Summary
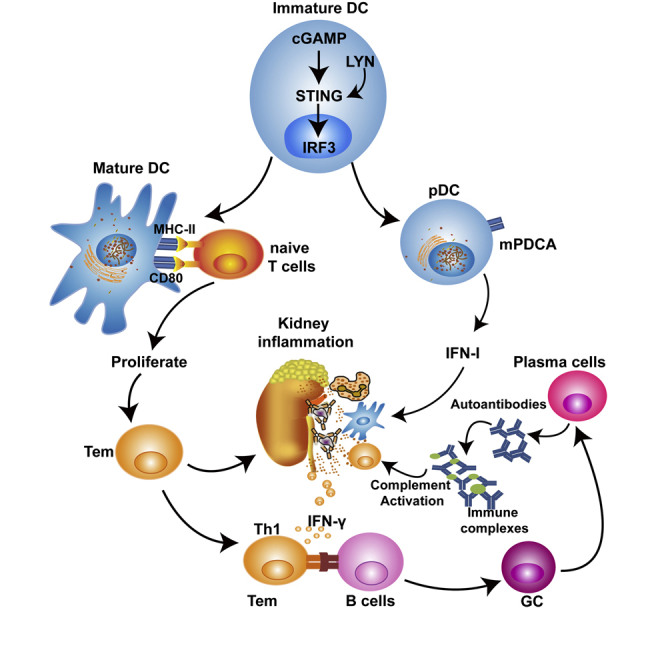
Signaling through stimulator of interferon genes (STING) leads to the production of type I interferons (IFN-Is) and inflammatory cytokines. A gain-of-function mutation in STING was identified in an autoinflammatory disease (STING-associated vasculopathy with onset in infancy; SAVI). The expression of cyclic GMP-AMP, DNA-activated cGAS-STING pathway, increased in a proportion of patients with SLE. The STING signaling pathway may be a candidate for targeted therapy in SLE. Here, we demonstrated that disruption of STING signaling ameliorated lupus development in Fcgr2b-deficient mice. Activation of STING promoted maturation of conventional dendritic cells and differentiation of plasmacytoid dendritic cells via LYN interaction and phosphorylation. The inhibition of LYN decreased the differentiation of STING-activated dendritic cells. Adoptive transfer of STING-activated bone marrow-derived dendritic cells into the FCGR2B and STING double-deficiency mice restored lupus phenotypes. These findings provide evidence that the inhibition of STING signaling may be a candidate targeted treatment for a subset of patients with SLE.
Subject Areas: Immunology, Molecular Genetics, Molecular Biology
Graphical Abstract
Highlights
-
•
STING constitutively activates in the Fcgr2b-deficient lupus mice
-
•
Signaling through STING-LYN interaction promotes DC differentiation
-
•
Inhibition of STING pathway disrupts the lupus phenotypes
-
•
STING mediates lupus disease via the activation of dendritic cells
Immunology; Molecular Genetics; Molecular Biology
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease with characteristics of autoantibody production and immune complex deposition that lead to severe inflammation and fatal glomerulonephritis. The heterogeneity of lupus disease has been shown through several mouse models of lupus disease, suggesting a variety of unique mechanisms participating in its pathogenesis. Type I interferon (IFN-I) is known to play significant roles in SLE pathogenesis (Muskardin and Niewold, 2018). In many patients with SLE, the expression of interferon-inducible genes is increased in their peripheral blood mononuclear cells (Baechler et al., 2003). Nucleic acid-sensing pathways are the main contributors of IFN-I production (Paludan, 2015). Several studies suggest that inappropriate recognition of self-nucleic acids can induce the production of IFN-I and promote SLE disease (Muskardin and Niewold, 2018).
Nucleic acids derived from extracellular sources are sensed via endosomal Toll-like-receptors (TLRs), whereas the recognition of cytosolic nucleic acids is independent of TLRs (Keating et al., 2011). The activation of TLRs, such as TLR7 and TLR9, by endosomal nucleic acids, leads to type I interferon production (Asselin-Paturel et al., 2005). Spontaneous duplication of Tlr7 causes autoimmune lupus phenotypes in Yaa-carrying BXSB mice (Pisitkun et al., 2006). Overexpression of Tlr7 promotes autoimmunity through dendritic cell proliferation, whereas the deletion of Tlr7 in lupus-prone MRL/lpr mice diminishes autoantibody and immune activation (Christensen et al., 2006; Deane et al., 2007). However, blocking TLR-mediated signaling by anti-malarial drugs can only treat SLE with mild disease activity (Sacre et al., 2012; Zeidi et al., 2019). Thus, investigation of other nucleic acid sensor pathways involved in lupus development could offer a more significant therapeutic opportunity.
Several cytosolic DNA sensors can induce IFN-I production, with cyclic GMP-AMP synthase (cGAS) being the major one (Sun et al., 2013). Cytosolic DNA sensing is also essential for innate immune signaling, and dysregulation of this process can cause autoimmune and inflammatory diseases (Yan, 2017). Stimulator of interferon genes (STING), also known as transmembrane protein 173 (TMEM173), is a cytoplasmic adaptor protein that acts downstream of cGAS to enhance IFN-I production (Ishikawa et al., 2009). The loss-of-function mutations in a DNA-specific exonuclease gene TREX1, resulting in increased cytosolic DNA levels, are observed in the type I interferonopathies Aicardi-Goutieres syndrome (AGS) and chilblain lupus (Crow et al., 2006; Günther et al., 2013). Consistent with these scenarios in human, Trex1-deficient mice exhibit fatal inflammation and autoimmunity (Morita et al., 2004; Stetson et al., 2008). Inhibition of the STING pathway in these mice improves their inflammatory condition and survival (Ahn et al., 2014). Moreover, the absence of STING rescues embryonic lethality and arthritis development in another nuclease knockout model, i.e., DNase II-deficient mice (Ahn et al., 2012).
The spontaneous lupus mouse models commonly used to study SLE pathogenesis are MRL/lpr, NZBxNZW.F1, and BxSB (Theofilopoulos and Dixon, 1985). Since these models possess different genetic backgrounds, each model could develop lupus with unique pathogenesis (Murphy and Roths, 1979; Pisitkun et al., 2006; Theofilopoulos and Dixon, 1985). Surprisingly, the absence of STING in MRL/lpr mice does not improve lupus phenotypes but instead promotes more inflammation (Sharma et al., 2015). Furthermore, knocking down the IFN receptor gene Ifnar1 in MRL/lpr mice aggravates lymphoproliferation, autoantibody production, and end-organ damage (Hron and Peng, 2004; Nickerson et al., 2010). Although the expression of cyclic GMP-AMP, DNA-activated cGAS-STING pathway, activated IFN-I, and increased in a proportion of patients with SLE (An et al., 2017), the data from lupus mouse models reveal the differential roles of STING in lupus pathogenesis depending on the models. Therefore, further studies in a relevant animal model that reflects human lupus are required to circumvent these conflicting data.
A comprehensive genetic analysis has identified FCGR2B as a susceptibility gene in patients with SLE (Zhu et al., 2016). The deletion of the Fcgr2b gene causes a lupus-like disease in genetic susceptibility to autoimmune development. The Fcgr2b−/− mice created in 129 strain with subsequently backcrossed into C57BL/6 develop overt autoreactivity and fatal lupus disease while the deletion of Fcgr2b in C57BL/6 mice showed only autoantibody production (Bolland and Ravetch, 2000; Boross et al., 2011). The 129-derived Sle16 covering the Nba2 interval region is an autoimmune susceptibility locus, which contains the Fcgr2b, Slam family, interferon-inducible Ifi200 family genes (Boross et al., 2011; Choubey, 2012; Sato-Hayashizaki et al., 2011). Among the Ifi200 family, the Ifi202 shows the highest expression in the splenocytes from Nba2 carrying mice (Rozzo et al., 2001). The Ifi202 is a candidate lupus susceptibility gene, and its human homolog IFI16 shows the association with SLE (Kimkong et al., 2010). Also, IFI16 signals through STING to initiate IFN-I production (Choubey and Panchanathan, 2008; Unterholzner et al., 2010). Based on the genetic background of the 129-derived locus, STING may play a significant role in the pathogenesis of the 129/B6.Fcgr2b−/− lupus mice.
In this work, we showed an increase in Sting expression of the 129/B6.Fcgr2b−/− mice. Disruption of STING signaling rescued lupus phenotypes of the 129/B6.Fcgr2b−/− mice. Stimulation of STING promoted dendritic cell maturation and plasmacytoid dendritic cell differentiation. After STING activation, LYN was phosphorylated and recruited to interact with STING. Inhibition of LYN diminished STING-driven differentiation of dendritic cells. The adoptive transfer of STING-activated bone marrow-derived dendritic cells (BMDCs) into the double-deficiency (Fcgr2b−/−.Stinggt/gt) mice restored the lupus phenotypes. The data suggested that STING signaling in the dendritic cells initiated the autoimmune development in the 129/B6.Fcgr2b−/− mice. STING is a promising therapeutic target for lupus disease.
Results
Loss of the Stimulator of Type I Interferon Genes (STING) Increases Survival of Fcgr2b−/− Lupus Mice
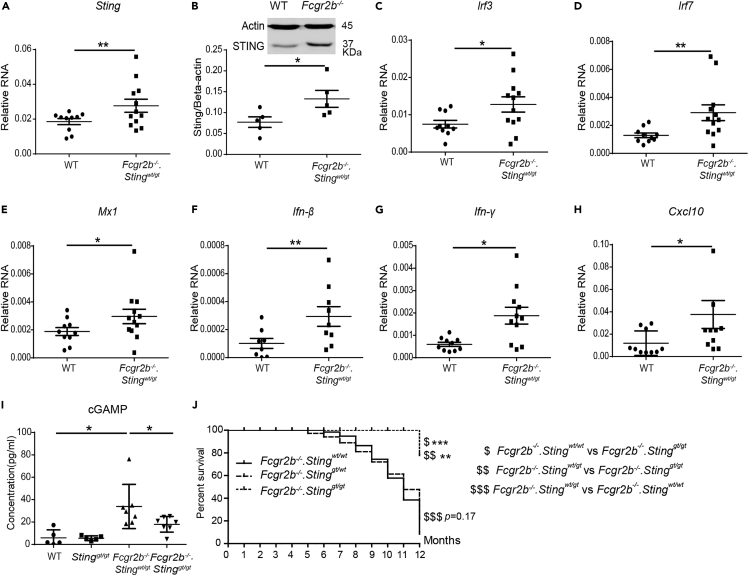
First, we confirmed that 129/B6.Fcgr2b−/− mice (or Fcgr2b−/− in short) showed the increase of Sting mRNA expression and protein expression in the spleen (Figures 1A and 1B). We further observed the significant rise of mRNA expression of interferon-inducible genes (Irf3, Irf7, Mx1) (Ifn-γ, Ifn-β, and Cxcl10) and in the spleen of the Fcgr2b−/− mice (Figures 1C–1H). To determine whether the Sting signaling is required for lupus development in the Fcgr2b−/− mice, we generated the double deficiency of Fcgr2b and Sting together with control littermates. The Fcgr2b−/− mice were crossed with the C57BL/6.Sting deficiency or Goldenticket mice (Stinggt), which behave as a functional knockout of STING (Sauer et al., 2011). Furthermore, we detected the increase of cGAMP in the splenocytes of the Fcgr2b−/−.Stingwt/gt mice but not in the double-deficient mice (Figure 1I). The double-deficient mice (Fcgr2b−/−.Stinggt/gt) showed a higher survival rate compared with the Fcgr2b−/− with homozygote Sting WT mice (Figure 1J). Also, the Fcgr2b−/− with heterozygote of Sting (Stingwt/gt) showed similarity in phenotypes and survival with Fcgr2b−/− with wild-type Sting (Stingwt/wt).
Figure 1.
Loss of the Stimulator of Type I Interferon Genes (STING) Increases Survival of Fcgr2b−/− Lupus Mice
(A and C–H) Gene expression profiles from spleens of wild-type and Fcgr2b−/− mice at the age of 6 months were tested by real-time PCR (N = 10–12 per group). The relative RNA expressions (normalized by actin) of (A) Sting, (C) Irf3, (D) Irf7, (E) Mx1, (F) Ifn-β, (G) Ifn-γ, and (H) Cxcl10 are shown.
(B) Isolated splenocytes were analyzed for STING protein expression by western blot. Data are representative of three mice per group. Quantification of the intensity was normalized by actin (N = 3 per group).
(I) The concentration of cGAMP from isolated splenocytes (N = 5–7).
(J) The Fcgr2b-deficient mice were crossed with Sting-deficient mice (Stinggt/gt) to generate the double-deficient mice (Fcgr2b−/−. Stinggt/gt) and littermate controls. The survival curve of the mice was observed for up to 12 months (N = 14 per group).
Error bars indicate SEM; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. The dollar sign ($) shown the comparison between the group.
STING Signaling Pathway Promotes Autoantibody Production and Glomerulonephritis in the Fcgr2b−/− Lupus Mice
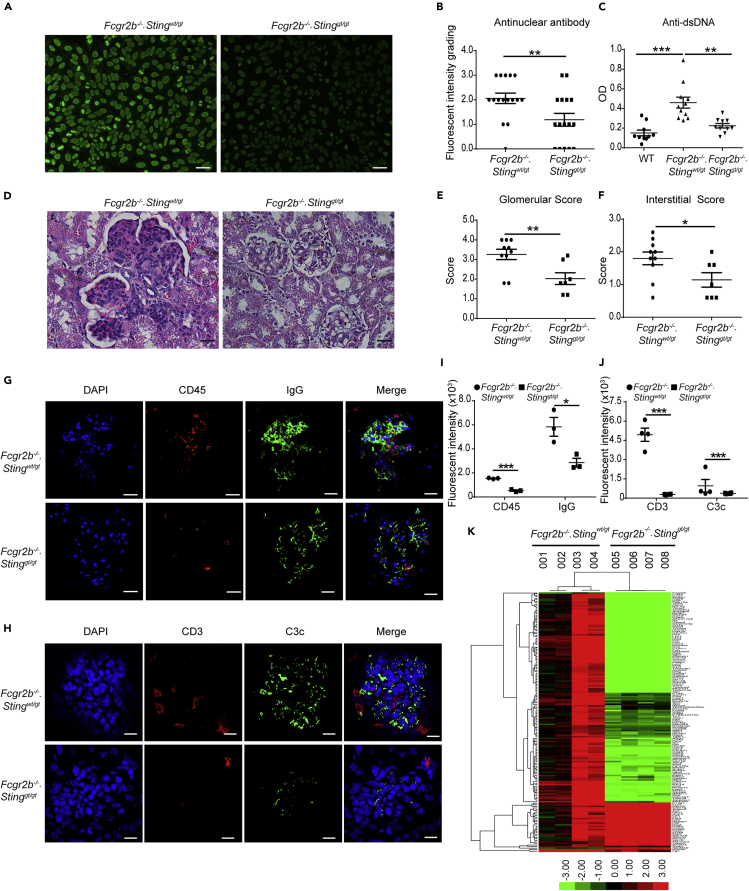
The lupus phenotypes of the double-deficient mice were examined and compared with littermate controls. The levels of the anti-nuclear antibody (ANA) and anti-dsDNA antibody from the sera of the double-deficient mice (Fcgr2b−/−.Stinggt/gt) were significantly lower than in the Fcgr2b−/− mice (Figures 2A–2C). The kidneys of Fcgr2b−/− mice showed pathology of diffuse proliferative glomerulonephritis, which did not present in the Fcgr2b−/−.Stinggt/gt mice (Figure 2D). The glomerular and interstitial scores in the kidneys of Fcgr2b−/− mice were significantly higher than in the double-deficient mice (Figures 2E and 2F). Consistent with the pathology, the immunofluorescence staining showed fewer CD45+ cells and IgG deposition in the kidneys of Fcgr2b−/−.Stinggt/gt mice (Figures 2G and 2I). The cell types infiltrated in the kidneys of the Fcgr2b−/− mice were CD3+ and CD11c+ cells, which significantly reduced in the double-deficient mice (Figures 2H, 2J, S1B, and S1C. Related to Figure 2).
Figure 2.
STING Signaling Pathway Promotes Autoantibody Production and Glomerulonephritis in the Fcgr2b−/− Lupus Mice
(A)The anti-nuclear antibodies (ANA) were detected in the serum (dilution 1:800) using the immunofluorescence staining on Hep-2 cells (A). Data are representative of eight mice per group (scale bar, 20 μm).
(B) Semi-quantification of ANA was graded by fluorescence intensity (N = 8 mice per group).
(C) Anti-dsDNA from sera (dilution 1:100) of Fcgr2b−/− and Fcgr2b−/−. Stinggt/gt was detected by ELISA (N = 10–11 per group).
(D) Kidney sections of Fcgr2b−/− and Fcgr2b−/−. Stinggt/gt mice (6–8 months old) were stained with H&E. Data are representative of 7–10 mice per group (scale bar, 25 μm).
(E–H) (E and F) Glomerular scores and interstitial scores of kidney sections were blindly graded (N = 7–10 per group). Immunofluorescence staining of the kidneys from Fcgr2b−/− and Fcgr2b−/−. Stinggt/gt mice show in (G) IgG (green), CD45 (red), and DAPI (blue) and (H) C3c (green), CD3 (red), and DAPI (blue). Data are representative of 3–4 mice per group (scale bar, 10 μm).
(I and J) The quantitative immunofluorescence signal (I) CD45, and IgG, (J) CD3 and C3c (N = 3–4 mice per group). Data are shown as mean ± SEM; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
(K) A heatmap of microarray data from the kidneys of Fcgr2b−/− and Fcgr2b−/−. Stinggt/gt mice show that the interferon signature genes significantly changed in the Fcgr2b−/−mice (N = 4 mice per group). Data shown in log2 (sample/wild-type).
We further looked at the gene expression profiles in the kidney of these mice and found a significantly different expression (Figure S1A. Related to Figure 2). The expression of interferon-inducible genes in the kidney of these mice was higher in the Fcgr2b−/− mice, especially in the ones with greater severity (#003, 004), and there was a significant reduction of interferon-inducible genes in the kidneys of Fcgr2b−/−.Stinggt/gt mice (Figure 2K). However, not all of these interferon-inducible genes decreased in the Fcgr2b−/−.Stinggt/gt mice (Figure 2K). These data suggested that STING-dependent pathology mediated by both interferon and non-interferon signaling and not all of the interferon signaling in the Fcgr2b−/− mice contributed by the STING pathway.
STING Is Essential for Inflammatory Phenotypes of the Fcgr2b−/− Lupus Mice
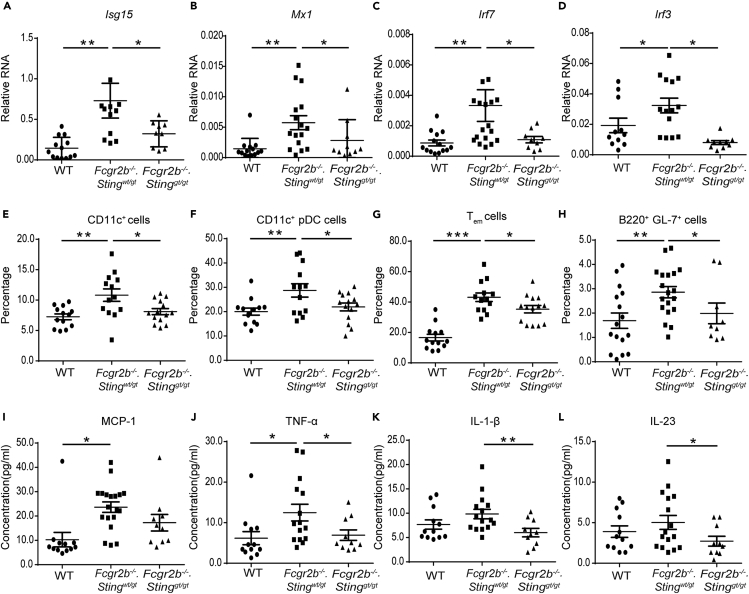
The expression of interferon-inducible genes and interferon regulatory factors in the kidneys was confirmed by real-time PCR. The expressions of Isg15, Mx1, Irf7, and Irf3 were upregulated in the Fcgr2b−/− mice and downregulated in the absence of STING (Figures 3A–3D). Also, the expression of Irf5, the lupus susceptibility gene, which upregulated in the kidneys of the Fcgr2b−/− mice, was Sting dependent (Figure S2A. Related to Figure 3). The splenocytes were analyzed from the mice at the age of 6–7 months to characterize the immunophenotypes. The expansion of dendritic cells (CD11c+) and plasmacytoid dendritic cells (CD11c+PDCA+) in the Fcgr2b−/− mice diminished in the absence of Sting (Figures 3E and 3F). The reduction of T effector memory cells (CD3+CD4+CD62LloCD44hi), germinal center B cells (B220+GL7+), and CD4+ICOS+ cells in the double-deficient mice were detected (Figures 3G, 3H, and S2B. Related to Figure 3). The percentage of IAb+ B cells significantly reduced in the double-deficient mice (Figure S2C. Related to Figure 3). However, the expansion of plasma cells did not show the difference between single and double-deficient mice (Figure S2D. Related to Figure 3). Besides, the percentage of CD11b+CD11c− and F480+ cells did not increase in both Fcgr2b−/−.Stingwt/gt mice and double-deficient mice (Figures S2E and S2F. Related to Figure 3). Furthermore, the sera levels of MCP-1 and TNF-α from the Fcgr2b−/−.Stingwt/gt mice were significantly increased compared with WT mice (Figures 3I and 3J), whereas IL-1β and IL-23 did not show significant changes (Figures 3K and 3L). However, the levels of TNF-α, IL-1β, and IL-23 from the Fcgr2b−/− mice significantly decreased in the absence of STING (Figures 3J–3L). These data suggested that STING mediated the inflammatory process in the Fcgr2b-deficient lupus mice.
Figure 3.
STING is Essential for Inflammatory Phenotypes of the Fcgr2b−/− Lupus Mice
(A–D) The relative RNA expression (normalized by actin) of (A) Isg15, (B) Mx1, (C) Irf7, and (D) Irf3 from the kidneys of wild-type, Fcgr2b−/−. Stingwt/gt, and Fcgr2b−/−. Stinggt/gt mice at the age of 6 months are shown (N = 10–17 per group).
(E–H) Flow cytometry analysis of splenocytes isolated from wild-type, Fcgr2b−/−. Stingwt/gt, and Fcgr2b−/−. Stinggt/gt mice at the age of 6–7 months (N = 13–14 per group). Data are shown in the percentage of (E) CD11c+, (F) plasmacytoid dendritic cells (pDC), (G) Tem (CD3+CD4+CD44hiCD62Llo), and (H) B220+GL7+ cells.
(I–L) The sera cytokines of wild-type, Fcgr2b−/−. Stingwt/gt, and Fcgr2b−/−. Stinggt/gt mice at the age of 6 months were analyzed by cytometric bead array. Serum cytokines of (I) MCP-1, (J) TNF-α, (K) IL-1β, and (L) IL-23 (N = 10–15 per group).
Data are shown as mean ± SEM; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
STING-Activated Dendritic Cells Induce the Proliferation of Naive CD4+ T Cells
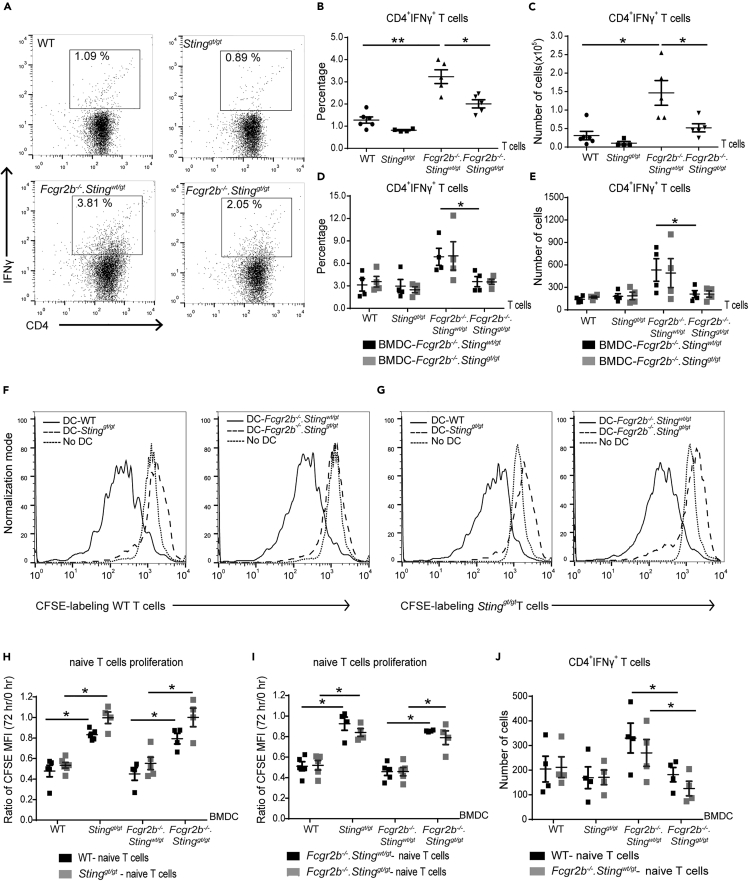
The splenocytes of Fcgr2b−/− mice showed an increase of effector memory T cells (Tem) and Ifng expression (Figures 3G and 1G). We hypothesized that the high proportion of Tem in the Fcgr2b−/− mice might contribute to the increase of Ifng expression. We performed the intercellular staining of IFN-γ to test this assumption. The IFN-γ+CD4+ T cells from the lymph nodes of Fcgr2b−/− mice were higher than wild-type and double-deficient mice (Figures 4A–4C). These IFN-γ+CD4+ T cells isolated from lymph nodes were primed in vivo by DCs. The reduction of IFN-γ+CD4+ T cells in the double-deficient mice could suggest that either STING in T cells or DCs could play a role in the in vivo Th1 skewing.
Figure 4.
STING-Activated Dendritic Cells Induce the Proliferation of Naive CD4+ T Cells
(A–C) Flow cytometry analysis of (A and B) intracellular staining of IFN-γ-producing CD4+ T cells isolated from lymph nodes of wild-type, Stinggt/gt, Fcgr2b−/−.Stingwt/gt, and Fcgr2b−/−.Stinggt/gt mice at the age of 6–7 months. (A) Data are representative of 4–5 mice per group. (B) The percentage of IFN-γ+CD4+ T cells and (C) the number of IFN-γ+CD4+ T cells (N = 4–5 per group).
(D and E) The isolated CD4+ T cells were co-cultured with stimulated BMDC for 6 h. The x axis shows the genotypes that CD4+ T cells were isolated. (D) The percentage and (E) the number of intracellular IFN-γ-producing CD4+ cells after co-culturing with DMXAA-activated BMDC from Fcgr2b−/−.Stingwt/gt and Fcgr2b−/−.Stinggt/gt (6–7 months old) for 6 h (N = 4–5).
(F–J) Co-culture of naive T cells with DMXAA-activated BMDC from wild-type, Stinggt/gt, Fcgr2b−/−.Stingwt/gt, and Fcgr2b−/−.Stinggt/gt mice for 72 h. The x axis shows the genotypes that BMDCs were isolated. (F and G) The histogram of CFSE labeling T cells in the co-culture with BMDCs. Data are representative of 4–5 mice per group. (H and I) CFSE dilution of isolated naive T cells showed in the ratio of mean fluorescence intensity (MFI) at 72 h/initial labeling (time 0), and (J) the total numbers of IFN-γ+CD4+ T cells (N = 4 per group).
Data are shown as mean ± SEM; ∗p < 0.05, and ∗∗p < 0.01.
Sting deficiency reduced the DC expansion in the spleen of Fcgr2b−/− mice (Figure 3E). We hypothesized that DC of the Fcgr2b−/− mice might promote the expansion of Tem and IFN-γ+ T cells. Thus, we co-cultured T cells with BMDC to check if the STING-expressing DC could directly influence the IFN-γ production in T cells. The co-culture for 6 h between CD4+ T cells (from every genotype) and STING-activated BMDC from either Fcgr2b−/− or double-deficient mice showed similar numbers of IFN-γ+CD4+ T cells (Figures 4D and 4E). However, the number of IFN-γ+CD4+ T cells isolated from Fcgr2b−/− mice in the co-culture with BMDC was higher than the isolated CD4+ cells from double-knockout mice regardless of STING expression on BMDC (Figures 4D and 4E). These data suggested that T cell priming of whole CD4+ T cells by DCs was not affected by the presence or absence of STING because Th1 cells in Fcgr2b−/− T cells were fully differentiated in vivo. This experiment suggested that STING-expressing DC could not promote the primed T cells in vivo to produce more IFN-γ.
Next, we tested if STING-expressing DC could prime naive T cells to proliferate and differentiate into IFN-γ-producing T cells. The purified naive T cells were labeled with CFSE and co-cultured with STING-activated BMDC for 72 h. The CFSE dilution assay showed that STING-expressing DC induced the proliferation of naive T cells regardless of STING expression on T cells (Figures 4F–4I). Interestingly, only BMDC from the Fcgr2b−/− mice induced the differentiation of Th1 cells regardless of the STING expression on naive T cells (Figure 4J). These co-culture experiments indicated that intrinsic STING expression on DC, but not on T cells, promoted Th1 differentiation. The data suggested that STING activation promoted the maturation of DC in the Fcgr2b−/− mice, which subsequently primed the naive CD4+ T cells to proliferate and become the IFN-γ-producing T cells.
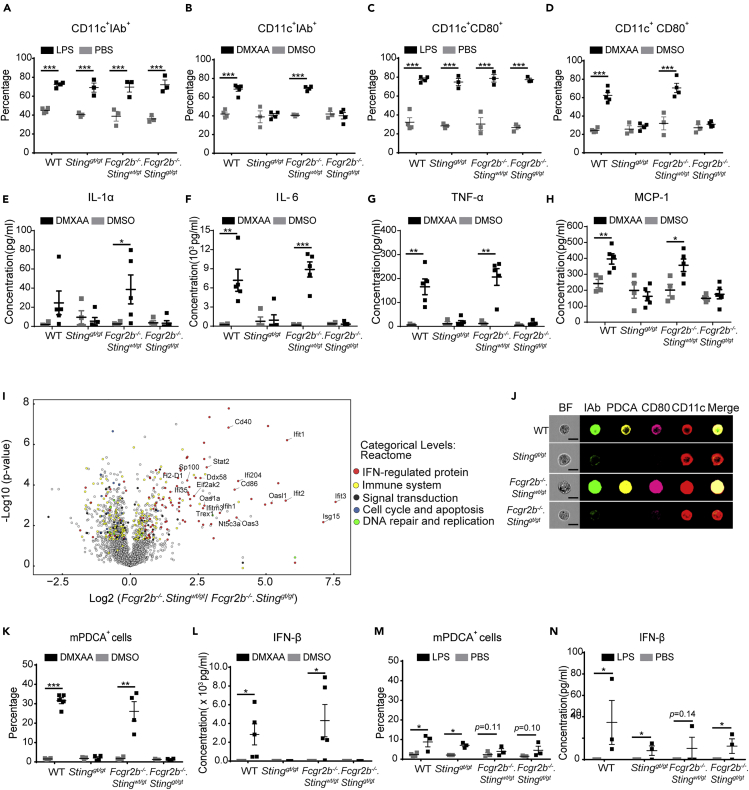
STING Activation Promotes the Maturation of Dendritic Cells and the Differentiation of Plasmacytoid Dendritic Cells
The cGAS/STING pathway is essential for DC activation (Marinho et al., 2018). STING-activating DC can induce naive T cells to proliferate and produce IFN-γ (Figures 4H–4J), which suggested STING may enhance the maturation of DC to become professional antigen-presenting cells. We hypothesized that STING signaling could mediate the expansion of DC in the Fcgr2b−/− mice. The bone-marrow-derived dendritic cells (BMDCs) were differentiated into immature DCs and subsequently stimulated with STING ligands (DMXAA), DMSO, and LPS (as a control) to assess if STING played a role in DC maturation. The LPS control induced the immature DC to increase the expression of MHC-II (IA-b) and CD80, which suggested the phenotypes of mature DC, from both Sting-sufficient and Sting-deficient mice (Figures 5A and 5C). The immature DC from wild-type and Fcgr2b−/− mice also showed the increasing percentage of IA-b+ and CD80+ DC cells after DMXAA stimulation; the Sting-deficient mice did not develop these mature phenotypes (Figures 5B and 5D). The supernatant from BMDC culture with DMXAA stimulation showed an increase in the concentration of IL-1α, IL-6, TNF-α, and MCP-1 in the wild-type and Fcgr2b−/− mice but not in Sting-deficient mice and double-deficient mice (Figures 5E–5H).
Figure 5.
STING Activation Promotes the Maturation of Dendritic Cells and the Differentiation of Plasmacytoid Dendritic Cells
Bone marrows were isolated from wild-type, Stinggt/gt, Fcgr2b−/−. Stingwt/gt, and Fcgr2b−/−. Stinggt/gt mice at the age of 6 months.
(A–D) IL-4 and G-CSF differentiated bone marrow-derived dendritic cells (BMDC) for 5 days were stimulated with LPS or DMXAA for 24 h. Flow cytometry analysis shows the percentage of (A and B) CD11c+ IAb+ cells and (C and D) CD11c+CD80+ cells.
(E–H) Supernatants were collected and analyzed after DMXAA stimulation for 24 h. Cytometric bead array shows the levels of (E) IL-1α, (F) IL-6, (G) TNF-α, and (H) MCP-1.
(I) Volcano plot of protein expressions from proteomic analysis of DMXAA-activated BMDC of Fcgr2b−/−. Stingwt/gt, and Fcgr2b−/−.Stinggt/gt mice at the age of 6–7 months (N = 4 per group).
(J) Imaging flow cytometry of DMXAA-activated BMDC shows the representative staining of IAb (green), mPDCA (yellow), CD80 (pink), and CD11c (red) (N = 3 mice per group).
(K and M) The percentage of pDC (PDCA+ cells) after (K) DMXAA activation and (M) LPS activation for 24 h (N = 3–4 per group).
(L and N) The level of IFN-β from the culture supernatant of activated BMDC with (L) DMXAA and (N) LPS (N = 5 per group).
Data are shown as mean ± SEM; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
To better understand the mechanisms of STING in DC differentiation, we performed the proteomic analysis of STING-activated BMDC in the Fcgr2b−/− mice compared with the double-deficient mice. The volcano plot showed the proteins that were highly expressed were interferon-regulated proteins (Figure 5I and Table S1. Lists of up and down of regulated proteins. Related to Figure 5). This finding may result from the increase of IFN-I production in the culture medium, which could upregulate the interferon-regulated proteins. We hypothesized that STING might promote the differentiation of pDC (a significant producer of IFN-I). The in vitro culture of BMDC with DMXAA and LPS (as a control) showed a significant increase in pDC and IFN-β with DMXAA but not with LPS stimulation (Figures 5K–5N). Also, we demonstrated the morphology of these cells by the imaging flow cytometry and found the pDCs expressed CD80 as well (Figure 5J). To confirm that activation of STING via another ligand results in DC differentiation, we stimulated the BMDC with cGAMP. The stimulation of STING with cGAMP increased the amount of CD11c+IAb+ (Figure S3A. Related to Figure 5), CD11c+CD80+ (Figure S2B. Related to Figure 5), and mPDCA+ cells (Figure S3C. Related to Figure 5). These data suggested that the STING signaling pathway mediated DC maturation and pDC differentiation.
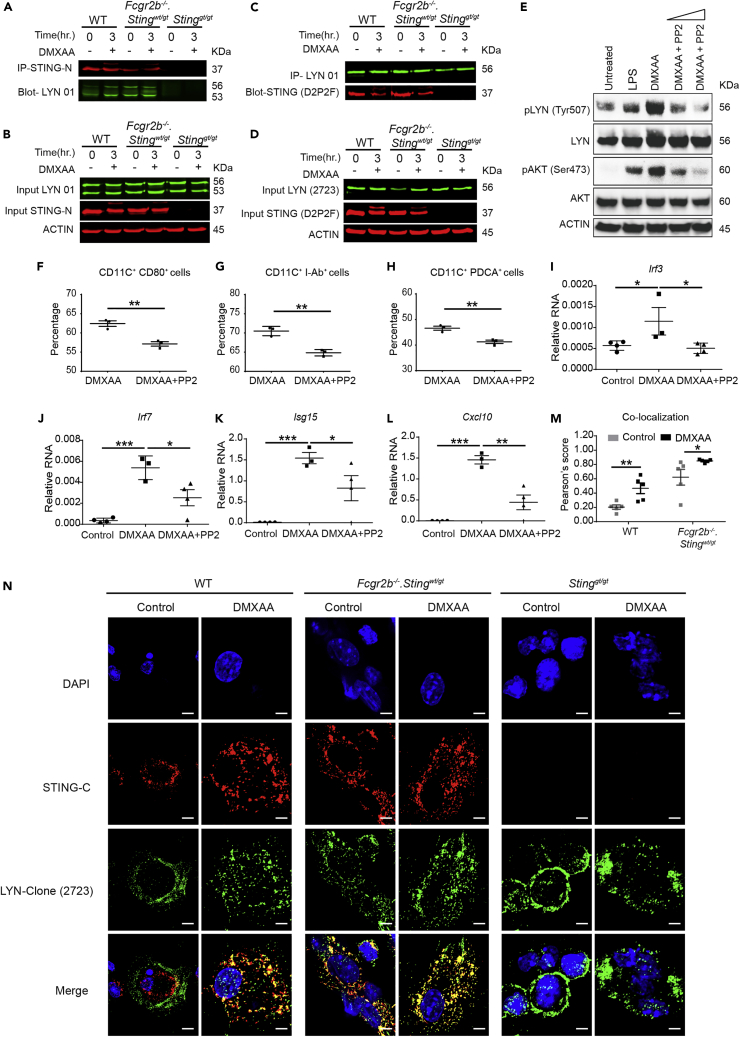
STING Activation Induced DC Maturation and Promoted the Interaction between LYN and STING in DC
STING-interacting proteins were identified by immunoprecipitation (IP) using the STING antibody that targets the N-terminal region of the protein. STING-activating BMDC with DMXAA for 3 h was immunoprecipitated and analyzed by mass spectrometry (Table S2. Lists of STING interacting proteins. Related to Figure 6). Among the proteins detected, LYN, a member of Src family kinases, has been shown to function in the maturation of DC and pDC response (Chu and Lowell, 2005; Dallari et al., 2017). Immunoprecipitation confirmed that LYN interacted with STING after DMXAA stimulation in WT BMDC, whereas LYN constitutively interacted with STING in the Fcgr2b-deficient BMDC (Figures 6A and 6C). This interaction could result from the intrinsic activation of STING in the Fcgr2b−/− mice. This activation did not change the protein abundance of LYN and STING in total cell lysate (Figures 6B and 6D). Also, the western blot from both IP and cell lysate showed another fainting band of STING after the activation; this protein was identified by mass spectrometry as the phosphorylation of STING (Ser357) (Figures S4A–S4B. Related to Figure 6). The activation of STING increased the phosphorylation of LYN (Tyr507) and AKT (Ser473), which were inhibited by PP2 (Figures 6E and S5D–S5E. Related to Figure 6).
Figure 6.
STING Activation Induced DC Maturation and Promoted the Interaction between LYN and STING in DC
(A–D) Fluorescent western blot shows (A) the immunoprecipitation (IP) with STING-N (red) and blots with Lyn (green) and (B) cell lysate of activated BMDC with DMXAA at 0 and 3 h (C) A reverse IP using the Lyn antibody and blot with STING antibody and (D) cell lysate of activated BMDC with DMXAA at 0 and 3 h. Data show a representative of four experiments.
(E–L) (E) Western blot analysis of Sting-activated BMDC with or without PP2 inhibitor showed the phosphorylation of Lyn (Try507) and Akt (Ser473). Data are representative of three mice per group. Sting-activated BMDCs were cultured with Lyn inhibitor (PP2) and analyzed by (F–H) flow cytometry shows the percentage of (F) CD80+CD11c+, (G) I-Ab+CD11c+, and (H) PDCA+CD11c+ cells (N = 3 per group), and (I–L) the relative RNA expression (normalized by actin) of (I) Irf3, (J) Irf7, (K) Isg15, and (L) Cxcl10 are shown (N = 4 per group).
(M and N) Confocal microscopy of DMXAA-activated BMDC from WT, Stinggt/gt, and Fcgr2b−/−. Stingwt/gt - mice for 6 h. (M) The quantification of colocalization signals between STING and Lyn (N = 5 per group). Data are shown as mean ± SEM; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. (N) Immunofluorescence staining of BMDC shows Lyn (green), STING (red), and DAPI (blue) (scale bar, 20 μm). Data show a representative of five experiments.
Lyn kinase regulates DC maturation, and genetic deletion of Lyn ablates pDC (Chu and Lowell, 2005; Dallari et al., 2017). We hypothesized that STING promoted DC maturation and pDC differentiation through LYN signaling pathway, and the inhibition of LYN should affect the STING-induced BMDC differentiation. The in vitro data showed that pan SFK inhibitor PP2 decreased STING-mediated expression of IAb and CD80 on conventional DC (Figures 6F, 6G, and S4C. Related to Figure 6) and the differentiation of pDC (Figure 6H). Next, we tested whether PP2 inhibited STING-mediated signaling. The PP2 decreased the mRNA expression of Irf3, Irf7, Isg15, and Cxcl10 in the STING-stimulated BMDC (Figures 6I–6L). These data suggested that PP2 treatment reduced the enhanced expression of ISG via STING activation.
To confirm the physical interaction between STING and LYN, we identified the colocalization of STING and LYN in the BMDC using two different clones of anti-STING and anti-LYN antibodies (Figures 6N and S5A. Related to Figure 6). The quantification of fluorescence signaling showed a significant increase in colocalization of STING and LYN upon STING stimulation (Figures 6M and S5C. Related to Figure 6). The Fcgr2b-deficient BMDC constitutively showed a certain degree of the colocalization between STING and LYN, whereas the activation of STING promoted more interaction (Figure 6N). Also, FYN, a member of the Src family kinases (SFKs), has been shown to have functional role in pDC maturation and PP2 inhibited both LYN and FYN signaling (Dallari et al., 2017). Therefore, we identified if FYN colocalized with STING and found that FYN did not colocalize with STING (Figure S5B. Related to Figure 6). These data suggested that activation of STING induced LYN interaction and mediated maturation and differentiation of conventional DCs and pDCs.
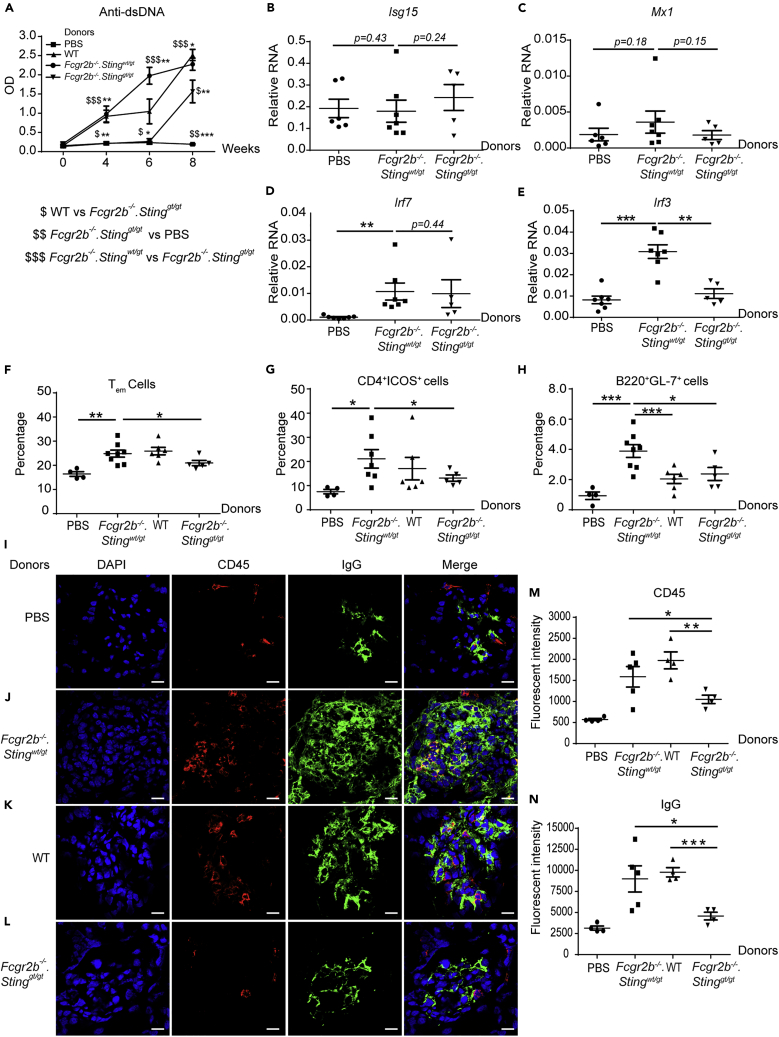
Adoptive Transfer of Sting-Expressing BMDC Induces Lupus Development in the Fcgr2b−/−.Stinggt/gt Mice
The STING signaling pathway activated the immature BMDC to differentiate into the mature DC and pDC. The dendritic cells are significant producers of inflammatory cytokines and capable of promoting T cell proliferation and differentiation. We proposed that STING may induce the lupus disease by initially acting through the DC activation. We performed the adoptive transfer of the STING-activated BMDC derived from Fcgr2b−/− mice into WT recipient mice to test this hypothesis. The recipient WT mice developed the autoimmune phenotypes, including the production of anti-dsDNA (Figure S6A. Related to Figure 7), expansion of Tem (Figure S6B. Related to Figure 7), CD4+ICOS+ (Figure S6C. Related to Figure 7), CD138+ (Figure S6D. Related to Figure 7), germinal center B cells (Figure S6E. Related to Figure 7), and minimal immune complex deposition in the kidneys (Figures S6F–S6G. Related to Figure 7). Although only minimal IgG deposition was detected, these data suggested that STING-activated BMDC can induce autoimmunity. The background of WT recipient mice may not promote the overt phenotypes of autoimmune disease.
Figure 7.
Adoptive Transfer of Sting-Expressing BMDC Induces Lupus Development in the Fcgr2b−/−.Stinggt/gt Mice
DMXAA-activated BMDC from Fcgr2b−/−.Stingwt/gt, WT, and Fcgr2b−/−.Stinggt/gt were transferred into the recipient mice (Fcgr2b−/−.Stinggt/gt).
(A) The level of anti-dsDNA from the sera (1:100) measured by ELISA (N = 5–10 per group). The dollar sign ($) shows the comparison between the groups.
(B–E) The relative RNA expressions (normalized by actin) of (B) Isg15, (C) Mx1, (D) Irf7, and (E) Irf3 in the kidney of the mice receiving BMDC are shown (N = 5–6 per group).
(F–H) Flow cytometry analysis of recipient splenocytes after BMDC transferred every 2 weeks for four times shows the percentage of (F) Tem (CD4+CD44hiCD62Llo), (G) CD4+ICOS+ cells, (H) B220+GL7+ cells (N = 5–10 per group).
(I–L) Immunofluorescence staining of the kidney from the Fcgr2b−/−.Stinggt/gt recipient mice after the transfer with (I) PBS control, DMXAA-activated BMDC from (J) Fcgr2b−/−.Stingwt/gt, (K) WT, and (L) Fcgr2b−/−.Stinggt/gt. The confocal microscope shows DAPI (blue), CD45 (red), and IgG (green). The representative of four experiments (scale bar, 10 μm).
(M and N) The quantification of fluorescence intensity of (M) CD45, and (N) IgG staining.
Data are shown as mean ± SEM; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Next, we performed the reconstitution experiment by adoptive transfer of STING-activated BMDC into the double-deficient mice. The level of anti-dsDNA significantly increased in the mice receiving Sting-sufficient BMDC compared with those receiving Sting-deficient BMDC and control (Figure 7A). The transfer of BMDC in the double-deficient mice did not increase the expression of Isg15, Mx1, and Irf7 (Figures 7B–7D); however, Irf3 increased in the kidney of the recipient mice (Figure 7E).
The analysis of spleens showed an increase in the percentage of Tem and CD4+ICOS+ in recipient mice that received STING-activated BMDC derived from WT or Fcgr2b−/−.Stingwt/gt mice when compared with the PBS control and double-deficient BMDC injection group (Figures 7F and 7G). These data suggested that T cell phenotypes required STING expression in BMDC. Interestingly, only Sting-sufficient BMDC from Fcgr2b−/-, but not WT mice, induced the spontaneous germinal center B cell formation. Also, Sting-deficient BMDC from Fcgr2b−/- did not increase germinal center B cell (Figure 7H). Next, we examined the immunofluorescence staining to identify the immune complexes at the kidney of the recipient. The recipient of Sting-sufficient BMDC showed an increase of IgG deposition and CD45+ cell infiltration, whereas Sting-deficient BMDC did not (Figures 7I–7N).
Nevertheless, the Fcgr2b−/− BMDC induced more immune complexes and CD45+ cells in the kidneys (Figures 7J and 7K). The results suggested that the restoration of the STING signaling pathway in dendritic cells is essential for lupus development in the Fcgr2b−/−.Stinggt/gt mice. The Sting-sufficient BMDC induced the lupus phenotypes in the double-deficient mice via the activation of T and B cells, which led to autoantibody production rather than promote type I IFN signaling.
Discussion
A gain-of-function mutation in STING has been identified as a gene responsible for a subpopulation of patients with SLE, and STING-dependent interferon-inducible genes correlated with disease activity (Kato et al., 2018; Konig et al., 2017). However, there are no functional data of STING in a lupus mouse model that are relevant to human SLE. The 129/B6.Fcgr2b−/− mice carrying Nba2 region expressed constitutively Ifi202. The CD19+ cells from B6.Nba2 show the increase of Ifi202 and the decrease of Sting expression (Panchanathan et al., 2013). However, the overexpression of Ifi202 can activate the Sting-dependent IFN-I response and the 129/B6.Fcgr2b−/− mice increase the expression of IFN-β (Brunette et al., 2012; Panchanathan et al., 2011). Here, we detected the high expression of Sting, Ifn-β, and interferon-inducible genes from the spleen of these mice. The activation of cGAS leads to cGAMP production and subsequently activates STING signaling (Sun et al., 2013; Wu et al., 2013). The increase of cGAMP in the 129/B6.Fcgr2b−/− mice raised the question of the natural origin of DNA that stimulates the cGAS-STING pathway. The Fcgr2b−/− mice showed the cGAMP overproduction, whereas the disruption of STING decreased the level of cGAMP in the Fcgr2b−/− mice. On the contrary to a previous study, the increase of cGAMP in Trex1 and DnaseII-deficient mice was upregulated in the absence of STING (Gao et al., 2015). TREX1 degrades double-stranded DNA, whereas oxidized DNA is resistant to TREX1-mediated degradation (Gehrke et al., 2013; Grieves et al., 2015). Also, DNASE II cleaves native dsDNA but works less effectively for denaturized DNA (Laukova et al., 2020). Moreover, the mitochondrial DNA (mtDNA) releasing into the cytosol can trigger the IFN-I response and accelerates the severity of a mouse model of lupus disease (Kim et al., 2019). The mtDNA released into the cytosol activates the cGAS-STING pathway in cisplatin-induced tubular inflammation (Maekawa et al., 2019). Since the absence of STING reduced the level of cGAMP in the Fcgr2b−/− mice, but not in the Trex1−/− and DnaseII−/− mice, the intrinsic DNA origin that constitutively activates the STING-cGAS pathway should be different among these autoimmune mouse models. The Fcgr2b−/−.Stinggt/gt mice did not develop overt kidney inflammation; thus, it is likely to release less amount of oxidized mtDNA. The cytosolic DNA that constitutively activates STING-cGAS pathway in the Fcgr2b−/− mice may originate from stress-induced mitochondrial DNA leakage from inflammation. These findings suggested that specific types of DNA from different compartments could be sensed and activated through the STING-cGAS pathway.
Although STING functions as a negative regulator in the Mrl/lpr lupus mice (Sharma et al., 2015), our data show that STING is required for the lupus development in the 129/B6.Fcgr2b−/− mice. STING may also play a crucial role in other lupus mouse models, which contained the Nba2 region. The survival of the 129/B6.Fcgr2b−/− mice depend on autoantibody production and glomerulonephritis (Bolland et al., 2002; Pisitkun et al., 2012). STING is required for the antibody production induced by cyclic-di-GMP in vitro (Walker et al., 2018). These data suggested that STING facilitated the autoantibody production, inflammatory cell infiltration, and glomerulonephritis in the 129/B6.Fcgr2b−/− mice. The expression of interferon-inducible genes associated with SLE disease activity (Feng et al., 2006). We detected the very high expression of IFN-inducible genes in the kidneys of 129/B6.Fcgr2b−/− mice showed severe pathology. The absence of STING signaling in the Fcgr2b−/− mice partly decreased the expression of interferon-inducible genes in the kidney. The kidneys in the experiment were not perfused; thus, we cannot conclude that the IFN signature expressed in the kidney derived from the blood or kidney. These data suggested that other nucleic acid sensors may promote the type I interferon production or signaling in the Fcgr2b−/− mice as well, and STING-dependent lupus phenotypes do not mediate only through type-I interferon pathway.
STING expresses and functions differentially depending on the cell types. STING signals synergistically with B cell receptor signaling to promote antibody response (Walker et al., 2018). Our results showed that spontaneous germinal center B cells and MHC-II expression in the Fcgr2b−/− mice were Sting dependent. However, plasma cell expansion was Sting independent. These data suggested STING may contribute to the autoantibody production through memory B cells. STING also activates T cells, which induced type I IFN production and mediated cell death (Larkin et al., 2017). Nevertheless, we found that the increase of T effector memory (Tem) in the Fcgr2b−/− mice was Sting dependent. The expansion of Tem may directly mediate through the interaction with antigen-presenting cells, not via Sting signaling in T cells.
STING agonist (DMXAA)-treated mice show the increased expression of CD80, CD86, and MHC-II on DC, suggesting the mature phenotypes of DC as the antigen-presenting cells (APCs) (Curran et al., 2016). We observed the reduction of DC expansion in the Fcgr2b−/− mice, which depended on STING signaling. We confirmed that STING was required for DC maturation and cytokine production. These DCs became professional APCs and could promote T cell differentiation. The IFN-γ-producing CD4+ cells in the spleen of the Fcgr2b−/− mice were reduced in the absence of STING. The Sting-expressing DCs derived from WT and Fcgr2b−/− mice stimulated naive T cells to proliferate; however, the ability of T cells to differentiate and produce IFN-γ did not depend on intrinsic Sting expression on T cells. Interestingly, only DCs from the Fcgr2b−/− mice can increase the IFN-γ production in CD4+ T cells. These data suggested the DC from the Fcgr2b−/− mice have the intrinsic property that promotes the generation of IFN-γ-producing CD4+ T cells.
The cGAS-STING signaling can activate human pDCs to produce IFN-I, and knockdown of Sting using siRNA in CAL-1 cells can cause the reduction of IFN response (Bode et al., 2016). The proteomic data showed the upregulation of interferon-regulated protein after STING activation with DMXAA, which implied that the culture environment should enrich with type-I IFN. STING activation led to phosphorylation of Ser357 of mouse Sting (homolog Ser358 in human Sting), and this site is phosphorylated by TBK1 which subsequently promoting type I IFN production (Tanaka and Chen, 2012; Zhong et al., 2008). The identification of pDC after STING activation uncovered the role of STING in the differentiation of pDC. These data revealed that STING was essential for the generation of pDCs. Besides, our study identified several STING-interacting proteins by mass spectrometry. Lyn kinase has been shown to have role in the differentiation of pDC (Dallari et al., 2017). The recruitment of LYN to STING after DMXAA stimulation suggested STING-mediated signaling through Lyn kinase. Also, the proteomics data of STING-activated BMDC showed a significant increase of phosphoinositide 3-kinase adapter protein 1 (Pik3ap1) and receptor of activated protein C kinase 1 (Rack1) (Table S1. Lists of up- and down-regulated proteins. Related to Figure 5). Pik3ap1 is an adaptor that signals to the phosphoinositide 3-kinase (PI3K) (Aiba et al., 2008). LYN and RACK1 are co-immunoprecipitated in membrane complexes (Sutton et al., 2013). Also, RACK1 silencing affected the phosphorylation of AKT (Liu et al., 2017). Our data suggest that the downstream of STING-LYN signaling mediated through the PI3K-AKT pathway. The inhibition of Lyn kinase with PP2 inhibitor during STING activation diminished DC maturation and pDC differentiation. The PP2 also is a broad inhibitor of other Src kinase family. However, we could identify the interaction of LYN and STING by mass spectrometry, immunoprecipitation, and colocalization during STING activation. All of these data suggested STING-mediated differentiation of BMDC probably through the LYN signaling pathway.
The duration of the transfer experiment was 8 weeks. Thus, the phenotypes that have changed would not be apparent lupus, but still, the hallmarks of lupus disease settled down in the recipient mice. However, the increase of anti-dsDNA in the serum, immune complex deposition, and inflammatory cell infiltration in the kidney were detected in the recipient mice when transferring the STING-sufficient BMDC into the double-deficient recipient mice.
The depletion of pDC ameliorates the autoimmune phenotypes in BXSB lupus-prone mice and B6.Nba2 mice (Davison and Jørgensen, 2015; Rowland et al., 2014). Our data strongly suggested STING involving in DC function both DC maturation and pDC differentiation. Adoptive transfer of Sting-sufficient BMDCs can induce autoantibody production and immune complex deposition regardless of the Fcgr2b status. However, the absence of Fcg2b in the BMDC can accelerate the severity of autoimmune phenotypes and notably increase inflammatory cell infiltration in the kidney of the double-deficient recipient mice.
The transfer of Sting-sufficient Fcgr2b−/− BMDC led to an increase of Irf3 expression but not Isg15, Mx1, and Irf7. Irf3 is a transcription factor downstream of STING signaling pathway (Burdette et al., 2011). These data suggested that Irf3 expression in the kidney was STING-dependent BMDC, whereas other ISGs may depend on the intrinsic STING expression of the resident cells in the kidney. Besides, the transfer of Sting-sufficient Fcgr2b−/−BMDC could increase the effector T cell and germinal center B cells in the spleens of the recipient mice. These data suggested that STING expression on BMDC is essential for the initiation of autoimmunity in the double-deficient mice via the activation of T and B cells, which led to autoantibody production rather than the promotion of type I IFN signaling.
In summary, this study established the vital function of STING in the autoimmune Fcgr2b−/− lupus mouse model, thus providing a reliable tool for future mechanistic and preclinical studies of STING in SLE. These findings provide proof of the concept that inhibition of STING signaling may be a candidate targeted treatment for a subset of patients with SLE.
Limitations of the Study
This study identified the functional role of LYN in the differentiation of BMDC based on the PP2 inhibitor, which is a broad inhibitor for the Src kinase family. The Lyn-deficient BMDC will be solid proof to conclude that STING mediated DC differentiation via LYN kinase. However, the identification of LYN that immunoprecipitated with STING in BMDC by mass spectrometry but not other members of the Src family suggested the interaction of LYN and STING in the BMDC. Furthermore, we showed the vital role of STING in the Fcgr2b-deficient mice, one of the lupus mice models. The study of STING function in the human SLE in the future research would suggest the promising target to inhibit STING signaling in the treatment of SLE.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to the Lead Contact, Dr. Prapaporn Pisitkun (Prapaporn.pis@mahidol.ac.th).
Materials Availability
Materials are available from the corresponding author on a reasonable request.
Data and Code Availability
The microarray data are available at Gene Expression Omnibus: GSE142594 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE142594.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRoteomics IDEntifications (PRIDE) partner repository with the dataset identifier PXD019239.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the Thailand Research Fund (TRF), Thailand, RSA5980023 to P.P., International Network for Lupus Research (IRN59W0004) from TRF, Thailand, to P.P., T.P., and P.W., the National Research Council of Thailand (NRCT), Thailand, to Chulalongkorn University (2015-2018) and Chulalongkorn University, Thailand, GB-CU-61-25-30-15 to T.P. and P.P., and Chulalongkorn Academic Advancement into the 2nd Century (CUAASC) Project to T.P. T.P. was supported by Thailand Research Fund (TRF), Thailand, for Research Career Development Grant (RSA6280026). J.M. was supported by TRF, International Network for Lupus Research, Thailand, (IRN59W0004).
Author Contributions
A.T. performed experiments, interpreted data, co-directed the study, and wrote the manuscript. T. Prabakaran performed and analyzed the in vitro functional assays with inhibitor. M.T. and T.B. provided experimental assistance for flow cytometry. J.M. performed the proteomics analysis. P.W. provided experimental assistance for mass spectrometry. N.C. provided experimental support for confocal microscopy. T.S. provided analysis assistance for microarray. A.L. analyzed and scored histopathology of tissue sections. S.P. provided reagents and expertise related to Sting signaling, co-directed the study, and edited the manuscript. T Pisitkun contributed reagents and expertise related to mass spectrometry, co-directed the study, and edited the manuscript. P.P. performed experiments, interpreted data, directed the studies, and wrote the manuscript.
Declaration of Interests
The authors declare no conflict of interest.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101530.
Contributor Information
Trairak Pisitkun, Email: pisitkut@nhlbi.nih.gov.
Prapaporn Pisitkun, Email: prapaporn.pis@mahidol.ac.th.
Supplemental Information
References
- Ahn J., Gutman D., Saijo S., Barber G.N. STING manifests self DNA-dependent inflammatory disease. Proc. Natl. Acad. Sci. U S A. 2012;109:19386–19391. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J., Ruiz P., Barber G.N. Intrinsic self-DNA triggers inflammatory disease dependent on STING. J. Immunol. 2014;193:4634–4642. doi: 10.4049/jimmunol.1401337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba Y., Kameyama M., Yamazaki T., Tedder T.F., Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- An J., Durcan L., Karr R.M., Briggs T.A., Rice G.I., Teal T.H., Woodward J.J., Elkon K.B. Expression of cyclic GMP-AMP synthase in patients with systemic lupus erythematosus. Arthritis Rheum. 2017;69:800–807. doi: 10.1002/art.40002. [DOI] [PubMed] [Google Scholar]
- Asselin-Paturel C., Brizard G., Chemin K., Boonstra A., O'Garra A., Vicari A., Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Ortmann W.A., Espe K.J., Shark K.B., Grande W.J., Hughes K.M., Kapur V. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode C., Fox M., Tewary P., Steinhagen A., Ellerkmann R.K., Klinman D., Baumgarten G., Hornung V., Steinhagen F. Human plasmacytoid dentritic cells elicit a Type I Interferon response by sensing DNA via the cGAS-STING signaling pathway. Eur. J. Immunol. 2016;46:1615–1621. doi: 10.1002/eji.201546113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolland S., Ravetch J.V. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- Bolland S., Yim Y.-S., Tus K., Wakeland E.K., Ravetch J.V. Genetic modifiers of systemic lupus erythematosus in FcγRIIB−/− mice. J. Exp. Med. 2002;195:1167–1174. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boross P., Arandhara V.L., Martin-Ramirez J., Santiago-Raber M.-L., Carlucci F., Flierman R., van der Kaa J., Breukel C., Claassens J.W.C., Camps M. The inhibiting Fc receptor for IgG, FcγRIIB, is a modifier of autoimmune susceptibility. J. Immunol. 2011;187:1304. doi: 10.4049/jimmunol.1101194. [DOI] [PubMed] [Google Scholar]
- Brunette R.L., Young J.M., Whitley D.G., Brodsky I.E., Malik H.S., Stetson D.B. Extensive evolutionary and functional diversity among mammalian AIM2-like receptors. J. Exp. Med. 2012;209:1969–1983. doi: 10.1084/jem.20121960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdette D.L., Monroe K.M., Sotelo-Troha K., Iwig J.S., Eckert B., Hyodo M., Hayakawa Y., Vance R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey D. Interferon-inducible Ifi200-family genes as modifiers of lupus susceptibility. Immunol. Lett. 2012;147:10–17. doi: 10.1016/j.imlet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choubey D., Panchanathan R. Interferon-inducible Ifi200-family genes in systemic lupus erythematosus. Immunol. Lett. 2008;119:32–41. doi: 10.1016/j.imlet.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S.R., Shupe J., Nickerson K., Kashgarian M., Flavell Richard A., Shlomchik M.J. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Chu C.-L., Lowell C.A. The Lyn tyrosine kinase differentially regulates dendritic cell generation and maturation. J. Immunol. 2005;175:2880. doi: 10.4049/jimmunol.175.5.2880. [DOI] [PubMed] [Google Scholar]
- Crow Y.J., Hayward B.E., Parmar R., Robins P., Leitch A., Ali M., Black D.N., van Bokhoven H., Brunner H.G., Hamel B.C. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat. Genet. 2006;38:917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- Curran E., Chen X., Corrales L., Kline D.E., Dubensky T.W., Jr., Duttagupta P., Kortylewski M., Kline J. STING pathway activation stimulates potent immunity against acute myeloid leukemia. Cell Rep. 2016;15:2357–2366. doi: 10.1016/j.celrep.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallari S., Macal M., Loureiro M.E., Jo Y., Swanson L., Hesser C., Ghosh P., Zuniga E.I. Src family kinases Fyn and Lyn are constitutively activated and mediate plasmacytoid dendritic cell responses. Nat. Commun. 2017;8:14830. doi: 10.1038/ncomms14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison L.M., Jørgensen T.N. Sialic acid–binding immunoglobulin-type lectin H–positive plasmacytoid dendritic cells drive spontaneous lupus-like disease development in B6.Nba2 mice. Arthritis Rheumatol. 2015;67:1012–1022. doi: 10.1002/art.38989. [DOI] [PubMed] [Google Scholar]
- Deane J.A., Pisitkun P., Barrett R.S., Feigenbaum L., Town T., Ward J.M., Flavell R.A., Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Wu H., Grossman J.M., Hanvivadhanakul P., FitzGerald J.D., Park G.S., Dong X., Chen W., Kim M.H., Weng H.H. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2951–2962. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- Gao D., Li T., Li X.D., Chen X., Li Q.Z., Wight-Carter M., Chen Z.J. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl. Acad. Sci. U S A. 2015;112:E5699–E5705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke N., Mertens C., Zillinger T., Wenzel J., Bald T., Zahn S., Tuting T., Hartmann G., Barchet W. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–495. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Grieves J.L., Fye J.M., Harvey S., Grayson J.M., Hollis T., Perrino F.W. Exonuclease TREX1 degrades double-stranded DNA to prevent spontaneous lupus-like inflammatory disease. Proc. Natl. Acad. Sci. U S A. 2015;112:5117–5122. doi: 10.1073/pnas.1423804112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther C., Hillebrand M., Brunk J., Lee-Kirsch M.A. Systemic involvement in TREX1-associated familial chilblain lupus. J. Am. Acad. Dermatol. 2013;69:e179–e181. doi: 10.1016/j.jaad.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Hron J.D., Peng S.L. Type I IFN protects against murine lupus. J. Immunol. 2004;173:2134. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Park J., Takamatsu H., Konaka H., Aoki W., Aburaya S., Ueda M., Nishide M., Koyama S., Hayama Y. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann. Rheum. Dis. 2018;77:1507–1515. doi: 10.1136/annrheumdis-2018-212988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating S.E., Baran M., Bowie A.G. Cytosolic DNA sensors regulating type I interferon induction. Trends Immunol. 2011;32:574–581. doi: 10.1016/j.it.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Kim J., Gupta R., Blanco L.P., Yang S., Shteinfer-Kuzmine A., Wang K., Zhu J., Yoon H.E., Wang X., Kerkhofs M. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366:1531–1536. doi: 10.1126/science.aav4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimkong I., Avihingsanon Y., Hirankarn N. Association of IFI200 gene polymorphisms with susceptibility to systemic lupus erythematosus. J. Rheumatol. 2010;37:1544–1547. doi: 10.3899/jrheum.091255. [DOI] [PubMed] [Google Scholar]
- Konig N., Fiehn C., Wolf C., Schuster M., Cura Costa E., Tungler V., Alvarez H.A., Chara O., Engel K., Goldbach-Mansky R. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 2017;76:468–472. doi: 10.1136/annrheumdis-2016-209841. [DOI] [PubMed] [Google Scholar]
- Larkin B., Ilyukha V., Sorokin M., Buzdin A., Vannier E., Poltorak A. Cutting edge: activation of STING in T cells induces type I IFN responses and cell death. J. Immunol. 2017;199:397–402. doi: 10.4049/jimmunol.1601999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukova L., Konecna B., Janovicova L., Vlkova B., Celec P. Deoxyribonucleases and their applications in biomedicine. Biomolecules. 2020;10:1036. doi: 10.3390/biom10071036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Wang C., Chen P., Wang L., Cheng Y. RACK1 promotes radiation resistance in esophageal cancer via regulating AKT pathway and Bcl-2 expression. Biochem. Biophysical Res. Commun. 2017;491:622–628. doi: 10.1016/j.bbrc.2017.07.153. [DOI] [PubMed] [Google Scholar]
- Maekawa H., Inoue T., Ouchi H., Jao T.M., Inoue R., Nishi H., Fujii R., Ishidate F., Tanaka T., Tanaka Y. Mitochondrial damage causes inflammation via cGAS-STING signaling in acute kidney injury. Cell Rep. 2019;29:1261–1273 e1266. doi: 10.1016/j.celrep.2019.09.050. [DOI] [PubMed] [Google Scholar]
- Marinho F.V., Benmerzoug S., Rose S., Campos P.C., Marques J.T., Báfica A., Barber G., Ryffel B., Oliveira S.C., Quesniaux V.F.J. The cGAS/STING pathway is important for dendritic cell activation but is not essential to induce protective immunity against Mycobacterium tuberculosis infection. J. Innate Immun. 2018;10:239–252. doi: 10.1159/000488952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M., Stamp G., Robins P., Dulic A., Rosewell I., Hrivnak G., Daly G., Lindahl T., Barnes D.E. Gene-targeted mice lacking the Trex1 (DNase III) 3'-->5' DNA exonuclease develop inflammatory myocarditis. Mol. Cell. Biol. 2004;24:6719–6727. doi: 10.1128/MCB.24.15.6719-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E.D., Roths J.B. A y chromosome associated factor in strain bxsb producing accelerated autoimmunity and lymphoproliferation. Arthritis Rheum. 1979;22:1188–1194. doi: 10.1002/art.1780221105. [DOI] [PubMed] [Google Scholar]
- Muskardin T.L.W., Niewold T.B. Type I interferon in rheumatic diseases. Nat. Rev. Rheumatol. 2018;14:214–228. doi: 10.1038/nrrheum.2018.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K.M., Christensen S.R., Shupe J., Kashgarian M., Kim D., Elkon K., Shlomchik M.J. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 2010;184:1840. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paludan S.R. Activation and regulation of DNA-driven immune responses. Microbiol. Mol. Biol. Rev. 2015;79:225. doi: 10.1128/MMBR.00061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan R., Liu H., Xin D., Choubey D. Identification of a negative feedback loop between cyclic di-GMP-induced levels of IFI16 and p202 cytosolic DNA sensors and STING. Innate Immun. 2013;20:751–759. doi: 10.1177/1753425913507097. [DOI] [PubMed] [Google Scholar]
- Panchanathan R., Shen H., Duan X., Rathinam V.A.K., Erickson L.D., Fitzgerald K.A., Choubey D. Aim2 deficiency in mice suppresses the expression of the inhibitory Fcgamma receptor (FcgammaRIIB) through the induction of the IFN-inducible p202, a lupus susceptibility protein. J. Immunol. 2011;186:6762–6770. doi: 10.4049/jimmunol.1003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun P., Deane J.A., Difilippantonio M.J., Tarasenko T., Satterthwaite A.B., Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- Pisitkun P., Ha H.-L., Wang H., Claudio E., Tivy C.C., Zhou H., Mayadas T.N., Illei G.G., Siebenlist U. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37:1104–1115. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland S.L., Riggs J.M., Gilfillan S., Bugatti M., Vermi W., Kolbeck R., Unanue E.R., Sanjuan M.A., Colonna M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 2014;211:1977–1991. doi: 10.1084/jem.20132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzo S.J., Allard J.D., Choubey D., Vyse T.J., Izui S., Peltz G., Kotzin B.L. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- Sacre K., Criswell L.A., McCune J.M. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res. Ther. 2012;14:R155. doi: 10.1186/ar3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato-Hayashizaki A., Ohtsuji M., Lin Q., Hou R., Ohtsuji N., Nishikawa K., Tsurui H., Sudo K., Ono M., Izui S. Presumptive role of 129 strain–derived Sle16 locus in rheumatoid arthritis in a new mouse model with Fcγ receptor type IIb–deficient C57BL/6 genetic background. Arthritis Rheum. 2011;63:2930–2938. doi: 10.1002/art.30485. [DOI] [PubMed] [Google Scholar]
- Sauer J.-D., Sotelo-Troha K., von Moltke J., Monroe K.M., Rae C.S., Brubaker S.W., Hyodo M., Hayakawa Y., Woodward J.J., Portnoy D.A., Vance R.E. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 2011;79:688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Campbell A.M., Chan J., Schattgen S.A., Orlowski G.M., Nayar R., Huyler A.H., Nündel K., Mohan C., Berg L.J. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc. Natl. Acad. Sci. U S A. 2015;112:E710–E717. doi: 10.1073/pnas.1420217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D.B., Ko J.S., Heidmann T., Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton P., Borgia J.A., Bonomi P., Plate J.M.D. Lyn, a Src family kinase, regulates activation of epidermal growth factor receptors in lung adenocarcinoma cells. Mol. Cancer. 2013;12:76. doi: 10.1186/1476-4598-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Chen Z.J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 2012;5:ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A.N., Dixon F.J. Murine models of systemic lupus erythematosus. In: Dixon F.J., editor. Advances in Immunology. Elsevier; 1985. pp. 269–390. [DOI] [PubMed] [Google Scholar]
- Unterholzner L., Keating S.E., Baran M., Horan K.A., Jensen S.B., Sharma S., Sirois C.M., Jin T., Latz E., Xiao T.S. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010;11:997. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M.M., Crute B.W., Cambier J.C., Getahun A. B cell–intrinsic STING signaling triggers cell activation, synergizes with B cell receptor signals, and promotes antibody responses. J. Immunol. 2018;201:2641. doi: 10.4049/jimmunol.1701405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Sun L., Chen X., Du F., Shi H., Chen C., Chen Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N. Immune diseases associated with TREX1 and STING dysfunction. J. Interferon Cytokine Res. 2017;37:198–206. doi: 10.1089/jir.2016.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidi M., Kim H.J., Werth V.P. Increased myeloid dendritic cells and TNF-α expression predicts poor response to hydroxychloroquine in cutaneous lupus erythematosus. J. Invest. Dermatol. 2019;139:324–332. doi: 10.1016/j.jid.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong B., Yang Y., Li S., Wang Y.-Y., Li Y., Diao F., Lei C., He X., Zhang L., Tien P., Shu H.-B. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Zhu X.W., Wang Y., Wei Y.H., Zhao P.P., Wang X.B., Rong J.J., Zhong W.Y., Zhang X.W., Wang L., Zheng H.F. Comprehensive assessment of the association between FCGRs polymorphisms and the risk of systemic lupus erythematosus: evidence from a Meta-Analysis. Sci. Rep. 2016;6:31617. doi: 10.1038/srep31617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microarray data are available at Gene Expression Omnibus: GSE142594 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE142594.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRoteomics IDEntifications (PRIDE) partner repository with the dataset identifier PXD019239.