Abstract
Background:
Primary lymphoma of bone (PLB) is an extremely rare malignancy arising in the skeletal system. There is no consensus over the best definition of PLB. Most of the published articles are single-institutional retrospective studies with a limited sample size. The rarity of PLB and discrepancies on diagnostic criteria has resulted in a vague understanding of PLB.
Methods
We retrospectively analyzed the clinical characteristics and prognostic factors of 2558 PLB patients who were registered in the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2016. Survival rates were calculated using the Kaplan–Meier method. The effects of various factors on survival outcomes were analyzed by using the log-rank test. Univariate and multivariate analyses were conducted by using the Cox proportional hazards model to determine independent prognostic factors.
Results:
The median follow-up time of all eligible patients was 58 months. There seemed no sex preponderance in PLB incidence. The most involved sites are axial skeletons. The most common histological subtype was diffuse large B-cell lymphoma. The 3-, 5-, 10-, and 20-year overall survival (OS) rates were 70.70%, 65.70%, 54.40% and 39.50%, respectively. PLB patients whose primary tumor sites were appendicular and craniofacial skeletons had a significant survival advantage [hazard ratio (HR) = 0.694, 95% confidence interval (CI) 0.552–0.872; HR = 0.729, 95% CI 0.597–0.889, respectively] over those with axial skeletons as primary tumor sites. Patients with Hodgkin lymphoma, non-Hodgkin lymphoma (NHL)–mature B-cell lymphoma, and NHL-precursor-cell lymphoblastic lymphoma also had a significant OS advantage (HR = 0.392, 95% CI 0.200–0.771; HR = 0.826, 95% CI 0.700–0.973; and HR = 0.453, 95% CI 0.223–0.923, respectively). Patients with Ann Arbor stage III–IV at diagnosis were at higher risk of death than those with stage I–II (HR = 1.348, 95% CI 1.107–1.641). Chemotherapy was an independent favorable prognostic factor (HR = 0.734, 95% CI 0.605–0.890).
Conclusions:
Primary anatomic site, histology type, higher Ann Arbor stage and chemotherapy were independent prognostic factors. Chemotherapy played a pivotal role in PLB treatment.
Keywords: primary lymphoma of bone, prognosis, SEER, survival, therapeutic modality
Introduction
Primary lymphoma of bone (PLB), a rare hematological malignancy arising in the skeletal system, constitutes approximately 5% of extranodal lymphomas, less than 1% of all non-Hodgkin lymphomas (NHLs) and 3–7% of all malignant bone tumors.1 PLB is putatively correlated with HIV infection, osteomyelitis, chemotherapy, and some autoimmune disease.2 Diagnostic criteria for defining and classifying PLB have varied over time. According to World Health Organization classification of bone and soft-tissue tumors, PLB is defined as a single skeletal neoplasm composed of malignant lymphoid cells without regional lymph-node invasion, or bone lesions without invasion on visceral tissue or lymph node. Up till now, there is no consensus on the best definition of PLB. The definition in previous studies varied depending on different authors. Some studies only enrolled patients with Ann Arbor stage I and stage II at diagnosis, while other studies also enrolled patients with stage III and stage IV.1,3 PLB in pediatric patients is considered another clinical entity which is markedly different from its adult counterpart.4,5
The most commonly observed symptom is bony pain and swelling (80–95%), followed by tumor mass (30–40%) and pathological fracture (15–20%). The most frequently involved sites are the axial skeletons, yet every bone throughout the body is the potential place for PLB tumorigenesis.2,6 It is difficult to distinguish PLB from other kinds of primary bone tumors including chondrosarcoma and Ewing’s sarcoma in that radiographic results of PLB are not specific. Survival outcomes of PLB were considered brighter than other types of primary bone cancers. Moreover, previous studies reported that 5-year overall survival (OS) rate ranged from 36.0% to 88.3%, since different studies adopted different diagnostic criteria.6–10 Due to the rarity of PLB, the existing relevant literature consists mainly of single-institutional studies with small sample sizes, thus leading to an ambiguous description of clinical features, management, and prognosis. Selecting the optimal therapeutic strategy remains enigmatic because there have been no prospective clinical trials conducted regarding PLB. Herein, we present a series of 2558 PLB patients who were registered in the Surveillance, Epidemiology and End Results (SEER) database to explore patient demographics, pathological characteristics, therapeutic options, survival outcomes, and prognostic factors, thus shedding more light on this rare bone cancer.
Methods
Information regarding PLB patients between 1973 and 2016 were extracted from the SEER database, which is a population-based cancer registry supported by the National Cancer Institute of the United States (US). The SEER database covers approximately 28% of the US population, holding annually uploaded data on patient demographics, tumor pathology, anatomic sites of tumor, stage at diagnosis, first course of treatment modalities and the follow-up vital status. Our present study was exempted from institutional review board because the SEER database is available to the public and contains completely anonymized patient information.
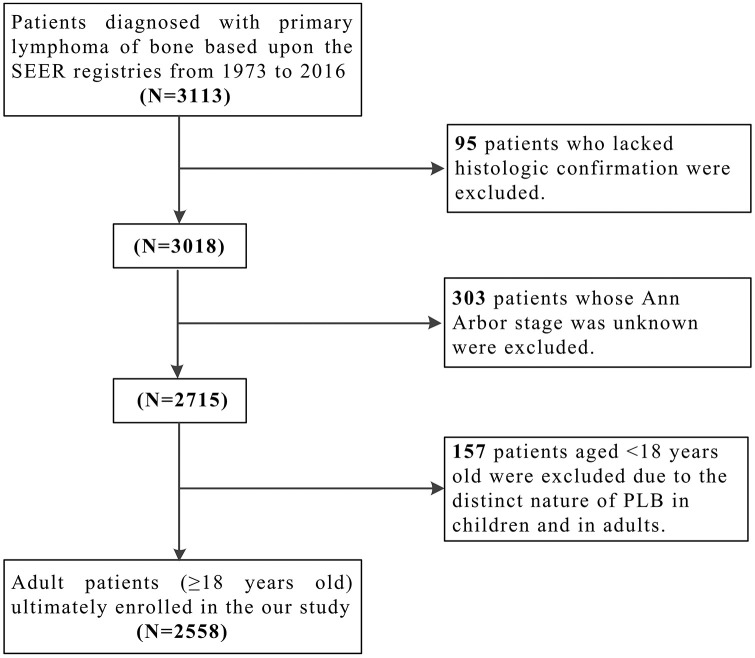
The flowchart of identification process is shown in Figure 1. A total of 3113 PLB patients were identified from the SEER database, of whom 2558 cases (82.17%) with complete survival information were eligible for further analysis. Inclusion criteria were as follows: (a) anatomic site of the primary tumor localized on the skeletal system [International Classification of Diseases (ICD)-O-3: C40.0–C41.9]; (b) histological type limited to lymphoma (ICD-O-3 histology codes: 9590–9738); (c) malignant behavior (ICD-O-3 behavior code: 3). Exclusion criteria contained: (a) patients without histological confirmation (diagnostic confirmation codes: 2, 4, 5, 6, 7, 8, 9); (b) patients with unclear information (stages, treatment modalities, age, sex, etc.); (c) patients aged under 18-years old (due to the potentially different natural history of disease). There were two endpoint events in this study. OS was calculated from pathological diagnosis to the date of last follow up, or death from any causes. Disease-specific survival (DSS) was defined as the time interval from diagnosis to the date of last follow up, or death caused by PLB.
Figure 1.
Flow diagram of the selection process for the patient cohort.
PLB, primary lymphoma of bone; SEER, Surveillance, Epidemiology, and End Results database.
All statistical analyses were performed with software SPSS (Version 26.0, SPSS Inc, Chicago, IL, USA). The influence of clinical and therapeutic variables on survival outcome was assessed by comparing the Kaplan–Meier survival curves through log-rank test. Multivariate analyses on DSS and OS were performed with a Cox proportional hazard regression model by incorporating variables that were statistically significant in univariate analysis. All significance tests were two tailed with p < 0.05 considered statistically significant.
Results
A total of 2558 patients with PLB were finally enrolled in our study, including 1251 men and 1307 women (0.957:1). The distribution of histologic subtypes of PLB was demonstrated in Table 1. The most frequently observed histological subtype was diffuse large B-cell lymphoma (DLBCL; n = 1703, 66.58%), followed by follicular lymphoma (n = 166, 6.49%). The distribution of primary involved skeletal sites of PLB is shown in Table 2. The most commonly involved site was vertebral column (n = 767, 29.98%), followed by the long bones of lower limb and associated joints (n = 597, 23.34%). Information about PLB patient demographic and variables is summarized in Table 3. Median age at diagnosis was 59.59 years old (range 18–100 years), and 86% of patients were White (n = 2189, 85.6%). The vast majority of PLB patients (n = 2534, 99.1%) suffered from NHL; 2082 of NHL–PLB patients were diagnosed with mature B-cell lymphoma (81.4%) and only 56 of NHL–PLB patients were diagnosed with mature T- and natural killer (NK)-cell lymphoma (2.2%).While patients who suffered from Hodgkin lymphoma (HL) accounted for merely 0.9% (n = 24). A total of 1430 cases (55.9%) had primary axial bone lesions (including vertebral column, rib, sternum, clavicle, pelvic bones, sacrum, coccyx, and associated joints), while 223 cases (8.7%) had craniofacial bone lesions (including mandible, bones of skull and face and associated joints) and 905 cases (35.4%) had appendicular bone lesions (including long and short bones of upper and lower limbs, scapula, and associated joints). Based upon Ann Arbor stage at diagnosis, PLB patients were categorized into four groups. That was stage I (n = 1367, 53.4%), stage II (n = 285, 11.1%), stage III (n = 58, 2.3%) and stage IV (n = 848, 33.2%), respectively. Most of patients underwent radiation and/or received chemotherapy therapy as initial treatment (1391 and 1941 patients, respectively). Of all patients, merely 618 cases (24.2%) received surgery.
Table 1.
The distribution of histologic subtypes in PLB.
| Histologic type (ICD-O-3) | Number | Percentage |
|---|---|---|
| Diffuse large B-cell lymphoma, NOS (9680) | 1703 | 66.58% |
| Non-Hodgkin lymphoma, NOS (9591) | 314 | 12.28% |
| Follicular lymphoma (9698) | 166 | 6.49% |
| Lymphoid neoplasm, NOS (9590) | 111 | 4.34% |
| Chronic/small lymphocytic, NOS (9670) | 49 | 1.92% |
| Anaplastic large cell lymphoma, T-cell and null cell type (9714) | 44 | 1.72% |
| Burkitt lymphoma, NOS (9687) | 34 | 1.33% |
| Marginal zone B-cell lymphoma, NOS (9699) | 30 | 1.17% |
| Precursor NHL, NOS (9727) | 29 | 1.14% |
| Lymphoplasmacytic lymphoma (9671) | 24 | 0.94% |
| Classical Hodgkin lymphoma, NOS (9650) | 17 | 0.66% |
| Peripheral T-cell lymphoma, NOS (9702) | 12 | 0.47% |
| Mantle-cell lymphoma (9673) | 9 | 0.35% |
| Nodular sclerosis classical Hodgkin lymphoma (9663) | 7 | 0.27% |
| NHL, NOS, T-cell (9684) | 5 | 0.20% |
| NK/T-cell lymphoma, nasal and nasal-type (9719) | 2 | 0.08% |
| Primary effusion lymphoma (9678) | 1 | 0.04% |
| Composite Hodgkin lymphoma and NHL (9596) | 1 | 0.04% |
| Total patients with PLB | 2558 | 100.00% |
ICD, International Classification of Diseases; NHL, non-Hodgkin lymphoma; NK, natural killer (cell); NOS, not otherwise specified; PLB, primary lymphoma of bone.
Table 2.
The distribution of primary anatomic sites in PLB.
| Primary anatomic sites (ICD site code) | Number | Percentage |
|---|---|---|
| Vertebral column (C41.2) | 767 | 29.98% |
| Long bones of lower limb and associated joints (C40.2) | 597 | 23.34% |
| Pelvic bones, sacrum, coccyx and associated joints (C41.4) | 356 | 13.92% |
| Long bones of upper limb, scapula, and associated joints (C40.0) | 258 | 10.09% |
| Bone, NOS (C41.9) | 181 | 7.08% |
| Bones of skull and face and associated joints (C41.0) | 130 | 5.08% |
| Rib, sternum, clavicle and associated joints (C41.3) | 104 | 4.07% |
| Mandible (C41.1) | 93 | 3.64% |
| Short bones of lower limb and associated joints (C40.3) | 41 | 1.60% |
| Overlap bones, joints, and cartilage (C41.8) | 19 | 0.74% |
| Bone of limb, NOS (C40.9) | 9 | 0.35% |
| Overlap of bones, joints, and cartilage of limbs (C40.8) | 3 | 0.12% |
| Total patients with PLB | 2558 | 100.00% |
ICD, International Classification of Diseases; NOS, not otherwise specified; PLB, primary lymphoma of bone.
Table 3.
Demographic and clinical characteristics of the adult PLB patients.
| Characteristic | Number | Percentage | |
|---|---|---|---|
| Sex | |||
| Female | 1251 | 48.9% | |
| Male | 1307 | 51.1% | |
| Marital status | |||
| Unmarried | 1152 | 45.2% | |
| Married | 1403 | 54.8% | |
| Age | |||
| 20–40 | 462 | 18.1% | |
| 40–60 | 674 | 26.3% | |
| 60–80 | 1021 | 39.9% | |
| 80– | 401 | 15.7% | |
| Race | |||
| White | 2189 | 85.6% | |
| Black | 206 | 8.1% | |
| American Indian/Alaska native | 23 | 0.9% | |
| Asian or Pacific Islander | 140 | 5.5% | |
| Lymphoma type | |||
| Hodgkin lymphoma | 24 | 0.9% | |
| Non-Hodgkin lymphoma | 2534 | 99.1% | |
| Primary site | |||
| Axial | 1430 | 55.9% | |
| Appendicular | 905 | 35.4% | |
| Craniofacial | 223 | 8.7% | |
| Laterality | |||
| Bilateral, single primary | 32 | 1.3% | |
| Left: origin of primary | 607 | 23.7% | |
| Right: origin of primary | 555 | 21.7% | |
| Not a paired site | 1364 | 53.3% | |
| Histologic type: broad groupings | |||
| ICD-O-3:9590-9599 | Malignant lymphomas, NOS or diffuse | 367 | 14.3% |
| ICD-O-3:9650-9669 | Hodgkin lymphomas | 24 | 0.9% |
| ICD-O-3;9670-9699 | NHL– mature B-cell lymphomas | 2082 | 81.4% |
| ICD-O-3:9700-9719 | NHL–mature T- and NK-cell lymphomas | 56 | 2.2% |
| ICD-O-3:9720-9729 | NHL-precursor-cell lymphoblastic lymphoma | 29 | 1.1% |
| Ann Arbor stage | |||
| Stage I | 1367 | 53.4% | |
| Stage II | 285 | 11.1% | |
| Stage III | 58 | 2.3% | |
| Stage IV | 848 | 33.2% | |
| Number of bone lesions | |||
| Single | 2328 | 91.0% | |
| Multiple (⩾2) | 230 | 9.0% | |
| Radiation sequence with surgery | |||
| No radiation and/or cancer-directed surgery | 2202 | 86.1% | |
| Radiation after surgery | 339 | 13.3% | |
| Radiation prior to surgery | 12 | 0.5% | |
| Radiation before and after surgery | 5 | 0.2% | |
| Surgery | |||
| No | 1940 | 75.8% | |
| Yes | 618 | 24.2% | |
| Radiation | |||
| No | 1167 | 45.6% | |
| Yes | 1391 | 54.4% | |
| Chemotherapy | |||
| No | 617 | 24.1% | |
| Yes | 1941 | 75.9% | |
| Overall survival | |||
| Censored | 1440 | 56.3% | |
| Dead | 1118 | 43.7% | |
| Disease-specific survival | |||
| Censored | 1833 | 71.7% | |
| Dead | 725 | 28.3% | |
| Year of diagnosis | |||
| 1975–1986 | 111 | 4.3% | |
| 1986–1996 | 340 | 13.3% | |
| 1996–2006 | 890 | 34.8% | |
| 2006–2016 | 1217 | 47.6% | |
ICD, International Classification of Diseases; NHL, non-Hodgkin lymphoma; NK, natural killer (cell); NOS, not otherwise specified; PLB, primary lymphoma of bone.
The median follow-up time for all eligible patients was 58 months (range 0–401 months). The Kaplan–Meier curves of OS and DSS are shown in Figure 2. The OS rates of 3, 5, 10 and 20 years were 70.70%, 65.70%, 54.40% and 39.50%, respectively. At the corresponding time point, DSS rates were 76.80%, 73.60%, 68.10% and 61.00%, respectively. In the univariate assessment, sex (p = 0.005), primary site (p < 0.001), lateral position (p = 0.001), histological records: broad grouping (p < 0.001), Ann Arbor stage (p < 0.001), the number of lesions (p = 0.027), surgery (p = 0.004), radiation (p < 0.001), and chemotherapy (p < 0.001) are the possible predictive factors of OS (Table 4).
Figure 2.
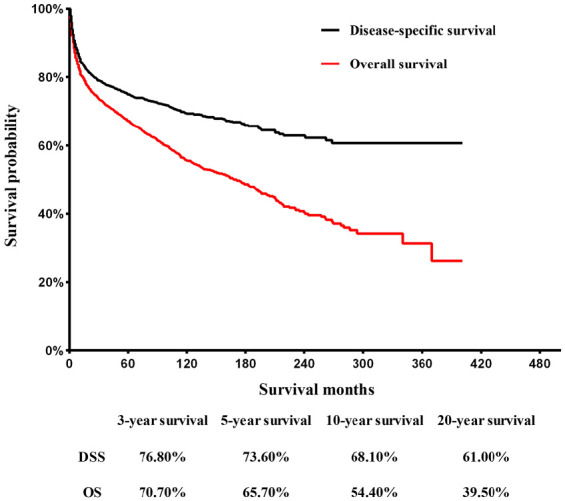
Kaplan–Meier curves of overall survival and disease-specific survival for adult patients with primary lymphoma of bone.
DSS, disease-specific survival; OS, overall survival.
Table 4.
Univariate analysis of overall survival.
| Characteristic | Median survival (95% CI) | HR (95% CI) |
p |
|---|---|---|---|
| Sex | |||
| Female | 134.0 (160.3–107.7) | Reference | |
| Male | 176.0 (149.4–202.6) | 0.843 (0.750–0.949) | 0.005 |
| Age | 0.143 | ||
| 20–39 | 157.0 (108.5–205.5) | Reference | – |
| 40–59 | 136.0 (105.2–166.8) | 1.073 (0.898–1.283) | 0.437 |
| 60–79 | 162.0 (124.4–199.6) | 1.010 (0.855–1.195) | 0.903 |
| >80 | 179.0 (143.2–214.8) | 0.855 (0.693–1.055) | 0.144 |
| Race | 0.108 | ||
| White | 157.0 (136.5–177.5) | Reference | – |
| Black | 183.0 (150.5–215.5) | 0.740 (0.583–0.941) | 0.114 |
| American Indian/Alaska native | 131.0 (81.2–180.8) | 0.965 (0.517–1.798) | 0.910 |
| Asian or pacific islander | 144.0 (73.7–214.3) | 0.967 (0.748–1.250) | 0.798 |
| Lymphoma type | |||
| Hodgkin lymphoma | 183.0 (95.1–270.9) | Reference | – |
| Non-Hodgkin lymphoma | 162.0 (142.9–181.1) | 1.478 (0.767–2.848) | 0.243 |
| Primary site | 0.000 | ||
| Axial | 114.0 (93.6–134.4) | Reference | – |
| Appendicular | 204.0 (112.7–295.3) | 0.685 (0.515–0.860) | 0.001 |
| Craniofacial | 219.0 (177.1–260.9) | 0.697 (0.612–0.793) | 0.000 |
| Laterality | 0.001 | ||
| Bilateral, single primary | 113.0 (39.5–186.5) | Reference | – |
| Left: origin of primary | 211.0 (175.6–246.4) | 0.776 (0.460–1.307) | 0.340 |
| Right: origin of primary | 163.0 (122.1–203.9) | 0.835 (0.495–1.408) | 0.498 |
| Not a paired site | 124.0 (101.6–146.4) | 1.027 (0.615–1.713) | 0.919 |
| Histologic type: broad groupings | 0.000 | ||
| Malignant lymphomas, NOS or diffuse | 92.0 (61.2–122.8) | Reference | / |
| Hodgkin lymphomas | 183.0 (95.1–270.9) | 0.520 (0.267–1.014) | 0.055 |
| NHL–mature B-cell lymphomas | 167.0 (145.3–188.7) | 0.740 (0.635–0.863) | 0.000 |
| NHL–mature T- and NK-cell lymphomas | 181.5 (141.6–221.4) | 0.618 (0.390–0.979) | 0.040 |
| NHL-precursor-cell lymphoblastic lymphoma | 239.6 (186.8–292.4) | 0.395 (0.195–0.800) | 0.010 |
| Ann Arbor stage | 0.000 | ||
| Stage I–II | 191.0 (166.5–215.5) | Reference | – |
| Stage III–IV | 107.0 (87.2–126.8) | 1.078 (1.324–1.681) | 0.000 |
| Number of lesions | |||
| Single | 167.0 (144.9–189.1) | Reference | – |
| Multiple (⩾2) | 104.0 (75.0–133.0) | 1.242 (1.024–1.505) | 0.027 |
| Surgery | |||
| No | 179.0 (157.3–200.7) | Reference | – |
| Yes | 114.0 (87.8–140.2) | 1.212 (1.064–1.379) | 0.004 |
| Radiation | |||
| No | 107.0 (89.7–124.3) | Reference | – |
| Yes | 225.0 (119.8–244.3) | 0.630 (0.559–0.710) | 0.000 |
| Chemotherapy | |||
| No | 80.0 (59.8–100.2) | Reference | – |
| Yes | 202 (180.1–223.3) | 0.619 (0.546–0.702) | 0.000 |
| Year of diagnosis | 0.522 | ||
| 1975–1986 | 175.0 (95.8–254.2) | Reference | – |
| 1986–1996 | 139.0 (101.7–176.3) | 0.911 (0.665–1.247) | 0.560 |
| 1996–2006 | 164.0 (128.0–200.0) | 0.828 (0.619–1.106) | 0.202 |
| 2006–2016 | 159.0 (129.3–188.7) | 0.872 (0.656–1.159) | 0.346 |
Bolded numerals indicate statistical significance.
CI, confidence interval; HR, hazard ratio; NHL, non-Hodgkin lymphoma; NK, natural killer (cell); NOS, not otherwise specified.
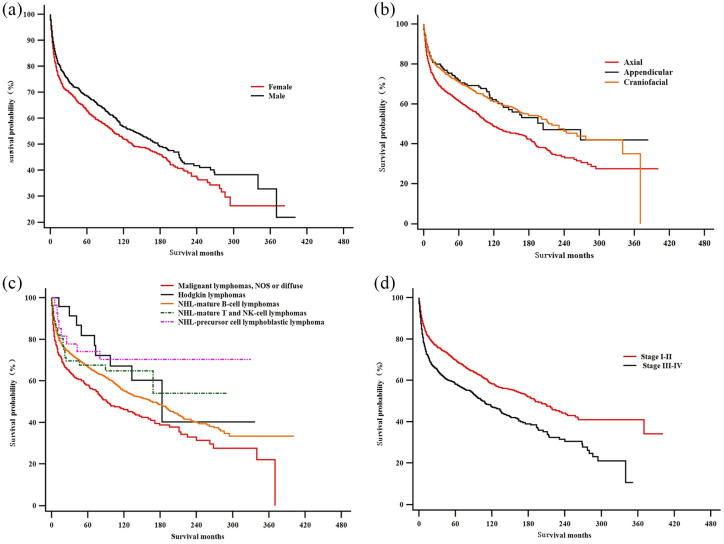
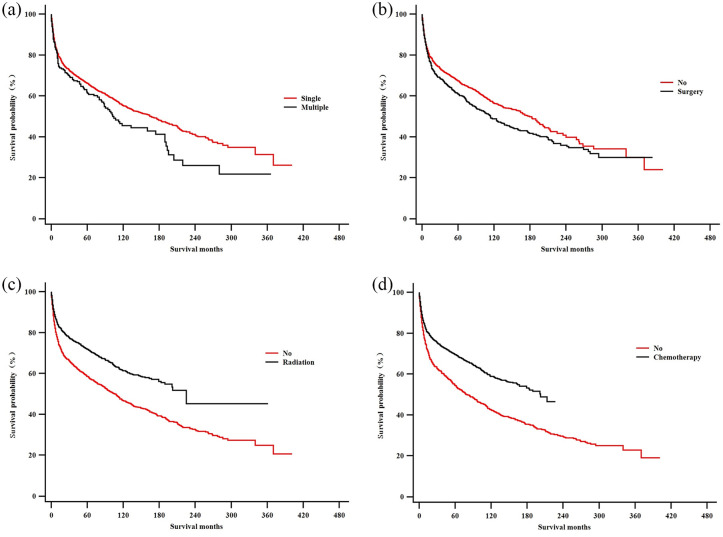
As demonstrated in Figures 3 and 4, Kaplan–Meier survival curves gave a detailed description of the associations between various factors and PLB prognosis. According to our results, sex did not seem to be one of those factors. Primary site could influence the prognosis of PLB, since patients with axial neoplasm had a bleaker prognosis than patients whose primary tumor sites were at the appendicular and craniofacial skeletons. The actual laterality of primary sites (left/right or bilateral) did not seem correlated with prognosis. Patients with malignant lymphoma [not otherwise specified (NOS) or diffuse] have shorter survival period than those with mature B-cell lymphomas and HLs. Patients with lower Ann Arbor stage (stage I–II) at diagnosis exhibited a remarkable survival advantage over those with higher Ann Arbor stage (stage III–IV). As to therapeutic approaches, chemotherapy and radiation therapy benefited PLB patients, while surgery did not prove to extend patient survival.
Figure 3.
Kaplan–Meier estimate of overall survival by subgroup analysis: (a) sex; (b) primary anatomic sites; (c) histological subtype; and (d) Ann Arbor stage.
NHL, non-Hodgkin lymphoma; NK, natural killer (cell); NOS, not otherwise specified.
Figure 4.
Kaplan–Meier estimate of overall survival by subgroup analysis: (a) number of osseous lesions; (b) Surgery; (c) radiotherapy; and (d) chemotherapy.
As revealed in Table 5, multivariate analysis showed that the primary site, histological classification, Ann Arbor stage, and chemotherapy were independent prognostic factors. As to classification of tumor, patients with HL, NHL–mature B-cell lymphomas and NHL-precursor-cell lymphoblastic lymphoma had a significant OS advantage [hazard ratio (HR) = 0.392, 95% confidence interval (CI) 0.200–0.771; HR = 0.826, 95% CI 0.700–0.973; and HR = 0.453, 95% CI 0.223–0.923, respectively]. In terms of primary sites, patients with primary appendicular and craniofacial tumor had a significant survival advantage (HR = 0.694, 95% CI 0.552–0.872; HR = 0.729, 95% CI 0.597–0.889, respectively) over those with axial tumor. Patients with higher stage (stage III– IV) at diagnosis were at higher risk of death than those with lower stage (stage I–II) at diagnosis, yielding an HR of 1.348 (95% CI 1.107–1.641). Surgical treatment and radiotherapy proved not to be a protective factor of patients’ long-term survival (p > 0.05), but chemotherapy was an independent favorable prognostic factor (HR = 0.734, 95% CI 0.605–0.890). The multivariate analysis of DSS was similar to the results of OS analysis.
Table 5.
Multivariate analysis of disease-specific survival and overall survival.
| Characteristic | Disease-specific survival |
Overall survival |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.896 (0.749–1.072) | 0.229 | 0.881 (0.763–1.017) | 0.084 |
| Primary site | ||||
| Axial | Reference | – | Reference | |
| Appendicular | 0.626 (0.466–0.841) | 0.002 | 0.694 (0.552–0.872) | 0.002 |
| Craniofacial | 0.708 (0.553–0.908) | 0.006 | 0.729 (0.597–0.889) | 0.002 |
| Laterality | ||||
| Bilateral, single primary | Reference | – | Reference | |
| Left: origin of primary | 0.930 (0.489–1.771) | 0.826 | 0.898 (0.532–1.519) | 0.689 |
| Right: origin of primary | 0.853 (0.446–1.631) | 0.631 | 0.953 (0.563–1.613) | 0.859 |
| Not a paired site | 0.920 (0.486–1.744) | 0.798 | 0.935 (0.555–1.577) | 0.802 |
| Histologic type: broad groupings | ||||
| Malignant lymphomas, NOS or diffuse | Reference | – | Reference | – |
| Hodgkin lymphomas | 0.184 (0.058–0.583) | 0.004 | 0.392 (0.200–0.771) | 0.007 |
| NHL–mature B-cell lymphomas | 0.828 (0.676–1.014) | 0.067 | 0.826 (0.700–0.973) | 0.023 |
| NHL–mature T- and NK-cell lymphomas | 0.518 (0.269–0.999) | 0.050 | 0.680 (0.424–1.090) | 0.109 |
| NHL-precursor-cell lymphoblastic lymphoma | 0.536 (0.235–1.221) | 0.137 | 0.453 (0.223–0.923) | 0.029 |
| Ann Arbor stage | ||||
| Stage I–II | Reference | – | Reference | – |
| Stage III–IV | 1.635 (1.281–2.086) | 0.000 | 1.348 (1.107–1.641) | 0.03 |
| Number of bone lesions | ||||
| Single | Reference | – | Reference | – |
| Multiple (⩾2) | 1.122 (0.878–1.435) | 0.357 | 1.201 (0.990–1.457) | 0.064 |
| Surgery | ||||
| No | Reference | – | Reference | – |
| Yes | 1.162 (1.064–1.379) | 0.101 | 1.129 (0.977–1.304) | 0.101 |
| Radiation | ||||
| No | Reference | – | Reference | – |
| Yes | 0.996 (0.559–0.710) | 0.981 | 0.934 (0.730–1.195) | 0.587 |
| Chemotherapy | ||||
| No | Reference | – | Reference | – |
| Yes | 0.641 (0.546–0.702) | 0.000 | 0.734 (0.605–0.890) | 0.002 |
CI, confidence interval; HR, hazard ratio; NHL, non-Hodgkin lymphoma; NK, natural killer (cell); NOS, not otherwise specified.
Discussion
PLB has the characteristics of non-specific clinical manifestations but responds well to chemotherapy. DLBCL accounts for approximately 80% of all PLB histological subtypes.2 Due to the low incidence of PLB, clinicopathological characteristics and therapeutic options are yet to be further investigated. Our present study analyzed 2558 cases in the SEER database, where most of the patients are White. Compared with the previous studies,6,10–16 our study achieved consistent conclusions. The majority of the PLB patients were those with NHL. The median age at diagnosis was over 50-years old. Axial skeletons were the most involved sites. Chemotherapy and radiotherapy are recognized as the main treatment options for PLB. In terms of prognosis, higher Ann Arbor stage and multifocal disease at diagnosis were the unfavorable factors. Previous studies reported that there were more male PLB patients than female patients,10–16 but our results were based upon a larger sample size, and indicated that the ratio of male to female PLB patients was close to 1:1. That is to say, the incidence of PLB has no sex predilection. Meanwhile, through further log-rank test, we found that the prognosis of female PLB patients seems to be worse than that of the male patients.
The past 2 decades witnessed the wide administration of anthracycline-containing chemotherapy with subsequent consolidative irradiation for the treatment of PLB.6,11,14,17–19A study conducted on 78 PLB patients with pathological fracture at presentation confirmed that anthracycline-based chemotherapy followed by irradiation proved to be the optimal treatment sequence, while the inverse sequence of these two modalities was correlated with bleaker survival outcome. Initial surgery did not help to inhibit the tumor and did not extend survival.20 In clinical practice, surgery is often applied for diagnostic biopsy, pathological fractures, and spinal decompression with the aim of improving the quality of life. The role of surgery in PLB tumor control and treatment warrants further verification. Multiple studies have revealed that a combined regime can achieve a higher OS rate and have clarified that a combinative use of chemotherapy and radiotherapy might be the best therapeutic option for PBL.7,13,21–23 A multicenter retrospective study verified that 116 PLB patients diagnosed at an early stage (stages I and II) had a brighter prognosis and can benefit greatly from adequate radiotherapy dose (40 Gy) alone, chemotherapy alone and the combined modalities.11 Another retrospective study enrolled 102 PLB patients with DLBCL. In comparison with the non-radiotherapy group, patients who received consolidative radiotherapy after standard chemotherapy achieved excellent survival outcomes, yielding both markedly improved 5-year progression-free survival (PFS) rate (88% versus 63%, p = 0.0069) and OS rate (91% versus 68%, p = 0.0064).24
However, the IELSG-14 study concluded that whether they received subsequent radiotherapy or not, PLB patients with DLBCL subtype had an encouraging prognosis when administered with the anthracycline-based therapeutic regimen. The addition of subsequent consolidative radiotherapy with intensified doses and enlarged involved fields to initial chemotherapy was not correlated with improved survival outcome.25 A retrospective study on 52 PLB patients demonstrated that the complete response rate in the radiotherapy-alone group and chemotherapy with/without radiation group were 64% and 85%, along with the relapse rate of 57% and 6%, respectively.26 Beal et al. revealed that the 5-year OS rate of PLB patients treated with a combination of chemotherapy and radiotherapy was not superior to that of patients who received chemotherapy alone.10 A retrospective study on 61 Chinese PLB patients demonstrated that chemotherapy played a pivotal role in PLB treatment, and chemotherapy alone was also not inferior to the combined therapeutic modality.19
Since the majority of PLB is pathologically categorized into DLBCL, cyclophosphamide, doxorubicin, vincristine, and prednisone or rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone are currently the main treatment regimens. Beal et al.10 reported that PFS and OS of PLB subtype with CD20-positive B-cell lymphoma have been greatly improved by combining rituximab. Yuste et al.15 found that PFS increased from 52% to 88% by adding rituximab into conventional chemotherapy. Bisphosphonates can inhibit the activity of osteoclast and are currently applied in multiple myeloma and metastatic bone lesions in prostate cancer, lung cancer, and prostate cancer. PLB patients have tendency toward osteolytic lesions, and even pathological fracture. Bisphosphonates can also be used in the context of hypercalcemia to prevent bone destruction.27
There existed several limitations in our present study. First, lymphomas contain a series of highly heterogenous diseases and thus PLB consists of various histological subtypes. Second, the dates of information retrieval from SEER spanned a long period of time, ranging from 1973 to 2016, which witnessed the changes in diagnostic criteria and the rapid advancement of treatment approaches. Third, the inherent drawbacks of the SEER database are unavoidable. The SEER database neither collects nor records information regarding disease progression, relapse or recurrence, infection, comorbidities, and complications. Besides, the SEER database lacks the important information about individual patient, including Eastern Cooperative Oncology Group Performance Status, international prognostic index score, tumor size, lactate dehydrogenase, and many other laboratory test results. Lastly, the specific therapeutic regimens, drug doses, administration frequency, and radiation doses were also not recorded in the SEER database.
Despite the abovementioned limitations, this study, to the best of our knowledge, represents the largest retrospective PLB cohort till now. We have found that there was no sex predilection in PLB occurrence. Multivariate analysis revealed that primary anatomic site, histological classification, Ann Arbor stage and chemotherapy were closely associated with PLB prognosis. Chemotherapy played a pivotal role in PLB treatment. It remains still controversial whether chemotherapy in combination with radiotherapy is superior to chemotherapy alone. The optimal treatment strategies, including agents with novel mechanisms of action and radiation doses and fields, warrant further verification in future studies and clinical trials.
Acknowledgments
Great appreciation should be accorded to all the researchers and staff of the SEER Program for their hard work in collecting patient information and maintaining the database.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Data availability statement: All data regarding patient information were acquired from the SEER database and will be made available upon request by correspondence to Dr Yan-Hua Zheng.
Funding: The author(s) disclose receipt of the following financial support for the research, authorship, and/or publication of this article: this study was granted by the National Natural Science Foundation of China (81970190 to Professor Gao, 81900207 to Dr Xu) and the Innovative Chain (Group) in Key Industry of Shaan’xi Province of China (2019ZDLSF02-02 to Professor Gao).
ORCID iDs: Tian-Qi Xu  https://orcid.org/0000-0003-1015-783X
https://orcid.org/0000-0003-1015-783X
Yan-Hua Zheng  https://orcid.org/0000-0002-7527-8248
https://orcid.org/0000-0002-7527-8248
Contributor Information
Chen-Xin Liu, Institute of Orthopedics, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China.
Tian-Qi Xu, Department of Hematology, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China.
Li Xu, Department of Hematology, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China.
Pan-Pan Wang, Institute of Pediatrics, The Second Affiliated Hospital of Shaanxi University of Chinese Medicine, Xi’an, Shaanxi Province, China.
Chun Cao, Department of Hematology, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi, China.
Guang-Xun Gao, Department of Hematology, Xijing Hospital, Fourth Military Medical University, 127 Chang’le West Road, Xi’an, Shaanxi 710032, PR China.
Yan-Hua Zheng, Department of Hematology, Xijing Hospital, Fourth Military Medical University, 127 Chang’le West Road, Xi’an, Shaanxi 710032, PR China.
References
- 1. Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013, (4th edition). Pathology 2014; 46: 95–104. [DOI] [PubMed] [Google Scholar]
- 2. Messina C, Christie D, Zucca E, et al. Primary and secondary bone lymphomas. Cancer Treat Rev 2015; 41: 235–246. [DOI] [PubMed] [Google Scholar]
- 3. Fletcher CD. The evolving classification of soft tissue tumours - an update based on the new 2013 WHO classification. Histopathology 2014; 64: 2–11. [DOI] [PubMed] [Google Scholar]
- 4. Glotzbecker MP, Kersun LS, Choi JK, et al. Primary non-Hodgkin’s lymphoma of bone in children. J Bone Joint Surg Am 2006; 88: 583–594. [DOI] [PubMed] [Google Scholar]
- 5. Chisholm KM, Ohgami RS, Tan B, et al. Primary lymphoma of bone in the pediatric and young adult population. Hum Pathol 2017; 60: 1–10. [DOI] [PubMed] [Google Scholar]
- 6. Ramadan KM, Shenkier T, Sehn LH, et al. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: a population-based study of successively treated cohorts from the British Columbia cancer agency. Ann Oncol 2007; 18: 129–135. [DOI] [PubMed] [Google Scholar]
- 7. Barbieri E, Cammelli S, Mauro F, et al. Primary non-Hodgkin’s lymphoma of the bone: treatment and analysis of prognostic factors for stage I and stage II. Int J Radiat Oncol Biol Phys 2004; 59: 760–764. [DOI] [PubMed] [Google Scholar]
- 8. Hsieh PP, Tseng HH, Chang ST, et al. Primary non-Hodgkin’s lymphoma of bone: a rare disorder with high frequency of T-cell phenotype in southern Taiwan. Leuk Lymphoma 2006; 47: 65–70. [DOI] [PubMed] [Google Scholar]
- 9. Heyning FH, Hogendoorn PC, Kramer MH, et al. Primary non-Hodgkin’s lymphoma of bone: a clinicopathological investigation of 60 cases. Leukemia 1999; 13: 2094–2098. [DOI] [PubMed] [Google Scholar]
- 10. Beal K, Allen L, Yahalom J. Primary bone lymphoma: treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer 2006; 106: 2652–2656. [DOI] [PubMed] [Google Scholar]
- 11. Cai L, Stauder MC, Zhang YJ, et al. Early-stage primary bone lymphoma: a retrospective, multicenter Rare Cancer Network (RCN) Study. Int J Radiat Oncol Biol Phys 2012; 83: 284–291. [DOI] [PubMed] [Google Scholar]
- 12. Alencar A, Pitcher D, Byrne G, et al. Primary bone lymphoma—the University of Miami experience. Leuk Lymphoma 2010; 51: 39–49. [DOI] [PubMed] [Google Scholar]
- 13. Baar J, Burkes RL, Bell R, et al. Primary non-Hodgkin’s lymphoma of bone. A clinicopathologic study. Cancer 1994; 73: 1194–1199. [DOI] [PubMed] [Google Scholar]
- 14. Matikas A, Briasoulis A, Tzannou I, et al. Primary bone lymphoma: a retrospective analysis of 22 patients treated in a single tertiary center. Acta Haematol 2013; 130: 291–296. [DOI] [PubMed] [Google Scholar]
- 15. Yuste AL, Segura A, Lopez-Tendero P, et al. Primary lymphoma of bone: a clinico-pathological review and analysis of prognostic factors. Leuk Lymphoma 2004; 45: 853–855. [DOI] [PubMed] [Google Scholar]
- 16. Wu H, Bui MM, Leston DG, et al. Clinical characteristics and prognostic factors of bone lymphomas: focus on the clinical significance of multifocal bone involvement by primary bone large B-cell lymphomas. BMC Cancer 2014; 14: 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pellegrini C, Gandolfi L, Quirini F, et al. Primary bone lymphoma: evaluation of chemoimmunotherapy as front-line treatment in 21 patients. Clin Lymphoma Myeloma Leuk 2011; 11: 321–325. [DOI] [PubMed] [Google Scholar]
- 18. Horsman JM, Thomas J, Hough R, et al. Primary bone lymphoma: a retrospective analysis. Int J Oncol 2006; 28: 1571–1575. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Zhu J, Song Y, et al. Clinical characterization and outcome of primary bone lymphoma: a retrospective study of 61 Chinese patients. Sci Rep 2016; 6: 28834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Govi S, Christie D, Messina C, et al. The clinical features, management and prognostic effects of pathological fractures in a multicenter series of 373 patients with diffuse large B-cell lymphoma of the bone. Ann Oncol 2014; 25: 176–181. [DOI] [PubMed] [Google Scholar]
- 21. Dubey P, Ha CS, Besa PC, et al. Localized primary malignant lymphoma of bone. Int J Radiat Oncol Biol Phys 1997; 37: 1087–1093. [DOI] [PubMed] [Google Scholar]
- 22. Catlett JP, Williams SA, O’Connor SC, et al. Primary lymphoma of bone: an institutional experience. Leuk Lymphoma 2008; 49: 2125–2132. [DOI] [PubMed] [Google Scholar]
- 23. Fidias P, Spiro I, Sobczak ML, et al. Long-term results of combined modality therapy in primary bone lymphomas. Int J Radiat Oncol Biol Phys 1999; 45: 1213–1218. [DOI] [PubMed] [Google Scholar]
- 24. Tao R, Allen PK, Rodriguez A, et al. Benefit of consolidative radiation therapy for primary bone diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys 2015; 92: 122–129. [DOI] [PubMed] [Google Scholar]
- 25. Bruno Ventre M, Ferreri AJ, Gospodarowicz M, et al. Clinical features, management, and prognosis of an international series of 161 patients with limited-stage diffuse large B-cell lymphoma of the bone (the IELSG-14 study). Oncologist 2014; 19: 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zinzani PL, Carrillo G, Ascani S, et al. Primary bone lymphoma: experience with 52 patients. Haematologica 2003; 88: 280–285. [PubMed] [Google Scholar]
- 27. Takasaki H, Kanamori H, Takabayashi M, et al. Non-Hodgkin’s lymphoma presenting as multiple bone lesions and hypercalcemia. Am J Hematol 2006; 81: 439–442. [DOI] [PubMed] [Google Scholar]