Abstract
Despite an abundant literature on gold nanoparticles use for biomedicine, only a few of the gold-based nanodevices are currently tested in clinical trials, and none of them are approved by health agencies. Conversely, ionic gold has been used for decades to treat human rheumatoid arthritis and benefits from 70-y hindsight on medical use. With a view to open up new perspectives in gold nanoparticles research and medical use, we revisit here the literature on therapeutic gold salts. We first summarize the literature on gold salt pharmacokinetics, therapeutic effects, adverse reactions, and the present repurposing of these ancient drugs. Owing to these readings, we evidence the existence of a common metabolism of gold nanoparticles and gold ions and propose to use gold salts as a “shortcut” to assess the long-term effects of gold nanoparticles, such as their fate and toxicity, which remain challenging questions nowadays. Moreover, one of gold salts side effects (i.e., a blue discoloration of the skin exposed to light) leads us to propose a strategy to biosynthesize large gold nanoparticles from gold salts using light irradiation. These hypotheses, which will be further investigated in the near future, open up new avenues in the field of ionic gold and gold nanoparticles-based therapies.
Keywords: gold nanoparticles, therapeutic gold salts, drug repurposing, nanomedicine, nanotoxicity
Gold nanoparticles (GNPs) emerged over the last decades due to their potential for biomedical use as imaging probes, drug delivery systems, or therapeutic agents. GNP use has been mostly documented in preclinical cancer research, but other prospective therapeutic approaches were also reported for treatment of Alzheimer and Parkinson diseases (1, 2), HIV/AIDS (3), obesity and diabetes (4), tissue engineering (5), and ophthalmology (6) as well as chemical and biological sensing (7). So far, GNPs-based technologies are still in preclinical development, with only few translations to clinical trials compared with the huge amount of scientific literature devoted to GNPs.
Nevertheless, the use of gold in medicine is not limited to GNPs, as gold compounds were used long before the advent of nanomedicine. Robert Koch discovered in 1890 that gold cyanide was toxic for the tuberculosis bacillus in vitro (8). Even if it appeared that gold cyanide was ineffective against tuberculosis in vivo, this discovery laid the first stone in the clinical use of gold and launched investigations related to the biological actions and effects of this precious metal on diverse pathologies (9). In this context, Jacques Forestier evidenced in 1929 that ionic gold compounds relieve joint pain of patients suffering from rheumatoid arthritis and sometimes lead to complete remission (10). Thereafter, gold salts therapy, also known as chrysotherapy, had been used until the 1990s, after which presumably less toxic and more efficient treatments developed.
The medical use of GNPs and gold salts has evolved in distinct fields of research, as chrysotherapy discontinued when GNPs-based medicine emerged. This separation in time and purpose probably explains why acquired knowledge on gold salt use has been poorly exploited when GNPs came under the light. Our group recently revealed that GNPs could be degraded by cells, which results in the formation of intralysosomal gold deposit with a specific nanostructure (11). Surprisingly, similar structures, called aurosomes, were evidenced 50 y ago (12). This similarity highlights an irrefutable relationship between the fate of ionic gold and GNPs, evidencing the link between gold-based therapies.
In this review, we focus on specific fundamental questions: what can we learn from the rich and wide scientific literature about the medical use of gold salts? Could this knowledge be exploited to better understand the fate of GNPs and to optimize GNP medical use? After a brief presentation of modern challenges in nanomedicine, we will summarize the considerable work that was performed with medical gold salts through 70 y. Acquired information will be presented from the nanomedicine perspective, in the attempt to help understanding of the metabolism and use of gold in medicine.
GNPs in Medicine
GNPs Properties and Applications.
The interest for GNPs in medicine stems from their particular physicochemical properties, which make them unique nanoplatforms for vectorization, imaging, diagnostic, and therapy. Due to their very stable crystalline form, it is generally accepted that large GNPs (2 nm) are poorly reactive, hardly corrodible, and poorly degradable. These features make GNPs more stable than other metallic nanoparticles in biological medium (13). In addition, their strong affinity for sulfurized compounds enables a variety of chemical grafts at the nanoparticle surface, which can be functionalized with drugs, polymers, peptides, antibodies, or antifouling species (14). This versatility is particularly useful for drug delivery and specific targeting of tissues (15).
GNPs are also attractive for biomedicine because of their high density. Gold is particularly dense in its crystalline form, which makes it useful for radiation absorption (16). As an example, GNPs strongly absorb X-ray radiation, which enables localization of them in complex organisms by X-ray imaging or tomography, or use of them in therapy as radiosensitizers.
Finally, GNPs are also attractive for biomedicine because of their plasmonic properties. Briefly, the valence electrons of GNPs can enter in resonance with an external electromagnetic field, leading to a strong absorption of the radiation in the visible range (16). This absorption can be modulated by nanoparticles shape, size, and functionalization and covers a range going from 500 to 1,000 nm. This wide window of absorption wavelength overlaps the tissue transparency windows (near infrared [NIR]1: 650 to 900 nm; NIR2: 1,000 to 1,300 nm; NIR3: 1,500 to 1,800 nm), which enables photoactivation of GNPs inside tissues (19). This photoactivation can then induce nanoparticles heating (photothermal therapy), the production of radical species at its surface (photodynamic therapy), the creation of acoustic waves (photoacoustic imaging), the emission of photons (single- or two-photon luminescence), or the enhancement of the electric field at the nanoparticle surface (surface-enhanced Raman spectroscopy and surface plasmon enhanced fluorescence) (20, 21).
GNP medical applications have already been the subject of numerous reviews and will not be further detailed here, but additional information could be found in the following works: refs. 16 and 22–24.
Current Challenges in Gold-Based Nanomedicine.
Despite promising applications involving GNPs, major concerns hamper their use in humans. GNP fate in the organism is complex and has not been elucidated. Empirical evidence indicates that as soon as the nanoparticles enter the body, their surface is surrounded by a protein corona. The composition of this corona depends on surrounding bodily fluids and on chemical and physical parameters such as GNP’s coating, size, and shape (25, 26). Depending on nanoparticle physicochemical properties and the protein corona, the biodistribution of GNPs in the organism may vary (24). GNPs can be eliminated from the body in the urine after kidney filtration if they are sufficiently small (generally below 8 nm, including the coating and the protein corona) (27). Most often, the opsonized nanoparticles are taken up by the monocyte–macrophage system with massive accumulation in the liver (24).
GNPs are then internalized by cells and accumulate into the lysosomes, but little is known about their fate inside this degradation organelle. The evidence of GNP biodegradation in cells and organism is recent and suggests that even persistent nanoparticles evolve in intracellular medium (11, 28). These observations raise new questions about the long-term fate of nanoparticles, as their structure evolves after degradation. Particularly, if GNPs were previously considered as permanent residents within the accumulation tissues, their degradation products could possibly be transferred to other cell types or organs or could be eliminated through different pathways than the original nanoparticles. Overall, the whole GNPs life cycle needs to be better understood, from its early stages to late ones, to optimize the medical use of GNPs.
Moreover, nanoparticles toxicity is still a subject of major concern, as it depends on numerous parameters, including nanoparticles composition, shape, size, coating, charge, hydrophobicity, mechanical properties, solubility, and reactivity (29). Furthermore, nanoparticles toxicity also depends on their path in the organism and the different biological environment they will encountered (biofluids, intracellular media, inclusion in biovesicles…). Therefore, each type of nanoparticle should be evaluated separately and systematically for its different routes of administration. Importantly, as gold-based nanomaterials are slowly degraded, toxicological assessments must be conducted over extended periods of time.
In addition to these general considerations, the use of GNPs in a medical frame opens up new questioning. To be medically relevant, GNPs have to target specific tissues (e.g., tumor or brain–blood barrier penetration). Nanoparticles can accumulate within the tumor through passive targeting, mostly by the so-called enhanced permeability and retention effect (30). The passive targeting to tumors, which was believed to occur due to the diffusion/translocation of nanoparticles through the leaky blood vessels that irrigate the tumor, is now increasingly subject to disillusionment (31, 32). Concomitantly, the active targeting through surface functionalization of nanoparticles has so far led to little if any improvement compared with passive targeting because the protein corona tends to occult specific functional groups (33, 34). In addition, the intrinsic structure of the tumor, which is characterized by a dense extracellular matrix and fibrotic tissue, hampers the penetration of nanoparticles and limits their access to targeted tumoral cells (31, 32).
The second feature that limits the use of nanoparticles in medicine is the evolution of their properties in biological medium. Nanoparticles are designed to have optimal therapeutic properties in model media, but the surrounding biological media and their biotransformations can dramatically affect their properties. GNP plasmonic properties highly depend on nanoparticles surface environment and aggregation state, which are drastically affected by its confinement within the acidic lysosome medium. Studies indicate that GNPs tend to aggregate within the lysosome, which modifies their optical properties and alters their activation under radiation (35, 36). The optimization of nanoparticles properties in intracellular medium thus remains challenging.
All these constraints limit the approval of GNPs for clinical trials and commercial use. Altogether, 57 nanotechnology-based drugs were approved by American or European health agencies according to recent publications, and 90 are subject to clinical trials, which is low compared with thousands of scientific articles published every year (37, 38). Most of them are organic nanoparticles or iron oxide nanoparticles. However, no gold-based nanoparticles have been approved by European and American health agencies, and only four clinical trials have been conducted and are currently in phase I or II. Three of them concern Aurolase therapy, developed by the company Nanospectra Biosciences. These gold-coated silica nanoparticles are developed for cancer therapy against head and neck tumors (National Clinical Trial [NCT]01679470), lung tumors (NCT00848042), and prostate tumors (NCT02680535). Moreover, a GNP-based nucleic acid platform is also being testing against glioblastoma (NCT03020017).
Contrary to GNPs, gold salts are approved by most public health agencies, were used by patients for decades, and are nowadays still subject to many clinical trials. This long experience of the use of gold in medicine can thus open perspectives for gold-based nanodrugs.
The Medical Use of Ionic Gold
Ionic Gold as Rheumatology Treatment.
Gold salts were mainly used to treat rheumatoid arthritis but have also been tested for other types of rheumatologic diseases, such as psoriatic arthritis (39), juvenile idiopathic arthritis (40), lupus erythematosus (41), or Sjogren syndrome (42). In the case of rheumatoid arthritis, 70 to 75% of patients responded to the treatment, which explains the use of gold salts for decades (43).
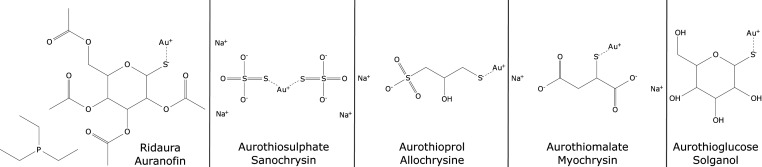
Auranofin, allochrysin, sanochrysin, myochrysin, and solganol are the different gold salts-based drugs (Fig. 1). Except auranofin, all salts were administrated by intramuscular injection, with a dose ranging from 25 to 100 mg per injection. Auranofin was administrated orally in 3-mg tablets because of its hydrophobicity, which gives it the ability to cross the gastrointestinal barrier (43).
Fig. 1.
Therapeutic gold salts structures and most common names. Structures have been drawn according to PubChem database (https://pubchem.ncbi.nlm.nih.gov/).
The treatment was divided in two phases; the first one consisted of one injection per week or two pills per day during 2 to 3 mo, reaching an overall intake of 1 to 1.5 g. This first phase of treatment corresponds to the time lapse required for the patients to observe a decrease of their joint pain. After that, the frequency of drug administration could be decreased. By the end of the treatment, the patients could be exposed to several grams of gold, attaining up to 10 g (44). The beneficial effects of the treatment gradually slowed down and stopped when it was interrupted or ceased.
Gold Salts Pharmacokinetics.
After oral or parenteral administration, gold salts spread within the body via the blood, after diffusion from the muscle to the bloodstream in the case of injectable gold salts, or by crossing the gastric barrier in the case of auranofin (45). The concentration of gold in the plasma peaks 2 h after administration for all gold salts (43, 46). The auranofin peak has a lower intensity than injectable gold salts, as only 15 to 33% of the dose reaches the blood (43, 47, 48). Within the blood, gold ions are mainly bound to albumin Cys-64 amino acid (80% of gold in blood in case of auranofin and 95% of gold for injectable gold salts), the remaining part being fixed to globulins or to red blood cells (43, 46, 49, 50). In the case of sodium aurothiomalate, it has been shown that the thiolated ligand is detached from gold within a few hours (51). The half-life time of gold in blood is between 5 and 7 d for injectable gold and between 11 and 33 d for auranofin (43, 46). This half-life time increases with repeated injections up to 250 d, and it takes 40 to 80 d for the gold concentration in blood to decrease back to its normal level after exposure (46, 52).
In human patients who received reiterated injections, traces of gold can be found in numerous tissues, among which are the kidney, lymph node, adrenal tissue, liver, and synovium (53, 54). Gold can also be found in the brain, gonads, or eyes (53–57). As these last organs are separated from the rest of the body by specific barriers, such findings are rather surprising. Importantly, gold was found in tissues 23 y after the end of the treatment, which underlines that gold is never completely eliminated from the organism (53).
Concerning its elimination from the body, gold is preferentially eliminated in urine or excreted via the bile into the feces (75 and 25% of the eliminated amount, respectively, for injectable gold salts; 15 and 85%, respectively, for auranofin) (43, 46). Gold can still be found in urine several months after the last injection (46, 58). Overall, it is estimated that 40% of the dose is eliminated from the body for injectable gold and 80% for auranofin (46).
In summary, even if some slight differences exist between injectable hydrophilic salts and ingestible hydrophobic salts, both lose their ligands within hours and distribute throughout the body. The half-lives in the blood, distribution within the organism, and elimination evolve after reiterated dose administration. This evolution of gold pharmacokinetics can explain the time lapse and injected dose (i.e., 1 to 6 mo and 1 to 1.5 g) that are necessary for the treatment to become efficient, as the accumulation in joints might not be sufficient after the first injection (43).
Aurosome Formation from Residual Gold.
Gold has been shown to persist in the body for years after the end of the treatment, despite its elimination over time. More precisely, gold accumulates inside cells, which are mainly phagocytic cells, such as neutrophils and macrophages. It forms gold deposits inside the cell lysosomes, which had previously been called aurosomes (59).
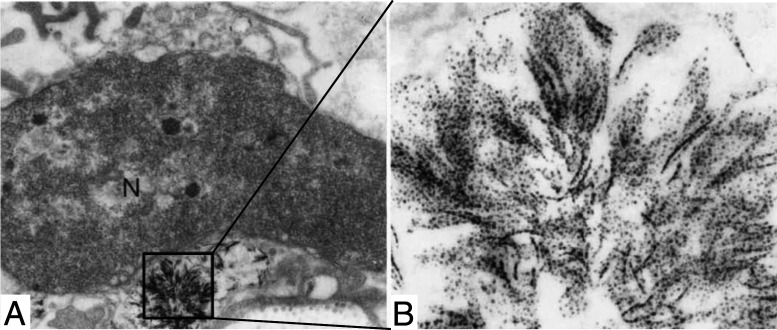
Ghadially (12) extensively described aurosomes, which appear as dense vesicles, presenting lamellar, filamentous, or rod-shaped structures composed of small particles with 5-nm diameter (Fig. 2). Such ultrastructures were observed in diverse organs (synovial membrane, articular cartilage, liver, kidney, lung, and skin), as well as in different animal species (rat, rabbit, and human) (12). Moreover, these structures occurred independently of the route of administration (intraarticular, intramuscular, and oral) or the type of administrated gold salt (12). Aurosomes can be observed about 3 d after the first injection to rabbits and can still be found years after the end of the treatment (60).
Fig. 2.
(A and B) Aurosomes observed in patient’s buttocks skin after chrysotherapy. Magnifications: (A) × 12,000; (B) × 136,000. Reprinted by permission from ref. 44, Springer Nature: Archives of Dermatological Research, copyright (1990).
Studies of aurosomes by energy-dispersive spectroscopy revealed that they are composed of gold, associated with sulfur with Au/S atomic ratios around 1.5 (61). In parallel, X-rays absorption spectroscopies showed that gold is at the oxidation state +1 and surrounded by an average 2.2 sulfur atoms per gold atom, at an average distance of 2.30 Å (50).
The nature of the proteins surrounding the gold particles and their mechanism of assembly into sheets, rods, or filaments were also questioned. It has been shown that an important fraction of injected gold happened to be bound to metallothioneins in the liver and kidneys after injection to rats (62). Metallothioneins are cysteine-rich small proteins that are implicated in metal detoxification and homeostasis and that are able to bind gold (63). X-ray characterization of metallothionein–gold complexes revealed that gold is surrounded by an average of 2.4 sulfur atoms situated at 2.29 Å from the gold atom, which is comparable with what was observed in intracellular aurosomes (50). Moreover, the use of metallothionein–gold complex as tag to replace gold NPs immunostaining in electron microscopy shows that these objects look like 1- to 2-nm particles, which are highly similar to aurosomes building blocks (64). These observations offer an insight in the composition of the particles observed in aurosomes, even if they do not offer any clue on the formation of such typical ultrastructures.
Therapeutic Effects of Ionic Gold.
The therapeutic activity of gold salts has been subject to multiple discussions at the climax of their use and is still not completely understood. It is generally considered that the anti-inflammatory effect of gold salts is mainly due to gold itself and not to the thiolate ligands, even if thiolated compounds have minor positive effects on rheumatoid arthritis (51). Moreover, the beneficial effects of gold salts ceased when the treatment stopped, while aurosomes persist in the body after the end of the treatment. Thus, it can be assumed that aurosomes are not therapeutically active, contrary to ionic gold.
However, it is hard to attribute its therapeutic effects to a given gold compound. It is still unknown whether gold is active in its +1 or +3 oxidation state, as its redox state may vary between extra- and intracellular media. Moreover, a wide variety of ligands can bind to gold ions (65). Hence, the question of the active form of gold in terms of ligands and oxidative state remains open.
Takahashi et al. (66) proposed two mechanisms to explain the action of gold salt at the molecule and protein levels. First, gold binds to thiol-containing proteins. Such a process could disturb enzyme–substrate or receptor–ligand recognition and affect many proteins. Second, gold ions can be sequentially oxidized in Au3+ by ROS, then reduced to Au+ by reacting with lysosomal enzymes. This oscillation between the two oxidation states of gold ions would result in both a decrease of oxidative stress and an inhibition of lysosomal protease activity. The first hypothesis is supported by the reported effects of gold thiolated ligands and thiolated drugs, such as d-penicillamine, tiopronin, or pyritinol, on rheumatoid arthritis, which were also found to decrease joint pain (43). As thiolated compounds have the ability to bind other thiols by disulfide bridges, they could play the same role as gold, as suggested in the first proposed mechanism. On the other hand, the second mechanism is not fully convincing, as auranofin decreased ROS level in cells, but such effect was not observed for sodium aurothiomalate (67, 68).
At the cell or the tissue level, several mechanisms were deemed responsible for the therapeutic activity of gold salts. The results were occasionally contradictory between studies, and several studies were limited to in vitro measurements or were performed on questionable animal models, so they have to be considered with caution (69). Overall, the therapeutic action of gold salts on patients suffering from rheumatoid arthritis can be summarized as follows. First, they can decrease oxidative stress, through ROS scavenging and/or inhibition of oxidative stress-related enzymes, such as thioredoxin reductase (68, 70). Second, they can inhibit lysosomal enzymes (71–73). Third, they can limit the adhesion and infiltration of monocytes in the synovium by inhibiting E-selectin and/or prostaglandin synthesis by epithelial cells (74–77). Fourth, they can prevent T lymphocyte proliferation by inhibiting protein kinase C synthesis and/or altering T lymphocytes and monocytes communication (78–80). Finally, they can inhibit collagenase production and/or activity (81, 82). At the systemic level, it results in a decrease of joint pain and swelling (83, 84) and a decline in the concentration of immunoglobulins and rheumatoid factor in the blood (85).
Side Effects and Toxicity of Gold Salts.
Gold therapy can trigger a wide variety of adverse reactions, impacting numerous organs. Adverse reactions include severe toxic effects (86, 87) and can rarely even lead to patient death (88). It has to be underlined that two types of side effects should be discriminated. First, there are side effects that disappear with the discontinuation of treatment. These side effects are the ones that could take the most severe forms, but in most cases, they are reversible. The injection of gold salt could continue most of the time after recovery (46). The second type of effects is long-lasting nonsevere side effects that are irreversible and that reflect the intratissular accumulation of gold over time.
Severe side effects are diverse and can affect skin, mucosa, kidney, blood, bone marrow, lung, nervous system, or liver (details and references are in SI Appendix, Table S1). However, prevalence is hardly assessable as adverse effects could be attributed to gold therapy or to the autoimmune disease itself.
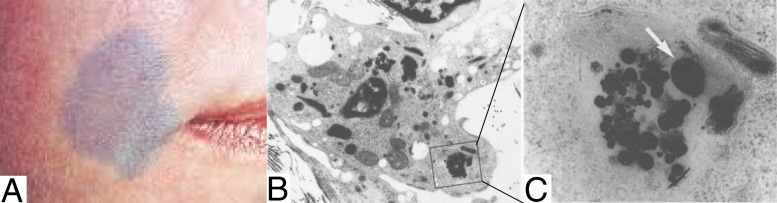
Irreversible adverse reactions generally occur at cumulated doses higher than 1.5 g and can take two forms. One of these effects is a bluish to gray skin discoloration that is called chrysiasis (Fig. 3A) (89–91). While this side effect can be disturbing to the patient, no pain or toxicity is associated with chrysiasis, and it is not associated with other skin damage (92).
Fig. 3.
(A) Case of skin chrysiasis that appears after laser exposure. Reprinted with permission from ref. 94. (B and C) Aurosomes recrystallization observed in patient’s face skin after chrysotherapy. Large biosynthesized nanoparticle is indicated by a white arrow. Magnifications: (B) × 5,400; (C) × 57,000. Reprinted by permission from ref. 44, Springer Nature: Archives of Dermatological Research, copyright (1990).
Chrysiasis was related to light exposure: first, it appears mainly on exposed skin (for example, on the face or hands), probably because of sunlight exposure (44, 93). Moreover, with the growing use of lasers for diverse skin treatments, it appears that persons who were treated with gold salts could develop chrysiasis after exposure to red to near-infrared pulsed lasers (694 to 1,064 nm) (94–98). Benn et al. (44) suggested that light induces a crystallization of gold residues, as assessed in patient skin biopsies that showed that light-exposed skin cells contain large crystalline particles instead of aurosomes (Fig. 3 B and C). This is in accordance with the blue discoloration observed, as GNPs are colored and could be red to blue according to their size, shape, and aggregation state. Moreover, direct injection of GNPs to a patient has been found to result in the same blue coloration of the skin (99).
The other long-term side effect of chrysotherapy is the appearance of a golden ring around the cornea called ocular or corneal chrysiasis (57, 100, 101). The emergence of such structures does not affect the vision nor does it generate pain. Observations of gold deposits show that they mostly take the form of aurosomes, as observed by electron microscopy (102).
Overall, two types of side effects have been described, exhibiting reversible or irreversible character. By comparing the kinetics of the side effects’ appearance/disappearance and the metabolism of gold previously described, we can consider that most toxic effects are due to the early forms of gold (i.e., supposedly ionic gold). On the contrary, dermal and ocular chrysiasis and long-term effects could be related to the presence of aurosome in tissues and their recrystallization under light exposure.
Drug Repurposing of Gold-Based Drugs.
New use of ancient drugs is raising lots of interest nowadays, with emerging strategies of drug repurposing to optimize new treatment development from already approved derisked compounds. Gold salts are no exception, particularly auranofin. Two fields are predominant in these publications and patents. The first one is the use of gold salts against viruses, bacteria, or parasites. The second one is their use for cancer therapy, for which gold salts are administrated with other anticancerous drug for additive or synergic beneficial effects. Publications and patents related to gold compounds repurposing are summarized in SI Appendix, Table S2.
Interestingly, if some modes of action are identical to previously reported effects on rheumatoid arthritis, such as modification of immune cell adhesion properties or impact on the immune response (103–105), most effects are attributed to the impact of gold salts on thioredoxin reductase. Thioredoxin reductase is a seleno-enzyme implicated in the response to oxidative stress, which is inhibited by gold salts in various organisms (e.g., humans, bacteria, or parasites) (106–108). Hence, therapeutic gold salts can alter selenium metabolism and/or H2O2 capture (109–111). Moreover, protein kinase C ioa or kallikrein-related peptidase 6 are also targeted by gold salts treatments (112, 113). Generally, the therapeutic mechanisms of gold salts are still unclear, and no unifying theory has been proposed so far to our knowledge despite the high number of targets and mechanisms described.
The repurposing strategies that have been suggested so far exploit the biological action of ionic gold. We suggest here to take advantage of the different forms of gold that have been described so far (i.e., ionic gold, aurosomes, and GNPs). Degradation and recrystallization processes enable the translation from one form to another, which offers new therapeutic perspectives that will now be described.
Building Bridges between GNPs and Ionic Gold
From GNPs to Aurosomes.
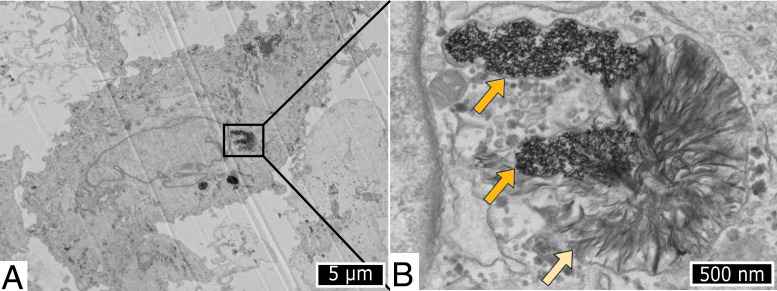
As previously mentioned, we recently found a parallel between gold salts and nanogold therapies, as aurosomes were observed inside cells in vitro after exposure to GNPs (11). Briefly, primary human fibroblasts were exposed to three sizes of citrate-coated GNPs (diameters of 4, 17, and 22 nm), and cells were observed by transmission electron microscopy over a period of 6 mo following nanoparticles exposure. Two weeks after nanoparticles incubation, gold-containing degradation products were observed within lysosomes containing 4-nm GNPs (Fig. 4). These degradation products appeared as diffuse curved structures and were found to be two-dimensional (2D) structures thanks to electron tomography. We also revealed that these 2D structures were composed of self-assembled crystalline clusters with an average diameter of 2.5 nm. Moreover, elemental analysis revealed that gold was associated with sulfur within these degradation products, while sulfur was not present in the areas containing the original GNPs. The study of the transcriptomic response to GNPs over time strongly suggests that the sulfur signal observed from degradation products can be due to cysteine-containing protein and more precisely, to metallothioneins. Similar gold structures were observed when cells were exposed to 17- and 22-nm nanoparticles, but the occurrence of such degradation products was visible only after several months postexposure, suggesting slower degradation of larger GNPs.
Fig. 4.
(A and B) Transmission electron microscopy observation of human fibroblast incubated with 4-nm GNPs and observed 2 wk after incubation. The dark orange arrows indicate nondegraded GNPs, while light orange arrow shows the structure formed after GNP degradation. Reproduced from ref. 11.
Interestingly, we discovered that the chemical features and the morphology of degradation products originating from GNPs were identical to aurosomes formed after the administration of gold salts. This was the first indication that there is a common evolution of gold inside the cell, independently of its original chemical form (ionic or crystalline). This finding is particularly interesting because the long-term fate of GNPs could be understood in view of a shared metabolism for the different gold species. The experience acquired after administration of gold salts could thus be used in gold-based nanotherapies. For example, studies suggest that gold can be eliminated in urines several months and even up to 1 y after the last injection of gold salts (46, 58). Knowing that after such a long period after injection, gold is resident in the tissue, this finding could indicate that ionic gold or aurosomes themselves can be released into the bloodstream after aurosome formation. Moreover, after in the bloodstream, gold, regardless of its chemical form, can then be eliminated by the kidneys. Probably the aurosomes formed after nanoparticles degradation could follow the same route over time, meaning that gold can be exocytosed and eliminated with time. Although this hypothesis demands further investigations to be confirmed in vivo, it introduces a novel perspective on the long-term fate of GNPs, which are believed to remain in tissues after eliminated from the bloodstream.
The presumably shared fate of GNPs and gold salts also provides new avenues to explore the aging and toxicity of GNPs. Indeed, the pharmacokinetics of gold salts and the concomitant adverse reaction strongly suggest that aurosomes are not toxic for patients. To a certain extent, this fact is reassuring in regard to the long-term toxicity of nanoparticles after they are degraded. However, this presumption should be taken with caution since there are no long-term studies to date on the development of diseases after gold salt treatment, while it is known that metal deposits are found in cancer and neurodegenerative diseases, without any consensus in their physiopathological implication (114).
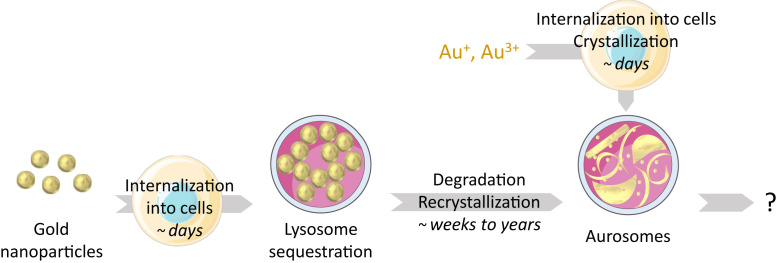
More generally, using gold salts could be considered as an alternative to study the long-term effects of GNPs while limiting the duration of the experiments. As gold salts rapidly form aurosomes within tissues, they could be used as a “shortcut” to determine the ultimate fate of GNPs and to distinguish the effects related to the nanocrystal’s size, shape, protein corona, mechanical properties, or surface reactivity with respect to those related to the gold element (Fig. 5).
Fig. 5.
Illustration of cellular metabolism of GNPs and ionic gold, with estimative period for each phenomenon. Illustrations credit: Servier Medical Art (http://smart.servier.com/), which is licensed under CC BY 3.0.
Overall, the formation of aurosomes with well-conserved 2D structures and curvature ubiquitously present in different species, tissues, and cells suggests a general response to gold overload in lysosomes. For therapeutic use of gold, it is of crucial importance to unravel the mechanism of aurosome formation and their evolution over time. The discovery of these ubiquitous gold structures opens up new perspectives for the nanotoxicology field and the biomedical applications of gold compounds.
From Aurosome to GNPs.
Previous studies indicate that gold can crystallize within cells, forming in situ biosynthesized GNPs. This process has been mostly observed in bacteria, plants, and algae after exposure of these organisms to ionic gold (115–117). Regarding mammal-mediated biosynthesis, several studies describe in vitro and in vivo nanoparticles synthesis after exposure of cells or mice to ionic gold, mostly HAuCl4. Nevertheless, published studies do not compare aurosome formation and biosynthesized GNPs, showing that the data obtained in chrysotherapy remain unknown to the majority of scientists operating within nanotechnology fields. However, described data are in agreement with aurosomes ultrastructure, as studies on GNPs biosynthesis describe the formation of crystalline gold clusters with diameters ranging from 1.8 to 3.5 nm, which are in the same order of magnitude as the clusters that constitute the aurosomes (118).
It has to be stressed that a second biosynthesis phenomenon emerged from the literature on chrysotherapy, which is light-mediated biosynthesis. The observation of chrysiasis, a blue discoloration of the skin after exposure to solar light or laser, strongly suggests that larger crystalline GNPs can be formed under light exposure. Biopsies of discolored skin support this hypothesis, as dense and large granules that look very different from aurosomes were observed by electron microscopy (Fig. 3 B and C) (44).
This light-induced crystallization seems to be caused by the instability of aurosomes upon irradiation. Recent studies described that thiol-coated clusters tend to be destabilized by the electron beam during transmission electron microscopy observations, forming larger GNPs with time (119). The same process was confirmed by our own observations on aurosomes. Fig. 6 A–D presents a series of micrographs that were taken sequentially after concentration of the electron beam on the studied area. It can be clearly seen that the electron beam triggers a reorganization of nanostructures into clusters. This observation raises question about the real nature of the aurosomes and the reality of the existence of the previously described self-assembled clusters. Nevertheless, this finding also suggests an instability of aurosomes with respect to external irradiation, leading to the formation of the previously described “granules.” Fig. 6E summarizes the biotranformations of gold inside cells and the transition between its three identified states: ionic gold, aurosomes, and GNPs.
Fig. 6.
(A–D) Aurosomes recrystallization under the electron beam operating at 80 keV during transmission electron microscopy observation. The time lapse between two images is about 2 min. The aurosomes were obtained from 4-nm GNPs 6 mo after incubation in primary human fibroblasts. Original nondegraded nanoparticles can be seen at the right bottom corner. (E) Biotransitions between the different forms of gold, summarizing ancient and recent literature. Illustration credit: Servier Medical Art (http://smart.servier.com/), which is licensed under CC BY 3.0.
The in situ formation of GNP could result in enormous advantages in nanomedicine. First, the injection of ionic gold could drastically improve the penetration of gold compounds inside the tumor. While GNPs can be rapidly opsonized and taken up by liver macrophages or could be blocked by the fibrotic tissues and dense extracellular matrix around the tumor, gold ions would avoid opsonization and diffuse much more easily within the tumor. After the ions would penetrate the tumor, the zone of interest could be exposed to a laser operating at wavelengths in the tissue transparency windows. Indeed, laser-triggered chrysiasis was described for lasers with wavelengths varying from 694 to 1,064 nm. This would allow the formation of GNPs inside the tumor itself and not in peripheral tissues. Moreover, this synthesis would occur directly within the lysosomes of target cells, limiting nanoparticles transfer to other organs. The remotely activated biosynthesized GNPs could subsequently be used as therapeutic agents for photothermal or photodynamic therapy.
We thus hypothesize that the administration of ionic gold followed by light-activated nanoparticles formation in situ within targeted areas could present a paradigm shift to current nanoparticle-based medicines and a solution to the issue of low GNPs delivery to tumor. We also emphasize that gold salts are approved by health agencies, which would facilitate clinical trials. Despite mentioned advantages and exciting perspectives, much work will be required to achieve, control, and characterize remotely activated light-induced intracellular biosynthesis of GNPs. While nanoparticles formation has thus far only been observed by accident and was considered as a side effect of chrysotherapy, we could indeed exploit this phenomenon in nanomedicine.
Gold Metabolism Transcriptomic Signature.
From a more fundamental point of view, the convergent fate of GNPs and ionic gold suggests a common metabolism for these two forms and a similar cellular response to different gold compounds. The transcriptomic response to GNPs was previously described by our group during GNPs degradation and formation of aurosomes in primary fibroblasts (11). Briefly, we reported that oxidative species were created within the lysosome, which was associated with NADPH production and was balanced by oxidative stress response via nuclear factor erythroid 2 pathway. In parallel, metallothioneins and glutathione were up-regulated and implicated in the clustering of ionic gold.
To what extent can we compare the transcriptomics response to GNP intracellular degradation and recrystallization as aurosomes to the transcriptomics response to gold salts? We looked for publicly available transcriptomic data on cell response to therapeutic ionic gold compounds. Using the National Center for Biotechnology Information database Gene Expression Omnibus, we found one study that describes the transcriptomic response of three human B cell lines (OCI-Ly3, OCI-Ly7, and U2932) to 92 drugs, including auranofin (120). The effect of the drug was followed 6, 12, and 24 h after exposure. The methodology for this analysis can be found in SI Appendix.
To identify the response to auranofin independently of the time of exposure and cell type, the differential expression was first calculated for each condition and then averaged over the three cells lines and times of studies. Twenty of the 20,218 studied genes were found to have an average differential expression over one in log2 fold change. The comparison between these 20 genes and the transcriptomic answer to GNPs during their biodegradation reveals that 12 genes were shared between the two conditions (i.e., auranofin and 4-nm GNPs exposure). Interestingly, these genes encode for the response to oxidative stress (NQO1, OSGIN1, TXND1, HMOX1, SLC48A1), metallothioneins (MT1G, MT1E, MT1X), glutathione (SLC7A11, GCLM), and two proteins that intervene in gene expression regulation (SQSTM1, PIR). On the contrary, no gene had an average differential expression below –1 in log fold change, indicating that no gene is clearly down-regulated by auranofin.
This comparison between cellular response to ionic gold and to GNPs clearly evidences the involvement of similar genes, even if different cell types were studied (i.e., B cells and fibroblasts, respectively). This result supports the concept of “gold metabolism” as a common response shared by different forms of gold and relies on known detoxification process, such as oxidative stress proteins and metallothioneins. However, both oxidative stress and metallothioneins are involved in the response to metal ions, which makes it difficult to determine which genes are highly specific to gold and which ones are shared with other metals. This underlines how this similarity between ionic and nanoparticulate gold cellular response must be further investigated and compared with other metals’ response in order to have a more precise definition of gold metabolism.
Conclusion
While the scientific literature overflows with suggestions for various prospective biomedical applications of GNPs for therapy, imaging, or drug delivery, only a few of the GNPs-based systems are tested in clinical trials, and none of them are actually approved by health agencies. Numerous questions remain on the fate of GNPs after medical use and on their potential toxicity. Previous works evidenced that GNPs and therapeutic gold salts end up as aurosomes (i.e., gold deposits within the lysosomes), which exhibit a clearly identifiable ultrastructure. This structural convergence motivated us to vastly review the literature dedicated to therapeutic gold salts use in rheumatology, focusing on gold salts pharmacokinetics, their therapeutic action, their side effects, and the potential repurposing of these drugs.
The thorough assessment of published literature enabled us to draw hypotheses on the long-term fate and toxicity of GNPs. Moreover, the data gathered from this past literature not only suggest that we can anticipate the fate of GNPs, and we put forward the use of gold salts as a surrogate approach to evaluate GNP fate and toxicity in the long term. Importantly, we herein emphasize a singular phenomenon, the light-induced biosynthesis of GNPs within tissues. This phenomenon results in the formation of large GNPs from ionic precursor, unlike previously observed biosynthesis phenomenon that results in tiny gold clusters. This remotely activated light-induced biosynthesis can be triggered locally by a laser and elicits the appearance of a blue coloration of the tissues. This phenomenon could be of great importance to design GNPs-based therapies and imaging using gold salts that were already approved by health agencies. We also note that gold metabolism can be identified from the commonly expressed genes that are observed after GNPs or gold salt exposure, implying notably metallothioneins and oxidative stress. Overall, this revisited review of past and forgotten literature provides new insights into a wide diversity of questions, ranging from the current concerns of GNP medical use to new therapeutic strategies using gold, and fundamental findings on gold management at the cellular level.
Data Availability
All study data are included in the article, SI Appendix, and Dataset S1.
Supplementary Material
Acknowledgments
A.B. received a PhD fellowship from the doctoral school Physique en Ile de France. We acknowledge financial support from Agence Nationale de la Recherche Grants CarGold-16-CE09-026, CycLys-18-CE09-0015-01, and Coligomere-18-CE06-0006; French National Research Program for Environmental and Occupational Health of Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail Grant 2018/1/007; and the European Union’s Horizon 2020 Research and Innovation Program Grant 801305. We are grateful to Prof. Frederic Liote, Prof. Jacques-Éric Gottenberg, Dr. Fabienne Arboit-Braun, Thomas Jacquemont, and Pierre Bost for fruitful discussion and to Mylène Brunet and Céline Benoit for their help with literature research.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2007285117/-/DCSupplemental.
References
- 1.Gao N., Sun H., Dong K., Ren J., Qu X., Gold-nanoparticle-based multifunctional amyloid-beta inhibitor against Alzheimer’s disease. Chemistry 21, 829–835 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Hu K., et al., Neuroprotective effect of gold nanoparticles composites in Parkinson’s disease model. Nanomed. Nanotechnol. Biol. Med. 14, 1123–1136 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Di Gianvincenzo P., et al., Gold nanoparticles capped with sulfate-ended ligands as anti-HIV agents. Bioorg. Med. Chem. Lett 20, 2718–2721 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Lee J. H., et al., Targeted hyaluronate–hollow gold nanosphere conjugate for anti-obesity photothermal lipolysis. ACS Biomater. Sci. Eng. 3, 3646–3653 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Vial S., Reis R. L., Oliveira J. M., Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid State Mater. Sci. 21, 92–112 (2017). [Google Scholar]
- 6.Masse F., Ouellette M., Lamoureux G., Boisselier E., Gold nanoparticles in ophthalmology. Med. Res. Rev. 39, 302–327 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Saha K., Agasti S. S., Kim C., Li X., Rotello V. M., Gold nanoparticles in chemical and biological sensing. Chem. Rev. 112, 2739–2779 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibier P., Dr. Joch’s discovery. N. Am. Rev. 151, 726–731 (1890). [Google Scholar]
- 9.Benedek T. G., The history of gold therapy for tuberculosis. J. Hist. Med. Allied Sci. 59, 50–89 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Forestier J., The treatment of rheumatoid arthritis with gold salts injections. Lancet 219, 441–444 (1932). [Google Scholar]
- 11.Balfourier A., et al., Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 117, 103–113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghadially F. N., Ultrastructural Pathology of the Cell and Matrix: A Text and Atlas of Physiological and Pathological Alterations in the Fine Structure of Cellular and Extracellular Components (Butterworth-Heinemann, 1988). [Google Scholar]
- 13.Feliu N., et al., In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 45, 2440–2457 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Sapsford K. E., et al., Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 113, 1904–2074 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Ghosh P., Han G., De M., Kim C. K., Rotello V. M., Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 60, 1307–1315 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Louis C., Pluchery O., Gold Nanoparticles for Physics, Chemistry and Biology (World Scientific, 2012). [Google Scholar]
- 17.Cole L. E., Ross R. D., Tilley J. M., Vargo-Gogola T., Roeder R. K., Gold nanoparticles as contrast agents in X-ray imaging and computed tomography. Nanomedicine 10, 321–341 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Haume K., et al., Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 7, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X., Jain P. K., El-Sayed I. H., El-Sayed M. A., Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Laser Med. Sci. 23, 217–228 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Bucharskaya A. B., et al., “Gold nanoparticle-based technologies in photothermal/photodynamic treatment: The challenges and prospects” in Nanotechnology and Biosensors, Nikolelis D., Nikoleli G. P., Eds. (Elsevier, 2018), pp. 151–173. [Google Scholar]
- 21.Wu Y., Ali M. R., Chen K., Fang N., El-Sayed M. A., Gold nanoparticles in biological optical imaging. Nano Today 24, 120–140 (2019). [Google Scholar]
- 22.Dykman L., Khlebtsov N., Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 41, 2256–2282 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Dreaden E. C., Alkilany A. M., Huang X., Murphy C. J., El-Sayed M. A., The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 41, 2740–2779 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dykman L., Khlebtsov N., Gold Nanoparticles in Biomedical Applications (CRC Press, 2017). [DOI] [PubMed] [Google Scholar]
- 25.Piella J., Bastús N. G., Puntes V., Size-dependent protein–nanoparticle interactions in citrate-stabilized gold nanoparticles: The emergence of the protein corona. Bioconjugate Chem. 28, 88–97 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Xu M., et al., How entanglement of different physicochemical properties complicates the prediction of in vitro and in vivo interactions of gold nanoparticles. ACS Nano 12, 10104–10113 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Semmler-Behnke M., et al., Biodistribution of 1.4-and 18-nm gold particles in rats. Small 4, 2108–2111 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Kolosnjaj-Tabi J.et al., The one year fate of iron oxide coated gold nanoparticles in mice. ACS Nano 9, 7925–7939 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Sukhanova A., et al., Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 13, 44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greish K., “Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting” in Cancer Nanotechnology: Methods and Protocols, Grobmyer S. R., Moudgil B. M., Eds. (Methods in Molecular Biology, Humana Press, New York, NY, 2010), pp. 25–37. [DOI] [PubMed] [Google Scholar]
- 31.Nichols J. W., Bae Y. H., EPR: Evidence and fallacy. J. Contr. Release 190, 451–464 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Danhier F., To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Contr. Release 244, 108–121 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Mirshafiee V., Mahmoudi M., Lou K., Cheng J., Kraft M. L., Protein corona significantly reduces active targeting yield. Chem. Commun. 49, 2557–2559 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelm S., et al., Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016). [Google Scholar]
- 35.Hainfeld J. F., et al., Infrared-transparent gold nanoparticles converted by tumors to infrared absorbers cure tumors in mice by photothermal therapy. PloS One 9, e88414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espinosa A., et al., Cancer cell internalization of gold nanostars impacts their photothermal efficiency in vitro and in vivo: Toward a plasmonic thermal fingerprint in tumoral environment. Adv. Healthcare Mater. 5, 1040–1048 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Bobo D., Robinson K. J., Islam J., Thurecht K. J., Corrie S. R., Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 33, 2373–2387 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Anselmo A. C., Mitragotri S., Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 4, e10143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salvarani C., et al., Clinical response to auranofin in patients with psoriatic arthritis. Clin. Rheumatol. 8, 54–57 (1989). [DOI] [PubMed] [Google Scholar]
- 40.Giannini E. H., Brewer E. J. Jr, Kuzmina N., Shaikov A., Wallin B., Auranofin in the treatment of juvenile rheumatoid arthritis. Arthritis Rheum. 33, 466–476 (1990). [DOI] [PubMed] [Google Scholar]
- 41.Dalziel K., et al., Treatment of chronic discoid lupus erythematosus with an oral gold compound (auranofin). Br. J. Dermatol. 115, 211–216 (1986). [DOI] [PubMed] [Google Scholar]
- 42.Gordon M. H., Tiger L. H., Ehrlich G. E., Gold reactions are not more common in Sjögren’s syndrome. Ann. Intern. Med. 82, 47–49 (1975). [DOI] [PubMed] [Google Scholar]
- 43.Bardin T., Kuntz D., Thérapeutique Rhumatologique (Médecine Sciences Publications, 1995). [Google Scholar]
- 44.Benn H. P., von Gaudecker B., Czank M., Loeffler H., Crystalline and amorphous gold in chrysiasis. Arch. Dermatol. Res. 282, 172–178 (1990). [DOI] [PubMed] [Google Scholar]
- 45.Kamel H., et al., A comparison of tissue gold levels in Guinea-pigs after treatment with myocrisin injected intramuscularly and triethylphosphine gold chloride and myocrisin administered orally. Agents Actions 8, 546–550 (1978). [DOI] [PubMed] [Google Scholar]
- 46.Goodman Gilman A., Hardman J., Limbird L., Goodman & Gilman’s The Pharmacological Basis of Therapeutics (McGraw-Hill, ed. 9, 1996). [Google Scholar]
- 47.Mascarenhas B. R., Granda J. L., Freyberg R. H., Gold metabolism in patients with rheumatoid arthritis treated with gold compounds-reinvestigated. Arthritis Rheum. 15, 391–402 (1972). [DOI] [PubMed] [Google Scholar]
- 48.Lewis A. J., Walz D. T., “1 Immunopharmacology of gold” in Progress in Medicinal Chemistry, Ellis G. P., West G. B., Eds. (Elsevier, 1982), vol. 19, pp. 1–58. [DOI] [PubMed] [Google Scholar]
- 49.McQueen E., Dykes P., Transport of gold in the body. Ann. Rheumatic Dis. 28, 437 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elder R. C., Eidsness M. K., Synchrotron X-ray studies of metal-based drugs and metabolites. Chem. Rev. 87, 1027–1046 (1987). [Google Scholar]
- 51.Jellum E., Munthe E., Guldal G., Aaseth J., Fate of the gold and the thiomalate part after intramuscular administration of aurothiomalate to mice. Ann. Rheumatic Dis. 39, 155–158 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linck J., “Hépatite aurique cholestatique persistante: à propos d’une observation syndrome d’hypersensibilité aux sels d’or et relation avec le système HLA” PhD thesis, Université Henri Poincaré, Nancy, France (2005).
- 53.Vernon-Roberts B., Dore J., Jessop J., Henderson W., Selective concentration and localization of gold in macrophages of synovial and other tissues during and after chrysotherapy in rheumatoid patients. Ann. Rheumatic Dis. 35, 477–486 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gottlieb N. L., Smith P. M., Smith E. M., Tissue gold concentration in a rheumatoid arthritic receiving chrysotherapy. Arthritis Rheum. 15, 16–22 (1972). [DOI] [PubMed] [Google Scholar]
- 55.Thompson D. M., Pegelow C. H., Singsen B. H., Powars D. R., Hanson V., Neutropenia associated with chrysotherapy for juvenile rheumatoid arthritis. J. Pediatr. 93, 871–875 (1978). [DOI] [PubMed] [Google Scholar]
- 56.Grubb B. R., Matthews D. O., Bentley P., Ocular chrysiasis: Accumulation of gold in the rabbit eye. Curr. Eye Res. 5, 891–893 (1986). [DOI] [PubMed] [Google Scholar]
- 57.Gottlieb N. L., Major J. C., Ocular chrysiasis correlated with gold concentrations in the crystalline lens during chrysotherapy. Arthritis Rheum. 21, 704–708 (1978). [DOI] [PubMed] [Google Scholar]
- 58.Freyberg R., Block W. D., Levey S., Metabolism, toxicity and manner of action of gold compounds used in the treatment of arthritis. I. human plasma and synovial fluid concentration and urinary excretion of gold during and following treatment with gold sodium thiomalate, gold sodium thiosulfate, and colloidal gold sulfide. J. Clin. Invest. 20, 401–412 (1941). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghadially F., The aurosome. J. Rheumatol. 5, 45–50 (1979). [PubMed] [Google Scholar]
- 60.Ghadially F., Lalonde J., Thomas I., Massey K., Long-term effects of myochrysine on the synovial membrane and aurosomes. J. Pathol. 125, 219–224 (1978). [DOI] [PubMed] [Google Scholar]
- 61.Nakamura H., Igarashi M., Localization of gold in synovial membrane of rheumatoid arthritis treated with sodium aurothiomalate. studies by electron microscope and electron probe X-ray microanalysis. Ann. Rheumatic Dis. 36, 209–215 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma R., McQueen E., The binding of gold to cytosolic proteins of the rat liver and kidney tissues: Metallothioneins. Biochem. Pharmacol. 29, 2017–2021 (1980). [DOI] [PubMed] [Google Scholar]
- 63.Stillman M. J., Metallothioneins. Coord. Chem. Rev. 144, 461–511 (1995). [Google Scholar]
- 64.Mercogliano C. P., DeRosier D. J., Concatenated metallothionein as a clonable gold label for electron microscopy. J. Struct. Biol. 160, 70–82 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eisler R., Chrysotherapy: A synoptic review. Inflamm. Res. 52, 487–501 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Takahashi K., Griem P., Goebel C., Gonzalez J., Gleichmann E., The antirheumatic drug gold, a coin with two faces: Au (i) and Au (iii). Desired and undesired effects on the immune system. Met. Base. Drugs 1, 483–496 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davis P., Johnston C., Miller C. L., Wong K., Effects of gold compounds on the function of phagocytic cells. Arthritis Rheum. 26, 82–86 (1983). [DOI] [PubMed] [Google Scholar]
- 68.Miyachi Y., Yoshioka A., Imamura S., Niwa Y., Anti-oxidant effects of gold compounds. Br. J. Dermatol. 116, 39–46 (1987). [DOI] [PubMed] [Google Scholar]
- 69.Brown D., Smith W., The chemistry of the gold drugs used in the treatment of rheumatoid arthritis. Chem. Soc. Rev. 9, 217–240 (1980). [Google Scholar]
- 70.Gromer S., Arscott L. D., Williams C. H., Schirmer R. H., Becker K., Human placenta thioredoxin reductase isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 273, 20096–20101 (1998). [DOI] [PubMed] [Google Scholar]
- 71.Ennis R. S., Granda J. L., Posner A. S., Effect of gold salts and other drugs on the release and activity of lysosomal hydrolases. Arthritis Rheum. 11, 756–764 (1968). [DOI] [PubMed] [Google Scholar]
- 72.Paltemaa S., The inhibition of lysosomal enzymes by gold salts in human synovial fluid cells. Acta Rheumatol. Scand. 14, 161–168 (1968). [DOI] [PubMed] [Google Scholar]
- 73.Persellin R. H., Ziff M., The effect of gold salt on lysosomal enzymes of the peritoneal macrophage. Arthritis Rheum. 9, 57–65 (1966). [DOI] [PubMed] [Google Scholar]
- 74.Heimbürger M., Lerner R., Palmblad J., Effects of antirheumatic drugs on adhesiveness of endothelial cells and neutrophils. Biochem. Pharmacol. 56, 1661–1669 (1998). [DOI] [PubMed] [Google Scholar]
- 75.Yanni G., Nabil M., Farahat M., Poston R., Panayi G., Intramuscular gold decreases cytokine expression and macrophage numbers in the rheumatoid synovial membrane. Ann. Rheumatic Dis. 53, 315–322 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bratt J., Belcher J., Vercellotti G. M., Palmblad J., Effects of anti-rheumatic gold salts on nf-b mobilization and tumour necrosis factor-alpha (tnf-)-induced neutrophil-dependent cytotoxicity for human endothelial cells. Clin. Exp. Immunol. 120, 79–84 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stone K., Mather S., Gibson P., Selective inhibition of prostaglandin biosynthesis by gold salts and phenylbutazone. Prostaglandins 10, 241–251 (1975). [DOI] [PubMed] [Google Scholar]
- 78.Hashimoto K., Whitehurst C., Matsubara T., Hirohata K., Lipsky P., Immunomodulatory effects of therapeutic gold compounds. Gold sodium thiomalate inhibits the activity of T cell protein kinase C. J. Clin. Invest. 89, 1839–1848 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lipsky P. E., Ziff M., Inhibition of antigen-and mitogen-induced human lymphocyte proliferation by gold compounds. J. Clin. Invest. 59, 455–466 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ugai K., Ziff M., Lipsky P. E., Gold-induced changes in the morphology and functional capabilities of human monocytes. Arthritis Rheum. 22, 1352–1360 (1979). [DOI] [PubMed] [Google Scholar]
- 81.Spalding D. M., Darby W. L., Heck L. W., Alterations in macrophage collagenase secretion induced by gold sodium thiomalate. Arthritis Rheum. 29, 75–81 (1986). [DOI] [PubMed] [Google Scholar]
- 82.Mallya S., Van Wart H., Mechanism of inhibition of human neutrophil collagenase by gold (i) chrysotherapeutic compounds. Interaction at a heavy metal binding site. J. Biol. Chem. 264, 1594–1601 (1989). [PubMed] [Google Scholar]
- 83.Srinivasan R., Miller B. L., Paulus H. E., Long-term chrysotherapy in rheumatoid arthritis. Arthritis Rheum. 22, 105–110 (1979). [DOI] [PubMed] [Google Scholar]
- 84.Sigler J. W., et al., Gold salts in the treatment of rheumatoid arthritis: A double-blind study. Ann. Intern. Med. 80, 21–26 (1974). [DOI] [PubMed] [Google Scholar]
- 85.Lorber A., Simon T., Leeb J., Peter A., Wilcox S., Chrysotherapy. Suppression of immunoglobulin synthesis. Arthritis Rheum. 21, 785–791 (1978). [DOI] [PubMed] [Google Scholar]
- 86.Kean W., Anastassiades T., Long term chrysotherapy. Arthritis Rheum. 22, 495–501 (1979). [DOI] [PubMed] [Google Scholar]
- 87.Davis P., Gold therapy in the treatment of rheumatoid arthritis. Can. Fam. Physician 34, 445–447 (1988). [Google Scholar]
- 88.McCarty D. J., Brill J. M., Harrop D., Aplastic anemia secondary to gold-salt therapy: Report of fatal case and a review of literature. J. Am. Med. Assoc. 179, 655–657 (1962). [Google Scholar]
- 89.Smith R., Cawley M., Chrysiasis. Br. J. Rheumatol. 36, 3–5 (1997). [DOI] [PubMed] [Google Scholar]
- 90.Larsen F. S., Bøye H., Hage E., Chrysiasis: Electron microscopic studies and X-ray microanalysis. Clin. Exp. Dermatol. 9, 174–180 (1984). [DOI] [PubMed] [Google Scholar]
- 91.Pelachyk J., Bergfeld W., McMahon J., Chrysiasis following gold therapy for rheumatoid arthritis: Ultrastructural analysis with X-ray energy spectroscopy. J. Cutan. Pathol. 11, 491–494 (1984). [DOI] [PubMed] [Google Scholar]
- 92.Smith R., et al., Chrysiasis revisited: A clinical and pathological study. Br. J. Dermatol. 133, 671–678 (1995). [DOI] [PubMed] [Google Scholar]
- 93.Fleming C. J., Salisbury E. L., Kirwan P., Painter D. M., Bametson R. S., Chrysiasis after low-dose gold and UV light exposure. J. Am. Acad. Dermatol. 34, 349–351 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Almoallim H., Klinkhoff A. V., Arthur A. B., Rivers J. K., Chalmers A., Laser induced chrysiasis: Disfiguring hyperpigmentation following q-switched laser therapy in a woman previously treated with gold. J. Rheumatol. 33, 620–621 (2006). [PubMed] [Google Scholar]
- 95.Cohen P. R., Ross E. V., Q-switched alexandrite laser-induced chrysiasis. J. Clin. Aesthetic Dermatol. 8, 48–53 (2015). [PMC free article] [PubMed] [Google Scholar]
- 96.Geist D. E., Phillips T. J., Development of chrysiasis after q-switched ruby laser treatment of solar lentigines. J. Am. Acad. Dermatol. 55, S59–S60 (2006). [DOI] [PubMed] [Google Scholar]
- 97.Trotter M. J., Tron V. A., Hollingdale J., Rivers J. K., Localized chrysiasis induced by laser therapy. Arch. Dermatol. 131, 1411–1414 (1995). [PubMed] [Google Scholar]
- 98.Yun P. L., Arndt K. A., Anderson R. R., Q-switched laser-induced chrysiasis treated with long-pulsed laser. Arch. Dermatol. 138, 1012–1014 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Schamberg J. F., Chrysoderma: A permanent gold staining of the skin. Arch. Dermatol. Syphilol. 18, 862–867 (1928). [Google Scholar]
- 100.McCormick S. A., DiBartolomeo A. G., Raju V., Schwab I. R., Ocular chrysiasis. Ophthalmology 92, 1432–1435 (1985). [DOI] [PubMed] [Google Scholar]
- 101.Hashimoto A., Maeda Y., Itō H., Okazaki M., Hara T., Corneal chrysiasis. A clinical study in rheumatoid arthritis patients receiving gold therapy. Arthritis Rheum. 15, 309–312 (1972). [DOI] [PubMed] [Google Scholar]
- 102.Kincaid M. C., Green W. R., Hoover R. E., Schenck P. H., Ocular chrysiasis. Arch. Ophthalmol. 100, 1791–1794 (1982). [DOI] [PubMed] [Google Scholar]
- 103.Tozawa K., et al., Gold compounds inhibit adhesion of human cancer cells to vascular endothelial cells. Canc. Lett. 196, 93–100 (2003). [DOI] [PubMed] [Google Scholar]
- 104.Kang K. W., Kim N., Yoon K. R., “Pharmaceutical composition comprising gold-containing agent for preventing or treating liver fibrosis or liver cirrhosis.” US Patent 20170266219A1 (2015).
- 105.Koch A. E., Cho M., Burrows J., Leibovich S. J., Polverini P. J., Inhibition of production of macrophage-derived angiogenic activity by the anti-rheumatic agents gold sodium thiomalate and auranofin. Biochem. Biophys. Res. Commun. 154, 205–212 (1988). [DOI] [PubMed] [Google Scholar]
- 106.Marzano C., et al., Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic. Biol. Med. 42, 872–881 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Caroli A., Simeoni S., Lepore R., Tramontano A., Via A., Investigation of a potential mechanism for the inhibition of SMTGR by auranofin and its implications for Plasmodium falciparum inhibition. Biochem. Biophys. Res. Commun. 417, 576–581 (2012). [DOI] [PubMed] [Google Scholar]
- 108.Harbut M. B., et al., Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. U.S.A. 112, 4453–4458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jackson-Rosario S., et al., Auranofin disrupts selenium metabolism in clostridium difficile by forming a stable Au–Se adduct. JBIC J. Biol. Inorganic Chem. 14, 507–519 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rigobello M. P., Folda A., Baldoin M. C., Scutari G., Bindoli A., Effect of auranofin on the mitochondrial generation of hydrogen peroxide. Role of thioredoxin reductase. Free Radic. Res. 39, 687–695 (2005). [DOI] [PubMed] [Google Scholar]
- 111.Madeira J. M., Bajwa E., Stuart M. J., Hashioka S., Klegeris A., Gold drug auranofin could reduce neuroinflammation by inhibiting microglia cytotoxic secretions and primed respiratory burst. J. Neuroimmunol. 276, 71–79 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Erdogan E., et al., Aurothiomalate inhibits transformed growth by targeting the pb1 domain of protein kinase C . J. Biol. Chem. 281, 28450–28459 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Kim T. W., et al., Kallikrein-related peptidase 6 induces chemotherapeutic resistance by attenuating auranofin-induced cell death through activation of autophagy in gastric cancer. Oncotarget 7, 85332–85348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jomova K., Valko M., Advances in metal-induced oxidative stress and human disease. Toxicology 283, 65–87 (2011). [DOI] [PubMed] [Google Scholar]
- 115.Kumar V., Yadav S. K., Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol.: Internat. Res. Process, Environ. Clean Technol. 84, 151–157 (2009). [Google Scholar]
- 116.Asmathunisha N., Kathiresan K., A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf. B Biointerf. 103, 283–287 (2013). [DOI] [PubMed] [Google Scholar]
- 117.Hulkoti N. I., Taranath T., Biosynthesis of nanoparticles using microbes—a review. Colloids Surf. B Biointerf. 121, 474–483 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Rehman F. U., Jiang H., Selke M., Wang X., Mammalian cells: A unique scaffold for in situ biosynthesis of metallic nanomaterials and biomedical applications. J. Mater. Chem. B 6, 6501–6514 (2018). [DOI] [PubMed] [Google Scholar]
- 119.Kumawat M. K., Thakur M., Lakkakula J. R., Divakaran D., Srivastava R., Evolution of thiol-capped gold nanoclusters into larger gold nanoparticles under electron beam irradiation. Micron 95, 1–6 (2017). [DOI] [PubMed] [Google Scholar]
- 120.Woo J. H., et al., Elucidating compound mechanism of action by network perturbation analysis. Cell 162, 441–451 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article, SI Appendix, and Dataset S1.