Significance
Biology is built of organic molecules, which originate primarily from the reduction of CO2 through several carbon-fixation pathways. Only one of these—the Wood–Ljungdahl acetyl-CoA pathway—is energetically profitable overall and present in both Archaea and Bacteria, making it relevant to studies of the origin of life. We used geologically pertinent, life-like microfluidic pH gradients across freshly deposited Fe(Ni)S precipitates to demonstrate the first step of this pathway: the otherwise unfavorable production of formate (HCOO–) from CO2 and H2. By separating CO2 and H2 into acidic and alkaline conditions—as they would have been in early-Earth alkaline hydrothermal vents—we demonstrate a mild indirect electrochemical mechanism of pH-driven carbon fixation relevant to life’s emergence, industry, and environmental chemistry.
Keywords: carbon fixation, origin of life, membranes, catalysis, electrochemistry
Abstract
All life on Earth is built of organic molecules, so the primordial sources of reduced carbon remain a major open question in studies of the origin of life. A variant of the alkaline-hydrothermal-vent theory for life’s emergence suggests that organics could have been produced by the reduction of CO2 via H2 oxidation, facilitated by geologically sustained pH gradients. The process would be an abiotic analog—and proposed evolutionary predecessor—of the Wood–Ljungdahl acetyl-CoA pathway of modern archaea and bacteria. The first energetic bottleneck of the pathway involves the endergonic reduction of CO2 with H2 to formate (HCOO–), which has proven elusive in mild abiotic settings. Here we show the reduction of CO2 with H2 at room temperature under moderate pressures (1.5 bar), driven by microfluidic pH gradients across inorganic Fe(Ni)S precipitates. Isotopic labeling with 13C confirmed formate production. Separately, deuterium (2H) labeling indicated that electron transfer to CO2 does not occur via direct hydrogenation with H2 but instead, freshly deposited Fe(Ni)S precipitates appear to facilitate electron transfer in an electrochemical-cell mechanism with two distinct half-reactions. Decreasing the pH gradient significantly, removing H2, or eliminating the precipitate yielded no detectable product. Our work demonstrates the feasibility of spatially separated yet electrically coupled geochemical reactions as drivers of otherwise endergonic processes. Beyond corroborating the ability of early-Earth alkaline hydrothermal systems to couple carbon reduction to hydrogen oxidation through biologically relevant mechanisms, these results may also be of significance for industrial and environmental applications, where other redox reactions could be facilitated using similarly mild approaches.
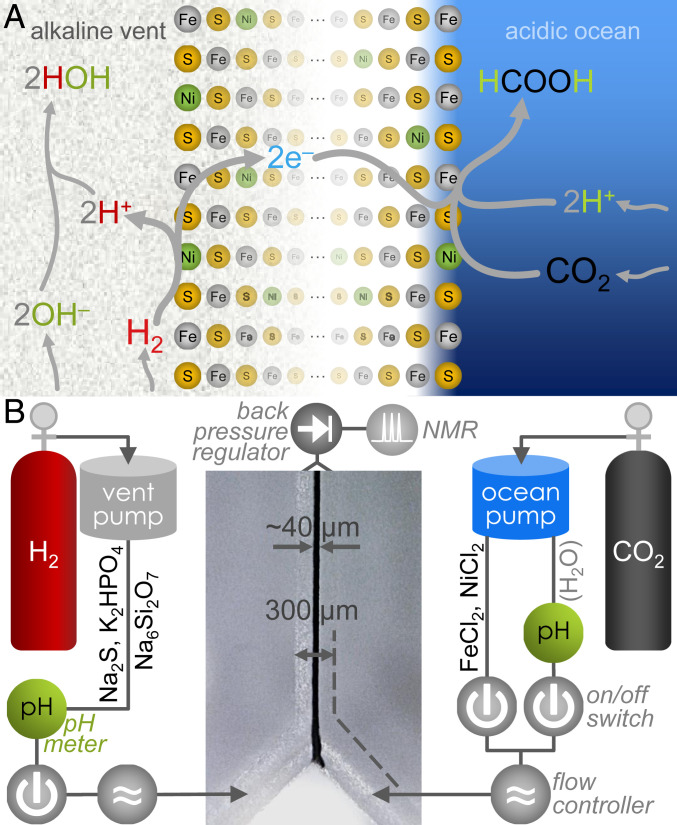
A dependable supply of reduced organic molecules is essential for any scenario of the origin of life. On the early Earth, one way in which this supply could have been attained was by reduction of CO2 with H2 (1–6). Geological studies indicate that CO2 was at comparatively high concentrations in the ocean during the Hadean eon, whereas H2 was the product of multiple processes in the Earth’s crust and would have emanated as part of the efflux of alkaline hydrothermal vents (3, 7–11). The model suggests that on meeting at the vent–ocean interface, the reaction between the two dissolved gases would have produced hydrocarbons, which would in turn take roles in the transition from geochemistry to biochemistry (2, 12). Under standard conditions (1 atm, 25 °C, pH 7), the reaction between CO2 and H2 to produce formate (HCOO–) is thermodynamically disfavored, with ΔG0’ = +3.5 kJ mol–1 (13, 14). In ancient alkaline vents, however (Fig. 1A), H2 was present in the OH–-rich effluent of the vent, which would have favored its oxidation to water (1). Conversely, CO2 would have been dissolved in the relatively acidic ocean, which facilitated protonation in its reduction to HCOO– (1). Assisted by Fe(Ni)S minerals precipitated at the interface, a pH gradient of more than three units should have been enough to increase the viability of the reaction by ∼180 mV, thereby rendering it favorable (15, 16).
Fig. 1.
Proposed mechanism of pH-driven CO2 reduction with H2 across a conducting Fe(Ni)S barrier, and schematic of the reactor. (A) Under alkaline-vent conditions, the oxidation of H2 (Left) is favored by the alkaline pH due to the presence of hydroxide ions (OH–) that react exergonically in the production of water. Released electrons would travel across the micrometers- to centimeters-thick Fe(Ni)S network (53) (Center) to the more oxidizing acidic solution on the ocean side. There they meet dissolved CO2 and a relatively high concentration of protons (H+), favoring the production of formic acid (HCOOH) or formate (HCOO–). This electrochemical system enables the overall reaction between H2 and CO2, which is not observed under standard reaction conditions. (B) Diagram of the reactor, with embedded micrograph of a reaction run with precipitate at the interface. Further details are provided in the main text and SI Appendix, Fig. S2.
Once produced, and depending on local conditions, formate would have had ample abiotic chemical potential. For example, formyl groups have been shown to yield intermediates of both the reverse citric acid (Krebs) cycle (17) and the Wood–Ljungdahl (WL) reductive acetyl-CoA pathway (18), offering a possible entry route into early biological metabolism. Alternatively, on heating in the presence of ammonia—itself also a predicted component of alkaline-vent effluents—formate yields formamide [HC(O)NH2], a highly reactive molecule central to a separate major theory for the emergence of life (19). Further reaction of this latter mixture yields hydrogen cyanide (HCN), which is also at the core of a major alternative origin-of-life scenario (20). In turn, dehydration of formate yields carbon monoxide (CO), which features prominently in a separate hydrothermal-vent model for the origin of life (21).
While there were multiple sources of reduced carbon on the early Earth and multiple plausible environments that would have hosted rich chemistries (17–25), the alkaline hydrothermal scenario described above can be especially appealing because of its resemblance to the WL pathway of carbon fixation (SI Appendix, Fig. S1) (2, 26). Highlighting its potential relevance to studies of the emergence of life, the WL process is the only one of six known biological carbon-fixation pathways that releases energy overall instead of consuming it, and variations of it are present in extant members of both Archaea (methanogens) and Bacteria (acetogens) (2, 4, 6, 12, 13, 27). The first step in the pathway is the reduction of CO2 with H2 to produce formate (HCOO–, or its dehydrated electronic equivalent, CO). This reaction is endergonic, so multiple members of both Archaea and Bacteria use either electron bifurcation or a chemiosmotic pH gradient across the cell membrane to power the otherwise unfavorable step (14, 28, 29). However, in the absence of cellular coupling mechanisms such as electron bifurcation or chemiosmosis, this first endergonic step is a key energetic bottleneck in the WL pathway and remains a major open question in studies of the origin of biological carbon fixation.
Here we show the abiotic indirect reduction of CO2 to HCOO– with H2, driven by a microfluidic pH gradient across freshly deposited Fe(Ni)S precipitates, via a mechanism that resembles—and may have been the evolutionary predecessor of—the decoupled electron flow of the WL pathway.
Results
Precipitation of Fe(Ni)S at the Interface in a Gas-Driven Y-Shaped Microfluidic Reactor.
Aiming to coincide with fluid compositions in previous work (5, 30, 31), and as detailed in SI Appendix, Table S1, we prepared an alkaline vent-simulant fluid containing Na2S (100 mM), K2HPO4 (10 mM), and Na2Si3O7 (10 mM) in deaerated water. The counterpart ocean analog fluid contained FeCl2 (50 mM) and NiCl2 (5 mM). The two fluids were introduced at the inlets of a Y-shaped borosilicate microfluidic reactor (Fig. 1B). Ambient pressures of H2 and CO2 have proven insufficient to drive CO2 reduction (31, 32), so instead of attempting to dissolve either gas by bubbling before the reaction, here we used gas pressure-driven microfluidic pumps. The alkaline fluid was pushed with H2 at 1.5 bar, and the ocean analog was pushed with CO2 at the same pressure (see reactor schematic in Fig. 1B and photograph in SI Appendix, Fig. S2). We split each reactor run into two consecutive stages, the first for deposition of the Fe(Ni)S precipitates at the interface between the two fluids and the second (referred to as “postprecipitation” below) for attempting the reaction between CO2 and H2 (or other reagents, as detailed where relevant). Confluence of the ocean and vent analogs over 15 to 60 s in the precipitation stage yielded a 30- to 60-µm-wide precipitate at the interface between the two fluids, visible under a digital 200× optical microscope (Fig. 1B, Center). Removing metals from the ocean side following precipitation prevented the precipitate from growing to the point of occluding the reactor channel.
Detection of Formate and Confirmation with 13C Isotopic Labeling.
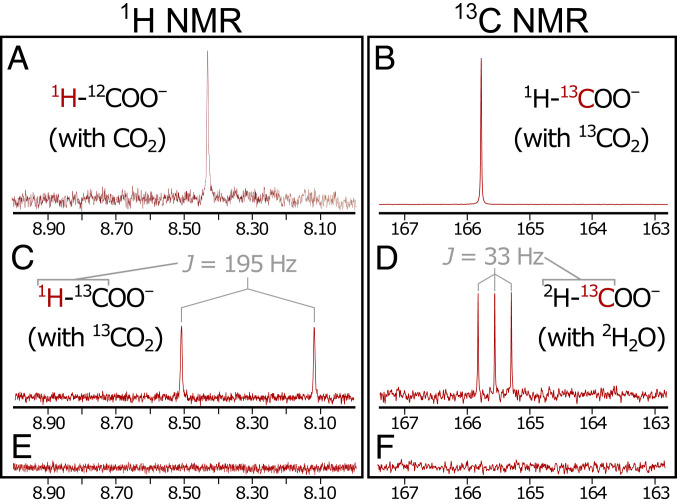
Following formation of the precipitate, and to prevent further precipitation from clogging the microfluidic channels (32), in the second (postprecipitation) stage we switched the ocean fluid to pure deaerated H2O, pushed by CO2 (Fig. 1B, Right). The vent simulant was as before, with Na2S, K2HPO4, and Na2Si3O7, pushed by H2. Using in-line pH meters, we determined the pH of the ingoing fluids at the point of entry (shortly before entering the reactor; Fig. 1B), finding the ocean simulant at pH 3.9 and the vent simulant at pH 12.3. At a flow rate of 5 µL/min for each input, the residence time of fluids within the central channel was ∼3.3 s, so we allowed the system to flow for at least 2 min before collecting output. We then collected a single (i.e., mixed) output of the reactor over the subsequent 50 min and later analyzed it via NMR spectroscopy. Under these conditions, we detected HCOO– by both 1H and 13C NMR (Table 1, experiment 1), at an average concentration of 1.5 µM. Singlet peaks in the 1H (8.42 ppm; Fig. 2A) and 13C (165.8 ppm; SI Appendix, Fig. S3) NMR spectra matched samples of pure (>98%) formic acid (SI Appendix, Fig. S4). Keeping the ocean solution free of metal salts from the start—i.e., in the absence of the precipitate—gave no detectable product (SI Appendix). Running both the precipitation and reaction stages with isotopically enriched (99% 13C) 13CO2 (Table 1, experiment 2) gave a stronger singlet in the 13C spectrum (165.8 ppm; Fig. 2B) and yielded the expected splitting of the formyl singlet into a doublet in the 1H spectrum (J = 195 Hz) due to 1H–13C coupling in the formyl group (Fig. 2C).
Table 1.
Mechanistic analysis of CO2 reduction
| Experiment | Vent driving gas | Ocean driving gas | Ocean solvent | Product detected |
| 1 | H2 | CO2 | H2O | HCOO– |
| 2 | H2 | 13CO2 | H2O | H13COO– |
| 3 | N2 | CO2 | H2O | n.d. |
| 4 | D2 | CO2 | H2O | HCOO– |
| 5 | H2 | CO2 | D2O | DCOO– |
n.d., none detected. For precipitation, the ocean fluid contained FeCl2 (50 mM) and NiCl2 (5 mM) in deaerated H2O or D2O (as shown); no solutes other than the driving gas (CO2) were included in the ocean fluid postprecipitation. Vent fluid contained Na2S (100 mM), K2HPO4 (10 mM), and Na2Si3O7 (10 mM) both during and following precipitation. The ocean and vent fluid pumps were driven by the gases shown, each at 1.5 bar and a flow rate of 5 µL/min. For relevant experiments from this table, quantification is listed in Table 2 and was achieved through 1H NMR by integrating the formyl singlet compared with a known concentration of internal standard. Most entries in this table could not be quantified in this fashion, because deuterium labeling resulted in loss of the 1H signal (experiment 5), the formyl singlet was split into a doublet by the 13C labeling (experiment 2), or no formate was detected (experiment 3).
Fig. 2.
1H and 13C NMR spectra of produced formate. (A and B) Singlets in the 1H (A) and 13C (B) NMR spectra demonstrate the production of formate in our system. (C) With isotopically labeled 13CO2, coupling between 1H and 13C produced an expected doublet in the 1H spectrum. (D) Replacing regular water with deuterated 2H2O on the ocean side yielded a triplet in the 13C spectrum due to coupling between the 2H and 13C nuclei. (E and F) No formate was detected on replacing H2 with N2 as the vent-driving gas. Corresponding entries in Table 1 are A:1, B:2, C:2, D:5. E:3, and F:3.
H2 appears to be necessary for CO2 reduction. With the vent-side fluid driven by N2 instead of H2 (i.e., in the absence of H2 both during and following precipitation), we observed no reduction products (experiment 3 and Fig. 2 E and F).
Evidence for an Indirect pH-Dependent Electrochemical Mechanism of CO2 Reduction Driven by H2 Oxidation.
In search of mechanistic insight, we conducted deuterium (2H, or D)-labeling experiments (Table 1, experiments 4 and 5), using the isotopic variants throughout the entire experiment. Regardless of whether we used unlabeled H2 (experiment 1) or D2 (experiment 4) to drive the vent-side pump, we observed only non-isotopically labeled HCOO– in the efflux, suggesting that CO2 reduction may be occurring exclusively on the ocean side. Conversely, with D2O used in place of regular H2O on the ocean side, and with unlabeled H2 driving the vent-side pump (experiment 5), we detected only deuterated formate (DCOO–), as evidenced by a triplet in 13C NMR, J = 33 Hz, and the lack of any other discernible peaks (Fig. 2D). This further confirms that the CO2 reduction here matches the isotopic composition of the ocean side, not that of the vent side.
We next explored the role of the inherent pH gradient of the simulated submarine alkaline hydrothermal system. The successful reductions reported in Table 1 proceeded with an initial ocean analog pH of 3.9 and a vent analog pH of 12.3. On mixing, this initial ΔpH of 8.4 units would have inevitably dropped, but, as we have previously shown, pH gradients of multiple units hold successfully over time across microfluidic scales, particularly in the presence of a precipitate at the interface (30). We aimed to determine whether such a pH gradient was required in our reduction system to facilitate the oxidation of H2 on the alkaline side and the reduction of CO2 on the acidic side (Fig. 1A). Following precipitation under the same conditions as above for experiment 1 in Table 1 (repeated in Table 2), we evaluated the effects of various changes to the pH and composition of each of the two fluids (Table 2). Replacing the vent simulant with pure H2O driven by H2 afforded no product (Table 2, experiment 6). Likewise, leaving Na2S, K2HPO4, and Na2Si3O7 in the vent solution and H2 as the driver gas, but acidifying the vent solution with HCl to pH 3.9 and pH 7.0, resulted in no detectable formate production (Table 2, experiments 7 and 8, respectively). Adding 100 mM Na2CO3 to the ocean fluid while still using CO2 as the driving gas (Table 2, experiment 9) raised the ocean-side pH to 9.8, and no product was detected under these conditions. Removing silicate from the vent side after precipitation still yielded formate (Table 2, experiment 10), as did removing both silicate and phosphate to have only Na2S (Table 2, experiment 11). With only K2HPO4 in the vent side postprecipitation, we detected only trace amounts of formate (below our limit of quantification of 0.37 µM; see SI Appendix, Methods and Table S2), possibly due to the insufficiently alkaline pH of 9.1 (Table 2, experiment 12). The more alkaline K3PO4 raised the pH to 12.1 and led to production of considerably more formate (Table 2, experiment 13).
Table 2.
Exploration of the role of the microfluidic pH gradient across the mineral precipitate in CO2 reduction
| Experiment | Vent-side pH | Ocean-side pH | Vent-side postprecipitation solute* | Ocean-side postprecipitation solute/gas† | Formate concentration, μM‡ |
| 1§ | 12.3 | 3.9 | Na2S/K2HPO4/Na2Si3O7 | None/CO2 | 1.5 (1.66; 1.40) |
| 6 | 7.0 | 3.9 | None | None/CO2 | n.d. |
| 7¶ | 7.0 | 3.9 | Na2S/K2HPO4/Na2Si3O7 | None/CO2 | n.d. |
| 8¶ | 3.9 | 3.9 | Na2S/K2HPO4/Na2Si3O7 | None/CO2 | n.d. |
| 9 | 12.3 | 9.8 | Na2S/K2HPO4/Na2Si3O7 | Na2CO3/CO2 | n.d. |
| 10 | 12.3 | 3.9 | Na2S/K2HPO4 | None/CO2 | 1.5 (1.33; 1.69) |
| 11 | 12.6 | 3.9 | Na2S | None/CO2 | 1.8 (2.12; 1.44) |
| 12 | 9.1 | 3.9 | K2HPO4 | None/CO2 | (<0.37)# |
| 13 | 12.1 | 3.9 | K3PO4 | None/CO2 | 0.9 (1.04; 0.76) |
n.d., none detected.
Vent fluid concentrations for precipitation in all reactions were as follows: Na2S, 100 mM; K2HPO4, 10 mM; Na2Si3O7, 10 mM. The same concentrations were used postprecipitation, as relevant. K3PO4 (100 mM) was used postprecipitation in experiment 14. In all reactions, both during and after precipitation, vent fluids were driven by H2 at 1.5 bar and a flow rate of 5 µL/min.
Ocean fluid for precipitation was composed of FeCl2 (50 mM) and NiCl2 (5 mM) in H2O, pushed by CO2 (1.5 bar) at a flow rate of 5 µL/min. Following precipitation, the ocean fluid for reaction was changed to contain no solutes besides dissolved CO2 from the driving gas, except for experiment 9 with Na2CO3 (100 mM).
Average calculated concentrations listed, with results from duplicate samples in parentheses.
Same as in Table 1.
Vent fluid titrated with 1 M HCl to pH 7.0 (experiment 7) or pH 3.9 (experiment 8).
Peak was observable, but concentration was below the limit of quantification (0.37 µM; SI Appendix, Fig. S16).
While we cannot preclude the possibility of precipitate-bound sulfide acting as a reductant in addition to H2, these results simultaneously confirm the role of the pH gradient and show that a continuous supply of aqueous sulfide is not required in our system; this contrasts with the continued requirement for H2 (Table 1, experiment 3). Removing Ni from the ocean precipitation fluid (experiment 14) produced only small amounts of formate (below our limit of quantification of 0.37 µM). Conversely, replacing Fe to leave Ni as the only metal in the ocean precipitation fluid (NiCl2, 55 mM; experiment 15) yielded 1.4 µM formate, pointing toward a crucial role for Ni within the precipitates. Removing both FeCl2 and NiCl2 from the ocean fluid expectedly did not produce a precipitate, and did not result in the production of detectable formate (experiment 16).
NMR spectra and further analyses for all experiments discussed here are presented in SI Appendix, Figs. S3–S20. Before-and-after images of the precipitates in a number of the reactions are provided in SI Appendix, Fig. S21.
Discussion
Here we report the abiotic pH-driven reduction of CO2 to formate with H2 in a minimalistic microfluidic replicant of an alkaline hydrothermal system. Removing the pH gradient entirely or significantly reducing it—either by acidifying the vent fluid or alkalinizing the ocean fluid—yielded no detectable formate. With the acidic ocean side fixed at pH 3.9, we observed greater formate yields with increasing vent-side pH. In experiments 7, 12, 13, 1, and 11 in Table 2, as the vent-side pH rose (7.0 < 9.1 < 12.1 < 12.3 < 12.6, respectively), so did the average formate concentration measured (undetected < below 0.37 µM < 0.9 µM < 1.5 µM < 1.8 µM).
Removing the precipitate entirely also yielded no product. Without Ni in the ocean precipitation fluid, the yield dropped below our limit of quantification, suggesting that Ni is a crucial part of the precipitate for the reduction mechanism operating here. Given that Ni can typically serve as a more efficient classical and ionic hydrogenation catalyst than Fe (33), the requirement for Ni within the precipitate is not surprising. Although we do not invoke such a direct hydrogenation mechanism here, the indirect electrochemical mechanism that we propose in Fig. 1A would rely on the metal center for similar catalytic functions as those required during classical or ionic hydrogenations.
Removing H2 entirely by replacing it with N2 as the vent-driving gas, while still keeping Na2S in the vent fluid, also led to no detectable product (experiment 3). Conversely, we did observe successful formate production with Na2S absent from the vent fluid postprecipitation and H2 as the vent-driving gas (experiment 14). Taken together, these results suggest that H2 is a necessary reagent in our mechanism and that sulfide is insufficient as a reducing agent. It is also noteworthy that experiment 11, which lacked K2HPO4 and Na2Si3O7 in the vent solution at the postprecipitation stage, yielded a higher concentration of formate than experiment 1 despite containing the same concentration of sulfide; we attribute this result to the higher vent-side pH in the absence of K2HPO4 and Na2Si3O7. While we cannot presently affirm that the precipitates are acting only as catalysts, overall our results suggest that H2 is the main electron donor, that a large pH gradient is necessary for its oxidation, and that sulfide is insufficient (and might not be required) as an electron donor.
We used 1.5 bar pressures of both H2 and CO2, which are nominally not too dissimilar to the ambient-pressure bubbling used in similar earlier work that did not yield any detectable reduced carbon (31, 32). In those experiments, the two gases were dissolved into the fluids before the reactions, with inevitable loss by volatilization, particularly of H2. Recent calculations suggest that atmospheric pressures of H2 and CO2 are only marginally insufficient to drive the reaction to formate (32). Therefore, we infer that the positive results reported in this work are most plausibly due to the advantages of the gas-driven system used here, which likely resulted in higher dissolved gas concentrations than the atmospheric pressure bubbling performed previously (31, 32). Chiefly, the ability to keep the gases consistently within the system, as well as to more reliably keep the fluids anoxic, may suffice as an explanation for the positive results observed here compared with previous attempts.
Alternative Mechanisms for the Reduction of CO2 Linked to H2 Oxidation.
Several CO2-reduction mechanisms are possible for a laminar-flow system such as the one that we have used. We briefly explore a number of them below and discuss why we deem them less likely than the electrochemical mechanism proposed in Fig. 1A. Further details are presented in SI Appendix, Figs. S22–S27.
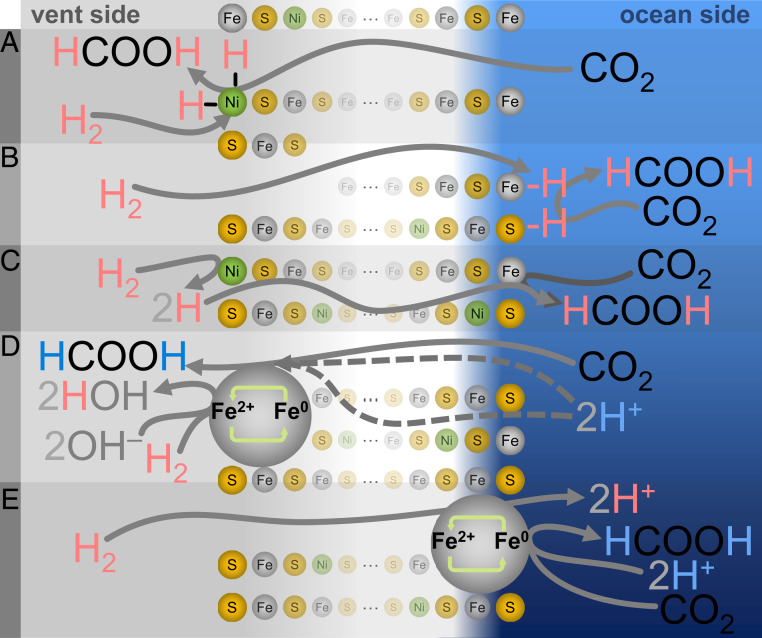
Perhaps the most chemically intuitive—albeit least biochemically homologous—mechanisms of carbon reduction possible here would be direct hydrogenations (Fig. 3 A–C), in which hydrogen from H2 would be transferred directly to CO2 (34–38) either as atomic hydrogen (classical hydrogenation) or as hydride (ionic hydrogenation). Most simply, the product in such a mechanism should match the isotopic signature of the H2/D2 vent gas. Instead, the formate produced here matches only the isotopic makeup of the ocean-side water, regardless of the composition of the vent-side gas or water. In these direct-hydrogenation mechanisms, adsorbed hydrogen species could in principle exchange with the surrounding fluid, such that the original isotopic signature is lost (39). However, any such process would imply the migration of significant amounts of fluid across the precipitate. The substantial mixing of fluids involved should have produced a scrambled H/D formyl signal, which we did not observe, all but ruling out any direct hydrogenation mechanisms.
Fig. 3.
Alternative CO2 reduction mechanisms. (A–C) Classical hydrogenations with CO2 permeability (A), H2 permeability (B), or passage of dissociatively adsorbed atomic H (C) are all unlikely: isotopic labeling (Table 1) indicates that the formyl H derives from ocean-side water rather than H2 from the vent side. (D) Localized redox cycling with CO2 and permeability of H+ (either through a pore or anhydrously through the precipitate) are both unlikely, because in our fully hydrated system, H+/D+ would exchange with local vent water, giving a mixed or exchanged isotopic signal that we do not observe. (E) H2 permeability is also an unlikely mechanism here, because H2 oxidation is much less favored on the acidic (ocean) side compared with the alkaline (vent) side, as demonstrated by the pH exploration data in Table 2. More details are provided in the text and SI Appendix, Figs. S22–S27.
Alternatively, the hydrogen atoms in the resultant formate might not derive directly from H2. Instead, the mechanism may proceed via redox cycling of an edge or corner Fe or Ni atom (M2+ ⇄ M0), wherein a metal is first reduced by H2 (leaving two protons to dilute away) and then the metal transfers the acquired electrons on to CO2, with accompanying proton abstraction from the local aqueous environment (Fig. 3 C–E). Noteworthy for any mechanisms that rely on proton transfer, none of our experiments contained acids added to the ocean side. The acidic pH of 3.9 that we report was achieved solely by dissolution of CO2 in water. Thus, any ocean-side protons must derive from the dissociation of carbonic acid via:
When our reaction was conducted with D2O (experiment 5) as the ocean-side solvent, we found only DCOO– in the efflux, suggesting that CO2 reduction did not occur on the vent side, which had normal H2 as the feed gas and normal H2O as the solvent and thus would have yielded unlabeled HCOO– as the product. This was confirmed in experiment 4 with D2 as the vent-driving gas, which yielded only unlabeled HCOO–.
Several scenarios for such a localized redox cycling are possible (Fig. 3 D and E), but because all require colocation, none could feasibly offer the exclusively ocean-side isotopic signature that we observe. Specifically, if H+ were to cross the precipitate (Fig. 3D; dotted line through pore), it would likely exchange with the isotopic signature of the vent-side water, and we did not observe such a fully exchanged or even mixed signal in the isotopic labeling experiments. Passage of H+ by anhydrous proton conductivity through the mineral lattice (Fig. 3D; dotted line through precipitate) could potentially explain the lack of H+/D+ signal exchange, but such anhydrous proton conductivity would be highly unlikely in an otherwise fully aqueous system. Alternatively, this possible redox cycling could instead be driven by H2 crossing to the ocean side (Fig. 3E); however, this is also unlikely, given that H2 oxidation is considerably less favored on the acidic (ocean) side compared with the alkaline (vent) side, as demonstrated by the pH dependence of our reactions.
As an alternative to the foregoing catalytic mechanisms (classical and ionic hydrogenations, or localized redox cycling), a noncatalytic (i.e., stoichiometric) precipitate oxidation could be driving CO2 reduction, with electrons provided by either free or bound metals or sulfide. However, the lack of reaction in the aqueous sulfide-containing but H2-free experiment 3 suggests that there is a requirement for a continuous supply of electrons from H2, not from dissolved sulfide or the precipitate itself.
Together with the strong pH dependence of the reactions shown in Table 2, these observations suggest that the reduction of CO2 here proceeds via an indirect electrochemical mechanism in which electrons from H2 oxidation on the alkaline vent side are shuttled across the Fe(Ni)S precipitates toward CO2 on the acidic ocean side (Fig. 1A). There the reacting CO2 picks up a proton from the local water, yielding formate with the isotopic signature of the ocean side.
Venturi Pull Inside Hydrothermal Pores Would Bring Ocean Fluids into the Vent System.
We show that production of organics could have occurred on the ocean side of an ancient alkaline hydrothermal-vent system. This raises the question of whether these organics would simply dilute away into the ocean before they could assume any biochemical role (40). It is also conspicuously unlike the WL pathway (32), in which carbon reduction occurs inside the cell. Because the microscopic structure of hydrothermal vents is typically highly porous and reticulated (9, 41), we hypothesize that ocean fluids could have been actively pulled into the microfluidic system due to Venturi-effect suction. Once inside, the ocean’s carbonic fluids could react with electrons being transferred across the vent’s catalytic minerals, and fresh precipitation could also occur further in as the two fluids meet. We have developed a computational finite-elements simulation to explore this prediction (SI Appendix, Fig. S28 and accompanying text in SI Appendix). Our results show that a system of size similar to our 300-µm-wide reactor would indeed lead to the type of microfluidic confluence of reagents that we have shown here and elsewhere (30, 31, 42). Notably, such an effect is not limited to submarine alkaline vents and will likely occur within porous hydrothermal systems at any location and depth, lending itself to multiple geochemical scenarios for the emergence of life.
Batch vs. Flow Reactors.
An alternative one-pot batch (rather than microfluidic) system for the reduction of CO2 with H2 has been reported recently (43), elaborating on previous results (18, 44, 45). Using more highly reduced minerals (Fe3Ni), higher pressures (10 bar H2), and higher temperatures (100 °C) than those used here, the batch system generates significantly higher concentrations of formate, as well as multiple further reduction products (including acetate, methanol, and pyruvate), all at a formate production rate (5.21 × 10−9 mol/s) four orders of magnitude higher than that reported here (2.5 × 10−13 mol/s). Several of these reduction products (e.g., acetate, pyruvate) represent an as-yet unachieved benchmark for microfluidic systems: the formation of new C–C bonds.
In a closer comparison to our system, when the mineral Fe3S4 was used (43) at a pressure of 1.6 bar H2 and temperature of 20 °C, the production rate (1.35 × 10−11 mol/s) was two orders of magnitude higher than that for our system. The differences between the two systems are crucial. The pH-driven CO2 reduction demonstrated here, along with the possibility of thermal-driven concentration increases in small pores (46–48), make microfluidic reactors attractive despite the presently lower yields. The two types of systems thus provide complementary compelling evidence for organics arising under anoxic alkaline hydrothermal vent conditions, and both must be explored further as potential sources for life’s first molecules.
Fates of Formate beyond the WL Pathway.
While we speculatively favor the continuity between geochemistry and biochemistry offered by the WL pathway of carbon fixation, it is worth noting that formate can readily yield formamide, hydrogen cyanide, and carbon monoxide, each of which is at the core of major alternative scenarios for the origin of life (19–21). Therefore, our results may extend beyond the alkaline hydrothermal vent theory or may link it to other scenarios, a set of possibilities that remain to be explored.
Conclusions
We report the pH-driven reduction of CO2 with H2 in the first step of a geologically plausible analog—and proposed evolutionary predecessor (2)—of the WL pathway of carbon fixation. These results tie in with previous findings that alkaline vent-like pH gradients can be kept at a microscale across Fe(Ni)S precipitates (30), that these pH gradients can provide the necessary overpotentials to drive the reaction between H2 and CO2 (15), that low pressures of the two gases are insufficient for reaction (31, 32), that heat gradients in hydrothermal systems can drive concentration increases by thermophoresis and capillary flow at gas–liquid interfaces (46–48), and that redox and pH gradients can drive early bioenergetics (49, 50) and amino acid synthesis (51).
Both dissolved hydrogen gas and a significant pH gradient of the type and polarity found at alkaline hydrothermal vents appear to be necessary driving forces in our system. The exact sources and sinks of H2—and corresponding concentrations of the dissolved gas—on present-day and early-Earth hydrothermal systems remain areas of intensive research (11, 52). Our results suggest that compartmentalization and pH gradients may become more important at lower H2 concentrations (52). Furthermore, our findings suggest an electrochemical mechanism whereby dissolved H2 oxidizes on the alkaline vent side and its electrons travel through the catalytic precipitate network toward the acidic ocean side, where they reduce dissolved CO2 to formate. Experimental confirmation of this hypothesis has proven elusive to date (5, 31, 32). Since this is the first endergonic hurdle in the reduction of CO2 via the sole energetically profitable CO2 fixation pathway known, our findings provide a geologically credible route to a plausible origin of carbon fixation at the emergence of life on the early Earth and potentially on water-rock planetary bodies elsewhere.
Methods
Minimal simulants of alkaline-vent and oceanic fluids were driven into respective tips of a Y-shaped borosilicate reactor. To maximize the amount of gases dissolved, we used gas pressure-driven microfluidic pumps (Mitos P-Pump; Dolomite Microfluidics). The ocean fluid was pushed with CO2, and the vent fluid was pushed with H2, both at 1.5 bar. The reaction was undertaken in an anaerobic N2-purged glovebox. Single (mixed) effluxes were collected and analyzed using 1H and 13C NMR. Quantification was achieved with 1H NMR. Further descriptions of experimental, analytical, and computational methods used in this study are provided in SI Appendix.
Supplementary Material
Acknowledgments
R.H., L.M.B., and V.S. acknowledge support from the NASA Maine Space Grant Consortium (SG-19-14 and SG-20-19). R.H. is supported by NSF Award 1415189 and acknowledges support from the Japan Society for the Promotion of Science (JSPS) (FY2016-PE-16047). The research reported in this paper was supported by an Institutional Development Award from the National Institute of General Medical Sciences of the NIH (P20GM103423). S.E.M. is supported by NSF Award 1724300. L.M.B.’s portion of this work was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration. V.S. acknowledges support from the JSPS (FY2016-PE-16721), the European Molecular Biology Organization (ALTF-1455-2015), the Institute for Advanced Study in Berlin, and the Gerstner Family Foundation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002659117/-/DCSupplemental.
Data Availability.
All data necessary for the replication of this work, including descriptions of the reactor and fluid compositions, are available in the main text and SI Appendix.
References
- 1.Russell M. J., Hall A. J., From geochemistry to biochemistry: Chemiosmotic coupling and transition element clusters in the onset of life and photosynthesis. Geochemical News 113, 6–12 (2002). [Google Scholar]
- 2.Russell M. J., Martin W., The rocky roots of the acetyl-CoA pathway. Trends Biochem. Sci. 29, 358–363 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Sleep N. H., Bird D. K., Pope E. C., Serpentinite and the dawn of life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 2857–2869 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braakman R., Smith E., The emergence and early evolution of biological carbon-fixation. PLoS Comput. Biol. 8, e1002455 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herschy B.et al., An origin-of-life reactor to simulate alkaline hydrothermal vents. J. Mol. Evol. 79, 213–227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sojo V., Herschy B., Whicher A., Camprubí E., Lane N., The origin of life in alkaline hydrothermal vents. Astrobiology 16, 181–197 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Sleep N. H., Meibom A., Fridriksson T., Coleman R. G., Bird D. K., H2-rich fluids from serpentinization: Geochemical and biotic implications. Proc. Natl. Acad. Sci. U.S.A. 101, 12818–12823 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley D. S., Baross J. A., Delaney J. R., Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu. Rev. Earth Planet. Sci. 30, 385–491 (2002). [Google Scholar]
- 9.Kelley D. S.et al., A serpentinite-hosted ecosystem: The lost city hydrothermal field. Science 307, 1428–1434 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Zgonnik V., The occurrence and geoscience of natural hydrogen: A comprehensive review. Earth-Sci. Rev. 203, 103140 (2020). [Google Scholar]
- 11.Worman S. L., Pratson L. F., Karson J. A., Schlesinger W. H., Abiotic hydrogen (H2) sources and sinks near the Mid-Ocean Ridge (MOR) with implications for the subseafloor biosphere. Proc. Natl. Acad. Sci. U.S.A. 117, 13283–13293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitschke W., Russell M. J., Beating the acetyl coenzyme A-pathway to the origin of life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thauer R. K., Jungermann K., Decker K., Energy conservation in chemotrophic anaerobic bacteria. Bacteriol. Rev. 41, 100–180 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maden B. E., Tetrahydrofolate and tetrahydromethanopterin compared: Functionally distinct carriers in C1 metabolism. Biochem. J. 350, 609–629 (2000). [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi A.et al., Electrochemical CO2 reduction by Ni-containing iron sulfides: How is CO2 electrochemically reduced at bisulfide-bearing deep-sea hydrothermal precipitates? Electrochim. Acta 141, 311–318 (2014). [Google Scholar]
- 16.Ooka H., McGlynn S. E., Nakamura R., Electrochemistry at deep-sea hydrothermal vents: Utilization of the thermodynamic driving force towards the autotrophic origin of life. ChemElectroChem 6, 1316–1323 (2019). [Google Scholar]
- 17.Muchowska K. B.et al., Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 1, 1716–1721 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varma S. J., Muchowska K. B., Chatelain P., Moran J., Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat. Ecol. Evol. 2, 1019–1024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saladino R., Crestini C., Pino S., Costanzo G., Di Mauro E., Formamide and the origin of life. Phys. Life Rev. 9, 84–104 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Patel B. H., Percivalle C., Ritson D. J., Duffy C. D., Sutherland J. D., Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber C., Wächtershäuser G., Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 276, 245–247 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Bernstein M., Prebiotic materials from on and off the early Earth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1689–1700, discussion 1700–1702 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasek M., Lauretta D., Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Orig. Life Evol. Biosph. 38, 5–21 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Ferus M.et al., High energy radical chemistry formation of HCN-rich atmospheres on early Earth. Sci. Rep. 7, 6275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitadai N., Maruyama S., Origins of building blocks of life: A review. Geosci. Front. 9, 1117–1153 (2018). [Google Scholar]
- 26.Poehlein A.et al., An ancient pathway combining carbon dioxide fixation with the generation and utilization of a sodium ion gradient for ATP synthesis. PLoS One 7, e33439 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood H. G., Life with CO or CO2 and H2 as a source of carbon and energy. FASEB J. 5, 156–163 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Thauer R. K., Kaster A.-K., Seedorf H., Buckel W., Hedderich R., Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6, 579–591 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Schuchmann K., Müller V., Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 12, 809–821 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Möller F. M., Kriegel F., Kieß M., Sojo V., Braun D., Steep pH gradients and directed colloid transport in a microfluidic alkaline hydrothermal pore. Angew. Chem. Int. Ed. 56, 2340–2344 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Sojo V., Ohno A., McGlynn S. E., Yamada Y. M. A., Nakamura R., Microfluidic reactors for carbon fixation under ambient-pressure alkaline-hydrothermal-vent conditions. Life 9, 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasiliadou R., Dimov N., Szita N., Jordan S. F., Lane N., Possible mechanisms of CO2 reduction by H2 via prebiotic vectorial electrochemistry. Interface Focus 9, 20190073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vries J. G., Ed., Catalytic Reduction in Organic Synthesis, (Thieme, 2018), Vol. 1. [Google Scholar]
- 34.Horiuti J., Ogden G., Polanyi M., Catalytic replacement of haplogen by diplogen in benzene. Trans. Faraday Soc. 30, 663–665 (1934). [Google Scholar]
- 35.Hudson R., Rivière A., Cirtiu C. M., Luska K. L., Moores A., Iron-iron oxide core-shell nanoparticles are active and magnetically recyclable olefin and alkyne hydrogenation catalysts in protic and aqueous media. Chem. Commun. 48, 3360–3362 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Peng G., Sibener S. J., Schatz G. C., Ceyer S. T., Mavrikakis M., CO2 hydrogenation to formic acid on Ni(111). J. Phys. Chem. 116, 3001–3006 (2012). [Google Scholar]
- 37.Kelsen V.et al., The use of ultrasmall iron(0) nanoparticles as catalysts for the selective hydrogenation of unsaturated C-C bonds. Chem. Commun. 49, 3416–3418 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Hudson R.et al., Highly efficient iron(0) nanoparticle-catalyzed hydrogenation in water in flow. Green Chem. 15, 2141–2148 (2013). [Google Scholar]
- 39.Miller S. L., Rittenberg D., The catalysis of the H2-D2O exchange by aqueous buffer solutions. J. Am. Chem. Soc. 80, 64–65 (1958). [Google Scholar]
- 40.Wächtershäuser G., In praise of error. J. Mol. Evol. 82, 75–80 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Ludwig K. A., Kelley D. S., Butterfield D. A., Nelson B. K., Früh-Green G., Formation and evolution of carbonate chimneys at the lost city hydrothermal field. Geochim. Cosmochim. Acta 70, 3625–3645 (2006). [Google Scholar]
- 42.Wang Q., Steinbock O., Materials synthesis and catalysis in microfluidic devices: Prebiotic chemistry in mineral membranes. ChemCatChem 12, 63–74 (2019). [Google Scholar]
- 43.Preiner M.et al., A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 4, 534–542 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Seewald J. S., Zolotov M. Y., McCollom T., Experimental investigation of single carbon compounds under hydrothermal conditions. Geochim. Cosmochim. Acta 70, 446–460 (2006). [Google Scholar]
- 45.He C., Tian G., Liu Z., Feng S., A mild hydrothermal route to fix carbon dioxide to simple carboxylic acids. Org. Lett. 12, 649–651 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Duhr S., Braun D., Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. U.S.A. 103, 19678–19682 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baaske P.et al., Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc. Natl. Acad. Sci. U.S.A. 104, 9346–9351 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morasch M.et al., Heated gas bubbles enrich, crystallize, dry, phosphorylate and encapsulate prebiotic molecules. Nat. Chem. 11, 779–788 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Sojo V., Pomiankowski A., Lane N., A bioenergetic basis for membrane divergence in archaea and bacteria. PLoS Biol. 12, e1001926 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West T., Sojo V., Pomiankowski A., Lane N., The origin of heredity in protocells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barge L. M., Flores E., Baum M. M., VanderVelde D. G., Russell M. J., Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems. Proc. Natl. Acad. Sci. U.S.A. 116, 4828–4833 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tutolo B. M., Seyfried W. E. Jr., Tosca N. J., A seawater throttle on H2 production in Precambrian serpentinizing systems. Proc. Natl. Acad. Sci. U.S.A. 117, 14756–14763 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamura R.et al., Electrical current generation across a black smoker chimney. Angew. Chem. Int. Ed. Engl. 49, 7692–7694 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data necessary for the replication of this work, including descriptions of the reactor and fluid compositions, are available in the main text and SI Appendix.