Highlights
-
•
Investigation of A. indica, M. oleifera, M. koenigii and P. guajava extracts against MRSA biofilm.
-
•
Pet. ether extract of A. indica show superior antibiofilm activity.
-
•
Extracts are non-toxic with minimal hemolytic properties.
Keywords: Plant extracts, Antimicrobial activity, Biofilm, Staphylococcus aureus, Anti-biofilm activity
Abstract
Biofilms are multi-species bacterial communities with complex structures that create antibiotic resistance, cause life-threatening infections, thereby considerable economic loss; needed new approaches. Medicinal plants are focused as new alternatives for their therapeutic and antimicrobial effects. Our present study, Azadirachta indica, Moringa oleifera, Murraya koenigii, and Psidium guajava extracts were investigated against MRSA. The preliminary antimicrobial study showed pet. ether extract of A. indica and ethanolic extract of P. guajava showed a MIC value of 125 μg/mL and MBC value of 500 μg/mL. These extracts showed biofilm inhibition in the range of 60.0–83.9 % and did not possess any hemolytic activity to the human erythrocytes. The plant species investigated in this study had different degrees of antibiofilm activity against MRSA. However, we suggest that A. indica and P. guajava are promising candidates and further investigation is needed to isolate the antimicrobial compounds for the management of MRSA and its mechanism of activity.
1. Introduction
Microorganisms in a human host adhere, arrange, and protect themselves according to the environments that allow cells to survive in hostile environments and disperse planktonic cells to colonize new niches [[1], [2], [3]]. For its survival, bacteria form biofilm by setting up communities through quorum sensing. In quorum sensing communication, bacteria secrete particular signaling molecules with specific genes which include virulence genes also [4]. Receptors (specific transcription regulatory proteins) receive these signals and activate the expression of target genes through DNA binding [5,6]. It is well known that biofilms are formed by different species of sessile microorganisms; biofilm bacteria differ in their cellular physiology and their genes when compared to planktonic bacteria. This biofilm forms synergistic micro consortia and enables quorum sensing within the cells enclosed in the extracellular polymeric substance (EPS) layer, which in turn empowers horizontal gene transfer. These formed biofilms reduce the penetration of antibacterial agents and antibiotics, eventually leading to develop antibacterial/antibiotic resistance up to 500–5000 times greater than usual concentrations and host immune defenses [[7], [8], [9], [10]]. Mature biofilms will intermittently disperse planktonic bacterial cells to the environments through interaction and wait for the occasion to invade a new host [1,11,12].
The National Institutes of Health (NIH) estimated that 70 % of microbial infections are chronic due to the formation of biofilms [13]. Moreover, biofilms contaminations major hospital devices; moreover heating, drying, cleaners, and detergents cannot remove these biofilms completely [14]. Methicillin-resistant Staphylococcus aureus (MRSA) is a hospital-acquired infectious pathogen which, now has become endemic and reflects in different categories such as HA-MRSA (healthcare-associated MRSA), CA-MRSA (community-associated MRSA) and LA-MRSA (livestock-associated). MRSA infections are life-threatening and have become a significant/super challenge in public health all over the world with an emergence of multidrug-resistant strains during 21st century. In addition MRSA is known to cause various skin infections and respiratory diseases [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]].
Different antibiotics were developed for eradicating various diseases; however, biofilms have been reported to be less sensitive against these developed antimicrobial agents [31,32]. Additionally, the major disadvantages of the antibiotics are disruption of the species composition in the intestinal flora and other side effects that include fever, nausea, and diarrhea [33].
Plants have historically served as the most significant source for new leads in drug discovery and development. World Health Organization (WHO) estimated, nearly 75–80 % of the world population still depends on herbs and other traditional medicines for their primary health care needs [34]. Since ancient times, plant extracts and their biologically active compounds have been utilized to treat several diseases and illnesses.
Plants such as Moringa oleifera Lam, Azadirachta indica A.Juss, Murraya koenigii (L.) Sprengel and Psidium guajava Lpossess a rich number of phytoconstituents and also possess many potential therapeutic activities. These plants are widely used in the traditional systems of medicine. Azadirachta indica (A. indica) leaves contain various phytoconstituents like nimbanene, nimbiol, nimbin, 6-desacetylnimbinene, nimbolide, nimbandiol, n-hexacosanol, ascorbic acid, amino acid, 7-desacetyl-7-benzoylgedunin, 7-desacetyl-7-benzoylazadiradione, 17-hydroxyazadiradione, ß-sitosterol, and Quercetin. Earlier studies have reported that A. indica has established noteworthy therapeutic activities like antiarthritic, anti-inflammatory, hypoglycemic, antipyretic, antiulcer, cytotoxicity activities, and antimicrobial activity against Staphylococcus aureus, Salmonella typhi (S.typhi), Escherichia coli (E.coli), Bacillus pumilus (B. pumilus) and Pseudomonas aeruginosa (P.aeruginosa) [[35], [36], [37]]. Moringa oleifera (M. oleifera) leaves contain a wide range of phytoconstituents such as omega-3, omega-6, palmitic acid, linoleic acid, protein, ß-carotene, vitamin C, minerals like potassium and calcium. Various parts of M. oleifera showed therapeutic activities like lipid-lowering, antitumor, antiepileptic, antipyretic, antiulcer, diuretic, antispasmodic, anti-inflammatory, antidiabetic, antifungal, hepatoprotective, antioxidant, and antibacterial activities against Bacillus subtilis (B.subtilis), E.coli, S. aureus, P. aeruginosa, Enterobacter aerogenes, Aspergillus niger and Candida albicans [[38], [39], [40]]. Leaves of Murraya koenigii (M. koenigii) contain carbohydrates, proteins, minerals, fiber, carotene, vitamin C, glycosides, oxalic acid, secondary metabolites like triterpenoid (tetrahydromahanmbine, cyclomahanimbine), coumarine (murrayone imperatoxin), alkaloids (murrayaline, murrayastine, pypayafolinecarbazole) and other compounds (mahanimbicine, phebalosin, bicyclomahanimbicine). These phytoconstituents showed a variety of pharmacological activities such as anti-inflammatory, analgesic, antioxidant, antitumor, hepatoprotective, antidiarrheal, and antimicrobial activities against S. aureus and B.subtilis [41]. Major phytoconstituents of Psidium guajava (P. guajava) leaves are isoflavonoids, phenolic compounds, rutin, catechin, epicatechin, kaempferol, and naringenin. Different parts of the plant have shown various therapeutic activities like antioxidant, anti-spasmodic, antioxidant, anticancer, hepatoprotective, antidiabetic, analgesic, anti-inflammatory, anti-diarrhea, anti-stomachache and antimicrobial activities against S. aureus, S. Typhi, E. coli and P. aeruginosa [37,42].
Considering the antimicrobial properties of these plants, we have attempted to investigate the antimicrobial and anti-biofilm potential of these plant extracts against the MRSA. Therefore, our study is aimed to investigate new compounds for defeating the microbial drug resistance, especially in biofilm structures, and to evaluate their anti-biofilm potential. The results of this investigation may provide newer leads for the successful treatment of MRSA biofilm infections.
2. Materials and methods
2.1. Materials required
Petroleum Ether and Ethanol (Obtained from Sisco Research Laboratories, India), Phosphate Buffer saline (pH 7.4), Mannitol Sorbitol agar, Muller Hinten agar, Nutrient broth, Luria Bertani broth and Crystal Violet dye (obtained from HiMedia Laboratories), distilled water. All the apparatus were washed with distilled water before doing experiments. All the experiments were triplicated.
2.2. Collection, processing and extraction of plants
Leaves from the plants, namely, Murraya koenigii, Moringa oleifera, Azadirachta indica, and Psidium guajava (Fig. 1) were collected from Ramanathapuram district, Tamil Nadu, India. The specimen for the proposed study was confirmed by Dr. S. Soosairaj, Assistant Professor, Department of Botany, St. Joseph’s College, Tiruchirappalli. Voucher specimens were prepared and were coded as SJCBOT2518, SJCBOT2519, SJCBOT2520 and SJCBOT2521 for Murraya koenigii, Moringa oleifera, Azadirachta indica, and Psidium guajava respectively. The leaves were collected in August and washed in running tap water to remove the adhered dust on the leaf surfaces. The washed leaves were then air-dried in shade for 15–20 days to remove moisture. The air-dried leaves were coarsely powdered and stored in an airtight container until extraction.
Fig. 1.
Leaves of the selected medicinal plants Azadirachta indica, Moringa oleifera, Murraya koenigii and Psidium guajava.
About 50 g of coarse powder of the leaves was extracted successively with 200 mL of petroleum ether (60−80 °C) for 8 h, followed by 200 mL of 99 % ethanol for 18 h in a Soxhlet apparatus. The solvents from the extracts were removed using a rotary vacuum evaporator. The resulting extracts were concentrated and dried in a hot air oven at 40 °C for 24 h.
2.3. Preliminary phytochemical analysis
Petroleum extracts and ethanol extracts were analyzed for the presence of secondary metabolite phytoconstituents namely, alkaloids, flavonoids, tannins, phenol, hydroxyanthroquinones, triterpenoids, steroids and saponins as per the earlier standard phytochemical test procedures [43,44]. The methods and procedures employed in the phytochemical tests are shown in Table 1.
Table 1.
Preliminary phytochemical screening and analysis.
| Phytoconstituent | Test | Testing procedure | Inference |
Azadirachta indica |
Moringa oleifera |
Murraya koenigii |
Psidium guajava |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P.E | Ethanol | P.E | Ethanol | P.E | Ethanol | P.E | Ethanol | ||||
| Alkaloids | Mayer | Sample + Dragendorff’s reagent | Reddish brown ppt. | + | + | + | + | + | + | + | + |
| Hager | Sample + Mayer’s reagent | White ppt. | + | + | + | + | + | + | + | + | |
| Ferric chloride | Sample + Hager’s reagent | Yellow ppt. | – | + | + | + | + | + | + | – | |
| Flavonoids | Ferric chloride | Sample + Ferric chloride | Yellow color, turns colorless with addition of 2 M HCl | – | + | + | + | + | + | + | – |
| Alkaline reagent | Sample + 5% NaOH | Dark green or greenish blue | – | + | + | + | + | + | – | – | |
| Tannin | Ferric chloride | Sample + 10 % lead acetate | Bulky white ppt. | – | + | + | + | + | + | + | – |
| Lead acetate | Sample + Ferric chloride | Dark green (condensed tannin) or greenish blue (hydrolysable tannin) | – | + | – | + | – | + | – | + | |
| Phenols | Ferric chloride | Sample + Ferric chloride | Dark green or bluish green | + | + | + | + | + | + | + | – |
| Hydroxyanthroquinones | Potassium hydroxide | Sample + 10 % Potassium hydroxide | Red color | – | – | – | – | – | – | – | + |
| Triterpenoids | Acidic reagent | Sample + acetic anhydride + Conc. H2SO4 | Violet/pink or red | + | – | – | + | + | + | – | + |
| Steroids | Acidic reagent | Sample + drop of Conc. H2SO4 along sides of test tubes | Blue or green rings | + | – | + | – | + | + | + | + |
| Saponins | Foam test | Sample + distilled water (agitated for 15 min) | Foam Formation | + | – | – | + | + | – | – | + |
2.4. Bacterial isolates
A total of 27 isolates were collected from Medical Microbiology Laboratory, Bharathidasan University, Tiruchirappalli, India. Finally S. aureus species were identified with coagulase test and mannitol fermentation tests.
2.4.1. Detection of MRSA
Cefoxitin disk diffusion (30 μg) method was used to detect the methicillin resistant S. aureus. At the concentration of 0.5 McFarland standard suspension of the isolate was prepared and created lawn culture on Mueller-Hinton agar plate (MHA). Then the cefoxitin disc was kept on the lawn culture and allowed to dry for 5 min. This agar plate was aerobically incubated at 35ᵒ C for 18 h to allow the grow of bacteria and measured the zone diameters. The zone of inhibition diameter is ≤21 mm were considered as methicillin resistant S. aureus (MRSA) whereas the diameter ≥22 mm indicates methicillin susceptible S. aureus (MSSA) [45,46].
2.5. Evaluation of antimicrobial activity
The antimicrobial studies for the extracts were performed using MRSA (obtained from Medical Microbiology Laboratory, Bharathidasan University, Tiruchirappalli, India). MRSA was grown on mannitol sorbitol agar medium (selective differential medium with high sodium chloride content) for further studies [47]. The antibiotic susceptibility of the MRSA isolated strain was tested against antibiotics such as Vancomycin and Ciprofloxacin.
2.5.1. Assessment of Minimum inhibitory concentration and Minimum bactericidal concentration
Minimum Inhibitory Concentration (MIC) of the extract was determined by the micro broth dilution method. Different concentrations of plant extract from 2 mg/mL to 125 μg/mL (2000 μg, 1000 μg, 500 μg, 250 μg and 125 μg) were prepared and transferred into test tubes. Then 100 μL of MRSA culture (106 CFU/mL) was added to each test tube and incubated at 37 °C for 24 h. After incubation, the samples were analyzed in a UV spectrophotometer at 595 nm to determine the MIC. MIC is defined as the lowest concentration of the extract that completely inhibits the growth of microorganisms.
For the determination of Minimum Bactericidal Concentration (MBC), 50 μL from each test tube that showed no visible growth was re-inoculated on Mueller Hinton (MH) agar plates. Then the plates were incubated at 37 °C for 12 h. MBC is defined as the lowest concentration of extracts showing no bacterial growth in the MH agar plates.
2.6. Optimization of biofilm formation
To optimize the formation of biofilm by MRSA, small glass pieces of dimension 5 mm × 5 mm were used as an abiotic surface for the attachment. The required time duration for the formation of biofilm was optimized by transferring the inoculated culture into a 24-well glass plate and incubated at 37 °C. The formation of biofilm was monitored and observed through a light microscope at different time intervals such as 24, 36, and 48 h [48].
2.7. Anti-biofilm studies
The anti-biofilm effect was assessed through light microscopy and quantitative determination of biofilm formation was determined by crystal violet staining method.
Luria Bertani (LB) medium was used, which was inoculated with MRSA culture. Inoculated culture (2 mL) whose OD value is above 0.3 was added into the 24 well plates containing a glass piece in each well. Then, 1 mg/mL and 2 mg/mL of the extracts were added to each well and incubated at 37 °C for about 48 h with positive control and negative control. The culture without the extract served as positive control and LB without culture served as the negative control. After incubation, the contents in wells were removed and the glass pieces were washed twice with sterile Phosphate Buffered Saline (PBS). The biofilm of the bacterial cells that adhered to the glass pieces was stained by using 1% crystal violet and incubated for 30 min. After 30 min incubation, plates were washed with sterile distilled water to remove the excess stain. The glass pieces were then viewed under a light microscope at 40 X magnification. The results were interpreted by comparing the extracts with positive and negative control. After analysis, the dye bound with the cells was solubilized in 95 % ethanol for 15 min. The de-stained solution from each glass piece was measured at absorbance 595 nm, taking the de-stained solution of negative control glass piece as blank. The results were interpreted in such a way that more number of cells absorb more amount of dye, which in turn gives a high optical density value.
The percentage of biofilm inhibition was found by employing the following formula:
2.8. In vitro hemolytic activity
In vitro hemolytic activity was performed by the spectrophotometer method. About 5 mL of human blood (from healthy volunteers with informed concern) was taken for this study. The blood was washed 4 times with PBS by centrifugation. About 500 μL of erythrocytes were mixed with 500 μL of 4 mg/mL concentration of plant extracts; such that the final concentration of the extracts will be 2 mg/mL. The mixture was then incubated at 37 °C for 1 h in an incubator. After incubation, the mixture was then centrifuged at 10,000 rpm for 10 min. The supernatant having free hemoglobin was collected and the absorbance was measured at 540 nm. As tap water is hypotonic, it can cause the lysis of cells. Hence tap water was served as a positive control; (i.e.) maximum hemolysis. PBS was used as the negative control; (i.e.) minimal hemolysis. The extracts were investigated for its hemolytic activity by comparing them with positive and negative controls [[49], [50], [51], [52]].
2.9. Statistical analysis
All tests were conducted in triplicate. Data are reported as means ± standard deviation (SD). Results were analyzed statically (P < 0.001) by using Graph Pad Prism Version 6.0.
3. Results and discussion
3.1. Phytochemical analysis of the selected medicinal plant's extracts
The qualitative phytochemical screening results showed that the extracts contain phytoconstituents such as alkaloids, flavonoids, tannins, phenols, anthroquinones, triterpenoids, steroids, and saponins. The phytochemical analysis observations of the extracts are shown in Table 1.
3.2. Detection of MRSA
To detect MRSA, disc diffusion method is a simple and it can be easily performed in microbiological laboratories. Earlier study results indicated that cefoxitin disk diffusion is sensitive and specific for determination of MRSA when compare to Oxacillin screen agar method [45]. Among the 27 isolates, only one was identified as MRSA, hence, it has been utilized for further studies.
3.3. Evaluation of antimicrobial activity
3.3.1. Antibiotic susceptibility testing
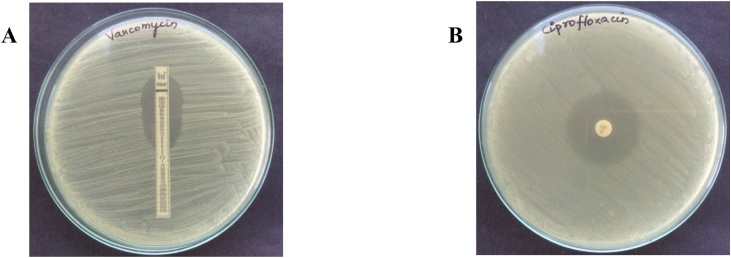
Susceptibility testing is performed to assess whether the antibiotics can inhibit the growth of the isolate. The disk diffusion method is the simplest, cost effective and most frequently used technique and it has been considered as gold standard for confirming the susceptibility of bacteria against the antibiotics [53]. Presently, vancomycin remains the most important first-line therapy for severe MRSA infection; whereas ciprofloxacin is used to treat Gram + ve bacteria (S. aureus) infections. Therefore, antibiotics such as vancomycin and ciprofloxacin were choosen and performed the antibiotic susceptibility testing by disk diffusion method aginst this MRSA isolate. Vancomycin showed inhibition at 4 μg/disc, whereas ciprofloxacin showed inhibtion at 5 μg/disc. The MRSA isolate was susceptible with vancomycin and ciprofloxacin and the observed findings for antibiotic susceptibility testing are shown in Fig. 2.
Fig. 2.
A. Antibiotic susceptibility testing - vancomycin at 4 μg/mL. B. Antibiotic susceptibility testing - ciprofloxacin at 5 μg/mL.
3.3.2. Assessment of Minimum inhibitory concentration and Minimum bactericidal concentration
The potential inhibitory activity of the extract was detected against the MRSA. The MIC test result showed that these extracts had inhibitory effects on the MRSA ranging from 125 μg/mL to 1000 μg/mL. Based on the observed results, pet.ether extract of A. indica and ethanol extract of P. guajava showed better inhibitory effects on the MRSA when compared to other extracts.
The MBC test results showed that these extracts have bactericidal activity on the MRSA ranging from 500 μg/mL to >2 mg/mL. The MIC and MBC results are tabulated in Table 2. The MBC results revealed that pet.ether extract of A. indica and ethanol extract of P. guajava showed the lowest MBC readings with the value of 500 μg/mL. The MBC results of these extracts inveterate their bactericidal activity, which is generally two to four times greater than their corresponding MIC values.
Table 2.
MIC and MBC of the selected medicinal plant extracts.
| Solvent | Extracts | MIC (μg/mL) | MBC (μg/mL) |
|---|---|---|---|
| Petroleum ether | A. indica | 125 ± 0.00 | 500 ± 0.00 |
| M. oleifera | 500 ± 0.00 | >2000 | |
| M. koenigii | 500 ± 0.00 | >2000 | |
| P. guajava | 250 ± 0.00 | 2000 ± 0.00 | |
| Ethanol | A. indica | 125 ± 0.00 | 2000 ± 0.00 |
| M. oleifera | 250 ± 0.00 | 500 ± 0.00 | |
| M. koenigii | 1000 ± 0.00 | >2000 | |
| P. guajava | 125 ± 0.00 | 500 ± 0.00 | |
MIC- Minimum inhibitory concentration.
MBC- Minimum bactericidal concentration.
Data are represented as mean MIC ± SD & mean MBC ± SD.
Plant extracts usually have a large array of phytoconstituents. Among the different phytoconstituents, terpenoids and flavonoids are the major groups of phytoconstituents present naturally in plants that possess good antimicrobial properties. The phytochemical investigation of these extracts reveals that these two phytoconstituents such as terpenoids and flavonoids are present in the pet. ether extract of A. indica and ethanol extract of P. guajava. The mechanism of action of terpenoids for its antibacterial effect is predominantly based on disruption of lipophilic compounds in the cell wall membrane which eventually leads to the inhibition of respiration, followed by the blockade in the ion transport process [54]. The mechanism of action of flavonoids for its antibacterial effect is by altering the electrical potential leads to damage to the energy system of the microorganism [55].
3.4. Biofilm formation
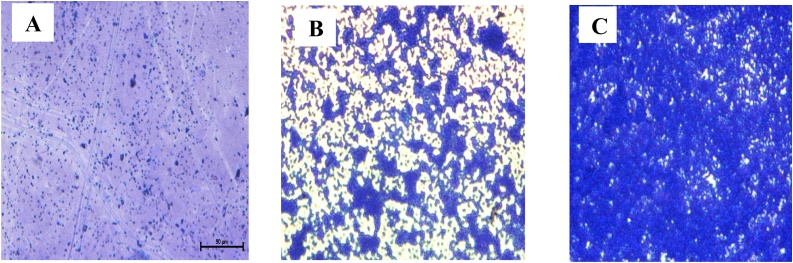
The period for the formation of biofilm was assessed through a light microscope at different time intervals such as 24, 36, and 48 h. Different stages in the formation of biofilm are attachment, aggregation, and mat formation, at different time intervals from 24 h to 48 h. In this study, at 24 h of incubation, the planktonic cells get attached to the abiotic surface (glass piece). At 36 h the planktonic cells start to aggregate and at 48 h of incubation, the complete biofilm was formed. The observed results revealed that the ability of the bacterial to form biofilms was influenced by the increased time duration of the incubation period. The light microscopic images for the formation of biofilm at different time intervals are shown in Fig. 3.
Fig. 3.
Light microscopic image of biofilm formation at different time intervals. A. Attachment of cells at 24 h. B. Aggregation of cells at 36 h. C. Biofilm formation or mat formation at 48 h.
3.5. Anti-biofilm studies
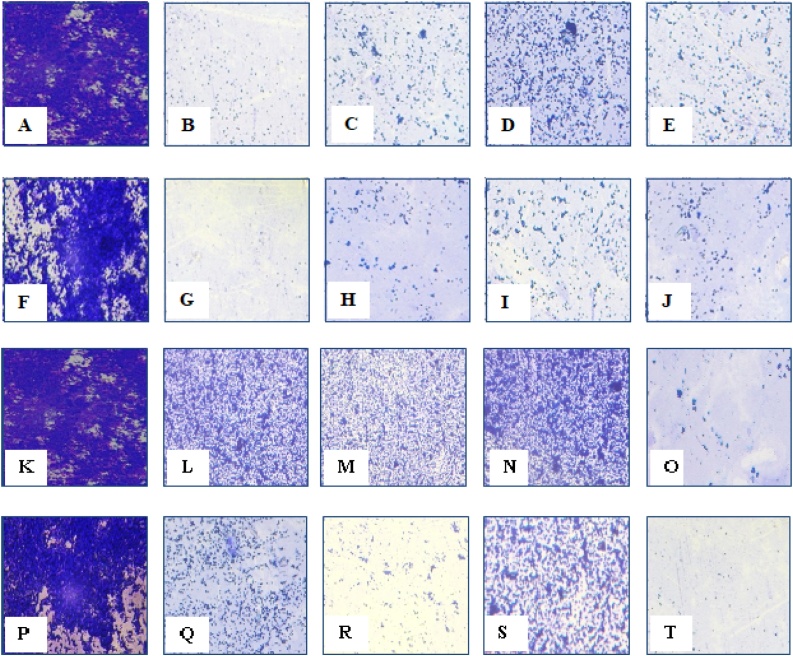
The anti-biofilm effect of the extracts was evaluated both qualitatively and quantitatively by light microscopy and crystal violet staining method at two different concentrations such as 1 mg/mL and 2 mg/mL. Light microscopy was used as a qualitative method. In the control, most of the areas were covered with a biofilm comprising clusters of bacteria. Non-aggregation of the cell was observed with the extracts. This qualitative finding was further investigated with the crystal violet staining method by comparing the absorbance between the controls and the extracts. The basic principle of this assay is based on the penetration of the dye into the cell membranes which eventually binds with the extracellular molecule having a negative charge. It also affects the cell surface molecules and bacteria’s extracellular polysaccharides. A significant (P < 0.001) reduction in inhibition of biofilm formation by pet. ether extract of A. indica (83.8 ± 1.02 %) and ethanolic extract of P. guajava (80.0 %±0.86 %) were observed, which is comparatively higher than that of the other extracts. The other extracts showed an inhibition percentage of around 30–60 %. The biofilm inhibition results are shown in Table 3.
Table 3.
Percentage of biofilm inhibition.
| Solvent | Extracts | Percentage of biofilm inhibition |
|
|---|---|---|---|
| 1 mg/mL | 2 mg/mL | ||
| Petroleum Ether | A. indica | 68.9 ± 0.64 | 83.8 ± 1.02 |
| M. oleifera | 34.86 ± 0.52 | 59.9 ± 0.62 | |
| M. koenigii | 57.67 ± 0.77 | 63.7 ± 0.73 | |
| P. guajava | 25.2 ± 0.53 | 62.9 ± 0.48 | |
| Ethanol | A. indica | 28.8 ± 0.82 | 43.0 ± 0.60 |
| M. oleifera | 34.86 ± 0.91 | 51.4 ± 0.93 | |
| M. koenigii | 27.5 ± 1.05 | 44.9 ± 0.38 | |
| P. guajava | 76.83 ± 0.56 | 80.0 %±0.86 | |
Data are represented as mean of percentage of biofilm inhibition ± SD; negative control group (without culture), positive control (without extract).
Quorum sensing (QS) is a signaling mechanism to establish communication between bacterial complexes, through the cell to cell signaling. Several strategies are adopted to interfere with the quorum sensing of the bacterial complex, namely, signal molecule inactivation, including quorum sensing disruption through inhibition of signal molecule biosynthesis and blockade of signal transduction [56,57]. It is reported that phytoconstituents such as flavonoids and terpenoids have potent anti-biofilm activity [58,59]. The phytochemical investigation results revealed that phytoconstituents such as terpenoids and flavonoids are present in these extracts.
The mechanism of flavonoids for its anti-biofilm activity is based on damaging the synthesis, secretion, signal transmission, antagonistic activity, and inhibition of receptor binding which leads to the shutdown of quorum sensing [60]. Also, the mechanism of terpenoids for its anti-biofilm activity is based on altering the cell membrane fatty acid composition, which causes the hydrophobicity of cells leads to biofilm eradication. Additionally, synergistic and antagonistic effects of other phytoconstituents may also have apparent anti-biofilm activity. The qualitative anti-biofilm effect of the extracts and controls through light microscopic study images are shown in Fig. 4.
Fig. 4.
Light microscopic image [Magnification 40X] of Biofilm inhibition by petroleum ether extracts.
3.6. In vitro hemolytic activity
The hemolytic activity provides primary information on the cytotoxicity of the bioactive molecules on the healthy cells. Assessment of in vitro hemolytic activity by UV spectrophotometric method is an easy and effective technique to assess the cytotoxicity of the bioactive molecules [50]. Erythrocytes have been widely used for the assessment of hemolytic activity and it has been considered to be the major target, which is affected by the concentration of the bioactive molecules in the extract [61]. The advantages of using erythrocyte as a model for hemolytic activity are the easy availability and isolation of cells from the blood. Moreover, this property is similar to other cell membranes [50].
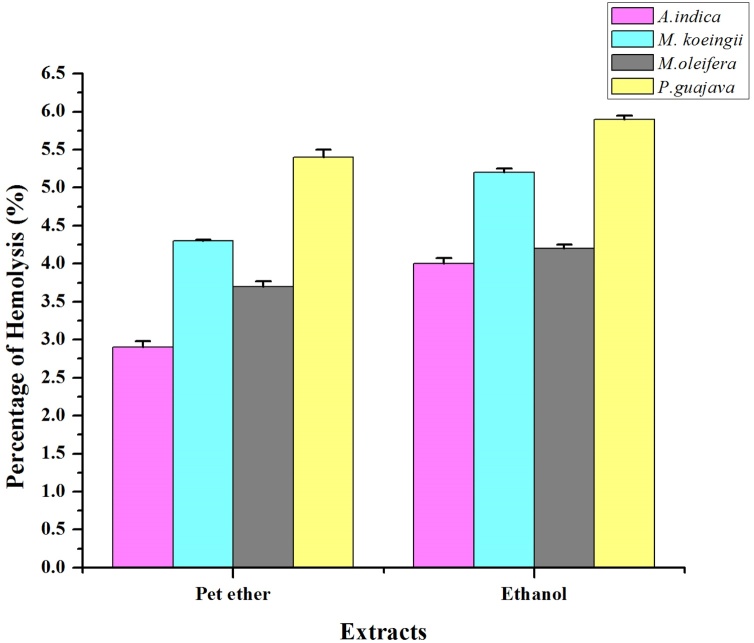
The hemolytic activity of the extracts was evaluated using human erythrocytes, by comparing the optical density of the extracts (Extract + human erythrocytes suspended in PBS) with the maximal hemolytic control (Tap water+Human erythrocytes suspended in PBS) and minimal hemolytic control (PBS+Human erythrocytes). The plant extracts hemolytic activity is expressed in percentage of hemolysis and reported as mean ± standard deviation of three replicates. The findings revealed that the extracts were non-toxic and have minimal hemolytic activity. This minimal hemolytic activity could be due to the presence of saponins and phenols in the extracts. Besides, saponins alter the erythrocyte membrane and promote the formation of methemoglobin through the oxidation of hemoglobin, which occurs due to the hemolysis by phenols [52]. This might also be the reason for the minimal hemolytic activity of the extracts. The comparative graphical representation of the extracts with controls of the hemolytic activity is shown in Fig. 5.
Fig. 5.
Hemolytic activity of 500 μL of erythrocytes+500 μL of 4 mg/mL concentration of plant extracts.
Data is represented as mean ± standard deviation (n = 3).
4. Summary
Biofilm formation by pathogenic microbes has become a serious threat to mankind since it is one of the primary contributing factors for drug resistance. Although, there are several reported surveys regarding antimicrobial activity, yet, there is a lack of authoritative studies concerning its effect on bacterial biofilm formation. This study is focused mainly on the anti-biofilm potential of selected medicinal plants such as A. indica, M. oleifera, M. koenigii, and P. guajava which are commonly found as garden plants, against the human selective pathogenic organism, MRSA.
Formation of biofilm by MRSA strain was optimized at different time intervals, a complete biofilm formation was observed at 48 h. Subsequently, inhibitory studies were performed by treating these extracts with the culture. Among these 8 extracts (4 plants extracted with 2 solvents), pet. ether extract of A. indica and ethanolic extract of P. guajava showed better antimicrobial and anti-biofilm activity compared to the other extracts.
The hemolytic activity of these extracts was determined by comparing it with the maximal and minimal hemolysis control. The findings revealed that the extracts were non-toxic and have a very minimal hemolytic activity.
Future perspectives: Although, several factors contribute towards the antimicrobial property of the crude extracts. Further, there is a need to purify and isolate specific phytoconstituents that are responsible for the antimicrobial and anti-biofilm activities. The above findings could serve as a better alternative treatment shortly for this human selective pathogenic organism MRSA.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors would like to thank Dr. K. Natarajaseenivasan, Professor, Department of Microbiology, Bharathidasan University, Tiruchirappalli for providing Laboratory facility to carry out this research work.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00523.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Lizana J.A., Lopez S., Marchal A., Serrano U., Velasco D., Urgel M.E. 2020. Use of Plant Extracts to Block Bacterial Biofilm Formation.http://digital.csic.es/bitstream/10261/100138/1/use_plant_Lizana.pdf (Assessed on September 2018) [Google Scholar]
- 2.Druschel G.K., Baker B.J., Gihring T.M., Banfield J.F. Acid mine drainage biogeochemistry at Iron mountain, California. Geochem. Trans. 2004;5:13–32. doi: 10.1186/1467-4866-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph R.L. Prosthetic joint infections: bane of orthopedists. Clin. Infect. Dis. 2003;36:1157–1161. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- 4.Antunes L.C., Ferreira R.B., Buckner M.M., Finlay B.B. Quorum sensing in bacterial virulence. Microbiology. 2010;156:2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- 5.Bhardwaj A.K., Vinothkumar K., Rajpara N. Bacterial quorum sensing inhibitors: attractive alternatives for control of infectious pathogens showing multiple drug resistance. Recent Pat. Antiinfect. Drug Discov. 2013;8:68–83. doi: 10.2174/1574891x11308010012. [DOI] [PubMed] [Google Scholar]
- 6.Karbasizade V., Dehghan P., Sichani M.M., Shahanipoor K., Sepahvand S., Jafari R. Evaluation of three plant extracts against biofilm formation and expression of quorum sensing regulated virulence factors in Pseudomonas aeruginosa. Pak. J. Pharm. Sci. 2017;30:585–589. [PubMed] [Google Scholar]
- 7.Pratiwi S.U.T., Lagendijk E.L., Hertiani T., Weert S.D., Van Den Hondel C.A.M.J.J. Antimicrobial effects of Indonesian medicinal plants extracts on planktonic and biofilm growth of Pseudomonas aeruginosa and Staphylococcus aureus. J. Hortic. 2015;2:119. doi: 10.4172/2376-0354.1000119. 2. [DOI] [Google Scholar]
- 8.Yarwood J.M., Schlievert P.M. Quorum sensing in staphylococcus infections. J. Clin. Invest. 2003;112:1620–1625. doi: 10.1172/JCI20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Percival S.L., Suleman L., Vuotto C., Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J. Med. Microbiol. 2015;64:323–334. doi: 10.1099/jmm.0.000032. [DOI] [PubMed] [Google Scholar]
- 10.Grant S.S., Hung D.T. Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response. Virulence. 2013;4:273–283. doi: 10.4161/viru.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolan R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall-stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 13.Shanmugapriya P., Roziahanim M. Chemical analysis, inhibition of biofilm formation and biofilm eradication potential of Euphorbia hirta L. against clinical isolates and standard strains. BMC Complement. Altern. Med. 2013;13:346. doi: 10.1186/1472-6882-13-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branda S.S., Vik S., Friedman L., Kolter R. Biofilms: the matrix revisited. Trends Microbiol. 2005;13:20–26. doi: 10.1016/j.tim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Green B.N., Johnson C.D., Egan J.T., Rosenthal M., Griffith E.A., Evans M.W. Methicillin-resistant Staphylococcus aureus: an overview for manual therapists. J. Chiropr. Med. 2012;11:64–76. doi: 10.1016/j.jcm.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karchmer A.W., Bayer A.S. Methicillin-resistant Staphylococcus aureus: an evolving clinical challenge. Clin. Infect. Dis. 2008;46:S342–S343. doi: 10.1086/533589. [DOI] [PubMed] [Google Scholar]
- 17.Gordon R.J., Lowy F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008;46:S350–9. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasricha J., Harbarth S., Koessler T., Camus V., Schrenzel J., Cohen G. Methicillin-resistant Staphylococcus aureus risk profiling: who are we missing? Antimicrob. Resist. Infect. Control. 2013;2:17. doi: 10.1186/2047-2994-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kock R., Becker K., Cookson B., Van Gemert-Pijnen J.E., Harbarth S., Kluytmans J. Methicillin-resistant staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill. 2010;15:19688. doi: 10.2807/ese.15.41.19688-en. [DOI] [PubMed] [Google Scholar]
- 20.Maksoud M.A., El-Shokry M., Ismail G., Hafez S., El-Kholy A., Attia E. Methicillin-resistant Staphylococcus aureus recovered from healthcare and community associated infections in Egypt. Int. J. Bacteriol. 2016 doi: 10.1155/2016/5751785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumari J., Shenoy S.M., Baliga S., Chakrapani M., Bhat G.K. Healthcare associated Methicillin-sesistant Staphylococcus aureus clinical characteristics and antibiotic resistance profile with emphasis on macrolide-lincosamide-streptogramin B resistance. Sultan Qaboos Univ. Med. J. 2016;16:e175–e181. doi: 10.18295/squmj.2016.16.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Z., Peng Y., Chen X., Bi J., Li Y., Ye X. Healthcare associated infections of methicillin-sesistant Staphylococcus aureus: a case-control-control study. PLoS One. 2015;10:e0140604. doi: 10.1371/journal.pone.0140604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David M.Z., Daum R.S. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang H., Flynn N.M., King J.H., Monchaud C., Morita M., Cohen S.H. Comparisons of community-associated methicillin-resistant staphylococcus aureus (MRSA) and hospital-associated MSRA infections in Sacramento, California. J. Clin. Microbiol. 2006;44:2423–2427. doi: 10.1128/JCM.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen J., Petersen A., Larsen A.R., Sieber R.N., Stegger M., Koch A. Emergence of livestock-associated methicillin-resistant Staphylococcus aureus bloodstream infections in Denmark. Clin. Infect. Dis. 2017;7:1072–1076. doi: 10.1093/cid/cix504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma M., Nunez-Garcia J., Kearns A.M., Doumith M., Butaye P.R., Argudín M.A. Livestock associated methicillin resistant Staphylococcus aureus (LA-MRSA) clonal complex (CC) 398 isolated from UK animals belong to European lineages. Front. Microbiol. 2016;7:1741. doi: 10.3389/fmicb.2016.01741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Cleef B.A., Monnet D.L., Voss A., Krziwanek K., Allerberger F., Struelens M. Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg. Infect. Dis. 2011;7:502–505. doi: 10.3201/eid1703.101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin L., Mao X., Sun Y., Cui H. Antibacterial mechanism of artemisinin / beta-cyclodextrins against methicillin-resistant Staphylococcus aureus (MRSA) Microb. Pathog. 2018;118:66–73. doi: 10.1016/j.micpath.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Cui H., Li W., Li C., Vittayapadung S., Lin L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphycoccus aureus biofilm. Biofouling. 2016;32:215–225. doi: 10.1080/08927014.2015.1134516. [DOI] [PubMed] [Google Scholar]
- 30.Hu W., Li C., Dai J., Cui H., Lin L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA) Ind. Crops Prod. 2019;130:34–41. [Google Scholar]
- 31.Parsek M., Greenberg Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2004;13:3–27. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Kamal G.D., Pfaller M.A., Rempe L.E., Jebson P.J.R. Reduced intravascular catheter infection by antibiotic bonding. A prospective, randomized, controlled trial. JAMA. 1991;265:2364–2368. [PubMed] [Google Scholar]
- 33.Cecilia J., Sonja L., Charlotta E., Janet K.J. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 34.Kunle O.F., Egharevba H.O., Ahmadu P.O. Standardization of herbal medicines - a review. Int. J. Biodivers. Conserv. 2012;4:101–112. [Google Scholar]
- 35.Alzohairy M.A. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid. Based Complement. Altern. Med. 2016;11:1–11. doi: 10.1155/2016/7382506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maragathavalli S., Brindha S., Kaviyarasi N.S., Annadurai B., Gangwar S.K. Antimicrobial activity in leaf extract of neem (Azadirachta indica Linn.) Int. J. Sci. Nat. 2012;3:110–113. [Google Scholar]
- 37.Rasool M., Malik A., Arooj M., Alam M.Z., Alam Q., Awan M. Evaluation of antimicrobial activity of ethanolic extracts of Azadirachta indica and Psidium guajava against clinically important bacteria at varying pH and temperature. Biomed. Res. 2017;28:134–139. [Google Scholar]
- 38.Anwar F., Latif S., Ashraf M., Gilani A.H. Moringa oleifera: a food plant with multiple medicinal uses. Phytother. Res. 2007;21:17–25. doi: 10.1002/ptr.2023. [DOI] [PubMed] [Google Scholar]
- 39.Saini R.K., Sivanesan I., Keum Y.S. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016;6:203. doi: 10.1007/s13205-016-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lakshmana Prabu S., Umamaheswari A., Puratchikody A. Phytopharmacological potential of the natural gift Moringa oleifera Lam and its therapeutic application: an overview. Asian Pac. J. Trop. Med. 2019;12:485–498. [Google Scholar]
- 41.Gahlawat D.K., Jakhar S., Dahiya P. Murraya koenigii (L.) Spreng: an ethnobotanical, phytochemical and pharmacological review. J. Pharmacog. Phytochem. 2014;3:109–119. [Google Scholar]
- 42.Barbalho S.M., Farinazzi-Machado F.M.V., Goulart R.D.A., Brunnati A.C.S., Ottoboni A.M.M.B., Nicolau C.C.T. Psidium guajava (Guava): a plant of multipurpose medicinal applications. Med. Aromat. Plants. 2012;1:104. [Google Scholar]
- 43.Raaman N. New India Publishing Agency; New Delhi: 2006. Phytochemical Techniques. [Google Scholar]
- 44.Kokate C.K., Purohit A.P., Gokhale S.B. 47th ed. Nirali Prakashan Publication; India: 2011. Pharmacognosy. [Google Scholar]
- 45.Alipour F., Ahmadi M., Javadi S. Evaluation of different methods to detect methicillin resistance in Staphylococcus aureus (MRSA) J. Inf. Publ. Health. 2014;7:186–191. doi: 10.1016/j.jiph.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Kateete D.P., Kimani C.N., Katabazi F.A., Okeng A., Okee M.S., Nanteza A. Identification of Staphylococcus aureus: DNase and Mannitol salt agar improve the efficiency of the tube coagulase test. Ann. Clin. Microbiol. Antimicrob. 2010;9:23. doi: 10.1186/1476-0711-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.2012. OIE Terrestrial Manual - Laboratory Methodologies for Bacterial Antimicrobial Susceptibility Testing; pp. 1309–1321. [Google Scholar]
- 48.Quelemes P.V., Perfeito M.L., Guimarães M.A., dos Santos R.C., Lima D.F., Nascimento C. Effect of neem (Azadirachta indica A.Juss) leaf extract on resistant staphylococcus aureus biofilm formation and schistosoma mansoni worms. J. Ethnopharmacol. 2015;175:287–294. doi: 10.1016/j.jep.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization; Geneva: 1998. Quality Control Methods for Medicinal Plant Materials; pp. 43–44. [Google Scholar]
- 50.Zohra M., Fawzia A. Hemolytic activity of different herbal extracts used in Algeria. Int. J. Pharma Sci. Res. 2014;5:495–500. [Google Scholar]
- 51.Kumar G., Karthik L., Rao K.V.B. Haemolytic activity of Indian medicinal plants toward human erythrocytes: an in vitro study. Elixir Appl. Bot. 2011;40:5534–5537. [Google Scholar]
- 52.de Oliveria V.M.A., Carneiro A.L.B., Cauper G.S.B., Pohlit A.M. In vitro screening of amazonian plants for hemolytic activity and inhibition of platelet aggregation in human blood. Acta Amazon. 2009;39:973–980. [Google Scholar]
- 53.Khan Z.A., Siddiqui M.F., Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics. 2019;9:49. doi: 10.3390/diagnostics9020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cown M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Policegoudra R., Rehna K., Rao L.J., Aradhya S. Antimicrobial, antioxidant, cytotoxicity and platelet aggregation inhibitory activity of a novel molecule isolated and characterized from mango ginger (Curcuma amada Roxb.) rhizome. J. Biosci. 2010;35:231–240. doi: 10.1007/s12038-010-0027-1. [DOI] [PubMed] [Google Scholar]
- 56.Hartmann A., Rothballer M., Hense B.A., Schroder P. Bacterial quorum sensing compounds are important modulators of microbeplant interactions. Front. Plant Sci. 2014;5:131–142. doi: 10.3389/fpls.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rampioni G., Leoni L., Williams P. The art of antibacterial warfare: deception through interference with quorum sensing-mediated communication. Bioorg. Chem. 2014;55:60–68. doi: 10.1016/j.bioorg.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Kuzma L., Rozalski M., Walencka E., Rozalska B., Wysokinska H. Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: salvipisone as a potential anti-biofilm agent active against antibiotic resistant staphylococci. Phytomedicine. 2007;14:31–35. doi: 10.1016/j.phymed.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Jain A., Parihar D.K. Antibacterial, biofilm dispersal and antibiofilm potential of alkaloids and flavonoids of Curcuma. Biocatal. Agric. Biotechnol. 2018;16:677–682. [Google Scholar]
- 60.Nazzaro F.F., Coppola R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013;14:12607–12619. doi: 10.3390/ijms140612607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.James O., Alewo I.M. In vitro antihemolytic activity of Gymnema sylvestre extracts against hydrogen peroxide (H2O2) induced haemolysis in human erythrocytes. Am. J. Phytomed. Clin. Ther. 2014;2:861–869. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.