Significance
Far-red photosystem II (FR-PSII) contains a small number of FR-chlorophylls (-f or -d) with the rest (∼85%) being chlorophyll-a molecules. Here ultrafast studies on FR-PSII support a model in which the primary electron donor is a FR-chlorophyll (P720, likely in the ChlD1 position), while the second electron donor is chlorophyll-a at the PD1 position, forming PD1+•. Excitation energy transfer from chlorophyll-a to the FR-chlorophylls is ultrafast. The excited state of FR-chlorophyll remains highly localized, i.e., P720* does not share the excitation with the chlorophyll-a pigments. This is markedly different from both the conventional, chlorophyll-a PSII, and the chlorophyll-d PSII of Acaryochloris marina. The entropic and site-energy differences result in efficient but apparently slower stabilization of the charge-separated state.
Keywords: chlorophyll-f, photosystem II, photosynthesis
Abstract
The recently discovered, chlorophyll-f-containing, far-red photosystem II (FR-PSII) supports far-red light photosynthesis. Participation and kinetics of spectrally shifted far-red pigments are directly observable and separated from that of bulk chlorophyll-a. We present an ultrafast transient absorption study of FR-PSII, investigating energy transfer and charge separation processes. Results show a rapid subpicosecond energy transfer from chlorophyll-a to the long-wavelength chlorophylls-f/d. The data demonstrate the decay of an ∼720-nm negative feature on the picosecond-to-nanosecond timescales, coinciding with charge separation, secondary electron transfer, and stimulated emission decay. An ∼675-nm bleach attributed to the loss of chl-a absorption due to the formation of a cation radical, PD1+•, is only fully developed in the nanosecond spectra, indicating an unusually delayed formation. A major spectral feature on the nanosecond timescale at 725 nm is attributed to an electrochromic blue shift of a FR-chlorophyll among the reaction center pigments. These time-resolved observations provide direct experimental support for the model of Nürnberg et al. [D. J. Nürnberg et al., Science 360, 1210–1213 (2018)], in which the primary electron donor is a FR-chlorophyll and the secondary donor is chlorophyll-a (PD1 of the central chlorophyll pair). Efficient charge separation also occurs using selective excitation of long-wavelength chlorophylls-f/d, and the localization of the excited state on P720* points to a smaller (entropic) energy loss compared to conventional PSII, where the excited state is shared over all of the chlorin pigments. This has important repercussions on understanding the overall energetics of excitation energy transfer and charge separation reactions in FR-PSII.
Photosystem II (PSII) is a pigment–protein complex found in plants, algae, and cyanobacteria. It plays a major role in photosynthesis as the water/plastoquinone photooxidoreductase, responsible for the water-splitting reaction that puts oxygen into the atmosphere (1). The structure and function of PSII have been extensively investigated using a wide range of methods including X-ray crystallography (2–6). PSII is made up of a central pair of near-symmetrical protein subunits, D1 and D2, containing the cofactors involved in photochemical charge separation, water oxidation, and quinone reduction. The central D1/D2 subunits are surrounded by the CP43 and CP47 antenna subunits (2–6). Excitation by light of a chlorophyll in PSII is shared between the chlorophylls and the pheophytins through excitation transfer. When a redox active chlorophyll in the D1/D2 reaction center is excited, charge separation occurs. The central reaction center chlorin pigments, which are collectively known as P680, are made up of four chlorophyll-a (chl-a) molecules (PD1, PD2, ChlD1, and ChlD2) and two pheophytin-a molecules (PheoD1 and PheoD2). The order and timing of charge separation and electron transfer remain rather uncertain despite extensive research (2–4).
Several lines of evidence, including ultrafast kinetic studies, indicate that charge separation proceeds at least in part via the formation of ChlD1+•PheoD1−• as the first radical pair (RP1) (3, 4, 7–12). It is also thought RP1 could be a distribution of ChlD1+•PheoD1−• in some centers and PD1+•ChlD1−• in others (13, 14). However, the view that RP1 in PSII is PD1+•ChlD1−• in all of the centers is still advocated (15). This model was originally based on the photochemistry in the well-characterized purple bacterial reaction center, where RP1 is P+B−, P is a special pair of (bacterio)chlorophylls over which the cation is shared and B is a monomeric (bacterio)chlorophyll (16). Irrespective of the identity of RP1, the secondary radical pair (RP2) in PSII is thought to be PD1+•PheoD1−•. The electron transfer from PheoD1−• to QA, leads to formation of PD1+•QA−•, RP3 (2–4, 9, 15). This uncertainty in the order and timing of the charge separation process is compounded by uncertainty over the rates and reversibility of excitation energy transfer (17).
These difficulties arise in large part from the fact that all of the 37 chlorins in PSII cores (35 chlorophylls-a and two pheophytins-a) are essentially the same color. This means 1) there is little scope for wavelength selectivity; 2) spectral deconvolution is near intractable; 3) there is little driving force for directionality of excitation energy transfer; and consequently, 4) excitation is shared over all of the pigments.
In 2010, a new form of chlorophyll was discovered in cyanobacteria: chlorophyll-f (chl-f) (18). Chl-f differs from chl-a chemically through the substitution of the methyl group at the C-2 position by a formyl group, causing the Qy absorption band to shift to significantly longer wavelengths, e.g., from 670 nm to 706 nm in methanol (18). This pigment is synthesized when some species of cyanobacteria grow in environments where most of the visible spectrum is shaded but far-red and near-infrared light is abundant. Under these conditions a variant form of PSII is expressed (19). In the case of Chroococcidiopsis thermalis PCC 7203, this FR-PSII contains 4 chl-f, 1 chl-d, and ∼30 chl-a and the long-wavelength chlorophylls were shown not only to be antenna pigments, but also to be responsible for primary charge separation (12).
Similar rates of PSII activity measured for FR-PSII excited with visible and far-red light at both room temperature and cryogenic temperatures showed that a far-red light chlorophyll, either chl-f or chl-d, was the primary electron donor (12). In the light-minus-dark 77 K difference absorption spectrum, a blue shift centered at 727 nm was attributed to long-wavelength ChlD1, because in conventional chlorophyll-a-containing PSII, ChlD1 gives rise to the dominant blue shift (∼680 nm) upon QA−• formation. A similar shift was seen when PheoD1−• was formed. These observations suggested that ChlD1 is the long-wavelength chlorophyll in FR-PSII that acts as the primary donor. This was supported by the finding that FR-PSII is highly luminescent, suggesting a smaller energy gap between P* (the excited state of the primary donor) and the primary radical pair (RP1), consistent with a long-wavelength primary donor (12).
Fitting of the 1.8 K magnetic circular dichroism (MCD) and absorption spectra of isolated FR-PSII indicated five long-wavelength chlorophylls with absorption maxima at 721, 727, 734, 737, and 749 nm. The primary donor, giving rise to the 727-nm band shift and suggested to be ChlD1, could not be definitively assigned to either chl-d or chl-f but chl-f was considered the more likely candidate (12).
The presence of five FR-chlorophylls, which were not only shifted from the bulk absorption of chlorophyll-a but also distinguishable from each other, provides a unique opportunity to resolve the primary events in PSII (12). This unprecedented alleviation of the spectral congestion that has complicated spectral and mechanistic assignments in PSII research, makes FR-PSII particularly attractive for a range of spectroscopic studies. In addition to understanding the novel FR-PSII itself, these studies could provide insights relevant to conventional chl-a PSII. The only previous subnanosecond time-resolved studies of FR-PSII used time-correlated single-photon counting fluorescence measurements in far-red light grown, intact cells of Halomicronema hongdechloris (20, 21). Here we report a femtosecond-to-nanosecond visible transient absorption (TA) study of energy transfer and charge separation kinetics from what is at present the only published preparation of isolated FR-PSII cores (12). This material was isolated from FR-grown C. thermalis and has been the subject of detailed biochemical and biophysical analyses (12).
Results
Transient absorption measurements were performed with two types of PSII core complexes: 1) from C. thermalis grown under far-red light conditions, FR-PSII, and 2) from Thermosynechococcus elongatus grown in white light, WL-PSII. The experimental data for the WL-PSII, which are shown and described in detail in SI Appendix, were similar to those reported in the literature.
Transient Absorption Spectra and Lifetime Maps of FR-PSII.
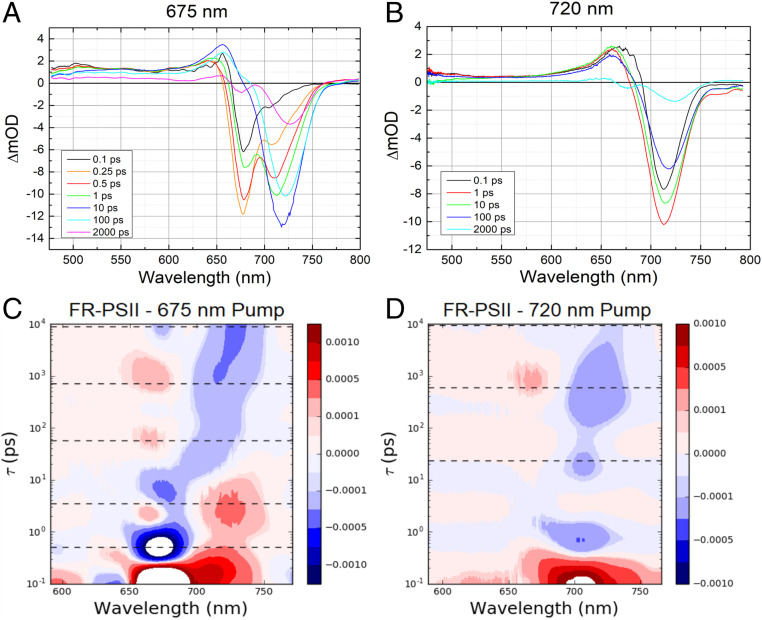
The FR-PSII spectra were obtained for two excitation wavelengths, 675 nm and 720 nm. The 675-nm excitation is absorbed primarily by the chl-a pigments (Fig. 1A), whereas 720-nm excitation is absorbed only by the long-wavelength pigments, chl-f and chl-d (Fig. 1B). The transient absorption spectra at selected delays for both excitation wavelengths are shown in Fig. 1 A and B. Panels C and D of Fig. 1 show the corresponding lifetime density maps.
Fig. 1.
(Upper) FR-PSII transient absorption spectra at selected delays with (A) 675-nm and (B) 720-nm pump wavelengths. The delay times are between 0.1 ps and 2,000 ps as color coded in the legends. (Lower) Lifetime density maps of FR-PSII TA data for (C) 675-nm and (D) 720-nm excitations. Positive amplitudes, represented in red, indicate increased bleaching/stimulated emission or decay of absorption. Negative amplitudes, represented in blue, indicate increased absorption or decay of bleaching/stimulated emission. The dashed lines correspond to the lifetimes associated with the sequential models used to fit the TA data (Fig. 3).
Close to time 0, the 675-nm excitation resulted in a difference spectrum showing one major negative feature at 677 nm attributed to 1) a ground state bleach (GSB) of chlorophyll-a and 2) stimulated emission (SE) band. In addition, an excited state absorption (ESA) at 655 nm and broadband absorption in the 500- to 650-nm region (Fig. 1A black spectrum) are evident. Those features are quite similar to those seen in WL-PSII (SI Appendix, Fig. S2), except that the FR-PSII spectrum also shows a small GSB/SE band at 705 nm, the short-wavelength edge of the FR-chlorophyll absorption.
In the 0.25-ps difference spectrum (Fig. 1A), the minor GSB/SE band at ∼705 nm increased in amplitude and its peak shifted by 4 nm to 709 nm. The amplitude of the main GSB/SE band at 677 nm also increased in this spectrum. In contrast, the 0.5-ps spectrum shows a decrease in the amplitude of the main GSB/SE band, while the amplitude of the 709-nm GSB/SE band continued to increase.
In the 1-ps spectrum, the GSB/SE band at ∼677 nm decreased further, while the 712-nm GSB/SE band increased further dominating the spectrum. By 10 ps, the GSB/SE in the 670- to 680-nm region had completely decayed, leaving a positive band at 656 nm and a major GSB/SE band at 720 nm as the main features in the spectrum (Fig. 1A).
At 100 ps, the amplitudes of the positive band at 656 nm and the ∼720-nm GSB/SE decreased, the latter shifting further to the red, 722 nm. In the 2,000-ps spectrum, the long-wavelength feature is at 727 nm and a small GSB at 675 nm is present (Fig. 1A).
The lifetime density maps shown in Fig. 1 C and D are the result of lifetime density analysis performed on the TA data using a semicontinuous distribution of ∼100 lifetimes in the 100-fs to 10-ns range (22). The maps provide a model-free analysis of the wavelength dependence of the TA kinetics with a choice of regularization technique that is particularly well suited to the highly heterogenous photosynthetic dynamics (22). The lifetime density map in Fig. 1C transforms the full dataset generated with 675-nm excitation of FR-PSII. The features described in the transient absorption spectra (Fig. 1A) are reflected in the maps. Interestingly, a second positive feature at 2 to 3 ps can be noted in the 660- to 670-nm region in addition to the positive peak in the 720- to 740-nm region that correspond to the bleach and stimulated emission in the transient absorption spectrum. This feature indicates a decay in the excited state absorption in the region, which could be due to an early charge separation.
The lifetime density map in Fig. 1C also shows two positive peaks centered at 670 nm, one at 60 ps, the other at 1,000 ps. These could reflect the lifetimes associated with the appearance of the 675-nm GSB in the long delay spectrum. The discontinuities in the 10-ps and 100-ps spectra (Fig. 1A) at ∼670 nm could reflect the early onset of the 675-nm bleach.
Fig. 1B shows transient absorption spectra of FR-PSII using 720-nm excitation. Compared to the data with 675-nm excitation, there is much less spectral evolution. The initial negative feature (GSB/SE) at 713 nm and the positive peak at 660 to 663 nm dominate the spectrum at delays close to time 0. There is also broadband absorption in the 500- to 650-nm region (Fig. 1 B and D).
There are no major modifications in the spectra at 1 ps and 10 ps apart from a small change in the shape of the features. In the 100-ps spectrum, a shift of the main GSB/SE band to 717 nm is seen. The spectrum at 2,000-ps delay is very similar to that observed for 675-nm excitation and consists of two negative features, the more intense one at 725 nm and a smaller one at 675 nm (Fig. 1B).
Consequently, the TA data collected with 720-nm excitation produced a much simpler lifetime map (Fig. 1D). The major GSB/SE band is reflected in a positive peak appearing around 715 nm on a subpicosecond scale in the lifetime density map (Fig. 1D). There are three distinct negative peaks with ∼0.7-ps, 20-ps, and 400-ps time constants in the same wavelength region. The positive peak seen at ∼670 nm at ∼1,000 ps in the lifetime density map with 720-nm excitation (Fig. 1D), appears to correspond to a similar peak at ∼670 nm with a similar lifetime when 675-nm excitation was used (Fig. 1C).
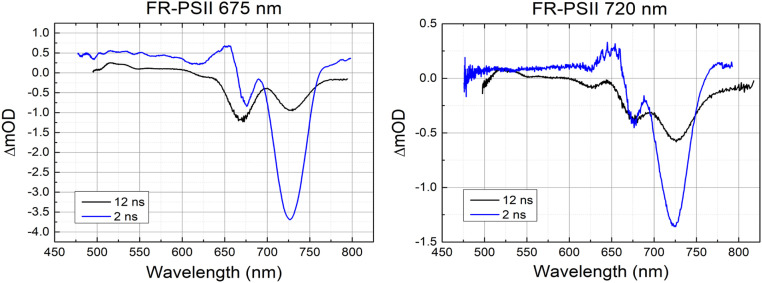
Fig. 2 shows the transient absorption spectra of FR-PSII at 2-ns and 12-ns delays. It is expected that contributions arising from stimulated emission should greatly decrease or fully decay at the longer times. Comparing the 2-ns and 12-ns spectra, both negative features at 675 nm and 725 nm are still present in the 12-ns spectrum despite the decay in the 725-nm band. The presence of both bands at 12 ns indicates that they both are associated with the charge-separated state, presumably RP3, P D1+•QA−•. The spectra appear to be similar for both excitation wavelengths, but with 675-nm excitation resulting in a larger amplitude presumably due to its much bigger absorption cross-section compared to that at 720 nm.
Fig. 2.
Comparison of 2-ns and 12-ns TA spectra of FR-PSII at 675-nm excitation (Left) and 720-nm excitation (Right).
The 12-ns spectra show significant decrease in the positive peak in the 650-nm region and thus, the state responsible for it had largely decayed by this time point. This observation supports the assignment of the positive 655-nm band in long delay spectra to excited state absorption. The excited state absorption that decayed in this time window corresponds to the decay of the stimulated emission at 725 nm (Fig. 2).
Furthermore, the position of the 675-nm bleach in the 12-ns spectrum with 675-nm excitation (Fig. 2, Left) is slightly blue shifted relative to the 2-ns delay measurement. This could be a result of the decayed overlapping positive peak or an electrochromic effect. However, it should be noted that due to the long delay introduced in the probe optical path, the precision of wavelength calibration in the 12-ns data are lower than in the data obtained at shorter delays.
TA spectra were also obtained at 27 ns, a time where the fluorescence should be virtually absent (SI Appendix, Fig. S5). The spectra were similar to the 12-ns spectra but with further decay of the 725-nm band, indicating a contribution from stimulated emission even at 12 ns. A residual stimulated emission contribution from chl-f at 12 ns is also inferred from the presence of the negative feature at 725 nm when the sample translator was switched off, i.e., with closed centers (SI Appendix, Fig. S8).
Comparison of the raw transient absorption data of WL-PSII (SI Appendix, Fig. S2) and FR-PSII at 675-nm excitation (Fig. 1A) shows that the FR-PSII spectra are only similar to those of WL-PSII at very early delays. Within the first 10 ps, the dominant feature in WL-PSII data, the GSB/SE at 675 nm, is fully replaced by the GSB/SE at 720 nm in FR-PSII. It is clear that most of the excitation energy is transferred from chl-a pigments to chl-f/d on the 10-ps timescale.
The improved spectral resolution of FR-PSII due to distinct absorption features of the FR-chlorophyll playing antenna and redox roles allows the spectral evolution of distinct bands to be followed providing easier assignments of charge-separated states compared with the WL-PSII spectra.
Homogeneous Modeling.
Although a homogeneous (sequential) modeling of the TA datasets does not reflect the complex dynamics of the excitation energy transfer and charge separation in PSII cores, it can provide useful insights and inform more sophisticated models. The resulting compartments that represent the discrete set of time constants that describe the data will have heterogeneous contributions, but the separation is based on the statistical significance of the amplitudes of the spectral dynamics. The aim is therefore to analyze the spectra that replace each other sequentially in time. Thus, sequential compartment models were applied to both WL- and FR-PSII data, with the number of compartments chosen using singular value decomposition (23) and the correspondence of the resulting time constants to the lifetime maps. The homogeneous model for the WL-PSII 675-nm excitation data are presented in SI Appendix, Fig. S4, while those for FR-PSII are shown in Fig. 3. The resulting time constants are also shown in the lifetime maps in Fig. 1 C and D.
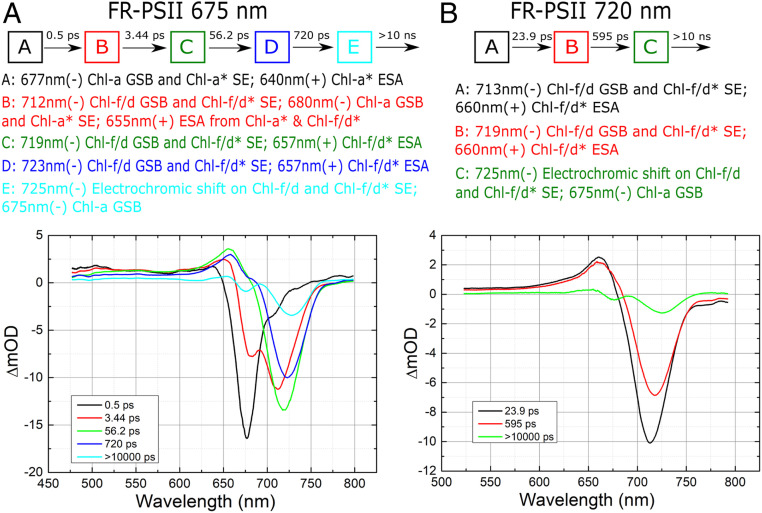
Fig. 3.
(A) Results of sequential five-compartment fit to FR-PSII 675-nm pump data. The time constants are: 0.5 ps, 3.4 ps, 56 ps, 720 ps, and >10,000 ps. (B) Results of sequential three-compartment fit to FR-PSII 720-nm pump data. The time constants are 23 ps, 595 ps, and >10,000 ps. Above each figure is a representation of the model compartments and a list of the main spectral features with their principal physical assignments in each compartment. Assignments of the features to specific pigments in PSII are suggested in Discussion. GSB, ground state bleach; SE, stimulated emission; ESA, excited state absorption.
Fig. 3A shows the globally fitted homogeneous spectra obtained for the TA data of FR-PSII with 675-nm excitation using five compartments. The spectrum in compartment A with a time constant of 0.5 ps has similar features as the equivalent spectrum in the WL-PSII sequential fit (SI Appendix, Fig. S4): a major GSB/SE band around 677 nm, excited state absorption around 640 nm, broad absorption in the 500- to 650-nm region and around 780 nm, and stimulated emission around 740 nm. The spectrum is thus dominated by features attributed to the formation of excited state chl-a antenna.
The compartment B spectrum, with a time-constant of 3.4 ps, shows significant changes: the main GSB/SE band around 677 nm in the compartment A spectrum decreased in B and was replaced by GSB/SE at 712 nm, indicating a loss of Qy ground state absorption of a far-red chlorophyll. This change of the major GSB/SE band position is attributed to the transfer of excitation energy from chl-a antenna to chl-f/d. The transition occurring from compartment A to B is rapid, with a subpicosecond time constant, indicating that the chl-f/d pigments involved are close to the excited chl-a pigments. The chl-f/d bleached at short times are thus likely to be part of the antenna of PSII core complex, perhaps a linker between the chl-a pigments and the other longer wavelength chlorophylls including the photochemical trap, as suggested earlier (12). This rapid downhill energy transfer process is kinetically comparable to other downhill light-harvesting processes in the literature in purple bacterial antenna (e.g., ref. 24), plant LHCII (25), and most pertinently from chl-a to chl-f in FR-PSI (26, 27).
In the spectrum of compartment C (with a 56-ps time constant), the GSB/SE in the 670- to 680-nm region seen in the earlier compartments, which was initially indicative of the presence of excited state chl-a, had fully decayed. The spectrum is dominated by a chl-f/d GSB/SE at 719 nm, and a positive band at 657 nm (Fig. 3A). The positive band at 657 nm may represent broad excited state absorption of the long-wavelength chlorophyll (see below). At this point all excitation energy has been transferred to FR pigments. The 56-ps time constant is close to the 50-ps time constant observed in WL-PSII data (SI Appendix, Fig. S4). A slight decrease in the broad excited state absorption around 500 nm, compared to that in compartments A and B, may indicate radical pair formation. Thus, the compartment C spectrum may reflect the formation of the charge-separated state (RP1).The negative feature at 720 nm clearly arises from the long-wavelength chlorophyll, but it could contain contributions from the loss of ground state absorption due to the presence of excited state and/or cation state as well as from stimulated emission (see below).
The spectrum of the next compartment, D (720 ps), is similar to that of compartment C, except the negative band is further shifted to 723 nm and there is a reduction of all band amplitudes. At such a long delay (720 ps), charge separation would have occurred in WL-PSII (8, 9, 17, 28–30) and thus might be expected in FR-PSII. There is a small feature around 675 nm, which could be caused by a decrease of the ground state absorption of chlorophyll-a and/or pheophytin due to formation of a chlorophyll-a cation radical (PD1+•) and/or the expected pheophytin anion radical (PheoD1−•). However, given the small size of the 675-nm bleach, if there is a contribution from a chl-a cation (e.g., PD1+• see below), it is not yet fully formed.
Compartments C and D, with time constants of 56 ps and 720 ps, are both much shorter than the fluorescence lifetime (a few nanoseconds) of the isolated chl-f/d pigment (31); thus, the events occurring in these lifetimes are likely to be steps in energy transfer and charge separation rather than radiative decay of the excitation energy.
In the spectrum of compartment E, the 675-nm bleach reaches its full amplitude and the long-wavelength band is further shifted to 725 nm (Fig. 3A). A small but noticeable broad absorption around 775 nm present in this compartment spectrum is tentatively attributed to a chlorophyll cation. It is similar to the broadband absorption beyond 700 nm observed in WL-PSII spectra (SI Appendix, Fig. S4). In WL-PSII, the radical pair formed at this time is RP3, PD1+•QA−•, which is stable on the nanosecond timescale. While other assignments of the two negative features are possible (e.g., loss of chl-f/d and chl-a ground state absorptions due to formation of chl-f/d+• in equilibrium with a chl-a+•, or loss of chl-f/d ground state absorption due to formation of a chl-f/d+• in addition to an electrochromic shift on a chl-a), we favor the conventional assignment of the final radical pair state to PD1+•QA−•, with the 675-nm bleach attributed to the formation of PD1+• and the 725-nm feature to an electrochromic blue shift caused by the charges on PD1+•QA−•. This fits with the report of an electrochromic blue shift of a 727-nm chlorophyll due to the charge on QA−• formed at cryogenic temperatures (12). Our assignment also fits with the model of Nürnberg et al. (12), in which a chl-f/d acts as the primary donor (in the ChlD1 location) and a chl-a in the PD1 position acts as the secondary donor.
When 720-nm excitation is used, the sequential fit results are much less complex than with 675-nm excitation, and a three-compartment model is sufficient to describe the data (Fig. 3B). The spectra of the three compartments (A, B, and C) using 720-nm excitation are very similar to those of compartments C, D, and E in the 675-nm excitation sequential fit. It seems clear that the reactions occurring are the same except for the transfer of excitation from chl-a antenna to chl-f, which occurs when chl-a is excited by 675-nm light.
The expected Pheo Qx bleach around 546 nm is not evident in the ultrafast FR-PSII spectra (Fig. 3) but a Pheo Qx bandshift does appear to be present in the nanosecond spectra (Fig. 2). Its presence fits with the reports of two pheophytin-a molecules per PSII and the QA−•-induced bandshift from the PheoD1 Qx band at 546 nm in the low temperature absorption difference spectra (12). This shows that in FR-PSII, the PheoD1 occupies its usual position and thus likely plays its usual role as the primary electron acceptor. The absence of clear changes from Pheo−• in the Qx region at 546 nm and only weak bleaching in the Qy region at ∼670 nm in the ultrafast kinetics (in the 10-ps and 100-ps spectra in Fig. 1A as pointed out earlier) might be explained by a change in the kinetics of its formation and/or decay. This could occur if electron transfer to Pheo were slower or if the rate of transfer from Pheo−• to QA were faster. The ∼546-nm spectral feature in the nanosecond spectra suggests the former possibility (Fig. 2).
Discussion
The transient absorption spectra presented here provide a number of clear insights into primary charge separation in FR-PSII. These insights rely on the unprecedented spectral resolution intrinsic to this recently discovered system.
Excitation Energy Transfer from chl-a* to chl-f/d.
Comparisons of the FR-PSII spectra obtained by exciting chl-a (675 nm) with those obtained when directly exciting chl-f/d (720 nm) show marked differences that are straightforward to interpret. The pigment bleaching associated with the formation of chl-a* in the antenna is followed by the ultrafast transfer of the excitation energy to the chl-f/d. This occurs on the subpicosecond-to-a few picoseconds timescale. Once that has occurred, the evolution of the spectra in time matches what occurs when chl-f/d is excited directly using 720-nm light.
When the long-wavelength chlorophylls are excited, either directly or from excitation energy transfer from chl-a, the excitation remains localized on the chl-f/d without redistributing back to the chlorophylls-a. Given the energy difference between chl-a and the long-wavelength chlorophylls (∼40 nm to ∼100 meV), this is understandable, but the possibility existed that the two sets of pigments were closely interacting or were tuned to span the energy gap and thus would have allowed back transfer. This does not appear to occur, which fits with the distribution of wavelengths for the far-red chlorophylls reported earlier (12).
The evolution of the long-wavelength feature in time consists of a shift to longer wavelengths: from 705 nm to 727 nm after downhill excitation transfer from chl-a* (with 675-nm excitation), and from 713 nm to 725 nm when excitation was directly into chl-f/d (with 720-nm excitation). The shortest wavelengths (705 to 709 nm) are associated with the fastest transfer. Nevertheless, at later times (1 ps; Fig. 1A) the band position is still at a relatively short wavelength, 712 nm, but with time it moves to longer wavelengths, ending up at about ∼725 nm (in the nanosecond spectra; Fig. 2).
The ∼712-nm GSB/SE band may reflect the excitation on the chl-f/d that has the shortest wavelength. Fittings of the absorption spectra at 1.8 K indicated that there is only one long-wavelength chlorophyll with an absorption maximum at a shorter wavelength than the electrochromically shifted chl-f/d in the presence of QA−• and Pheo−• (12). The electrochromically shifted chlorophyll was attributed to the primary electron donor, while the far-red pigment with the shortest wavelength was suggested to be the chl-d, because of its intrinsic absorption properties. The shortest wavelength chlorophyll of the five chlorophylls-f/d was suggested to have a role as a “linker” pigment, acting as a physical and energetic bridge between the other four long-wavelength chlorophylls and the chl-a and/or the allophycocyanin antenna (12). The present data are consistent with such a role.
We note the absorption peak positions for the long-wavelength chlorophylls at 1.8 K appeared to be shifted further to long wavelengths (by ∼7 nm) compared to their positions at room temperature (12). The current study, which is done at room temperature, appears to fit with the previous room temperature position for the bandshift at 720 nm. The discussion of this feature and the proposed linker chlorophyll f/d at ∼712 nm, takes this temperature-induced shift into account when comparing with the 1.8 K spectra.
Excited State Decay.
The spectra collected on the few-to-tens of nanoseconds timescale showed a decrease in the long-wavelength negative feature. Light emission from an excited state chlorophyll will be detected as stimulated emission in the wavelength range of spontaneous fluorescence. Chlorophyll fluorescence in photosynthesis is expected to decay in ∼5 ns (32). It is thus likely that some of the reduction in the amplitude of the negative feature at 725 nm is associated with the decay of stimulated emission from the excited state of the long-wavelength chlorophyll contributing to the feature. This could reflect a small fraction of centers that are closed (where QA−• is present before the flash), although tests showed that the centers were primarily open (SI Appendix). In order to ensure that excited state decay was complete, additional measurements with an even longer delay time of 27 ns were made, and this spectrum still exhibited the negative feature at 725 nm. This confirmed its assignment to a feature associated with the final radical pair state.
Charge Separation.
The spectral evolution of the long-wavelength features at both excitation wavelengths (675 nm and 720 nm) demonstrate the direct involvement of chlorophyll-f in the primary charge separation reactions, a view that was recently proposed (12) and that challenged the original consensus that chlorophyll-f only functions as an antenna pigment (33).
The spectra taken at the longest times and attributed to PD1+•QA−•, contain two negative features at 675 nm and 725 nm. In the context of the existing model, these are assigned to the loss of ground state absorption of chl-a at 675 nm due to the formation of the cation radical PD1+•, and to the long-wavelength trough (725 nm) of an electrochromic shift on the absorption of a FR-chlorophyll centered at 720 nm and proposed to be ChlD1 (12).
The model proposes that the FR-ChlD1 is the primary donor, while a chl-a PD1 is the secondary donor and bearer of the final chlorophyll cation radical. The kinetic measurements are consistent with this model.
The data indicate that the final radical pair does show a bleach at 675 nm, which matches the expected wavelength of absorption for PD1. This is considered direct evidence of the secondary donor at the PD1 position remaining a chlorophyll-a in FR-PSII. The bandshift on the far-red ChlD1 fits well with the steady-state spectra (12) and with what was seen for the PD1+•QA−• radical pair spectrum in Acaryochloris marina PSII, which is also modeled as a chl-a PD1 cation with a chl-d ChlD1 bandshift (34). Therefore, the presence of the electrochromic shift at 725 nm in the nanosecond spectra is strong support for a far-red chlorophyll acting as the primary donor, probably at the ChlD1 position (12) and provides additional evidence for the still-contested model that assigns a primary donor role to ChlD1 in conventional PSII (3, 4, 15, 7–14).
Although there are signs of the emergence of the 675-nm bleach at 10 ps and 100 ps (Fig. 1A), it is still a minor component in the compartmental spectrum with τ = 720 ps (Fig. 3A) and it is not fully developed until the 2-ns spectra (Fig. 1 A and B). The lifetime map (Fig. 1C) indicates an increased bleaching in the 675-nm region at around 60 ps, and then again after 1 ns, which is apparently the lifetime associated with the full development of the bleach. Thus, it appears that the PD1+• is formed later than is generally assumed for WL-PSII which is on the order of a few tens of picoseconds. In fact, the earlier weaker bleach in the 670-nm region may arise from the formation of Pheo−•, which is expected to be present in the earlier radical pair(s).
While the QA−•-induced bandshift on PheoD1 Qx absorption at 546 nm seemed to be present in the nanosecond spectra, in accordance with low temperature difference spectra (12), this change and that expected from the bleach associated with anion radical formation were not resolved in the ultrafast data (Fig. 3). A possible explanation could be that the electron transfer kinetics are modified in FR-PSII such that transient accumulation is significantly reduced. This kinetic spread could also explain the weakness of the bleach in the Qy region caused by the presence of PheoD1−• which is expected in RP1 and RP2.
The excitation energy transfer and charge separation kinetics in FR-PSII might be a manifestation of different subpopulations of centers undergoing charge separation over a range of lifetimes. This would make it more difficult to detect the bleaching and bandshift of the Pheo Qx band in the spectra at specific delay points. One scenario that could cause such heterogeneity would be the existence of different pathways of excitation energy transfer prior to charge separation. Rapid charge separation would be expected when excitation energy arrives directly at the long-wavelength (∼720 nm) primary donor. Slower charge separation would be expected if equilibration between antenna chl-f pigments occurs followed by a slower, uphill excitation energy transfer to the 720-nm trap.
It is also worth considering that electron donation from PD1 to ChlD1+• may become more favorable by electron transfer from Pheo−• to QA since this diminishes the stabilizing influence of the adjacent charges on ChlD1+•Pheo−•. Thus, the formation of PD1+•QA−• as the final radical pair, RP3, may be slow and may not occur as a single discrete electron transfer event. This additional distribution of charge separation kinetics in FR-PSII could also explain the reduced amplitude of the 675-nm bleach associated with the formation of PD1+• in the 2-ns spectra of FR-PSII (Fig. 2) compared to WL-PSII (SI Appendix, Fig. S2B), implying a lower concentration of RP3 state at this time point and a slower rate of formation.
The smaller amount of energy available in FR-PSII appears to modify the steps of charge separation, allowing them to be distinguished. The observation here of the slow PD1+• formation is something that was not possible to detect in WL-PSII because of the overlapping absorption bands of chl-a pigments. The possibility exists that this reflects a similar situation occurring in WL-PSII on a faster timescale than in FR-PSII but slower than generally assumed and obscured by spectral overlap.
Materials and Methods
Sample.
Sample preparation was as described in (12). WL-PSII cores were isolated from T. elongatus with His-tagged CP43, and purified by nickel-nitrilotriacetic acid (Ni-NTA) affinity chromatography essentially as described in ref. 35. Cells were grown in liquid DTN medium at 45 °C under white light of 30 µE m−2 s−1.
FR-PSII cores were isolated and purified by sucrose density gradient followed by ion exchange chromatography as described in ref. 12. C. thermalis PCC 7203 cells were grown in liquid BG11 medium at 30 °C under far-red light (750 nm) of 45 µE m−2 s−1. All samples were in a buffer of 50 mM 2-(N-Morpholino)ethane-sulphonic acid (MES)-NaOH, 5 mM CaCl2, 10 mM MgCl2 and 0.04% (wt/vol) n-dodecyl-β-d-maltoside (β-DDM) (pH 6.5).
The samples were transferred to a cell (Harrick Scientific) with 1-mm thickness and 25-mm diameter CaF2 windows and a 25-µm spacer. The purity of the sample was assessed by absorption and fluorescence measurements. The absorption spectra for both WL- and FR-PSII samples are presented in SI Appendix, Fig. S1. The optical density at 675 nm was typically 0.6 to 0.7. All measurements were performed at room temperature.
Setup.
The setup for visible TA measurements was described previously (36). Briefly, the 800-nm output from Ti-Sapphire laser (Spectra Physics, Hurricane, 90 fs, 1 kHz, 0.85 mW) was divided between an optical parametric amplifier (OPA, Spectra Physics, OPA-800C) and a white-light generator. The OPA produced three pump wavelengths used in the experiment: 675 nm, 663 nm, and 720 nm. The typical bandwidth of the pump radiation was around 20 nm and the energy reaching the sample was below 10 nJ. From the beam diameter of 0.175 mm at the sample position, this energy corresponds to 0.04 W/cm2 or 42 μJ/cm2. The delay between the pump and the probe pulses was varied by delaying the pump pulse using a retroreflector mounted on a delay line (M-IMS400CCHA, Newport). Positive delay measurements were subtracted from a background negative delay (−100 ps) measurement representing a 1-ms spectrum with inverted amplitude. The 12-ns and 27-ns measurements were collected by introducing a long delay line using multiple optical mirrors to create additional beam path. To avoid repeated exposure of the same sample volume, the sample was continuously moved in a Lissajous pattern using a home-built sample translator at ST = 6 sample translation speed.
The white light was generated using a sapphire plate and spanned the spectral region from 400 to above 800 nm. The probe light was dispersed by a home-built prism spectrometer and registered using 1,024 pixel charge-coupled device camera at a 1-kHz frame rate on a single-shot basis.
The data were analyzed using Global Analysis (23, 37) and Lifetime Density (38, 39) methods. Specifically, freely available Glotaran (40) and the Global analysis toolkit (37) were employed for this purpose. A custom Python code was used for lifetime density maps (22). The number of compartments in the results of sequential analysis was chosen based on fitting statistics and residuals.
Supplementary Material
Acknowledgments
This work was supported by the Leverhulme Trust awards RPG-2014-126 and RPG-2018-372 (to J.J.v.T.); Biotechnology and Biological Sciences Research Council grants BB/L011506/1, BB/R001383/1, and BB/R00921X/1 (to A.W.R.); a Wolfson Merit Award from the Royal Society (to A.W.R.); and Deutsche Forschungsgemeinschaft award NU 421/1-1 (to D.J.N.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2006016117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Cardona T., Murray J. W., Rutherford A. W., Origin and evolution of water oxidation before the last common ancestor of the cyanobacteria. Mol. Biol. Evol. 32, 1310–1328 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dau H., Zaharieva I., Principles, efficiency, and blueprint character of solar-energy conversion in photosynthetic water oxidation. Acc. Chem. Res. 42, 1861–1870 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Rappaport F., Diner B. A., Primary photochemistry and energetics leading to the oxidation of the (Mn)4Ca cluster and to the evolution of molecular oxygen in Photosystem II. Coord. Chem. Rev. 252, 259–272 (2008). [Google Scholar]
- 4.Cardona T., Sedoud A., Cox N., Rutherford A. W., Charge separation in photosystem II: A comparative and evolutionary overview. Biochim. Biophys. Acta 1817, 26–43 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Ferreira K. N., Iverson T. M., Maghlaoui K., Barber J., Iwata S., Architecture of the photosynthetic oxygen-evolving center. Science 303, 1831–1838 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Umena Y., Kawakami K., Shen J.-R., Kamiya N., Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55–60 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Prokhorenko V. I., Holzwarth A. R., Primary processes and structure of the photosystem II reaction center: A photon echo study. J. Phys. Chem. B 104, 11563–11578 (2000). [Google Scholar]
- 8.Groot M. L.et al., Initial electron donor and acceptor in isolated photosystem II reaction centers identified with femtosecond mid-IR spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 102, 13087–13092 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holzwarth A. R.et al., Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: Pheophytin is the primary electron acceptor. Proc. Natl. Acad. Sci. U.S.A. 103, 6895–6900 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diner B. A., Rappaport F., Structure, dynamics, and energetics of the primary photochemistry of photosystem II of oxygenic photosynthesis. Annu. Rev. Plant Biol. 53, 551–580 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Kawashima K., Ishikita H., Energetic insights into two electron transfer pathways in light-driven energy-converting enzymes. Chem. Sci. (Camb.) 9, 4083–4092 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nürnberg D. J.et al., Photochemistry beyond the red limit in chlorophyll f-containing photosystems. Science 360, 1210–1213 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Romero E., van Stokkum I. H. M., Novoderezhkin V. I., Dekker J. P., van Grondelle R., Two different charge separation pathways in photosystem II. Biochemistry 49, 4300–4307 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Romero E.et al., Mixed exciton-charge-transfer states in photosystem II: Stark spectroscopy on site-directed mutants. Biophys. J. 103, 185–194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mamedov M., Govindjee, Nadtochenko V., Semenov A., Primary electron transfer processes in photosynthetic reaction centers from oxygenic organisms. Photosynth. Res 125, 51–63 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Zinth W., Wachtveitl J., The first picoseconds in bacterial photosynthesis–Ultrafast electron transfer for the efficient conversion of light energy. ChemPhysChem 6, 871–880 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Miloslavina Y.et al., Charge separation kinetics in intact photosystem II core particles is trap-limited. A picosecond fluorescence study. Biochemistry 45, 2436–2442 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Chen M.et al., A red-shifted chlorophyll. Science 329, 1318–1319 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Gan F.et al., Extensive remodeling of a cyanobacterial photosynthetic apparatus in far-red light. Science 345, 1312–1317 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Tomo T., Shinoda T., Chen M., Allakhverdiev S. I., Akimoto S., Energy transfer processes in chlorophyll f-containing cyanobacteria using time-resolved fluorescence spectroscopy on intact cells. Biochim. Biophys. Acta 1837, 1484–1489 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Schmitt F. J.et al., Photosynthesis supported by a chlorophyll f-dependent, entropy-driven uphill energy transfer in Halomicronema hongdechloris cells adapted to far-red light. Photosynth. Res. 139, 1–17 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Dorlhiac G. F., Fare C., van Thor J. J., PyLDM–An open source package for lifetime density analysis of time-resolved spectroscopic data. PLOS Comput. Biol. 13, e1005528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Stokkum I. H. M., Larsen D. S., van Grondelle R., Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta 1657, 82–104 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Shreve A. P., Trautman J. K., Frank H. A., Owens T. G., Albrecht A. C., Femtosecond energy-transfer processes in the B800-850 light-harvesting complex of Rhodobacter sphaeroides 2.4.1. Biochim. Biophys. Acta Bioenerg. 1058, 280–288 (1991). [DOI] [PubMed] [Google Scholar]
- 25.Kleima F. J.et al., Energy transfer in LHCII monomers at 77K studied by sub-picosecond transient absorption spectroscopy. Biochemistry 36, 15262–15268 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Kaucikas M., Nürnberg D., Dorlhiac G., Rutherford A. W., van Thor J. J., Femtosecond visible transient Absorption spectroscopy of chlorophyll f-containing photosystem I. Biophys. J. 112, 234–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamzam N., Kaucikas M., Nürnberg D. J., Rutherford A. W., van Thor J. J., Femtosecond infrared spectroscopy of chlorophyll f-containing photosystem I. Phys. Chem. Chem. Phys. 21, 1224–1234 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Nuijs A. M., van Gorkom H. J., Plijter J. J., Duysens L. N. M., Primary-charge separation and excitation of chlorophyll a in photosystem II particles from spinach as studied by picosecond absorbance-difference spectroscopy. Biochim. Biophys. Acta Bioenerg. 848, 167–175 (1986). [Google Scholar]
- 29.Schatz G. H., Brock H., Holzwarth A. R., Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density. Proc. Natl. Acad. Sci. U.S.A. 84, 8414–8418 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlowicz N. P., Groot M.-L., van Stokkum I. H. M., Breton J., van Grondelle R., Charge separation and energy transfer in the photosystem II core complex studied by femtosecond midinfrared spectroscopy. Biophys. J. 93, 2732–2742 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niedzwiedzki D. M., Liu H., Chen M., Blankenship R. E., Excited state properties of chlorophyll f in organic solvents at ambient and cryogenic temperatures. Photosynth. Res. 121, 25–34 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Kalaji H. M.et al., Experimental in vivo measurements of light emission in plants: A perspective dedicated to David Walker. Photosynth. Res. 114, 69–96 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Allakhverdiev S. I.et al., Chlorophylls d and f and their role in primary photosynthetic processes of cyanobacteria. Biochemistry (Mosc.) 81, 201–212 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Schlodder E.et al., Both chlorophylls a and d are essential for the photochemistry in photosystem II of the cyanobacteria, Acaryochloris marina. Biochim. Biophys. Acta 1767, 589–595 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Sugiura M., Inoue Y., Highly purified thermo-stable oxygen-evolving photosystem II core complex from the thermophilic cyanobacterium Synechococcus elongatus having His-tagged CP43. Plant Cell Physiol. 40, 1219–1231 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Fitzpatrick A. E., Lincoln C. N., van Wilderen L. J. G. W., van Thor J. J., Pump-dump-probe and pump-repump-probe ultrafast spectroscopy resolves cross section of an early ground state intermediate and stimulated emission in the photoreactions of the Pr ground state of the cyanobacterial phytochrome Cph1. J. Phys. Chem. B 116, 1077–1088 (2012). [DOI] [PubMed] [Google Scholar]
- 37.van Wilderen L. J. G. W., Lincoln C. N., van Thor J. J., Modelling multi-pulse population dynamics from ultrafast spectroscopy. PLoS One 6, e17373 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slavov C., Hartmann H., Wachtveitl J., Implementation and evaluation of data analysis strategies for time-resolved optical spectroscopy. Anal. Chem. 87, 2328–2336 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Croce R., Müller M. G., Bassi R., Holzwarth A. R., Carotenoid-to-chlorophyll energy transfer in recombinant major light-harvesting complex (LHCII) of higher plants. I. Femtosecond transient absorption measurements. Biophys. J. 80, 901–915 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snellenburg J. J., Laptenok S. P., Seger R., Mullen K. M., van Stokkum I. H. M., Glotaran : A java -based graphical user interface for the R package TIMP. J. Stat. Softw. 49, 1–22 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.