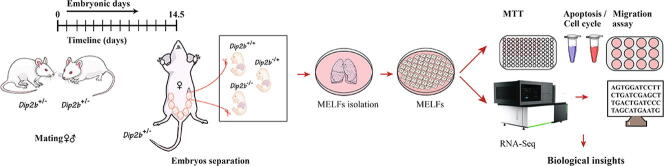
Graphical abstract
Keywords: Dip2B knockout, MELFs, Transcriptome, DEG, Gene ontology, KEGG
Abstract
Disco-interacting protein 2 homolog B (Dip2B) is a member of Dip2 family encoded by Dip2b gene. Dip2B has been reported to regulate murine epithelial KIT+ progenitor cell expansion and differentiation epigenetically via exosomal miRNA targeting during salivary gland organogenesis. However, its molecular functions, cellular activities and biological process remain unstudied. Here, we investigated the transcriptome of Dip2B-deficient mouse embryonic lung fibroblasts (MELFs) isolated from E14.5 embryos by RNA-Seq. Expression profiling identified 1369 and 1104 differentially expressed genes (DEGs) from Dip2b−/− and Dip2b+/− MELFs in comparisons to wild-type (Dip2b+/+). Functional clustering of DEGs revealed that many gene ontology terms belong to membrane activities such as ‘integral component of plasma membrane’, and ‘ion channel activity’, suggesting possible roles of Dip2B in membrane integrity and membrane function. KEGG pathway analysis revealed that multiple metabolic pathways are affected in Dip2b−/− and Dip2b+/− when compared to Dip2b+/+ MELFs. These include ‘protein digestion and absorption’, ‘pancreatic secretion’ and ‘steroid hormone synthesis pathway’. These results suggest that Dip2B may play important roles in metabolism. Molecular function analysis shows transcription factors including Hox-genes, bHLH-genes, and Forkhead-genes are significantly down-regulated in Dip2b−/− MELFs. These genes are critical in embryo development and cell differentiation. In addition, Dip2B-deficient MELFs demonstrated a reduction in cell proliferation and migration, and an increase in apoptosis. All results indicate that Dip2B plays multiple roles in cell proliferation, migration and apoptosis during embryogenesis and may participate in control of metabolism. This study provides valuable information for further understanding of the function and regulatory mechanisms of Dip2B.
1. Introduction
Disco gene was identified as a transcription factor with two C2H2 type zinc finger domains that involve neuronal connection in visual system of Drosophila melanogaster [1], [2]. By using yeast two-hybrid system, Mukhopadhyay et al. [3] identified a protein that interacts with Disco and named disco-interacting protein 2 (Dip2). Dip2 is highly conserved from insects to mammals and evolved into three different proteins, DIP2A, DIP2B and DIP2C in mammals [3]. Based on amino acid sequences, Dip2B has three putative functional domains, a binding domain for the transcriptional regulator DMAP1 (DNMT1-associated protein 1), an AMP-binding domain and an adenylate-forming domain [4]. Study suggests that Dip2B may play important roles in DNA methylation and metabolism. Dip2B may epigenetically control cell proliferation and differentiation through DNA methylation [4]. Dip2B deficiency has been associated with mental retardation and developmental delay [4], [5]. A small RNA miR-133b-3p can down-regulate Dip2B and epigenetically repress genes for KIT+K5 progenitor cell expansion [6]. A correlation in methylation status between colorectal cancer and aberrant miR-133b expression was also reported [7]. However, the mechanism underlining most of Dip2B’s roles including on cell proliferation, cell differentiation are still not clear.
RNA sequencing (RNA-Seq) takes advantage of the second-generation sequencing technology and is a powerful approach for studying global gene expression profile in a particular cell or tissue. It helps us to understand the regulatory network of genes and pathways [8], [9]. Fibroblasts cells were identified as ubiquitous mesenchymal cells that play many essential roles including tissue repair and wound healing [10]. Embryonic fibroblasts are highly diversified and have potential to differentiate into different cell types. Mouse embryonic lung fibroblasts (MELFs) cells from genetically manipulated mouse models have been used to study the molecular mechanism of genes and their regulatory networks, especially those genes that their ablations resulted in mouse prenatal lethality. To explore the potential role of Dip2B, MELFs were isolated from embryos of homozygous knockout (Dip2b−/−), heterozygous (Dip2b+/−) and wild type (Dip2b+/+) at E14.5. Genes and pathways under Dip2B regulation were investigated.
2. Materials and methods
2.1. Animals
Mouse study has been approved by Institutional Animal Care and Use Committee for Animal Experimental Ethics Committee of Northeast Normal University with approval number of (NENU/IACUC, AP2018011) and carried out in accordance with the Guide for Care and Use of Laboratory Animals of National Institutes of Health as well. Mice were housed in a pathogen-free facility in Northeast Normal University with temperature at 21 ± 1 °C, humidity 30–60%, 12:12 light/dark cycles and free access to water and food.
2.2. Isolation and culture of lung MELFs
MELFs were isolated from mouse embryos obtained by inter-crossing of Dip2b+/− transgenic mice. Dip2b+/− pregnant mouse at E14.5 day post-coitus (p.c.) was euthanized by cervical dislocation and soaked in 70% ethanol for 5 min, then the uterine horns were dissected out and rinsed in 70% EtOH. Uterine horns were then transferred into petri dish and the embryos were separated individually. Visceral organs of each embryo were removed except lungs. Lungs were washed in 1× PBS and placed in a new Petri dish. Lung tissues were gently minced using a sterile razor blade till possible to pipette. The tissues were then incubated with 0.25% trypsin (Sigma Aldrich) at 37 °C. Trypsin was inactivated 15 min later by adding Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Shanghai, China) containing 10% fetal bovine serum (Sigma, St Louis, USA) and 1% penicillin/streptomycin (Invitrogen Life Technologies, USA). Plates were coated with 0.2% gelatin (Gelatin from bovine skin, Type B, Sigma) for 2 h. The cells were then cultured at 37 °C in a humidified incubator with 5% CO2.
2.3. Hematoxylin and eosin staining (H&E)
Mice embryos from E19.5 were dissected out from uterus and fixed with 4% paraformaldehyde (PFA) (Sigma-Aldrich; EMD Millipore) for overnight at 4 °C. Fixed tissues were then washed three times in 1x PBS and processed in 70% ethanol. Tissues were dehydrated with a series of graded alcohols, cleared in xylene, and embedded in paraffin. Six-µm thick sections were obtained, mounted on glass slides and stained with Hematoxylin and eosin using standard protocol. Images were taken using microscope (Olympus, Tokyo, Japan).
2.4. Cell proliferation assay by MTT
Methylthiazolyldiphenyl-tetrazolium (MTT, Sigma) test was performed on Dip2b−/−, Dip2b+/− and Dip2b+/+ MELFs to measure the proliferation and cell viability. MELFs were detached in 0.25% trypsin/EDTA and seeded into 96-well plates with 5 × 103 cells per well in 200 μl DMEM containing 10% FBS. Cells were incubated at 37 °C and 5% CO2 for 24 h and allowed to grow to 70–80% confluence. Then 20 μl of MTT (5 mg/ml in 1× PBS) was added to each well and incubated for 4hrs. The medium was discarded and 100 μl of dimethyl sulfoxide (DMSO) added to each well. Optical density was analyzed at 490 nm using (BioTek Instruments, Winooski, VT). A total of 4 replicate for each sample was prepared and experiment repeated 3 times.
2.5. Cell apoptosis analysis by FITC-Annexin-V and PI
Apoptosis was detected using FITC-Annexin-V and PI staining kit (Cat #630109, Takara, Japan) according to manufacturer’s instructions. Briefly, Dip2b−/−, Dip2b−/+ and WT MELFs were seeded in 6-well plates at a concentration of 3 × 105 cells/well. MELFs were trypsinized and washed twice with 1X PBS. A 5 μl Annexin V-FITC and 10 μl propidium iodide (PI) at 50 μg/ml in 1× binding buffer (10 mM HEPES/pH 7.4, 140 mM NaOH, 2.5 mM CaCl2) were added for 15 min at room temperature in the dark. Apoptotic cells were analyzed using a Becton-Dickinson FACScan cytofluorometer (Mansfield, MA). Both early (Annexin V-positive, PI negative) and late (Annexin V-positive and PI-positive) were considered as apoptotic cells.
2.6. Cell cycle analysis
Dip2b+/+, Dip2b+/−and Dip2b−/− MELFs were seeded in 6-well plates at a density of 2 × 105 cells/well. Cells were trypsinized and washed twice with 1× PBS. Then cells were fixed with 70% ice-cold EtOH overnight. Fixed cells were treated with RNase (200 μg/ml) for 2hrs at RT, followed by staining with 500 μl PI (50 μg/ml) (Cat#630109, Takara, Japan). Cells were transferred to FACS tubes and incubated for 30 min at RT in dark. Afterwards, cells were subjected to FACS analysis using Caliber flow cytometer (Becton Dickinson, USA). Dead cells and cell debris were excluded.
2.7. Cell migration assay
Cells were grown in 6-well culture plates. Confluent monolayer was scratched using a 200 μl pipette tips and medium replaced with fresh medium to remove cell debris. Cells were allowed to grow for 48 h. Photographs were taken with an inverted microscope (Olympus, Tokyo, Japan). Digital straight lines were drawn on the borders of scratches and distance of cell growth with time was recorded.
2.8. Preparation of RNA-Seq libraries
Total RNA was purified from MELFs of Dip2b+/+, Dip2b+/− and Dip2b−/− using RNAiso plus reagent (Takara, Dalian, China) according to manufacturer’s protocol. RNA concentration and quality were determined using NanoDrop 2000 (Thermo Fisher Scientific, USA) and Bioanalyzer (Agilent Technologies, USA). Messenger RNA was purified using oligo (dT)-attached magnetic beads, fragmented before cDNA Synthesis.
2.9. RNA-Seq data processing and identification of differentially expressed genes (DEGs)
Low quality reads, adaptor-only and reads with more than one unknown base (N) were removed through SOAPnuke software [11] to obtain clean reads. Q20 (%), Q30 (%) and GC content (%) were calculated. More than 1.14 Gigabyte clean reads were obtained as FASTQ format from each library. Reads were assembled into longer transcripts and mapped to reference genome using HISAT (Hierarchical Indexing for Spliced Alignment of Transcripts) [12] and Bowtie2 tool [13].
The level of transcripts was quantified and presented as paired-end RNA-Seq FPKM (Fragments Per Kilobase per Million mapped reads) normalized reads. Differentially expressed genes (DEGs) were identified by comparison of two different libraries using Possion distribution [14] and Expectation-Maximization (RSEM) softwares [15].
2.10. Gene ontology and Kyoto Encyclopedia of genes and genome pathways analysis
DEGs with FDR of ≤0.01 and absolute value of FC ≥ 2 (Two-fold change) were considered significant for further analysis. Gene ontology (GO) annotation enrichment analysis of DEGs was implemented using GOseq R package software and estimated by hypergeometric test. DEGs were also used to identify the enriched Kyoto Encyclopedia of Genes and Genome (KEGG) pathways [16].
2.11. Validation of RNA-Seq results by quantitative real-time PCR (qPCR)
One μg of total RNA was reverse-transcribed into first-strand complementary DNA (cDNA) with Prime Script RT Reagent Kit (Perfect Real Time, TaKaRa, Dalian, China) according to the manufacturer’s instructions. Quantitative real-time PCR (qPCR) was performed with 50 ng of cDNA using One-Step SYBR PrimeScript™ RT-PCR kit (Takara, Dalian, China). All reactions were performed in triplicate. All primers were initially evaluated for efficiency using relative standard curve and electrophoresis on gel.
2.12. Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.01 (GraphPad Software Inc). Significant differences between groups were evaluated using Student’s t test. P-values were two-sided and P-values <0.05 was considered statistically significant.
3. Results and discussion
3.1. Dip2b mRNA expression level of Dip2B-deficient MELFs
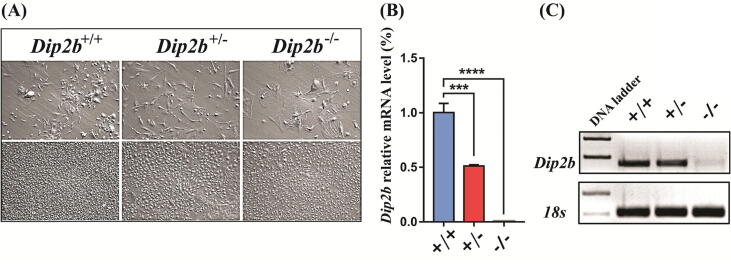
Since Dip2b−/− mice dies postnatally and lung development seems the major cause (Supplementary Fig. 1), intercrosses of Dip2b+/− mice were used to prepare MELFs and total RNAs from all three genotypes, Dip2b−/−, Dip2b+/− and Dip2b+/+at E14.5 (Fig. 1A). Dip2b mRNA expression in MELFs was confirmed by quantitative real-time PCR (qPCR) (Fig. 1B, C). Results show expected decrease of Dip2b mRNA expression. The mRNAs from MELFs were prepared for RNA-Seq.
Fig. 1.
MELF cell isolation and Dip2b mRNA expression analysis by qPCR (A) Cell images of MELF cultures at low (Top penal) and high (Bottom panel) density. (B) Relative expression levels of Dip2b mRNA in MELFs by qPCR. (C) Gel electrophoresis image showing PCR products.
3.2. Gene expression profiling of MELFs under Dip2B
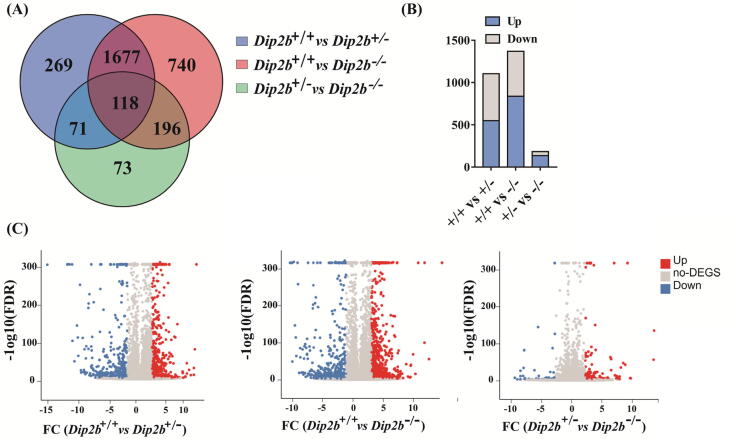
To study the potential biological role of Dip2B, three libraries were generated from Dip2b−/−, Dip2b+/− and Dip2b+/+MELFs and analyzed on BGISEQ-500 platform. The raw data of RNA-Seq is accessible at the Sequence Read Archive (SRA) database in NCBI with the following link (https://www.ncbi.nlm.nih.gov/bioproject/ PRJNA647133/) under the accession number PRJNA647133. Clean reads were mapped to Mus musculus reference genome (GRCm38.p6/NCBI, GCF_000001635.26). As shown in Table 1, ~97% of total mapping was acquired and >80% was uniquely mapped, indicating the reliability of sequencing data. Table 2 is summarizing the sequencing reads among samples. Normalized FPKM was calculated and expression level distribution shown in (Supplementary Fig. 2A). The overlapped genes were identified 1677 and 118 genes differentially expressed in both homozygous and heterozygous MELFs. 269 unique DEGs were expressed in Dip2b+/−, 740 unique DEGs were expressed in Dip2b−/− vs to Dip2b+/+ and 73 unique DEGs were expressed in Dip2b+/− vs to Dip2b−/− MELFs respectively (Fig. 2A).
Table 1.
Summary of sequencing data processing.
| Sample | Total Mapping (%) | Uniquely Mapping (%) |
|---|---|---|
| Dip2b+/+ | 97.06 | 79.67 |
| Dip2b+/− | 97.08 | 80.82 |
| Dip2b−/− | 96.98 | 81.42 |
Table 2.
Statistical summary of filtered reads.
| Sample | Total Raw Reads (M) | Total Clean Reads (M) | Total Clean Bases (Gb) | Clean Reads Q20 | Clean Reads Q30 | Clean Reads Ratio |
|---|---|---|---|---|---|---|
| Dip2b+/+ | 23.38 | 22.7 | 1.14 | 99.12 | 96.3 | 97.1 |
| Dip2b+/− | 23.51 | 22.92 | 1.15 | 99.19 | 96.75 | 97.5 |
| Dip2b−/− | 23.44 | 22.81 | 1.14 | 99.1 | 96.55 | 97.3 |
Fig. 2.
Overview of gene expression profiling. (A) Venn diagram showing unique and overlapping DEGs between heterozygous and homozygous MELFs (FC ≥ 1 and FDR ≤ 0.001). (B) Number of up- and down-regulated DEGs in Dip2b−/− and Dip2b+/− vs to Dip2b+/+ (C) Volcano plots highlighting significant DEG among three comparative samples. Each dot in plot corresponds to one differentially expressed gene, the y-axis represents −log10 (FDR) and the x-axis displays the differences of FC values in samples. Blue and red dots represent up- and down-regulated differentially expressed genes, whereas gray dots indicate genes with no change. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Comparisons between Dip2b−/− vs Dip2b+/+ and Dip2b+/− vs Dip2b+/+ identified 1369 and 1104 differentially expressed genes (DEGs) based on fold change ≥2 and adjusted FDR ≤0.01. Among them, 839 and 549 are significantly up- and 530 and 555 significantly down-regulated (Fig. 2B). Volcano plot and heatmap are used to show the significant DEGs (Fig. 2C and Supplementary Fig. 2B). Fifty of the most up- and down-regulated DEGs are listed in Table 3 and Supplementary Table 1. Among them, six genes Ear2, Entpd4, Fpr2, Hist2h3c1, Zfp967 and Gm14296 are the most significantly up-regulated while 6 genes Hmga1b, Havcr1, Pax2, Pax8, Hoxa10, Hoxa11 and Hoxac10 are the most significantly down-regulated.
Table 3.
The 50 most differentially expressed genes between Dip2b+/+ vs Dip2b−/− (Fold change ≥ 2, FDR < 0.01).
| Up-regulated genes | FC ≥ 2 | FDR | P value | Down-regulated genes | FC < 2 | FDR | P value |
|---|---|---|---|---|---|---|---|
| Ear1 | 13.11374217 | 0 | 0 | Pax8 | −10.82813648 | 2.23E-12 | 4.84E-13 |
| Ear10 | 11.05934446 | 5.87E-53 | 4.20E-54 | Pax2 | −10.60316268 | 6.36E-13 | 1.34E-13 |
| Ear2 | 10.37424254 | 0 | 0 | Havcr1 | −10.48850965 | 1.70E-104 | 6.57E-106 |
| Fpr2 | 10.35974956 | 7.82E-95 | 3.25E-96 | Sprr2f | −10.46147945 | 1.11E-46 | 8.74E-48 |
| Eno1b | 9.370435927 | 0 | 0 | Hoxa10 | −10.24317398 | 1.20E-302 | 1.60E-304 |
| Ndufa11b | 8.939579214 | 7.79E-15 | 1.50E-15 | Hoxa11 | −10.05799172 | 1.94E-139 | 5.62E-141 |
| Entpd4 | 8.569855608 | 2.50E-65 | 1.47E-66 | Hoxd10 | −9.920352855 | 2.22E-108 | 8.23E-110 |
| Sell | 8.22881869 | 3.05E-38 | 2.83E-39 | ccdc198 | −9.840777924 | 6.09E-61 | 3.81E-62 |
| Zfp967 | 8.184875343 | 6.81E-36 | 6.67E-37 | Hmga1b | −9.63828506 | 0 | 0 |
| Hamp2 | 8.159871337 | 9.74E-07 | 3.02E-07 | Hoxc10 | −9.564784619 | 1.18E-245 | 1.93E-247 |
| Wfdc21 | 8.06608919 | 1.89E-06 | 5.98E-07 | Tff1 | −9.398743692 | 2.19E-17 | 3.80E-18 |
| Stfa1 | 8.027905997 | 7.06E-06 | 2.34E-06 | Hoxc8 | −9.221587121 | 1.08E-80 | 5.25E-82 |
| Hist2h3c1 | 7.768184325 | 6.95E-08 | 1.97E-08 | Hoxc9 | −9.204571144 | 1.32E-57 | 8.74E-59 |
| Gm14296 | 7.491853096 | 5.23E-40 | 4.70E-41 | Hist2h3c2 | −9.022367813 | 2.75E-18 | 4.60E-19 |
| Hist2h2aa2 | 7 | 3.55E-04 | 1.41E-04 | Clca1 | −8.854868383 | 1.19E-77 | 5.97E-79 |
| Adora3 | 6.845490051 | 2.05E-15 | 3.83E-16 | Reg1 | −8.764871591 | 1.38E-18 | 2.28E-19 |
| Evi2 | 6.584962501 | 1.53E-21 | 2.27E-22 | Tff2 | −8.717676423 | 3.48E-13 | 7.19E-14 |
| Mstn | 6.584962501 | 7.79E-15 | 1.50E-15 | Cyp4a12b | −8.632995197 | 2.87E-54 | 2.01E-55 |
| Gm39743 | 6.539158811 | 1.75E-10 | 4.22E-11 | Spaca7 | −8.581200582 | 8.74E-17 | 1.55E-17 |
| Ang5 | 6.539158811 | 6.80E-04 | 2.79E-04 | Hoxd11 | −8.558420713 | 1.49E-85 | 6.78E-87 |
| Ly6k | 6.491853096 | 3.55E-04 | 1.41E-04 | Gsta1 | −8.471675214 | 4.37E-17 | 7.67E-18 |
| Bmx | 6.459431619 | 3.99E-15 | 7.58E-16 | Pkp1 | −8.438791853 | 2.82E-91 | 1.21E-92 |
| Cd177 | 6.459431619 | 1.14E-13 | 2.31E-14 | Hist1h4m | −8.361943774 | 3.12E-07 | 9.29E-08 |
| Lipf | 6.303780748 | 3.65E-06 | 1.18E-06 | Gm14434 | −8.290018847 | 1.33E-81 | 6.36E-83 |
| Gm14548 | 6.28128611 | 9.11E-75 | 4.75E-76 | Hoxd9 | −8.162391329 | 1.74E-66 | 1.01E-67 |
| Gm46223 | 6.247927513 | 4.88E-09 | 1.28E-09 | Klhl1 | −8.154818109 | 2.26E-64 | 1.35E-65 |
| LOC100041057 | 6.209453366 | 3.65E-06 | 1.18E-06 | Clca3b | −8.142107057 | 6.44E-105 | 2.47E-106 |
| Ear6 | 6.189824559 | 3.90E-21 | 5.85E-22 | Spink8 | −8.06608919 | 3.34E-10 | 8.17E-11 |
| DXBay18 | 6.169925001 | 1.22E-11 | 2.75E-12 | Clec18a | −8.033423002 | 7.99E-54 | 5.63E-55 |
| Ms4a8a | 6.161887682 | 5.48E-20 | 8.53E-21 | Gm8210 | −7.990827547 | 0 | 0 |
| Gm44805 | 6.14974712 | 0.008947464 | 0.00428538 | Il24 | −7.948367232 | 1.39E-15 | 2.59E-16 |
| Tex45 | 6.132713922 | 3.28E-33 | 3.39E-34 | Prr32 | −7.77478706 | 8.75E-14 | 1.76E-14 |
| Serpinb10 | 6.125843933 | 2.50E-68 | 1.40E-69 | Malrd1 | −7.768184325 | 6.05E-85 | 2.77E-86 |
| Cyp4f18 | 6.076815597 | 7.19E-25 | 9.49E-26 | Gc | −7.707359132 | 1.36E-21 | 2.00E-22 |
| A530032D15Rik | 6.044394119 | 3.55E-04 | 1.41E-04 | Dmbt1 | −7.693486957 | 6.44E-75 | 3.35E-76 |
| LOC677525 | 6.044394119 | 3.55E-04 | 1.41E-04 | Apela | −7.607330314 | 8.46E-11 | 2.00E-11 |
| Sod3 | 6.042856338 | 0 | 0 | Hist1h3c | −7.54689446 | 7.07E-05 | 2.58E-05 |
| F13a1 | 6.005624549 | 0 | 0 | Hoxd4 | −7.459431619 | 8.27E-26 | 1.06E-26 |
| Prss16 | 5.930737338 | 1.34E-07 | 3.89E-08 | Pmp2 | −7.434628228 | 1.75E-13 | 3.56E-14 |
| Awat1 | 5.906890596 | 5.07E-05 | 1.82E-05 | Gsdmc2 | −7.400879436 | 4.25E-23 | 5.94E-24 |
| Gm36504 | 5.882643049 | 9.74E-07 | 3.02E-07 | Pitx2 | −7.309855263 | 2.70E-43 | 2.26E-44 |
| Rnase2b | 5.832890014 | 0.008942223 | 0.00428538 | Kcnj16 | −7.235216462 | 2.60E-183 | 5.72E-185 |
| 2010005H15Rik | 5.817623258 | 1.43E-22 | 2.04E-23 | Scin | −7.192292814 | 6.79E-193 | 1.41E-194 |
| Gm5796 | 5.754887502 | 1.36E-05 | 4.64E-06 | Dcdc2a | −7.144266046 | 0 | 0 |
| Gm9733 | 5.754887502 | 0.008949561 | 0.00428538 | Dclk3 | −7.14068778 | 5.49E-243 | 9.12E-245 |
| Gpr15 | 5.727920455 | 3.55E-04 | 1.41E-04 | Spns3 | −7.108524457 | 3.34E-10 | 8.17E-11 |
| Fhl5 | 5.700439718 | 0.002477977 | 0.001093626 | Dnajc19-ps | −7.098032083 | 2.70E-04 | 1.06E-04 |
| Klrb1b | 5.700439718 | 9.74E-07 | 3.02E-07 | Hoxd8 | −7.096275911 | 1.84E-82 | 8.73E-84 |
| Mcemp1 | 5.698247727 | 1.99E-110 | 7.28E-112 | Lhx1 | −7.055282436 | 5.42E-21 | 8.18E-22 |
| Sftpa1 | 5.672425342 | 6.37E-29 | 7.41E-30 | Cltrn | −7.044394119 | 1.32E-09 | 3.34E-10 |
3.3. Gene ontology (GO) analysis of Dip2b-regulated DEGs.
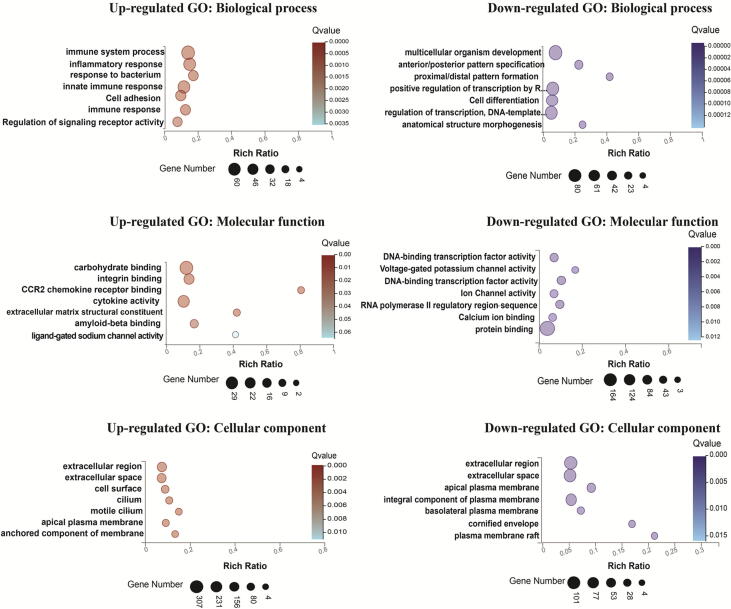
In order to identify the potential biological roles of Dip2B in embryonic stages, DEGs identified from comparison of Dip2B-deficient and WT MELFs were used for GO analysis. GO is classified into three independent categories, biological process (BP), molecular function (MF) and cellular component (CC). Each DEG could be assigned to one or more GO terms. A total of 1369 and 1104 DEGs with FC ≥ 2 and FDR < 0.01 were identified from comparisons of Dip2b−/− vs Dip2b+/+ and Dip2b+/− vs Dip2b+/+ MELFs. GO analysis found 28 BP, 17 MF and 12 CC significantly annotated (Fig. 3, Supplementary Fig. 3 and Supplementary Fig. 4). Annotated BP categories include ‘cell adhesion’, ‘cell differentiation’, ‘regulation of signaling receptor activity’, ‘multicellular organism development’. MF categories include ‘ion channel activity’, ‘ligand-gated sodium channel activity’, ‘voltage-gated potassium channel activity’, ‘extracellular matrix structural constituent’, ‘calcium ion binding’ and ‘DNA binding transcription activity’. DEGs-annotated CC categories are ‘extracellular region’, ‘integral component of plasma membrane’, ‘anchored component of plasma membrane’, ‘basolateral plasma membrane’ ‘cornified envelop’, ‘cell surface’ and ‘apical plasma membrane’ (Supplementary Table 2). Based on DAVID (Database for Annotation, Visualization and Integrated Discovery [17], [18]), the functional annotation clustering tool revealed that a list of cluster terms from BP, MF, and CC categories were mainly enriched with DEGs related to membrane structure and its activities (Supplementary Table 3). All the results suggest that Dip2B may play important roles in cell to cell interactions, membrane integrity and membrane activities which are critical for cell differentiation and function.
Fig. 3.
GO analysis. Top seven GO terms of up- and down-regulated DEGs of Dip2b+/+ vs Dip2b−/− (FC ≥ 2, FDR < 0.01).
Dip2B-deficient MELFs resulted in upregulation of 60 (Dip2b−/− vs Dip2b+/+) and 52 (Dip2b+/− vs Dip2b+/+) DEGs that are involved in immune system and inflammatory responses respectively. In immune system process, 43 genes were enriched in innate immune response, 14 genes in defense response to virus and 11 genes in adaptive immune response (Supplementary Table 4, Supplementary Table 5). In inflammatory response category, most of the genes are chemotaxis genes including (Fpr2, Fpr1, Ccr2, Cxcl15, Ccl9, Ccl6, Ccl11, Cxcl2, Ccl22, S100a8, Ccl12, Ccr5, Ccl7, Pik3cg, Ccl2, Cxcl3), 12 genes in neutrophil chemotaxis, 7 genes in Eosinophil chemotaxis, 8 genes in monocyte chemotaxis and 8 genes in lymphocyte chemotaxis (Supplementary Table 6), indicating the importance of Dip2B in regulation of cell migration in immune responses.
3.4. KEGG pathway analysis on Dip2B-regulated DEGs
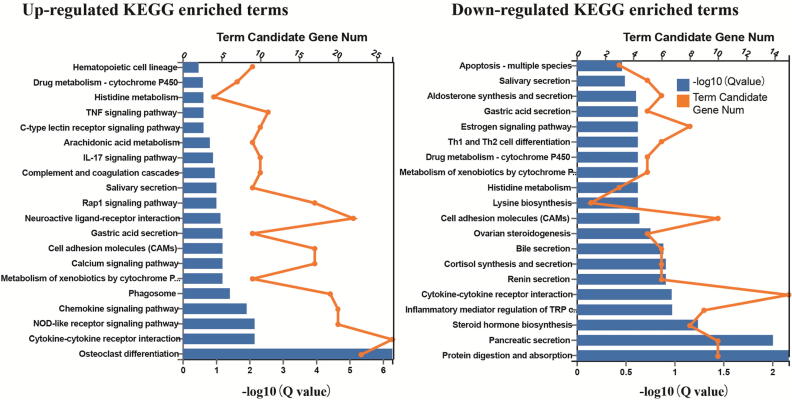
To elucidate the potential biological pathways under Dip2B regulation, KEGG pathway analysis was performed for Dip2B-regulated DEGs. KEGG classification revealed that ‘signal transduction’ and ‘immune system’ terms have the highest number of DEGs (Supplementary Fig. 5). The Q value <0.05 was considered significantly represented and KEGG pathways were enriched based on FC ≥ 2 and FDR < 0.01. Top 20 most enriched pathways between Dip2b−/− vs Dip2b+/+and Dip2b+/+ vs Dip2b+/− are shown in Fig. 4 and Supplementary Fig. 6. Among them, ‘ko04974 protein digestion and absorption’, ‘ko04924 renin secretion’, ‘ko04972 pancreatic secretion’, ‘ko04060 cytokines-cytokines receptor interaction’ and ‘ko00140 steroid hormone synthesis’ metabolism pathways are the most significantly down-regulated pathways, whereas ‘ko04380 Osteoclast differentiation’, ‘ko04621 NOD-like receptor signaling pathway’ and ‘ko04145 Phagosome’ were the most up-regulated pathways (Supplementary Table 7a and 7b). Results demonstrate that multiple pathways are under Dip2B regulation and confirms that Dip2B is important in regulating membrane activities and be involved in regulation of metabolism.
Fig. 4.
KEGG pathway analysis of DEGs from Dip2b+/+ vs Dip2b−/− MELFs. Adjusted p-values (Q-values) depicting significant enrichment (q-value < 0.05).
3.5. Hox gene family are regulated by Dip2B
Homeodomain proteins encoded by Hox genes are responsible for regulating expression of many target genes involved in cell proliferation and differentiation [19], [20], [21], [22]. These genes are also known to be involved in cancers and controlled by DNA methylation [23], [24]. Among differentially expressed transcription factors (TFs), homeobox (Hox) gene family was the most dysregulated genes in Dip2B-deficient MELFs (38 down- and 6 up-regulated genes in both heterozygous and homozygous MELFs). Results strongly suggest that Dip2B may regulate embryo development and differentiation through Hox gene expression. Dip2B contains DMAP1 binding domain and may regulate Hox gene transcription by DNA methylation [4]. Several TFs that belong to zinc finger (zf-C2H2), basic helix-loop-helix (bHLH) and Fox proteins are also significantly dysregulated. TFs with fold change ≥ 2 and FDR < 0.01 are listed in Table 4 and Supplementary Tables 8 and 9.
Table 4.
List of differentially expressed TFs between Dip2b−/− and Dip2b+/+ (FC ≥ 2, FDR < 0.01).
| TF symbol | Number of dysregulated genes | Rich Ratio | P value | Q value |
|---|---|---|---|---|
| Homeobox | 44 | 0.195556 | 9.21E-10 | 2.67E-08 |
| zf-C2H2 | 15 | 0.026978 | 1 | 1 |
| bHLH | 11 | 0.102804 | 0.235915 | 0.484594 |
| Fork_head | 10 | 0.227273 | 0.001856 | 0.026911 |
| HMG | 5 | 0.098039 | 0.39226 | 0.531165 |
| TF_bZIP | 4 | 0.076923 | 0.614627 | 0.742674 |
| Tub | 3 | 0.6 | 0.004512 | 0.043619 |
| ETS | 3 | 0.111111 | 0.372 | 0.531165 |
| AP-2 | 2 | 0.4 | 0.054668 | 0.396346 |
| ESR-like | 2 | 0.222222 | 0.159449 | 0.420366 |
| PAX | 2 | 0.222222 | 0.159449 | 0.420366 |
| SAND | 2 | 0.25 | 0.130642 | 0.420366 |
3.6. Dip2B regulates cell proliferation
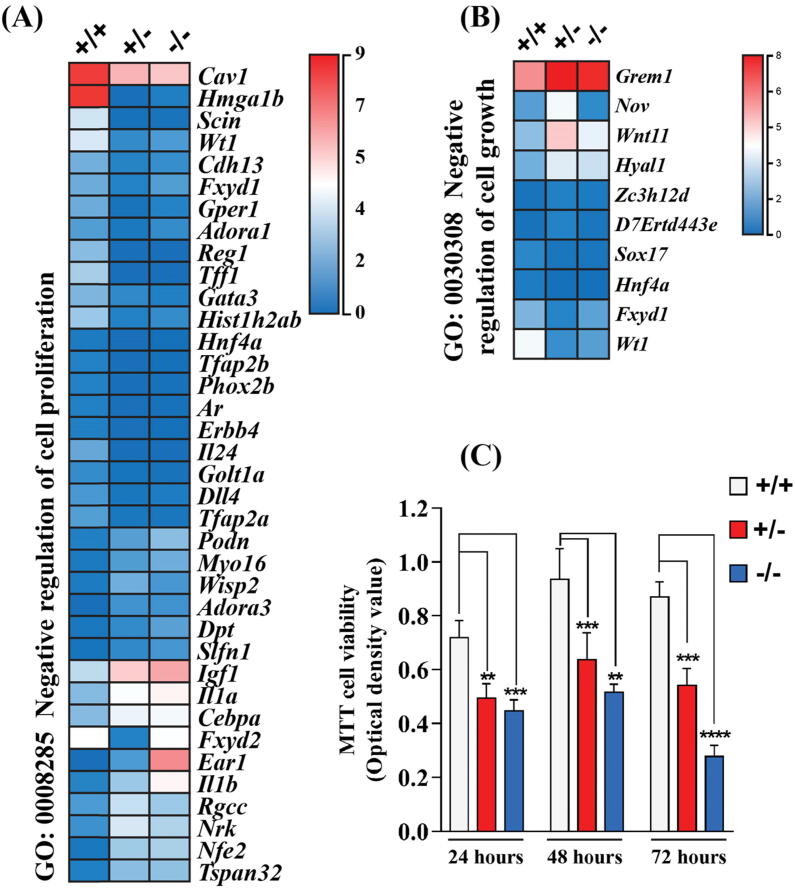
Cell proliferation is an important process during development and tissue maintenance [25]. GO analysis shows that DEGs were significantly enriched in ‘GO:0008285 ~negative regulation of cell proliferation’ and ‘GO:0030308 ~negative regulation of cell growth’, indicating Dip2B knockout reduces cell proliferation and growth (Fig. 5A, B). Genes including Adora3, Il1b, Slfn1 and Nrk [26], [27], [28], [29], [30] responsible for inhibition of cell proliferation were significantly up-regulated. To validate the role of Dip2B in cell proliferation, isolated MELFs were subjected to MTT viability assay. As shown in Fig. 5C, growth rates of MELFs from Dip2b−/− and Dip2b+/− are significantly slower than cells from Dip2b+/+ (p < 0.01), and more obvious in Dip2b−/− MELFs (p < 0.001). Result suggests that Dip2B may be a critical factor in regulation of cell proliferation.
Fig. 5.
Effect of Dip2B on cell proliferation and growth. Heatmap for DEGs associated with GO biological process terms of (A) ‘negative regulation of cell proliferation’ and (B) ‘negative regulation of cell growth’. (C) MTT viability assay of MELFs derived from Dip2b−/−, Dip2b+/− and Dip2b+/+.
3.7. Dip2B regulates apoptosis and cell cycle
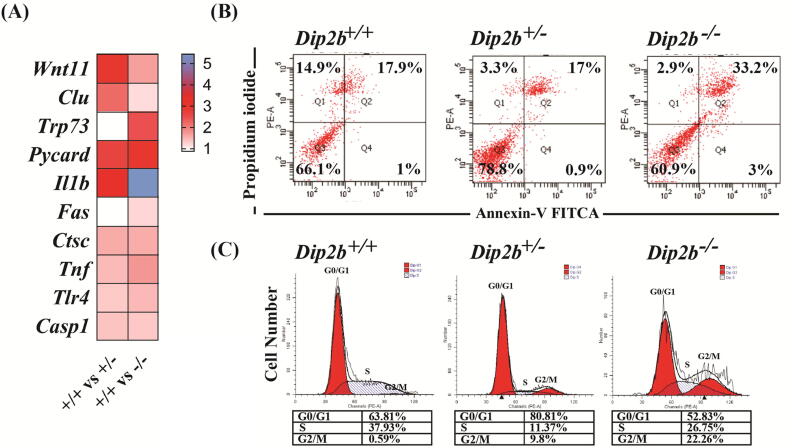
GO analysis shows that apoptosis and cell cycle are regulated by Dip2B. Several DEGs were found significantly enriched in ‘positive regulation of apoptotic process’, such as up-regulation of Wnt11, Pycard, Il1b, Trp73, Clu and Fas [27], [31], [32], [33], [34], [35]. To verify whether Dip2B affects apoptosis, cells were analyzed using Annexin V and PI staining and cell cycle by flow cytometry (Fig. 6A). Results indicate that percentage of later apoptotic MELFs is significantly increased in Dip2b−/− in comparison to Dip2b+/− heterozygous or WT MELFs. The early apoptotic proportions in Dip2b−/+ MELFs are similar to Dip2b+/+ MELFs but increased later (Fig. 6B). Dip2b−/− MELFs exhibited an increase in G2/M population in cell-cycle profiles (Fig. 6C). Results confirm that Dip2B can significantly inhibit MELF proliferation and induce apoptosis and cell cycle arrest.
Fig. 6.
Apoptosis and cell cycle analysis. (A) Heatmap for DEGs associated with GO terms ‘positive regulation of apoptotic process’. (B) Apoptotic cells measured using a Becton-Dickinson FACScan cytofluorometer. (C) Histogram of flow cytometric analysis of cell cycle showing the distribution of cell phase.
3.8. Dip2B regulates cell migration
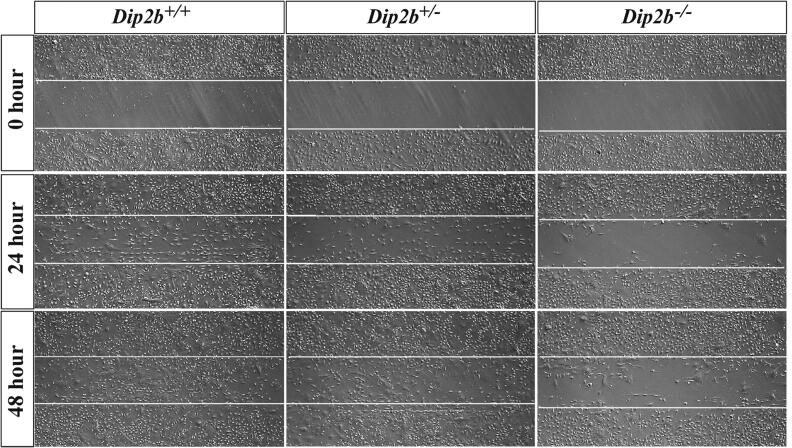
Transcriptomic data revealed that Dip2B is involved in promotion of cell migration. Knockout of Dip2B results in significantly down-regulation of Cdh2, Podxl, L1cam, Bcar1, Tdgf1 and Hbegf [36], [37], [38], [39], [40], [41] that are known for promoting cell migration (Table 5). Genes including Podn, Ptprf and Adora3 [26], [42], [43] responsible for inhibition of cell migration were up-regulated. To confirm the effect of Dip2B in cell migration, MELFs were allowed to grow to confluence and scratch wounds were made. Gap closing were recorded at different time points. Results show that Dip2b−/− MELFs was significantly slower in migration than that of Dip2b+/− and Dip2b+/+ MELFs (Fig. 7).
Table 5.
Differentially expressed genes involved in cell migration.
| Gene symbol |
Dip2b+/+ vs Dip2b+/− |
Dip2b+/+ vs Dip2b−/− |
||||
|---|---|---|---|---|---|---|
| FC ≥ 2 | FDR | P value | FC ≥ 2 | FDR | P value | |
| Cdh2 | −2.212266596 | 0 | 0 | −2.974162444 | 0 | 0 |
| Podxl | −1.835315003 | 5.00E-51 | 2.81E-52 | −2.297219108 | 2.88E-67 | 1.65E-68 |
| L1cam | −1.975442896 | 1.38E-97 | 4.16E-99 | −2.256855831 | 1.74E-115 | 6.12E-117 |
| Bcar1 | −1.223105744 | 0 | 0 | −1.223105744 | 2.66E-05 | 3.28E-06 |
| Tdgf1 | −6.794415866 | 2.73E-12 | 4.78E-13 | −6.794415866 | 2.73E-12 | 5.94E-13 |
| Vil1 | −7.658211483 | 7.92E-33 | 2.30E-37 | −6.073248982 | 7.92E-33 | 8.27E-34 |
| Adra2a | −3.195256291 | 3.47E-108 | 3.99E-85 | −4.445799753 | 3.47E-108 | 1.29E-109 |
| Erbb4 | −3.841302254 | 7.09E-24 | 5.32E-24 | −4.426264755 | 7.09E-24 | 9.66E-25 |
| Tfap2a | −3.145677455 | 6.38E-24 | 3.12E-21 | −3.660250628 | 6.38E-24 | 8.68E-25 |
| Lgr6 | −2.220729372 | 1.16E-139 | 9.02E-90 | −3.42003818 | 1.16E-139 | 3.33E-141 |
| Sema5b | −1.247927513 | 5.76E-04 | 0.0794798 | −3.247927513 | 5.76E-04 | 2.35E-04 |
| Hbegf | −2.279689532 | 0 | 0 | −2.713977039 | 0 | 0 |
| Cav1 | −2.302638712 | 0 | 0 | −2.604238401 | 0 | 0 |
| Gper1 | −4.812498225 | 1.93E-18 | 1.58E-34 | −2.379538818 | 1.93E-18 | 3.20E-19 |
| Sema3g | −2.212993723 | 2.63E-06 | 1.97E-06 | −2.350497247 | 2.63E-06 | 8.44E-07 |
| Podxl | −1.835315003 | 2.88E-67 | 2.81E-52 | −2.297219108 | 2.88E-67 | 1.65E-68 |
| Pdgfd | −2.460097222 | 6.32E-90 | 4.99E-93 | −2.242945253 | 6.32E-90 | 2.74E-91 |
| Sema6a | −1.808903709 | 2.14E-71 | 3.83E-57 | −2.191760803 | 2.14E-71 | 1.16E-72 |
| Col18a1 | −1.61403128 | 0 | 0 | −2.074086318 | 0 | 0 |
| Sema7a | −1.018378529 | 4.99E-47 | 1.49E-18 | −2.025796001 | 4.99E-47 | 3.92E-48 |
| F2rl1 | −2.152928449 | 8.34E-270 | 1.25E-299 | −2.003572503 | 8.34E-270 | 1.23E-271 |
| Fam107a | −3 | 1.56E-04 | 4.89E-05 | −1.192645078 | 0.038533376 | 0.0208544 |
| Ntrk3 | −2.237039197 | 5.97E-04 | 2.03E-04 | −1.137503524 | 0.025580458 | 0.01329282 |
| Podn | 2.162271429 | 3.41E-12 | 5.72E-13 | 3.402098444 | 3.81E-44 | 3.14E-45 |
| Ptprf | 0.567122422 | 2.89E-54 | 1.55E-55 | 1.018699606 | 1.21E-204 | 2.37E-206 |
| Adora3 | 6.906890596 | 4.77E-16 | 6.58E-17 | 6.845490051 | 2.05E-15 | 3.83E-16 |
Fig. 7.
Scratching assay for cell migration. Images were taken at 0, 24, 48hrs post-scratching.
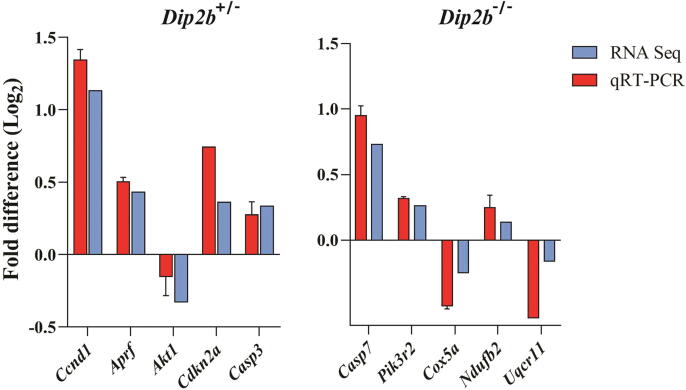
3.9. Verification of RNA-Seq results
Ten important DEGs were validated by qPCR. Overall, qPCR results are highly consistent with the RNA-Seq results (Fig. 8). Primer sequences are listed in Supplementary Table 10.
Fig. 8.
Gene expression validation of DEGs from RNA-Seq by qPCR.
4. Conclusions
In this study, MELFs were isolated from Dip2b knockout mouse embryos at E14.5. Dip2b+/+, Dip2b+/− and Dip2b−/− MELFs were examined for genome-wide gene expression. DEGs were identified and analyzed by GO and KEGG for Dip2B-regulated bioprocesses and pathways. The most enriched bioprocesses and pathways were confirmed that include cell proliferation, cell cycle, cell apoptosis and cell migration.
GO analysis showed that DEGs were mostly annotated to membrane-related GO terms including ‘integral component of plasma membrane’, ‘anchored component of plasma membrane’, ‘basolateral plasma membrane’, ‘ion channel activity’, and ‘voltage-gated potassium channel activity, whereas KEGG pathways enriched DEGs are related to metabolism including protein digestion and absorption’, ‘renin secretion’, ‘pancreatic secretion’, and ‘steroid hormone synthesis’. Results demonstrate that Dip2B promotes cell proliferation, increases cell migration and inhibits cell apoptosis. Knockout Dip2B leads to cell arrest at G2/M phase. Results indicate that Dip2B is involved in multiple biological processes and pathways. Dip2B may regulate many important biologic functions that are highly correlated with development, differentiation and morphogenesis. Dip2B caused upregulation of multiple genes involves in innate and adaptive immune response and inflammatory response. Our analysis has identified upregulation of potential leukocyte chemotaxis genes (Ccl9, Ccl6, Ccl11, Ccl22, Ccl12, Ccr5, Ccl7, Ccl2, Cxcl2, Cxcl3, Cxcl15), highlighting the potential role of Dip2B in regulation of the immune cell mobilization and inflammatory responses. A number of transcription factors such as homeobox (Hox) gene family, zinc finger (zf-C2H2), basic helix-loop-helix (bHLH) and Fox proteins were found to be significantly dysregulated. The most dysregulated TFs was Homeobox (Hox) genes suggesting that Dip2B may regulate cell differentiation and embryo development. Together, these information are valuable for further deciphering Dip2B roles in development and disease.
Acknowledgments
Acknowledgments
We are thankful to Ms. Huiyan Wu for mouse colony management.
Funding
This work is partially supported by Natural Science Foundation of Jilin Province (20160101344JC and 20200201127JC), National Natural Science Foundation of China (81270953) and Science and Technology Project of Jilin Provincial Education Department (JJKH20180023KJ). The funders had no roles in any decision to publish or manuscript preparation.
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2020.08.030.
Contributor Information
Xuechao Feng, Email: fengxc997@nenu.edu.cn.
Yaowu Zheng, Email: zhengyw442@nenu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Steller H., Fischbach K.F., Rubin G.M. disconnected: A locus required for neuronal pathway formation in the visual system of drosophila. Cell. 1987 doi: 10.1016/0092-8674(87)90180-2. [DOI] [PubMed] [Google Scholar]
- 2.Campos A.R., Lee K.J., Steller H. Establishment of neuronal connectivity during development of the Drosophila larval visual system. J Neurobiol. 1995 doi: 10.1002/neu.480280305. [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay M., Pelka P., DeSousa D., Kablar B., Schindler A., Rudnicki M.A. Cloning, genomic organization and expression pattern of a novel Drosophila gene, the disco-interacting protein 2 (dip2), and its murine homolog. Gene. 2002 doi: 10.1016/S0378-1119(02)00694-7. [DOI] [PubMed] [Google Scholar]
- 4.Winnepenninckx B., Debacker K., Ramsay J., Smeets D., Smits A., FitzPatrick D.R. CGG-repeat expansion in the DIP2B gene is associated with the fragile site FRA12A on chromosome 12q13.1. Am J Hum Genet. 2007 doi: 10.1086/510800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debacker K., Frank K.R. Fragile sites and human disease. Hum Mol Genet. 2007 doi: 10.1093/hmg/ddm136. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T., Lombaert I.M.A., Hauser B.R., Patel V.N., Hoffman M.P. Exosomal MicroRNA transport from salivary mesenchyme regulates epithelial progenitor expansion during organogenesis. Dev Cell. 2017 doi: 10.1016/j.devcel.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv L., Zhou J., Lin C., Hu G., Yi L., Du J. DNA methylation is involved in the aberrant expression of miR-133b in colorectal cancer cells. Oncol Lett. 2015 doi: 10.3892/ol.2015.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozsolak F., Milos P.M. RNA sequencing: Advances, challenges and opportunities. Nat Rev Genet. 2011 doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickrell J.K., Marioni J.C., Pai A.A., Degner J.F., Engelhardt B.E., Nkadori E. Understanding mechanisms underlying human gene expression variation with RNA sequencing. Nature. 2010 doi: 10.1038/nature08872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal P.K., Sassi S., Lan L., Au P., Halvorsen S.C., Fukumura D. Mouse embryonic fibroblasts exhibit extensive developmental and phenotypic diversity. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1522401112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cock P.J.A., Fields C.J., Goto N., Heuer M.L., Rice P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkp1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D., Langmead B., Salzberg S.L. HISAT: A fast spliced aligner with low memory requirements. Nat Methods. 2015 doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012 doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audic S., Claverie J.M. The significance of digital gene expression profiles. Genome Res. 1997 doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- 15.Li B., Dewey C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011 doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanehisa M., Araki M., Goto S., Hattori M., Hirakawa M., Itoh M. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang D.W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009 doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 19.Boncinelli E. Homeobox genes and disease. Curr Opin Genet Dev. 1997 doi: 10.1016/S0959-437X(97)80146-3. [DOI] [PubMed] [Google Scholar]
- 20.Cillo C., Cantile M., Faiella A., Boncinelli E. Homeobox genes in normal and malignant cells. J Cell Physiol. 2001 doi: 10.1002/jcp.1115. [DOI] [PubMed] [Google Scholar]
- 21.Holland P.W.H., Booth H.A.F., Bruford E.A. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007 doi: 10.1186/1741-7007-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y., Westphal H. Homeobox genes and human genetic disorders. Curr Mol Med. 2005 doi: 10.2174/1566524023363077. [DOI] [PubMed] [Google Scholar]
- 23.Li B., Huang Q., Wei G.H. The role of hox transcription factors in cancer predisposition and progression. Cancers (Basel) 2019 doi: 10.3390/cancers11040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatlekar S., Fields J.Z., Boman B.M. Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int. 2018 doi: 10.1155/2018/3569493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaldis P. Quo Vadis cell growth and division? Front Cell Dev Biol. 2016 doi: 10.3389/fcell.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohana G., Bar-Yehuda S., Arich A., Madi L., Dreznick Z., Rath-Wolfson L. Inhibition of primary colon carcinoma growth and liver metastasis by the A3 adenosine receptor agonist CF101. Br J Cancer. 2003 doi: 10.1038/sj.bjc.6601315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maier J.A., Statuto M., Ragnotti G. Endogenous interleukin 1 alpha must be transported to the nucleus to exert its activity in human endothelial cells. Mol Cell Biol. 1994 doi: 10.1128/mcb.14.3.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady G., Boggan L., Bowie A., O’Neill L.A.J. Schlafen-1 causes a cell cycle arrest by inhibiting induction of cyclin D1. J Biol Chem. 2005 doi: 10.1074/jbc.M500435200. [DOI] [PubMed] [Google Scholar]
- 29.Kuang C.Y., Yang T.H., Zhang Y., Zhang L., Wu Q. Schlafen 1 inhibits the proliferation and tube formation of endothelial progenitor cells. PLoS ONE. 2014 doi: 10.1371/journal.pone.0109711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Q.H., He H.Y., Shi Y.F., Lu H., Zhang X.J. Overexpression of acetylcholinesterase inhibited cell proliferation and promoted apoptosis in NRK cells. Acta Pharmacol Sin. 2004 [PubMed] [Google Scholar]
- 31.Lee J.M., Kim J.Y., Cho K.W., Lee M.J., Cho S.W., Kwak S. Wnt11/Fgfr1b cross-talk modulates the fate of cells in palate development. Dev Biol. 2008 doi: 10.1016/j.ydbio.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 32.Martinon F., Hofmann K., Tschopp J. The pyrin domain: A possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr Biol. 2001 doi: 10.1016/S0960-9822(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 33.Stiewe T., Putzer B.M. Role of the p53-homologue p73 in E2F1-induced apoptosis. Nat Genet. 2000 doi: 10.1038/82617. [DOI] [PubMed] [Google Scholar]
- 34.Chaiwatanasirikul K.A., Sala A. The tumour-suppressive function of CLU is explained by its localisation and interaction with HSP60. Cell Death Dis. 2011 doi: 10.1038/cddis.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaufmann T., Strasser A., Jost P.J. Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012 doi: 10.1038/cdd.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bremmer F., Schallenberg S., Jarry H., Küffer S., Kaulfuss S., Burfeind P. Role of N-cadherin in proliferation, migration, and invasion of germ cell tumours. Oncotarget. 2015 doi: 10.18632/oncotarget.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernández D., Horrillo A., Alquezar C., González-Manchón C., Parrilla R., Ayuso M.S. Control of cell adhesion and migration by podocalyxin. Implication of Rac1 and Cdc42. Biochem Biophys Res Commun. 2013 doi: 10.1016/j.bbrc.2013.01.112. [DOI] [PubMed] [Google Scholar]
- 38.Mechtersheimer S., Gutwein P., Agmon-Levin N., Stoeck A., Oleszewski M., Riedle S. Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J Cell Biol. 2001 doi: 10.1083/jcb.200101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tikhmyanova N., Little J.L., Golemis E.A. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.N. Behrens A Nkx2-5 regulates Tdgf1 (Cripto) early during cardiac development. J Clin Exp Cardiol. 2013 doi: 10.4172/2155-9880.s11-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mine N., Iwamoto R., Mekada E. HB-EGF promotes epithelial cell migration in eyelid development. Development. 2005 doi: 10.1242/dev.02030. [DOI] [PubMed] [Google Scholar]
- 42.Hutter Randolph, Giannarelli Chiara, Huang Li, Badimon Juan, Klotman Paul. Overexpression of the human form of the novel extracellular matrix protein podocan inhibits migration and proliferation of human vascular smooth muscle cells via down-regulation of the Wnt-pathway. Am Heart Assoc. 2011:A16488. [Google Scholar]
- 43.Soulières D., Hirsch F.R., Shepherd F.A., Bordogna W., Delmar P., Shames D.S. PTPRF expression as a potential prognostic/predictive marker for treatment with erlotinib in non-small-cell lung cancer. J Thorac Oncol. 2015 doi: 10.1097/JTO.0000000000000624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.