Abstract
Background:
Azithromycin 1% and 1.5% ophthalmic preparations are used widely in clinical practice for the treatment of signs and symptoms of eye diseases. The aim of this study was to render conclusive evidence by comparing the efficacy of azithromycin 1% and 1.5% over tobramycin 0.3% ophthalmic solutions for the treatment of eye diseases in a short duration in terms of bacterial resolution, the cure rate, and resolving clinical sign and symptoms.
Methods:
Systematic searches were performed in the electronic database (MEDLINE, Embase, Emcare, CINAHL, Scopus, PubMed, ProQuest, and Web of Science) and other sources. Multicenter randomized controlled trial studies conducted in English were identified and screened. Analysis of individual studies was conducted using the OpenMeta-analyst and Review Manager Version 5.3 software.
Results:
Eleven studies were included in the systematic review and meta-analysis. In clinical cure rate, azithromycin 1% and 1.5% eye drops were more effective than tobramycin 0.3% eye drops in short duration dosing (⩽5 days) with a twice-a-day regimen (relative risk = 1.13; 95% confidence interval: 1.008, 1.28), whereas on increased duration (>5 days), azithromycin is almost similarly as effective as tobramycin (relative risk = 1.007; 95% confidence interval: 0.96, 1.05). There was no significant difference in efficacy of bacterial resolution of azithromycin (1%, 1.5%) eye drops compared to tobramycin (0.3%) eye drops (relative risk = 0.99; 95% confidence interval: 0.96, 1.018). Azithromycin eye drops are effective in improving the signs and symptoms of eye disease.
Conclusion:
Azithromycin 1% or 1.5% is more effective in the clinical cure rate of eye disease than tobramycin 0.3% eye drops in ⩽5 days of treatment. It is also the best choice of treatment for improving the signs and symptoms of eye disease. So that we recommend clinicians to use azithromycin 1% or 1.5% eye drops.
Systematic Review Registration:
PROSPERO 2019 CRD42019139911
Keywords: Azithromycin, eye diseases, eye drops, tobramycin, efficacy
Introduction
Azithromycin is an acid-stable orally administered macrolide antimicrobial drug, structurally related to erythromycin, with a similar spectrum of antimicrobial activity.1 But it is particularly noted for its activity against several gram-negative organisms. Based on in vitro data, azithromycin is more active than erythromycin, clarithromycin, and roxithromycin against Haemophilus influenzae. It shows similar activity to erythromycin, clarithromycin, and roxithromycin against Moraxella catarrhalis (with good activity against β-lactamase-positive strains of this organism) and Streptococcus pneumoniae.2
Several clinical trials have proven that a 5-day course of azithromycin administered once a day is equally efficacious to a 7- to 14-day course of other commonly used oral antimicrobials, administered 2–4 times a day, for the treatment of upper and lower respiratory tract and skin and skin structure infections. Urethritis and cervicitis caused by chlamydia are treated with a single 1-g dose of azithromycin. Trials have shown azithromycin’s adverse-effect profile to be equal or even superior to that of other agents, with only 0.7% of patients discontinuing therapy versus 2.6% for comparable drugs. Azithromycin’s primary role in the near future will be in the community setting. Although its use in the hospital may be limited, this drug will be a convenient therapeutic option to have on hand in the emergency room and outpatient clinic. Azithromycin may also be used in the future to treat opportunistic infections in immunocompromised patients.3
Azithromycin is recently adapted for topical use in ophthalmology. It is effective against the most frequent pathogens found in bacterial conjunctivitis, gram-positive, and gram-negative bacteria.4 The clinical efficacy and microbial eradication of 1% and 1.5% azithromycin ophthalmic solutions were found to have comparable results for treatment of different eye diseases like purulent bacterial conjunctivitis,5,6 Meibomian gland dysfunction, blepharitis,7 and papulopustular rosacea.8 Most of the individualized randomized controlled trial (RCT) studies proved that azithromycin ophthalmic solutions are effective for the treatment of the above-mentioned eye diseases when compared with other antibiotics and a different route. Despite individual studies, there have been no conclusive investigations about the efficacy of ophthalmic solution in clinical cure rate and bacterial resolution. Therefore, this study aimed to provide conclusive evidence on the efficacy of azithromycin ophthalmic solutions compared to tobramycin eye drops for the treatment of eye diseases, in terms of clinical cure rate, bacterial resolution, and resolving clinical sign and symptoms of eye diseases.
Materials and methods
Protocol and registration
Study protocol and search strategy
The protocol of this study has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) with ID: CRD42019139911. The whole search was conducted by the investigators (B.M.A./lecturer and researcher, T.W./assistant professor). The authors were certified in comprehensive systematic searching techniques and comprehensive systematic review and meta-analysis.
Sources of studies and searching strategies
The systematic searches were conducted from both electronic and other gray literature sources. An electronic database such as MEDLINE (Ovid), Embase (Ovid), Emcare (Ovid), CINAHL (EBSCOhost), Scopus, PubMed, ProQuest, Web of Science, and Cochrane Central Register of Controlled Trials was searched. For unpublished studies and gray literature, WorldCat, Mednar, Google, and Google Scholar were used. Advanced search strategies were applied to each database using search strings, constructed from indexing terms, text words, and key terms of adapting from the review questions. For example, the following search strategy was used on PubMed: efficacy [MeSH] OR “treatment outcome” AND azithromycin [MeSH] OR Tobramycin AND “ophthalmic solution” [MeSH] OR “eye drop.” To identify ongoing trials, multiple World Health Organization (WHO) trial registries were searched (see Supplemental Additional File 1). We do not have any regional or time restrictions.
Study selection procedure
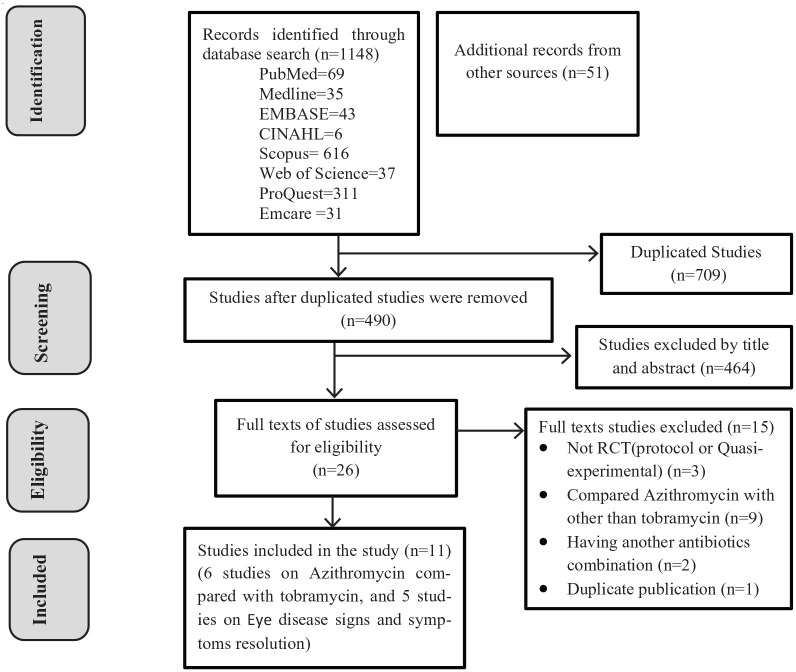
We included RCTs and controlled clinical trials that have been conducted in all age groups and written in English, irrespective of duration/time limitation. Studies with abstract only and not able to access the full article were excluded. Observational studies, reviews, commentaries, editorials, case series/reports, and patient stories were not included in the systematic review. Articles extracted from different sources were exported to EndNote X8 citation manager, and duplicates were removed. The authors (B.M.A. and T.W.) screened the title and abstracts of the studies with predefined inclusion criteria independently. These authors also independently collect full texts and evaluate for the eligibility to be included for final analysis by considering study subjects, language, study designs, quality, and outcome. Totally 1509 articles were searched. Of these, 490 articles were screened by title and abstract. After thorough screening, 26 studies were assessed for full. Finally, 11 studies were included in the final analysis (Figure 1). The study was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist (see Supplemental Additional File 2).
Figure 1.
Flow diagram showing the selection process of included studies.
Description of the outcomes of the systematic review and meta-analysis
Based on our systematic review and meta-analysis questions, we considered three outcome variables to be achieved by the review. The primary outcome variables were clinical cure rate, bacterial resolution, and resolution of clinical signs and symptoms of different eye diseases after treatment by azithromycin ophthalmic solutions in comparison with tobramycin eye drops.
Assessment of methodological quality (risk of bias assessment)
The quality assessment (critical appraisal) was performed by the authors (B.M.A. and T.W.) independently using Joanna Briggs Institute (JBI) critical appraisal tool for Randomized Controlled Trials9 (see Supplemental Additional File 3). The tool has 13 questions. It has yes, no questions, and 1 was given for yes and 0 for no. The scores were summed up and changed to percentages. Studies with ⩾50% were included for the meta-analysis. Special focus was given to clear statement of the objective of the study, the randomness of participant selection, identification of study participants, and preciseness of measurement of outcomes of interest and use of appropriate statistical analysis method, as well as documentation of sources of bias or confounding. The risk of bias in the included studies was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials10 (Table 1). During critical appraisal and inclusion of the studies, the discrepancies that arose between the authors were solved by consensus.
Table 1.
Risk of bias of included studies.
| Author | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Selective reporting (reporting bias) | Blinding (performance bias) | Incomplete outcome data (attrition bias) | Other sources of bias (other bias) | Overall decision on quality of study |
|---|---|---|---|---|---|---|---|
| Abelson et al.5 | Low | Low | Low | Low | Low | Low | Low |
| Bremond-Gignac et al.6 | Low | Low | Low | Low | Low | Low | Low |
| Cochereau et al.11 | Low | Low | Low | Low | Low | Low | Low |
| Denis et al.12 | Low | Low | Low | Low | Low | Low | Low |
| Protzko et al.13 | Low | Low | Unclear | Low | Low | Low | Moderate |
| Robert et al.14 | Low | Low | Low | Low | Low | Low | Low |
| Bakar Demircay et al.8 | Low | Unclear | Low | Low | Low | Low | Low |
| Haque et al.7 | Low | Low | Low | Low | Low | Low | Low |
| Luchs15 | Low | Low | Low | Low | Low | Low | Low |
| Yildiz et al.16 | Low | Low | Low | Low | Low | Low | Low |
| Opitz and Tyler17 | Low | Low | Low | Low | Low | Low | Low |
Data extraction and recording
Data (containing author, year, the aims of the study, study design, outcome of the study, participants, sample size, interventions, and key findings) were abstracted by using a template prepared in Microsoft Word 2016 (Tables 2 and 3). Of this, the findings (the raw numerical data) of selected studies were extracted by the authors (B.M.A. and T.W.) independently and stored using the data extraction template on Microsoft Excel (2016) spreadsheet.
Table 2.
Descriptions of included studies for evaluating the efficacy of azithromycin as compared to tobramycin eye drops.
| Study description of included studies | |||||
|---|---|---|---|---|---|
| Author, country | Aim of the study | Study design/outcome/participants | Sample size | Interventions | Key finding |
| Abelson et al.,5 USA | To evaluate the efficacy of an ophthalmic formulation of 1% azithromycin and demonstrate equivalence with 0.3% tobramycin ophthalmic solution | Prospective, randomized, active-controlled, double-masked, phase 3 trial/the efficacy of 1% azithromycin compared to tobramycin/bacteriologically confirmed participants | CR I = 159 C = 157 T = 316 BR I = 159 C = 157 T = 316 |
IG: Participants received 1% azithromycin for 5 days CG: Participants received tobramycin 0.3% ophthalmic solution |
CR: 127/159 CR: 123/157 BR:140/159 BR: 148/157 |
| Bremond-Gignac et al.,6 France, Germany, Italy, Poland, Portugal, Romania, Algeria, and Tunisia | To determine the efficacy and safety of azithromycin 1.5% eye drops in a pediatric population with purulent bacterial conjunctivitis | Multicentre, international, RCT/efficacy and safety of azithromycin/children (from 1 day to 18 years old) with purulent bacterial conjunctivitis, defined by mild to severe bulbar conjunctival injection and purulent discharge in at least one eye | CR I = 102 C = 101 T = 203 BR I = 102 C = 101 T = 203 |
IG: Azithromycin 1.5% eye drops (one drop twice daily) CG: Tobramycin 0.3% eye drops regimen (every 2 h for 2 days, then 4 times daily for 5 days) |
Day 3 CR: 48/102 CR: 29/101 Day 7 CR: 91/102 CR: 79/101 Day 7 BR: 85/102 BR: 80/101 |
| Cochereau et al.,11 France, India, Bulgaria, Guinea Conakry, Morocco, Portugal, Romania, and Tunisia | To compare the efficacy and safety of azithromycin 1.5% eye drops, for 3 days with tobramycin 0.3% for 7 days to treat purulent bacterial conjunctivitis | Multicenter, investigator-masked RCT/efficacy of azithromycin 1.5% for 3 days compared to tobramycin for 7 days/children and adults with purulent bacterial conjunctivitis | CR I = 245 C = 226 T = 471 BR Day 3 I = 237 C = 216 T = 453 Day 9 I = 236 C = 223 T = 459 |
IG: Participants received either azithromycin 1.5% twice daily for 3 days CG: Tobramycin 0.3%, one drop every 2 h for 2 days, then 4 times daily for 5 days |
Day 9 CR: 215/245 CR: 202/226 BR Day 3 BR: 202/237 BR: 181/216 Day 9 BR: 219/236 BR: 211/223 |
| Denis et al.,12 France, Bulgaria, Guinea Conakry, India, Morocco, Portugal, Romania, and Tunisia | To compare antibacterial efficacy of topically applied azithromycin 1.5% with tobramycin 0.3% in a multicenter, randomized, investigator-masked study for the treatment of purulent bacterial conjunctivitis | Multicenter, investigator-masked RCT/BR of topical therapy with azithromycin 1.5%/children, adult, infant, and newborn patients at least 1 day of age and diagnosed with purulent bacterial conjunctivitis | BR Day 3 I = 237 C = 216 T = 453 Day 9 I = 236 C = 223 T = 459 |
IG: Azithromycin 1.5% eye drops, one drop twice daily for 3 days CG: Tobramycin 0.3% eye drops, one drop every 2 h while awake up to 8 times a day for 2 days, then one drop 4 times daily for 5 days. |
BR Day 3 BR: 204/239 BR: 183/218 Day 9 BR: 221/238 BR: 213/225 |
| Protzko et al.,13 USA | To compare the safety and tolerability of 1.0% azithromycin in a polymeric mucoadhesive delivery system with 0.3% tobramycin ophthalmic solution for the treatment of bacterial conjunctivitis | Prospective, randomized, active-controlled, double-masked, phase 3 trial/safety, efficacy, and tolerability of 1% azithromycin/subjects with a clinical diagnosis of bacterial conjunctivitis at 47 sites | I = 159 C = 157 T = 316 |
IG: 1% Azithromycin twice a day on days 1 and 2 and daily on days 3–5 CG: 0.3% Tobramycin |
Bacterial eradication rate Day 5 BR: 147/159 BR: 147/157 |
| Robert et al.,14 France | To compare the clinical efficacy (signs and symptoms) and safety of azithromycin 1.5% eye drops with tobramycin 0.3%. | Multicenter, investigator-masked RCT/efficacy and safety of 1.5% azithromycin compared to 0.3% tobramycin/patients with purulent bacterial conjunctivitis | CR I = 245 C = 226 T = 471 |
IG: Azithromycin 1.5% twice daily for 3 days CG: Patients received tobramycin 0.3%, one drop every 2 h for 2 days, then 4 times daily for 5 days |
Clinical CR Day 9 CR: 216/246 CR: 203/227 |
BR: bacterial resolution; C: control; CG: control group; CR: cure rate; I: intervention; IG: intervention group; T: total; RCT: randomized control trial.
Table 3.
Descriptions of included studies for clinical sign and symptoms of eye disease.
| Author | Eye symptom scores | Eyelid finding scores | Conjunctival hyperemia | Schirmer test (mm) | Meibomian gland secretion | TBUT (sc) | Ocular surface staining scores | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Mean ± SD (median) |
After Mean ± SD (median) |
Before Mean ± SD (median) |
After Mean ± SD (median) |
Before Mean ± SD (median) |
After Mean ± SD (median) |
Before Mean ± SD (median) |
After Mean ± SD (median) |
Before Mean ± SD (median) |
After Mean ± SD (median) |
Before Mean ± SD (median) |
After Mean ± SD (median) |
Before Mean ± SD (median) |
After Mean ± SD (median) |
|
| Bakar et al.8 | 2.22 ± 1.98 (2.00) | 0.28 ± 0.15 (0.00) | 2.72 ± 1.01 (3.00) | 1.44 ± 0.98 (1.00) | 1.39 ± 0.60 (1.00) | 0.94 ± 0.72 (1.00) | 19.11 ± 7.98 (20.0) | 23.72 ± 8.09 (25.0) | 7.78 ± 3.52 (8.00) | 9.06 ± 2.41 (10.5) | 0.88 ± 0.90 (1.00) | 0.88 ± 0.83 (0.00) | ||
| Haque et al.7 | 2.22 ± 0.72 (2.00) | 0.82 ± 0.96 (0.0) | 2.0 (0.00) 2.0 |
1.2 (0.47)1.0 | 1.8 (0.50) 2.0 |
1.3 (0.56)1.0 | 1.88 ± 0.57 (1.76) | 1.64 ± 0.73 (1.39) | 1.36 ± 0.66 (1.00) | 1.3 ± 0.57 (1.00) | ||||
| Luchs15 | 3.2 ± 0.65 | 1.1 ± 0.64 | 1.97 ± 0.77 | 0.67 ± 0.61 | 2.5 ± 0.92 | 0.8 ± 0.43 | ||||||||
| Yildiz et al.16 | 2.33 ± 0.49 | 0.62 ± 0.65 | 2.20 ± 0.56 | 0.61 ± 0.65 | 1.80 ± 0.56 | 0.47 ± 0.52 | 17.73 ± 3.56 | 20.46 ± 1.98 | 2.13 ± 0.64 | 0.54 ± 0.52 | 7.87 ± 1.51 | 8.39 ± 1.26 | 1.60 ± 0.51 | 1.00 ± 0.58 |
| Opitz and Tyler17 | 2.73 ± 0.89 | 2.21 ± 0.78 | 11.54 ± 7.33 | 14.31 ± 9.53 | 2.44 ± 0.65 | 1.62 ± 0.57 | 4.37 ± 1.67 | 6.58 ± 2.84 | 3.65 ± 3.06 | 0.62 ± 0.80 | ||||
TBUT (sc): the tear breakup time in seconds; SD: standard deviation.
Strategy for data analysis and assessment of certainty in the findings
Data synthesis and statistical analysis were carried out by the authors (B.M.A. and T.W.). Summary statistics (pooled effect sizes) in relative risk (RR) ratios with 95% confidence intervals (CIs) were calculated for clinical cure rate and bacterial resolution, of azithromycin compared to tobramycin eye drops, using OpenMeta-analyst software. Review manager Version 5.3 software was also used for the standardized mean difference (SMD) of ocular sign and symptom resolution after azithromycin treatment. Forest plots were used to graphically present the meta-analysis results.
The presence of statistical heterogeneity was checked by using the chi-square test (Cochran’s Q test) at p value ⩽ 0.05. The level of heterogeneity among the studies was quantified using the I2 statistic described by Higgins and Thompson18 and p value. A low p value (less than 0.10) or a large I2 statistic (I2 > 75%) was considered as evidence of significant heterogeneity. Sensitivity analysis was employed to decrease the heterogeneity. A fixed-effect model was used. Publication bias was explored using visual inspection of the funnel plot. Besides, Egger’s regression was carried out to check the symmetry of the funnel plot.19 Approximately, symmetric funnel plots would indicate a “low risk,” whereas asymmetric funnel plots would indicate a “high risk” of publication bias.
Results
A total of 11 RCT studies conducted in different regions of the world were included in this systematic review and meta-analysis. Initially, we got 1199 articles through both electronic databases and other sources. From the identified articles, 709 of them were removed due to duplications, and the remaining 490 articles were screened by title and abstract. Of these, 464 of the studies were excluded since the titles and abstracts did not coincide with our study. The full texts of the 26 studies were reviewed for eligibility and 15 of them were excluded due to inconsistent, incomplete, different outcomes and duplicate publication. Finally, 11 studies were critically appraised for the quality and included in the final analysis. The data were first presented using a narrative synthesis and followed by the meta-analysis result. Summary tables of the included studies were also included (Figure 1, Tables 2 and 3).
The cure rate of azithromycin (1% and 1.5%) compared to tobramycin (0.3%) eye drops
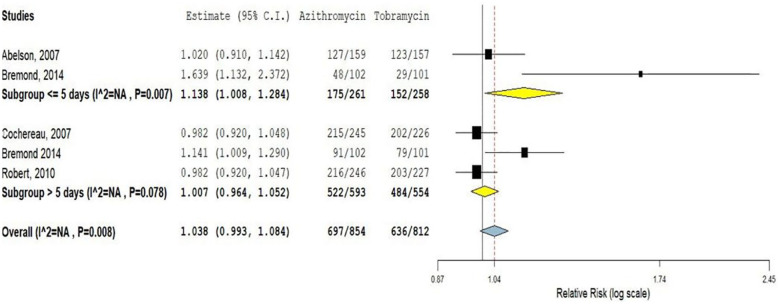
Five multicenter RCTs were included, which was conducted in different countries to compare the clinical efficacy of azithromycin 1% and 1.5% ophthalmic solutions in comparison with tobramycin 0.3% eye drop. All the included studies used 1% and 1.5% azithromycin as the intervention compared with 0.3% tobramycin in the control arms. Two of the five studies reported the clinical cure rate of azithromycin ophthalmic solutions is more effective than tobramycin eye drop.5,6,11,14 In another way, the rest three studies were reported that there was no significant difference in efficacy.6,11 This meta-analysis was based on the duration of treatment, which indicated that azithromycin 1% and 1.5% eye drops provide a more rapid clinical cure than tobramycin 0.3% eye drops in a twice-a-day dosing regimen for short duration (⩽5 days) use (RR = 1.13; 95% CI: 1.008, 1.28). Whereas on increased duration (>5 days), azithromycin is as effective as tobramycin (RR = 1.007; 95% CI: 0.96, 1.05; Figure 2).
Figure 2.
Clinical cure rate of azithromycin ophthalmic solutions compared to tobramycin eye drop.
Bacterial resolution of azithromycin (1%, 1.5%) eye drops compared to tobramycin (0.3%) eye drops
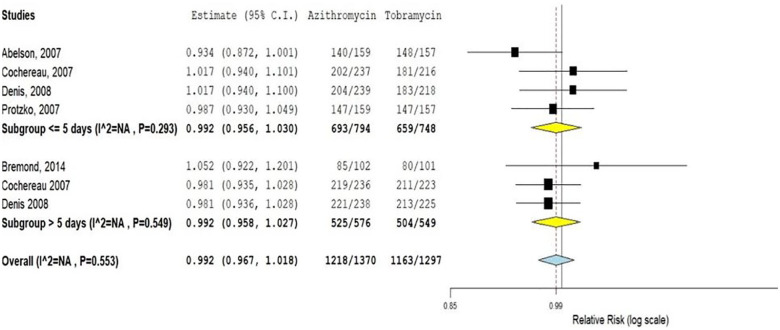
Five of the six RCTs reported the bacterial resolution rate of azithromycin compared to tobramycin eye drops.5,6,11–13 The overall finding of these studies showed that there is no statistically significant difference in bacterial resolution between azithromycin and tobramycin eye drops (RR = 0.99; 95% CI: 0.96, 1.01).
We also analyzed the effect of duration of treatment on the bacterial resolution rate between these two drugs by subgroup analysis. The result indicates that on short (⩽5 days; RR = 0.99; 95% CI: 0.95, 1.03) and long (>5 days; RR = 0.99; 95% CI: 0.95, 1.02) duration treatments, the bacterial resolution rate of azithromycin eye drops is almost similar with that of tobramycin (Figure 3).
Figure 3.
Bacterial resolution rate of azithromycin ophthalmic solutions compared to tobramycin eye drop.
Efficacy of azithromycin ophthalmic solutions (1%, 1.5%) on clinical signs and symptoms
Five RCTs were conducted to evaluate the effects of azithromycin ophthalmic solutions for resolving ocular signs and symptoms due to different eye diseases. Four of the five studies took subjects with blepharitis,7,15–17 and one study reported the effect of azithromycin on patients with papulopustular rosacea.8 The efficacy of azithromycin on different clinical signs and symptoms is presented as follows.
Eye symptom scores
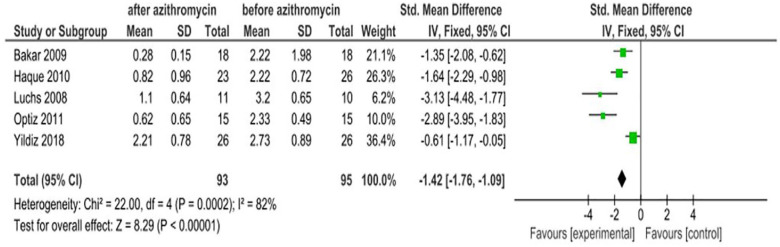
All five studies reported the effect of azithromycin ophthalmic solutions on eye symptom scores. The pooled estimate of the studies showed that azithromycin eye drops are effective in improving eye symptom scores with an SMD of −1.42 (95% CI: −1.76, −1.09). The intervention group was in favor of after treatment with an azithromycin eye drop and the control group favors before treatment. Hence, the interpretation is inverse. The finding revealed there was significant statistical heterogeneity (I2 = 82%, χ2 = 22, p = 0.0002; Figure 4).
Figure 4.
Effect of azithromycin eye drops (1%, 1.5%) on eye symptom scores.
Eyelid finding scores
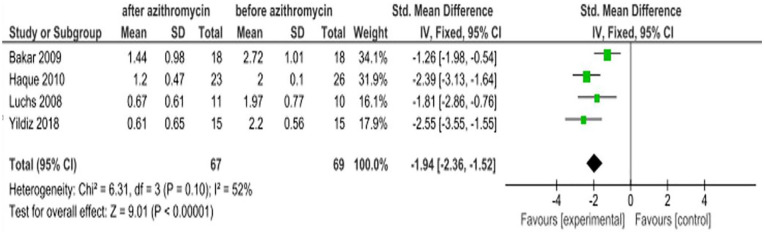
Four of the studies 8,7,16,17 reported the clinical efficacy of azithromycin ophthalmic solutions in improving eyelid finding scores. The SMD of these studies indicates that there is a statistically significant improvement in the severity of eyelid finding scores with an SMD of −1.94 (95% CI: −2.36, −1.52) The intervention group was in favor of after treatment with an azithromycin eye drop and the control group favors before treatment. Hence, the interpretation is inverse (Figure 5).
Figure 5.
Effect of azithromycin eye drops (1%, 1.5%) on eyelid finding scores.
Meibomian gland secretion
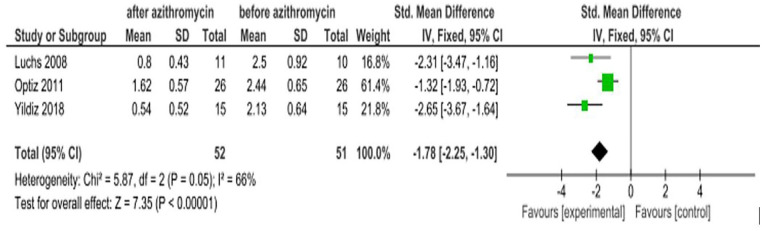
Blepharitis, Meibomian gland dysfunction, and other ocular diseases lead to dry eye due to decreased secretions.20–22 Three RCTs6,15,16 were found to report the effect of azithromycin on improving Meibomian gland secretion. The pooled estimate of the studies revealed that patients treated with azithromycin showed statistically significant improvement in Meibomian gland secretion with an SMD of −1.78 (95% CI: −2.25, −1.30). The intervention group was in favor of after treatment with an azithromycin eye drop and the control group favors before treatment. Hence, the interpretation is inverse (Figure 6).
Figure 6.
Effect of azithromycin eye drops (1%, 1.5%) on Meibomian gland secretion.
Ocular surface staining scores
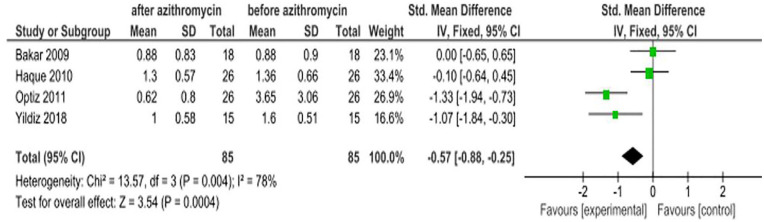
Fluorescein and rose bengal dyes have been reported to be advantageous for measuring ocular surface staining scores.23 Four studies8,7,16,17 evaluated the efficacy of azithromycin ophthalmic solutions on ocular surface staining scores, and the overall result indicated that azithromycin is effective in improving the staining scores due to some eye disease with an SMD of −0.57 (95% CI: −0.88, −0.25; Figure 7).
Figure 7.
Effect of azithromycin eye drops (1%, 1.5%) on ocular surface staining scores.
Tear breakup time in seconds
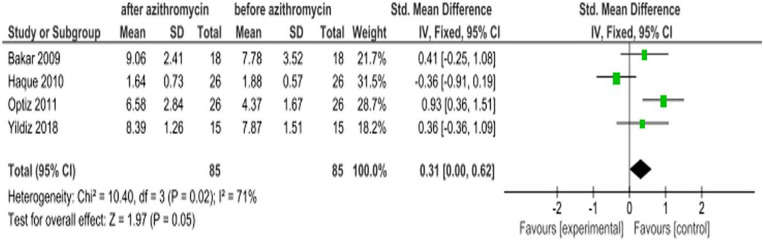
The tear breakup time (TBUT) is recorded as the number of seconds that elapse between the last blink and the appearance of the first dry spot in the tear film. A TBUT under 10 s is considered abnormal.24 The overall effect of azithromycin treatment from four RCTs showed that TBUT was not significantly improved with the SMD of 0.31 (95% CI: 0.00, 0.62; Figure 8).
Figure 8.
Effect of azithromycin eye drops (1%, 1.5%) on TBUT.
Schirmer test in millimeters
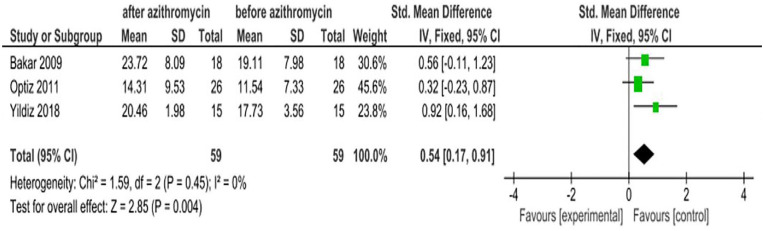
The Schirmer test has been widely used for the assessment of the adequacy of tear production. This test is used when a person experiences very dry eyes or excessive watering of the eyes.25 Results from three RCT studies showed that there is an increase in the Schirmer test value after study subjects are treated with azithromycin eye drops. The pooled estimate of these studies is inconsistent with individual studies with an SMD of 0.54 (95% CI: 0.17, 0.91; Figure 9).
Figure 9.
Effect of azithromycin eye drops (1%, 1.5%) on Schirmer test in millimeters.
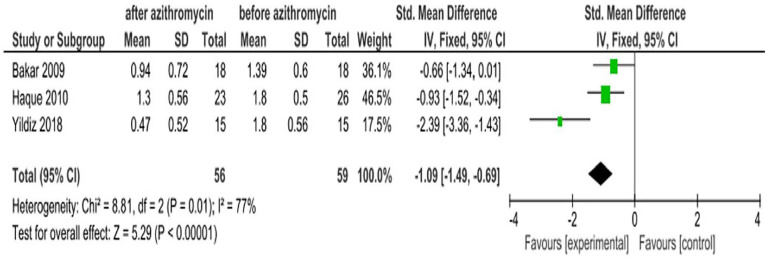
Conjunctival hyperemia
Conjunctival hyperemia is dilation and redness of the conjunctival blood vessels secondary to eye diseases. The outline of hyperemia often appears with the greatest redness at the fornices and declines moving toward the limbus.26 Three studies8,7,16 reported the effect of azithromycin eye drops for conjunctival hyperemia due to different eye diseases. The pooled estimate of these studies indicates azithromycin ophthalmic solutions were effective in decreasing conjunctival hyperemia with an SMD of −1.09 (95% CI: −1.49, −0.69; Figure 10).
Figure 10.
Effect of azithromycin eye drops (1%, 1.5%) on reducing conjunctival hyperemia.
Discussion
This systemic review and meta-analysis presented the concluded evidence on the efficacy of azithromycin ophthalmic solutions for the treatment of eye diseases compared to tobramycin eye drops in terms of clinical cure rate, bacterial resolution, and resolving clinical sign and symptoms of eye diseases by summarizing primary RCTs. A total of 11 studies that have been critically appraised using the JBI assessment checklist, undertaken in different countries in the world, were identified and included. Even though we identified and included similar studies, the duration of the drug and its efficacy were different, we performed subgroup analysis. The studies conducted in a non-English language and incomplete abstract were excluded.
This study infers that azithromycin ophthalmic solution is a better treatment choice than tobramycin eye drop to rapidly improving eye diseases, mainly in less than or equal to 5-day treatment for 2 times or more dosing per day. The finding is supported by a multicenter, international, randomized, investigator-masked study which declared azithromycin was superior to tobramycin in clinical cure rate on day 3.6 Similarly, it is also consistent with a study done on the microbiologic efficacy of 3-day treatment with azithromycin 1.5% eye drops for purulent bacterial conjunctivitis.12
Similarly, many studies clearly show azithromycin 1.5% for 3 days (six drops) was as effective and safe as tobramycin for 7 days (36 drops).11,13,14 The azithromycin 1.5% regimen produced a rapid resolution of cardinal signs of purulent bacterial conjunctivitis with a more convenient dosage regimen. Such improved convenience is likely to improve compliance and lessen the burden of illness for patients and carers.27 More azithromycin than tobramycin patients presented an early clinical cure at day 3. Due to its twice-daily dosing regimen for 3 days, azithromycin represents a step forward in the management of purulent bacterial conjunctivitis, especially in children. This new anti-infective product has the advantage of a short treatment course which could lead to an improvement in patient compliance.6,11,12 Azithromycin 1% is safe and can be administered in a regimen of less frequent doses than can tobramycin.14
However, it is contradicted by a randomized trial study, which was reported that bacterial eradication was lower in the 1% azithromycin eye drop treatment group than in the tobramycin group.5 Despite this, most of the studies were on the support of the efficacy of the azithromycin eye drop in the clinical cure rate in short durations over tobramycin eye drop.
The azithromycin 1.5% regimen produced a rapid resolution of cardinal signs and symptoms of eye disease. Significant improvement was verified on the eye symptom scores,8,7,15 eyelid finding scores, Meibomian gland secretion,8,7,16 ocular surface staining scores,7,8,16 TBUT in seconds,7,8,16 Schirmer test in millimeters,8,16 and conjunctival hyperemia8,7,16 with less than 5-day azithromycin ophthalmic solution treatment.
Conclusion
Azithromycin 1% and 1.5% is as effective as tobramycin 0.3% in the clinical cure rate for less than 5 days of treatment. It is also the best choice of treatment for improving the signs and symptoms of eye disease: eye symptom scores, eyelid finding scores, Meibomian gland secretion, ocular surface staining scores, and conjunctival hyperemia. It is the safest drug for topical use. In addition, azithromycin has longer ocular drug residence time, less frequent dosing, and an increase in patient compliance. Therefore, this makes azithromycin the most ideal topical antibiotic over tobramycin eye drops to get a good result in a short duration. So that we recommend clinicians to use azithromycin 1% or 1.5% eye drops for the treatments of eye diseases to get good results.
Strengths and limitations
The strength of this study is that the included studies are RCTs conducted in different contexts and settings. The strengths of this meta-analysis include a broad literature search, screening and data extraction performed in duplicate, careful exclusion of studies with overlapping populations, and the final summary result depends on critically appraised studies. The limitations of this systematic review and meta-analysis is it did not include studies conducted in languages other than English. In addition, the included studies have significant heterogeneity, which was due to the variation between studies in characteristics of the study population, medical and nonmedical factors as a reason for the variation between studies.
Supplemental Material
Supplemental material, Additional_file_1_2 for Efficacy of azithromycin 1% and 1.5% ophthalmic solutions compared to tobramycin 0.3% eye drops: A systematic review and meta-analysis by Birhanu Motbaynor Alemu and Teshager Worku in SAGE Open Medicine
Supplemental material, Additional_file_2_1 for Efficacy of azithromycin 1% and 1.5% ophthalmic solutions compared to tobramycin 0.3% eye drops: A systematic review and meta-analysis by Birhanu Motbaynor Alemu and Teshager Worku in SAGE Open Medicine
Supplemental material, Additional_file_3_1 for Efficacy of azithromycin 1% and 1.5% ophthalmic solutions compared to tobramycin 0.3% eye drops: A systematic review and meta-analysis by Birhanu Motbaynor Alemu and Teshager Worku in SAGE Open Medicine
Acknowledgments
We would like to address our deepest gratitude to the authors of the included studies for this systematic review and meta-analysis. Our deepest gratitude also goes to the staff of Haramaya University, College of Health and Medical Sciences who gave us technical support.
Footnotes
Author contributions: B.M.A. and T.W. conceived and designed the review. Both authors carried out the draft of the manuscript; developed the search strings; screened and selected studies; carried out analysis and interpretation; rigorously reviewed the manuscript; and read and approved the final version of the manuscript. B.M.A. is the guarantor of the review.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Birhanu Motbaynor Alemu  https://orcid.org/0000-0001-6018-2596
https://orcid.org/0000-0001-6018-2596
Teshager Worku  https://orcid.org/0000-0002-6153-7819
https://orcid.org/0000-0002-6153-7819
Supplemental material: Supplemental material for this article is available online.
References
- 1. Peters DH, Friedel HA, McTavish DJD. Azithromycin: a review of its antimicrobial activity, pharmacokinetic properties and clinical efficacy. Drugs 1992; 44(5): 750–799. [DOI] [PubMed] [Google Scholar]
- 2. Dunn CJ, Barradell LB. Azithromycin: a review of its pharmacological properties and use as 3-day therapy in respiratory tract infections. Drugs 1996; 51(3): 483–505. [DOI] [PubMed] [Google Scholar]
- 3. Ballow CH, Amsden GW. Azithromycin: the first azalide antibiotic. Ann Pharmacother 1992; 26(10): 1253–1261. [DOI] [PubMed] [Google Scholar]
- 4. Ambroziak AM, Szaflik JP, Hapunik A. [Evaluation of effectiveness and tolerance of treatment with azithromycin 1.5% eye drops in bacterial conjunctivitis]. Klin Oczna 2009; 111(1–3): 46–49 (in French). [PubMed] [Google Scholar]
- 5. Abelson M, Protzko E, Shapiro A, et al. A randomized trial assessing the clinical efficacy and microbial eradication of 1% azithromycin ophthalmic solution vs tobramycin in adult and pediatric subjects with bacterial conjunctivitis. Clin Ophthalmol 2007; 1(2): 177–182. [PMC free article] [PubMed] [Google Scholar]
- 6. Bremond-Gignac D, Nezzar H, Bianchi PE, et al. Efficacy and safety of azithromycin 1.5% eye drops in paediatric population with purulent bacterial conjunctivitis. Br J Ophthalmol 2014; 98(6): 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haque RM, Torkildsen GL, Brubaker K, et al. Multicenter open-label study evaluating the efficacy of azithromycin ophthalmic solution 1% on the signs and symptoms of subjects with blepharitis. Cornea 2010; 29(8): 871–877. [DOI] [PubMed] [Google Scholar]
- 8. Bakar Demircay ÖZ, Toker E, Cakir S. Ocular signs, symptoms and tear function tests of papulopustular rosacea patients receiving azithromycin. J Eur Acad Dermatol Venereol 2009; 23(5): 544–549. [DOI] [PubMed] [Google Scholar]
- 9. Tufanaru CMZ, Aromataris E, Campbell J, et al. Systematic reviews of effectiveness. In: Aromataris E, Munn Z. (eds) Joanna Briggs Institute reviewer’s manual. The Joanna Briggs Institute, 2017, https://wiki.jbi.global/display/MANUAL/Chapter+3%3A+Systematic+reviews+of+effectiveness [Google Scholar]
- 10. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cochereau I, Meddeb-Ouertani A, Khairallah M, et al. 3-day treatment with azithromycin 1.5% eye drops versus 7-day treatment with tobramycin 0.3% for purulent bacterial conjunctivitis: multicentre, randomised and controlled trial in adults and children. Br J Ophthalmol 2007; 91(4): 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Denis F, Chaumeil C, Goldschmidt P, et al. Microbiological efficacy of 3-day treatment with azithromycin 1.5% eye-drops for purulent bacterial conjunctivitis. Eur J Ophthalmol 2008; 18(6): 858–868. [DOI] [PubMed] [Google Scholar]
- 13. Protzko E, Bowman L, Abelson M, et al. Phase 3 safety comparisons for 1.0% azithromycin in polymeric mucoadhesive eye drops versus 0.3% tobramycin eye drops for bacterial conjunctivitis. Invest Ophthalmol Vis Sci 2007; 48(8): 3425–3429. [DOI] [PubMed] [Google Scholar]
- 14. Robert PY, Bourcier T, Meddeb-Ouertani A, et al. [Efficacy assessment of azithromycin 1.5% eye drops versus tobramycin 0.3% on clinical signs of purulent bacterial conjunctivitis]. J Fr Ophtalmol 2010; 33(4): 241–248. [DOI] [PubMed] [Google Scholar]
- 15. Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv Ther 2008; 25(9): 858–870. [DOI] [PubMed] [Google Scholar]
- 16. Yildiz E, Yenerel NM, Turan-Yardimci A, et al. Comparison of the clinical efficacy of topical and systemic azithromycin treatment for posterior blepharitis. J Ocul Pharmacol Ther 2018; 34(4): 365–372. [DOI] [PubMed] [Google Scholar]
- 17. Opitz DL, Tyler KF. Efficacy of azithromycin 1% ophthalmic solution for treatment of ocular surface disease from posterior blepharitis. Clin Exp Optom 2011; 94(2): 200–206. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- 19. Shi X, Nie C, Shi S, et al. Effect comparison between Egger’s test and Begg’s test in publication bias diagnosis in meta-analyses: evidence from a pilot survey. Int J Res Stud Biosci 2017; 5: 14–20. [Google Scholar]
- 20. Doan S, Gabison E, Chiambaretta F, et al. Efficacy of azithromycin 1.5% eye drops in childhood ocular rosacea with phlyctenular blepharokeratoconjunctivitis. J Ophthal Inflam Infect 2013; 3(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hosseini K, Lindstrom RL, Foulks G, et al. A randomized, double-masked, parallel-group, comparative study to evaluate the clinical efficacy and safety of 1% azithromycin-0.1% dexamethasone combination compared to 1% azithromycin alone, 0.1% dexamethasone alone, and vehicle in the treatment of subjects with blepharitis. Clin Ophthalmol 2016; 10: 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Satitpitakul V, Ratanawongphaibul K, Kasetsuwan N, et al. Efficacy of azithromycin 1.5% eyedrops vs oral doxycycline in meibomian gland dysfunction: a randomized trial. Graefes Arch Clin Exp Ophthalmol 2019; 257(6): 1289–1294. [DOI] [PubMed] [Google Scholar]
- 23. Korb DR, Herman JP, Finnemore VM, et al. An evaluation of the efficacy of fluorescein, rose bengal, lissamine green, and a new dye mixture for ocular surface staining. Eye Contact Lens 2008; 34(1): 61–64. [DOI] [PubMed] [Google Scholar]
- 24. Golding TR, Bruce AS, Mainstone JCJC. Relationship between tear-meniscus parameters and tear-film breakup. Cornea 1997; 16(6): 649–661. [PubMed] [Google Scholar]
- 25. Cho P, Yap M. Schirmer test: I: a review. Optom Vis Sci 1993; 70(2): 152–156. [DOI] [PubMed] [Google Scholar]
- 26. Hyperemia BJ. Conjunctival. In: Schmidt-Erfurth U, Kohnen T. (eds) Encyclopedia of ophthalmology. Berlin; Heidelberg: Springer, 2014, pp. 27–57. [Google Scholar]
- 27. Bremond-Gignac D, Messaoud R, Lazreg S, et al. A 3-day regimen with azithromycin 1.5% eyedrops for the treatment of purulent bacterial conjunctivitis in children: efficacy on clinical signs and impact on the burden of illness. Clin Ophthalmol 2015; 9: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Additional_file_1_2 for Efficacy of azithromycin 1% and 1.5% ophthalmic solutions compared to tobramycin 0.3% eye drops: A systematic review and meta-analysis by Birhanu Motbaynor Alemu and Teshager Worku in SAGE Open Medicine
Supplemental material, Additional_file_2_1 for Efficacy of azithromycin 1% and 1.5% ophthalmic solutions compared to tobramycin 0.3% eye drops: A systematic review and meta-analysis by Birhanu Motbaynor Alemu and Teshager Worku in SAGE Open Medicine
Supplemental material, Additional_file_3_1 for Efficacy of azithromycin 1% and 1.5% ophthalmic solutions compared to tobramycin 0.3% eye drops: A systematic review and meta-analysis by Birhanu Motbaynor Alemu and Teshager Worku in SAGE Open Medicine