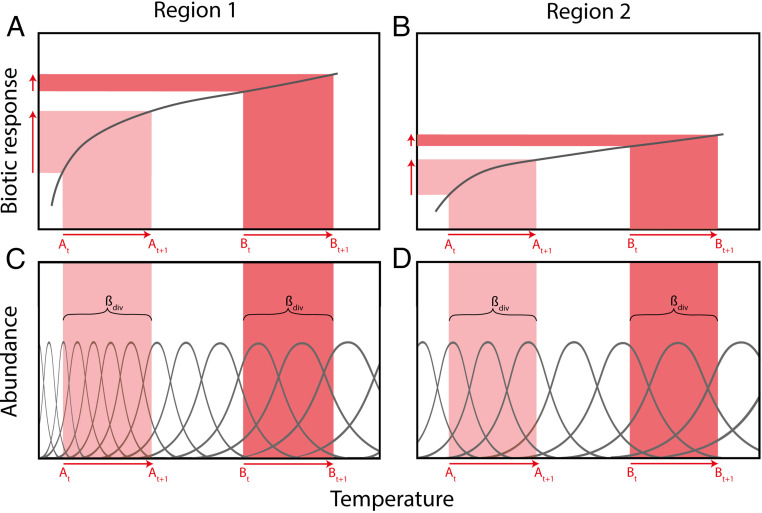
We argue that climate context dependencies arise from three ubiquitous complexities in how biological systems respond to the environment: First, responses to climate change are often due to changes in biotic interactions mediated by climate, rather than direct physiological response to the climate factors per se, especially at the warmer/lagging edge of species’ climatic ranges (15, 17–20). Second, biological responses, whether to biotic or abiotic factors, are generally nonlinear, which will cause the rate and direction of response to a given change in that factor to depend on the ambient value of that factor (Fig. 1A), as has been repeatedly documented for temperature responses (3, 16, 21). Third, biological responses are generally affected by multiple and interacting environmental drivers, which implies that response to one factor depends on variation in another, as exemplified by the differential responses to climate warming found in dry vs. wet regions (Fig. 1A vs. Fig. 1B), which also has consequences at the community level (Fig. 1C vs. Fig. 1D) (22, 23).
Fig. 1.
Conceptual model of the possible sources of context dependencies in biotic responses to climate change. (A) Nonlinear biological responses to climate lead to differences in the consequences of a given temperature change (from t to t + 1) at different positions along a climate gradient (A vs. B). (B) As in A, but with different response functions under different climatic contexts (for example, region 2 representing a wetter climate relative to a drier region 1). (C) At the community level, nonlinear biotic responses predict variable rates of community turnover, colonizations, and local extinctions, in response to a given climatic change (from t to t + 1)) at different positions along a climate gradient (A vs. B). (D) As in C, but with a lower rate of community turnover along the gradient in region 2 in response to the same climatic change (e.g., from At to At + 1 in D vs. C).
We propose that these complexities can be simplified, and generality of responses to climate change broadened, by rescaling the climatic predictor variables in biological terms, that is, by taking the plant’s eye view on the environment. To do this, we argue that the predominance of indirect effects of climate mediated through biotic interactions may actually represent an opportunity rather than a challenge. This is because many different environmental factors influence biotic interactions through the same underlying biological mechanisms, and these diverse environmental factors should then be reflected in the same biological variables. For example, in herbaceous plant communities, either warmer or wetter conditions often lead to increased biomass or height (24, 25), which leads to increased competition for light, which, in turn, determines which species are able to persist in the community. In this example, biomass or height serves as a proxy for the magnitude and mode of competitive interactions that determine community responses such as species composition or diversity. Because biomass integrates the effect of multiple abiotic drivers, for example, temperature and precipitation, it collapses the nonlinear and nonadditive effects of these, as well as any other correlated factors, to a single dimension that reflects the actual biological mechanisms driving the ecological response.
The use of easily measured static biotic variables to predict and quantify ecological processes has a long history. For example, plant ecologists have used biomass or standing crop to predict the magnitude of competitive or facilitative effects on plant growth (26, 27) or of herbivore effects on vegetation diversity (28). Similarly, species diversity has been used as a predictor of invasion success by exotics, or of ecosystem resilience (29, 30), and Wisz et al. (17) suggest using proxies of species’ interactions in species distribution models. The specific appropriate proxies of ecological processes will inevitably differ among systems, and may change along environmental gradients. We argue that rescaling the abiotic environment in terms of biotic proxies should nevertheless provide opportunities to simplify models of response to climate change because this approach targets an intermediate level of complexity between phenomenological approaches using environmental factors directly to predict community responses and more complex mechanistic models that explicitly incorporate both environmental effects on ecological processes and the consequences of those processes for communities. In this study, we test the hypothesis that community responses to climate change are better explained by rescaling climate change with easily measured proxies of biotic interactions than by direct metrics of climate change itself. That is, exploring community response to climate change through such integrative proxies will eliminate the complex interaction terms that characterize climate context dependencies and, importantly, enhance our understanding of the specific processes driving the community response.
We use a biotic rescaling approach to explore and explain variation in local-scale extinction and colonization rates in the face of climate change because these rates express the population- and community-level consequences of taxon-specific differences in individual-level vital rates of recruitment and survival. Thus, they encapsulate both the direct effects of climate on individual fitness and the indirect effects mediated through changes in species interactions.
We measured colonization and local extinction responses to climate change across different climate contexts using a macroecological experiment, sensu ref. 31, where entire grassland plant communities were transplanted in the direction projected by regional climate models (32) into new climates beyond current range limits of some of the component species (Fig. 2). Importantly, unlike in situ climate change experiments, the community transplant approach experimentally enhances proximity of the communities undergoing climate change to propagules of potential novel species (20), which should enhance both colonization and biotically driven extinction events. This provides opportunities to explore determinants of variation in these community-level processes independent of landscape-level processes such as dispersal limitation and lags, which are undoubtedly also important in colonization and range expansions on real landscapes (33, 34). The transplanted communities are otherwise exposed to realistic local-scale dispersal mechanisms, community interactions, and climatic contexts.
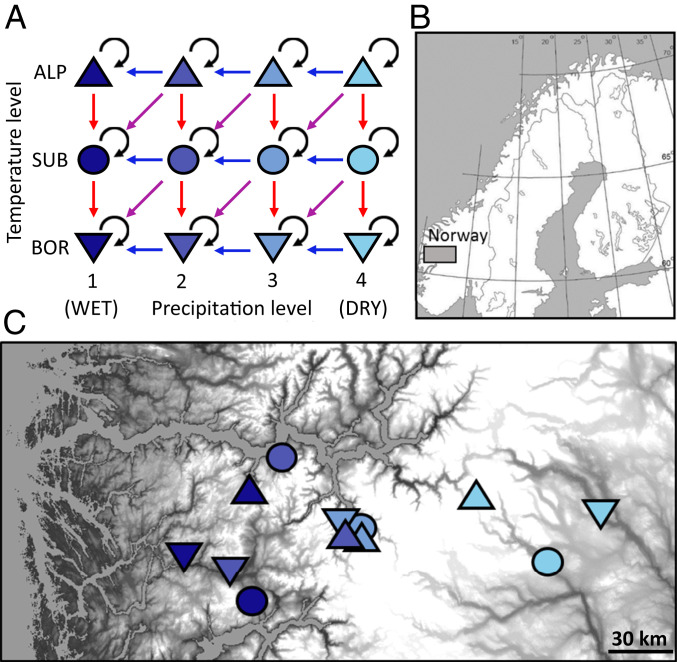
Fig. 2.
Design and location of the replicated macroecological turf transplant experiment. (A) Climate grid with three temperature levels (mean growing season temperature ca. 10.5 °C [BOR, Boreal] to 6.5 °C [ALP, Alpine]) and four precipitation levels (mean annual precipitation ca. 700 mm [1] to 2,900 mm [4]). Experimental transplantation of vegetation turfs between sites indicated by arrows (red, warmer; blue, wetter; purple, warmer and wetter; black, local transplants). Each treatment thus has an origin site and a destination site. (B) Location of study area in Norway. (C) Location of sites within the study area, with grayscale reflecting elevation (0 m to 2,405 m above sea level) Symbols are as in A.
The replication of the experiment across multiple sites along regional climatic gradients allows us to explore the extent to which general proxies of biological mechanisms can account for system-specific environmental context dependencies. We use a unique study system in southern Norway, where complex topography and careful site selection allows separation of orthogonal gradients of temperature and precipitation (Fig. 2). Previous experimental work in this system has shown that biotic interactions are important regulators of colonization−extinction dynamics (35–38), with the magnitude of these interaction effects driven especially by graminoid and bryophyte abundance, consistent with other work in similar systems (39, 40). Several potential proxies of biotic interactions change in consistent ways across the landscape (35, 41), suggesting they hold potential for predicting variation in colonization and extinction rates across the region.
We test the following specific predictions arising from our general hypothesis: 1) In alpine grassland vegetation, increases in both temperature and precipitation generally drive increasing plant−plant competition for light, which causes declines in colonization rates and increases in extinction rates. Thus, proxies of these interactions, such as vegetation cover or height, should be strong predictors of colonization and extinction rates in response to climate change, regardless of ambient climate or the specific climatic change treatment. Further, 2) incorporating these proxies into analyses of climate change effects should reduce climate context dependencies in extinction and colonization rates and therefore simplify models of response to climate change.
Materials and Methods
Experimental Design and Measurements.
In 2009, we established a climatic grid of 12 sites across three temperature and four precipitation levels spanning ∼4 °C in growing season temperature and ∼2,200 mm of annual precipitation (SI Appendix, Table S1). Sites were selected based on gridded climate data downscaled at 0.1-km resolution, which was verified by monitoring a number of climatic and environmental variables through local weather stations established at each site for the duration of the experiment (SI Appendix, Extended Methods). Careful site selection ensured that other factors such as grazing regime and history, bedrock, vegetation type and structure, slope, and exposure were kept as constant as possible (41). The sites were originally grazed by free-range domestic and wild ungulate grazers, but were fenced and mowed annually to protect experimental installations while mimicking past disturbance regimes.
At each site, we established five replicate experimental blocks, each containing up to five 25 × 25 cm plots randomly assigned to up to five treatments: transplanting to warmer, wetter, or warmer and wetter climates; transplanting locally within blocks (to control for the transplanting itself); and an untouched control (Fig. 2). These treatments correspond to the 35 arrows in Fig. 2 plus 12 control turfs, giving a total of 47 turf site and treatment combinations, which were replicated five times each, thus 235 turfs or observational units in total. All transplant turfs (n = 175) were dug up from the origin site and transplanted to destination sites at the end of the 2009 growing season.
Vegetation was surveyed at peak growing season in 2009, 2011, 2012, and 2013, using Lid and Lid (42) for nomenclature. At each census in each turf, we visually estimated percentage cover of each vascular plant species, as well as of total vascular plants, bryophytes, lichens, litter, and bare soil, using a 5 × 5 cm grid overlay. The mean heights of the vascular vegetation and of bryophytes were measured at four fixed points per turf. We quantified the number of new species (turf colonizations) and species lost (local extinctions) per turf between 2009 and 2013. Cover of forbs, graminoids, woody plants, and new species (colonists) were calculated at each census as the sum of all appropriate species. See SI Appendix, Extended Methods for details on the site selection, transplanting, data collection, and data management, and ref. 43 for the data used in the analyses.
Statistical Analyses.
To visualize overall community composition changes in response to climate change, we performed site-level canonical constrained ordination analyses (CCA) (44). We used the warming and/or wetting treatments as constraints and plotted individual turf ordination scores for each treatment and year with their associated destination site controls (Fig. 2).
To investigate the effects of climate change treatments on colonization and extinction, quantify the climatic context dependence of these effects, and test possible biological explanations, we carried out a series of regression analyses (generalized linear model [GLM]) (45). Initial Poisson regressions showed that the number of colonists was independent of species richness (χ2 test of the effect of richness in model with climate treatment and context accounted for: deviance = 0.89, P = 0.34), whereas the number of extinctions strongly depended on species richness (deviance = 113.4, P < 0.001). For subsequent analyses, we therefore used Poisson regression for colonization (species counts), and logistic regression for extinction (proportion of original species extinct). These analyses used a reduced “full factorial dataset” to achieve a balanced design for the statistical testing, that is, the six origin sites that had all three transplant treatments represented (Fig. 2). To investigate the importance of site-specific variation and the nested structure of the data, we compared the GLMs to corresponding models with origin site as an additional random effect (generalized linear mixed model [GLMM]) (46, 47). The estimated random effects were generally small (sometimes zero). The fixed effects parts of the GLMMs were quantitatively very similar to the GLMs, leading to the same qualitative conclusions. For extinction, some of the GLMMs did not properly converge, and, for colonization, the GLMMs had higher AICs (Akaike information criterion [AIC], used for model selection) (48), than the GLMs, suggesting that the site effects were obsolete. We therefore only present the results of GLMs.
We developed separate regression models for observed colonizations or extinctions in the full factorial dataset with two different sets of independent variables, corresponding to our predictions.
-
1)
The climate-only model includes three groups of climate-related factors: 1) climate change treatments (warming, wetting, warming and wetting), 2) ambient climate at the origin site (temperature, precipitation), and 3) climate context dependencies (interactions between climate change treatments and ambient climate).
-
2)
The biotic interactions and climate model includes the climate factors as above plus six potential proxies of interactions measured at the end of the 4-year study period: vegetation height, graminoid cover, forb cover, bryophyte height, bryophyte cover, and novel colonist species cover. These were selected based on a substantial literature suggesting they are useful potential proxies of interactions in grassland plant communities (e.g., refs 10, 20, 21, 39, 40, 49–52). These variables were standardized by SD and centered to 0 to facilitate comparison of effect sizes (SI Appendix, Table S2).
To test our predictions, we made a full climate-only model including all climate variables (set 1) and selected a model for biotic interactions and climate (set 2) based on forward selection with AIC (48) according to our predictions: we first selected variables representing biotic interactions, then main effects for climate change or ambient climate, and lastly climate interactions. Any selected interactions involving biotic interactions were included with those main effects.
Results
Biodiversity Responses to Climate Change across the Landscape.
Colonization and extinction rates in the control and local transplant turfs are low but nonzero, reflecting background levels of local-scale community turnover across the landscape (Fig. 3 and SI Appendix, Fig. S1).
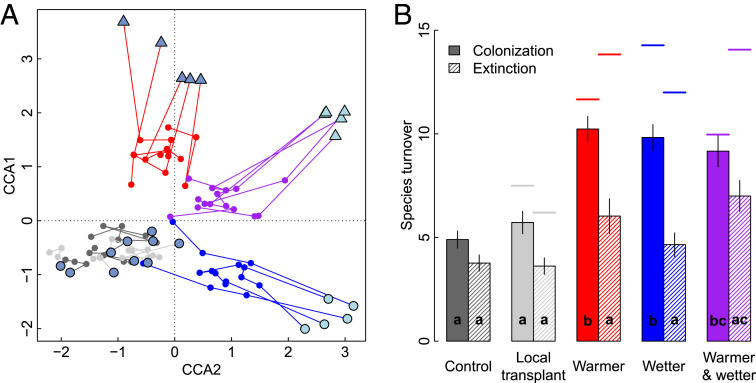
Fig. 3.
Community composition change over 4 y with climate change treatments at one site (A), and colonization and extinction rates across sites (B). (A) CCA of turf-level plant community composition at site SUB2 (Fig. 1), with symbols for each replicate turf in 2009 and lines with points for three subsequent years (2011, 2012, and 2013; symbols and colors as in Fig. 1). The plot is rotated to match the orientation of the climate gradients in Fig. 1. (B) Mean number of colonizations or extinctions (±SE) per plot between 2009 and 2013 for each climate change treatment, averaged across all of the six origin sites where all treatments were represented. Bars denoted with different letters are significantly different (Tukey's HSD [honestly significant difference] test, P < 0.05). Horizontal lines above each bar represent the mean number of colonizations or extinctions needed for complete convergence of transplants to destination controls for that treatment (i.e., if the biotic “distance-to-go” in response to a climatic change was fully realized, e.g., from At to At + 1 in Fig. 1C). Note that horizontal lines in the local transplant treatment reflect the background within-site community turnover.
With experimental climate change, both colonization and extinction rates increased considerably (Fig. 3B), and transplanted communities gradually converged toward the species composition of the destination communities for all climate change treatments and sites, albeit with considerable variability in rates (Fig. 3A and SI Appendix, Fig. S2 and Table S3). This community convergence happened largely through colonization by new species, which occurred at significantly higher rates in all climate change treatments relative to controls, so that, after only 4 years, the transplanted turfs had acquired most of the colonization events needed for complete convergence (Fig. 3B). In contrast, extinctions lagged considerably, and fewer than half of expected extinctions had been realized after 4 years (Fig. 3B).
These effects of climate change varied strongly across the ambient climate grid (Fig. 4 A and B; “climate” models in SI Appendix, Table S4). In fact, the interactions between climate change and ambient climate account for as much deviance in both colonization and extinction as the main effects of climate change itself (climate models, Fig. 5 and SI Appendix, Table S5). Specifically, colonization increased more in response to wetting in warmer regions, while responses along the precipitation gradient were even more complex (Fig. 4A and SI Appendix, Table S4). In contrast, extinction increased more in response to wetting in the drier parts of the climate grid and in response to warming in colder regions (Fig. 4B and SI Appendix, Table S4). The warmer and wetter treatment followed the general patterns of wetting alone, but yielded higher extinction rates overall (Fig. 4B and SI Appendix, Table S4).
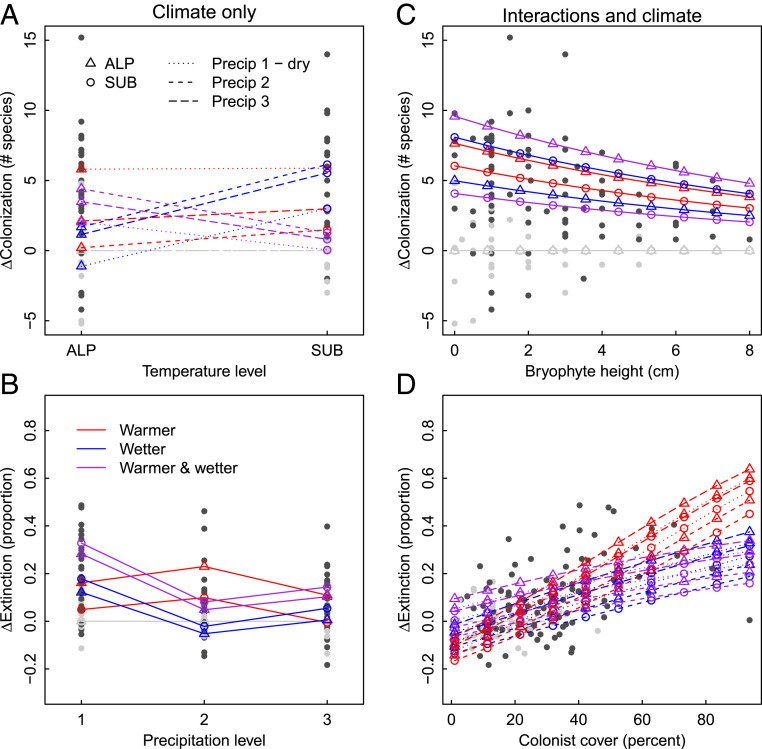
Fig. 4.
The role of climate context (A and B) and biotic interactions (C and D) in moderating colonization (A and C) and extinction (B and D) rates in response to climatic changes, based on a series of GLMs on the full factorial data set. ΔColonization is expressed as the difference between the treatment and origin control turfs in cumulative number of new species appearing per turf between 2009 and 2013. ΔExtinction is calculated as the difference between the treatment and origin control plots in the proportion of the species present in 2009 that disappeared between 2009 and 2013. See SI Appendix, Fig. S1 for background colonization and extinction rates in the controls. For biotic interactions, ΔColonization and ΔExtinction are plotted against two of the community metrics tested; coefficients for other significant community metrics are shown in SI Appendix, Table S3, and deviances are explained by the models in Fig. 5 and SI Appendix, Table S5. Black dots (n = 120) represent the five replicate plots for each transplant treatment in the six sites in which all climate change treatments are represented. Colors indicate climate change treatment, symbols indicate temperature levels, and line types indicate precipitation levels (see legends on A and B). Gray lines and symbols indicate control plots. In cases where the climatic changes or contexts are not significant (SI Appendix, Table S3), the associated colors, symbols, or line types are not differentiated on the panel.
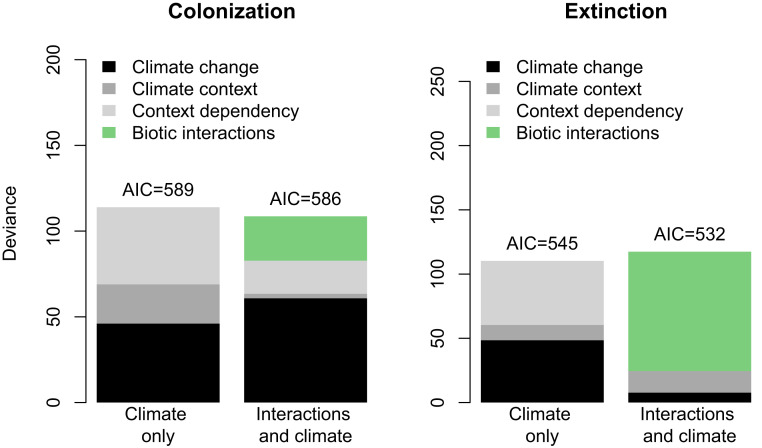
Fig. 5.
Accounting for climate context dependencies in colonization and extinction rates via proxies of biotic interactions. The deviance accounted for by the climate variables alone (climate change, climate context, and climate context dependency) is shown on the Left. The Right depicts the full models including both climate and proxies of biotic interactions (SI Appendix, Fig. S2). The models were constructed using forward selection, based on AIC, within three groups of variables which were combined as follows: First, we included variables representing biotic interactions (where relevant), then main effects for climate change or context, and, lastly, climate interactions. Any selected interactions involving biotic interactions were included with those main effects. Because models with biotic proxies are more complex, we also show AICs to allow comparison of the explanatory power of each model. More detailed breakdown of deviances for model components is in SI Appendix, Table S5.
Rescaling Abiotic Gradients in Biotic Terms.
The biotic proxies of species interactions varied across the ambient climate grid. Vegetation height, and hence assumed intensity of competition for light, increases toward warmer sites and, somewhat surprisingly, drier sites, while bryophyte height increases toward warmer sites (SI Appendix, Fig. S3 and Table S6). Underlying this is a switch in dominance from forbs in the warm/dry corner of the grid to graminoids in both the warm/wet and cold/dry corners of the grid. The two groups codominate the fourth cold/wet corner.
Consistent with prediction 1, biotic proxies of the intensity of plant−plant interactions explain substantial variation in colonization and, especially, extinction rates, indicating they do reflect some of the mechanisms that drive biodiversity in our study system. Specifically, colonization decreased while extinction increased as bryophytes were taller, both for controls across all ambient climates (SI Appendix, Fig. S1) and under all climate change treatments (Fig. 4C and SI Appendix, Table S4). On the other hand, some biotic interactions seem to differ in importance among climate change treatments. For example, extinction rates increased with cover of new colonists, but significantly so only in the warming treatment (Fig. 4D and SI Appendix, Table S4).
Consistent with prediction 2, incorporating these proxies of interactions completely eliminates all climate context dependencies for extinction, and considerably reduces them for colonization, both in terms of effect sizes (see “biotic interaction” models in SI Appendix, Table S4) and deviance accounted for (Fig. 5 and SI Appendix, Table S5). For colonization, the effects of ambient climate are also greatly reduced, suggesting the pattern of declining background colonization rates in the lower-elevation controls (SI Appendix, Fig. S1) was driven primarily by increasing biotic interactions (SI Appendix, Table S4 and Fig. S1 and Fig. 5). While the total deviance explained is similar between the biotic interactions and climate-only models, the model with biotic interactions is much simpler and hence more general, due to the loss of statistical interaction terms involving climate (SI Appendix, Table S4).
Discussion
Across the landscape, our experimental community transplants converged relatively rapidly toward the species composition of the new destination climates (see also ref. 53), consistent with the extensive literature reporting community shifts in response to experimental warming (3, 10) and species range shifts in response to recent climate change (5, 6). The community shifts were largely driven by increases in colonization rates above the background levels, as might be expected given that experimentally transplanting communities to new climates eliminated the need for long-distance dispersal of the novel species appropriate under the new climate. In contrast, while extinctions in transplanted communities were also higher than background levels, they lagged considerably behind colonizations, and many species characteristic of the original climate were still present after 4 y, lending experimental evidence to the many observational studies of species ranges that report extinction lags under climate change (16, 33, 34, 54).
In this study, we focus on understanding the considerable variation across the landscape in colonization events and local extinction rates in relation to a given magnitude of climate change, often described as “context dependency” or “baseline condition dependency” in the recent literature (3–10, 13, 16, 21). By taking the plant’s eye view through rescaling climate in terms of proxies of the underlying ecological processes, we were able to account for and explain much of this variation, and we found that it was related to systematic trends in the nature and strength of biotic interactions across the landscape.
The Importance of Biotic Interactions.
Consistent with our first prediction, we found that biotic interactions were major drivers of local colonization and, especially, extinction rates, regardless of the ambient baseline climate, or the experimental climate change treatments. Our candidate list of proxies of biotic interactions was based on community attributes known to be important mediators of competitive effects in similar systems: vascular plant and bryophyte height; cover of forbs, graminoids, and bryophytes; and cover of novel competitors. For example, the height and cover of vascular plants are proxies for plant−plant competition for light (49, 50), and graminoids have been suggested to be particularly important in mediating increased competitive effects in response to climatic warming in arctic and alpine systems (10, 21, 40). Recently, novel interactions by colonists from warmer climates have been put forward as an important indirect effect of global climate change, again, especially for arctic and alpine biodiversity (20). Bryophytes can have important impacts on both vascular and nonvascular components of the vegetation through modifying the soil microclimate, intercepting precipitation and nutrients, and interfering with seed recruitment and plant regeneration (39, 51, 52).
The overall weaker impact of biotic interactions on colonization than on extinction makes sense if the species in the destination environment (i.e., the warmer-climate species colonizing the transplanted turfs) are closer to their niche optima and better competitors in that environment than the species subjected to the climate change (i.e., the transplanted colder-climate adapted community). This stronger biotic effect on extinctions lends experimental support to the often-stated interpretation that interactions are especially important in determining dynamics at the warmer edge of species ranges, where extinctions should be the dominant process under anthropogenic climate change (6, 19, 55).
Although our results are consistent with the argument that biotic interactions are important, the specific mechanisms are not necessarily those expected. Notably, interactions from vascular plants do not impede colonization by novel species, in contrast to findings from numerous removal experiments (39, 40, 56, 57), including in this system (36, 58, 59). Similarly, overall vascular plant cover had no significant effect on extinction rates. While our study does not experimentally manipulate interactions per se, the lack of strong relationships of colonization and extinction to measures of vascular plant abundance suggest that the individual-level interactions measured in many experiments do not necessarily scale up to population dynamics and community turnover. This is likely because most experiments are comparisons of extremes, that is, vegetation is present at natural abundance or completely absent. In contrast, climate change drives more subtle changes in vegetation structure and abundance (38). Further, competitive effects are rarely linear with the density of competitors (60); if the changes in density observed occur within flatter parts of competitive response curves, weaker impacts would be expected (see also Fig. 1).
Instead of vascular plants, we found bryophytes and specific groups of vascular plants to be important. Going from no bryophytes to an 8-cm-tall mat reduced colonization by almost half. This is in line with the few available experiments exploring interactions from bryophytes, which document substantial effects on recruitment probabilities of vascular plants in boreal and alpine systems (39, 52). Extinction also increased with bryophyte height, but the strongest predictor of local extinction rates was the cover of novel colonist species. This finding adds experimental evidence to support recent research pointing to the role of novel interactions in driving species extinctions under climate warming (20). Our results suggest that future experimental work on the effects of interactions on response to climate change should move beyond studies of interactions among extant vascular plants and simple removals of all vegetation to explicitly include and disentangle the roles of a broader range of plant functional types, explore effects of more subtle changes in biomass, and incorporate density dependence.
Biotic Interactions Explain Context-Dependent Climate Change Responses.
Across the landscape, context dependencies in climate change responses explained as much variation in local colonization or extinction rates in our study system as did the main effects of climate change itself. The specific patterns underlying these context dependencies parallel previous observations and experiments in mountainous regions (3–6, 8–10, 21). Our second prediction stated that a major cause of these context dependencies is change in the intensity and nature of biotic interactions along temperature and precipitation gradients (61, 62). In line with this prediction, we found that incorporating proxies for biotic interactions in the models for local extinction rates completely eliminates all climate context dependencies, as well as much of the effect of the climate change treatments themselves. Indeed, incorporating just two such proxies, bryophyte cover and new colonist species cover, eliminates five complex interaction terms, and can account for ∼80% of the explained deviance in extinctions. Importantly, incorporating these biotic interactions does not explain any additional deviance relative to the climate-only model, reinforcing the conclusion that biotic interactions are a main underlying cause of the complex and context-dependent effects on extinction found when the climatic variables are used directly as explanatory variables (Fig. 1). We thus provide experimental support to earlier work that implicated species’ interactions as the underlying process of climate change responses, especially at the lagging edges of species ranges, where extinction is the main process under anthropogenic climate change (35, 63). The ability of biotic interactions to account for context dependency of colonization was significant but weaker, consistent with the overall weaker effect of interactions on colonization, as discussed above.
An important implication of our results is that using a single proxy of interaction intensity, such as the biomass of vascular plant competitors used in many of the experiments testing the stress gradient hypothesis (64, 65), will not capture the full richness of mechanisms of interactions. For example, our study was conducted in a boreal oceanic region, where bryophytes constitute a significant component of the biomass and play important ecological roles. Vascular plant height could well be more important in more-temperate grassland systems, while proxies of canopy structure such as Leaf Area Index might be important in temperate forests. A better understanding of generalities as well as variation in which proxies of interactions are important in what environments could lead to greater understanding of how the nature of biotic interactions changes across environments. Such broad understanding could either be validated in detailed and necessarily more restricted field experiments on interactions, or could suggest new more general hypotheses to be tested experimentally, for example, through replicated experiments (66).
Our results also suggest two additional important directions of further research to understand the role of interactions in response both to landscape variation in ambient climate and to changes in climate. First, we need to correct the significant mismatch between the bulk of experimental studies to date, and what is actually needed to understand and to forecast community response to climate change. Rather than experiments that simply contrast intact communities with removals of all vegetation, we need experiments that explicitly disentangle roles of the key functional components of the vegetation, especially nonvascular plants or novel competitors, and that look at more subtle effects of changing interaction intensity (60). Second, we need to assess the response of multiple species to interactions at multiple life history stages or over longer time periods, to enable assessment of population and community-level consequences, rather than single species and single components of fitness as response variables (35, 37, 67–70). These more detailed experiments could then lead to developing more easily measured proxies of interactions appropriate for particular systems, as well as facilitate new generalizations. For example, which vegetation components are important competitors or facilitators in different environments, and when during the plant life history are these effects realized?
Generalizing the Biotic Rescaling Approach.
Scaling by biotic proxies of underlying processes and responses, rather than by the environmental gradients per se, allows us to compare responses to climate change across systems differing in realized niche breadths or other aspects of underlying organism−environment relationships (Fig. 1). The approaches described here are applicable to any study of ecological responses to the environment, not simply context dependencies or climate change responses. The key to rescaling gradients in biological terms is to identify appropriate proxies for relevant community processes. We focused on plant−plant interactions because of their well-documented importance in grasslands and relatively simple proxies. However, other kinds of processes and therefore biotic proxies are likely to be appropriate in other systems. For example, trophic interactions are likely important in many systems, for which proxies such as defense chemistry or tissue nutrient content proxies might be predictive (71, 72). More generally, community-level functional trait values (e.g., community-weighted means or distributions) can relate predictably to a suite of ecological processes (e.g., refs. 50 and 73). While it is now quite common to use trait-based approaches to understand individual to community-level responses to the abiotic or biotic environment, our biotic rescaling approach is rooted in an “effect traits” rather than a “response traits” model (38, 74, 75), thus exploiting opportunities to use traits as predictors of specific biotic processes in affecting the biotic response of interest.
Implications for Understanding and Forecasting Climate Change Responses.
We argue that, because biodiversity rarely responds in direct and linear ways to climate variables, analytical or forecasting models that use these climate variables directly as predictors of biodiversity responses will inevitably be complex and therefore not very general. On the other hand, explicitly incorporating the specific indirect and nonlinear biological mechanisms that link biodiversity to climate would demand impossible amounts of data. For example, multiple authors have called for incorporating species interactions into models of response to climate change (15, 17), but it is clear that detailed knowledge of species-specific interaction networks and how these change with abiotic environments can only be obtained for a very limited set of empirical case studies and study systems (17). Our approach of using biological proxies to rescale climate gradients provides an intermediate level of complexity that does not require vast amounts of data but is detailed enough to both reveal mechanisms and enhance generality in prediction.
Supplementary Material
Acknowledgments
We thank land owners for permission to set up the experiments, the Norwegian Research Council (Grants 184912 and 244525) for funding, and Eric Meineri, John Guittar, Siri L. Olsen, Joachim Töpper, and numerous other collaborators, technicians, field assistants, and students for help with data collection and comments on earlier versions of the paper.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003377117/-/DCSupplemental.
Data Availability.
The plant community, climate, and site data used in this paper are deposited in a public Open Science Framework (OSF) project, https://osf.io/8y4mk/ (43). All relevant study data and metadata can be found in the article, OSF, and SI Appendix.
References
- 1.Pacifici M.et al., Assessing species vulnerability to climate change. Nat. Clim. Change 5, 215–224 (2015). [Google Scholar]
- 2.Scheffers B. R.et al., The broad footprint of climate change from genes to biomes to people. Science 354, aaf7671 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Steinbauer M. J.et al., Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556, 231–234 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Cahill A. E.et al., Causes of warm-edge range limits: Systematic review, proximate factors and implications for climate change. J. Biogeogr. 41, 429–442 (2014). [Google Scholar]
- 5.Grytnes J.-A.et al., Identifying the driving factors behind observed elevational range shifts on European mountains. Glob. Ecol. Biogeogr. 23, 876–884 (2014). [Google Scholar]
- 6.Lenoir J., Svenning J.-C., Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 38, 15–28 (2015). [Google Scholar]
- 7.Klanderud K., Birks H. J. B., Recent increases in species richness and shifts in altitudinal distributions of Norwegian mountain plants. Holocene 13, 1–6 (2003). [Google Scholar]
- 8.Pauli H.et al., Recent plant diversity changes on Europe’s mountain summits. Science 336, 353–355 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Bütof A.et al., The responses of grassland plants to experimentally simulated climate change depend on land use and region. Glob. Change Biol. 18, 127–137 (2012). [Google Scholar]
- 10.Elmendorf S. C.et al., Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecol. Lett. 15, 164–175 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Bjorkman A. D.et al., Status and trends in Arctic vegetation: Evidence from experimental warming and long-term monitoring. Ambio 49, 678–692 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers-Smith I. H.et al., Complexity revealed in the greening of the Arctic. Nat. Clim. Change 10, 106–117 (2020). [Google Scholar]
- 13.Antão L. H.et al., Temperature-related biodiversity change across temperate marine and terrestrial systems. Nat. Ecol. Evol. 4, 927–933 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Dickinson M. G., Orme C. D. L., Suttle K. B., Mace G. M., Separating sensitivity from exposure in assessing extinction risk from climate change. Sci. Rep. 4, 6898 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban M. C.et al., Improving the forecast for biodiversity under climate change. Science 353, aad8466 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Bertrand R.et al., Ecological constraints increase the climatic debt in forests. Nat. Commun. 7, 12643 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisz M. S.et al., The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. Camb. Philos. Soc. 88, 15–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaarlejärvi E., Eskelinen A., Olofsson J., Herbivores rescue diversity in warming tundra by modulating trait-dependent species losses and gains. Nat. Commun. 8, 419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ettinger A., HilleRisLambers J., Competition and facilitation may lead to asymmetric range shift dynamics with climate change. Glob. Change Biol. 23, 3921–3933 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Alexander J. M., Diez J. M., Levine J. M., Novel competitors shape species’ responses to climate change. Nature 525, 515–518 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Elmendorf S. C.et al., Plot-scale evidence of tundra vegetation change and links to recent summer warming. Nat. Clim. Change 2, 453–457 (2012). [Google Scholar]
- 22.Bindoff N. L., et al., “Detection and attribution of climate change: From global to regional” in Climate Change 2013: The Physical Science Basis, Stocker T. F., et al., Eds. (Cambridge University Press, 2013), pp. 867−952. [Google Scholar]
- 23.Pachauri R. K., et al., Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, (Intergovernmental Panel on Climate Change, 2014). [Google Scholar]
- 24.Wang N.et al., “Effects of climate warming on carbon fluxes in grasslands—A global meta-analysis” in Glob. Change Biol., (2019), Vol. 25, pp. 1839–1851. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z., Dijkstra P., Koch G. W., Peñuelas J., Hungate B. A., Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Change Biol. 17, 927–942 (2011). [Google Scholar]
- 26.Grime J. P., Competitive exclusion in herbaceous vegetation. Nature 242, 344–347 (1973). [Google Scholar]
- 27.Michalet R.et al., Do biotic interactions shape both sides of the humped-back model of species richness in plant communities? Ecol. Lett. 9, 767–773 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Bakker E. S., Ritchie M. E., Olff H., Milchunas D. G., Knops J. M. H., Herbivore impact on grassland plant diversity depends on habitat productivity and herbivore size. Ecol. Lett. 9, 780–788 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Drenovsky R. E.et al., A functional trait perspective on plant invasion. Ann. Bot. 110, 141–153 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliver T. H.et al., Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 30, 673–684 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Alexander J. M., Experiments link competition and climate change responses. J. Veg. Sci. 27, 217–218 (2016). [Google Scholar]
- 32.Hanssen-Bauer I., et al. , “Climate in Norway 2100—A knowledge base for climate adaptation” (Report 1-2017, The Norwegian Centre for Climate Services, 2017).
- 33.Bertrand R.et al., Changes in plant community composition lag behind climate warming in lowland forests. Nature 479, 517–520 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Dullinger S.et al., Extinction debt of high-mountain plants under twenty-first-century climate change. Nat. Clim. Change 2, 619–622 (2012). [Google Scholar]
- 35.Olsen S. L., Töpper J. P., Skarpaas O., From facilitation to competition: Temperature-diven shift in dominant plant interactions affects population dynamics in seminatural grasslands. Glob. Change Biol. 22, 1915–1926 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Klanderud K., Meineri E., Töpper J., Biotic interaction effects on seedling recruitment along bioclimatic gradients: testing the stress-gradient hypothesis. J. Veg. Sci. 28, 347–356 (2017). [Google Scholar]
- 37.Töpper J. P.et al., The devil is in the detail: Nonadditive and context-dependent plant population responses to increasing temperature and precipitation. Glob. Change Biol. 24, 4657–4666 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Meineri E., Klanderud K., Guittar J., Goldberg D. E., Vandvik V., Functional traits, not productivity, predict alpine plant community openness to seedling recruitment under climatic warming. Oikos 129, 13–23 (2019). [Google Scholar]
- 39.Soudzilovskaia N. A.et al., How do bryophytes govern generative recruitment of vascular plants? New Phytol. 190, 1019–1031 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Elumeeva T. G., Aksenova A. A., Onipchenko V. G., Werger M. J. A., Effects of herbaceous plant functional groups on the dynamics and structure of an alpine lichen heath: The results of a removal experiment. Plant Ecol. 219, 1435–1447 (2018). [Google Scholar]
- 41.Klanderud K., Vandvik V., Goldberg D., The importance of biotic vs. abiotic drivers of local plant community composition along regional bioclimatic gradients. PLoS One 10, e0130205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lid J., Lid D. T., Norsk Flora (Norwegian Flora), (Samlaget, Oslo, Norway, 2005). [Google Scholar]
- 43.Vandvik V., et al. , Data for: Biotic rescaling reveals importance of species interactions for variation in biodiversity responses to climate change (Vandvik et al. 2020). Open Science Foundation (OSF). https://osf.io/8y4mk/. Deposited 7 August 2020. [DOI] [PMC free article] [PubMed]
- 44.Ter Braak C. J. F., The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetatio 69, 69–77 (1987). [Google Scholar]
- 45.McCullagh J. A., Nelder P., Generalized Linear Models, (Chapman and Hall, 1989). [Google Scholar]
- 46.Zuur A., Ieno E. N., Walker N., Saveliev A. A., Smith G. M., Mixed Effects Models and Extensions in Ecology with R, (Springer Science & Business Media, 2009). [Google Scholar]
- 47.Bates D., Mächler M., Bolker B., Walker S.. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 48.Akaike H., New look at statistical-model identification. IEEE Trans. Automat. Contr. AC19, 716–723 (1974). [Google Scholar]
- 49.Pérez-Harguindeguy N.et al., New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234 (2013). [Google Scholar]
- 50.Díaz S.et al., The global spectrum of plant form and function. Nature 529, 167–171 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Cornelissen J. H. C., Lang S. I., Soudzilovskaia N. A., During H. J., Comparative cryptogam ecology: A review of bryophyte and lichen traits that drive biogeochemistry. Ann. Bot. 99, 987–1001 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lett S., Nilsson M.-C., Wardle D. A., Dorrepaal E., Bryophyte traits explain climate-warming effects on tree seedling establishment. J. Ecol. 105, 496–506 (2017). [Google Scholar]
- 53.Guittar J., Goldberg D., Klanderud K., Telford R. J., Vandvik V., Can trait patterns along gradients predict plant community responses to climate change? Ecology 97, 2791–2801 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Alexander J. M.et al., Lags in the response of mountain plant communities to climate change. Glob. Change Biol. 24, 563–579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.HilleRisLambers J., Harsch M. A., Ettinger A. K., Ford K. R., Theobald E. J., How will biotic interactions influence climate change-induced range shifts? Ann. N. Y. Acad. Sci. 1297, 112–125 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Sundqvist M. K.et al., Responses of tundra plant community carbon flux to experimental warming, dominant species removal and elevation. Funct. Ecol. 34, 1497–1506 (2020). [Google Scholar]
- 57.Vandvik V., Elven R., Töpper J., Seedling recruitment in subalpine grassland forbs: Predicting field regeneration behaviour from lab germination responses. Botany 95, 73–88 (2017). [Google Scholar]
- 58.Tingstad L., Olsen S. L., Klanderud K., Vandvik V., Ohlson M., Temperature, precipitation and biotic interactions as determinants of tree seedling recruitment across the tree line ecotone. Oecologia 179, 599–608 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Meineri E., Spindelböck J., Vandvik V., Seedling emergence responds to both seed source and recruitment site climates: A climate change experiment combining transplant and gradient approaches. Plant Ecol. 214, 607–619 (2013). [Google Scholar]
- 60.Goldberg D. E., Scheiner S. M., “ANOVA and ANCOVA: field competition experiments” in Design and Analysis of Ecological Experiments, Scheiner S., Gurevitch J., Eds. (Chapman & Hall, 2001), pp. 69–93. [Google Scholar]
- 61.Callaway R. M.et al., Positive interactions among alpine plants increase with stress. Nature 417, 844–848 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Choler P., Michalet R., Callaway R. M., Facilitation and competition on gradients in alpine plant communities. Ecology 82, 3295–3308 (2001). [Google Scholar]
- 63.He Q., Bertness M. D., Altieri A. H., Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 16, 695–706 (2013). [DOI] [PubMed] [Google Scholar]
- 64.le Roux P. C., McGeoch M. A., Interaction intensity and importance along two stress gradients: Adding shape to the stress-gradient hypothesis. Oecologia 162, 733–745 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Armas C., Rodríguez-Echeverría S., Pugnaire F. I., A field test of the stress-gradient hypothesis along an aridity gradient: Facilitation and the stress-gradient hypothesis. J. Veg. Sci. 22, 818–827 (2011). [Google Scholar]
- 66.Alexander J. M., Diez J. M., Hart S. P., Levine J. M., When climate reshuffles competitors: A call for experimental macroecology. Trends Ecol. Evol. 31, 831–841 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levine J. M., HilleRisLambers J., The importance of niches for the maintenance of species diversity. Nature 461, 254–257 (2009). [DOI] [PubMed] [Google Scholar]
- 68.Adler P. B., Ellner S. P., Levine J. M., Coexistence of perennial plants: An embarrassment of niches. Ecol. Lett. 13, 1019–1029 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Chu C., Adler P. B., Large niche differences emerge at the recruitment stage to stabilize grassland coexistence. Ecol. Monogr. 85, 373–392 (2015). [Google Scholar]
- 70.Guittar J., et al., Dispersal dynamics and local filtering vary with climate across a grassland landscape. bioRxiv:567586 (22 August 2019).
- 71.Roslin T.et al., Higher predation risk for insect prey at low latitudes and elevations. Science 356, 742–744 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Wardle D. A.et al., Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633 (2004). [DOI] [PubMed] [Google Scholar]
- 73.de Bello F.et al., Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 19, 2873–2893 (2010). [Google Scholar]
- 74.Lavorel S., Garnier E., Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 16, 545–556 (2002). [Google Scholar]
- 75.Suding K. N., Lavorel S., Chapin F. S., Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Change Biol. 14, 1125–1140 (2008). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The plant community, climate, and site data used in this paper are deposited in a public Open Science Framework (OSF) project, https://osf.io/8y4mk/ (43). All relevant study data and metadata can be found in the article, OSF, and SI Appendix.