Significance
Rhodopsin mutations account for ∼30% of autosomal-dominant retinitis pigmentosa cases. While dozens of distinct mutations have been identified, the reported detriments that they cause in cell culture are not necessarily all consistent across studies and have often not been evaluated in animal models. Even when an animal model is available, it is typically not straightforward to isolate mutant-rhodopsin signals from related endogenous-rhodopsin signals. Here, we introduce an approach employing a largely “functionally silenced” endogenous rhodopsin in rods as background for isolating activity due to D190N-rhodopsin (D190N-Rho). We conclude that, despite D190N-Rho’s elevated molecular rate constant of spontaneous activation, it does not increase the overall cellular dark noise of the rod, thus excluding its increased noise as the reason for disease.
Keywords: D190N-rhodopsin, spontaneous isomerization, protein misfolding, night blindness, retinitis pigmentosa
Abstract
Numerous rhodopsin mutations have been implicated in night blindness and retinal degeneration, often with unclear etiology. D190N-rhodopsin (D190N-Rho) is a well-known inherited human mutation causing retinitis pigmentosa. Both higher-than-normal spontaneous-isomerization activity and misfolding/mistargeting of the mutant protein have been proposed as causes of the disease, but neither explanation has been thoroughly examined. We replaced wild-type rhodopsin (WT-Rho) in RhoD190N/WT mouse rods with a largely “functionally silenced” rhodopsin mutant to isolate electrical responses triggered by D190N-Rho activity, and found that D190N-Rho at the single-molecule level indeed isomerizes more frequently than WT-Rho by over an order of magnitude. Importantly, however, this higher molecular dark activity does not translate into an overall higher cellular dark noise, owing to diminished D190N-Rho content in the rod outer segment. Separately, we found that much of the degeneration and shortened outer-segment length of RhoD190N/WT mouse rods was not averted by ablating rod transducin in phototransduction—also consistent with D190N-Rho’s higher isomerization activity not being the primary cause of disease. Instead, the low pigment content, shortened outer-segment length, and a moderate unfolded protein response implicate protein misfolding as the major pathogenic problem. Finally, D190N-Rho also provided some insight into the mechanism of spontaneous pigment excitation.
Our dark-adapted visual system has an exceedingly low threshold for perceiving light (1), imparted largely by rhodopsin in several ways, namely, the high density of rhodopsin packed into each rod to ensure a high photon-capture ability (2), rhodopsin’s substantial signal amplification to provide a sizeable single-photon response (3–5), and rhodopsin’s low spontaneous excitation to minimize false signaling, or dark noise (6, 7). As the most abundant protein in rods, rhodopsin also supports the structure of the outer segment, sustaining photoreceptor health and survival (8–11). Various forms of night blindness and retinal degeneration have been linked to single-amino acid substitutions in rhodopsin (12). For example, G90D-Rho has been found to elevate spontaneous activity to such an extent as to produce rod desensitization similar to light adaptation (13–15). Other familial rhodopsin mutations have been similarly speculated to elevate dark noise to produce desensitization or even cell damage (16–19). Certain mutations may cause deficits in signal amplification (20), and still others could lead to protein misfolding/mistargeting, ultimately resulting in cell death and vision loss (21–24). Much of what is known about rhodopsin mutants has come from in vitro studies of heterologously expressed mutants (25–30). Thus, with only a few animal models generated, the exact deleterious effects of many rhodopsin mutations in rods remain obscure or unsolved.
D190N-rhodopsin (D190N-Rho) is a well-known mutation and exemplifies a case of longstanding mechanistic uncertainty, in that past studies have led to a debate over this mutant pigment’s reduced ability to fold properly (26, 27) versus the possibility of higher-than-normal dark noise in a live mammalian rod (16, 19, 31, 32), or both. This mutation was found in several families with autosomal-dominant retinitis pigmentosa (26, 27, 33, 34), with heterozygous (RhoD190N/WT) patients first reporting poor night vision early in life (33) and subsequently showing retinal degeneration (by age ∼50, for example) (32–34). The pathology for homozygous (RhoD190N/D190N) patients is unknown because they have not been reported in clinical studies. Broader interest in D190N-Rho came from the high conservation of rhodopsin’s native D190 amino acid residue across rod and cone pigments and from its intriguing structural position in the protein—forming, together with another highly conserved residue (R177), a salt bridge directly above the chromophore-binding pocket (31). Biochemical experiments suggested that disruption of this salt bridge in D190N-Rho destabilized dark-state rhodopsin to give the mutant pigment a spontaneous isomerization rate higher than normal (16, 31, 35). However, these in vitro observations may not provide direct information about the situation in vivo.
Recently, a D190N-Rho knockin mouse was generated (32), but it did not readily allow isolation of D190N-Rho activity from endogenous wild-type (WT)-Rho activity in RhoD190N/WT heterozygous animals. At the same time, RhoD190N/D190N homozygous rods degenerate very rapidly (19), making recordings infeasible already at eye opening. To largely silence endogenous WT-Rho activity while maintaining outer-segment structure for recording, we bred RhoD190N/D190N with RhoREY/REY mice (3) to obtain RhoD190N/REY mice so as to replace WT-Rho with REY-Rho. In REY-Rho, the amino acid sequence ERY in the G-protein (transducin)–binding motif has been changed to REY, causing no apparent change in light absorption, only a small change in pigment-protein expression, but a huge reduction (by many thousands of times) in the coupling efficiency between pigment and transducin, rendering the pigment largely “functionally silenced” (3, 20). This genotype allows us to isolate D190N-Rho’s single-photon response, and to also quantify its content as well as its dark spontaneous activity in RhoD190N/REY rods. At the same time, such measurements provide an opportunity for us to check D190N-Rho’s activity against our recently developed physicochemical theory of spontaneous pigment isomerization (7). Finally, the general strategy described here may be useful for elucidating the disease mechanisms of some other rhodopsin mutations implicated in night blindness and retinal degeneration.
Results
RhoD190N/WT Mouse Rods Had Lower Flash Sensitivity and Faster Response Kinetics than RhoWT/WT Rods.
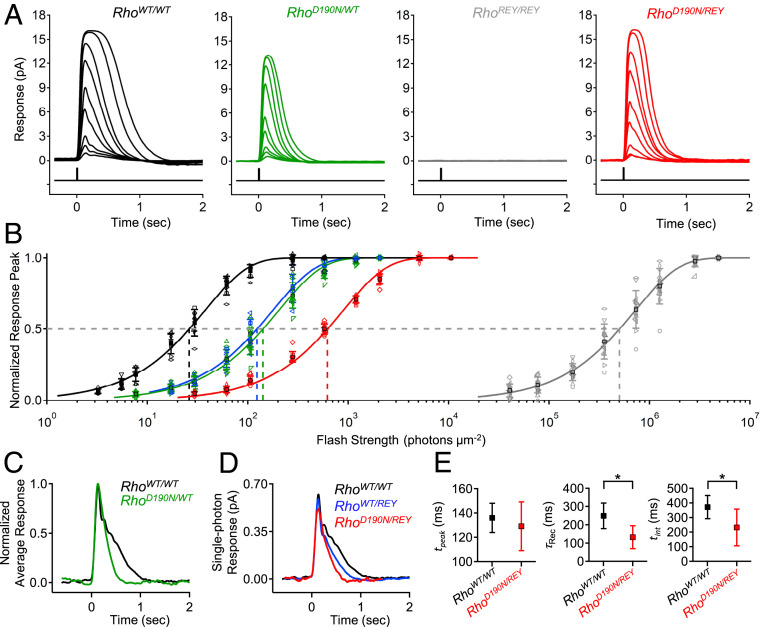
Because the D190N mutation typically occurs in the heterozygous state in patients, we started by measuring flash responses from single RhoD190N/WT mouse rods (referred to as RhoD190N/+ in ref. 32). Compared to RhoWT/WT (C57BL/6J) rods (Fig. 1A, black), RhoD190N/WT rods showed flash responses with faster kinetics (Fig. 1A, green), a smaller saturated-response amplitude (Fig. 1A and SI Appendix, Table S1A), and ∼5.5-fold lower sensitivity based on the shift in intensity–response relation along the flash intensity axis (Fig. 1B). These effects were broadly in line with previous electroretinographic data from RhoD190N/WT mice and humans (32–34). The comparison between the normalized, averaged dim-flash responses from RhoWT/WT and from RhoD190N/WT rods is given in Fig. 1C, showing the latter’s faster response decay. One cannot, however, isolate the behavior of the D190N-Rho single-photon response from that of WT-Rho in RhoD190N/WT rods because the fluctuation analysis for extracting the single-photon response requires a homogeneous population of pigment molecules, which is not the case here.
Fig. 1.
Lower flash sensitivity and faster response kinetics of RhoD190N/WT rods compared to WT, and isolation of D190N-Rho responses in RhoD190N/REY. (A) Flash response families for RhoWT/WT (C57BL/6J, black), RhoD190N/WT (green), RhoREY/REY (gray), and RhoD190N/REY (red) rods. REY-Rho responses were not observed in RhoREY/REY rods in a flash response family of ∼30 to 10,000 photons⋅µm−2⋅s−1, while D190N-Rho responses were robust in RhoD190N/REY rods over the same range. (B) Intensity–response relations of normalized response peak versus flash strength for each genotype shown in A in addition to that of RhoWT/REY rods. The solid curves are fits to a saturating-exponential function (Methods) with half-saturating flash strengths (ρ) of 26 (RhoWT/WT), 142 (RhoD190N/WT), 124 (RhoWT/REY), 622 (RhoD190N/REY), and 502,754 (RhoREY/REY) photons⋅µm−2. (C) Normalized, averaged dim-flash responses for RhoWT/WT rods (black) and RhoD190N/WT rods (green). (D) Single-photon responses (obtained from quantal fluctuation analysis) of WT-Rho in RhoWT/WT rods (black), D190N-Rho in RhoD190N/REY rods (red), and WT-Rho in RhoWT/REY (blue). (E, Left) Time-to-peak (tpeak) of single-photon responses from WT-Rho (black) and D190N-Rho (red); tpeak = 136 ± 12 ms (RhoWT/WT; n = 9 cells) and 129 ± 20 ms (RhoD190N/REY; n = 12 cells). (E, Center) Recovery time constant (τRec) from fitting the final exponential decline of the single-photon response; τRec = 249 ± 70 ms (RhoWT/WT), 132 ± 63 ms (RhoD190N/REY). (E, Right) Integration time (tint) of single-photon response (defined as the time integral of single-photon response divided by transient peak amplitude), tint = 371 ± 79 ms (RhoWT/WT), 232 ± 126 ms (RhoD190N/REY). The single stars mark statistical significance of 0.0001 ≤ P ≤ 0.05 from Student’s t tests. There was no statistically significant difference in time-to-peak.
Isolation of the D190N-Rho Response.
To isolate D190N-Rho responses without removing WT-Rho, which otherwise would compromise the structural support provided by rhodopsin in the outer segment (9), we instead silenced WT-Rho signaling (although not fully) by replacing it with a mutant rhodopsin, REY-Rho, which has a disrupted G-protein–binding site (3, 20) (see Introduction]. In RhoREY/REY mouse rods, REY-Rho is expressed at near-normal levels (3), which helps to maintain outer-segment structure for recordings. The 500-nm light absorption by detergent-solubilized whole RhoREY/REY retinae likewise demonstrated that the REY-Rho content, at 0.31 ± 0.02 nmol per retina (n = 4 retinae), was similar to that for RhoWT/WT mice (9). There is no change in protein level of any phototransduction signaling component (3). However, RhoREY/REY rods were ∼16,000-fold less sensitive than RhoWT/WT rods (Fig. 1 A and B). The reason for such low sensitivity is that REY-Rho, upon absorbing a photon, triggers phototransduction with such an exceedingly low probability of success that, even when successful, no more than a single downstream transducin/phosphodiesterase effector complex is activated, giving a relatively tiny electrical response (3). As such, we found no clearly detectable responses from RhoREY/REY rods at flash strengths up to ∼10,000 photons⋅μm−2 (Fig. 1A, gray; see legend), although, at high enough intensities, the rarely successful and tiny single-REY-Rho–triggered events still summed to give measurable macroscopic responses (Fig. 1B, gray). At a flash intensity of ∼30 photons⋅μm−2, the light responses elicited from RhoD190N/REY rods were composed practically entirely of photosignals from D190N-Rho (Fig. 1 A and B, red). RhoWT/REY rods, in comparison, showed a higher sensitivity than RhoD190N/REY rods (Fig. 1B, blue). Fluctuation analysis of a response ensemble elicited from RhoD190N/REY rods by repeated, identical dim flashes demonstrated that D190N-Rho’s single-photon response was actually fairly similar to that for WT-Rho in RhoWT/WT rods (Fig. 1D). Both responses had very similar rising phases and times-to-peak (tpeak) (Fig. 1D), but D190N-Rho responses decayed ∼40% faster, with a shorter recovery time constant (τRec) and a shorter integration time (tint) (Fig. 1 D and E).
One possible reason for the faster response decline in RhoD190N/REY rods was the alleged higher dark spontaneous activity of D190N-Rho compared to WT-Rho, acting as an “equivalent background light” to trigger adaptation as does real background light (see Introduction). The puzzle, however, is that such an adaptation to background light is typically accompanied by a reduction in the single-photon response amplitude (36, 37), which was surprisingly insignificant here (Fig. 1D and SI Appendix, Table S1A). Interestingly, WT-Rho’s single-photon response in heterozygous rhodopsin-knockout RhoWT/− rods [commonly referred to as Rho+/− (9)] happens to also have a faster response decline and an unaffected peak amplitude (10, 38). Such a resemblance in single-photon response kinetics between RhoWT/− and RhoD190N/REY suggests that they may have a common cause, namely, a lower pigment content in the rods. RhoWT/− rods have ∼50% of the rhodopsin content in RhoWT/WT (9). Associated with this lower rhodopsin content is a ∼30% shorter outer segment and a smaller interdiskal space in RhoWT/− rods, which have been proposed to result in a faster response decline by accelerating Ca2+-mediated negative feedback (10, 11). Similarly, the outer-segment length of RhoWT/REY rods was ∼11% shorter, and also accompanied by a faster response decay (Fig. 1D, blue; SI Appendix, Table S1A). We found even shorter outer-segment lengths in the RhoD190N/REY and RhoD190N/WT rods that we recorded from, both being ∼40% shorter than in RhoWT/WT rods (SI Appendix, Table S1A). All in all, the overall rhodopsin content in RhoD190N/REY and RhoD190N/WT rods may indeed be lower than normal.
Measurement of D190N-Rho Content in RhoD190N/REY Rods.
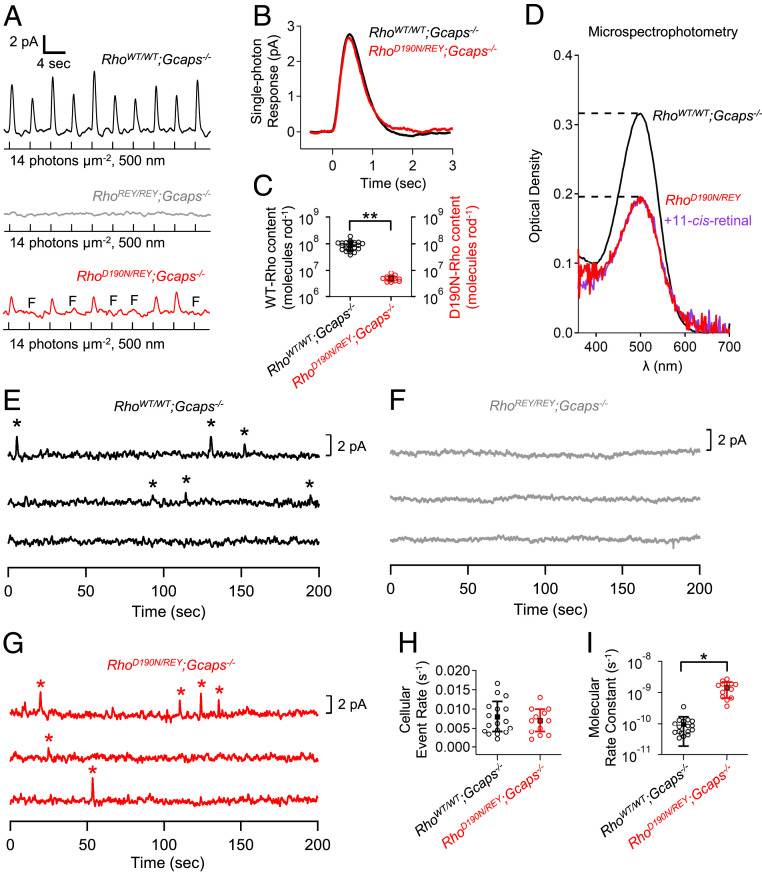
To quantify D190N-Rho content in RhoD190N/REY rods, we bred these mice into the Gcaps−/− background to give the RhoD190N/REY;Gcaps−/− genotype, which facilitates the measurement of D190N-Rho’s single-photon responses by increasing their amplitude to approximately fivefold as large through removing a major Ca2+-mediated negative feedback (39–41). Incidentally, we found that removing this feedback mechanism resulted in RhoD190N/REY;Gcaps−/− rods having similar, instead of faster, single-photon response kinetics compared to RhoWT/WT;Gcaps−/− rods (Fig. 2B and SI Appendix, Table S1B; see also Fig. 3B). This observation may fit with the proposal mentioned earlier that Ca2+-mediated negative feedback underlies the faster response decline in RhoD190N/REY rods (see Fig. 1D).
Fig. 2.
Pigment content and spontaneous activity of RhoD190N/REY rods. (A) Repeated dim flashes at the same flash strength (14 photons⋅μm−2) on RhoWT/WT;Gcaps−/− rods (black) reliably triggered responses, while RhoREY/REY;Gcaps−/− rods (gray) did not show any discernible responses because of severely weakened interaction between REY-Rho and G protein. RhoD190N/REY;Gcaps−/− rods (red) exhibited a higher probability of failure (red, response failures marked by “F”) compared to RhoWT/WT;Gcaps−/−. (B) Single-photon responses from WT-Rho in RhoWT/WT;Gcaps−/− and D190N-Rho in RhoD190N/REY;Gcaps−/− rods. Waveforms are ensemble averages from all cells of each genotype. (C) Functional rhodopsin content in each genotype based on the measured probability of failure (Methods). The double stars mark statistical significance of P < 0.0001. (D) Microspectrophotometry measuring optical density in a group of outer segments extending from a piece of retina. Peak optical density was 0.316 ± 0.087 in RhoWT/WT;Gcaps−/−. Data were reproduced from ref. 3 because the corresponding measurements were not made with RhoWT/WT;Gcaps+/+ rods. There is no reported difference in WT-Rho content between the Gcaps−/− and Gcaps+/+ backgrounds. Peak optical density was 0.187 ± 0.037 in RhoD190N/REY;Gcaps+/+ (red; n = 4 retinal regions, 2 animals). To assess any excess apo-opsin content in RhoD190N/REY, rods were exposed to excess 11-cis-retinal (50 µM for 15 min; SI Appendix, Supplementary Methods) and optical density was measured again (purple). Peak optical density did not significantly increase after retinal exposure, 0.196 ± 0.028 (n = 4 retinal regions, 2 animals; P = 0.62, Student’s t test). (E and F) WT-Rho produced discrete dark-noise events (indicated with stars) in RhoWT/WT;Gcaps−/− rods, which were functionally silenced by replacing WT-Rho with REY-Rho in RhoREY/REY;Gcaps−/− rods. (G) Discrete noise events from D190N-Rho could be clearly identified to determine their frequency in RhoD190N/REY;Gcaps−/− rods. (H) Cellular rate of spontaneous-isomerization events in RhoWT/WT;Gcaps−/− and RhoD190N/REY;Gcaps−/−. (I) Molecular rate constant of spontaneous isomerization for WT-Rho in RhoWT/WT;Gcaps−/− and for D190N-Rho in RhoD190N/REY;Gcaps−/− rods. The single star marks statistical significance of 0.0001 ≤ P ≤ 0.05.
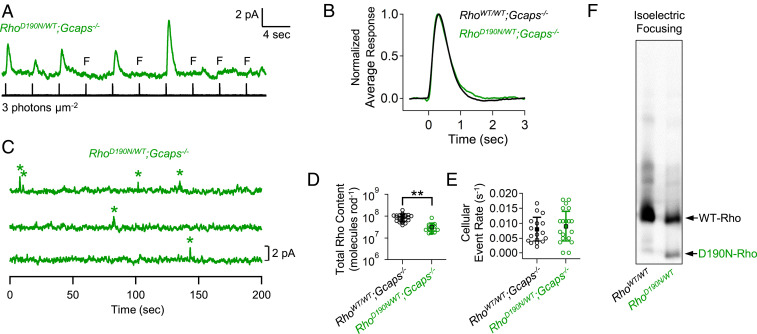
Fig. 3.
Pigment content and spontaneous activity of RhoD190N/WT rods. (A) Series of dim-flash responses from RhoD190N/WT;Gcaps−/− (green) rods. (B) Normalized ensemble averages of dim-flash responses from RhoWT/WT;Gcaps−/− and RhoD190N/WT;Gcaps−/− rods (n = 23 and 16 rods, respectively). (C) Dark noise from a RhoD190N/WT;Gcaps−/− rod with discrete events marked by stars. (D) Functional pigment content estimated from the probability of failure (Methods) in RhoWT/WT;Gcaps−/− (same as in Fig. 2C) and RhoD190N/WT;Gcaps−/− rods. Double stars mark statistical significance of P < 0.0001. (E) Cellular rate of spontaneous isomerization events. Each open symbol represents the estimate from an individual rod. There was not a significant difference in cellular event rate between these genotypes. (F) Isoelectric focusing blot showing distinct D190N-Rho (∼35% of total pigment) and WT-Rho (∼65% of total pigment) bands.
To estimate the D190N-Rho content in RhoD190N/REY rods, we delivered repeated, identical dim flashes to a rod (Fig. 2A) and measured the probability of failure (i.e., the fraction of flash trials that elicited no detectable responses). Based on the Poisson distribution of photon absorption (42) (Methods), this probability of failure readily gives an estimate of the number of rhodopsin molecules capable of triggering normal-sized single-photon responses (i.e., excluding REY-Rho and also mistargeted/misfolded, thus nonfunctional, D190N-Rho). As such, we obtained (4.8 ± 1.3) × 106 functional D190N-Rho molecules in a RhoD190N/REY;Gcaps−/− rod (Fig. 2C, red; n = 12 rods). The same flash strength did not elicit noticeable responses in RhoREY/REY;Gcaps−/− rods owing to REY-Rho’s extremely poor efficiency to interact with transducin and to its small associated single-photon responses (Fig. 2A, gray). The same probability-of-failure experiment on RhoWT/WT;Gcaps−/− rods gave (8.8 ± 3.4) × 107 Rho molecules in a WT rod (Fig. 2C, black; n = 23 rods). Thus, the functional D190N-Rho content in RhoD190N/REY;Gcaps−/− was only 5.5% of the WT-Rho content in RhoWT/WT;Gcaps−/− rods (Fig. 2C). The low pigment content in RhoD190N/REY;Gcaps−/− rods was confirmed independently by microspectrophotometry (Fig. 2D), and in RhoD190N/REY rods by physiological action spectra as well as biochemical isoelectric-focusing experiments (SI Appendix, Supplementary Text and Fig. S1).
To check whether any of the diminished functional D190N-Rho pigment content was simply due to a weaker binding of chromophore to D190N-apo-opsin, thus reducing holopigment formation, we exposed dark-adapted RhoD190N/REY rods to excess chromophore (50 μM 11-cis-retinal for 15 min), but found no increase in holo-pigment content as evaluated by microspectrophotometry (Fig. 2D). There was also no sign of extra apo-opsin noise (3) in dark-adapted RhoD190N/REY;Gcaps−/− rods; their steady noise variance (0.14 ± 0.08 pA2, n = 5 rods) was similar to that in RhoWT/WT;Gcaps−/− rods (0.17 ± 0.11 pA2; n = 17).
Molecular Spontaneous Activity of D190N-Rho Measured in RhoD190N/REY Rods.
As pointed out earlier, RhoD190/REY rods offer the opportunity to isolate D190N-Rho activity from WT-Rho activity. Fig. 2 E–G show dark recordings from RhoWT/WT;Gcaps−/−, RhoREY/REY;Gcaps−/−, and RhoD190/REY;Gcaps−/− rods. RhoWT/WT;Gcaps−/− rods exhibited occasional dark events (marked by stars) resembling normal-sized single-photon responses. From collected data, we obtained an average cellular spontaneous-isomerization rate at 37 °C of 0.008 ± 0.004 s−1⋅cell−1 (n = 17 rods; Fig. 2H, black), similar to past measurements (7, 39–41). There were no prominent dark events in RhoREY/REY;Gcaps−/− rods, as expected. RhoD190/REY;Gcaps−/− rods showed similar dark events as RhoWT/WT;Gcaps−/− rods, with collected data giving 0.007 ± 0.003 s−1⋅cell−1 (n = 12 rods; Fig. 2H, red). Dividing the average cellular isomerization rates in RhoWT/WT;Gcaps−/− rods and RhoD190/REY;Gcaps−/− rods by their respective functional pigment content described in the previous section, we obtained the molecular spontaneous activities at 37 °C, being ∼9.1 × 10−11 s−1 for WT-Rho and ∼1.5 × 10−9 s−1 for D190N-Rho (Fig. 2I). Hence, D190N-Rho’s spontaneous molecular isomerization rate is 16-fold as high as WT-Rho’s. However, the resulting dark noise at the cellular level is practically unchanged, owing to the low D190N-Rho content in RhoD190N/REY rods. Incidentally, the molecular spontaneous activity of WT-Rho appeared to be similar in RhoWT/REY;Gcaps−/− and in RhoWT/WT;Gcaps−/− rods, as one might expect (SI Appendix, Fig. S2F).
RhoD190N/WT Genotype.
With the RhoD190/REY genotype, it is straightforward to quantify D190N-Rho’s molecular spontaneous activity with electrophysiology. However, because the RhoD190N/WT genotype is exactly what corresponds to that of human patients, we repeated the same measurements on this genotype with the additional help of biochemistry to estimate D190N-Rho content. From the probability-of-failure experiment with identical dim flashes (Fig. 3A), we estimated the total functional pigment content (i.e., D190N-Rho and WT-Rho together) in RhoD190N/WT;Gcaps−/− rods to be (3.2 ± 1.6) × 107 molecules⋅rod−1 (Fig. 3D, green; n = 16 rods), also lower than in RhoWT/WT;Gcaps−/− rods (Fig. 3D, black). Next, from dark-noise measurements (Fig. 3C), the cellular rate of spontaneous events of RhoD190N/WT;Gcaps−/− rods was 0.009 ± 0.005 s−1 (Fig. 3E, green; n = 16 rods), also similar to that of RhoWT/WT;Gcaps−/− rods—perhaps not surprising, given the findings from RhoD190/REY;Gcaps−/− rods in the previous section. The similar kinetics in the dim-flash responses between RhoWT/WT;Gcaps−/− and RhoD190N/WT;Gcaps−/− rods have been commented before in connection with Fig. 2B.
To estimate the content ratio of D190N-Rho to WT-Rho molecules in RhoD190N/WT rods, we employed the biochemical method of isoelectric focusing (43, 44), which separated the two rhodopsin molecular species according to their distinct isoelectric points. In this genotype, we found the pigment content to consist of 35 ± 4% D190N-Rho and 65 ± 4% WT-Rho (Fig. 3F; n = 5 retinae), or a ratio of roughly one D190N-Rho for every two WT-Rho molecules.
Using the spontaneous molecular rate constant of 9.1 × 10−11 s−1 for WT-Rho from the previous section, we obtained, after some calculations from the above measurements, a molecular rate constant of 6.3 × 10−10 s−1 for D190N-Rho, about a factor of 2 less than that obtained from RhoD190/REY;Gcaps−/− rods described above. Considering the rather round-about way of making calculations from the above RhoD190N/WT rod measurements, such a discrepancy is not major.
In summary, in the RhoD190N/WT disease genotype, as in the RhoD190/REY genotype, D190N-Rho’s content is likewise low enough that, despite its much higher molecular isomerization rate than WT-Rho, the cellular isomerization rate is no greater than normal.
D190N-Rho’s Elevated Spontaneous Activity Is Not High Enough to Trigger Adaptation.
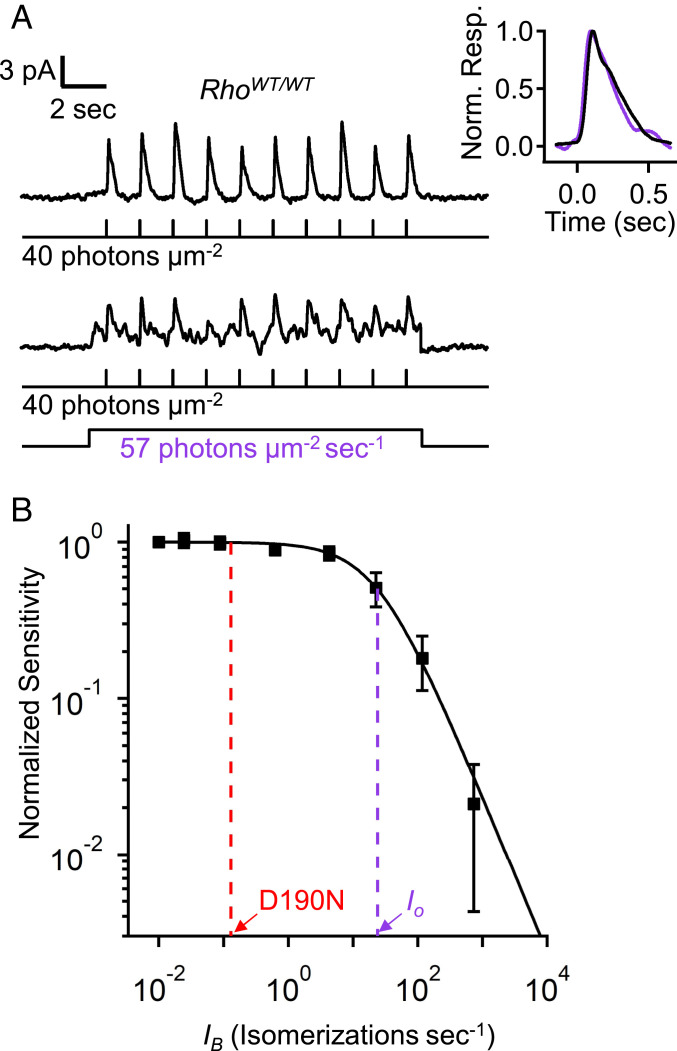
With so much past speculation about D190N-Rho and other noisy rhodopsin mutants (16, 19, 45) causing “equivalent-light adaptation,” we were curious about how high rhodopsin’s spontaneous-isomerization rate would have to be in order to desensitize mouse rods substantially. Fig. 4A shows the effect of steady background light on flash sensitivity in RhoWT/WT (C57BL/6J) rods. Dim-flash sensitivity was reduced to ∼50% by a background light of 57 photons⋅µm−2⋅s−1, calculated to trigger ∼22 Rho* s−1 based on the effective collecting area of mouse rods (3). This steady background light did not significantly change flash response kinetics (Fig. 4 A, Inset). Furthermore, from the Weber–Fechner behavior of background light adaptation (Fig. 4B, solid curve and legend, with a background intensity that reduces flash sensitivity by half, Io, of ∼24 Rho* s−1) (see also refs. 39, 46), it could be seen that even if every WT-Rho molecule in a RhoWT/WT rod were replaced by D190N-Rho, giving 0.008 isomerizations⋅s−1⋅cell−1 × 16 = 0.13 isomerizations⋅s−1⋅cell−1, the flash sensitivity of mouse rods would hardly change (red dashed line, Fig. 4B). Indeed, in order for a rhodopsin mutant to desensitize mouse rods by a factor of 2 in darkness, its spontaneous pigment-isomerization rate would need to be ∼3,000-fold (=Io/0.008 Rho* s−1) higher than that of WT-Rho.
Fig. 4.
Magnitude of equivalent background light for producing mouse rod desensitization. (A) Flash-on-background experiment with RhoWT/WT;Gcaps+/+ rods showing flash responses in the absence (Top) and presence (Bottom) of steady background light that reduced sensitivity approximately by half. (Inset) Normalized and averaged flash-response waveforms in the absence (black) or presence (purple) of the background light. (B) Weber–Fechner relation [SF/SD = 1/(1 + IB/Io)] fit to flash sensitivity (SF) normalized to dark-adapted sensitivity (SF/SD) plotted against background light [IB, converted to isomerizations⋅s−1 by multiplying with the effective collective area of mouse rods, ∼0.4 μm2 (3)]. The intensity required to reduce sensitivity by half (Io, purple dashed line) was 63 photons⋅µm−2⋅s−1 (∼24 isomerizations⋅s−1). If D190N-Rho content in a mouse rod was as high as endogenous WT-Rho in WT rods, the “equivalent” steady light from spontaneous D190N-Rho isomerizations would be ∼0.13 isomerizations⋅s−1 (red dashed line), which would reduce a rod’s sensitivity by only ∼0.5%.
D190N-Rho Causes Retinal Degeneration through a Transducin-Independent Mechanism.
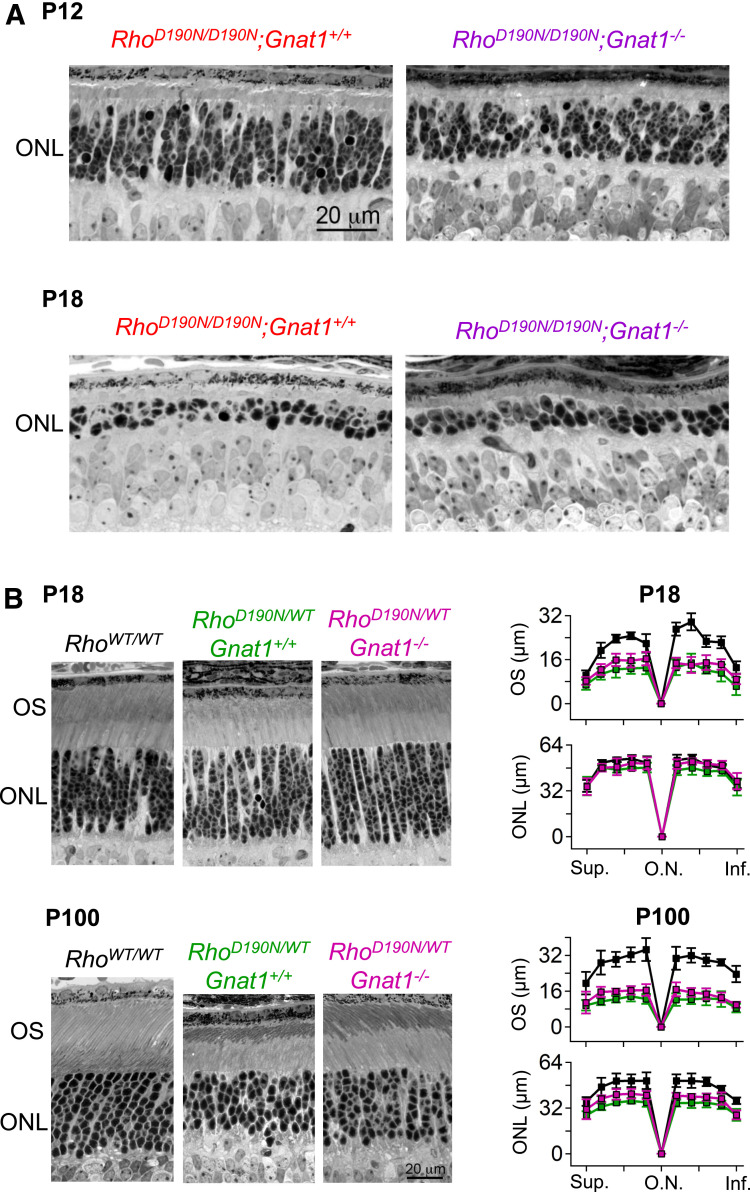
Given the normal cellular dark noise in RhoD190N/WT rods, we asked whether some other aberrant side effect of phototransduction by D190N-Rho via transducin would possibly lead to degeneration (47). We bred RhoD190N/D190N and RhoD190N/WT animals into the rod transducin-α–knockout (Gnat1−/−; ref. 48). There was severe degeneration in RhoD190N/D190N; Gnat1−/− rods, with no sign of improvement over RhoD190N/D190N; Gnat1+/+ rods at either postnatal day 12 (P12) or P18 (Fig. 5A). Broadly, the same was true for RhoD190N/WT rods at P18, except for a slight improvement (not statistically significant) in the outer-segment length with transducin being absent (Fig. 5 B, Upper Left, verified by morphometric measurements in Fig. 5 B, Upper Right). At P100, some loss of photoreceptor nuclei in RhoD190N/WT was quite obvious, with also no improvement in the Gnat1−/− background except again for a slightly longer (statistically insignificant) outer-segment length (Fig. 5 B, Lower Right). In summary, D190N-Rho signaling via transducin appears to be unrelated to rod degeneration.
Fig. 5.
D190N-Rho causes retinal degeneration by a transducin-independent mechanism. (A, Top Left) Representative retinal cross-section from a RhoD190N/D190N animal at eye opening, P12. The outer nuclear layer (ONL) was thinner than in P12 RhoWT/WT (see ref. 19). (A, Top Right) Cross-section from a RhoD190N/D190N;Gnat1−/− animal at eye opening, P12. (A, Bottom Left) Representative retinal cross-section from a RhoD190N/D190N animal at P18, when rod outer segments are fully mature in RhoWT/WT rods. Severe degeneration led to only two to three rods remaining in each ONL column. (A, Bottom Right) The ONL thickness was similar in RhoD190N/D190N;Gnat1−/− demonstrating that the Gnat1−/− background did not prevent degeneration. (B, Top Left) Histological sections at P18 from RhoWT/WT, RhoD190N/WT;Gnat1+/+ and RhoD190N/WT;Gnat1−/−. (B, Top Right) Outer nuclear layer thickness (ONL) and approximate outer segment length (OS) quantified along the superior–inferior axis along the central meridian (n = 4 to 9 animals; mean ± SD). (B, Bottom) Histological sections from P100 animals and the corresponding morphometric analysis. One-way ANOVA was calculated to detect differences in ONL thickness and OS length between the three groups, followed by Tukey HSD. RhoD190N/WT;Gnat1+/+ and RhoD190N/WT;Gnat1−/− groups showed no difference at all retinal regions in P18 and P100 sections, whereas both were significantly different from RhoWT/WT retinae in P18 OS length and in P100 ONL thickness and OS length (0.0001 ≤ P ≤ 0.05). Inf., inferior; O.N., optic nerve; Sup., superior.
Misfolding of D190N-Rho Induces Cellular Stress.
Having ruled out D190N-Rho noise as the cause of degeneration, we investigated the possible involvement of this protein’s misfolding. RhoD190N/D190N animals had very rapid photoreceptor cell death, with obvious degeneration already at eye opening (P12). By P21, only two rows of photoreceptor nuclei remained (19). This is even more severe than the reported degeneration in homozygous rhodopsin knockout mice (Rho−/−), in which most Rho−/− rod cell bodies survive until at least P30 (9). Such rapid degeneration suggests that D190N-Rho in some way exacerbates degeneration beyond simply a loss of structural support from diminished pigment content (see Introduction).
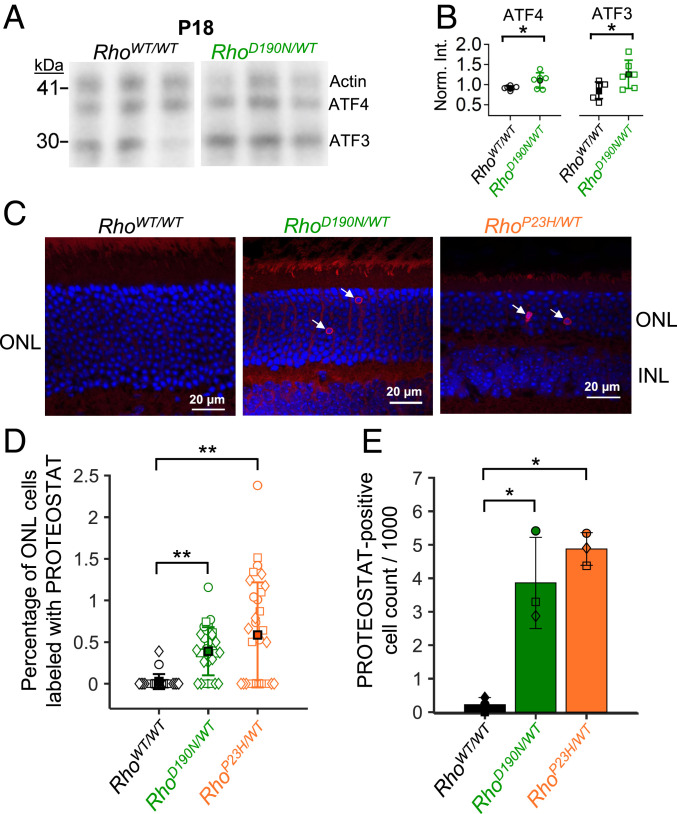
The diminution of pigment content in the outer segment in the presence of D190N-Rho suggests misfolding of a fraction of D190N-Rho molecules in the rod. Accordingly, we looked for signs of the unfolded protein response, which could cause cell death eventually through stress on the endoplasmic reticulum (21, 22, 49). Immunoblots for ATF-4 and ATF-3 [components of the PERK pathway of the unfolded protein response (22, 49)] showed a modest but significant up-regulation in retinal extracts from P18 RhoD190N/WT animals (Fig. 6 A and B). We also tested a dye that becomes fluorescent when trapped by misfolded-protein aggregates [PROTEOSTAT (50–52)] and found it to label more rod cell bodies in the outer nuclear layer of 1- to 2-mo RhoD190N/WT retinae than of RhoWT/WT controls (Fig. 6C). In some sections, this dye showed a weak background signal in the outer-segment and/or inner-segment layers as well as the outer plexiform layer, but the strongest and most consistent labeling was in some rod cell bodies. As a positive control (Fig. 6C), we did similar dye-labeling experiments on a mutant-rhodopsin knockin line, RhoP23H/WT (21), well known to show cell death through protein misfolding of P23H-Rho (53). Across all images, the average percentage of PROTEOSTAT-positive cells in each RhoWT/WT image was 0.03 ± 0.09% (Fig. 6D; n = 24 images) or a cell density in each animal of 0.22 ± 0.19 PROTEOSTAT-positive cells per 1,000 outer nuclear layer cells (Fig. 6E; n = 3 animals). In RhoD190N/WT retinal sections, the proportion of PROTEOSTAT-positive cells in each image and total cell densities in each animal were more than 10-fold higher than in RhoWT/WT controls, being 0.39 ± 0.29% (n = 29 images) and 3.86 ± 1.36 PROTEOSTAT-positive cells per 1,000 cells (n = 3 animals). In comparison, RhoP23H/WT retinal sections had 0.59 ± 0.63% labeled ONL cell bodies per image (n = 32 images) and 4.87 ± 0.49 PROTEOSTAT-positive cells per 1,000 outer nuclear layer cells (n = 3 animals). While PROTEOSTAT-positive cells were not abundant in RhoD190N/WT or even RhoP23H/WT animals, such sparse labeling has been reported for apoptotic cells in other mouse models of retinal degeneration (54).
Fig. 6.
Induction of the unfolded protein response and detection of misfolded protein aggregates. (A) Immunoblots of ATF4 and ATF3, which are involved in the unfolded protein response. Actin was costained for normalization. Blots from three animals each of P18 RhoWT/WT (Left) and RhoD190N/WT (Right) are shown. (B) Band intensity normalized to actin for each sample. Both ATF4 and ATF3 were significantly up-regulated in retinal extracts from P18 RhoD190N/WT;Gnat1+/+ animals (P < 0.05). (C) Cryosections and fluorescent labeling using PROTEOSTAT dye to stain RhoWT/WT (Left), RhoD190N/WT (Middle), and RhoP23H/WT (Right) retinae. (D) Percentage of PROTEOSTAT-positive cells across images. Each symbol represents the percentage from a single image as shown in C. Circles, squares, and diamonds represent data from each of three animals. (E) Overall density of PROTEOSTAT-positive cells per 1,000 cell bodies counted in the outer nuclear layer in each animal (n = 3 animals). Each symbol (circle, square, or diamond) represents the PROTEOSTAT-positive cell density from an individual animal. The single stars mark statistical significance of 0.0001 ≤ P ≤ 0.05, and the double stars mark P < 0.0001. Comparing RhoD190N/WT and RhoP23H/WT, there was not a statistically significant difference in the densities of PROTEOSTAT-positive cells.
Mechanism of D190N-Rho’s Elevated Spontaneous Activity.
Independent of D190N-Rho’s pathogenic mechanism, we were also interested in what underlies its heightened spontaneous activity. We previously developed a physicochemical theory that predicts quite well the measured spontaneous isomerization rates across WT rod and cone pigments of multiple animal species (7, 40). In this theory, two factors govern the molecular rate constant. The first is a pigment’s “critical wavelength,” λc, which is the photon wavelength shorter than which photoexcitation of the pigment becomes temperature insensitive, thus quantifying its ground-state isomerization energy barrier, Ea, according to Ea = hc/λc, with h being Planck’s constant and c the speed of light (7). We have found that the ratio λc/λmax, where λmax is the wavelength of maximum light absorption by the pigment, appears to be a fundamental constant equal to 0.84 ± 0.01 [n = 7 pigments (7)], although no disease-inducing mutant pigments have yet been tested up to now.
The second factor has to do with physical constraints in the pigment’s chromophore-binding pocket, which, in our theory, manifests as a proportionality constant equivalent to the “preexponential factor” in reaction rate theory in physical chemistry (7). Past measurements by us from several native rod and cone pigments have indicated that this proportionality constant is empirically ∼26 times higher for native cone pigments than for native rod pigments (7). Cone pigments are known to have a more accessible chromophore-binding pocket, exhibiting the property of spontaneous chromophore exchange in darkness not found in rod pigments (40, 55, 56). Accordingly, we have hypothesized a correlation between this dark-chromophore exchange property and a steric openness (i.e., less restrictiveness for molecular motion of the chromophore) of the binding pocket, hence giving a larger proportionality constant for spontaneous isomerization (7, 40). This correlation is only qualitative, however, in that a ∼26-fold difference in the multiplication factor does not have to be constant between all rod pigments and all cone pigments. After all, gradations in the openness of the chromophore binding pocket can in principle exist.
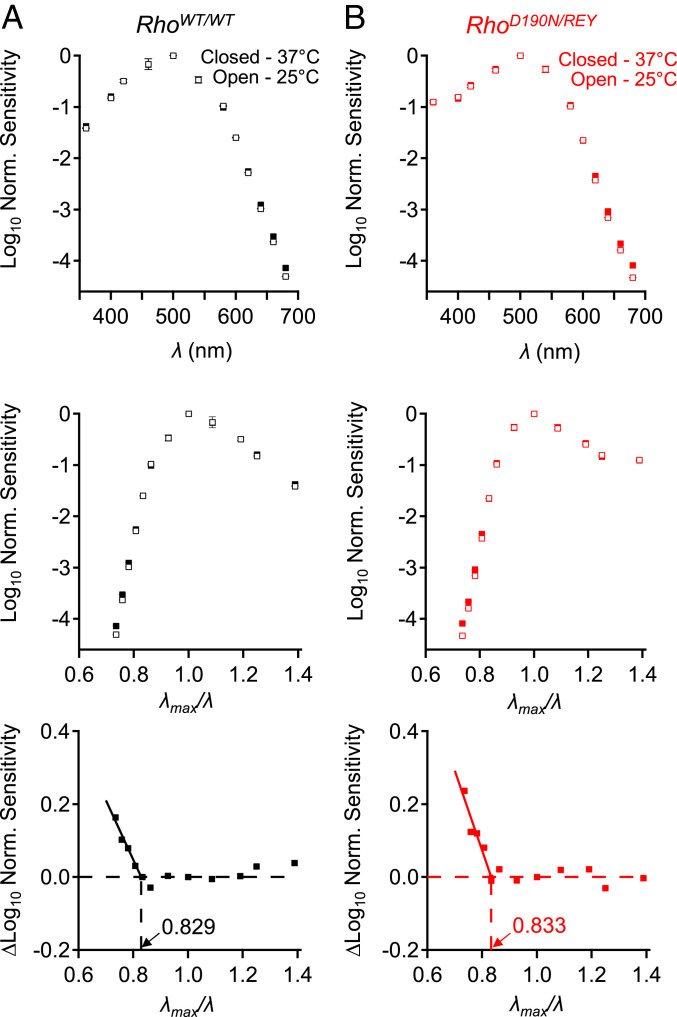
With the above background, the relevant factors determining the thermal isomerization of D190N-Rho in RhoD190N/REY can now be presented. By using dim-flash responses to measure rod spectral sensitivity at 37 and 25 °C, we found that the λc/λmax ratio for D190N-Rho in RhoD190N/REY rods (Fig. 7) indeed adheres to the universal constant of 0.84 ± 0.01 (7). We confirmed this parameter to be ∼0.83 for WT-Rho in RhoWT/WT rods (Fig. 7A), and the same for D190N-Rho in RhoD190N/REY rods (Fig. 7B), thus giving, together with a λmax of ∼500 nm for both WT-Rho and D190N-Rho, an isomerization energy barrier (Ea) of ∼47 kcal⋅mol−1.
Fig. 7.
The isomerization energy barrier is unchanged in D190N-Rho. (A, Top) Control experiments with RhoWT/WT (C57BL/6J) rods showing the effect of temperature on normalized action spectra at 25 °C (open symbols) and at 37 °C (closed symbols). (A, Middle) Plotting normalized sensitivity on a reciprocal-wavelength plot with λmax/λ as the abscissa. (A, Bottom) Subtraction of the 25 °C spectrum from the 37 °C spectrum shows enhancement of photosensitivity by heat at longer wavelengths (i.e., lower-energy photons). (B) Corresponding experiments with RhoD190N/REY rods. Peak sensitivity was at ∼500 nm for both WT-Rho and D190N-Rho. For RhoWT/WT rods, temperature enhancement began at λmax/λ = 0.829 corresponding to an isomerization energy barrier of ∼47.4 kcal⋅mol−1 in WT-Rho, and for RhoD190N/REY rods, λmax/λ = 0.833 indicating an isomerization energy barrier of ∼47.7 kcal⋅mol−1 in D190N-Rho.
Next, we checked the property of chromophore exchange in darkness in D190N-Rho. The molecular rate of chromophore exchange in WT-Rho is exceedingly low, being reported to be on average only once every 52 d at 37 °C (57), versus only hours at room temperature (40) for the relatively more “open” binding pocket of cone pigments. We heterologously expressed D190N-Rho in HEK293 cells and extracted the protein for in vitro spectroscopic measurements (SI Appendix, Supplementary Methods). We then tracked any exchange of native 11-cis-retinal with exogenous 9-cis-retinal in darkness as indicated by a gradual blueshift in λmax (40, 55, 56). We found that mouse D190N-Rho could not be reconstituted in vitro, perhaps due to some misfolding/mistargeting even at room temperature (SI Appendix, Fig. S3A), similar to what has been reported for human D190N-Rho in HEK293 cells (26). In contrast, bovine D190N-Rho could be reconstituted and incubated at 37 °C for up to 20 d, albeit with steady thermal deterioration (SI Appendix, Fig. S3 B and C).
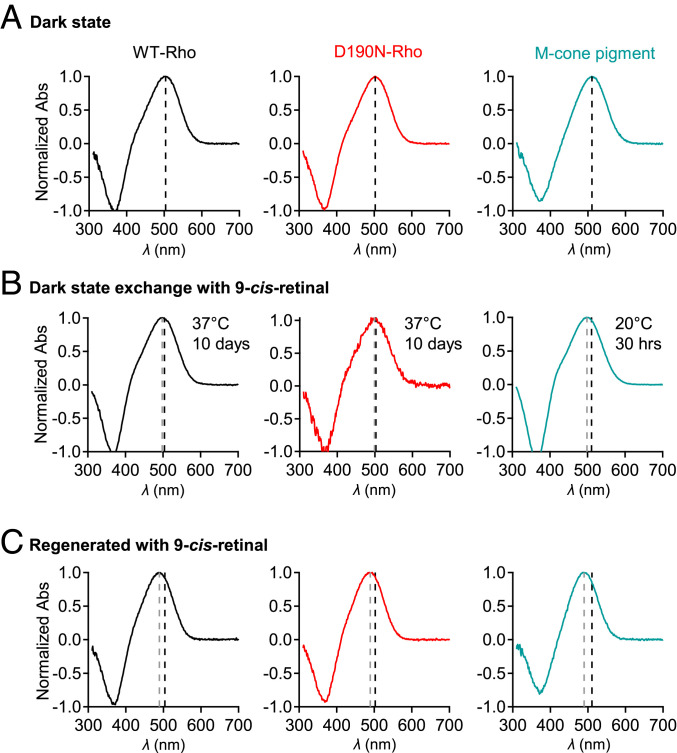
For chromophore exchange, parallel experiments were done on bovine WT-Rho, bovine D190N-Rho, and chicken M-cone pigment for comparison. Chicken M-cone pigment was used because of its lower rate of deterioration in vitro than for the mouse homolog. Dark-state bovine WT-Rho had a λmax of 503.5 nm (Fig. 8 A, Left) and shifted only slightly (by −5.2 nm) after a 10-d incubation at 37 °C with 9-cis-retinal to 498.3 ± 0.6 nm (Fig. 8 B, Left; n = 3). Dark-state bovine D190N-Rho’s λmax was 502.8 nm (Fig. 8 A, Center) and shifted only ever so slightly (by −3.3 nm) to 499.5 ± 1.8 nm (Fig. 8 B, Center; n = 3) after the same 9-cis-retinal treatment for 10 d. Contrastingly, chicken M-cone pigment showed a much faster and more obvious chromophore exchange, with its dark-state λmax of 510.5 nm (Fig. 8 A, Right) shifting (by −12.1 nm) after only 30 h at 20 °C to 498.4 ± 0.7 nm (Fig. 8 B, Right; n = 3). To demonstrate the maximal shifts, we fully reconstituted each pigment with 9-cis-retinal (Fig. 8C), which gave λmax shifts of −14.0, −13.3, and −20.5 nm for WT-Rho, D190N-Rho, and M-cone pigment, respectively. While these measurements were largely qualitative, D190N-Rho’s exceedingly slow chromophore exchange behavior clearly resembled that of WT-Rho.
Fig. 8.
Chromophore exchange in D190N-Rho. (A) Dark absorption spectra (normalized against peak optical density) of bovine WT-Rho (black), bovine D190N-Rho (red), and chicken M-cone pigment (teal), bound to 11-cis-retinal. The black dashed lines are λmax values for each pigment (503.8, 502.5, and 510.5 nm, respectively). (B) Chromophore exchange experiment involving exposure of bovine WT-Rho and D190N-Rho (for 10 d at 37 °C) or of chicken M-cone pigment (for 30 h at 20 °C) to 9-cis-retinal. The gray dashed lines are blue-shifted λmax after exchange of 9-cis-retinal for 11-cis-retinal after the labeled exposure times. The λmax shifts were −5.5 ± 0.6 nm (n = 3 preparations) for WT-Rho, −3.3 ± 1.8 nm (n = 3 preparations) for D190N-Rho, and −12.1 ± 0.7 nm (n = 3 preparations) for M-cone pigment. There was no significant difference between the λmax-shift values of D190N-Rho and WT-Rho (P = 0.16). (C) Maximally blue-shifted spectra from full regeneration with 9-cis-retinal, starting with respective opsin, giving λmax shifts to 489.5 nm (WT-Rho), 489 nm (D190N-Rho), and 490 nm (chicken M-cone pigment).
From the above experiments, it seems that the much higher spontaneous activity of D190N-Rho measured physiologically in live rods could not be explained by a shallowing of its isomerization energy barrier or from an open chromophore-binding pocket resembling that in cone pigments. On the other hand, a mutation such as D190N, which is situated directly above the chromophore-binding pocket, could conceivably still give more freedom of movement to the chromophore (hence a larger preexponential factor, albeit <26-fold) without necessarily opening the binding pocket enough to increase chromophore exchange as that shown by cone pigments. It is not yet known exactly how the “openness” of the chromophore-binding pocket and the preexponential factor from our pigment noise theory (7) are influenced by the bond energies in the hydrogen-bond network formed between D190 and neighboring residues within the chromophore-binding pocket (16, 31).
Discussion
Some rhodopsin mutations causing night blindness followed by retinal degeneration have been speculated to do so by producing a higher frequency of spontaneous isomerization events (reviewed in ref. 45). Spontaneous-isomerization noise is particularly amenable to physiological study (6, 7, 39, 40, 58), prompting us to examine this property regarding D190N-Rho. By silencing the endogenous rhodopsin signal sufficiently through replacing it with the mutant rhodopsin, REY-Rho, we were able to quantify D190N-Rho’s dark noise in mouse rods. We found that D190N-Rho indeed had over an order-of-magnitude higher molecular rate constant of spontaneous isomerization compared to WT-Rho. Nonetheless, because of the diminished D190N-Rho content in RhoD190N/REY and RhoD190N/WT animals, this elevation in molecular noise actually does not increase the overall cellular dark noise. Thus, the higher molecular D190N-Rho spontaneous activation compared to WT-Rho does not cause rod desensitization or, more importantly, underlie degeneration of RhoD190N/WT mouse rods. The lack of improvement of the condition in a transducin-knockout background further supported this conclusion. Assuming that the data from the genetic mouse model are valid for human patients, this rather surprising conclusion importantly demonstrates that in vitro measurements, albeit important for capturing certain salient protein properties, can still miss key subtleties that are evident only in live rods.
It would be interesting to know whether our understanding gained here applies to some other disease-causing rhodopsin mutants also thought to have higher spontaneous activity. In this regard, another extensively studied disease-causing rhodopsin mutation, G90D-Rho, has been found to destabilize a crucial ionic bond (E113 to K296) normally preventing dark-state WT-Rho from signaling (59), thus constitutively triggering activation of transducin by exposing the G-protein interaction site without canonical isomerization of 11-cis-retinal (30, 59, 60). Curiously, dark electrical noise did not appear substantially elevated in transgenic mouse rods expressing G90D-Rho despite signs of adaptation to an equivalent background light estimated to be ∼130 Rho* s−1⋅cell−1 (13, 14). Other disease-causing mutations, such as T94I-, A292E-, and A295V-Rho, have also been speculated to show dark-state constitutive activity without canonical chromophore isomerization (30, 45, 61), but these mutations lack animal models for in vivo confirmation. Mechanistic uncertainties likewise remain for G90V, E113Q, S186W, and K296G (16, 30, 45). As illustrated by G90D-Rho, constitutive pigment activity measured biochemically does not necessarily translate into a measurable elevation in dark electrical noise in intact photoreceptors. As a separate example, K296E-Rho lacks the chromophore linkage site and results in heightened constitutive apo-opsin activity in vitro (28). This mutation causes rod degeneration in vivo—not due to elevated dark noise—but primarily from rhodopsin being retained in the inner segment bound to arrestin, which induces cell stress because of the inability of apo-opsin to convert to dark-state rhodopsin (23, 24).
In the particularly debatable case of D190N-Rho (16, 19, 26, 27, 31, 35), our results suggest that the main phenotype is a reduction in the functional pigment content due to protein misfolding, resulting in a shortened outer-segment length and a faster response shutoff rather similar to heterozygous rhodopsin-knockout rods (9–11, 38). Similarly, isoelectric focusing experiments showed that D190N-Rho comprised approximately only one-third of the visual pigment in both RhoD190N/REY and RhoD190N/WT retinae. Of note, nonetheless, is that those D190N-Rho molecules that traffic successfully to the outer segment respond to light literally identically to WT-Rho, based on suction pipette recordings from RhoD190N/REY rods, Previous studies of RhoD190N/WT animals were unable to detect mistargeted D190N-Rho by immunostaining or immunoblots with anti-Rho antibodies (19, 32), perhaps due to rapid degradation of misfolded D190N-Rho. Such degradation could help explain why many RhoD190N/WT rods are able to tolerate the presence of D190N-Rho for months in mice (and decades in human patients) before the mutant pigment triggers rod death.
We found a modest induction of the unfolded protein response as well as occasional rods showing misfolded-protein aggregates accompanying the gradual degeneration in RhoD190N/WT retinae. In contrast, homozygous RhoD190N/D190N rods do not form an outer segment at all and the rate of retinal degeneration is even more rapid than Rho−/− rods (9, 19). Thus, possibly, the trafficking and/or stability of D190N-Rho is contingent on the presence of WT-Rho. On the other hand, the presence of D190N-Rho appears to impact negatively the overall Rho content in RhoD190N/WT rods. An important remaining question is what can be done to prevent vision loss. Our findings demonstrate that D190N-Rho does not desensitize rods through elevated spontaneous activity; instead, the mutation leads to disruption of rhodopsin’s structural support of the outer segment, loss of photon capture, and mild induction of the unfolded protein response. Gene therapy using CRISPR/Cas9 to correct the D190N mutation has been proposed as one possible solution (62), but it is important to keep in mind that early intervention might be required for maintaining healthy rod morphology.
Methods
Analyses of Flash Responses and Dark Noise.
Suction pipette recording was used to record flash responses and dark noise (SI Appendix, Supplementary Methods), 10 to 30 flash responses were measured at each flash strength (with fewer flashes for saturated responses). Transient peaks of mean responses were used to determine the intensity–response relation for each cell by fitting to a saturating-exponential function R/Rmax = 1 − exp(−IF × K), where R is response peak, Rmax is saturated-response peak, IF is flash strength (in photons per square micrometer), and K is a constant. The half-saturating flash strength, ρ, is given by ρ = loge(2)/K. To evaluate single-photon responses, we measured 30 to 100 dim-flash responses at an intensity within the linear foot of the intensity–response relation. Quantal analyses and determination of flash response parameters (integration time, time-to-peak, and recovery time constant) were carried out as described previously (3).
To measure thermal isomerization events in mouse rods, we needed extremely stable recording and low instrumental noise. Recordings were optimized using pipettes with a tip opening of ∼1.5 to 1 μm and a resistance of ∼7 to 8 MΩ in recording solutions at room temperature. To minimize the slow electronic drift, we used a custom-made chamber with an exit port lined with a piece of absorbent tissue paper (Kimberly-Clark Professional; Kimtech Science, Kimwipe) that would wick away solution at the same speed that it entered the chamber. The ground electrode holder (World Precision Instruments; MEH3S10) was filled with pipette solution and connected to the bath by a glass capillary filled with 1 M KCl in 3% agar gel. Dark noise was recorded in 10-min epochs. Discrete events were counted using custom MATLAB (MathWorks) software with the criteria of peak amplitude >30% of the single-photon response peak and duration of 50 to 200% of the integration time of the single-photon response measured from the same cell.
Estimating Rho Content from the Probability of Failure.
According to the Poisson distribution of photon absorption (42), the probability of failure (p0) is given by p0 = exp (−AC × IF), where AC is the effective collecting area of an outer segment (in square micrometers) and IF is flash strength (in photons per square micrometer). A failure was defined as a flash trial that did not result in a response reaching a criterion amplitude (typically ≥50% of the single-photon response peak) within a characteristic time window based on the average response waveform. The computed probability of failure was routinely verified by inspecting each flash response trial. AC is a function of effective-Rho content in the outer segment and Rho’s light absorption properties (3, 42), AC = V × δ × αλ × f × Q, where V is the volume of outer segment, δ is the concentration of functional Rho molecules in the outer segment (the unknown parameter, with units of molecules per cubic micrometer), αλ is the molecular extinction coefficient of Rho (63) oriented in an outer segment disk membrane [∼1.76 × 10−8 μm2 for WT-Rho based on microspectrophotometry (2, 3), with this parameter being 6% lower for D190N-Rho (16)], Q is the probability that an absorbed photon leads to isomerization [0.67 (63)], and f is a polarization factor due to the orientation of rhodopsin approximately perpendicular to the outer segment long axis [0.5 for transverse illumination with unpolarized light (42)]. Thus, the probability of failure can be used to estimate the number of effective Rho molecules (n) in a rod as follows:
| [1] |
| [2] |
| [3] |
Supplementary Material
Acknowledgments
We thank Drs. Chyuan-Sheng Lin (Columbia University) and Janis Lem (Tufts University) for generating the RhoD190N/D190N and Gnat1−/− mouse lines, respectively. We also thank Dr. Jeremy Nathans, Dr. Jacob S. Heng, and members of the K.-W.Y. laboratory for discussions. This work was supported by NIH Grants EY006837 (to K.-W.Y.); EY012155 and EY027193 (to J.C.); Vision Core Grant P30EY029220 (to the Roski Eye Institute at University of Southern California); EY018213 and EY024698; and the Crowley Family Fund (to S.H.T.); CREST, Japan Science and Technology Agency Grant JPMJCR1753 (to T.Y.); the António Champalimaud Vision Award, Portugal (to K.-W.Y.); NIH Grant NS050274 (Multiphoton Imaging Core at Johns Hopkins University); and the Visual Science Training Program Fellowship from the National Eye Institute, Grant EY007143 (to D.S.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2010417117/-/DCSupplemental.
Data Availability.
All study data are included in the article and SI Appendix.
References
- 1.Hecht S., Shlaer S., Pirenne M. H., Energy, quanta and vision. J. Gen. Physiol. 25, 819–840 (1942). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hárosi F. I., Absorption spectra and linear dichroism of some amphibian photoreceptors. J. Gen. Physiol. 66, 357–382 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yue W. W. S.et al., Elementary response triggered by transducin in retinal rods. Proc. Natl. Acad. Sci. U.S.A. 116, 5144–5153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leskov I. B. B.et al., The gain of rod phototransduction: Reconciliation of biochemical and electrophysiological measurements. Neuron 27, 525–537 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Arshavsky V. Y., Burns M. E., Current understanding of signal amplification in phototransduction. Cell. Logist. 4, e29390 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baylor D. A., Matthews G., Yau K. W., Two components of electrical dark noise in toad retinal rod outer segments. J. Physiol. 309, 591–621 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo D.-G., Yue W. W. S., Ala-Laurila P., Yau K.-W., Activation of visual pigments by light and heat. Science 332, 1307–1312 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries M. M.et al., Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat. Genet. 15, 216–219 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Lem J.et al., Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc. Natl. Acad. Sci. U.S.A. 96, 736–741 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makino C. L.et al., Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS One 7, e37832 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y.et al., Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J. Biol. Chem. 279, 48189–48196 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathans J., In the eye of the beholder: Visual pigments and inherited variation in human vision. Cell 78, 357–360 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Sieving P. A.et al., Constitutive “light” adaptation in rods from G90D rhodopsin: A mechanism for human congenital nightblindness without rod cell loss. J. Neurosci. 21, 5449–5460 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dizhoor A. M.et al., Night blindness and the mechanism of constitutive signaling of mutant G90D rhodopsin. J. Neurosci. 28, 11662–11672 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin S., Cornwall M. C., Oprian D. D., Opsin activation as a cause of congenital night blindness. Nat. Neurosci. 6, 731–735 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Liu M. Y.et al., Thermal stability of rhodopsin and progression of retinitis pigmentosa: Comparison of S186W and D190N rhodopsin mutants. J. Biol. Chem. 288, 17698–17712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeitz C.et al., Identification and functional characterization of a novel rhodopsin mutation associated with autosomal dominant CSNB. Invest. Ophthalmol. Vis. Sci. 49, 4105–4114 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Dryja T. P., Berson E. L., Rao V. R., Oprian D. D., Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat. Genet. 4, 280–283 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Sancho-Pelluz J.et al., Mechanisms of neurodegeneration in a preclinical autosomal dominant retinitis pigmentosa knock-in model with a RhoD190N mutation. Cell. Mol. Life Sci. 76, 3657–3665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franke R. R., König B., Sakmar T. P., Khorana H. G., Hofmann K. P., Rhodopsin mutants that bind but fail to activate transducin. Science 250, 123–125 (1990). [DOI] [PubMed] [Google Scholar]
- 21.Sakami S.et al., Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J. Biol. Chem. 286, 10551–10567 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Athanasiou D.et al., The role of the ER stress-response protein PERK in rhodopsin retinitis pigmentosa. Hum. Mol. Genet. 26, 4896–4905 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J.et al., Stable rhodopsin/arrestin complex leads to retinal degeneration in a transgenic mouse model of autosomal dominant retinitis pigmentosa. J. Neurosci. 26, 11929–11937 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moaven H.et al., Visual arrestin interaction with clathrin adaptor AP-2 regulates photoreceptor survival in the vertebrate retina. Proc. Natl. Acad. Sci. U.S.A. 110, 9463–9468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sung C. H.et al., Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc. Natl. Acad. Sci. U.S.A. 88, 6481–6485 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung C. H., Davenport C. M., Nathans J., Rhodopsin mutations responsible for autosomal dominant retinitis pigmentosa. Clustering of functional classes along the polypeptide chain. J. Biol. Chem. 268, 26645–26649 (1993). [PubMed] [Google Scholar]
- 27.Kaushal S., Khorana H. G., Structure and function in rhodopsin. 7. Point mutations associated with autosomal dominant retinitis pigmentosa. Biochemistry 33, 6121–6128 (1994). [DOI] [PubMed] [Google Scholar]
- 28.Robinson P. R., Cohen G. B., Zhukovsky E. A., Oprian D. D., Constitutively active mutants of rhodopsin. Neuron 9, 719–725 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Rao V. R., Cohen G. B., Oprian D. D., Rhodopsin mutation G90D and a molecular mechanism for congenital night blindness. Nature 367, 639–642 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Rao V. R., Oprian D. D., Activating mutations of rhodopsin and other G protein-coupled receptors. Annu. Rev. Biophys. Biomol. Struct. 25, 287–314 (1996). [DOI] [PubMed] [Google Scholar]
- 31.Janz J. M., Fay J. F., Farrens D. L., Stability of dark state rhodopsin is mediated by a conserved ion pair in intradiscal loop E-2. J. Biol. Chem. 278, 16982–16991 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Sancho-Pelluz J.et al., Mice with a D190N mutation in the gene encoding rhodopsin: A model for human autosomal-dominant retinitis pigmentosa. Mol. Med. 18, 549–555 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsui I., Chou C. L., Palmer N., Lin C. S., Tsang S. H., Phenotype-genotype correlations in autosomal dominant retinitis pigmentosa caused by RHO, D190N. Curr. Eye Res. 33, 1014–1022 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fishman G. A.et al., Ocular findings associated with rhodopsin gene codon 267 and codon 190 mutations in dominant retinitis pigmentosa. Arch. Ophthalmol. 110, 1582–1588 (1992). [DOI] [PubMed] [Google Scholar]
- 35.Janz J. M., Farrens D. L., Assessing structural elements that influence Schiff base stability: Mutants E113Q and D190N destabilize rhodopsin through different mechanisms. Vision Res. 43, 2991–3002 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Nakatani K., Yau K.-W., Calcium and light adaptation in retinal rods and cones. Nature 334, 69–71 (1988). [DOI] [PubMed] [Google Scholar]
- 37.Fain G. L., Matthews H. R., Cornwall M. C., Koutalos Y., Adaptation in vertebrate photoreceptors. Physiol. Rev. 81, 117–151 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Calvert P. D.et al., Membrane protein diffusion sets the speed of rod phototransduction. Nature 411, 90–94 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Fu Y., Kefalov V., Luo D.-G., Xue T., Yau K.-W., Quantal noise from human red cone pigment. Nat. Neurosci. 11, 565–571 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue W. W. S.et al., Spontaneous activation of visual pigments in relation to openness/closedness of chromophore-binding pocket. eLife 6, e18492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burns M. E., Mendez A., Chen J., Baylor D. A., Dynamics of cyclic GMP synthesis in retinal rods. Neuron 36, 81–91 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Baylor D. A., Lamb T. D., Yau K.-W., Responses of retinal rods to single photons. J. Physiol. 288, 613–634 (1979). [PMC free article] [PubMed] [Google Scholar]
- 43.Lokappa S., Cornwall M., Chen J., Isoelectric focusing to quantify rhodopsin phosphorylation in mouse retina. Bio Protoc. 9, e3300 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berry J.et al., Effect of rhodopsin phosphorylation on dark adaptation in mouse rods. J. Neurosci. 36, 6973–6987 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park P. S.-H., Constitutively active rhodopsin and retinal disease. Adv. Pharmacol. 70, 1–36 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan J., Woodruff M. L., Cilluffo M. C., Crouch R. K., Fain G. L., Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. J. Physiol. 568, 83–95 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majumder A.et al., Transducin translocation contributes to rod survival and enhances synaptic transmission from rods to rod bipolar cells. Proc. Natl. Acad. Sci. U.S.A. 110, 12468–12473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calvert P. D.et al., Phototransduction in transgenic mice after targeted deletion of the rod transducin alpha -subunit. Proc. Natl. Acad. Sci. U.S.A. 97, 13913–13918 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang T., Chen J., Induction of the unfolded protein response by constitutive G-protein signaling in rod photoreceptor cells. J. Biol. Chem. 289, 29310–29321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen D.et al., Novel cell- and tissue-based assays for detecting misfolded and aggregated protein accumulation within aggresomes and inclusion bodies. Cell Biochem. Biophys. 60, 173–185 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ou J.et al., iPSCs from a hibernator provide a platform for studying cold adaptation and its potential medical applications. Cell 173, 851–863.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jena K. K.et al., TRIM16 controls assembly and degradation of protein aggregates by modulating the p62-NRF2 axis and autophagy. EMBO J. 37, e98358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiang W. C.et al., Robust endoplasmic reticulum-associated degradation of rhodopsin precedes retinal degeneration. Mol. Neurobiol. 52, 679–695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portera-Cailliau C., Sung C. H., Nathans J., Adler R., Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc. Natl. Acad. Sci. U.S.A. 91, 974–978 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumoto H., Tokunaga F., Yoshizawa T., Accessibility of the iodopsin chromophore. Biochim. Biophys. Acta 404, 300–308 (1975). [DOI] [PubMed] [Google Scholar]
- 56.Kefalov V. J.et al., Breaking the covalent bond—a pigment property that contributes to desensitization in cones. Neuron 46, 879–890 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian H., Sakmar T. P., Huber T., Measurement of slow spontaneous release of 11-cis-retinal from rhodopsin. Biophys. J. 112, 153–161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kefalov V., Fu Y., Marsh-Armstrong N., Yau K.-W., Role of visual pigment properties in rod and cone phototransduction. Nature 425, 526–531 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singhal A.et al., Insights into congenital stationary night blindness based on the structure of G90D rhodopsin. EMBO Rep. 14, 520–526 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zvyaga T. A., Fahmy K., Siebert F., Sakmar T. P., Characterization of the mutant visual pigment responsible for congenital night blindness: A biochemical and Fourier-transform infrared spectroscopy study. Biochemistry 35, 7536–7545 (1996). [DOI] [PubMed] [Google Scholar]
- 61.Singhal A.et al., Structural role of the T94I rhodopsin mutation in congenital stationary night blindness. EMBO Rep. 17, 1431–1440 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai Y.-T.et al., Clustered regularly interspaced short palindromic repeats-based genome surgery for the treatment of autosomal dominant retinitis pigmentosa. Ophthalmology 125, 1421–1430 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dartnall H. J. A., “Photosensitivity” in Handbook of Sensory Physiology, Dartnall H. J. A., Ed. (Springer, New York, 1972), Vol. 7, pp. 122–145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and SI Appendix.