Graphical abstract
Keywords: Anacardic acid, Nanoparticles, Zein, Subacute toxicity, Micronucleus, Genotoxicity
Highlights
-
•
Novel anacardic acid loaded-zein nanoparticles were evaluated in terms of subacute toxicity and genotoxicity in Swiss mice.
-
•
The anacardic acid nanoparticles administered orally at 2.5 and 112.5 μg/Kg for 7 days did not present relevant toxicity.
-
•
These doses also presented a low frequency of micronuclei in the peripheral blood.
-
•
They showed good safety standards in mice under short-term treatments and could become novel therapeutic candidates.
Abstract
Anacardic acid extracted from cashew nut shells of Anacardium occidentale L has demonstrated important biological activities, such as antibacterial activity against the cariogenic specie Streptococcus mutans. Zein nanoparticles containing anacardic acid (9.375 μg/mL) were evaluated in terms of toxicity and genotoxicity in vivo. The subacute toxicity assay was used to evaluate the cumulative effects of the oral administration of nanoencapsulated anacardic acid at 2.25 and 112.5 μg/kg for 7 days in mice, simulating a mouth rinse short-term clinical course treatment. Blank zein nanoparticles and saline solution 0.9 % were used as negative controls. Peripheral blood samples were collected to evaluate the genotoxicity in polychromatic erythrocytes using the micronucleus test. The animals were anesthetized, euthanized and the target organs collected, weighed and submitted to histopathological analysis. Liver, kidney and spleen relative weights did not change. Nevertheless, stomach, lung and heart increased the relative weights in the group receiving the highest dose, in which occasional histopathological findings were also identified. Both doses maintained the micronucleus frequency within the normal range and the animals treated with the highest dose presented a discrete weight lost, which could explain the organs’ relative weight reductions. Blank and anacardic acid loaded zein nanoparticles were nontoxic when administered repeatedly for 7 days, as no relevant histopathological changes neither genotoxicity were observed. These preparations demonstrated limited toxicity under the conditions used in this study and could become an antibacterial alternative for preventing/treating oral infections in short-term treatments.
1. Introduction
Caries and periodontal disease can be prevented and controlled by mechanically removing the biofilm and using additional mouthwashes [1]. Chlorhexidine is currently considered the gold standard chemical antiplaque agent, although its prolonged use is associated to staining teeth and restorations, desquamation and pain in the mucosa, discoloration of the taste buds and taste changes [2] and the emergence of resistant species [3]. Therefore, medicinal plants and their phytochemicals are a potential source of therapeutic options for the treatment and prevention of biofilm- dependent oral diseases, considering several advantages, such as their biological properties, low cost and availability [4].
Anacardic acid is found as the major constituent extracted from cashew nut shells of Anacardium occidentale L., representing up to 70 % of its overall content [5]. It can be found as 4 distinct phenolic molecules whose difference remains in the unsaturation found in the 15-carbon aliphatic chain, correlated with the enhancement of biological properties [[6], [7], [8]].
This compound has gained attention in the last years in view of its biological activities, such as: anti-inflammatory [9,10], larvicide [5], antinoceptive [11], GABAA receptor-mediated anxiolytic [12], antioxidant [5,7] and antimicrobial [6], comprising oral bacteria [4,13]. Nonetheless, its low pH, high viscosity, hydrophobia and anti-aesthetic color discouraged its clinical use in dentistry. The incorporation in polymeric systems can overcome these disadvantages.
Polymeric nanoparticles have been extensively studied as drug delivery systems, providing drug protection, increased stability, controlled release, specific targeting and decreased toxicity [[14], [15], [16]]. Apart from overcoming the pharmacokinetic and pharmacodynamics limitations of many potential molecules, whose otherwise could be therapeutically useless, they may also be useful for enhanced permeability and retention effect, important features for local delivery in the biofilm control in the oral cavity [17]. Zein (Zea mays L) is a polymer biocompatible, biodegradable and highly hydrophobic [18,19]. It was used in the development of polymeric nanoparticles as a drug delivery system [[20], [21], [22]], incorporating several active compounds such as epigallocatechin gallate (EGCG) [18], curcumin [23,24], rutin [25], thymol [26] and glimepiride [20].
Anacardic acid loaded zein nanoparticles were able to improve important properties of this molecule, such as antioxidant and antibacterial activities against the cariogenic Streptococcus mutans in vitro [13].
Although several studies have attempted to evaluate the biological activities of anacardic acid, very little is found in the literature regarding its safety patterns. In view of the improved potential found in the anacardic acid nanoencapsulation and the potential use in the composition of mouthwashes to treat oral biofilms, the subacute toxicity and genotoxicity in vivo of anacardic acid loaded-zein nanoparticles administered orally to mice were investigated.
2. Materials e methods
2.1. Materials
Zein was purchased from Sigma-Aldrich® (St. Louis, MO, USA). Anacardic acid was extracted from cashew nut shell liquid of Anacardium occidentale. Deuterated solvents D2O 99.9 % and CD3OD 99.8 were purchased from Eurisotop (Saint-Aubin, France). Giemsa solution was purchased from Laborclin (Pinhais, Parana, Brazil). The anesthetics ketamine 10 % and xylazine 2 % were purchased from Venco (Londrina, Parana, Brazil). All the other reagents were pure grade and used as received.
2.2. Purification and characterization of anacardic acid
Anacardic acid was isolated from cashew nut shell liquid extracted and purified according to the method of Trevisan et al. [8], through the formation of precipitated calcium anacardate, acidification and extraction with hexane, followed by evaporation. The extracted compound (∼400 mM) was dissolved in 0.6 mL of CD3OD:D2O 90:10 (v/v) and submitted to Nuclear Magnetic Resonance (1H NMR) appreciation. 1H NMR spectrum was measured at 25 °C using a 17.6 T Bruker NEO-750 NMR spectrometer (750 MHz proton frequency) and processed with MestraNova® software version 12.0.
2.3. Preparation and characterization of nanoparticles
The formulations were obtained by nanoprecipitation of the protein according to our previously described method [13], using zein protein as a carrier and anacardic acid at 9.375 μg/mL (ZAa), the maximum loading reached. This concentration demonstrated the antibiofilm activity of ZAa against S. mutans in vitro [27]. Blank nanoparticles (ZB) were prepared in the same manner, except for the absence of drug, and used as control. The blank and loaded nanoparticles were appreciated morphologically by transmission electron microscopy (TEM) (JEOL JEM-2010, Electron Microscope) and characterized in terms of size (nm), polydispersity index (pdI), zeta potential (ζ) (mV) using a dynamic light scattering (DLS) analyzer (Zetasizer® Nano-ZS90, Malvern Instruments) before and after dilution.
Anacardic acid-loaded nanoparticles (ZAa) were administered in a volume of 0.3 mL. To obtain the lowest dose 2.25 μg/kg, freshly prepared nanoparticles (112.5 μg/kg) were diluted 1:50 in purified water. This dilution was chosen based on the possibility of ingestion of the residual amount left in the oral cavity after a daily administration. The blank nanoparticles were administered in a volume equivalent to the highest loaded dose.
2.4. Animals
Twenty-three female Swiss mice (Mus musculus), nulliparous, non-pregnant, with 25−30 g, were obtained from the bioterium of the Christus University Center, Fortaleza, Brazil. All animals were acclimated and kept under controlled temperature (20−25 °C) and relative humidity (50–60 %), following a 12 -h light-dark cycle. The animals were housed in cages with 4 or 5 animals and provided standard commercial diet and ad libitum drinking water. This study was based the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and was approved by the Animal Use Ethics Committee (CEUA) of Christus University Center under the protocol number 020/18.
2.5. Subacute toxicity
The cumulative effect of a short-term course treatment was assessed by a subacute toxicity assay for 7 consecutive days, according to the OECD 407 protocol [28] with some modifications, justified by the low doses administered, compatible to its effective dose [27] and the potential use as mouthwash in oral biofilm diseases prevention and control. ZAa nanoparticles were administered by oral gavage daily in two doses (ZAad1: 2.25 μg/kg and ZAad2: 112.5 μg/kg) (5 animals per group), while blank zein nanoparticles (ZB) and saline solution 0.9 % were administered in a volume equivalent to the highest dose to groups of four animals, each. The treatments are compiled in Table 1. The number of animals was based in another in vivo study focused in the antiproliferative activity of AA in experimental model of mice bearing human prostate tumor xenografts [29], which needed four animals by experimental group to reject the null hypothesis, similarly to the protocol used in this work.
Table 1.
Physical-chemical characterization of blank and anacardic acid loaded-zein nanoparticles.
| Formulation | Size (nm) | pdI | Zeta potential (mV) |
|---|---|---|---|
| ZAad2 | 381.6 ± 2.12 | 0.215 ± 0.02 | −15.9 ± 0.60 |
| ZAad1 (1:50) | 228.4 ± 1.609 | 0.245 ± 0.019 | −34.7 ± 1.61 |
| ZB | 376.5 ± 3.95 | 0.137 ± 0.01 | +6.56 ± 0.22 |
| ZB (1:50) | 785.1 ± 143.0 | 0.231 ± 0.022 | −14.1 ± 3.65 |
ZAad1, ZAad2 and ZB: anacardic acid loaded zein and blank nanoparticles, respectively. (1:50) means after 1:50 dilution in ultrapure water. Results are expressed as mean ± SD.
Following the daily administration, the animals were carefully observed in individual cages for 1 h, regarding their general behavior, signs or symptoms of toxicity such as tremors, convulsions, changes in posture and activity or death, according to Carvalho et al. [30]. The body mass of each animal (in g) was checked in the beginning and end of the experiment using a 2 digits electronic scale to appreciate the variation between groups.
Finally, the animals were anesthetized with ketamine 150 mg/kg and xylazine 50 mg/kg anesthetic mixture, euthanized and the target organs were removed to determine their relative weight (organ weight/animal body weight) and submitted to macroscopic and microscopic examination.
2.6. Morphological and histopathological evaluation
For the histological evaluation, the organs (liver, spleen, heart, lung and kidneys) were carefully analyzed in terms of mass, size, color changes and suggestive lesions such as organ structure, degenerative, necrosis and signs of inflammation [31]. Subsequently, they were stored in 10 % buffered formaldehyde for 48 h and subjected to sample preparation for histopathological purposes: dehydrated in crescent alcohol series, embedded in paraffin, sectioned in 4 μm thick slices, stained with hematoxylin-eosin [32] and assembled in Canada balsam for conventional microscopic analysis.
2.7. Genotoxicity evaluation
The genotoxic effects of ZAad1, ZAad2 and ZB were evaluated using the micronucleus test in polychromatic erythrocytes according to the method used by Carvalho et al. [30], which evaluated anacardic acid isolated, in agreement with OECD 474 protocol [33]. The negative and positive control groups received 0.9 % saline solution by oral gavage and doxorubicin (DXR) 15 mg/kg intraperitoneally, respectively. A blood drop was collected from each animal’s tail tip and dragged over a clean microscopic blade to obtain the blood smear, dried at room temperature, followed by fixation in absolute methanol for 5 min and a second drying at room temperature. The blades were stained with diluted Giemsa dye [34] for 20 min, washed to remove excess of dye, dried at room temperature and finally analyzed in an optical microscope (Opton TIM-2008) on the immersion objective (100x) [35].
Micronucleated polychromatic erythrocytes (PCEMNs) in peripheral blood samples were calculated from 2000 cells [35] and the micronucleus prevalence (in %) determined.
3. Results and discussion
3.1. Nanoparticles characterization
The nanoparticles showed adequate physical-chemical parameters: particle size bellow 500 nm, monodispersed (pdI <0.3) [21,22] and positive zeta potential for ZB and negative for ZAa, attributed to the salicylic acid groups [7]. The characteristics of the formulations obtained and after their dilution are compiled in Table 1.
Although blank nanoparticles doubled its size after dilution, it did not collapse (pdI = 0.231). This phenomenon can be attributed to a change in the nanoparticles conformation (confirmed by the zeta potential inversion: +6.56 to
-14.1 mV), as the protein depends of a certain proportion of ethanol to maintain its stability and nanoparticles reduced size. In the anacardic acid loaded nanoparticles size was reduced at almost half and remained stable after dilution (pdI 0.245 ± 0.019), while zeta potential reduced its value to -34.7 ± 1.61 mV and did not change their charge, indicating a more stable system. The appreciation of the nanoparticles by TEM, confirms the particle size values obtained by DLS, ranging from 300−490 nm for ZAad1 and ZB (Fig. 1). Both systems showed spherical and non-aggregated characteristics.
Fig. 1.
TEM images of the nanoparticle formulations before dilutions. a) ZAad2, Anacardic acid-loaded zein nanoparticles and b) ZB, Blank zein nanoparticles.
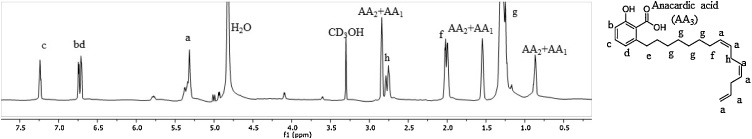
The 1H NMR spectrum of AA, naturally found in the cashew shells of Anacardium occidentale is shown in Fig. 2. The signal assignment confirms the solely presence of this compound in the purified supply used to prepare the nanoparticles. The monoene and diene aliphatic chain molecules were predominant and no impurities or their degradation products (such as cardol or cardanol) were evident.
Fig. 2.
¹H NMR spectrum of anacardic acids. The proton signal assignment is indicated with letters that corresponds to the molecule structure shown on the right.
3.2. Subacute toxicity
During the subacute toxicity experiment no behavioral changes, death or severe toxicity signs were observed. Apart from the negative control group (NC), all the other groups presented weighting loss, though they did not differ from each other (p > 0.05) (Table 2).
Table 2.
Body weight variation in mice submitted to subacute toxicity assay. Weight assessment was performed at the beginning and after 7 days (results are expressed as mean ± SD).
| Day | ZAad1 | ZAad2 | ZB | NC |
|---|---|---|---|---|
| 1 | 27.60 ± 2.30 | 26.60 ± 2.07 | 26.50 ± 2.38 | 25.50 ± 3.10 |
| 7 | 27.20 ± 2.28 | 22.40 ± 3.50 | 24.00 ± 2.44 | 25.75 ± 2.06 |
ZAad1: Anacardic acid loaded zein nanoparticles administered at 2.25 μg/kg; ZAad2: Anacardic acid loaded zein nanoparticles administered at 112.5 μg/kg; ZB: Blank zein nanoparticles.
NC: Negative control group, treated with sterile saline solution 0.9 %.
Carvalho et al. [30] found the lethal dose of anacardic acid and cashew nut oil to be above 2000 mg/kg in an acute toxicity study. In the same study the oral administration of anacardic acid at 300, 600 and 1000 mg/kg during 30 days did not incur in biochemical and/or hematological or body weight change, similarly to the results of Konan et al. [36] who studied the toxicity of A. occidentale leaves extract. The reduction in the body weight was found in the highest dose (ZAad2) (Table 2), although it has been unspecific and did not differ statically (p > 0.05) from the other groups.
In the relative weight analysis, no significant alterations were found in spleen (Fig. 3A), liver (Fig. 3B) and kidneys (Fig. 3C) in comparison with the negative control group (p > 0.05). However, an increment in the stomach (Fig. 3D), lung (Fig. 3E) and heart (Fig. 3F) (p < 0.05) relative weights of the ZAad2 group were found. This finding can be partly explained by the body weight reduction in this group; as the absolute organ weights (heart and lung) did not differ significantly from the negative control groups.
Fig. 3.
Target organs weight variation (organ weight/body weight x 100) in mice orally treated for 7-days with ZAad1, ZAad2, ZB nanoparticles and NC. (A) Spleen, (B) Liver, (C) Kidneys, (D) Stomach, (E) Lung and (F) Heart. (results are express as Mean ± SD).
**p < 0.01 ZAad2versus NC, ZB and ZAad1, *p < 0.05 ZAad2versus CN, ZB and ZAad1. ZAad1: Anacardic acid loaded zein nanoparticles administered at 2.25 μg/kg.
ZAad2: Anacardic acid loaded zein nanoparticles administered at 112.5 μg/kg; ZB: Blank zein nanoparticles.
NC: Negative control group, treated with sterile saline solution 0.9 %.
Carvalho et al. [30] reported an increment in the relative weight of spleen in female mice treated with anacardic acid (600 mg/kg) for 30 days. Nonetheless, when a single dose of anacardic acid (2000 mg/kg) was administered, this aspect was not observed. A reduction in the relative lung weight was also observed in treated male mice. Contrariwise, doses below 300 mg/kg have not produced signs of chronic toxicity. Even so, the doses used in that study are much superior than those administered (2.25 and 112.5 μg/kg) in this experimental protocol. Therefore, the changes observed are more likely to be related to the body weight reduction observed in the group treated with 112.5 μg/kg ZAad2 nanoparticles, as aforementioned.
3.3. Histopathological evaluation
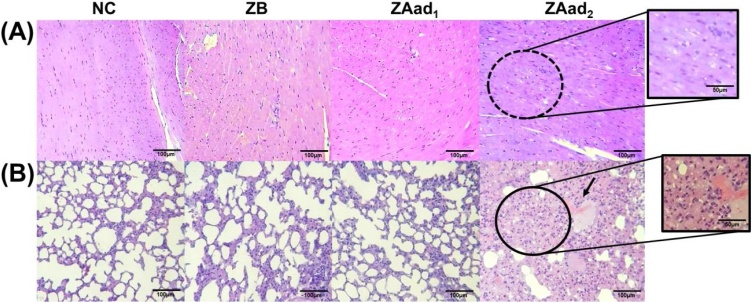
In the macroscopic analysis of the organs, no alterations were noticeable in the parameters color, texture, size or anatomical aspects. The spleen preserved follicles and the white and red pulps were intact in their constitution. No vascular congestion or inflammatory cells were found, only the common presence of megakaryocytes in all groups (Fig. 4A), demonstrating no relevant histopathological changes.
Fig. 4.
Photomicrographs of representative tissue sections (H&E) showing the histopathology: (A) spleen, (B) liver and (C) kidneys among mice groups NC (Negative control group, treated with sterile saline solution 0.9 %), ZB (Blank zein nanoparticles.), ZAad1 (Anacardic acid loaded zein nanoparticles administered at 2.25 μg/kg) and ZAad2 (Anacardic acid loaded zein nanoparticles administered at 112.5 μg/kg) treated for 7- days. Black arrows indicate the presence of megakaryocytes in all groups in the spleen; circle indicates focus of inflammatory cells in the liver; elliptical indicates the presence of tubular and interstitial hemorrhages and yellow arrow indicates the presence of hyaline cylinder.
Liver samples were constituted by hepatocyte cords without swelling, with portal and central lobular vein congestion, Kupffer cell hyperplasia, absence of micro or macro vesicular steatosis neither focal hepatocyte necrosis in any group. One animal from the groups NC and ZB presented signs of sinusoidal hemorrhage and in the groups NC and ZAad2 occasional focus of inflammation. In the groups ZAad1, ZAad2 and NC, mild to moderate hydropic degeneration was observed (Fig. 4B).
Tédong et al. [37] reported the hepatotoxicity of A. occidentale L leaves’ hexane extracts, causing vascular congestion, degeneration and necrosis in the dose of 14 g/kg. Nevertheless, the therapeutic doses associated with its biological properties: anti-inflammatory [9], antibacterial [38,39], anti-tumor [40], anti-ulcerative [41] and antioxidant [42] are considerably lower than those used in that study.
Kupffer cells have phagocytic purposes and their hyperplasia shows that all animals were exposed to hepatic oxidative stress [43]. Hydropic degeneration is a vacuolization in which the amount of intracellular water increases, caused by disturbance of ionic and fluid balance, commonly reversible and nonlethal process, but can lead to cellular degeneration (necrosis) in more severe cases [43,44], suggesting that anacardic acid may have contributed to some level of ionic unbalance.
The kidneys of all groups presented normal distribution of preserved glomerular structures in the cortex and renal medulla, without tubular epithelial swelling or vacuolar degeneration. No inflammatory cells and necrosis were observed. In ZAad1 and ZAad2 groups, mild tubular and interstitial hemorrhage was found, while the presence of hyaline cylinders could be observed solely in the highest dose (ZAad2) (Fig. 4C).
Alterations such as inflammation and mild hemorrhage in the renal tissues may occur and are considered occasional, but it can progress in severity to renal insufficiency and tubular necrosis [[45], [46], [47]]. Hyaline cylinders are formed from the accumulation of mucoproteins secreted by epithelial cells in the renal tubules and may be related to acute tubular irritation, nephritis, hemorrhages or changes in the renal flow [[48], [49], [50]].
Carvalho et al. [30] did not found hepatic or renal morphological alterations after acute and subacute anacardic acid administration in doses between 300 and 2000 mg/kg. Anacardic acid 0.1 % (p/p) has also been evaluated in rat supplementation diet for 29 days and no changes in the hepatic and renal markers were found [51]. Mice fed with a high-fat and high-sucrose diet treated orally with 500 μg/kg of AA slowed down the lipid accumulation rates in the liver and mitigated insulin resistance, demonstrating its protective effect in metabolic disorders [52].
The animals of all groups showed heart tissues predominantly represented by cardiac striated muscle, longitudinal and transverse fibers preserved with absence of hemosiderin pigments or inflammatory cells. Discrete areas of hemorrhage were noticed in three animals of ZAad2 from whose one presented vacuolization in the myocytes (Fig. 5A). This vacuolization in myocytes is considered a non-lethal degeneration process, characterized by clear spaces, ranging from 0.1–5 μm [53,54]. Nonetheless this aspect is more likely be related to cardiotoxicity when associated with other changes such as infiltration of inflammatory cells, necrosis and fibrosis [[55], [56], [57]], hemorrhagic spots accompanied by necrosis and the presence of macrophages and hemosiderin [58,59]. All these factors were absent, demonstrating only focal damage, not related to cardiotoxicity, and limited to the highest dose (ZAad2) treated group.
Fig. 5.
Photomicrographs of representative tissue sections (H&E) showing the histopathology: (A) heart and (B) lung among mice groups NC (Negative control group, treated with sterile saline solution 0.9 %), ZB (Blank zein nanoparticles.), ZAad1 (Anacardic acid loaded zein nanoparticles administered at 2.25 μg/kg) and ZAad2 (Anacardic acid loaded zein nanoparticles administered at 112.5 μg/kg) treated for 7-days. Dotted circle indicates vacuolation in the heart; Full circle indicates the presence of inflammatory cells and arrow indicating bleeding point in the lung.
Lung samples were represented in all groups by alveolar spaces with bronchioles, bronchi of preserved morphology and intact pleura. Occasional areas of edema or hemorrhage were observed in one individual animal of the groups treated with ZB, ZAad1 (lowest dose) and NC group, all without inflammatory cells, not configuring a relevant and treatment-related sign of toxicity. ZAad2 group showed moderate hemorrhagic points and occasional inflammatory cells (Fig. 5B). Despite its rarely occurrence, it may result from side effects of drugs that alter hemostatic mechanisms or disruption of capillaries [60,61]. Despite that, anacardic acid was able to decrease induced inflammatory and oxidative processes in mice treated orally in the doses of 50 a 250 mg/kg for 30 days, suggesting its possible protective activity in the lung [62].
Some histological changes in the stomach were found and its relative weight increased in the groups ZB, ZAad1 and ZAad2, corroborating with the histological changes observed, such as mucosal thickening of the fundus and disorganization (Fig. 6A and 6B). Moreover, no inflammatory infiltrate was observed in these groups (Fig. 4B), which could possibly be associated with the gastroprotective effect of anacardic acid [63] and zein nanoparticles, hence the blank formulations were consonant with this behavior.
Fig. 6.
Photomicrographs of representative tissue sections (H&E) showing the histopathology of the stomach: (A) cardia and (B) fund among mice NC (Negative control group, treated with sterile saline solution 0.9 %), ZB (Blank zein nanoparticles.), ZAad1 (Anacardic acid loaded zein nanoparticles administered at 2.25 μg/kg) and ZAad2 (Anacardic acid loaded zein nanoparticles administered at 112.5 μg/kg) treated for 7-days Black arrows indicate the thickness of gastric mucosa growth and red arrow indicates hemorrhagic point.
Unlike these aforementioned findings, where heart, lung and stomach organs showed relative weight increment and few toxicity signs in the higher dose tested (ZAad2), similar studies with anacardic acid and A. occidentale extracts did not observe apparent macroscopic or microscopic changes in the target organs analyzed in higher doses [30,36]. This fact could be attributed to the nanoencapsulation of anacardic acid, which may have contributed to enhance its bioavailability and thus exacerbate the side effects in the highest dose assayed. Further studies exploring the pharmacokinetics of different doses and also comparing the non-encapsulated to the nanoencapsulated anacardic acid are mandatory to confirm this hypothesis and could provide further insight for novel therapeutic approaches.
3.4. Genotoxicity assay
Clastogenesis or aneugenesis can occur during the process of erythrocyte maturation, resulting in micronucleus [31]. These changes can be identified in the analysis of immature (polychromatic) erythrocytes in peripheral blood or bone marrow samples [42].
No relevant difference on the micronucleus frequency was observed in polychromatic erythrocytes between treated and negative control groups. A very low frequency of micronucleus was found in immature (polychromatic) erythrocytes of both treated and control groups, which implies in non-genotoxic characteristics [36] (Table 3). The lowest dose (ZAad1) triggered a lower frequency of PCEMNs than the negative control (p > 0.05), blank zein nanoparticles (p > 0.05), ZAad2 (p > 0.05) and DXR (p < 0.001). DXR acts through the topoisomerase II inhibition, causing evident genotoxicity by damaging the DNA, used as positive control in micronucleus assays [[64], [65], [66]].
Table 3.
Frequency of micronucleated polychromatic erythrocytes in peripheral blood of mice treated with blank and anacardic acid loaded-zein nanoparticles.
| Groups | PCE (nº) | PCEMN (nº) | MN (%) |
|---|---|---|---|
| NC | 8000 | 21 | 0.26 ± 0.013a |
| ZB | 8000 | 20 | 0.25 ± 0.009a |
| ZAad1 | 8000 | 17 | 0.21 ± 0.012a |
| ZAad2 | 8000 | 25 | 0.31 ± 0.012a |
| DXR | 8000 | 140 | 1.75 ± 0.712b |
PCE, polychromatic erythrocytes, MN, frequencies of micronucleus, DXR, doxorubicin (15 mg/kg). One-way ANOVA followed by Tukey test. Different uppercase letters indicate statistical difference (p < 0.001).
Moreover, no difference between ZB and negative control group (p > 0.05) was found. Despite the absence of genotoxicity, the largest number of PCEMNs were found in the ZAad2 group, attributed to the highest concentration of nanoencapsulated anacardic acid and its transient toxicity. Despite this fact, the frequency of micronuclei was much lower compared to the DXR treated group, demonstrating its lack of genotoxicity.
Different doses of anacardic acid were evaluated up to 300 mg/kg, and no difference in the micronucleus frequency was observed in comparison to their controls [30,35]. A micronuclei frequency of 0.26 % was found in rats treated with 2000 mg/kg of hydroethanolic extract of leaves Anacardium occidentale, while the negative control had 0.11 % and over 1 % was found in the positive control group (cyclophosphamide – CYP 50 mg/kg) [36], these data are in agreement with those obtained in this study (Table 3). The highest frequency of micronucleus was found in the DXR-treated group (1.75 %), followed by the highest dose of anacardic acid loaded-zein nanoparticles (ZAad2 – 0.31 %), which is considered non-genotoxic under the treatment course used.
4. Conclusions
Novel blank and anacardic acid-loaded zein nanoparticles, designed for treating oral biofilms, were administered orally for 7 days in mice. Although, they did not show relevant signs of toxicity, an increment in the lung, heart and stomach relative weight was observed, which could be related to the weight loss observed in the nanoparticles treated groups. In view of the histopathological examination, none of these findings were correlated with high toxicity in these organs. In the genotoxic assay, zein nanoparticles containing anacardic acid, presented a very low frequency of micronucleus in the peripheral blood, especially at the lowest dose administered and are considered non-genotoxic. Long-term complementary tests are necessary to prove the absence of chronic toxicity and genotoxicity in view of the potential use of these formulations as an active ingredient of daily use mouthwashes to prevent and treat oral-biofilm related diseases, although they were found to be safe under short-term treatments.
CRediT authorship contribution statement
Jennifer Thayanne Cavalcante de Araújo: Methodology, Investigation, Validation, Visualization, Writing - original draft, Writing - review & editing. Laís Aragão Lima: Investigation. Everton Pantoja Vale: . Manuel Martin-Pastor: . Ramille Araújo Lima: Conceptualization, Methodology, Resources, Project administration, Supervision. Paulo Goberlânio de Barros Silva: Methodology, Resources, Investigation, Validation, Visualization, Writing - review & editing, Supervision. Francisco Fabio Oliveira de Sousa: Conceptualization, Writing - review & editing, Supervision, Resources, Funding acquisition.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The present work was accomplished with the support of the National Program of Cooperation in the Amazon – PROCAD/Amazon of Coordination of Superior Level Staff Improvement– CAPES/Brazil. We would like to thank Fundação de Amparo à Pesquisa do Estado do Amapá (FAPEAP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for supporting this research (EDITAL PRONEM, grant # 95/2018). This research also received financial support from PAPESQ Program (EDITAL Nº 14/2017) from Federal University of Amapa. We thank the Research Laboratory of Drugs (LPFar) of Federal Universty of Amapa for the physicochemical characterization measurements and Christus University Center (UNICHRISTUS) bioterium for supporting this research.
References
- 1.Takenaka S., Ohsumi T., Noiri Y. Evidence-based strategy for dental biofilms: current evidence of mouthwashes on dental biofilm and gingivitis. Jpn. Dent. Sci. Rev. 2019;55:33–40. doi: 10.1016/j.jdsr.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tartaglia G.M., Tadakamadla S.K., Connelly S.T., Sforza C., Martín C. Adverse events associated with home use of mouthrinses: a systematic review. Ther. Adv. Drug Saf. 2019;10 doi: 10.1177/2042098619854881. 204209861985488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cieplik F., Jakubovics N.S., Buchalla W., Maisch T., Hellwig E., Al-Ahmad A. Resistance Toward Chlorhexidine in Oral Bacteria – Is There Cause for Concern? Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Araújo J.C., De Castilho A.R.F., Lira A.B., Pereira A.V., De Azevêdo T.K.B., De Brito E.M.D.M., do M., Pereira S.V., de H., Pessôa L.F., Pereira J.V. Antibacterial activity against cariogenic bacteria and cytotoxic and genotoxic potential of Anacardium occidentale L. and Anadenanthera macrocarpa (Benth.) Brenan extracts. Arch. Oral Biol. 2018;85:113–119. doi: 10.1016/j.archoralbio.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira M.S.C., de Morais S.M., Magalhães D.V., Batista W.P., Vieira Í.G.P., Craveiro A.A., de Manezes J.E.S.A., Carvalho A.F.U., de Lima G.P.G. Antioxidant, larvicidal and antiacetylcholinesterase activities of cashew nut shell liquid constituents. Acta Trop. 2011;117:165–170. doi: 10.1016/j.actatropica.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Hamad F.B., Mubofu E.B. Potential biological applications of bio-based anacardic acids and their derivatives. Int. J. Mol. Sci. 2015;16:8569–8590. doi: 10.3390/ijms16048569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morais S., Silva K., Araujo H., Vieira I., Alves D., Fontenelle R., Silva A. Anacardic acid constituents from cashew nut shell liquid: NMR characterization and the effect of unsaturation on its biological activities. Pharmaceuticals. 2017;10:31. doi: 10.3390/ph10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trevisan M.T.S., Pfundstein B., Haubner R., Würtele G., Spiegelhalder B., Bartsch H., Owen R.W. Characterization of alkyl phenols in cashew (Anacardium occidentale) products and assay of their antioxidant capacity. Food Chem. Toxicol. 2006;44:188–197. doi: 10.1016/j.fct.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Mamidyala S.K., Ramu S., Huang J.X., Robertson A.A.B., Cooper M.A. Bioorganic & Medicinal Chemistry Letters Efficient synthesis of anacardic acid analogues and their antibacterial activities. Bioorg. Med. Chem. Lett. 2013;23:1667–1670. doi: 10.1016/j.bmcl.2013.01.074. [DOI] [PubMed] [Google Scholar]
- 10.Wisastra R., Ghizzoni M., Boltjes A., Haisma H.J., Dekker F.J. Anacardic acid derived salicylates are inhibitors or activators of lipoxygenases. Bioorganic Med. Chem. 2012;20:5027–5032. doi: 10.1016/j.bmc.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 11.da Silva D.P.B., Florentino I.F., da Silva Moreira L.K., Brito A.F., Carvalho V.V., Rodrigues M.F., Vasconcelos G.A., Vaz B.G., Pereira-Junior M.A., Fernandes K.F., Costa E.A. Chemical characterization and pharmacological assessment of polysaccharide free, standardized cashew gum extract (Anacardium occidentale L.) J. Ethnopharmacol. 2018;213:395–402. doi: 10.1016/j.jep.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Gomes Júnior A.L., Tchekalarova J.D., Machado Kda C., Moura A.K.S., Paz M.F.C.J., da Mata A.M.O.F., Nogueira T.R., Islam M.T., de M.A., Rios S., das Graças Lopes Citó A.M., Uddin S.J., Shilpi J.A., Das A.K., Lopes Lda S., de A.A., Melo-Cavalcante C. Anxiolytic effect of anacardic acids from cashew (Anacardium occidentale) nut shell in mice. IUBMB Life. 2018 doi: 10.1002/iub.1738. [DOI] [PubMed] [Google Scholar]
- 13.Sousa F.F.O., Araujo J.T.C. 2017. Nanopartículas de ácido anacárdico extraído do caju e seu uso como agente bactericida e larvicida. [Google Scholar]
- 14.Ma B.-L., Yin C., Zhang B.-K., Dai Y., Jia Y.-Q., Yang Y., Li Q., Shi R., Wang T.-M., Wu J.-S., Li Y.-Y., Lin G., Ma Y.-M. Naturally occurring proteinaceous nanoparticles in Coptidis Rhizoma extract act as concentration-dependent carriers that facilitate berberine absorption. Sci. Rep. 2016;6:20110. doi: 10.1038/srep20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., del M., Rodriguez-Torres P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.-S. Nano based drug delivery systems : recent developments and future prospects. J. Nanobiotechnology. 2018;16:1–33. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramtoola Z., Lyons P., Keohane K., Kerrigan S.W., Kirby B.P., Kelly J.G. Investigation of the interaction of biodegradable micro- and nanoparticulate drug delivery systems with platelets, J. Pharm. Pharmacol. 2011;63:26–32. doi: 10.1111/j.2042-7158.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S., Ghosh S., Sil P.C. Role of nanostructures in improvising oral medicine. Toxicol. Reports. 2019 doi: 10.1016/j.toxrep.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang J., Yan H., Wang X., Zhou Y., Gao X., Puligundla P., Wan X. Encapsulation of epigallocatechin gallate in zein / chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017;231:19–24. doi: 10.1016/j.foodchem.2017.02.106. [DOI] [PubMed] [Google Scholar]
- 19.Yong Z., Lili C., Feng L., Nianqiu S., Chunlei L., Xianghui Y., Yan C., Wei K. Design, fabrication and biomedical applications of zein-based nano / micro-carrier systems. Int. J. Pharm. 2016;513:191–210. doi: 10.1016/j.ijpharm.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed O.A.A., Zidan A., Khayat M. Mechanistic analysis of Zein nanoparticles/PLGA triblock in situ forming implants for glimepiride. Int. J. Nanomedicine. 2016;11:543. doi: 10.2147/IJN.S99731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F., Chen Y., Liu S., Qi J., Wang W., Wang C., Zhong R., Chen Z., Li X., Guan Y., Kong W., Zhang Y. Size-controlled fabrication of zein nano/microparticles by modified anti-solvent precipitation with/without sodium caseinate. Int. J. Nanomedicine. Volume. 2017;12:8197–8209. doi: 10.2147/IJN.S143733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sousa F.F.O., Luzardo-Alvarez A., Blanco-Mendez J., Otero-Espinar F.J., Martin-Pastor M., Sandez Macho I. Use of 1H NMR STD, WaterLOGSY, and Langmuir monolayer techniques for characterization of drug-zein protein complexes. Eur. J. Pharm. Biopharm. 2013;85:790–798. doi: 10.1016/j.ejpb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Chen S., Han Y., Sun C., Dai L., Yang S., Wei Y., Mao L., Yuan F., Gao Y. Effect of molecular weight of hyaluronan on zein-based nanoparticles: fabrication, structural characterization and delivery of curcumin. Carbohydr. Polym. 2018;201:599–607. doi: 10.1016/j.carbpol.2018.08.116. [DOI] [PubMed] [Google Scholar]
- 24.Wang T., Fernandez M.L., Luo Y., Hu S., Wang T., Fernandez M.L., Luo Y. 2016. Development of Tannic Acid Cross-linked Hollow Zein Nanoparticles As Potential Oral Delivery Vehicles for Curcumin Potential Oral Delivery Vehicles for Curcumin. [DOI] [Google Scholar]
- 25.Zhang S., Zhao H. Preparation and properties of zein – rutin composite nanoparticle / corn starch films. Carbohydr. Polym. 2017;169:385–392. doi: 10.1016/j.carbpol.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Niu Y., Luo Y., Ge M., Yang T., Lucy L., Wang Q. Fabrication, characterization and antimicrobial activities of thymol- loaded zein nanoparticles stabilized by sodium caseinate – chitosan hydrochloride double layers. Food Chem. 2014;142:269–275. doi: 10.1016/j.foodchem.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 27.Lima R.A., de Souza S.L.X., Lima L.A., Batista A.L.X., de Araújo J.T.C., Sousa F.F.O., Rolim J.P.M.L., Bandeira T.D.J.P.G. Antimicrobial effect of anacardic acid–loaded zein nanoparticles loaded on Streptococcus mutans biofilms. Braz. J. Microbiol. 2020 doi: 10.1007/s42770-020-00320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.OECD 407 . 2008. (Organization for Economic Cooperation and Development), Guidel. Fot Test. Chem. Repeated Dose 28-Day Oral Toxic. Study Rodents. Guidel. [Google Scholar]
- 29.Wu Y., He L., Zhang L., Chen J., Yi Z., Zhang J., Liu M. Anacardic acid (6-Pentadecylsalicylic acid) inhibits tumor angiogenesis by targeting src / FAK / rho GTPases signaling pathway. J. Pharmacol. Exp. Ther. 2011;339:403–411. doi: 10.1124/jpet.111.181891. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho A.L.N., Annoni R., Silva P.R.P., Borelli P., Fock R.A., Trevisan M.T.S., Mauad T. Acute, subacute toxicity and mutagenic effects of anacardic acids from cashew (Anacardium occidentale Linn.) in mice. J. Ethnopharmacol. 2011;135:730–736. doi: 10.1016/j.jep.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Oliveira M.C., Lemos L.M.S., De Oliveira R.G., Dall’Oglio E.L., de Sousa Júnior P.T., de D.T., Martins O. Evaluation of toxicity of Calophyllum brasiliense stem bark extract by in vivo and in vitro assays. J. Ethnopharmacol. 2014;155:30–38. doi: 10.1016/j.jep.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Peng C., Luo X., Li S., Sun H. Phenylephrine-induced cardiac hypertrophy is attenuated by a histone acetylase inhibitor anacardic acid in mice. Mol. Biosyst. 2017;13:714–724. doi: 10.1039/C6MB00692B. [DOI] [PubMed] [Google Scholar]
- 33.OECD 474 . Erythrocyte Micronucleus Test; Mamm: 2016. Guideline for the Testing of Chemicals. [Google Scholar]
- 34.Minist. Heal. 2009. Manual de Diagnóstico Laboratorial da Malária Manual de Diagnóstico. Série A. Normas e Manuais Técnicos. p. 118. [Google Scholar]
- 35.Vale E.P., do Rego L.R., Pureza D.D.N., de P.G., Silva B., de Sousa F.F.O., de M., Monteiro Neto A.B. Cytogenetic and toxicological effects of Punica granatum Linnaeus fruit peel hydroethanolic extract in mice. S. Afr. J. Bot. 2020;130:465–470. doi: 10.1016/j.sajb.2020.01.041. [DOI] [Google Scholar]
- 36.Konan N.A., Bacchi E.M., Lincopan N., Varela S.D., Varanda E.A. Acute, subacute toxicity and genotoxic effect of a hydroethanolic extract of the cashew (Anacardium occidentale L.) J. Ethnopharmacol. 2007;110:30–38. doi: 10.1016/j.jep.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Tédong L., Dzeufiet P.D.D., Dimo T., Asongalem E.A., Sokeng S.N., Flejou J.F., Callard P., Kamtchouing P. Acute and subchronic toxicity of Anacardium occidentale Linn (Anacardiaceae) leaves hexane extract in mice. Afr. J. Tradit. Complement. Altern. Med. 2007;4:140–147. doi: 10.4314/ajtcam.v4i2.31194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima R.A., Lima L.A., Santos S.V., Rodrigues L.K.A., Araújo J.T.C., Sousa F.F.O. Anti-biofilm activity of Anacardium occidentale chestnut extract. Caries Res. 2017;51:290–385. doi: 10.1159/000471777. [DOI] [PubMed] [Google Scholar]
- 39.Hemshekhar M., Sebastin Santhosh M., Kemparaju K., Girish K.S. Emerging roles of anacardic acid and its derivatives: a pharmacological overview. Basic Clin. Pharmacol. Toxicol. 2012;110:122–132. doi: 10.1111/j.1742-7843.2011.00833.x. [DOI] [PubMed] [Google Scholar]
- 40.Legut M., Lipka D., Filipczak N., Piwoni A., Kozubek A., Gubernator J. Anacardic acid enhances the anticancer activity of liposomal mitoxantrone towards melanoma cell lines – in vitro studies. Int. J. Nanomedicine. 2014;9:653–668. doi: 10.2147/IJN.S54911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shilpa P., Kaveri K., Salimath B.P. Anti-metastatic action of anacardic acid targets VEGF-induced signalling pathways in epithelial to mesenchymal transition. Drug Discov. Ther. 2015;9:53–65. doi: 10.5582/ddt.2014.01042. [DOI] [PubMed] [Google Scholar]
- 42.Encarnação S., De Mello-sampayo C., Graça N.A.G., Catarino L., Silva I.B.M., Lima B.S., Silva O.M.D. Total phenolic content, antioxidant activity and pre-clinical safety evaluation of an Anacardium occidentale stem bark Portuguese hypoglycemic traditional herbal preparation. Ind. Crops Prod. 2016;82:171–178. doi: 10.1016/j.indcrop.2015.11.001. [DOI] [Google Scholar]
- 43.Almansour M.I., Jarrar Y.B., Jarrar B.M. In vivo investigation on the chronic hepatoxicity induced by sertraline. Environ. Toxicol. Pharmacol. 2018;61:107–115. doi: 10.1016/j.etap.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Hayelom K., Mekbeb A., Eyasu M., Wondwossen E., Kelbesa U. Methanolic effect of Clerodendrum myricoides root extract on blood, liver and kidney tissues of mice. Afr. Health Sci. 2012;4:489–497. doi: 10.4314/ahs.v12i4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de M.C., Araújo P.M., Barcellos N.M.S., de P.M., Vieira A., Gouveia T.M., Guerra M.O., Peters V.M., Saúde-Guimarães D.A. Acute and sub chronic toxicity study of aqueous extract from the leaves and branches of Campomanesia velutina (Cambess) O. Berg. J. Ethnopharmacol. 2017;201:17–25. doi: 10.1016/j.jep.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 46.Kiss N., Hamar P. Histopathological evaluation of contrast-induced acute kidney injury rodent models. Biomed Res. Int. 2016;2016:1–15. doi: 10.1155/2016/3763250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sato N., Takahashi D., Chen S., Tsuchiya R., Mukoyama T., Yamagata S., Ogawa M., Yoshida M., Kondo S., Satoh N., Ueda S. Acute nephrotoxicity of aristolochic acids in mice. J. Pharm. Pharmacol. 2004;56:221–229. doi: 10.1211/0022357023051. [DOI] [PubMed] [Google Scholar]
- 48.Caleffi A., Lippi G. Cylindruria. Clin. Chem. Lab. Med. Rev. 2015:1–7. doi: 10.1515/cclm-2015-0480. [DOI] [PubMed] [Google Scholar]
- 49.Frazier K.S., Seely J.C., Hard G.C., Betton G., Burnett R., Nakatsuji S., Nishikawa A., Durchfeld-Meyer B., Bube A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol. Pathol. 2012;40:14–86. doi: 10.1177/0192623312438736. [DOI] [PubMed] [Google Scholar]
- 50.Ringsrud K.M. Casts in the urine sediment. Lab. Med. 2001;32:191–193. [Google Scholar]
- 51.Toyomizu M., Okamoto K., Ishibashi T., Nakatsu T., Akiba Y. Reducing effect of dietary anacardic acid on body fat pads in rats. Anim. Sci. J. 2003;74:499–504. [Google Scholar]
- 52.Chung S., Ju E., Choi S.H., Ho J., Hwang J. Anacardic acid mitigates liver fat accumulation and impaired glucose tolerance in mice fed a high-fat and high-sucrose diet. Food Sci. Nutr. 2020;8:796–804. doi: 10.1002/fsn3.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berridge B.R., Van Vleet J.F., Herman E. Elsevier Inc.; 2018. Cardiovascular System, in: Fundam. Toxicol. Pathol. pp. 153–194. [DOI] [Google Scholar]
- 54.Cross M.J., Berridge B.R., Clements P.J.M., Cove-Smith L., Force T.L., Hoffmann P., Holbrook M., Lyon A.R., Mellor H.R., Norris A.A., Pirmohamed M., Tugwood J.D., Sidaway J.E., Park B.K. Physiological, pharmacological and toxicological considerations of drug-induced structural cardic injury. Br. J. Pharmacol. 2015;172:957–974. doi: 10.1111/bph.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunnick J.K., Lieuallen W., Moyer C., Orzech D., Nyska A. Cardiac Damage in Rodents after Exposure to Bis (2-chloroethoxy) methane. Toxicol. Pathol. 2004;32:309–317. doi: 10.1080/01926230490431501. [DOI] [PubMed] [Google Scholar]
- 56.Dunnick J., Johnson J., Horton J., Nyska A. Bis (2-chloroethoxy) methane-induced mitochondrial and myofibrillar damage : short-term time-course study. Toxicol. Sci. 2004;81:243–252. doi: 10.1093/toxsci/kfh194. [DOI] [PubMed] [Google Scholar]
- 57.Nyska A., Murphy E., Foley J.F., Collins B.J., Petranka J., Howden R., Hanlon P., Dunnick J.K. Acute hemorrhagic myocardial necrosis and sudden death of rats exposed to a combination of ephedrine and caffeine. Toxicol. Sci. 2005;83:388–396. doi: 10.1093/toxsci/kfi034. [DOI] [PubMed] [Google Scholar]
- 58.Jokinen M.P., Lieuallen W.G., Johnson C.L., Dunnick J., Nyska A. Characterization of spontaneous and chemically induced cardiac lesions in rodent model systems: the national toxicology program experience. Cardiovasc. Toxicol. 2005;5:227–244. doi: 10.1385/CT:5:2:227. [DOI] [PubMed] [Google Scholar]
- 59.Jokinen M.P., Lieuallen W.G., Boyle M.C., Johnson C.L., Malarkey D.E., Nyska A. Morphologic aspects of rodent cardiotoxicity in a retrospective evaluation of national toxicology program studies. Toxicol. Pathol. 2011;39:850–860. doi: 10.1177/0192623311413788. [DOI] [PubMed] [Google Scholar]
- 60.Kumar K., Holden W.E. Drug- nduced pulmonary vascular disease- mechanisms and clinical patterns. Clin. Med. (Northfield. Il) 1986;145:343–349. [PMC free article] [PubMed] [Google Scholar]
- 61.Marchertienė I., Macas A., Karbonskienė A. Pulmonary edema and hemorrhage as complications of acute airway obstruction following anesthesia. Medicina (B. Aires) 2008;44:871. doi: 10.3390/medicina44110110. [DOI] [PubMed] [Google Scholar]
- 62.Carvalho A.L.N., Annoni R., Torres L.H.L., Santos A.C.C., Shimada A.L.B., Almeida F.M., Hebeda C.B., Lopes F.D.T.Q., Dolhnikoff M., Martins M.A., Silva L.F.F., Farsky S.H.P., Saldiva P.H.N., Ulrich C.M., Owen R.W., Marcourakis T., Trevisan M.T.S., Mauad T. Anacardic acids from cashew nuts ameliorate lung damage induced by exposure to diesel exhaust particles in mice, evidence-based complement. Altern. Med. 2013;2013:1–13. doi: 10.1155/2013/549879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morais T.C., Pinto N.B., Maria K., Carvalho M.B., Rios J.B., Maria N., Ricardo P.S., Teresa M., Trevisan S., Rao V.S., Santos F.A. Chemico-Biological Interactions Protective effect of anacardic acids from cashew (Anacardium occidentale) on ethanol-induced gastric damage in mice. Chem. Biol. Interact. 2010;183:264–269. doi: 10.1016/j.cbi.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Hajra S., Patra A.R., Basu A., Bhattacharya S. Prevention of doxorubicin (DOX) -induced genotoxicity and cardiotoxicity : effect of plant derived small molecule indole-3-carbinol (I3C) on oxidative stress and inflammation. Biomed. Pharmacother. 2018;101:228–243. doi: 10.1016/j.biopha.2018.02.088. [DOI] [PubMed] [Google Scholar]
- 65.Ribeiro J.C., Antunes L.M.G., Aissa A.F., Darin J.D.C., De Rosso V.V., Mercadante A.Z., de M., Bianchi L.P. Evaluation of the genotoxic and antigenotoxic effects after acute and subacute treatments with açai pulp (Euterpe oleracea Mart.) on mice using the erythrocytes micronucleus test and the comet assay. Mutat. Res. Toxicol. Environ. Mutagen. 2010;695:22–28. doi: 10.1016/j.mrgentox.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Vilas Boas G.R., Lemos J.M.R., De Oliveira M.W., Dos Santos R.C., Da Silva A.P.S., Bacha F.B., Ito C.N.A., Cornelius E.B., Lima F.B., Rodrigues A.M.S., Costa N.B., Bittencourt F.F., de Lima F.F., Paes M.M., Gubert P., Oesterreich S.A. Preclinical safety evaluation of the aqueous extract from Mangifera indica Linn. (Anacardiaceae): genotoxic, clastogenic and cytotoxic assessment in experimental models of genotoxicity in rats to predict potential human risks. J. Ethnopharmacol. 2019;243:1–12. doi: 10.1016/j.jep.2019.112086. [DOI] [PubMed] [Google Scholar]