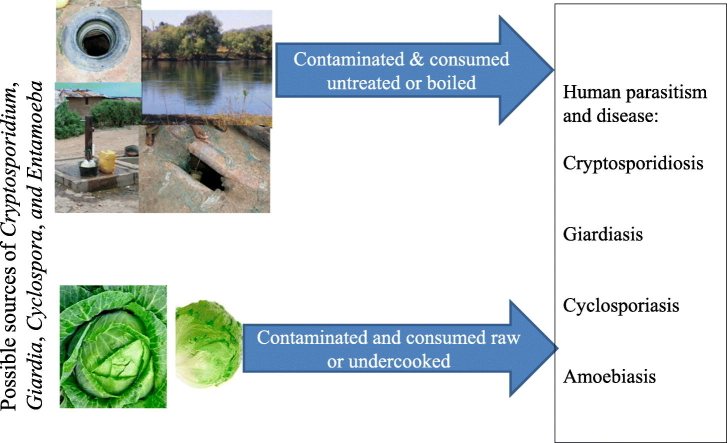
Abstract
Parasitic food-borne diseases, particularly those caused by the protozoan parasites Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba are increasingly becoming common and have received considerable attention in the last two decades. The ability of the transmission stages of the parasites to survive in the environment for prolonged periods, globalization of the food industry and changes in eating habits have contributed to the numbers of human infections. This systematic scoping review highlights these important water- and foodborne parasites in the African context, detailing the burden in African water sources, wastewater/effluents and fresh produce. A scoping review search targeting African countries was conducted in Medline, Web of science and African journals online as well as back referencing from included studies covering the period 1990 to January 2020. Out of 1134 studies, 68 were included in the review. The articles covered 17 out of 54 African countries. There were 39/68 studies reporting on water sources while the rest reported on fresh produce. Cryptosporidium prevalence ranged from 6 to 100% in surface water, 4 to 100% in tap water and up to 100% in wastewater and sludge. In fresh produce, Cryptosporidium was reported from five countries with prevalence of 0.8–75%. Giardia was reported in 47 out of 68 articles; prevalence ranged from 2.4% in surface water; 1% to over 70% in tap water; 28–100% in wastewater and 2% - 99% in fresh produce. Prevalence of Cyclospora cayetanensis was lower. Prevalence of Entamoeba was 78% in surface water; 100% in wastewater and up to 99% in fresh produce. This study finds that Africa is no exception to the risk presented by the subject parasites from water and/or food sources. Routine screening for these parasites particularly at household level and provision of adequate and safe drinking water would help to control the parasites.
Keywords: Cryptosporidium, Giardia, Cyclospora, Entamoeba, Food- and waterborne parasites, Africa
Graphical abstract
Highlights
-
•
African rural communities faced with clean water challenges.
-
•
African water bodies and sources contaminated with Cryptosporidium, Giardia, Cyclospora, and Entamoeba and other pathogens
-
•
Fresh produce are carriers of protozoan parasites Cryptosporidium, Giardia, Cyclospora, Entamoeba.
1. Introduction
Parasitic protozoa are ubiquitous in nature and have a (Abd El-Salam, 2012) worldwide distribution. They are responsible for epidemic and endemic human suffering in both the developed and developing countries (Cotruva et al., 2004). Some parasites are zoonotic in nature, therefore, occur in animals (Robertson and Gjerde, 2001); their occurrence on foods and water should be considered a public health concern. Food and waterborne parasites are generally under-recognised, but this scenario has changed over the years due to a number of food and water borne disease outbreaks attributed to parasites (Marshall et al., 1997). In the past, the risk of human infection with parasites was considered to be limited to distinct geographic regions due to food eating practices, parasites' adaptations to specific definitive hosts and intermediate hosts and environmental conditions (Orlandi et al., 2002; El Said Said, 2012). This is slowly changing due to the globalization of the food supply and availability of refrigerated food transport which has made it easier to transport various foods across borders (Dorny et al., 2009). Despite the inability of the parasitic protozoa to multiply in foods, they may survive in or on moist foods for months in cool (such as refrigerator) or damp environments, making it possible to convey infective stages of the parasite from one point to another (Dawson, 2005). This is especially true for Cryptosporidium oocysts and Giardia cysts which were shown to survive well when kept moist and refrigerated, at 4 °C (Utaaker et al., 2017).
Most of the sub-Saharan African population still lives without access to clean water and sanitary facilities (Sente et al., 2016) and in many African households, especially the rural dwellers, untreated water is used for various purposes including drinking, cooking, washing of fruits and other fresh produce, bathing, and swimming which exposes them to not only protozoan parasites but other pathogens as well (Yongsi, 2010; WHO, 2014). The possible contamination of drinking water with protozoan pathogens therefore poses a serious threat to millions of people in the developing world. Reports from periodic diarrhoeal disease outbreaks in the developed world have commonly been attributed to protozoan parasites such as Cryptosporidium sp. and Giardia duodenalis and to a lesser extent, Cyclospora cayetanensis and Entamoeba histolytica (Lane and Lloyd, 2002; Karanis et al., 2007), with Cryptosporidium species being responsible for majority of them. Most of the protozoan parasites use the faecal-oral route of transmission and infection can be direct (human to human, animal to human) or indirectly through contaminated drinking and recreational water, food and food products contaminated with infectious (oo) cysts or inhalation (Fayer et al., 2000; Ethelberg et al., 2009; Sponseller et al., 2014). Supplementary Table 1 (suppl1) summarises the parasites' transmission routes, brief life cycle, clinical signs and treatment. Very little is known about these important parasites with regard to food and water contamination in Africa, particularly sub-Saharan Africa. The faecal-oral route of transmission implies that humans can be infected through sewage contamination of drinking water sources by animal or human faeces (Lanata, 2003).
Other than limited access to clean water and sanitary facilities, other factors equally significantly contribute to the risk of transmission of food and waterborne parasites, especially among the highly susceptible individuals such as the aged, young children, malnourished and HIV infected (Dorny et al., 2009; Lund and O'Brien, 2012). Increase in the eating of raw and undercooked foods (Dorny et al., 2009) and eating food from street food vendors or open markets' caterers who do not always respect food safety probably play a significant role in the transmission of these parasites in the African setting (Kudah et al., 2018).
Cryptosporidium is an apicomplexan, intracellular, zoonotic parasite that causes cryptosporidiosis, a diarrhoeal disease of humans and animals (Fayer, 2010). It is frequently reported in waterborne outbreaks (Karanis et al., 2007). According to the World Health Organization, Cryptosporidium is the most common diarrhoea causing protozoan parasite worldwide (WHO/UNICEF, 2009). Severity and duration of symptoms are influenced by age, immune and nutritional status of the host, infective dose and genetics of the parasite. On the other hand, Giardia duodenalis is a protozoan flagellate that infects mammals including humans, and accounts for more than 250 million symptomatic human infections annually, with the less developed countries being the most afflicted (Einarsson et al., 2016). The parasite is a species complex, comprising eight genetic assemblages A-H (Caccio and Ryan, 2008). Assemblage A and B infects humans and other animals while C & D infects canids, E – hooved animals, F – cats, G rats and H – marine mammals (Minetti et al., 2016; Caccio et al., 2017). However, human infections with assemblages E and F have recently been reported, highlighting the zoonotic potential of the parasite (Abdel-Moein and Saeed, 2016; Fantinatti et al., 2016; Gelanew et al., 2007; Zahedi et al., 2017). Assemblages A and B are further subdivided into sub-assemblages AI, AII, AIII, AIV, BI, BII, BIII & BIV (Monis et al., 2003). Giardia duodenalis is commonly isolated in humans, with prevalence ranging from <10% (in developed countries) to up to around 30% or higher in countries with resource poor communities (Feng and Xiao, 2011; Einarsson et al., 2016). It is considered the most common protozoan parasite infecting the human intestines, causing an estimated 280 million cases each year (Lane and Lloyd, 2002). Giardia has been associated with several water- and foodborne outbreaks worldwide (Karanis et al., 2007; Baldursson and Karanis, 2011).
Cyclospora cayetanensis, another apicomplexan parasite, is an important waterborne parasite in industrialized countries (Herwaldt, 2000; Insulander et al., 2010) but it is likely that its effect on human health is underestimated. The parasite causes the disease cyclosporiasis. Host's age and immune status and endemicity in a particular region appear to influence severity of infection (Giangaspero and Gasser, 2019). Outbreaks of cyclosporiasis have increasingly been observed since the 1990s, particularly in North America and Asia. In 1996, more than 1000 cases, associated with eating raspberries, occurred in the United States of America and Canada (Herwaldt et al., 1997). Other outbreaks were associated with consumption of lettuce (CDC, 1997), or fresh basil (Hoge et al., 1995). During the period 2013–2014, over 900 people in the US were infected with C. cayetanensis linked to imported cilantro and salad mixes (CDC, 2014; Harvey, 2013). Because of these reports, the parasite has been considered of public health concern primarily in developed countries. Entamoeba, another protozoan parasite belongs to the phylum Sarcomastigophora, subphylum Sarcodina and has many species such as Entamoeba histolytica, E. dispar, E. coli, E. hartmanni, E. gingivalis, E. polecki, E. moshkovskii, Dientamoeba fragilis, Endolimax nana and Iodamoeba bütschlii. Among these, some are pathogenic to humans while others are commensals (Carrero et al., 2020). In this review, only E. histolytica/dispar is discussed. Even though clinical diagnosis of amoebiasis usually depends on the visualization of parasites by light microscopy of a wet smear or stained specimens, trophozoites and cysts of the pathogenic Entamoeba histolytica and non-pathogenic E. dispar are morphologically indistinguishable. Species identification requires use of molecular methods. Young age, pregnancy, malignancy, malnutrition, alcoholism, and prolonged corticosteroid use are some of the risk factors; and high-risk groups include travellers, immigrants, migrant workers, refugees, immunocompromised individuals, institutionalized individuals and, possibly, children in day-care centres (Salit et al., 2009; Carrero et al., 2020).
Despite the public health importance of these parasites, which has not extensively been highlighted in Africa, little is known about the occurrence and prevalence in food and water in Africa, the common modes through which infections occur.
1.1. The scoping review question
The subject protozoan parasites can infect humans from a variety of sources which can be broadly categorized as environment, animals and human sources. Studies looking at associations between risk factors and disease often sample humans to measure the rate of disease in relation to the selected risk factors. These studies do not detail the rate of occurrence of parasites in environmental and animal sources. This information is obtained when the individual potential sources of infection are sampled. Despite the many negative health effects of the four parasites (Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba), very little has been done to highlight the prevalence of the parasites in water and fresh produce in Africa. Additionally, limited studies have been conducted to determine the occurrence of the parasite in fresh produce which have been reported to be common vehicles for several parasites (Robertson and Gjerde, 2001). The aim of this review is therefore to provide an overview of evidence of Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba contamination on fruits and vegetables that are consumed raw and various water sources (and effluents) in Africa and highlight their potential for water and foodborne disease.
2. Methods
The study was conducted according to the methodology by the Joana Brigs Institute (JBI) (Peters et al., 2015) and the reporting guidelines of the Preferred Reporting System for Systematic Reviews and Meta-Analysis for scoping review Protocols (PRISMA ScR) (Tricco et al., 2018).
2.1. Inclusion criteria
Primary studies meeting the following criteria were included in this ScR; (1) all primary studies on the occurrence of the four parasites Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba in various water bodies/sources and fresh produce particularly fruits and vegetables with samples from Africa (any of the 54 countries), (2). study design - all peer reviewed observation studies (3) studies published from1990 up to January 2020 with no language restriction.
2.2. Exclusion criteria
This review excluded (1) editorials, systematic reviews or reviews covering the subject parasites with no primary data, (2) studies on the subject parasites but with a different population such as humans, animals, other environmental samples besides water, (3) samples outside Africa or with different outcomes and studies outside the targeted time frame.
2.3. Search strategy
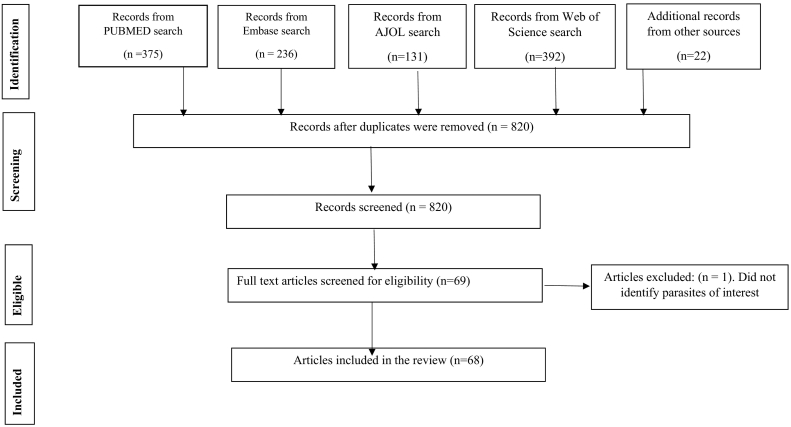
The search was done in Medline via PubMed, Web of Science via the Web of Knowledge, Embase and African Journals online databases. A search phrase was developed based on Medline index terms and adapted to the other three databases (CM) (suppl2). The literature search was conducted on February 13, 2020. In addition, reference lists in the included articles were searched to capture possible additional publications. Majority of publications were excluded based on the title and abstract for not meeting the inclusion criteria. Independently, JS and FM read through all the retrieved articles, one-by-one and selected those that, reported studies on Cryptosporidium, Giardia, Cyclospora and Entamoeba conducted in any one of the African Countries. CM was responsible for quality assurance. In the third step, JS searched all additional relevant articles cited in the list of references of each of the initially selected articles. The selection of articles for inclusion from the search list was done based on the inclusion and exclusion criteria. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for this study is presented in Fig. 1.
Fig. 1.
Diagram for a review on Protozoan parasites Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba in water and fresh produce 1990–January 2020.
2.4. Charting the results
Data extraction included basic information such as country, study site, author, publication year, study aims, sample type and sample size, diagnostic methods, number of positive samples, reported outcome measure such as prevalence or incidence, and incidental findings of other parasites. Prevalence was the major outcome measure and data has been presented according to parasite and sample type. In studies where prevalence was not given, it was estimated. Due to the heterogeneity of included studies, data was summarised and presented in tables without determining the overall average prevalence. Furthermore, incidental parasite isolations beyond the intended parasites were also reported.
3. Results
The results are presented in the following order; general findings (Section 3.1), prevalence and distribution of the four protozoan parasites (Cryptosporidium Section 3.2, Giardia Section 3.3, C. cayetanensis Section 3.4, Entamoeba histolytica/dispar Section 3.5) in water and sewage/effluent and, a combined prevalence and distribution of all four parasites in fresh produce (Section 3.6). Additionally, the prevalence of other parasites found in the various samples is presented (3.7, 3.8). As a number of studies did not provide prevalence estimates, these were calculated based on the absolute numbers provided and are presented as percentages.
3.1. General findings
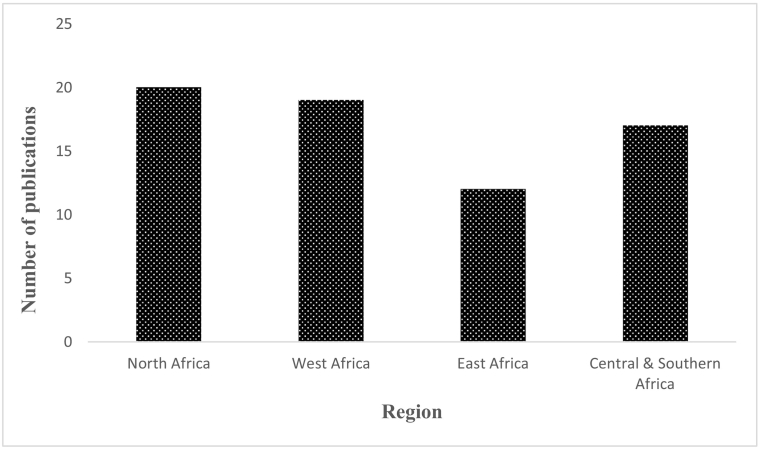
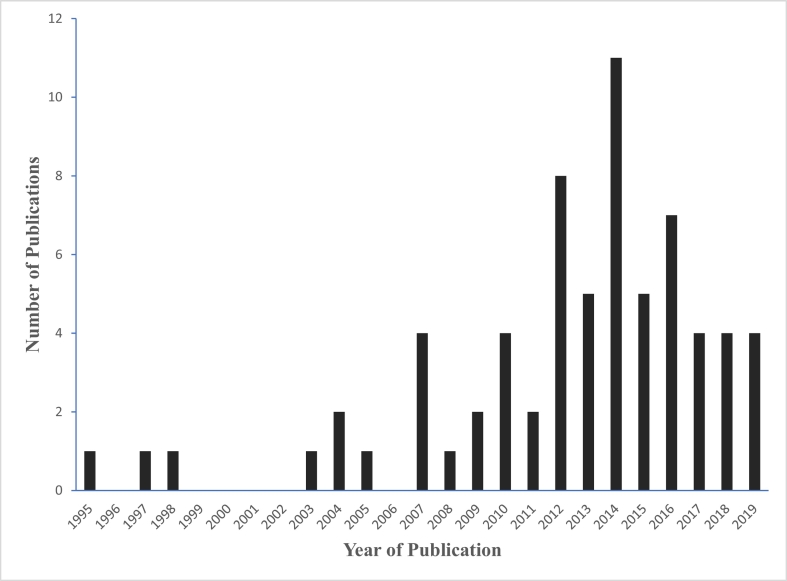
A total of 1134 scientific articles were retrieved from the data bases, of which 46 were included in the review. Additional publications (22) which were captured from the reference lists of the 46 included studies were also included giving a total of 68 articles. All the reviewed articles were published from 1990 to January 2020. Of these, 62 were full-length articles, three conference papers (Samie and Mashau, 2012; Petersen et al., 2014; Medani et al., 2016) and three theses (Duhain, 2011; Markos, 2013; Sampson, 2015). The publications were distributed throughout Africa covering 17 countries (Algeria, Burkina Faso, Cameroon, Cote d'Ivore, Egypt, Ethiopia, Ghana, Libya, Kenya, Nigeria, Rwanda, South Africa, Sudan, Tunisia, Uganda, Zambia and Zimbabwe) with North African countries accounting for the majority of the articles (29.4%; 20/68) while east Africa accounted for 17.6% (12/68) (Fig. 2). Egypt had the highest number of publications (11) followed by Ethiopia, Ghana, Nigeria and South Africa which had eight publications each. There were more publications from the year 2012 onwards with a peak in 2014 (Fig. 3). The studies assessed the occurrence and/or prevalence of Cryptosporidium or Giardia or Cyclospora or Entamoeba or a combination of the parasites in fresh vegetables, fruits, surface water (rivers, lakes, dams, wells, ponds), waste water (treated effluents, untreated effluents) and tap water. All publications, except one (Ssemanda et al., 2018) reported positive results for any of the parasites under review, either as a single parasite or in combination with the other parasites. A total of 51 publications reported Cryptosporidium, while 47 reported Giardia, 23 Entamoeba histolytica/dispar and 15 reported Cyclospora cayetanensis. Helminths and other protozoan parasites and various bacterial forms were also isolated in the various water sources, wastewater and sludge.
Fig. 2.
Number of publications on protozoan parasites in water and/or fresh produce according to region.
Fig. 3.
Number of publications on protozoan parasites in water and/or fresh produce in Africa.
Diagnostic methods used to identify the parasites varied per study. For Cryptosporidium and/or C. cayetanensis, various studies employed different diagnostic methods including modified Ziehl Neelsen, modified Bailengar, immunofluorescence, Auramine O-phenol, immunomagnetic separation and staining and EPA 1623. For Giardia and/or Entamoeba, wet smears (after a concentration method) with or without iodine stains, and Zinc Sulfate floatation were used to identify the cysts. For water samples, they were either filtered before concentration or directly concentrated before examination. A few studies (15) employed PCR in the analysis of the water.
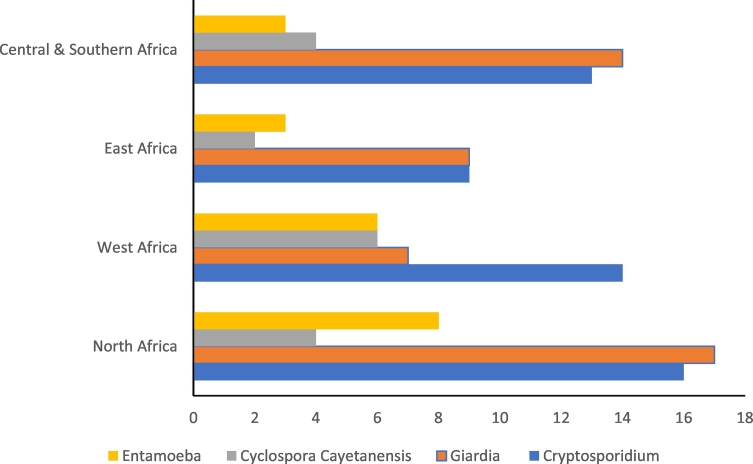
The highest number of articles reporting Cryptosporidium was from North Africa followed by West Africa and Central and Southern Africa (Fig. 4). Further, North Africa accounted for the highest number of studies reporting Giardia in various water bodies while East Africa had the least. East Africa also had the least number of publications reporting C. cayetanensis and Entamoeba (Fig. 4).
Fig. 4.
Number of publications on Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba in water and/or fresh produce in Africa according to region.
Fresh produce (fruits and vegetables) that are eaten raw varies from country to country. Carrots, onions, tomatoes, green pepper, lettuce, green pepper, parsley, watercress, tomato, cucumber and cabbage were the common vegetables reported to be eaten raw in the reviewed publications. Others, mostly from west African countries included green leaf (ugwu leaf, bitter leaf, sokoyokoto, igbagba), fluted pumpkin, waterleaf, morning glory solanum and curry (Alakpa et al., 2003; Chijioke et al., 2018). Fruits screened included mango, orange, lime, banana and cherry (Hassan et al., 2013; Tefera et al., 2014; Bekele et al., 2017; Chilo et al., 2018). Nineteen publications on contamination of fruits and vegetables were retrieved. Details of the parasite prevalence and distribution in various countries are described in Section 3.6.
3.2. Prevalence and distribution of Cryptosporidium in water and sewage/effluents
For purposes of this review, water samples were categorized into surface (if obtained from river, lakes, sea, pond or well), tap (if obtained from some form of conveyance such as a tap or hydraulic pump or borehole) or waste water when specified as such. Majority of the publications were on surface water (39/68). Table 1 summarises the distribution and reported estimates of Cryptosporidium in various water sources and sewage/effluents based on the specific African countries. Thirty-three of the 39 publications (85%) on various water sources reported occurrence of Cryptosporidium. The estimates are tabulated in Table 1. The reported prevalence of Cryptosporidium ranged from 6% (Sente et al., 2016) to over 58% (Grundlingh and De Wet, 2004; Ajeagah et al., 2010; Amenu et al., 2013) in surface water while the prevalence in tap water (sachet water was considered as tap water) ranged from 4% (Ndur et al., 2015) to 100% (Fikrie et al., 2008) (Table 1). Cryptosporidium oocysts were also recovered in wastewater, sludge and sewage (Table 1) with prevalence as high as 100% (Dungeni and Momba, 2010). The regional prevalence in various water sources and effluent/sewage was reported at 1–43% in North Africa,0.9–100% in West Africa, 7–100% in East Africa and 3–100% in Central and Southern Africa.
Table 1.
Parasite distribution in positive water samples among various countries in Africa.
3.3. Prevalence and distribution of Giardia in water and sewage/effluents
From all the articles captured in this review, Giardia was the second most common parasite after Cryptosporidium; with 47 out of 68 articles reporting the parasite. In publications reporting both Cryptosporidium and Giardia, Giardia appeared to have higher prevalence rates (Fig. 4). The prevalence in surface water varied from 2.4% (Khalifa et al., 2014) to 100% (Ajeagah et al., 2005, Ajeagah et al., 2010) while that in tap water ranged from 1% (Atnafu et al., 2012) to over 70% (Fikrie et al., 2008) (Table 2).
Table 2.
Prevalence and distribution of Giardia in water and sewage/effluents among various African countries.
Articles on Giardia in wastewater, sludge/sewage were from Algeria, Cote d'Ivore, South Africa and Tunisia; the parasite estimates are indicated in Table 2. Prevalence ranged from 28% (Ben Ayed et al., 2012) to 100% of the tested sites and/or samples (Dungeni and Momba, 2010; Hamaidi-Chergui et al., 2019). The regional prevalence in various water sources and effluent/sewage ranged from 2 to 100% in North Africa, 0 to 100% in Central and Southern Africa, 5 to 70% in West Africa, and 1 to 73% in East Africa.
3.4. Prevalence and distribution of Cyclospora cayetanensis in water and sewage/effluents
Like Cryptosporidium and Giardia, Cyclospora cayetanensis was isolated from various water sources, wastewater and fresh produce. In surface water, the prevalence of C. cayetanensis ranged from 0.2% (El-Shazly et al., 2007) to 22% (2/9) (Dalu et al., 2011). In tap water, the prevalence was low for all studies reporting the parasite while in wastewater, Ben Ayed et al. (2012) reported the highest prevalence of 45% (see Table 3). The same article by Ben Ayed et al. (2012) further reported a C. cayetanensis prevalence of 58% in sludge (Table 3). The regional prevalence in various water sources and effluent/sewage was 0.2–58% in North Africa, 0–22% in Central and Southern Africa and 5–7% in West Africa.
Table 3.
Prevalence and distribution of Cyclospora cayetanensis in water and sewage/effluents among African countries.
| Parasite | Water sample type | Country reported | Prevalence range | References |
|---|---|---|---|---|
| Cyclospora cayetanensis | Surface water | Egypt | 0.2–4% | El-Shazly et al., 2007; Khalifa et al., 2014 |
| Zimbabwe | 10–22% | Dalu et al., 2011; Mtapuri-Zinyowera et al., 2014 | ||
| Tap water | Ghana | 5–7% | Ndur et al., 2015; Tetteh-Quarcoo et al., 2016 | |
| Zimbabwe | 0% | Dalu et al., 2011 | ||
| Wastewater | South Africa | 15% | Samie and Mashau, 2012 | |
| Tunisia | 45% | Ben Ayed et al., 2012 | ||
| Sludge | Tunisia | 58% | Ben Ayed et al., 2012 |
3.5. Prevalence and distribution of Entamoeba histolytica/dispar in water and sewage/effluents
Most studies did not apply molecular methods to distinguish E. histolytica from E. dispar and results were therefore reported as positive for E. histolytica/dispar. Similar to the other parasites discussed above, Entamoeba histolytica/dispar was reported in various water bodies and sources (Table 4) and from all the four categorized regions (North, West, East and Central and Southern Africa) with prevalence ranging from 8 to 36% in West Africa, 0.3 to 100% in North Africa, and 0 to 78% in Southern Africa. In surface water, the prevalence ranged from 1% (El-Shazly et al., 2007) to 78% (Mtapuri-Zinyowera et al., 2014). No publication reported Entamoeba spp. oocysts in tap water. In wastewater, two articles reported a prevalence of 100% (Khouja et al., 2010; Hamaidi-Chergui et al., 2019) and Ben Ayed et al. (2009) also reported a 100% prevalence in sludge.
Table 4.
Prevalence and distribution of Entamoeba in water and sewage/effluents among various African countries.
| Parasite | Water sample type | Country reported | Prevalence range | References |
|---|---|---|---|---|
| E. histolytica | Surface water | Burkina Faso | 8% | Kpoda et al., 2015 |
| Cameroon | 16% | Nsoh et al., 2016 | ||
| Cote d'Ivore | 35% | Koffi et al., 2014 | ||
| Egypt | 1–3% | El-Shazly et al., 2007; Khalifa et al., 2014 | ||
| Nigeria | 36% | Bishop and Inabo, 2015 | ||
| Sudan | 0.3% | Shanan et al., 2015 | ||
| Zimbabwe | 13–78% | Dalu et al., 2011; Mtapuri-Zinyowera et al., 2014 | ||
| Tap | Zimbabwe | 0% | Dalu et al., 2011 | |
| Wastewater | Algeria | 100% | Khouja et al., 2010; Hamaidi-Chergui et al., 2019 | |
| Sewage/sludge | Tunisia | 50–100% | Ben Ayed et al., 2009; Khouja et al., 2010 |
3.6. Prevalence and distribution of Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba in fresh produce
Nineteen publications out of the 68 publications reported contamination of fruits and vegetables with the subject parasites. The prevalence of Cryptosporidium on fresh produce (fruits and vegetables) ranged from 0.8% (Tchounga et al., 2017) to over 80% (Saaed and Ongerth, 2019). Most publications that reported the parasites in vegetables/fruits were from West Africa (8/12), this review did not capture any article from the Central and Southern African region (Table 5). The prevalence in East, West and North Africa were reported at 5–13%, 0.8–75% and 29–81% respectively.
Table 5.
Prevalence and distribution of Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba in fresh produce among various African countries.
Vegetables and fruits.
Tiger nuts.
Publications reporting Giardia cysts from fruits and vegetables in various countries are indicated in Table 5. Giardia cysts were identified in over 50% of the studies (73.7%; 14/19). Prevalence rates ranged from 2% (3/150) (Amaechi et al., 2016) to over 99% (159/160) (Chijioke et al., 2018). The regional prevalence in North, West and East Africa ranged from 3 to 84%, 2 to 99% and 8 to 28% respectively. Similar studies from central and southern Africa appear to be lacking as no articles on the subject matter were captured in the search.
A few (five) publications reported C. cayetanensis in fruits and vegetables with relatively lower prevalence rates (range: 5%–23%) (Table 5).
Entamoeba histolytica/dispar was also isolated from fresh fruits and vegetables (Table 5) with varying prevalence ranging from 3% (Hassan et al., 2013) to 99% (Chijioke et al., 2018). West Africa accounted for most of the articles (Table 5).
3.7. Prevalence and distribution of other protozoan parasites in water, sewage/effluents & fresh produce
The subject protozoan parasites in this review were Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba. The reviewed articles also reported other protozoa as tabulated in Table 6 with varying prevalence rates. In surface water, the following protozoa were reported: Toxoplasma gondii, Sarcocystis spp., Isospora belli, Balantidium coli, Blastocystis hominis, Acanthamoeba, Entamoeba coli, Trichomonas vaginalis and Chilomastix mesnilli; in wastewater. Isospora belli and Eimeria spp. were reported while in tap water, only Sarcocystis was reported (Table 6). Balantidium coli, T. gondii and I. belli were also reported in vegetables (Table 6).
Table 6.
Prevalence and distribution of other protozoan parasites reported in water, sewage/effluents & fresh produce in African countries.
| Parasite | Sample type | Country reported | Prevalence range | References |
|---|---|---|---|---|
| Toxoplasma gondii | Surface water | Sudan | 0.7–81% | Shanan et al., 2015; Medani et al., 2016 |
| Blastocystis hominis | Surface water | Cameroon | 4% | Nsoh et al., 2016 |
| Egypt | 1% | El-Shazly et al., 2007 | ||
| Sudan | 0.2% | Shanan et al., 2015 | ||
| Acanthamoeba | Surface water | Egypt | 73% | Sakran et al., 2017 |
| Sudan | 1% | Shanan et al., 2015 | ||
| Trichomonas vaginalis | Surface water | Sudan | 0.2% | Shanan et al., 2015 |
| Sarcocystis | Surface water | Cameroon | 7% | Nsoh et al., 2016 |
| Isospora belli | Surface water | Cameroon | 4% | Nsoh et al., 2016 |
| Egypt | 0.5% | El-Shazly et al., 2007 | ||
| Balantidium coli | Surface water | Cameroon | 4% | Nsoh et al., 2016 |
| Chilomastix mesnilli | Surface water | Egypt | 0.1% | El-Shazly et al., 2007 |
| Entamoeba coli | Surface water | Burkina Faso | 6% | Kpoda et al., 2015 |
| Sarcocystis | Tap water (sachets) | Ghana | 67% | Kwakye-Nuako et al., 2007 |
| I. belli | Wastewater | South Africa | 18% | Samie and Mashau, 2012 |
| Eimeria spp. | Wastewater | Tunisia | 81% | Ben Ayed et al., 2012 |
| Sludge | Tunisia | 58% | Ben Ayed et al., 2012 | |
| Balantidium coli | Vegetables | Ghana | 14% | Kudah et al., 2018 |
| Vegetables | Nigeria | 3% | Amaechi et al., 2016 | |
| T. gondii | Vegetables | Nigeria | 3% | Tchounga et al., 2017 |
| I. belli | Vegetables | Nigeria | 2% | Hassan et al., 2013 |
3.8. Helminth parasites and other pathogens in water, sewage/effluents & fresh produce
Various helminth species were reported in all water types and in wastewater (Table 7). However, not all articles provided estimates for the specific parasite species, making it difficult to make comparisons. In surface water, Ascaris lumbricoides, Strongyloides stercoralis, Ancylostoma duodenale, Enterobius vermicularis, hookworm; Taenia spp., Hymenolepis nana were reported (Dalu et al., 2011; Bishop and Inabo, 2015; Mtapuri-Zinyowera et al., 2014; Kpoda et al., 2015; Ndur et al., 2015; Mohamed et al., 2016). In wastewater, Ascaris, Trichuris spp., H. nana, H. diminuta, E. vermicularis, Taenia spp. and Toxocara spp. were reported (Ben Ayed et al., 2009; Khouja et al., 2010; Hamaidi-Chergui et al., 2019).
Table 7.
Prevalence and distribution of helminth parasites reported in water, sewage/effluents & fresh produce in African countries.
| Helminths | Sample type | Country reported | Prevalence range | References |
|---|---|---|---|---|
| Ascaris lumbricoides | Surface water | Burkina Faso | 8% | Kpoda et al., 2015 |
| Tap (sachet) | Ghana | 10% | Ndur et al., 2015 | |
| Sprinkling water | Sudan | 29% | Mohamed et al., 2016 | |
| Tap, stored | Zimbabwe | 22% | Dalu et al., 2011 | |
| Wastewater | Algeria | 100% | Hamaidi-Chergui et al., 2019 | |
| Tunisia | 71% | Khouja et al., 2010 | ||
| Sewage | Tunisia | 100% | Ben Ayed et al., 2009 | |
| Vegetables | Egypt | 20% | El Said Said, 2012 | |
| Vegetables/fruits | Ethiopia | 7–32% | Tefera et al., 2014; Bekele et al., 2017; Chilo et al., 2018 | |
| Vegetables | Libya | 68% | Abougrain et al., 2010 | |
| Nigeria | 19–100% | Hassan et al., 2013; Amaechi et al., 2016; Tchounga et al., 2017; Chijioke et al., 2018 | ||
| Sudan | 3% | Mohamed et al., 2016 | ||
| Ancylostoma duodenale (hookworm) | Surface water | Burkina Faso | 31% | Kpoda et al., 2015 |
| Sprinkling water | Sudan | 6% | Mohamed et al., 2016 | |
| Tap, stored | Zimbabwe | 44% | Dalu et al., 2011 | |
| Vegetables | Burkina Faso | 20% | Kpoda et al., 2015 | |
| Tiger nuts | Ghana | 25% | Ayeh-Kumi et al., 2014 | |
| Vegetables | Ghana | 13% | Duedu et al., 2014 | |
| Sudan | 6% | Mohamed et al., 2016 | ||
| Nigeria | 12% | Tchounga et al., 2017 | ||
| Strongyloides stercolaris | Surface water | Burkina Faso | 3% | Kpoda et al., 2015 |
| Nigeria | 7% | Bishop and Inabo, 2015 | ||
| Tap (sachet) | Ghana | 5% | Ndur et al., 2015 | |
| Sprinkling water | Sudan | 43% | Mohamed et al., 2016 | |
| Tap, stored | Zimbabwe | 11% | Dalu et al., 2011 | |
| Vegetables/fruits | Ethiopia | 22% | Tefera et al., 2014 | |
| Tiger nuts | Ghana | 22% | Ayeh-Kumi et al., 2014 | |
| Vegetables | Ghana | 36–43% | Duedu et al., 2014; Kudah et al., 2018 | |
| Nigeria | 2–20%% | Hassan et al., 2013; Amaechi et al., 2016; Tchounga et al., 2017 | ||
| Sudan | 9% | Mohamed et al., 2016 | ||
| Enterobius vermicularis | Surface | Nigeria | 3% | Bishop and Inabo, 2015 |
| Wastewater &sludge | Tunisia | 37% | Khouja et al., 2010 | |
| Sewage | Tunisia | 100% | Ben Ayed et al., 2009 | |
| Vegetables | Ghana | 1% | Duedu et al., 2014 | |
| Nigeria | 12–89% | Amaechi et al., 2016; Chijioke et al., 2018 | ||
| Trichuris trichiura | Wastewater | Algeria | 100% | Hamaidi-Chergui et al., 2019 |
| Vegetables | Ghana | 2% | Duedu et al., 2014 | |
| Nigeria | 6–89% | Hassan et al., 2013; Amaechi et al., 2016; Chijioke et al., 2018 | ||
| Sudan | 3% | Mohamed et al., 2016 | ||
| Schistosoma spp. | Tap, stored | Zimbabwe | 22% | Dalu et al., 2011 |
| Vegetables | Nigeria | 44% | Chijioke et al., 2018 | |
| Taenia spp. | Wastewater | Algeria | 100% | Hamaidi-Chergui et al., 2019 |
| Wastewater & sludge | Tunisia | 14% | Khouja et al., 2010 | |
| Sewage | Tunisia | 47% | Ben Ayed et al., 2009 | |
| Vegetables | Libya | 22% | Abougrain et al., 2010 | |
| Nigeria | 2–67% | Hassan et al., 2013; Chijioke et al., 2018 | ||
| Toxocara spp. | Wastewater | Algeria | 100% | Hamaidi-Chergui et al., 2019 |
| Vegetables/fruits | Ethiopia | 15–16% | Tefera et al., 2014; Bekele et al., 2017 | |
| Vegetables | Libya | 16–26 | Abougrain et al., 2010 | |
| Hymenolepis nana | Wastewater | Algeria | 100% | Hamaidi-Chergui et al., 2019 |
| Wastewater & sludge | Tunisia | 14% | Khouja et al., 2010 | |
| Sewage | Tunisia | 18% | Ben Ayed et al., 2009 | |
| Vegetables | Egypt | 3% | El Said Said, 2012 | |
| Vegetables/fruits | Ethiopia | 8–16% | Tefera et al., 2014; Bekele et al., 2017; Chilo et al., 2018 | |
| Hymenolepis diminuta | Wastewater | Algeria | 100% | Hamaidi-Chergui et al., 2019 |
| Vegetables/fruits | Ethiopia | 1–8% | Tefera et al., 2014; Bekele et al., 2017 | |
| Fasciola spp. | Vegetables | Ghana | 0.2–7% | Duedu et al., 2014; Kudah et al., 2018 |
| Nigeria | 8–78% | Amaechi et al., 2016; Tchounga et al., 2017; Chijioke et al., 2018 |
From fresh produce, nematodes (A. lumbricoides, S. stercolaris, Ancylostoma duodenale, E. vermicularis, hookworm) were commonly reported in most publications cestodes (Taenia spp., Hymenolepis nana, H. diminuta) and trematodes (Fasciola spp., Schistosoma mansoni) were also reported (Table 7).
Bacterial forms were also reported in surface water by Amenu et al. (2013); Koffi et al. (2014); Khalifa et al. (2014) in Ethiopia, Cote d'Ivore and Egypt respectively; tap water in Zimbabwe, South Africa and Ghana (Dalu et al., 2011; Dobrowsky et al., 2014; Tetteh-Quarcoo et al., 2016) and waste water in Cote d'Ivore (Yapo et al., 2014). In vegetables, bacteria were reported from studies from Ghana and Ethiopia (Ayeh-Kumi et al., 2014; Chilo et al., 2018). One publication from Egypt reported viruses in tap water (Ali et al., 2004).
4. Discussion
This review has brought out important findings indicating the widespread contamination of African water bodies, tap water and fresh produce with protozoan parasites Cryptosporidium, Giardia, Cyclospora and Entamoeba. The review has also revealed the presence of the protozoa and other parasites in sewage and treated effluents. A significant number of articles have been published across the African continent on Cryptosporidium and Giardia in water, fruits and vegetables especially after the year 2010 (Fig. 3). There is, however, lack of uniformity in the design, determination of sample size and reporting of findings making it difficult to compare the various studies.
The review has further revealed increasing research on the waterborne protozoan parasites in various water sources and fresh produce over time. Publications were distributed throughout Africa with North African countries accounting for the majority of the articles while East Africa had the least number (Fig. 2). However, despite the clustering in the northern region, some countries produced more publications than others, for example, Egypt had 11 publications while there was only one from Algeria. The status was similar for east Africa where there is limited information in Uganda and Kenya compared to Ethiopia. There is need for more countrywide research in the various parts of the continent to appreciate the burden of the subject parasites in Africa.
Studies in surface water revealed widespread contamination with Cryptosporidium and Giardia, information which corroborates with studies in developed countries (Bouzid et al., 2008; Karanis et al., 2007). For Cryptosporidium, prevalence of over 50% (Grundlingh and De Wet, 2004; Amenu et al., 2013) were reported in most studies while Giardia prevalence was as high as 100% in some of the water samples tested (Ajeagah et al., 2005, Ajeagah et al., 2010). Despite the variations in sample size, it is still clear that surface African surface waters are contaminated with the two important waterborne parasites. Tap water was equally contaminated, including packaged water (in sachets) (Ndur et al., 2015). Even though no major waterborne outbreak of diarrhoeal disease linked to protozoa contamination of water has been reported in Africa, the two parasites have previously been associated with waterborne outbreaks in the USA and other parts of the world (MacKenzie et al., 1994; Karanis et al., 2007). The high burden of Cryptosporidium and Giardia demonstrated in the reviewed studies in surface water, untreated and treated effluents provide evidence necessary to carry out causation studies in Africa. It is possible that contamination of water bodies (some of which are sources of water for many households) and tap water by these parasites could be responsible for some of the diarrhoea disease in affected countries. Future human studies reporting Cryptosporidium and/or Giardia infections should endeavor to establish sources of infections as this will assist in coming up with targeted intervention measures.
The review also highlighted a high level of parasite contamination in wastewater (treated and untreated) and sewage/sludge. Wastewater as well as treated effluents were variably contaminated with the parasites under study. Wastewater (treated and untreated) has been reported to be increasingly being used in agriculture with the majority in untreated form in developing countries due to scarcity of water (Scott et al., 2010; Dickin et al., 2016). The levels of contamination exhibited in this review therefore raises concern. In places where wastewater may be used for vegetable cultivation, such contamination as seen in the reviewed publications poses a public health risk. It is important that such acts, where practiced, are discouraged and safe water should be provided to communities to safeguard their health. In the current review, 13 out of the 19 studies and 14 of the 19 studies on parasite contamination in vegetables and/or fruits that are normally eaten raw reported presence of Cryptosporidium and Giardia parasites, respectively. Cyclospora cayetanensis and E. histolytica were also detected (Table 5). With the known clinical consequences of human infection with these parasites especially in immunocompromised individuals (Supplementary Table 1) and the zoonotic potential of the parasites, it is important that people are sensitized about the risks of consuming raw or undercooked foods. Health education could therefore assist in preventing infections. Fresh produce can be contaminated with enteric pathogens throughout the process of planting to consumption. It is therefore, important that strict hygiene measures are advocated for in the production process as well as at household level to prevent human infections. Several publications also reported presence of other pathogens including helminths, other protozoa and bacteria in water, sewage and effluents and fruits and vegetables (Table 6, Table 7). It is evident that there is widespread helminths contamination especially in surface water and vegetables.
With the current evidence, it is important that research linking diarrhoea disease with contamination of water bodies/sources or fresh produce with protozoan parasites be carried out in Africa. This will provide evidence-based data on the various possible causes and sources of diarrhoea disease especially in children and immunocompromised individuals in disadvantaged communities. Furthermore, only 17 out of the 54 African countries have conducted studies on these parasites; more studies are necessary in other countries to provide conclusive evidence on the exact burden of the four protozoan parasites in water sources, sewage/effluents and fresh produce.
The limitation of this review is the possibility of failing to capture publications that are not indexed in the searched data bases. Further, only four databases were searched. However, we believe most were obtained from the reference lists of the reviewed publications and these sufficiently give a picture of the status of the target parasites in Africa. Secondly, some studies did not strictly follow the STROBE guidelines making it impossible to extract uniform data from the articles. Another limitation is that most studies did not perform molecular tests to distinguish E. histolytica and E dispar. The prevalence of E. histolytica may therefore be overestimated.
5. Conclusions and future perspectives
In many African communities, particularly those in rural areas, individuals have limited access to adequate and safe household water. This coupled with inadequate or lack of water treatment, poor hygiene practices, and lack of awareness and education programmes, significantly contributes to predisposition of many such communities to parasitic infections. With population growth in the midst of inadequate infrastructure, sanitary facilities and lack of a systematic way of determining the prevalence Cryptosporidium, Giardia, Cyclospora and Entamoeba histolytica/dispar from food and water sources, parasitism in humans in the African setting will continue to be a challenge. The findings in this review echo this. Despite the importance and popularity of swimming pools particularly in hotter months of year, reports of protozoan parasites in these waters poses a public health risk to users. Further, swimming in contaminated water bodies, a common practice in rural settings, adds to the public health risk to users of these facilities. Periodic screening for these parasites in treated and untreated raw water particularly at household level and provision of adequate safe drinking water is advocated for. This should be supported by education and awareness programmes on the importance of using clean and safe water at household level including washing of fresh produce. Active and passive surveillance should also be conducted, and efforts should be made to minimise dissemination of oocysts and cysts in the farming environment and via human waste management. To achieve meaningful outcomes, it is also important to note that diseases that may result from infection with the discussed parasites cannot be managed with one method alone; they require an integrated and one health approach.
The following are the supplementary data related to this article.
Summarised overview of Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba.
Supplementary material
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abd El-Salam M.M. Assessment of water quality of some swimming pools: a case study in Alexandria, Egypt. Environ. Monit. Assess. 2012;184:7395–7406. doi: 10.1007/s10661-011-2508-6. [DOI] [PubMed] [Google Scholar]
- Abdel-Moein K.A., Saeed H. The zoonotic potential of Giardia intestinalis assemblage E in rural settings. Parasitol. Res. 2016;115:3197–3202. doi: 10.1007/s00436-016-5081-7. [DOI] [PubMed] [Google Scholar]
- Abougrain A.K., Nahaisi M.H., Madi N.S., Saied M.M., Ghenghesh K.S. Parasitological contamination in salad vegetables in Tripoli-Libya. Food Control. 2010;21:760–762. doi: 10.1016/j.foodcont.2009.11.005. [DOI] [Google Scholar]
- Ajeagah A.G., Foto S.M., Njime M., Wouafo M., Moyou R.S., Nola M. Distribution of the Giardia sp. cysts in the Mfoundi water basin. (Cameroon): influence of the physical-chemical factors of the medium. J. Cameroon Academy of Sciences. 2005;5:85–90. [Google Scholar]
- Ajeagah G., Njine T., Foto S., Bilong C.B., Karanis P. Enumeration of Cryptosporidium sp. and Giardia sp. (oo)cysts in a tropical eutrophic lake. Int. J. Environ. Sci. Technol. 2007;4:223–232. (ISSN: 1735-1472) [Google Scholar]
- Ajeagah G.A., Njine T., Bilong Bilong C., Foto S.M., Marguerite W.N., Nola Moïse. Seasonal distribution of enteric opportunistic Cryptosporidium spp. oocysts and Giardia spp. cysts in a tropical water basin Cameroon. Water. 2010;2:44–57. [Google Scholar]
- Alakpa G.E., Clarke S.C., Fagbenro-Beyioku A.F. Cyclospora cayetanensis infection: vegetables and water as possible vehicles for its transmission in Lagos, Nigeria. Br. J. Biomed. Sci. 2003;60:113–116. doi: 10.1080/09674845.2003.11783686. [DOI] [PubMed] [Google Scholar]
- Ali M.A., Al-Herrawy A.Z., El-Hawaary S.E. Detection of enteric viruses, Giardia and Cryptosporidium in two different types of drinking water treatment facilities. Water Res. 2004;38:3931–3939. doi: 10.1016/j.watres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Amaechi E.C., Ohaeri C.C., Ukpai O.M., Adegbite R.A. Prevalence of parasitic contamination of salad vegetables in Ilorin, North Central, Nigeria. Momona Ethiopian J. Sci. 2016;8:136–145. doi: 10.4314/mejs.v8i2.3. [DOI] [Google Scholar]
- Amenu G., Menkir S., Gobena T. Microbiological quality of drinking water sources and water handling practices among rural communities of Dire Dawa Administrative Council. Int. J. Curr. Res. Aca. Rev. 2013;1:29–54. (ISSN: 2347-3215) [Google Scholar]
- Atnafu T., Kassa H., Keil C., Fikrie N., Leta S., Keil I. Presence, viability and determinants of Cryptosporidium oocysts and Giardia cysts in the Addis Ababa water supply and distribution system. Water Qual Expo Health. 2012;4:55–65. doi: 10.1007/s12403-012-0065-z. [DOI] [Google Scholar]
- Ayeh-Kumi P.F., Tetteh-Quarcoo P.B., Duedu K.O., Obeng A.S., Addo-Osafo K., Mortu S. A survey of pathogens associated with Cyperus esculentus L. (tiger nuts) tubers sold in a Ghanaian city. BMC Res. Notes. 2014;7:343. doi: 10.1186/1756-0500-7-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldursson S., Karanis P. Waterborne transmission of protozoan parasites: review of worldwide outbreaks - an update 2004–2010. Water Res. 2011;45:6603–6614. doi: 10.1016/j.watres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Bekele F., Tefera T., Biresaw G., Tsegaye Yohannes T. Parasitic contamination of raw vegetables and fruits collected from selected local markets in Arba Minch town, Southern Ethiopia. Infect. Dis. Poverty. 2017;6:19. doi: 10.1186/s40249-016-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ayed L., Schijven J., Alouini Z., Jemli M., Sabbahi S. Presence of parasitic protozoa and helminth in sewage and efficiency of sewage treatment in Tunisia. Parasitol. Res. 2009;105:393–406. doi: 10.1007/s00436-009-1396-y. [DOI] [PubMed] [Google Scholar]
- Ben Ayed L., Yang W., Widmer G., Cama V., Ortega Y., Xiao L. Survey and genetic characterization of wastewater in Tunisia for Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi, Cyclospora cayetanensis and Eimeria spp. J. Water Health. 2012;10:431–444. doi: 10.2166/wh.2012.204. [DOI] [PubMed] [Google Scholar]
- Bishop H.G., Inabo H.I. Incidence of Entamoeba Histolytica in well water in Samaru-Zaria, Nigeria. IJSRES. 2015;3(00):16–0022. doi: 10.12983/ijsres-2015-p0016-0022. [DOI] [Google Scholar]
- Bouzid M., Steverding D., Tyler K.M. Detection and surveillance of waterborne protozoan parasites. Curr. Opin. Biotechnol. 2008;19:302–306. doi: 10.1016/j.copbio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Caccio S.M., Ryan U. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 2008;160:75–80. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Caccio S.M., Lalle M., Svard S.G. Host specificity in the Giardia duodenalis species complex. Infect. Genet. Evol. 2017;17:30418. doi: 10.1016/j.meegid.2017.12.001. S1567-1348. [DOI] [PubMed] [Google Scholar]
- Carrero J.C., Reyes-López M., Serrano-Luna J., Shibayama M., Unzueta J., León-Sicairos N. Intestinal amoebiasis: 160 years of its first detection and still remains as a health problem in developing countries. Int. J. Medical Microbiol. 2020 doi: 10.1016/j.ijmm.2019.151358. [DOI] [PubMed] [Google Scholar]
- CDC Update: outbreaks of cyclosporiasis—United States and Canada, 1997. Morb. Mortal. Wkly Rep. 1997;46:521–523. [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; 2014. Cyclosporiasis Outbreak Investigations—United States, 2014. [Google Scholar]
- Chia P.N., Ukaga C.N., Yongabi K.A., Nwoke B.E.B., Tih P.M. Baseline study on the occurrence of Cryptosporidium spp. from streams water, after torrential rains in Bamenda. Cameroon GJBAHS. 2015;4:62–69. (ISSN: 2319 – 5584) [Google Scholar]
- Chijioke U.O., Onyemelukwe N., Ogboi S.J. Factors affecting the parasitic contamination of edible locally produced dry season leafy vegetables cultivated in south east Enugu, Nigeria. AJCEM. 2018;19:133–140. doi: 10.4314/ajcem.v19i2.9. [DOI] [Google Scholar]
- Chilo E., Berkessa T., Lulu Y., Tolossa G., Berkessa B. Parasitological survey and antimicrobial susceptibility patterns of microbial isolates from pre-washed fruits and vegetables collected in Mettu town, I/A/Bora zone, southwest Ethiopia. IAJPS. 2018;5:13040–13049. http://www.iajps.com ISSN: 2349-7750. [Google Scholar]
- Cotruva J.A., Durfour A., Rees G., Bartram J., Carr R., Cliver D.O. World Health Organisation, IWA Publishing; 2004. Waterborne Zoonoses: Identification, Causes and Control. [Google Scholar]
- Dalu T., Barson M., Nhiwatiwa T. Impact of intestinal microorganisms and protozoan parasites on drinking water quality in Harare, Zimbabwe. J. Water Sanit. Hyg. Dev. 2011;1:153–163. doi: 10.1186/s13071-017-2111-y. [DOI] [Google Scholar]
- Dawson D. Foodborne protozoan parasites. International J. Food Microbiol. 2005;103:207–227. doi: 10.1016/j.ijfoodmicro.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Dickin S.K., Schuster-Wallace C.J., Qadir M., Pizzacalla K. A review of health risks and pathways for exposure to wastewater use in agriculture. Environ. Health Perspect. 2016;124:900–909. doi: 10.1289/ehp.1509995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky P., De Kwaadsteniet M., Cloete T., Khan W. Distribution of indigenous bacterial pathogens and potential pathogens associated with roof-harvested rainwater. Appl. Environ. Microbiol. 2014;80:2307–2316. doi: 10.1128/AEM.04130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorny P., Praet N., Deckers N., Gabriel S. Emerging food-borne parasites. Vet. Parasitol. 2009;163:196–206. doi: 10.1016/j.vetpar.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Duedu K.O., Yarnie E.A., Tetteh-Quarcoo P.B., Attah S.K., Donkor E.S., Ayeh-Kumi P.F. A comparative survey of the prevalence of human parasites found in fresh vegetables sold in supermarkets and open-aired markets in Accra, Ghana. BMC Res. Notes. 2014;7:836. doi: 10.1186/1756-0500-7-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhain G.L.M.C. 2011. Occurrence of Cryptosporidium spp. in South African Irrigation Waters and Survival of Cryptosporidium parvum During Vegetable Processing. (MSc Thesis. 2011) [Google Scholar]
- Dungeni M., Momba M.N.B. The abundance of Cryptosporidium and Giardia spp. in treated effluents produced by four wastewater treatment plants in the Gauteng province of South Africa. Water SA. 2010;36:425–432. (ISSN 1816-7950) [Google Scholar]
- Einarsson E., Ma'ayeh S., Svard S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016;34:47–52. doi: 10.1016/j.mib.2016.07.019. [DOI] [PubMed] [Google Scholar]
- El Said Said D. Detection of parasites in commonly consumed raw vegetables. Alex J. Med. 2012;48:345–352. doi: 10.1016/j.ajme.2012.05.005. [DOI] [Google Scholar]
- El-Kowrany S.I., El-Zamarany E.A., El-Nouby K.A., El-Mehy D.A., Ali E.A.A., Othman A.A. Water pollution in the Middle Nile Delta, Egypt: an environmental study. J. Adv. Res. 2016;7:781–794. doi: 10.1016/j.jare.2015.11.005. [DOI] [Google Scholar]
- El-Shazly A.M., Elsheikha H.M., Soltan D.M., Mohammad K.A., Morsy T.A. Protozoal pollution of surface water sources in Dakahlia Governorate, Egypt. J. Egyptian Soc. Parasitol. 2007;37:51–64. (PMID:17580568) [PubMed] [Google Scholar]
- Ethelberg S., Lisby M., Vestergaard L.S., Enemark H.L., Olsen K.E.P., Stensvold C.R. A foodborne outbreak of Cryptosporidium hominis infection. Epidemiol. Infect. 2009;137:348–356. doi: 10.1017/S0950268808001817. [DOI] [PubMed] [Google Scholar]
- Fantinatti M., Bello A.R., Fernandes O., Da-Cruz A.M. Identification of Giardia lamblia assemblage E in humans points to a new anthropozoonotic cycle. J. Infect. Dis. 2016;214:1256–1259. doi: 10.1093/infdis/jiw361. [DOI] [PubMed] [Google Scholar]
- Fayer R. Taxonomy and species delimitation in Cryptosporidium. Exp. Parasitol. 2010;124:90–97. doi: 10.1016/j.exppara.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Fayer R., Morgan U.M., Upton S.J. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 2000;30:1305–1322. doi: 10.1016/s0020-7519(00)00135-1. [DOI] [PubMed] [Google Scholar]
- Feng Y., Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrie N., Hailu A., Belete H. Determination and enumeration of Cryptosporidium oocyst and Giardia cysts in Legedadi (Addis Ababa) municipal drinking water system. Ethiop. J. Health Dev. 2008;22:68–70. [Google Scholar]
- Gelanew T., Lalle M., Hailu A., Pozio E., Caccio S.M. Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop. 2007;102:92–99. doi: 10.1016/j.actatropica.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Giangaspero A., Gasser R.B. Human cyclosporiasis. Lancet Infect. Dis. 2019;19:e226–e236. doi: 10.1016/S1473-3099(18)30789-8. [DOI] [PubMed] [Google Scholar]
- Grundlingh M., De Wet C.M.E. The search for Cryptosporidium oocysts and Giardia cysts in source water used for purification. Water SA. 2004;30:33–36. doi: 10.4314/wsa.v30i5.5163. [DOI] [Google Scholar]
- Hamaidi-Chergui F., Errahmani M.B., Ouahchia C. Occurrence and removal of protozoan cysts and helminth eggs in the Médéa sewage treatment plant (south-east of Algiers) Ann. Parasitol. 2019;65:139–144. doi: 10.17420/ap6502.193. [DOI] [PubMed] [Google Scholar]
- Hamdy D., El-Badry A., Abd El Wahab W. Assessment of Giardia and Cryptosporidium assemblages/species and their viability in potable tap water in Beni-Suef, Egypt using nested PCR/RFLP and staining. Iran. J. Parasitol. 2019;14:368–378. http://ijpa.tums.ac.ir [PMC free article] [PubMed] [Google Scholar]
- Harvey R.R. Notes from the field: outbreaks of cyclosporiasis—United States, June August 2013. Morb. Mortal. Wkly Rep. 2013;62:862. [PMC free article] [PubMed] [Google Scholar]
- Hassan A., Farouk H., Abdul-Ghani R. Parasitological contamination of freshly eaten vegetables collected from local markets in Alexandria, Egypt: a preliminary study. Food Control. 2012;26:500e503. doi: 10.1016/j.foodcont.2012.01.033. [DOI] [Google Scholar]
- Hassan A., Farouk H., Abdul-Ghani R., Hassanein F. Contamination of irrigation systems of dental units with Cryptosporidium species in Alexandria, Egypt: a neglected disinfection pitfall. Risk Management and Healthcare Policy. 2012;5:93–95. doi: 10.2147/RMHP.S35257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A.A., Ojuromi O.T., Onyeahialam O. Presence of parasitic ova, cysts and larvae on common fresh fruits and vegetables sold at some major markets in Ibadan, Oyo State, Nigeria. Zoologist. 2013;11:40–45. ISSN 1596 972X. [Google Scholar]
- Herwaldt B. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin. Infect. Dis. 2000;31:1040–1057. doi: 10.1086/314051. [DOI] [PubMed] [Google Scholar]
- Herwaldt B.L., Ackers M.L., Cyclospora Working Group An outbreak in 1996 of cyclosporiasis associated with imported raspberries. N. Engl. J. Med. 1997;336:1548–1556. doi: 10.1056/NEJM199705293362202. [DOI] [PubMed] [Google Scholar]
- Hoge C.W., Schlim D.R., Ghimire M., Rabold J.G., Pandey P., Walch A. Placebo-controlled trial of co-trimoxazole for Cyclospora infections among travellers and foreign residents in Nepal. Lancet. 1995;345:691–693. doi: 10.1016/S0140-6736(95)90868-4. [DOI] [PubMed] [Google Scholar]
- Insulander M., Svenungsson B., Lebbad M., Karlsson L., de Jong B. A foodborne outbreak of Cyclospora infection in Stockholm, Sweden. Foodborne Pathog. Dis. 2010;7:1585–1587. doi: 10.1089/fpd.2010.0628. [DOI] [PubMed] [Google Scholar]
- Karanis P., Kourenti C., Smith H. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health. 2007;5:1e38. doi: 10.2166/wh.2006.002. (PMID: 17402277) [DOI] [PubMed] [Google Scholar]
- Kato S., Ascolillo L., Egas J., Elson L., Gostyla K., Naples L. Waterborne Cryptosporidium oocyst identification and genotyping: use of GIS for ecosystem studies in Kenya and Ecuador. J. Eukaryot. Microbiol. 2003;50(Suppl. 1):548–549. doi: 10.1111/j.1550-7408.2003.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Kelly P., Baboo K.S., Ndubani P., Nchito M., Okeowo N.P., Luo N.P. Cryptosporidiosis in adults in Lusaka, Zambia, and its relationship to oocyst contamination of drinking water. J. Infect. Dis. 1997;176:1120–1123. doi: 10.1086/516528. [DOI] [PubMed] [Google Scholar]
- Kfir R., Hilner C., Du Preez M., Bateman B. Studies on the prevalence of Giardia cysts and Cryptosporidium oocysts in South African water. Water Sci. Technol. 1995;31:435–438. http://hdl.handle.net/10204/579 [Google Scholar]
- Khalifa R., Ahmad A.K., Abdel-Hafeez E.H., Mosllem F.A. Present status of protozoan pathogens causing water-borne disease in northern part of El-Minia Governorate, Egypt. J. Egypt. Soc. Parasitol. 2014;240:1–8. doi: 10.12816/0007860. [DOI] [PubMed] [Google Scholar]
- Khouja L.B., Cama V., Xiao L. Parasitic contamination in wastewater and sludge samples in Tunisia using three different detection techniques. Parasitol. Res. 2010;107:109–116. doi: 10.1007/s00436-010-1844-8. [DOI] [PubMed] [Google Scholar]
- Koffi M., Anoh J., N'Djetchi M., Konan T., Djè Y. Genetic-based investigation of three prevalent waterborne protozoa parasites in drinking water sources in Daloa district in Côte d'Ivoire. J. Appl. Biosci. 2014;77:6534. doi: 10.4314/jab.v77i1.10. [DOI] [Google Scholar]
- Kpoda N.W., Oueda A., Somé Y.S.C., Cissé G., Maïga A.H., Kabré G.B. Physicochemical and parasitological quality of vegetables irrigation water in Ouagadougou city, Burkina-Faso. Afr. J. Microbiol. Res. 2015;9:307–317. doi: 10.5897/AJMR2014.7295. [DOI] [Google Scholar]
- Kudah C., Sovoe S., Baiden F. Parasitic contamination of commonly consumed vegetables in two markets in Ghana. Ghana Med. J. 2018;52:88–93. doi: 10.4314/gmj.v52i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakye-Nuako G., Borketey P., Mensah-Attipoe I., Asmah R., Ayeh-Kumi P. Sachet drinking water in Accra: the potential threats of transmission of enteric pathogenic protozoan organisms. Ghana Med. J. 2007;41:62–67. doi: 10.4314/gmj.v41i2.55303. (PMID: 17925844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanata C. Studies of food hygiene and diarrhoeal disease. Int. J. Environ. Health Res. 2003;13(Suppl. 1):S175eS183. doi: 10.1080/0960312031000102921. [DOI] [PubMed] [Google Scholar]
- Lane S., Lloyd D. Current trends in research into the waterborne parasite Giardia. Critical Rev. Microbiol. 2002;28:123–147. doi: 10.1080/1040-840291046713. [DOI] [PubMed] [Google Scholar]
- Lund B.M., O'Brien S.J. The occurrence and prevention of foodborne disease in vulnerable people. Foodborne Pathog. Dis. 2012;8:961–973. doi: 10.1089/fpd.2011.0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie W.R., Hoxie N.J., Proctor M.E., Gradus M.S., Blair K.A., Peterson D.E. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- Maikai B.V., Baba-Onoja E.B.T., Elisha I.A. Contamination of raw vegetables with Cryptosporidium oocysts in markets within Zaria metropolis, Kaduna State, Nigeria. Food Control. 2013;31:45–48. doi: 10.1016/j.foodcont.2012.09.032. [DOI] [Google Scholar]
- Markos M. 2013. Prevalence of Intestinal Protozoan Parasites among School Children in Durame District in Relation to Different Sources of Drinking Water, Southern Ethiopia. (MSc Thesis. 2013) [Google Scholar]
- Marshall M.M., Naumovitz D., Ortega Y., Sterling C.R. Waterborne protozoan pathogens. Clin. Microbiol. Rev. 1997;10:67–85. doi: 10.1128/cmr.10.1.67. (PMCID: PMC172915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medani M.Y.I., Khogali H., Rahman S.A., Khogali S. Poster Presentation, 17th International Congress on Infectious Diseases/International Journal of Infectious Diseases 45S. 2016. Biological pollution of drinking water ponds hafirs with Toxoplasma gondii, Giardia and Cryptosporidium spp in Eastern Sudan; pp. 1–477. [Google Scholar]
- Minetti C., Chalmers R.M., Beeching N.J., Probert C., Lamden K. Giardiasis. BMJ. 2016;355 doi: 10.1136/bmj.i5369. [DOI] [PubMed] [Google Scholar]
- Mohamed M.A., Siddig E.E., Elaagip A.H., Edris A.M.M., 3 Nasr A.A. Parasitic contamination of fresh vegetables sold at central markets in Khartoum state, Sudan. Ann. Clin. Microbiol. Antimicrob. 2016;15:17. doi: 10.1186/s12941-016-0133-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monis P.T., Andrews R.H., Mayrhofer G., Ey P.L. Genetic diversity within the morphological species Giardia intestinalis and its relationship to host origin. Infect. Genet. Evol. 2003;3(1):29–38. doi: 10.1016/s1567-1348(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Mtapuri-Zinyowera S., Ruhanya V., Midzi N., Berejena C., Chin'ombe N., Nziramasanga P. Human parasitic protozoa in drinking water sources in rural Zimbabwe and their link to HIV infection. Germs. 2014;4(4):86–91. doi: 10.11599/germs.2014.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchiri J.M., Ascolillo L., Mugambi M., Mutwiri T., Ward H.D., Naumova E.N. Seasonality of Cryptosporidium oocyst detection in surface waters of Meru, Kenya as determined by two isolation methods followed by PCR. J. Water Health. 2009;7:67–75. doi: 10.2166/wh.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndur S.A., Kuma J.S.Y., Buah W.K., Galley J.Y. Quality of sachet water produced at Tarkwa, Ghana. Ghana Mining J. 2015;15:22–34. [Google Scholar]
- Nsoh F.A., Wung B.A., Atashili J., Benjamin P.T., Marvlyn E., Ivo K.K. Prevalence, characteristics and correlates of enteric pathogenic protozoa in drinking water sources in Molyko and Bomaka, Cameroon: a cross-sectional study. BMC Microbiol. 2016;16:268. doi: 10.1186/s12866-016-0890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandi P.A., Chu D.M.T., Bier J.W., Jackson G.J. Parasites and the food supply. Food Technol. 2002;56:72–81. [Google Scholar]
- Peters M.D.J., Godfrey C.M., Khalil H., McInerney P., Parker D., Soares C.B. Guidance for conducting systematic scoping reviews. Methodology paper. Int. J. Evid. Based. Health. 2015;13:141–146. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- Petersen H.H., Enemark H.L., Sampson A., Dalsgaard A. 2014. Occurrence of Cryptosporidium spp. Oocysts in Low Quality Water and on Vegetables in Kumasi, Ghana. Conference Poster. [Google Scholar]
- Robertson L.J., Gjerde B. Occurrence of parasites on fruits and vegetables in Norway. J. Food Prot. 2001:1793–1798. doi: 10.4315/0362-028x-64.11.1793. (PMID: 11726161) [DOI] [PubMed] [Google Scholar]
- Saaed F.M.A., Ongerth J.E. Cryptosporidium oocyst and Giardia cyst contamination of salad vegetables in Kufra city, Libya. J. Acad. Res. 2019;13:62–75. [Google Scholar]
- Sakran T.F., El-Shahawy G.A., Shalaby M.A., Sabry H.Y., Pessant M., Matooq P.M. Detection rates of waterborne protozoa in water sources from Fayoum Governorate. PUJ. 2017;10(1 & 2) doi: 10.21608/PUJ.2017.4734. (ISSN: 1687-7942) [DOI] [Google Scholar]
- Salit I.E., Khairnar K., Gough K., Pillai D.R. A possible Ccuster of sexually transmitted Entamoeba histolytica: genetic analysis of a highly virulent strain. Clin. Infect. Dis. 2009;49:346–353. doi: 10.1086/600298. [DOI] [PubMed] [Google Scholar]
- Samie A., Mashau F. 2012. Distribution of Cryptosporidium Species and Effect of Treatment Process in Wastewater Treatment Plants in the Vhembe District, South Africa. Conference Paper. [Google Scholar]
- Samie A., Ntekele P. Genotypic detection and evaluation of the removal efficiency of Giardia duodenalis at municipal wastewater treatment plants in Northern South Africa. Trop. Biomed. 2014;31:122–133. (PMID: 24862052) [PubMed] [Google Scholar]
- Sampson E.A. 2015. Cryptosporidium spp Contamination and Risk Associated With the Irrigation of Lettuce With Contaminated Water in the Kumasi Metropolis of Ghana. (PhD Thesis. 2015) [Google Scholar]
- Scott C.A., Drechsel P., Raschid-Sally L., Bahri A., Mara D., Redwood M. Wastewater irrigation and health: Assessing and mitigating risk in low-income countries. In: Drechsel P., Scott C.A., Raschid-Sally L., Redwood M., Bahri A., editors. Wastewater Irrigation and Health: Challenges and Outlook for Mitigating Risks in Low-income Countries. Earthscan; London, UK: 2010. pp. 381–394. [Google Scholar]
- Sente C., Erume J., Naigaga I., Mulindwa J., Ochwo S., Magambo P.K. Prevalence of pathogenic free-living Amoeba and other protozoa in natural and communal piped tap water from Queen Elizabeth protected area, Uganda. Infect. Dis. Poverty. 2016;5:68. doi: 10.1186/s40249-016-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanan S., Abd H., Bayoumi M., Saeed A., Gunnar Sandström G. Prevalence of protozoa species in drinking and environmental water sources in Sudan. Biomed. Res. Int. 2015 doi: 10.1155/2015/345619. (5 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponseller J.K., Griffiths J.K., Tzipori S. The evolution of respiratory cryptosporidiosis: evidence for transmission by inhalation. Clin. Microbiol. Rev. 2014;27:575–586. doi: 10.1128/CMR.00115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssemanda J.N., Reij M.W., van Middendorp G., Bouw E., van der Plaats R., Franz E. Foodborne pathogens and their risk exposure factors associated with farm vegetables in Rwanda. Food Control. 2018;89:86–96. doi: 10.1016/j.foodcont.2017.12.034. [DOI] [Google Scholar]
- Tchounga K.S., Ajugwo A.O., Nsa M., Oshoma C.E., Dunga K.E., Ikenazo H. Prevalence of intestinal parasites in vegetables sold in some local markets in Port-Harcourt, Rivers-State, Nigeria. Arch. Microbiol. Immunol. 2017;1:41–49. [Google Scholar]
- Tefera T., Biruksew A., Mekonnen Z., Eshetu T. Parasitic contamination of fruits and vegetables collected from selected local markets of Jimma town, southwest Ethiopia. Int. Sch. Res. Not. 2014;2014 doi: 10.1155/2014/382715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh-Quarcoo P.B., Anim-Baidoo I., Attah S.K., BAbdl-Latif Baako B., Opintan J.A., Minamor A.A. Microbial content of (Bowl Water) used for communal handwashing in preschools within Accra Metropolis. Ghana Int. J. Microbiol. 2016 doi: 10.1155/2016/2617473. (8 pages) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco A.C., Lillie E., Zarin W., O'Brien K.K., Colquhoun H., Levac D. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- Uneke C.J., Uneke B.I. Occurrence of Cryptosporidium species in surface water in south-eastern Nigeria: the public health implication. Int. J. Health. 2007;7:1–6. [Google Scholar]
- Utaaker K.S., Skjerve E., Robertson L.J. Keeping it cool: survival of Giardia cysts and Cryptosporidium oocysts on lettuce leaves. Int. J. Food Microbiol. 2017;2255:1–57. doi: 10.1016/j.ijfoodmicro.2017.05.009. [DOI] [PubMed] [Google Scholar]
- WHO/UNICEF . World Health Organization/United Nations International Children’s Emergency Fund; Geneva/New York: 2009. Diarrhoea: Why children are still dying and what can be done.http://apps.who.int/iris/bitstream/ [Google Scholar]
- World Health Statistics World Health Organisation, Geneva. 2014. http://apps.who.int/iris/bitstream/10665/112738/1/9789240692671_eng.pdf
- Yapo R.I., Koné B., Bonfoh B., Cissé G., Zinsstag J., Nguyen-Viet H. Quantitative microbial risk assessment related to urban wastewater and lagoon water reuse in Abidjan, Côte d'Ivoire. J. Water Health. 2014;12:2. doi: 10.2166/wh.2013.051. [DOI] [PubMed] [Google Scholar]
- Yongsi H.B.N. Suffering for water, suffering from water: access to drinking water and associated health risks in Cameroon. J. Health Popul. Nutr. 2010;28:424–435. doi: 10.3329/jhpn.v28i5.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef M.Y., Khalifa A.M., el Azzouni M.Z. Detection of cryptosporidia in different water sources in Alexandria by monoclonal antibody test and modified Ziehl Neelsen stain. J. Egypt. Soc. Parasitol. 1998;28:487–496. (PMID:9707677) [PubMed] [Google Scholar]
- Zahedi A., Field D., Ryan U. Molecular typing of Giardia duodenalis in humans in Queensland - first report of Assemblage E. Parasitol. 2017;144:1154–1161. doi: 10.1017/S0031182017000439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summarised overview of Cryptosporidium, Giardia, Cyclospora cayetanensis and Entamoeba.
Supplementary material