Abstract
Background:
Preconditioning intensity, donor choice and graft-versus-host disease (GVHD) prophylaxis of allogeneic hematopoietic cell transplantation (allo-HCT) for advanced myelofibrosis (MF) have not been fully elucidated.
Methods:
Thirty-five patients with advanced MF were treated with reduced-intensity conditioning (RIC) allo-HCT. We searched for matched sibling donors first, followed by matched or mismatched unrelated donors and familial mismatched donors. Preconditioning regimen consisted of fludarabine (total 150 mg/m2) and busulfan (total 6.4 mg/kg) with total body irradiation ⩽400cGy.
Results:
All showed engraftments, but four showed either leukemic relapse or delayed graft failure. Two-year overall survival (OS) and non-relapse mortality (NRM) was 60.0% and 29.9%, respectively. Acute GVHD was observed in 19 patients, and grade III–IV acute GVHD (eight grade III and four grade IV) was higher in human leukocyte antigen (HLA)-mismatched donor HCT compared with HLA-matched HCT (70% versus 20%). Chronic GVHD was observed in 16 patients, and a cumulative incidence of severe chronic GVHD was 33% in HLA-mismatched donor HCT and 7.7% in HLA-matched HCT. Significant hepatic GVHD was observed in nine patients (five acute, four chronic) and six of them died. Multivariate analysis revealed inferior OS in HLA-mismatched donor HCT (hazard ratio (HR) = 6.40, 95% confidence interval (CI) 1.6–25.7, p = 0.009) and in patients with high ferritin level at the time of pre-conditioning period (HR = 7.22, 95% CI 1.9–27.5, p = 0.004), which were related to higher incidence of hepatic GVHD with high NRM rate.
Conclusion:
RIC allo-HCT can be a valid choice providing graft-versus-fibrosis effect for advanced MF patients. However, HLA-mismatched donor and high pre-HCT ferritin level related to fatal hepatic GVHD should be regarded as poor-risk parameters.
Keywords: allogeneic, hematopoietic cell transplantation, myelofibrosis, reduced intensity conditioning
Introduction
Although novel agents for myelofibrosis (MF) have been developed, few have been shown to produce morphologic, cytogenetic or molecular remissions, and drug resistance or significant toxicities are also challenging.1–3 Particularly for patients with advanced MF, only allogeneic hematopoietic cell transplantation (allo-HCT) has shown the potential for cure or prolongation of survival,4,5 and post-HCT outcomes have been much improved with the development of transplantation techniques. In the era of Janus kinase (JAK) inhibitors, transplantation may be delayed in good responders, but cytopenia is a major complication, and we confront refractory patients who should be treated with allo-HCT immediately.6 However, substantial risk of transplantation-related non-relapse mortality (NRM) is still a considerable obstacle, especially in elderly patients or in patients with severe comorbidity.6,7 In addition, the role of allo-HCT was shown to be limited in patients with far advanced MF, including leukemic transformation such as accelerated or blastic phase MF.8,9
For identification of transplantation-related parameters affecting survival outcomes, several comparative studies have been conducted. First, when the intensity of the conditioning regimen was studied, a myeloablative conditioning (MAC) regimen showed trends toward higher incidence of NRM,10 but overall outcomes were similar between a reduced-intensity conditioning (RIC) regimen and a MAC regimen.11 Interestingly, in both intensity regimens, the most affecting factor for survival outcome was a donor-related issue. Previous data showed that donors other than matched sibling donors (MSDs), which included matched unrelated donors (MUDs), mismatched unrelated donors (MMUDs) and haploidentical familial donors (HIDs), showed significantly poorer survival outcomes.10–14 Some recent data, however, showed that the outcomes of allo-HCT from unrelated or alternative donors were much improved these days along with the development of transplantation techniques.15
We performed allo-HCT using a RIC regimen for advanced MF patients with splenomegaly, refractory anemia or thrombocytopenia, significant constitutional symptoms, or leukemic transformation, and we sometimes tried a non-MAC regimen for elderly patients and/or patients with severe comorbidity. We achieved acceptable survival outcomes but also experienced unexpected transplantation-related complications and mortality. Here, we report transplantation outcomes in MF patients treated with RIC allo-HCT and suggest parameters affecting survival outcome in this cohort.
Patients and methods
Patients
From 2008 to 2018, a total of 35 consecutive adult patients with primary or secondary MF were treated with allo-HCT following RIC regimens in Catholic Hematology Hospital in South Korea. As many patients with transformation to secondary acute myeloid leukemia were referred to the acute leukemia department, we excluded patients with blastic phase MF in this study. This study was approved by the Institutional Review Board of The Catholic University of Korea (KC19RESI0428) and was conducted in accordance with the Declaration of Helsinki. Written informed consents were obtained from all patients.
Clinical parameters
We finally analyzed 35 patients treated with RIC regimen for identification of factors affecting outcomes. Among them, there were eight secondary MF cases following either polycythemia vera (n = 4) or essential thrombocytosis (n = 4). For the detection of karyotype, at least 20 metaphases from bone marrow (BM) cells were analyzed using the GTG banding method, and the International System for Cytogenetic Nomenclature16 and National Comprehensive Cancer Network were used as guidelines for classification. Spleen size was serially calculated by computed tomography, and complete blood count (CBC) and symptoms were assessed at every visit. Using those parameters, prognostic index was calculated using the International Prognostic Scoring System (IPSS), Dynamic IPSS (DIPSS) and DIPPS-plus at the time of diagnosis and before allogeneic-HCT. Since 2005, detection of JAK2V617F and JAK2 exon 12 gene mutations has been available in all patients. Except for JAK2 mutations, unfortunately, detection of CALR and MPL gene mutations were available starting in 2015. In addition, we focused on ferritin, and the highest levels were selected at the time of pre-conditioning period and D + 21 post-HCT.
Pre-transplant management and transplantation procedures
During follow-up before transplantation, transfusion was actively supported at the cut-off values of 7.5 g/dL for hemoglobin and 20.0 × 109/L for platelet count. In some patients without significant cytopenia, ruxolitinib was applied, and symptom assessment and CBC monitoring were actively performed at every visit. For patients with adverse-risk features such as symptomatic splenomegaly, refractory anemia or thrombocytopenia, significant constitutional symptoms, or leukemic transformation resulting in intermediate-2 or high-risk status based on the DIPPS-plus, we explained the need for allo-HCT and started preparing. We initially searched for available donors with a sequence of MSD and MUD and subsequently for alternative donors such as MMUD or HID. In all patients, we used peripheral blood as a stem cell source and RIC regimens which consisted of fludarabine (30 mg/m2 for 5 days) and busulfan (3.2 mg/kg for 2 days) with total body irradiation (TBI) 200–400 cGy. Anti-thymocyte globulin (ATG) was administered at a dose of 2.5–10 mg/kg according to the donor types (5 mg/kg or higher in unrelated or mismatched donor transplantation) and short course methotrexate and calcineurin inhibitors (cyclosporine for sibling donor transplants, tacrolimus for unrelated donor transplants) were used for graft-versus-host disease (GVHD) prophylaxis.
Statistical analysis
The main end points were overall survival (OS), NRM, and cumulative incidence of relapse or graft failure. Survival curves were plotted using the Kaplan–Meier method, and subgroups were compared by log-rank tests. Relapse, NRM and GVHD incidences were calculated using cumulative incidence estimates to accommodate the competing events, and subgroups were compared by the Gray test.17 The prognostic significance of covariates affecting the response rate was determined by multiple logistic regression, and covariates affecting OS were determined using the Cox proportional hazards regression model. Prognostic significances of covariates affecting NRM were determined using the Fine–Gray proportional hazards regression model. In these models, acute and chronic GVHD were considered as time-dependent covariates. Multivariate analyses were performed using variables with a p-value < 0.10 in prior univariate analyses. All statistical analyses were performed using ‘R’ software version 2.15.1 (R Foundation for Statistical Computing, 2012). Statistical significance was set at a p-value < 0.05.
Results
Baseline characteristics
We analyzed 35 patients with advanced MF (median age 55 years old, range 34–72) treated with allo-HCT following RIC regimen. They were divided into two subgroups according to the donor–recipient human leukocyte antigen (HLA) matched status (Table 1). There were 26 patients who received stem cells from an HLA-matched donor (16 from MSD and 10 from MUD), while nine received from an HLA-mismatched donor (five from MMUD and four from HID). Median age was 55 years old (range 34–72) in HLA-matched subgroup and 54 years old (range 54–68) in HLA-mismatched subgroup (p = 0.798), respectively. There were 16 (65.4%) male patients in HLA-matched subgroup and four (44.4%) in HLA-mismatched subgroup (p = 0.266), respectively. Between the two subgroups, except for a proportion of abnormal karyotype that was higher in the HLA-matched subgroup (42.3% versus 0%, p = 0.034), other various parameters were characteristically not different. Pre-HCT splenomegaly was observed in 16 (61.5%). The JAK2 mutation was observed in 16 (45.7%) patients (12 in HLA-matched, four in mismatched) and negativity for all mutations was observed in seven (20.0%) patients (six in HLA-matched, one in mismatched), respectively. Regarding the disease status, 19 (54.3%) were high-risk by IPSS at diagnosis, while 24 (68.6%) were high-risk by DIPPS-plus at the time of transplantation. There were four patients with accelerated phase MF harboring a blast count higher than 10%, and all of them were in the HLA-matched group. Ferritin levels at the time of pre-conditioning and post-HCT D + 21 and infused CD3+ and CD34+ cell counts were also not different between the two groups.
Table 1.
Baseline characteristics of MF patients treated with allogeneic-HCT following reduced-intensity conditioning regimens.
| HLA-match n = 26 |
HLA-mismatch n = 9 |
p (**<0.05) | |
|---|---|---|---|
| Age, years old, median | 55 (34–72) | 54 (54–68) | 0.798 |
| Age <40 years old | 17 (65.4%) | 6 (66.7%) | 0.706 |
| Age ⩾40 years old | 9 (34.6%) | 3 (33.3%) | |
| Gender (male) | 16 (61.5%) | 4 (44.4%) | 0.266 |
| Secondary MF | 6 (23.0%) | 2 (22.2%) | 1.000 |
| Post-PV MF | 3 (11.5%) | 1 (11.1%) | |
| Post-ET MF | 3 (11.5%) | 1 (11.1%) | |
| Splenomegaly | 18 (69.2%) | 5 (55.5%) | 0.712 |
| Splenectomy | 2 (7.7%) | 1 (11.1%) | |
| Pre-HCT splenomegaly | 16 (61.5%) | 4 (44.4%) | |
| Mutation status | |||
| Not assessed* | 4 (15.4%) | 2 (22.2%) | 0.950 |
| JAK2 | 12 (46.1%) | 4 (44.4%) | |
| CALR | 3 (11.5%) | 2 (22.2%) | |
| MPL | 1 (3.8%) | 0 (0.0%) | |
| Triple negative | 6 (23.0%) | 1 (11.1%) | |
| Cytogenetics | |||
| Normal | 15 (57.7%) | 9 (100%) | 0.034** |
| Abnormal | 11 (42.3%) | 0 (0.0%) | |
| IPSS at diagnosis | |||
| High | 15 (57.7%) | 4 (44.4%) | 0.873 |
| Intermediate-2 | 8 (30.8%) | 3 (33.3%) | |
| Intermediate-1 | 3 (11.5%) | 2 (22.2%) | |
| Dynamic IPSS plus at pre-HCT | |||
| High | 16 (61.5%) | 8 (88.9%) | 0.218 |
| Intermediate-2 | 10 (38.5%) | 1 (11.1%) | |
| Time to transplantation, months | 11.3 (2.9–180.6) | 8.8 (4.0–97.8) | 0.688 |
| HCT-CI | 0.784 | ||
| 0–1 | 10 (38.5%) | 3 (33.3%) | |
| ⩾2 | 16 (61.5%) | 6 (66.7%) | |
| Pre-HCT blast counts | 0.218 | ||
| Blast count 5–9% | 5 (19.2%) | 0 (0.0%) | |
| Blast count 10–19% | 4 (15.4%) | 0 (0.0%) | |
| Ferritin level, median, ng/mL | |||
| At the time of pre-conditioning | 1372 (19–5802) | 1473 (209–8376) | 0.971 |
| Post-transplant D + 21 | 2565 (573–68,951) | 3450 (2064–15,556) | 0.116 |
| Donor type | |||
| Matched sibling donor | 16 (61.5%) | 0 (0.0%) | – |
| Matched unrelated donor | 10 (38.4%) | 0 (0.0%) | |
| Mismatched unrelated donor | 0 (0.0%) | 5 (55.5%) | |
| Haploidentical donor | 0 (0.0%) | 4 (44.4%) | |
| ABO | |||
| Match | 16 (61.5%) | 3 (33.3%) | 0.266 |
| Major mismatch | 6 (23.0%) | 4 (44.4%) | |
| Minor mismatch | 4 (15.4%) | 2 (22.2%) | |
| CD34+ (× 106//kg) | 5.97 (2.6–11.8) | 7.99 (3.3–24.1) | 0.258 |
| CD3+ (× 106/kg) | 361.1 (105.9–748.0) | 265.1 (147.4–1022.4) | 0.270 |
All were JAK2-negative, while CALR and MPL mutations were not fully evaluated.
ET, essential thrombocytosis; HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic stem cell transplantation-comorbidity index; HLA, human leukocyte antigen; IPSS, international prognostic scoring system; MF, myelofibrosis; PV, polycythemia vera
CBC recovery, MF improvement and incidence of GVHD
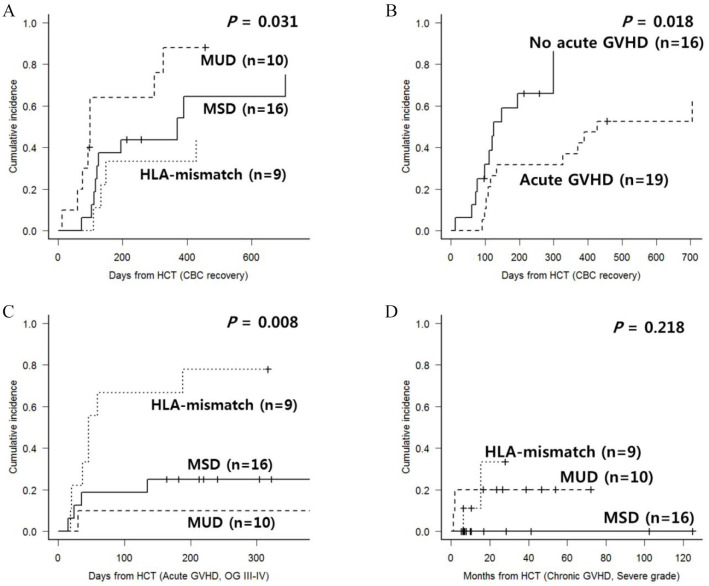
All analyzed patients showed successful engraftment. After engraftment, BM biopsy was performed at D + 30, 6 months, 12 months, 18 months and 2 years post-HCT. Among the 31 evaluable patients by BM biopsy, MF improvement by at least one grade was observed in 23 (74.2%) patients with a median duration of 122 days (range, 25–661), and recipient–donor HLA status was not a factor that affected MF improvement. We defined full CBC recovery as hemoglobin >10 g/dL, platelet >100 × 109/L and neutrophil count >1.0 × 109/L, and full CBC recovery was observed in 28 (80.0%) patients with a median duration of 299 days (range, 12–705). In the HLA-mismatched subgroup, the proportion of patients achieving full CBC recovery was smaller and the recovery was later compared with the HLA-matched subgroup (Figure 1(A)). Among various transplant-related parameters, patients with acute GVHD showed delayed full CBC recovery (Figure 1(B)). Acute GVHD was observed in 19 patients (one grade I, six grade II, eight grade III and four grade IV), and a cumulative incidence of grade III–IV acute GVHD was significantly higher after HLA-mismatched HCT (Figure 1(C), 70% versus 20%, p = 0.008). Chronic GVHD was observed in 16 patients (five mild, seven moderate, four severe), and a cumulative incidence of severe chronic GVHD was relatively higher after HLA-mismatched HCT (Figure 1(D), 33.3% versus 7.7%, p = 0.218). Significant hepatic GVHD was observed in nine patients (five acute and four chronic).
Figure 1.
(A) CBC recovery according to donor and HLA-matching status; (B) CBC recovery according to the occurrence of acute GVHD; (C) incidence of grade III–IV acute GVHD according to donor and HLA-matching status; and (D) incidence of severe chronic GVHD according to donor and HLA-matching status.
CBC, complete blood count; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; HLA, human leukocyte antigen; MSD, matched sibling donor; MUD, matched unrelated donor; OG, overall grade.
Clinical outcomes according to HLA disparity
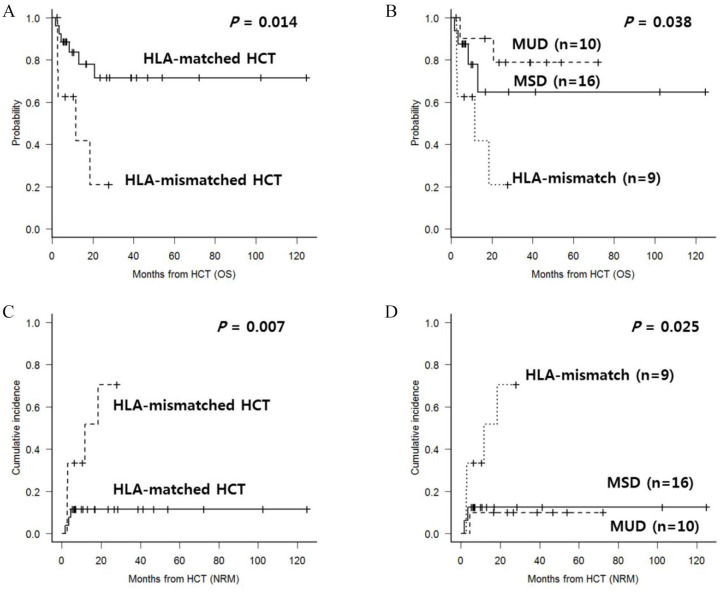
Of a total of 35 patients, one showed delayed GF and three relapsed with leukemic blasts in the formation of accelerated phase MF (overall 11.4%). A patient with GF was treated with a second allo-HCT, and the other two relapsed patients were treated with donor lymphocyte infusion, but all of them died due to GVHD or progressive disease. Apart from the patients with relapse or graft failure (GF), nine additional patients died due to transplantation-related complications with an estimated two-year NRM of 29.9%. Among these, six died due to fatal hepatic GVHD, one died of lung GVHD and two died due to septic pneumonia. After a median follow-up duration of 24.6 months (range, 6.1–124.8), two-year OS was 60.0%, and 56.2% for the triple-negative subgroup. We found that the HLA-mismatched group showed a significantly inferior survival outcome compared with the HLA-matched group (Figure 2(A), 20.8% versus 71.5%, p = 0.014). The difference between outcomes for MSD and MUD was not significant (Figure 2(B), 78.7% versus 64.2%, p = 0.862). A poor outcome was mainly caused by NRM, which was significantly higher in the HLA-mismatched group (Figure 2(C), 70.4% versus 11.5%, p = 0.007). Also, cumulative incidence of NRM was not significantly different between MSD and MUD (Figure 2(D), 12.5% versus 10.0%, p = 0.981). Final multivariate analysis (Table 2) showed that HLA-mismatched transplantation yielded poorer OS (hazard ratio (HR) = 6.40, 95% confidence interval (CI) 1.6–25.7, p = 0.009) and a higher NRM rate (HR = 12.1, 95% CI 1.8–77.6, p = 0.008) compared with the HLA-matched group.
Figure 2.
(A) OS according to HLA-match status; (B) OS according to donor types; (C) NRM according to HLA-match status; and (D) NRM according to donor types.
HCT, hematopoietic cell transplantation; HLA, human leukocyte antigen; MSD, matched sibling donor; MUD, matched unrelated donor; NRM, non-relapse mortality; OS, overall survival
Table 2.
Multivariate analysis of factors affecting overall survival.
| Variables | Overall survival |
Non-relapse mortality |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| % at 2 years | p | HR (95% CI) | p | % at 2 years | p | HR (95% CI) | p | |
| Age | ||||||||
| <40 years (n = 23) | 51.9 | 0.238 | 28.6 | 0.608 | ||||
| ⩾40 years (n = 12) | 78.6 | 21.4 | ||||||
| Gender | ||||||||
| Male (n = 20) | 62.2 | 0.683 | 30.0 | 0.232 | ||||
| Female (n = 15) | 54.0 | 25.2 | ||||||
| Karyotype | ||||||||
| Normal (n = 24) | 55.4 | 0.481 | 28.0 | 0.781 | ||||
| Abnormal (n = 11) | 81.8 | 18.2 | ||||||
| DIPSS-plus | ||||||||
| Intermediate-2 (n = 11) | 65.5 | 0.845 | 18.2 | 0.780 | ||||
| High (n = 24) | 58.3 | 28.6 | ||||||
| Pre-HCT blast count | ||||||||
| <5% (n = 26) | 56.7 | 0.706 | 34.0 | 0.266 | ||||
| ⩾5% (n = 9) | 66.7 | 11.1 | ||||||
| HCT-CI | ||||||||
| 0–1 (n = 13) | 72.5 | 0.360 | 26.0 | 0.784 | ||||
| ⩾2 (n = 22) | 49.7 | 30.9 | ||||||
| Splenomegaly at pre-HCT | ||||||||
| Splenomegaly (+) (n = 23) | 64.0 | 0.999 | 28.0 | 0.563 | ||||
| Splenomegaly (–) (n = 9) | 59.3 | 25.9 | ||||||
| Splenectomy (n = 3) | 50.0 | 0.0 | ||||||
| Mutation | ||||||||
| Not assessed (n = 6) | 66.7 | 0.987 | 33.3 | 0.818 | ||||
| No triple negative (n = 22) | 52.6 | 14.3 | ||||||
| Triple-negative (n = 7) | 64.3 | 30.4 | ||||||
| HLA-match | ||||||||
| Match (n = 26) | 71.5 | 0.014 | 1 | 11.5 | 0.007 | 1 | ||
| Mismatch (n = 9) | 20.8 | 6.40 (1.6–25.7) | 0.009 | 70.4 | 12.1 (1.8–77.6) | 0.008 | ||
| Ferritin level at the time of pre-conditioning | ||||||||
| <4000 ng/mL (n = 26) | 68.1 | 0.004 | 1 | 17.8 | 0.006 | 1 | ||
| ⩾4000 ng/mL (n = 9) | 33.3 | 7.22 (1.9–27.5) | 0.004 | 55.6 | 12.9 (3.4–48.9) | <0.001 | ||
| Acute GVHD | ||||||||
| Grade I–III (n = 23) | 72.1 | 0.026 | 8.7 | 0.006 | ||||
| Grade IV (n = 12) | 34.2 | 60.6 | ||||||
| Chronic GVHD | ||||||||
| Mild to moderate (n = 11) | 100.0 | 0.002 | 0.0 | 0.017 | ||||
| None/severe (n = 24) | 31.7 | 47.3 | ||||||
CI, confidence interval; DIPSS, Dynamic International Prognostic Scoring System; GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; HCT-CI, hematopoietic stem cell transplantation-comorbidity index; HLA, human leukocyte antigen; HR, hazard ratio
Parameters affecting outcomes after RIC allo-HCT
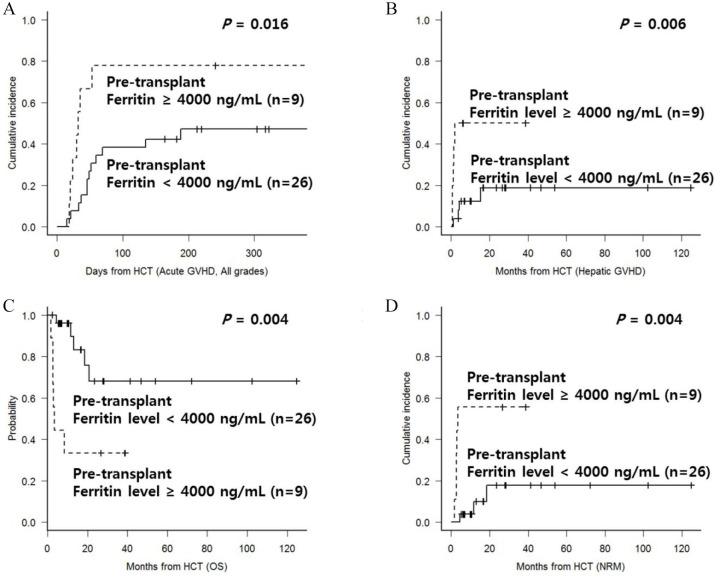
Multivariate analysis for parameters affecting OS and NRM was conducted. We found that age, karyotype abnormality at diagnosis, DIPPS-plus score, pre-HCT blast count, splenomegaly or splenectomy prior to HCT, and genetic mutations were not significant for transplantation outcomes in this cohort. In addition to HLA-mismatching as mentioned above, our data revealed that grade IV acute GVHD showed poorer OS with higher NRM, while patients with mild to moderate chronic GVHD showed superior OS with lower NRM compared with patients with either severe chronic GVHD or no chronic GVHD. We also determined that higher ferritin levels at the time of pre-conditioning period and post-HCT D + 21 were both associated with higher incidence of acute GVHD and hepatic GVHD, which was in turn associated with higher NRM rate. We calculated the significant cut-off of ferritin levels by using the receiver operation curve method and identified that higher ferritin levels at both time points were significantly associated with poor survival outcomes. Patients with ferritin ⩾4000 ng/mL at the time of pre-conditioning period showed a higher incidence of acute GVHD (Figure 3(A), 51.9% versus 19.6%, p = 0.016) and hepatic GVHD specifically (Figure 3(B), 55.6% versus 17.8%, p = 0.006). Finally, two-year OS was poorer in patients with ferritin ⩾ 4000 ng/mL at the time of the pre-conditioning period (Figure 3(C), 33.3% versus 68.1%, p = 0.004) with a higher two-year NRM rate (Figure 3(D), 55.6% versus 17.8%, p = 0.004) compared with patients with lower ferritin levels. Final multivariate analysis (Table 2) also revealed that higher ferritin levels at the time pre-conditioning showed poorer OS (HR = 7.22, 95% CI 1.9–27.5, p = 0.004) with a higher NRM rate (HR = 12.9, 95% CI 3.4–48.9, p < 0.001).
Figure 3.
(A) Cumulative incidence of acute GVHD according to ferritin level at the time of pre-transplant period; (B) cumulative incidence of any hepatic GVHD according to ferritin level at the time of pre-transplant period; (C) OS according to ferritin level at the time of pre-transplant period; and (D) NRM according to ferritin level at the time of pre-transplant period.
GVHD, graft-versus-host disease; HCT, hematopoietic cell transplantation; NRM, non-relapse mortality; OS, overall survival
Discussion
In early transplantations in MF patients, high risks for GF, GVHD, regimen-related toxicities, and higher NRM rates were significantly higher compared with other hematologic disorders, especially in elderly patients.18–22 Those previous reports also reported a significant proportion of GF in up to greater than 25% of patients, and many of the patients were treated with donor lymphocyte infusion due to incomplete chimerism. Therefore, transplantation outcomes according to the conditioning intensity and the donor types were analyzed in several studies.11 We cannot make a solid conclusion between MAC versus RIC regimens but RIC regimens are regarded as a feasible choice for patients with comorbid conditions, while many data revealed that unrelated or HLA-mismatched donors showed poor survival outcomes.11,12,23 Thus, we still confront severe complications after allo-HCT and the major affecting factors are significant GVHD, hepatotoxicity and HLA disparity, which are all suggested in our data.
We analyzed transplantation outcomes of our advanced MF patients treated with RIC allo-HCT and the estimated 2-year OS was 60%. We experienced only one delayed GF, while almost 30% of mortality was NRM, which mainly consisted of hepatic GVHD related to HLA-mismatched transplantation. Among them, nine patients who received stem cells from an HLA-mismatched donor showed poor survival outcomes. Fortunately, our data showed only one delayed GF treated with second transplantation. We suggest that the GF might possibly be overcome with a TBI-based conditioning regimen, which was similarly reported by previous Canadian data.24 However, the previous data and our data both showed increased significant GVHD and hepatotoxicity including hepatic GVHD, which also might be caused by TBI.
For hepatotoxicity, although a TBI-related regimen might be one of the possible causes, a pathophysiological mechanism of portal hypertension and venous thrombosis, or previous hepatic iron overload and busulfan-related issues are more likely to be considered.21,22 However, there was no case of hepatic sinusoidal obstruction syndrome in our cohort, which could be explained by several transplantation-related factors and should be studied. Nevertheless, high-incidence hepatic GVHD has emerged, especially with higher mortality. Therefore, we are planning to change our regimen to a melphalan-based regimen, which includes a reduction in the TBI dose or an increase in the dose of ATG, especially in cases of HLA-mismatched transplantation. For GVHD prophylaxis, many transplantation centers have recently used post-transplant cyclophosphamide (PTCY) method, especially in HID transplantation, and the incidence of GVHD is reported lower than previous methods.25 Based on this concept, we can also use PTCY concomitant with or instead of ATG, which can give totally different results.
Among those affecting factors which have been considered for hepatotoxicity, we focused on ferritin levels at the time of pre-conditioning period and at post-HCT D + 21, after which subsequent GVHD complications developed. We found that higher ferritin levels at both time points were associated with a higher incidence of subsequent acute and/or hepatic GVHD, which caused higher NRM rates, resulting in poorer OS. Iron overload is common in patients with hematological disease undergoing allo-HCT, and several studies have already shown that a high pre-HCT level of ferritin was related with early D + 100 mortality, poorer OS and higher incidence of acute GVHD or hepatotoxicity including sinusoidal obstruction syndromes or infectious complications.26,27 The cause of toxicity is attributed to the increased level of hydroxyl radicals by non-transferritin bound iron after exposure to toxic conditioning regimens,28,29 and the effects of iron chelation during pre- and post-HCT periods have been evaluated for reduction of those severe acute complications such as immune reconstitution.30,31 It may be the concern of future studies whether more meticulous iron chelation prior to transplantation could improve the transplant outcome.
Several recent studies already showed that leukemic transformations of myeloproliferative neoplasms are hardly cured even after allo-HCT.8,9 In our data, there were no patients with blastic phase MF with 20% or higher blast counts, but there were four patients with accelerated phase MF. Of them, two patients with nearly 20% blast counts relapsed and died within 6 months post-HCT, while patients whose blast counts were around 10% showed no relapse until 2 years post-HCT. Our observations suggest that patients who begin to show increased leukemic blasts should be actively monitored and should more actively prepare for allo-HCT. More preemptive approaches such as prophylactic donor lymphocyte infusion should be considered for these patients. Furthermore, as previous data showed with blastic phase MF, so called secondary acute myeloid leukemia is hardly cured even with conventional allo-HCT; additional novel agents or immunotherapy should be added with the development of sophisticated transplantation strategies.8
Driver mutations including JAK2, CALR and MPL and additional somatic deoxyribonucleic acid (DNA) mutations have been studied in many reports related to natural disease courses with a risk of thrombosis and transplantation outcomes.32–34 Although the role of mutations has yet to be clearly elucidated, survival outcomes of patients with more than three mutations, including ASXL1, EZH2, SRSF2 or IDH mutations, and patients in the triple-negative group are considered poor even after allo-HCT, while patients with the CALR mutation were found to have the best prognosis.33,35–37 Unfortunately, our data could not identify the role of mutations for survival outcomes after allo-HCT because not all driver mutations and detailed DNA mutations were fully evaluated in patients. Overall, no specific mutations or triple-negative status were associated with significantly better or poorer transplantation outcomes in this study, which revealed a 2-year OS of 56.2% in eight patients with triple-negative mutations.
Although the results originated from retrospective analysis and the role of genetic mutations could not be fully analyzed due to the unavailability of complete data, our observations were based on consistent and uniform strategies in terms of pre-HCT therapy, donor searching sequence, conditioning regimens, immunosuppressive agents and supportive care without significant change over time. In conclusion, our data showed that RIC allo-HCT can be a valid choice to provide the graft-versus-fibrosis effect for patients with advanced and triple-negative MF. However, HLA-mismatched donors and high ferritin levels were associated with a higher incidence of acute GVHD including hepatic GVHD and a higher NRM rate, which finally caused poor survival outcomes. Those factors should be deeply considered in current practice and in future clinical trials related to detailed genetic mutation studies.
Footnotes
Author contribution: JHY contributed to reviewing patients and writing the manuscript; GJM, SSP, SP, SEL, BSC, YJK supported the treatment course of those patients; and the manuscript was critically reviewed by HJK, CKM, SGC, JWL and SL. KSE, as a corresponding author, has been in charge of management for patients and wrote the manuscript.
Availability of data and materials: All data generated or analyzed during this study are included in this published article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: This research was conducted in accordance with the Institutional Review Board guidelines of the Catholic Medical Center (KC19RESI0428).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent: Written informed consent was obtained at the stage of transplantation in all patients.
ORCID iDs: Jae-Ho Yoon  https://orcid.org/0000-0002-2145-9131
https://orcid.org/0000-0002-2145-9131
Jong Wook Lee  https://orcid.org/0000-0003-2949-4166
https://orcid.org/0000-0003-2949-4166
Contributor Information
Jae-Ho Yoon, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Gi June Min, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Sung-Soo Park, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Silvia Park, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Sung-Eun Lee, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Byung-Sik Cho, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Yoo-Jin Kim, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Seok Lee, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Hee-Je Kim, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Chang-Ki Min, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Seok-Goo Cho, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Jong Wook Lee, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
Ki-Seong Eom, Department of Hematology, Catholic Hematology Hospital and Leukemia Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, 222, Banpo-daero, Seocho-gu, Seoul 06591, Republic of Korea.
References
- 1. Tefferi A. Primary myelofibrosis: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 2. Newberry KJ, Patel K, Masarova L, et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood 2017; 130: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polverelli N, Breccia M, Benevolo G, et al. Risk factors for infections in myelofibrosis: role of disease status and treatment. A multicenter study of 507 patients. Am J Hematol 2017; 92: 37–41. [DOI] [PubMed] [Google Scholar]
- 4. Kroger NM, Deeg JH, Olavarria E, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia 2015; 29: 2126–2133. [DOI] [PubMed] [Google Scholar]
- 5. Kekre N, Ho VT. Allogeneic hematopoietic stem cell transplantation for myelofibrosis and chronic myelomonocytic leukemia. Am J Hematol 2016; 91: 123–130. [DOI] [PubMed] [Google Scholar]
- 6. Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood 2012; 120: 1367–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mclornan DP, Mead AJ, Jackson G, et al. Allogeneic stem cell transplantation for myelofibrosis in 2012. Br J Haematol 2012; 157: 413–425. [DOI] [PubMed] [Google Scholar]
- 8. Scherber RM, Mesa RA. Managing myelofibrosis (MF) that “blasts” through: advancements in the treatment of relapsed/refractory and blast-phase MF. Hematology Am Soc Hematol Educ Program 2018; 2018: 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tefferi A, Mudireddy M, Mannelli F, et al. Blast phase myeloproliferative neoplasm: mayo-agimm study of 410 patients from two separate cohorts. Leukemia 2018; 32: 1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant 2010; 16: 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mclornan DP, Yakoub-Agha I, Robin M, et al. State-of-the-art review: allogeneic stem cell transplantation for myelofibrosis in 2019. Haematologica 2019; 104: 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta V, Malone AK, Hari PN, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2014; 20: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rondelli D, Goldberg JD, Isola L, et al. Mpd-Rc 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood 2014; 124: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keyzner A, Han S, Shapiro S, et al. Outcome of allogeneic hematopoietic stem cell transplantation for patients with chronic and advanced phase myelofibrosis. Biol Blood Marrow Transplant 2016; 22: 2180–2186. [DOI] [PubMed] [Google Scholar]
- 15. Bregante S, Dominietto A, Ghiso A, et al. Improved outcome of alternative donor transplantations in patients with myelofibrosis: from unrelated to haploidentical family donors. Biol Blood Marrow Transplant 2016; 22: 324–329. [DOI] [PubMed] [Google Scholar]
- 16. Shaffer LG, Slovak ML, Campbell LJ, et al. An international system for human cytogenetic nomenclature. Basel, Switzerland, 2009. [Google Scholar]
- 17. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141–1154. [Google Scholar]
- 18. Kroger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the chronic leukemia working party of the European group for blood and marrow transplantation. Blood 2009; 114: 5264–5270. [DOI] [PubMed] [Google Scholar]
- 19. Abelsson J, Merup M, Birgegard G, et al. The outcome of Allo-Hsct for 92 patients with myelofibrosis in the Nordic countries. Bone Marrow Transplant 2012; 47: 380–386. [DOI] [PubMed] [Google Scholar]
- 20. Ditschkowski M, Elmaagacli AH, Trenschel R, et al. Dynamic international prognostic scoring system scores, pre-transplant therapy and chronic graft-versus-host disease determine outcome after allogeneic hematopoietic stem cell transplantation for myelofibrosis. Haematologica 2012; 97: 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wong KM, Atenafu EG, Kim D, et al. Incidence and risk factors for early hepatotoxicity and its impact on survival in patients with myelofibrosis undergoing allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1589–1599. [DOI] [PubMed] [Google Scholar]
- 22. Rezvani AR, Mccune JS, Storer BE, et al. Cyclophosphamide followed by intravenous targeted busulfan for allogeneic hematopoietic cell transplantation: pharmacokinetics and clinical outcomes. Biol Blood Marrow Transplant 2013; 19: 1033–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ciftciler R, Goker H, Demiroglu H, et al. Comparison of myeloablative versus reduced-intensity conditioning regimens for allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia: a cohort study. Turk J Haematol 2019; 36: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shanavas M, Messner HA, Atenafu EG, et al. Allogeneic hematopoietic cell transplantation for myelofibrosis using fludarabine-, intravenous busulfan- and low-dose Tbi-based conditioning. Bone Marrow Transplant 2014; 49: 1162–1169. [DOI] [PubMed] [Google Scholar]
- 25. Teofili L, Chiusolo P, Valentini CG, et al. Bone marrow haploidentical transplant with post-transplantation cyclophosphamide: does graft cell content have an impact on main clinical outcomes? Cytotherapy 2020; 22: 158–165. [DOI] [PubMed] [Google Scholar]
- 26. Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2008; 42: 799–805. [DOI] [PubMed] [Google Scholar]
- 27. Maradei SC, Maiolino A, De Azevedo AM, et al. Serum ferritin as risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood 2009; 114: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 28. Bradley SJ, Gosriwitana I, Srichairatanakool S, et al. Non-transferrin-bound iron induced by myeloablative chemotherapy. Br J Haematol 1997; 99: 337–343. [DOI] [PubMed] [Google Scholar]
- 29. Sahlstedt L, Ebeling F, Von Bonsdorff L, et al. Non-transferrin-bound iron during allogeneic stem cell transplantation. Br J Haematol 2001; 113: 836–838. [DOI] [PubMed] [Google Scholar]
- 30. Mckay PJ, Murphy JA, Cameron S, et al. Iron overload and liver dysfunction after allogeneic or autologous bone marrow transplantation. Bone Marrow Transplant 1996; 17: 63–66. [PubMed] [Google Scholar]
- 31. Cho BS, Jeon YW, Hahn AR, et al. Improved survival outcomes and restoration of graft-vs-leukemia effect by Deferasirox after allogeneic stem cell transplantation in acute myeloid leukemia. Cancer Med 2019; 8: 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alchalby H, Badbaran A, Zabelina T, et al. Impact of Jak2v617f mutation status, allele burden, and clearance after allogeneic stem cell transplantation for myelofibrosis. Blood 2010; 116: 3572–3581. [DOI] [PubMed] [Google Scholar]
- 33. Panagiota V, Thol F, Markus B, et al. Prognostic effect of calreticulin mutations in patients with myelofibrosis after allogeneic hematopoietic stem cell transplantation. Leukemia 2014; 28: 1552–1555. [DOI] [PubMed] [Google Scholar]
- 34. Salit RB, Deeg HJ. Transplant decisions in patients with myelofibrosis: should mutations be the judge? Biol Blood Marrow Transplant 2018; 24: 649–658. [DOI] [PubMed] [Google Scholar]
- 35. Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the international working group for myelofibrosis research and treatment. Blood 2009; 113: 2895–290. [DOI] [PubMed] [Google Scholar]
- 36. Abbas S, Lugthart S, Kavelaars FG, et al. Acquired mutations in the genes encoding Idh1 and Idh2 both are recurrent aberrations in acute myeloid leukemia: prevalence and prognostic value. Blood 2010; 116: 2122–2126. [DOI] [PubMed] [Google Scholar]
- 37. Patel KP, Newberry KJ, Luthra R, et al. Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood 2015; 126: 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]