Significance
Recent structures of GPCRs in complex with G proteins provide important insights into G protein activation by family A and family B GPCRs; however, important questions remain. We don’t fully understand the mechanism of G protein coupling specificity or coupling promiscuity of some GPCRs. The β2AR preferentially couples to Gs and less efficiently to Gi, yet β2AR-Gi coupling has been shown to play important roles in cardiac physiology. To better understand the structural basis for the preferential coupling of the β2AR to Gs over Gi, we used NMR spectroscopy and supporting MD simulations to study the conformational changes in the intracellular surface of the β2AR. These studies reveal a distinct difference in intracellular loop 2 interactions with Gs and Gi1.
Keywords: GPCR, β2-adrenergic receptor, NMR spectroscopy, G protein coupling specificity
Abstract
The β2-adrenergic receptor (β2AR) is a prototypical G protein-coupled receptor (GPCR) that preferentially couples to the stimulatory G protein Gs and stimulates cAMP formation. Functional studies have shown that the β2AR also couples to inhibitory G protein Gi, activation of which inhibits cAMP formation [R. P. Xiao, Sci. STKE 2001, re15 (2001)]. A crystal structure of the β2AR-Gs complex revealed the interaction interface of β2AR-Gs and structural changes upon complex formation [S. G. Rasmussen et al., Nature 477, 549–555 (2011)], yet, the dynamic process of the β2AR signaling through Gs and its preferential coupling to Gs over Gi is still not fully understood. Here, we utilize solution nuclear magnetic resonance (NMR) spectroscopy and supporting molecular dynamics (MD) simulations to monitor the conformational changes in the G protein coupling interface of the β2AR in response to the full agonist BI-167107 and Gs and Gi1. These results show that BI-167107 stabilizes conformational changes in four transmembrane segments (TM4, TM5, TM6, and TM7) prior to coupling to a G protein, and that the agonist-bound receptor conformation is different from the G protein coupled state. While most of the conformational changes observed in the β2AR are qualitatively the same for Gs and Gi1, we detected distinct differences between the β2AR-Gs and the β2AR-Gi1 complex in intracellular loop 2 (ICL2). Interactions with ICL2 are essential for activation of Gs. These differences between the β2AR-Gs and β2AR-Gi1 complexes in ICL2 may be key determinants for G protein coupling selectivity.
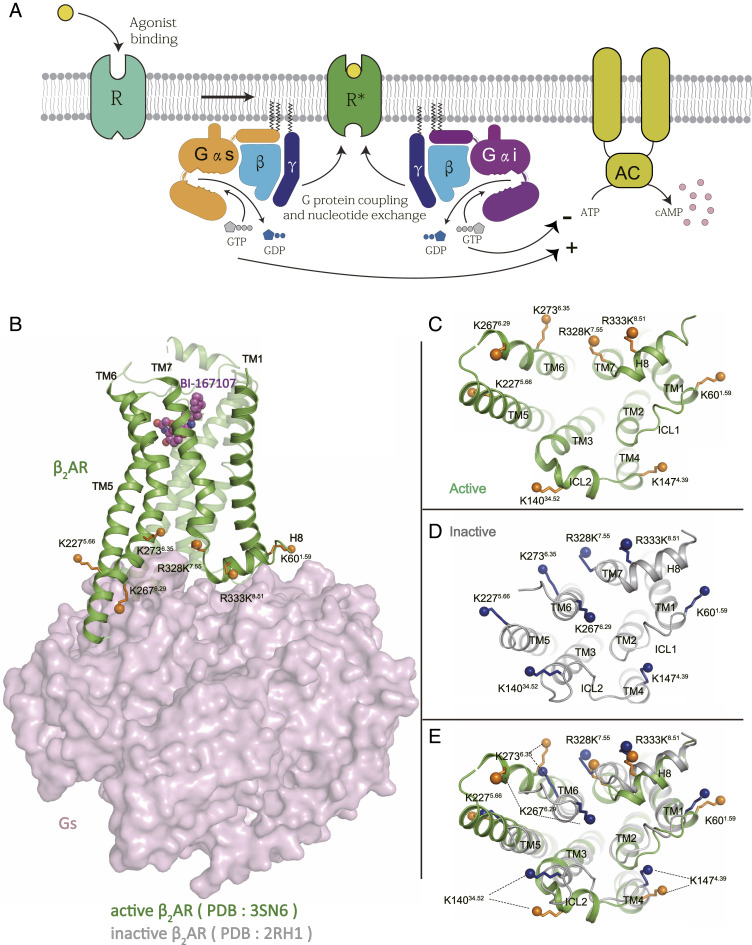
G protein-coupled receptors (GPCRs) are the largest family of membrane proteins responsible for most of the transmembrane signal transduction processes. The activation of GPCRs is mediated by diverse stimuli, including neurotransmitters, hormones, ions, and photons. It is now well known that GPCRs can activate different signaling pathways through coupling with distinct downstream transducers, for example G proteins and arrestins (1). It is generally accepted that GPCRs are highly dynamic proteins that can adopt multiple distinct conformations depending on the ligands, lipids, environments, and signaling transducers through a conformational selection mechanism (1, 2). The β2 adrenergic receptor (β2AR) has been an important model system for studying aspects of GPCR biology and pharmacology for more than 40 y. The β2AR can activate more than one G protein isoform, while preferentially coupling to the stimulatory G protein (Gs), which activates adenylyl cyclase, it also couples to the inhibitory G protein family (Gi/o) (3), which inhibits adenylyl cyclase (Fig. 1A).
Fig. 1.
The β2AR-G protein signaling pathways. (A) The agonist-bound β2AR activates either Gs or Gi heterotrimer, which stimulates or inhibits the adenylyl cyclase activity, respectively. (B) Structure of β2AR-Gs complex (PDB ID code: 3SN6), the lysine residues that are chosen as NMR probes are shown as solid spheres, other lysine residues were mutated to arginine as described in the text. (C–E) The lysine probes undergo conformational changes during the activation of the β2AR, as shown by cytoplasmic views of active β2AR (PDB ID code: 3SN6) (C), inactive β2AR (PDB ID code: 2RH1) (D), and the overlap of active and inactive β2AR (E).
The structure of the β2AR-Gs complex revealed the interaction between β2AR and Gs, and the structural changes that take place upon complex formation (4), yet, the dynamic process of β2AR signaling through Gs is still not fully understood. The structural features of the β2AR-Gs complex have been observed in GPCR-G protein complexes for Gi, Go, and G11 proteins determined by cryo-electron microscopy (cryo-EM) (5–7); however, the molecular mechanism of G protein coupling specificity is still unclear. This may in part be due to the fact that all of the GPCR-G protein complex structures solved so far are captured in a biochemically stable nucleotide-free state, while specificity determination may happen at an earlier stage of complex formation. Single-molecule studies of the β2AR coupling to Gs provide evidence for at least one intermediate state (8). The existence of intermediate states is further supported by monitoring β2AR-Gs complex formation using time-resolved radiolytic footprinting and hydrogen-deuterium exchange (9).
NMR spectroscopy has previously been used to study GPCR dynamics (10–16). Several site-specific isotopic labeling strategies have been shown to be feasible for studying conformational dynamics of GPCRs, such as 13C-dimethylated lysine, 13CH3-ε-methionine, and 19F-labeled cysteine (17). Previous NMR studies of the β2AR using these methods have revealed weak allosteric coupling between ligand binding domain and G protein coupling domain, and that the β2AR can adopt multiple conformational states during activation (11–13). However, due to the limitation of large molecular weight and difficulty in sample preparation, little is known about the conformational dynamics of the β2AR in response to different G protein subtypes. In this study, we utilized 13C-dimethylated lysine as an NMR probe to investigate the conformational changes of several sites in the intracellular G protein binding interface of the β2AR. 13C-dimethylated lysine is an ideal probe for this purpose because of its large, flexible side chain, enabling studies of larger protein complexes. Our studies show that in addition to TM6, agonist binding can also promote conformational changes in TM4, TM5, and TM7. We observe further structural changes in β2AR coupled to Gs and Gi1. While the structural changes stabilized by Gs and Gi1 are qualitatively similar in most cytoplasmic domains probed, we detected distinct differences between the β2AR-Gs and the β2AR-Gi1 complex in intracellular loop 2 (ICL2). In the inactive-state structure of the β2AR, ICL2 is a loop, while in the β2AR-Gs complex, ICL2 forms an α-helix that positions F13934.51 in ICL2 to engage a hydrophobic pocket in Gαs, thereby triggering nucleotide release (4).
Results
Design of Modified β2ARs for 13C-Dimethylated Lysine NMR Studies.
13C-dimethylated lysine probes have long served as excellent probes for NMR experiments due to high sensitivity and relatively long transverse relaxation times (18). The WT β2AR contains 16 lysine residues, most of which are located at the G protein coupling interface (Fig. 1 B–E and SI Appendix, Fig. S1A). Therefore, these lysine residues can be used as NMR reporters to monitor structural changes in response to agonists and G proteins.
However, previous studies have shown that the 13C-dimethylated lysine spectra of C-terminally truncated WT β2AR (β2AR-365N) was too crowded to make assignments (11), probably due to the similar chemical environments of these intracellular lysines. To avoid the NMR signals overlapping, we made a truncated lysine-free β2AR construct (β2AR-365N-zero-K) by mutating all lysines to arginines as previously reported (19) and removing the C-terminal 48 residues (SI Appendix, Fig. S1 A and D). Radio-ligand binding studies show that these modifications have only small effects on the affinity of antagonists and agonists (SI Appendix, Fig. S1C). A previous study showed that the lysine-free β2AR has functional properties that are comparable to WT β2AR in stimulation of adenylyl cyclase activity (19) and the recruitment of β-arrestin (20). The only reported effect of removing lysine is loss of ubiquitination and subsequent targeting receptor to lysosome for degradation (20). We then individually introduced eight lysines to different transmembrane segments in the intracellular domain of the β2AR based on the lysine-free construct (Fig. 1 B–E and SI Appendix, Fig. S1A). These eight residues were K601.59, K14034.52, K1474.39, K2275.56, K2676.29, K2736.35, R328K7.55, R333K8.51 (superscripts are Ballesteros and Weinstein numbering; ref. 21), located at the intracellular end of TM1, TM4, TM5, TM6, TM7, ICL2, and H8. The resulting eight constructs were expressed, purified, and labeled through reductive methylation (SI Appendix, Figs. S1B and S2A). Methylation efficiency was high, as determined by loss of labeling with fluorescamine, an amine reactive fluorophore (SI Appendix, Fig. S1B). We applied 1H-13C heteronuclear single quantum coherence spectroscopy (1H-13C HSQC) to each construct. The signal of each lysine was found at around 2.85 ppm in the 1H dimension and 45 ppm in the 13C dimension as compared to the spectrum of lysine-free β2AR (SI Appendix, Fig. S3 A–H). The signals are unlikely from the N terminus because the N-terminal Flag tag was removed after methylation. In order to make sure they are not residual N terminus signals due to incomplete protein digestion, we measured the signals from the methylated N terminus and they are in different positions from the lysine signals (SI Appendix, Fig. S3I). For each β2AR-365N-one-K construct, we obtained the 1H-13C HSQC spectra under the following conditions: unliganded (apo-state) β2AR, BI-167107-bound, carazolol-bound, BI-167107-bound β2AR in complex with Gs or Gi1 in the presence of 300 µM GDP (GsGDP or Gi1GDP), and BI-167107-bound β2AR in complex with nucleotide-free Gs or Gi1 (GsEMPTY or Gi1EMPTY). For these studies we used saturating concentrations of BI-167107, a potent full agonist with a dissociation half-life of 400 min and an association half-life of less than 4.4 min (12). For inverse agonist, we used a saturating concentration of carazolol. The very slow dissociation half-lives for these ligands ensures that they are bound throughout the NMR experiment.
There are crystal structures of carazolol-bound β2AR (22) and the BI-167107–bound β2AR-Gs complex (4); however, it has not been possible to crystalize native β2AR in the apo-state or bound to agonist alone, most likely due to the inherent instability of the receptor under these conditions. While β2AR also couples to Gi, there are no reported structures of a β2AR-Gi complex. Therefore, the NMR experiments provide structural insights into the apo-state, the agonist-bound β2AR, and the β2AR-Gi1 complex relative to available crystal structures. Figs. 2–4 show the comparison of 1H-13C HSQC spectra of the eight 13C-dimethylated lysine probes in TM1, ICL2, TM4, TM5, TM6, TM7, and Helix-8 in four different conditions: apo-state, inverse agonist-bound, agonist-bound, and agonist + GsEMPTY.
Fig. 2.
Conformational changes of TM1 and H8 during β2AR activation. (A and B) The 1H-13C HSQC spectra of K601.59 from TM1 (A) and K3338.51 from H8 (B). The one-dimensional (1D) slices of corresponding 1H-13C HSQC spectra in H dimension are shown at the bottom. (C–E) The local environments of the two probes are shown by side views in inactive (gray, C) and active conformations (green, D), as well as cytoplasmic views in inactive (gray, E) or active conformations (green, F). The agonist BI-167107 is abbreviated by BI.
Fig. 4.
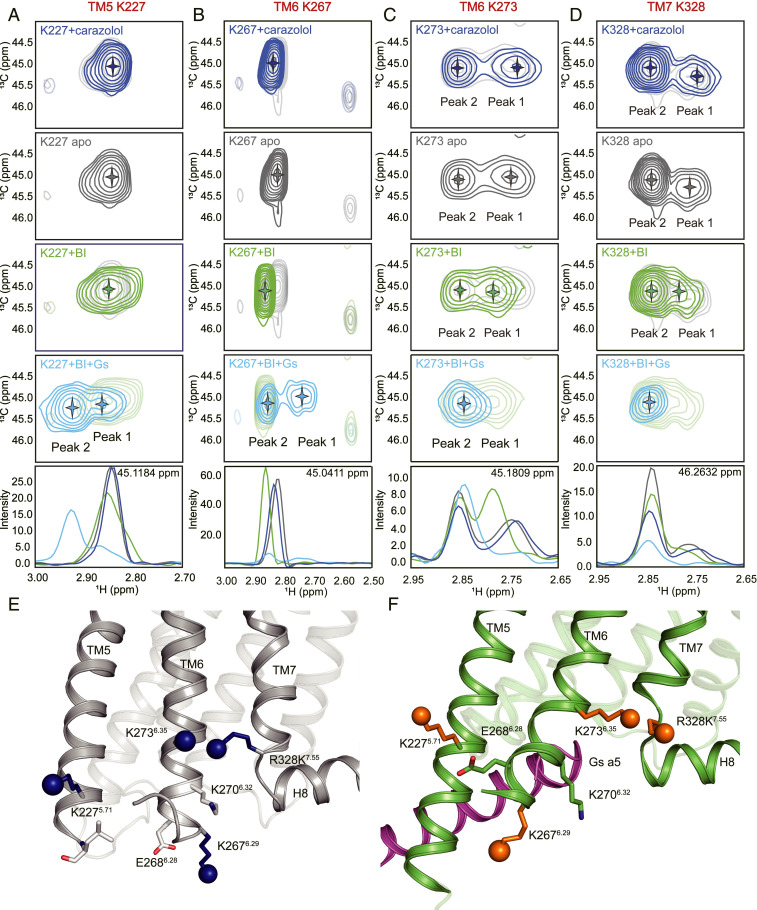
Conformational changes of TM5, TM6, and TM7 during β2AR activation. (A–D) The 1H-13C HSQC spectra of K2275.71 (A), K2676.29 (B), K2736.35 (C), and K3287.55 (D). The 1D slices of corresponding 1H-13C HSQC spectra in H dimension are shown at the bottom. The local environments of the four probes are shown in inactive (gray, E) and active (green, F) conformations. The α5 helix from Gsα is shown as purple cartoon. The agonist BI-167107 is abbreviated by BI.
Apo-β2AR Compared to Inverse Agonist-Bound States.
The β2AR exhibits basal activity for Gs activation in the apo-state. This activity can be suppressed by inverse agonists such as carazolol. Previous 13C-methionine and 19F NMR studies revealed relatively subtle conformational changes when comparing inverse-agonist stabilized inactive-state and the apo-state β2AR (12, 13). Consistent with these findings, our results show relatively small spectral changes in several domains of the intracellular surface in apo-state and the carazolol-stabilized inactive state. We observe the largest difference between the apo-state and carazolol-bound inactive state is in K601.59 (Fig. 2A), suggesting a change in its chemical environment possibly due to a change in polar interactions with the end of Helix 8 (Fig. 2 C–F). In the apo-state, K601.59 is represented by strong Peak 1 with a weak shoulder Peak 2. In carazolol-bound receptor we observe an up-field shift in Peak 1 and no change in Peak 2.
In the apo-state we observe two peaks for K1474.39 at the end of TM4 (Fig. 3), K2736.35 in TM6, and R328K7.55 in TM7 (Fig. 4). This suggests the existence of conformational heterogeneity in these receptor domains with exchange between two states on slow time scale (seconds or slower). In K2736.35 and R328K7.55 we observe very small up-field shifts in the position of Peak 1 in carazolol-bound receptor compared to the apo-state that are in the opposite direction observed for agonist (Fig. 4 C and D).
Fig. 3.
Conformational changes of ICL2 from inactive to active state. (A and B) 1H-13C HSQC spectra of K14034.52 (A) and K1474.39 (B). The 1D slices of corresponding 1H-13C HSQC spectra in H dimension are shown at the bottom. The local environment of the two probes are shown by side views in inactive (gray, C, E) and active (green, D, F) conformations. Gαs is shown in purple (D). The agonist BI-167107 is abbreviated by BI.
Conformational Changes following Agonist Binding and Gs Coupling.
To correlate structural changes observed by NMR with the β2AR-Gs crystal structure, we obtained spectra of the β2AR bound to nucleotide-free Gs (β2AR-GsEMPTY). β2AR-GsEMPTY was formed by adding the nucleotidase apyrase after complex formation to degrade any released GDP. In the β2AR-GsEMPTY crystal structure, the largest structural changes are the outward movement of TM5 and TM6 and inward movement of TM7, which allows the insertion of the α5 helix of Gαs (Fig. 1 C–E and SI Appendix, Fig. S4). We observed obvious spectral changes when comparing β2AR bound to agonist alone with the β2AR-GsEMPTY complex in all probes except K3338.51 in Helix 8 (Figs. 2–4). This may be due to a combined effect of direct Gs protein binding, as well as a fully active conformation stabilized only by agonist and Gs protein, but not agonist alone (13).
ICL1 and helix 8.
The spectrum of K601.59 at the end of TM1 is nearly identical for apo-state and agonist-bound state (Fig. 2A), suggesting little perturbation of conformational dynamics in the junction of TM1 and ICL1. ICL1 and Helix 8 have no direct interaction with Gs in the β2AR-GsEMPTY complex. The observation of intensity reduction upon coupling with Gs for K601.59 (Fig. 2A) and K3338.51 (Fig. 2B) may result from the slower tumbling due to the formation of complex with a much larger molecular weight. We do observe a change in the spectra of K601.59 upon coupling to Gs. In the inactive state K601.59 forms a polar interaction with E3388.56 in Helix 8 (Fig. 2 E and F). This is lost in the β2AR-GsEMPTY complex due to a small ∼1.5 Å movement of TM1 away from Helix 8 (Fig. 2 C and D). We also observe the appearance of a weak downfield peak in R333K8.51 in H8.
ICL2.
In the inactive state structure of the β2AR, ICL2 has no secondary structure while it is an alpha-helix in the β2AR-GsEMPTY complex (Fig. 3 C and D). The helix positions F13934.51 in ICL2 to engage a hydrophobic pocket formed by H41, V217, F376, C379, R380, and I383 in the Gαs subunit (Fig. 3D). This interaction is essential for Gs activation (4, 23, 24). The conformational changes detected in the NMR spectra of K1474.39 and K14034.52 may represent the chemical environment change during the transition of ICL2 from a loop to a helix (Fig. 3 A and B).
K1474.39 is located at the junction of TM4 and ICL2 (Fig. 1 C and D). In the inactive state, K1474.39 forms a hydrogen bond with the backbone carbonyl of Q6512.51 in ICL1 (Fig. 3E). This interaction is lost in the structure of β2AR-GsEMPTY (Fig. 3F). In the apo state, the two peaks likely represent an equilibrium between the formation (Peak 2) and disruption (Peak 1) of the hydrogen bond in slow exchange (Fig. 3B). The observation of one dominant peak for K1474.39 in the agonist-bound state compared to the two peaks in the apo-state most likely suggests faster exchange rate between the different conformations observed in the apo state (Fig. 3B). In β2AR-Gs complex we observe predominantly Peak 1, consistent with the loss of the hydrogen bond (Fig. 3B).
In contrast to the dynamics in the junction of TM4 and ICL2 observed in the apo and agonist-bound receptor, we observe a strong single peak for K14034.52 at the junction of ICL2 and TM3 (Fig. 3A). In the inactive state, K14034.52 forms a hydrogen bond with Q2295.58 in TM5, while in the β2AR-Gs complex it is adjacent to the β2AR-Gs interface (Fig. 3 C and D). Coupling with Gs dramatically alters the chemical environment of K14034.52, as evidenced by the appearance of a new downfield peak (labeled as peak2). Only a small fraction of Peak 1 remains following the formation of β2AR-GsEMPTY, consistent with a minor population of uncoupled β2AR (Fig. 3A).
TM5, 6, and 7.
While we observe spectral changes in K2275.56, K2676.29, K2736.35, and R328K7.55 upon agonist binding, further changes are observed following G protein coupling (Fig. 4 A–D), consistent with changes observed in TM5, TM6, and TM7 in the β2AR-Gs structure (Fig. 4 E and F). The slight broadening of the peak representing K2275.56 upon agonist binding suggests the appearance of two or more conformations in intermediate exchange at the intracellular side of TM5 (Fig. 4A). Upon coupling to Gs we see the appearance of a new downfield peak (Peak 2) and a weaker Peak 1 (Fig. 4A).
The two TM6 probes, K2676.29 and K2736.35, show significant differences in terms of peak patterns. In the apo and agonist-bound state K2736.35 is represented by two weak peaks, indicating that there are at least two conformations with a slow exchange rate, while K2676.29 is represented by a stronger single peak (Fig. 4 B and C). This is because K2676.29 is located at the end of TM6 and its side chain is expected to exhibit relatively high conformational flexibility, whereas K2736.35 is located in the transmembrane core and its side chain can interact with neighboring residues I2776.39 and R3287.55 (Fig. 4 E and F). Upon agonist binding, there is a downfield shift in the peak representing K2676.29. There are two peaks for K2676.29 in the β2AR-Gs complex (Fig. 4B). Peak 2 has the same chemical shift seen with agonist alone but with marked reduction in intensity. Of note, the dynamics of the cytoplasmic end of TM6 may impact the cytoplasmic end of TM5, given their close proximity (Fig. 4F). K2275.56 in TM5 is close enough to have a polar interaction with E2686.28 (Fig. 4F). Thus, the minor Peak 1 in K2275.56 and in K2676.29 may reflect different TM5–TM6 interactions in a small population of nucleotide-free β2AR-Gs complex.
It is interesting to note that the spectra of K2736.35 and R328K7.55 are similar (Fig. 4 C and D). This may be due to the close proximity of K2736.35 and R3287.55 observed in the inactive state crystal structures (Figs. 1 C and D and 4E) where R328K7.55 could form a hydrogen bond with the backbone carbonyl of K2706.32. This interaction would be lost upon coupling with Gs. For both K2736.35 and R328K7.55, agonist binding leads to a downfield shift in Peak 1, and Peak 1 is lost in the β2AR-Gs complex. Peak 2 may represent a conformation that is more exposed to the solvent, therefore Peak 2 is less affected by agonist-binding or G protein coupling than Peak 1.
Conformational Differences in Gs and Gi1 Coupling.
Recently, a number of GPCR-Gi/o complex structures have been determined by cryo-EM (5–7, 25–28). The Gs and β2AR interaction interface is composed by TM3, TM5, TM6, and ICL2. While Gαi/o interactions with TM3, TM5, and TM6 are similar in GPCR-Gi/o protein complexes, TM2, TM7, and H8 are also involved in formation of the A1 adenosine receptor (A1R)-Gi complex, and ICL2 and the junction between TM7 and H8 contribute to formation of the rhodopsin-Gi complex (SI Appendix, Fig. S5). For GPCR-Gi/o complexes, the outward displacement of TM6 is smaller than observed for the β2AR-Gs complex. The differences in the interaction interface for these complexes might contribute to G protein coupling preferences. As noted above, the β2AR can couple to both Gs and Gi. However, efforts toward obtaining the structure of β2AR-Gi complex have failed due to the relatively weak interaction and instability of the complex. As a consequence, little is known about differences in the interactions between β2AR and Gi compared with the β2AR-Gs complex.
To provide more structural insights into the basis of β2AR coupling selectivity for Gs over Gi, we next sought to compare β2AR binding with Gs and Gi by using the 1H-13C HSQC spectra of the β2AR-BI-167017-Gi1EMPTY complex (Fig. 5). The β2AR-Gi1EMPTY complex was formed by adding the nucleotidase apyrase after complex formation to degrade any released GDP. Fig. 5 compares the spectra of β2AR-GsEMPTY and β2AR-Gi1EMPTY complexes. Consistent with the previous observation that the β2AR-Gi1 complex is less stable than the β2AR-Gs complex (29), we observed smaller spectral changes in the β2AR-Gi1 complex sample. The NMR spectral changes of K2275.56, K2676.29, K2736.35, and R328K7.55 corresponding to the conformational changes of TM5, 6, and 7 are similar to those obtained from the β2AR-Gs complex; however, peak 2 of K2275.56 and K2736.35 and peak 1 of K2676.29 are notably weaker in the β2AR-Gi1 complex compared to the β2AR-Gs complex. However, it’s hard to discriminate whether the TM6 outward displacement of β2AR−Gi1 is smaller than that of β2AR−Gs based on the signal intensity difference observed by NMR.
Fig. 5.
Comparison of conformational changes in β2AR coupled to Gs or Gi1. BI is short for BI-167107. (A–H) The 1H-13C HSQC spectra of the eight probes from TM1 to H8. The eight probes are K601.59 (A), K14034.52 (B), K1474.39 (C), K2275.56 (D), K2676.29 (E), K2736.35 (F), R328K7.55 (G), and R333K8.51 (H). For each probe, the overlay of the 1H-13C HSQC spectra of agonist-bound β2AR (green) and β2AR-GsEMPTY (blue) are shown at the top, followed by the comparison of the 1H-13C HSQC spectra of agonist-bound β2AR (green) and β2AR-Gi1EMPTY (pink), then by the overlay of β2AR-GsEMPTY (blue) and β2AR-Gi1EMPTY (pink). The 1D slices of corresponding 1H-13C HSQC spectra in H dimension are shown at the bottom.
Remarkably, there are nearly no chemical shift changes for K14034.52 in ICL2 and K1474.39 in TM4 in the β2AR-Gi1 complex, which is in contrast to what is observed for β2AR-Gs complex (Fig. 5 B and C). The observed decrease of peak intensity is likely due to the increase in mass. The lack of spectral changes for K14034.52 and K1474.39 suggest that ICL2 does not form an alpha helix when coupled to Gi1 and may have only weak interactions with Gi1, possibly explaining the less efficient coupling and the overall instability of the β2AR-Gi1 complex.
As noted above, the insertion of F13934.51 in ICL2 of the β2AR into a hydrophobic pocket formed by H41, V217, F376, C379, and R380 in the Gαs is essential for GDP release (SI Appendix, Figs. S6A and S7A). Of these, only F376 is conserved as F336 in Gi (SI Appendix, Fig. S6 B and C). When comparing sequences of ICL2 from GPCRs that couple to Gs, Gi/o, Gq/11, and G12, position 34.51 is most often a L, F, I, or M for Gs coupled receptors, while a broader range of amino acids are found in Gi/o coupled receptors (SI Appendix, Fig. S6D). It is possible that interactions with 34.51 in ICL2 may be less important for coupling with Gi1 than Gs. This is in agreement with the recent structures of Gi in complex with the cannabinoid receptor subtype 1 (CB1) (27), the µ-opioid receptor (µOR) (6), and the A1R (7). For these receptors, the interactions between the amino acid at position 34.51 in ICL2 (L in CB1 and A1R, and V in µOR) and Gi are much weaker, interacting with only one or two side chains of Gi (SI Appendix, Fig. S7 B–D). However, this weaker interaction between ICL2 and Gi is not universal. For the neurotensin receptor subtype 1 (NTSR1), which couples promiscuously to Gi/o, Gs, and Gq/11 (30), F17434.51 in ICL2 packs into a pocket formed by four amino acids in Gi (SI Appendix, Fig. S7E). The importance of the amino acid in position 34.51 was initially demonstrated for the Gq/11 coupled M1 muscarinic receptor (M1R) where the substitution of L13134.51 for Ala in ICL2 led to a loss of Gq/11 coupling (24). In the recent cryo-EM structure of the M1R-G11 complex, L13134.51 packs into a pocket formed by four amino acids in G11 (28) (SI Appendix, Fig. S7F). Like Gs coupled GPCRs, L, F, I, or M are more commonly found at position 34.51 in Gq/11 coupled GPCRs (SI Appendix, Fig. S6D). While the evidence suggests that weak interactions between F13934.51 in ICL2 of the β2AR and Gi1 may account for poor coupling efficiency, we cannot exclude the possibility that β2AR simply couples less efficiently to Gi and the lack of strong engagement with ICL2 is a manifestation of that.
Similar to Gs, Gi1 binding also has little effect on R333K8.51 located at H8 (Fig. 5H). The decrease in intensity is likely due to the increased size of the complex. Notably, the decrease in intensity is greater for GsEMPTY compared to Gi1EMPTY, consistent with the formation of a more stable complex. The low intensity downfield peak observed in R333K8.51 with Gs is not observed with Gi1. The spectra of K601.59 in TM1 are similar for Gs and Gi1, with a reduction of intensity and the peak splitting into a slightly upfield-shifted peak and a smaller downfield peak (Fig. 5A). ICL1 and H8 are not observed to interact with Gs in the β2AR-Gs complex; however, the orientation of ICL1 relative to H8 changes upon complex formation with the loss of an electrostatic interaction between K601.59 in ICL1 and R328K7.55 in H8.
We have previously observed evidence for a transient intermediate state following the disruption of the β2AR-Gs complex by the addition of GDP (8). In an effort to detect this state by NMR spectroscopy, we formed β2AR-Gs and β2AR-Gi1 complexes in the presence of 300 µM GDP. As can be seen in SI Appendix, Figs. S8 and S9, we don’t observe a distinct state in the presence of GDP. The spectra are consistent with a mixture of nucleotide-free β2AR-Gs or β2AR-Gi1, and β2AR bound to agonist alone. Our inability to detect a distinct GDP-bound β2AR-Gs complex could be due to the insensitivity of our probes or to the fact that the amount of the intermediate may be too small to detect.
Molecular Dynamics Simulations Showed Different ICL2 Behavior in the β2AR-GiEMPTY and the β2AR-GsEMPTY Complex.
To investigate the dynamics at the receptor G protein interface, classical unbiased MD simulations of the β2AR-Gi1EMPTY and the β2AR-GsEMPTY complex were carried out. The β2AR-Gi1EMPTY model was built based on the β2AR-GsEMPTY complex (Protein Data Bank [PDB] ID code: 3SN6) (see Methods for more details) and as a result, ICL2 in the starting structures of both β2AR-Gi1EMPTY and β2AR-GsEMPTY complex are in an alpha-helical conformation. Three independent, 3-µs-long molecular dynamics (MD) simulations were carried. While the simulations are too short to observe unfolding of the secondary structure, the significantly higher rmsd of the ICL2 region of the β2AR-Gi1 complex compared to the β2AR-Gs complex suggests the starting alpha-helical structure of ICL2 is less stable in the β2AR-Gi1EMPTY structure. Of note, such changes are not observed for ICL1, H8, nor Gα(α5) when both systems are compared (Fig. 6 A–D). The only other structural elements at the receptor G protein interface with increased rmsd values are TM5 and TM6 (Fig. 6 E and F), consistent with the high dynamics of their cytoplasmic ends observed by chemical shift analysis in β2AR-Gi1EMPTY and in β2AR-GsEMPTY complexes. A close inspection of the MD simulations results suggests a higher flexibility of K14034.52 and K1474.39 from ICL2 for the β2AR-Gi1EMPTY compared to ICL2 from β2AR-GsEMPTY as measured by the average rms fluctuation (RMSF) (SI Appendix, Fig. S10). The average RMSF value for K14034.52 is two times higher and for K1474.39 is six times higher in β2AR-Gi1EMPTY than β2AR-GsEMPTY. The MD simulations results are in agreement with the NMR experimental observation that ICL2 plays different roles when β2AR couples to Gi or Gs.
Fig. 6.
Comparison of conformational changes measured by MD simulations in β2AR coupled to Gs or Gi. Backbone rmsd in Å extracted from 3 × 3-µs-long MD simulations of β2AR-Gi (pink) and β2AR-Gs (cyan) of structural elements at the receptor-G protein interface. (A) ICL1 of β2AR: E6212.48-Q6512.51. (B) ICL2 of β2AR: I1353.54-A1504.42. (C) H8 of β2AR: S3298.47-C3418.59. (D) α5 helix of Gαi and Gαs. (E) TM5 of β2AR: N1965.35-R239ICL3. (F) TM6 of β2AR: C2656.27-Q2996.61. The rmsd values were calculated relative to the respective structural element in the starting structure of β2AR-GiEMPTY and β2AR-GsEMPTY.
Discussion
We applied NMR spectroscopy to monitor structural changes in the cytoplasmic surface of the unliganded β2AR and in response to an inverse agonist, an agonist, Gs, and Gi. We have been unable to obtain crystal or cryo-EM structures of the apo-β2AR and agonist-bound β2AR due to its instability and possibly due to conformational heterogeneity. Our NMR studies provide further evidence for conformational heterogeneity as we observe more than one peak for K1474.39, K2736.35, and K3287.55 in apo and agonist-bound β2AR (Figs. 2–4). Agonist binding leads to changes in K1474.39 in ICL2, K2676.29, and K2736.35 in TM6, and in K3287.55 in TM7; however, additional changes are observed upon the addition of Gs and Gi1. This suggests that for the β2AR agonist, binding alone cannot fully stabilize the G protein bound conformation, as has been observed in previous fluorescence and double electron electron resonance spectroscopy experiments (8, 13). The structural changes stabilized by Gi1 and Gs are qualitatively the same for TM5, TM6, and TM7; however, our NMR studies and supporting MD simulations suggest that Gi1 does not promote the formation of an alpha helix in ICL2.
Methods
The receptors were expressed in Sf9 insect cells, purified by affinity column and size exclusion chromatography, and labeled by reductive methylation. NMR data were collected at 25 °C on a Burker Avance 800-MHz spectrometer equipped with a cryoprobe. The 1H-13C HSQC spectra were recorded with spectral width of 11,160.71428 Hz in the 1H-dimension (w1) and 14,492.7536 Hz in the 13C-dimension (w2) centered at 46 ppm. For all spectra, 1024 × 256 complex points were recorded and a relaxation delay of 2 s was used to allow spin to relax back to equilibrium. MD simulations were performed using Gromacs simulation package. Further details are proved in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the Beijing Advanced Innovation Center for Structural Biology, Tsinghua-Peking Joint Center for Life Science, Tsinghua University. C.J. acknowledges funding from the National Key R&D Program of China (Grant 2016YFA0501201). P.W.H. acknowledges funding by the Deutsche Forschungsgemeinschaft (German Research Foundation) through SFB1423, project 421152132 and HI1502/1-2, project 168703014; the Stiftung Charitè; and the Einstein Center Digital Future. All NMR spectra were obtained at the Beijing NMR Center and the NMR facility of the National Center for Protein Sciences at Peking University. We thank Jiawei Zhao for providing G protein P1 virus and suggestions. We thank the Gauss Center for Supercomputing (GCS) e.V. for funding this project by providing computing time on the GCS Supercomputer SuperMUC at Leibniz Supercomputing Centre. B.K.K. is a Chan Zuckerberg Biohub Investigator.
Footnotes
Competing interest statement: B.K.K. is co-founder of and consultant for ConfometRx, Inc.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2009786117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Hilger D., Masureel M., Kobilka B. K., Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 25, 4–12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manglik A., Kobilka B., The role of protein dynamics in GPCR function: Insights from the β2AR and rhodopsin. Curr. Opin. Cell Biol. 27, 136–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao R. P., Beta-adrenergic signaling in the heart: Dual coupling of the beta2-adrenergic receptor to G(s) and G(i) proteins. Sci. STKE 2001, re15 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen S. G.et al., Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang Y.et al., Cryo-EM structure of human rhodopsin bound to an inhibitory G protein. Nature 558, 553–558 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koehl A.et al., Structure of the µ-opioid receptor-Gi protein complex. Nature 558, 547–552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draper-Joyce C. J.et al., Structure of the adenosine-bound human adenosine A(1) receptor-G(i) complex. Nature 558, 559–563 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Gregorio G. G.et al., Single-molecule analysis of ligand efficacy in β2AR-G-protein activation. Nature 547, 68–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Y.et al., Assembly of a GPCR-G protein complex. Cell 177, 1232–1242.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casiraghi M., Banères J.-L., Catoire L. J., “NMR Spectroscopy for the Characterization of GPCR Energy Landscapes” in Structure and Function of GPCRs, Lebon G., Ed. (Springer International Publishing, 2019), pp. 27–52. [Google Scholar]
- 11.Bokoch M. P.et al., Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature 463, 108–112 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nygaard R.et al., The dynamic process of β(2)-adrenergic receptor activation. Cell 152, 532–542 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manglik A.et al., Structural insights into the dynamic process of β2-Adrenergic receptor signaling. Cell 161, 1101–1111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sounier R.et al., Propagation of conformational changes during μ-opioid receptor activation. Nature 524, 375–378 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J.et al., Conformational complexity and dynamics in a muscarinic receptor revealed by NMR spectroscopy. Mol. Cell 75, 53–65.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Ye L.et al., Mechanistic insights into allosteric regulation of the A2A adenosine G protein-coupled receptor by physiological cations. Nat. Commun. 9, 1372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleist A. B.et al., “Site-specific side chain labeling for NMR studies of G protein-coupled receptors” in Methods in Cell Biology, Shukla A. K., Ed. (Academic Press, 2019), Vol. 149, pp. 259–288. [Google Scholar]
- 18.Larda S. T., Bokoch M. P., Evanics F., Prosser R. S., Lysine methylation strategies for characterizing protein conformations by NMR. J. Biomol. NMR 54, 199–209 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Parola A. L., Lin S., Kobilka B. K., Site-specific fluorescence labeling of the beta2 adrenergic receptor amino terminus. Anal. Biochem. 254, 88–95 (1997). [DOI] [PubMed] [Google Scholar]
- 20.Xiao K., Shenoy S. K., Beta2-adrenergic receptor lysosomal trafficking is regulated by ubiquitination of lysyl residues in two distinct receptor domains. J. Biol. Chem. 286, 12785–12795 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballesteros J. A., Weinstein H., “Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors” in Methods in Neurosciences, Sealfon S. C., Ed. (Academic Press, 1995), Vol. 25, pp. 366–428. [Google Scholar]
- 22.Rosenbaum D. M.et al., GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 318, 1266–1273 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Liu X.et al., Mechanism of β2AR regulation by an intracellular positive allosteric modulator. Science 364, 1283–1287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moro O., Lameh J., Högger P., Sadée W., Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J. Biol. Chem. 268, 22273–22276 (1993). [PubMed] [Google Scholar]
- 25.García-Nafría J., Lee Y., Bai X., Carpenter B., Tate C. G., Cryo-EM structure of the adenosine A2A receptor coupled to an engineered heterotrimeric G protein. eLife 7, e35946 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Nafría J., Nehmé R., Edwards P. C., Tate C. G., Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go. Nature 558, 620–623 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar K. K.et al., Structure of a signaling cannabinoid receptor 1-G protein complex. Cell 176, 448–458.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda S., Qu Q., Robertson M. J., Skiniotis G., Kobilka B. K., Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552–557 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strohman M. J.et al., Local membrane charge regulates β2 adrenergic receptor coupling to Gi3. Nat. Commun. 10, 2234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besserer-Offroy É.et al., The signaling signature of the neurotensin type 1 receptor with endogenous ligands. Eur. J. Pharmacol. 805, 1–13 (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.