Abstract
Background:
Progesterone receptor (PgR) negative breast cancer (BC) is an aggressive subtype with poor prognosis and reduced response to endocrine treatments. Several studies have suggested that androgen receptor (AR) expression is associated with a favorable tumor biology, longer recurrence free survival (RFS), and overall survival. In the literature no data exist regarding the role of AR expression in early stage estrogen receptor (ER)+/PgR– BCs. The aim of this study was to evaluate the prognostic role of AR expression in this setting.
Patients and methods:
This is a monocentric retrospective study in which 208 patients who underwent surgical intervention for ER+/PgR−/Human Epidermal growth factor Receptor 2 (HER2)– BC were included. The primary objective was to analyze the relationship between AR expression and RFS.
Results:
At a median follow-up of 77 months, 75 patients (36%) had a disease relapse (all sites included). AR expression was significantly higher in patients who did not relapse compared with those who relapsed with an impact on RFS (hazard ratio [HR] = 0.99, p = 0.025). Patients with AR expression ⩾80% had a lower risk of relapse compared with those with AR <80% (HR = 0.53, p = 0.008). In addition, breast tumors with higher AR expression had good biological features (low ki67 and nuclear grade) compared with BCs with lower AR expression, at least partly explaining the different outcome.
Conclusions:
The results of this study support the potential prognostic role of AR in patients with ER+/PgR− BCs and may contribute to the identification of subgroups of high-risk patients.
Keywords: androgen receptor, early breast cancer, immunohistochemistry, progesterone receptor negative, relapse-free survival
Introduction
Breast cancer (BC) is classified according to estrogen (ER), progesterone (PgR), and Human Epidermal growth factor Receptor 2 (HER2) expression, each of them playing an important predictive and prognostic role.
PgR gene is estrogen-dependent, and most ER positive (+) tumors are also PgR+. On the other hand, almost all ER negative (−) cancers are PgR−. PgR pathway plays a role in mammary gland growth as well as in the development of BC.1 The receptor is expressed in two isoforms (PgR-A and PgR-B), whose relationship has a functional and morphogenic role.2 In clinical practice, the lack of PgR expression is a well-known marker of worse prognosis in luminal cancers; in tumors defined as ER+ and having ki67 >14%, the loss of PgR was identified as an adverse prognostic factor.3,4 In addition, luminal B cancers, classified as ER+, HER2−, ki67 >14% and PgR <20%, have worse prognosis than tumors with PgR >20%.5,6
Several studies demonstrated that ER and PgR expression can vary during cancer natural history and during treatments; indeed, ER expression may be reduced during endocrine therapy, in particular with tamoxifen and, in 50% of cases, its complete loss together with the development of resistance to endocrine treatments has been described.7,8
Androgen receptor (AR) is expressed in more than 85% of early stage BCs and in about 75% of metastatic BCs.9 Recent gene expression profiling studies have identified four different BC subgroups: luminal subtypes A and B, both of which are ER and/or PgR positive; the HER2 subtype; and the basal-like group.10 Collins et al. evaluated the expression of AR among these molecularly-defined categories of invasive BC and found that AR expression was present in 91% of luminal A cancers, 68% of luminal B cancers, 59% of HER2-type cancers, 32% of basal-like cancers, and in 32% of unclassified carcinomas (those that were ER, PgR, HER2, CK 5/6 and epidermal growth factor [EGFR] negative).11 As concerning tumor histotype, AR has been reported to be expressed in about 71% of ductal, 96% of lobular, 81% of mucinous and 100% of tubular cancers.12 Apocrine carcinomas are typically characterized by AR expression without ER and PgR expression and most of them fitted into the luminal AR (LAR) molecular subtype of triple negative BC. However, a small subset of LAR cancers lacks an apocrine morphology.13
In BCs expressing ER and AR, an AR/ER crosstalk exists and AR competes with ER to block ER cascade.14 In this setting, several studies have described that, in PgR− BCs, AR could have a pro-tumorigenic role, promoting the transcription of ER-dependent genes.15 On the other hand, other papers suggested that, in early BC, AR expression represents a positive prognostic factor associated with a longer relapse free survival (RFS), favorable biologic features (lower tumor size, nuclear grade, proliferation index and ki67), longer overall survival (OS) whereas AR hyper-expression (AR/ER ratio ⩾2) appears to be detrimental in patients treated with endocrine therapies.12,16–18 The results regarding the expression of AR in BC subtypes are heterogeneous.19–21 A recent analysis of the PREPARE and TECHNO trials was published, trying to investigate the predictive and/or prognostic role of AR expression in high-risk BC patients.22 In particular, in the phase III PREPARE trial, patients with high-risk primary BC stratified for tumors ⩾3 cm or inflammatory BC were randomly assigned to receive concurrent preoperative epirubicin/paclitaxel every 3 weeks or dose-dense and dose-escalated sequential epirubicin followed by paclitaxel every 2 weeks. All patients received three cycles of cyclophosphamide, methotrexate, and fluorouracil chemotherapy after surgery.23 The primary objective of the trial was to compare preoperative dose-dense chemotherapy with conventionally scheduled preoperative chemotherapy in terms of pathologic complete response, disease-free survival (DFS) and OS. In the phase III TECHNO trial, patients with HER2-overexpressing BC (⩾2 cm or inflammatory) received four 3-week cycles of epirubicin and cyclophosphamide followed by four 3-week cycles of paclitaxel and trastuzumab before surgery.24 Trastuzumab was continued after surgery to complete 1 year of treatment. The primary endpoint was pathologic complete response. Overall, high AR mRNA levels were found to be associated with lower pathological complete response rates (odds ratio [OR] = 0.77, 95% confidence interval [CI] = 0.67–0.88, p = 0.0002) but also with better prognosis in terms of DFS (hazard ratio [HR] = 0.57, 95% CI = 0.39−0.85, p = 0.0054) and OS (HR = 0.43, 95% CI = 0.26−0.71, p = 0.0011).22 In the PREPARE trial, survival differences for patients with high and low AR1 mRNA levels could only be seen in the standard chemotherapy arm but not in the dose-dense treatment arm (OS: HR = 0.41, 95% CI = 0.22−0.74 versus HR = 1.05, 95% CI = 0.52−2.13; p = 0.0459).
To our knowledge, in the literature no data are reported regarding the role of AR expression in early stage ER+/PgR− BCs. The aim of this study was to evaluate the prognostic role of AR expression in this setting after a long follow-up (median 77 months).
Patients and methods
Study setting and design
We report the results of a monocentric retrospective analysis in which consecutive patients who underwent surgical intervention for ER+/PgR−/HER2− BC at Istituti Clinici Scientifici Maugeri (Pavia, Italy) between January 2005 and December 2014 were included. Patients with a triple negative BC, apocrine histotype and immunohistochemistry (IHC) for HER2 3+ or HER2 2+ and fluorescence in situ hybridization test positive for amplification were excluded. According to the literature, BC was considered ER+ if an ER expression higher than 10% was seen immunohistochemically.25 Data collection ended the 30th June 2019 for the analysis.
Information including BC history, previous and subsequent treatments in (neo)adjuvant setting, menopausal status, staging at diagnosis, type of surgical intervention, response to treatments, tumor biology including histotype, grade, ki67, vascular invasion and lymphoplasmacytic infiltrate, relapse sites, and date of last follow-up or death were collected for each patient. AR expression was evaluated by IHC in each tumor surgical specimen. The research was conducted according to the principles of the Declaration of Helsinki and was approved by the Local Institutional Ethical Committee (ICS Maugeri IRCCS Pavia Ethic Committee; approval number 2409). All patients involved in this study signed an authorization form for the use of their data.
Study objectives
The primary objective of the study was the evaluation of the relationship between AR expression in tumor specimen and the RFS.
Secondary objectives were: the evaluation of the relationship between AR expression in the tumor specimen and OS; the correlation between AR/ER ratio and RFS; the correlation between AR/ER ratio and OS; the correlation between AR expression and the tumor biology including histotype, grade, ki67, vascular invasion, and lymphoplasmacytic infiltrate; the correlation between AR expression and patient characteristics, in particular stage, age at diagnosis, and menopausal status.
Study assessment and outcomes
As a primary objective, RFS was defined as the time between the diagnosis of BC and the evidence of first disease relapse (all sites included). As a secondary objective, OS was defined as the time between the diagnosis of BC and the date of cancer-specific death or to the last date of follow-up, estimated using the Kaplan−Meier method. Finally, multivariate and univariate analyses were conducted in order to identify factors potentially linked with AR expression and disease outcomes.
Immunohistochemically determination of AR expression
The expression of the AR on BC tissues was evaluated using IHC analysis. Surgical specimens were fixed in formalin and included in paraffin. For each sample, 3 µm sections were obtained. The IHC analysis was performed using VENTANA BenchMark Ultra (Ventana Medical Systems, Tucson, AZ, USA) with ultraView Universal DAB kit (Ventana Medical System).
A primary monoclonal rabbit antibody directed versus AR (SP107 Cell Marque, Ventana Medical System) and pre-diluted by the supplier was used. After immunostaining, the slides were counterstained with Harris hematoxylin de-hydrated and mounted for microscopic reading. Nuclear staining was expressed as the percentage of stained cells (0−100%) over the total number of viable cells. Two expert pathologists in BC (LV and MA) assessed the expression of AR together, and disagreement was resolved through consensus.
Statistical analysis
Sample size was calculated according to the primary endpoint. It was estimated that 207 patients were necessary to demonstrate the presence of a statistically significant difference in 5-year relapse probability in patients with high AR expression (presumed to be 15%) than patients with low or absent AR expression (presumed to be 35%), with a statistical power (1 − beta) of 90% assuming a two-sided confidence level (1 − alpha) of 95%. The sample size was calculated using OpenEpi.26 Continuous variables’ distribution was expressed in terms of median and interquartile range (25th−75th percentiles) since most of the variables deviated significantly from the normal distribution, and the categorical variables’ distribution was described by absolute and relative frequency (%). AR and ER distributions were discretized into two levels using Receiver Operating Characteristic (ROC) method and their median value. The presence of a statistically significant difference in terms of variable distribution by outcomes’ values was assessed by the non-parametric Wilcoxon rank sum test (for numeric explanatory variables) or by Fisher’s exact tests for categorical explanatory variables. The quantile regression with stepwise selection was applied to evaluate the impact of variables on non-Gaussian numeric dependent variables. The log-rank test was applied to compare survival profiles between AR groups; survival curves were defined by the Kaplan−Meier method. Cox regression was applied to estimate the time-dependent risk of the outcomes of interest. The statistical significance threshold was set to p < 0.05 for all analyses. All statistical procedures were performed by the R statistical software tool (https://www.r-project.org).
Results
We included 237 women with early stage ER+/PgR−/HER2− BC; 29 patients were excluded from the analysis because they were lost at follow-up (12/29) or AR expression evaluation could not be performed (17/29) because cancer tissue was no longer available. For these reasons, a total of 208 patients were analyzed; their baseline characteristics are reported in Table 1. AR expression was present in 184 patients (88.5%); among them, 5% had an AR expression ⩽5% whereas 29% had ⩾90% (Figure 1). The median AR expression value was 80%; about 90% of patients had an AR/ER ratio <2.
Table 1.
Patients baseline characteristics.
| Variable | Whole sample |
|---|---|
| Median age (range) | 65 (33−88) |
| Menopausal status | |
| Pre-menopausal | 23 (11%) |
| Post-menopausal | 185 (89%) |
| Stage | |
| I | 102 (49%) |
| II | 73 (35%) |
| III | 33 (16%) |
| ER | |
| ⩽10% | 16 (8%) |
| 10−60% | 23 (11%) |
| ⩾60% | 169 (81%) |
| AR | |
| 0 | 24 (12%) |
| ⩽5% | 11 (5%) |
| 5−90% | 112 (54%) |
| ⩾90% | 61 (29%) |
| Histotype | |
| NOS/Ductal | 131 (63%) |
| Lobular | 67 (32%) |
| Other | 10 (5%) |
| Grade | |
| G1 | 18 (9%) |
| G2 | 130 (62%) |
| G3 | 60 (29%) |
| Ki67 | |
| <20% | 148 (71%) |
| ⩾20% | 60 (29%) |
| Vascular invasion | |
| Absent | 83 (40%) |
| Mild | 68 (33%) |
| Diffuse | 13 (6%) |
| Not available | 44 (21%) |
| Lymphoplasmacytic infiltrate | |
| Low | 149 (72%) |
| Moderate | 13 (6%) |
| Not available | 46 (22%) |
| Type of surgical intervention | |
| Quadranctectomy + SLNB | 86 (41%) |
| Quadrantectomy + ALND | 58 (28%) |
| Mastectomy + SLNB | 15 (7%) |
| Mastectomy + ALND | 49 (24%) |
| Radiotherapy | |
| Yes | 141 (68%) |
| No | 67 (32%) |
| Neoadjuvant therapy | |
| No | 171 (82%) |
| Yes | 37 (18%) |
| Anthracycline | 15 (41%) |
| Taxane | 1 (3%) |
| Anthracycline + taxane | 9 (24%) |
| Endocrine treatment | 11 (30%) |
| Other (CMF) | 1 (2%) |
| Adjuvant chemotherapy | |
| No | 142 (68%) |
| Yes | 66 (32%) |
| Anthracycline | 24 (36%) |
| Taxane | 10 (15%) |
| Anthracycline + taxane | 23 (35%) |
| Other | 9 (14%) |
| Adjuvant endocrine therapy | |
| No | 20 (10%) |
| Yes | 188 (90%) |
| Tamoxifen | 19 (10%) |
| Aromatase inhibitors | 150 (80%) |
| Tamoxifen + LHRH analogue | 11 (6%) |
| Sequential | 8 (4%) |
ALND, axillary lymph node dissection; AR, androgen receptor; CMF, cyclophosphamide-methotrexate-fluorouracil; ER, estrogen receptor; LHRH, luteinizing hormone-releasing hormone; NOS, not otherwise specified; SLNB, sentinel lymph node biopsy; variable, analyzed variable; whole sample, variables’ distribution in the whole sample: median (25th−75th percentiles) or absolute and relative frequency (%).
Figure 1.
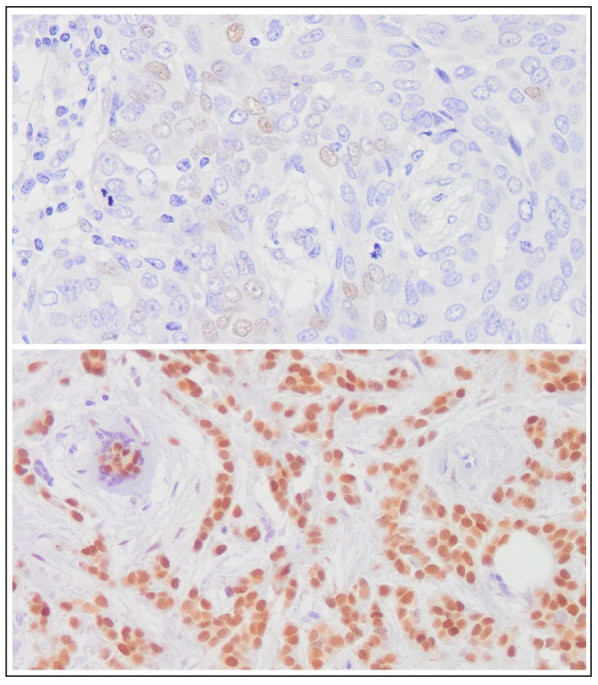
Representative histologic images showing immunohistochemical expression of androgen receptor in breast cancer tissue (upper image: androgen receptor expression ⩽5%; lower image: androgen receptor expression ⩾90%) (original magnification, ×100).
At a median follow-up of 77 months (range 18−168 months), 75 patients (36%) had a disease relapse. Among them, 32 patients (43%) had a visceral recurrence, 20 (27%) a bone progression, 18 patients (23%) a loco-regional relapse, and 5 patients (6%) a mediastinal lymph node progression.
We first tested for the presence of statistically significant differences in terms of AR and ER values distribution in relapsed and non-relapsed patients by the Wilcoxon rank sum test and found that the median AR expression was significantly higher in patients who did not relapse compared with those who had relapsed (median AR values = 80% versus 70%, respectively, p = 0.011) with an impact on RFS (HR = 0.99, p = 0.025) (Table 2). Similarly, ER expression was significantly higher in patients who did not relapse compared with the rest of the cohort (median ER values = 80% versus 70%, respectively, p = 0.038) but this data had no statistically significant impact on the probability of RFS (HR = 0.99, 95% CI = 0.99 − 1, p = 0.068).
Table 2.
Univariate analysis on relapse probability.
| Variable | Value | Relapse |
Relapse free survival |
|||
|---|---|---|---|---|---|---|
| No | Yes | p | HR (95% CI) | p | ||
| AR (%) | 80 (60−90) | 70 (12.5−80) | 0.011 | 0.99 (0.99−1) | 0.025 | |
| <80% | 52 (53.06%) | 46 (46.94%) | 0.002 | Baseline | ||
| ⩾80% | 81 (73.64%) | 29 (26.36%) | 0.53 (0.34−0.85) | 0.008 | ||
| ER (%) | 80 (70−80) | 70 (60−80) | 0.038 | 0.99 (0.98−1) | 0.068 | |
| <80% | 54 (40.6%) | 41 (54.67%) | 0.060 | Baseline | ||
| ⩾80% | 79 (59.4%) | 34 (45.33%) | 0.69 (0.44−1.09) | 0.116 | ||
| AR/ER ratio | 1 (0.8−1.12) | 0.89 (0.29−1.12) | 0.281 | 1.06 (0.88−1.27) | 0.536 | |
| <2 | 125 (64.77%) | 68 (35.23%) | 0.410 | Baseline | ||
| ⩾2 | 8 (53.33%) | 7 (47.67%) | 1.47 (0.67−3.20) | 0.334 | ||
| Grade | G1 − G2 | 97 (65.54%) | 51 (34.46%) | 0.524 | Baseline | |
| G3 | 36 (60%) | 24 (40%) | 1.24 (0.76−2.01) | 0.394 | ||
| Stage | I | 76 (81.72%) | 17 (18.28%) | 1 | Baseline | |
| yI | 6 (85.71%) | 1 (14.29%) | 0.89 (0.12−6.77) | 0.912 | ||
| II | 32 (55.17%) | 26 (44.83%) | 1 | Baseline | ||
| yII | 8 (53.33%) | 7 (46.67%) | 0.79 (0.34−1.83) | 0.581 | ||
| III | 10 (50%) | 10 (50%) | 0.022 | Baseline | ||
| yIII | 1 (7.69%) | 12 (92.31%) | 1.46 (0.6−3.52) | 0.405 | ||
| Vascular invasion | Absent | 64 (77.11%) | 19 (22.89%) | 0.061 | Baseline | |
| Mild | 42 (61.76%) | 26 (38.24%) | 1.80 (1−3.26) | 0.051 | ||
| Diffuse | 7 (53.85%) | 6 (46.15%) | 2.23 (0.89−5.6) | 0.086 | ||
| Histotype | NOS/ductal | 87 (66.41%) | 44 (33.59%) | 0.485 | Baseline | |
| Lobular | 39 (58.21%) | 28 (41.79%) | 1.13 (0.7−1.81) | 0.620 | ||
| Other | 7 (70.00%) | 3 (30.00%) | 0.89 (0.28−2.88) | 0.850 | ||
| ki67 | <20% | 103 (69.59%) | 45 (30.41%) | 0.011 | Baseline | |
| ⩾20% | 30 (50%) | 30 (50%) | 2.3 (1.44−3.66) | <0.001 | ||
AR, androgen receptor; baseline, intercept of the regression; CI, 95% confidence interval; ER, estrogen receptor; HR, hazard ratio; NOS, not otherwise specified; p = unadjusted p-value from Wilcoxon rank sum test, Chi-square test, or Fisher’s exact test as appropriate; value, value that the variable assumes; variable, analyzed variable; yI – yII – yIII, patients who performed neoadjuvant treatment.
After, it was tested whether an AR and ER value threshold could be informative in terms of RFS and OS. Both AR and ER were discretized according to their median value (AR = 80%, ER = 80%) and the prognostic role of these cut-off values had been assessed by univariate analysis. Results showed that patients with AR expression ⩾80% had a lower risk of relapse compared with those with an AR <80% (HR = 0.53, 95% CI = 0.34 – 0.85, p = 0.008), while women with an ER expression ⩾80% had no different risk of relapse compared with the rest of the cohort (HR = 0.694, 95% CI = 0.440 – 1.094, p = 0.116). Extremely similar thresholds to discriminate patients who relapsed from those who did not relapse had been obtained by applying the ROC method (ROC method: AR = 75% and ER = 75%). Of note, the two thresholds derived from the median method and the ROC method were able to distinguish the same patients: individuals with AR values ⩾75% (n = 110/208) also had AR values ⩾80% (n = 110/208) while patients with ER values ⩾75% (n = 113/208) also had ER values ⩾80% (n = 113/208).
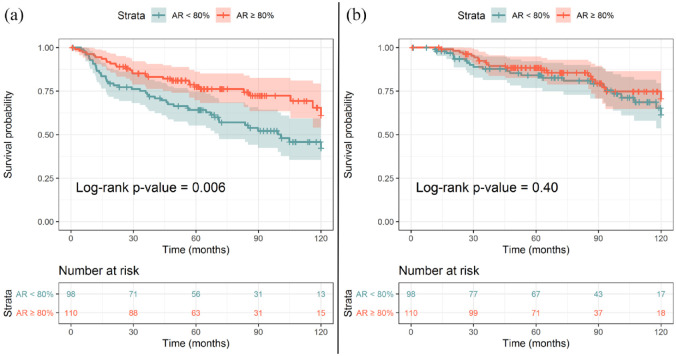
Patients with AR expression <80% had a median RFS of 8.3 months whereas the median RFS value was not reached in patients with AR ⩾80%. The Kaplan–Meier curve comparing the probability of RFS in the two cohorts (AR ⩾80% and AR <80%) is shown in Figure 2A (p = 0.007). Instead, no association between AR expression and OS was observed, as shown in Figure 2B (p = 0.40).
Figure 2.
Kaplan−Meier curve showing the relationship between androgen receptor expression and probability of relapse (A) and between androgen receptor expression and probability of survival (B). The numbers in each table describe the number of patients at risk by stratum at different time points. The shaded areas represent the 95% confidence interval of each curve. p-values were generated by the Log-rank test.
AR, androgen receptor.
We also evaluated if the AR/ER ratio could have a prognostic role; this variable was not informative for RFS (p > 0.05) but had a statistically significant association with OS (HR = 1.21, 95% CI = 1.01 – 1.45, p = 0.035). If discretized according to a threshold corresponding to a ratio of 2, no statistically significant difference in terms of AR/ER was observed neither with RFS nor with OS (p > 0.05).27
We then investigated if the presence of AR (defined as an AR expression ⩾5%) was associated with particular tumor biological characteristics compared with AR negative BCs and we found that patients with an AR expression ⩾5% had more frequently low lymphoplasmacytic infiltrate (p = 0.021), ki67 <20% (p = 0.016), and lobular histotype (p = 0.010) (Table 3). When considering an AR expression ⩾80%, tumors more frequently had a low ki67 (<20%, p = 0.001), a low tumor grade (p = 0.003), and they were more commonly related to lobular histotype (p = 0.003) (Table 3).
Table 3.
Biological and clinical characteristics according to AR expression.
| Variable | Value | AR |
Median AR |
||||
|---|---|---|---|---|---|---|---|
| ⩽5% | >5% | p | <80% | ⩾80% | p | ||
| Grade | G1-G2 | 21 (60%) | 127 (73.41%) | 0.110 | 60 (61.22%) | 88 (80%) | 0.003 |
| G3 | 14 (40%) | 46 (26.59%) | 38 (38.78%) | 22 (20%) | |||
| Stage | I | 13 (92.86%) | 80 (93.02%) | 1 | 37 (92.5%) | 56 (93.33%) | 1 |
| yI | 1 (7.14%) | 6 (6.98%) | 3 (7.5%) | 4 (6.67%) | |||
| II | 12 (92.31%) | 46 (76.67%) | 0.279 | 32 (80%) | 26 (78.79%) | 0.898 | |
| yII | 1 (7.69%) | 14 (23.33%) | 8 (20%) | 7 (21.21%) | |||
| III | 4 (50%) | 16 (64%) | 0.681 | 9 (52.94%) | 11 (68.75%) | 0.481 | |
| yIII | 4 (50%) | 9 (36%) | 8 (47.06%) | 5 (31.25%) | |||
| LPI infiltrate | low | 29 (82.86%) | 163 (94.22%) | 0.021 | 91 (92.86%) | 101 (91.82%) | 0.779 |
| high | 6 (17.14%) | 10 (5.78%) | 7 (7.14%) | 9 (8.18%) | |||
| Vascular invasion | absent | 12 (34.29%) | 71 (41.04%) | 0.770 | 41 (41.84%) | 42 (38.18%) | 0.611 |
| mild | 13 (37.14%) | 55 (31.79%) | 29 (29.59%) | 39 (35.45%) | |||
| diffuse | 2 (5.71%) | 11 (6.36%) | 5 (5.1%) | 8 (7.27%) | |||
| Histotype | NOS/ductal | 26 (74.29%) | 105 (60.69%) | 0.010 | 65 (66.33%) | 66 (60%) | 0.003 |
| lobular | 5 (14.29%) | 62 (35.84%) | 24 (24.49%) | 43 (39.09%) | |||
| other | 4 (11.43%) | 6 (3.47%) | 9 (9.18%) | 1 (0.91%) | |||
| ki67 | <20% | 19 (54.29%) | 129 (74.57%) | 0.016 | 59 (60.2%) | 89 (80.91%) | 0.001 |
| ⩾20% | 16 (45.71%) | 44 (25.43%) | 39 (39.8%) | 21 (19.09%) | |||
AR, androgen receptor; LPI, lymphoplasmacytic infiltrate; NOS, not otherwise specified; value, value that the variable assumes; variable, analyzed variable; yI – yII – yIII, patients who performed neoadjuvant treatment.
Variables’ distribution within AR category is described by absolute and relative frequency (%).
When performing multivariate analyses for RFS including all potential confounders (demographic and biological characteristics and type of treatments), neither AR nor ER expression reached statistical significance.
Moreover, in the whole cohort, the only other characteristics associated with a higher probability of relapse were ki67 ⩾20% (HR = 2.3, p < 0.001, 95% CI = 1.44–3.66) and presence of low or diffuse vascular invasion (HR = 1.80, 95% CI = 1.0–3.26, p = 0.051 and HR = 2.23, 95% CI = 0.89–5.60, p = 0.086, respectively) (Table 2). Stepwise quantile regression showed that ER expression correlated significantly with AR distribution (quantile regression coefficient = +0.80, SE = 0.26, p = 0.002) independently from the chemotherapy or endocrine treatment performed. The scatterplot in Figure 3 describes the correlation between ER and AR expression (Spearman coefficient = +35).
Figure 3.
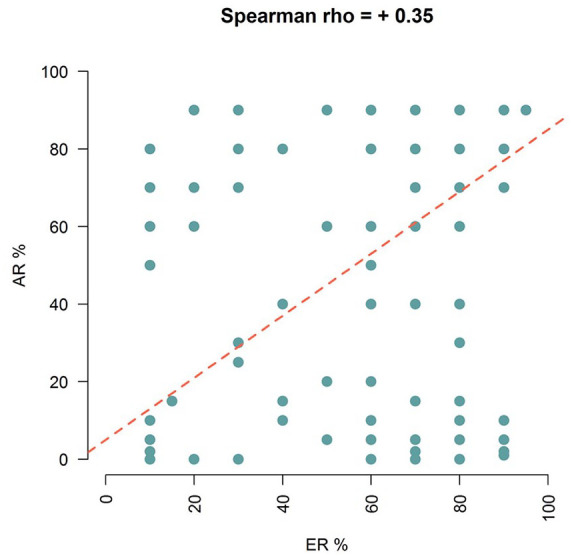
Scatterplot describing the correlation between estrogen receptor (ER) and androgen receptor (AR) expression. The quantile regression line is represented in red.
Multivariate Cox regression models including AR and ER expression as well as demographic factors, tumor characteristics, and type of therapies performed suggested that neither AR nor ER expression were informative with respect to the probability of survival (p > 0.05). The only variables associated with OS were disease relapse (HR = 4.85, 95% CI = 2.39–9.84, p < 0.001) and having performed adjuvant chemotherapy (HR = 0.38, 95% CI = 0.15–0.99, p = 0.048).
Discussion
To the best of our knowledge, this is the first study evaluating the potential prognostic role of AR in ER+/PgR–/HER2– BC. In the literature, this BC subtype has a worse survival compared with tumors with the same histotype and PgR+.28–31 PgR expression is linked to ER expression since PgR is an estrogen-regulated gene. For this reason, PgR absence may be due, at least in part, to the presence of a non-functional ER.7,32 This fact could explain why the subgroup of PgR– BC has a lower response to endocrine therapies.31 A recent study examined the clinical and biological features of BC women stratified according to ER and PgR tumor expression as double positive (ER+, PgR+), single positive (ER+, PgR–) and double negative (ER–, PgR–).33 The results confirm the poor prognosis of ER+/PgR–/HER2– BC patients that resulted in being similar to the triple negative subtype.
The increasing interest for the potential predictive and/or prognostic role of AR is well portrayed in the literature. Several retrospective studies described an association between high AR expression and older age at diagnosis, lower tumor grade, lower ki67, and smaller tumor size. In addition, AR seems to be involved in the development of drug resistance in luminal BC treated with aromatase inhibitors (AIs) or tamoxifen.34 AR and ER can compete for the binding to estrogen response elements (EREs) on specific genes. Therefore, the binding of AR to EREs reduces the estrogen proliferative action, thus inducing anti-proliferative effects.35 Conversely, ER can bind to androgen response elements, obtaining the opposite effect. This mechanism could explain the role of AR in the resistance to standard endocrine treatments. Moreover, a high AR expression can activate the EGFR, promoting an agonist effect of tamoxifen on the ER pathway. Recent meta-analysis demonstrated that AR expression is a positive prognostic factor independent from ER, being associated with a lower risk of disease recurrence in all BC subtypes and with a better OS in ER+ breast tumors.36–38 In our study, AR resulted in being expressed in about 90% of patients but with variable values. The majority of patients had an AR expression between 5% and 90% (54%), 29% an AR ⩾90%; 17% had an AR expression ⩽5%. At a median follow-up of 77 months, 75 patients (36%) experienced a disease relapse. Among them, 42% had a visceral recurrence suggesting a higher aggressiveness of this subgroup.33 This study also confirmed that in the ER+/PgR– BC subtype an AR expression >80% is a prognostic factor for RFS. In fact, in patients with AR ⩾80% BCs, risk recurrence is reduced by 47% (HR = 0.53, 95% CI = 0.34 – 0.85, p = 0.008). Post hoc statistical power calculations showed that the statistical power to detect the observed differences in terms of probability of relapse in patients with AR <80% (n = 46/98, 46.94%) and those with AR ⩾80% (n = 29/110, 26.36%) was 84% assuming a bilateral confidence level of 95%. The analysis of independent cohorts of patients will allow validation of these findings. Of note, extremely similar thresholds to discriminate patients who had relapsed from those who did not relapse can be obtained by applying the supervised ROC method (AR = 75%). The median value and the ROC method provided thresholds able to distinguish the same patients: the same individuals with AR values ⩾75% calculated with the ROC method (n = 110/208) also had AR values ⩾80% calculated as median value (n = 110/208).
Regarding the relationship between AR expression, type of endocrine treatment performed, and RFS, data in the literature are scarce with discordant results. In particular, a recent study described that patients with a high AR/ERα ratio (⩾2.0) or prostate-derived Ets factor (PDEF)/ERα ratio (⩾2.0) receiving adjuvant tamoxifen treatment had a two-fold increased risk of failure and that both the AR/ERα ratio and PDEF/ERα ratio were independently associated with the risk of tamoxifen treatment failure.27 On the other hand, the results of another trial evidenced that AR expression is not an informative biomarker for the selection of adjuvant endocrine therapy in postmenopausal women with ER+ BCs.39 In this study, no associations between the type of endocrine adjuvant treatment, AR expression, and RFS were evident.
The link between AR expression and RFS may vary according to ER expression.39 In the work by Castellano and colleagues, AR-ER co-expression was reported to be associated with a better outcome in terms of time to relapse and disease-specific survival.40 Two subsequent studies described that a high AR/ER ratio is detrimental although the AR/ER co-expression is related to a better prognosis.16,17 In the first work, Authors established that an AR/ER ratio ⩾2 identified a subgroup of patients with a four times higher probability of disease recurrence, in particular to lymph nodes, and a shorter DFS.16 In the other work, a high AR/ER ratio was associated with poor clinic-biological characteristics; in particular, higher tumor size at diagnosis, lymph nodes involvement, and higher tumor grade. In addition, an AR/ER ratio ⩾2 was related to a worse survival with shorter DFS.17 In our study, an AR/ER ratio ⩾2 was not associated with a higher risk of recurrence since statistical analysis did not identify an AR/ER ratio that could split the population in two different groups of patients with different prognosis. Moreover, no correlations between AR expression and OS were identified. In addition, from univariate analysis, patients with a ki67 ⩾20% had a higher probability to develop disease recurrence than patients with a ki67 <20% (HR = 2.3, 95% CI = 1.44 – 3.66, p < 0.001) as well as patients with tumor vascular invasion compared with patient with absent tumor vascular invasion (HR = 1.80, 95% CI = 1.0 – 3.26, p = 0.051 and HR = 2.23, 95% CI = 0.89 – 5.60, p = 0.086 for low and diffuse vascular invasion, respectively). We also found that, compared with AR <80%, AR+ (⩾80%) BCs had a higher probability to have lobular histotype while having a lower ki67 and tumor grade. On the other hand, 65% of the tumors with ki67 ⩾20% (luminal B like) and 63% poorly differentiated (G3) tumors had an AR expression <80%.
The retrospective design of the study limits its significance, but a long follow-up period was considered to strengthen our hypothesis. Nevertheless, these data might help in developing further prospective and mechanistic studies and add more information to the identification of subgroups of high-risk patients for daily decision making in clinical practice.
Conclusion
The results of this retrospective observational trial on early ER+/PgR– BC patients support the potential prognostic role of AR in this peculiar population. Breast tumors with high AR expression usually have good biological features (low ki67 and good nuclear differentiation) whereas BCs with lower AR expression often show adverse biological features, at least partly explaining the better outcome of patients with ER+/PgR– coupled with high AR expression BCs.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Barbara Tagliaferri, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Erica Quaquarini, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, via Maugeri 10, Pavia, 27100, Italy; Experimental Medicine School, University of Pavia, Pavia, 27100, Italy.
Raffaella Palumbo, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Emanuela Balletti, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Daniele Presti, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Alberto Malovini, Laboratory of Informatics and System Engineering for Clinical Research, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Manuela Agozzino, Operative Unit of Anatomic Pathology, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Cristina Maria Teragni, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Andrea Terzoni, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Antonio Bernardo, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Laura Villani, Operative Unit of Anatomic Pathology, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
Federico Sottotetti, Medical Oncology Unit, ICS Maugeri-IRCCS SpA SB, Pavia, Italy.
References
- 1. Obr AE, Edwards DP. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol 2012; 357: 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Axlund SD, Sartorius CA. Progesterone regulation of stem and progenitor cells in normal and malignant breast. Mol Cell Endocrinol 2012; 357: 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ades F, Zardavas D, Bozovic-Spasojevic I, et al. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. Clin Oncol 2011; 32: 2794–2803. [DOI] [PubMed] [Google Scholar]
- 4. Bardou VJ, Arpino G, Elledge RM, et al. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 2003; 21: 1973–1979. [DOI] [PubMed] [Google Scholar]
- 5. Rakha EA, El-Sayed ME, Green AR, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol 2007; 25: 4772–4778. [DOI] [PubMed] [Google Scholar]
- 6. Prat A, Cheang MCU, Martín M, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 2013; 31: 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hilton HN, Clarke CL, Graham JD. Estrogen and progesterone signalling in the normal breast and its implications for cancer development. Mol Cell Endocrinol 2018; 466: 2–14. [DOI] [PubMed] [Google Scholar]
- 8. Presti D, Quaquarini E. The PI3K/AKT/mTOR and CDK4/6 pathways in endocrine resistant HR+/HER2-metastatic breast cancer: biological mechanisms and new treatments. Cancers 2019; 11: 1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Honma N, Horii R, Iwase T, et al. Clinical importance of androgen receptor in breast cancer patients treated with adjuvant tamoxifen monotherapy. Breast Cancer 2013; 20: 323–330. [DOI] [PubMed] [Google Scholar]
- 10. Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? Clin Oncol 2005; 23: 7350–7360. [DOI] [PubMed] [Google Scholar]
- 11. Collins LC, Cole KS, Marotti JD, et al. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the nurses’ health study. Mod Pathol 2011; 24: 924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kraby MR, Valla M, Opdahl S, et al. The prognostic value of androgen receptors in breast cancer subtypes. Breast Cancer Res Treat 2018; 172: 283–296. [DOI] [PubMed] [Google Scholar]
- 13. Giovannelli P, Di Donato M, Galasso G, et al. The androgen receptor in breast cancer. Front Endocrinol 2018; 9: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basile D, Cinausero M, Iacono D, et al. Androgen receptor in estrogen receptor positive breast cancer: Beyond expression. Cancer Treat Rev 2017; 61: 15–22. [DOI] [PubMed] [Google Scholar]
- 15. Karamouzis MV, Papavassiliou KA, Adamopoulos C, et al. Targeting androgen/estrogen receptors crosstalk in cancer. Trends Cancer 2016; 2: 35–48. [DOI] [PubMed] [Google Scholar]
- 16. Peters KM, Edwards SL, Nair SS, et al. Androgen receptor expression predicts breast cancer survival: the role of genetic and epigenetic events. BMC Cancer 2002; 12: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cochrane DR, Bernales S, Jacobsen BM, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 2014; 16: R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rangel N, Rondon-Lagos M, Annaratone L, et al. The role of the AR/ER ratio in ER-positive breast cancer patients. Endocr Related Cancer 2018; 25: 163–172. [DOI] [PubMed] [Google Scholar]
- 19. Gerratana L, Basile D, Buono G, et al. Androgen receptor in triple negative breast cancer: a potential target for the targetless subtype. Cancer Treat Rev 2018; 68:102–110. [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Bi X, Huang Z, et al. The prognostic value of AR in HER2-enriched metastatic breast cancer. Endocr Relat Cancer 2020; 27: 199–208. [DOI] [PubMed] [Google Scholar]
- 21. Lamb CA, Vanzulli SI, Lanari C. Hormone receptors in breast cancer: more than estrogen receptors. Medicina 2019; 79: 540–545. [PubMed] [Google Scholar]
- 22. Untch M, Mobus V, Kuhn W, et al. Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol 2009; 27: 2938–2945. [DOI] [PubMed] [Google Scholar]
- 23. Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 2011; 29: 3351–3357. [DOI] [PubMed] [Google Scholar]
- 24. Witzel I, Loibl S, Wirtz R, et al. Androgen receptor expression and response to chemotherapy in breast cancer patients treated in the neoadjuvant TECHNO and PREPARE trial. Br J Cancer 2019; 121: 1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fujii T, Kogawa T, Dong W, et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer breast cancer. Ann Oncol 2017; 28: 2420–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. OpenEpi: sample size for X-sectional, cohort, and clinical trials, https://www.openepi.com/SampleSize/SSCohort.htm (accessed 12 March 2019).
- 27. Cao L, Xiang G, Liu F, et al. A high AR:ERα or PDEF:ERα ratio predicts a sub-optimal response to tamoxifen therapy in ERα-positive breast cancer. Cancer Chemother Pharmacol 2019; 84: 609–620. [DOI] [PubMed] [Google Scholar]
- 28. Purdie CA, Quinlan P, Jordan LB, et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: a population-based study. Br J Cancer 2013; 110: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caldarella A, Barchielli A. Prognostic role of progesterone receptor expression in a population-based analysis. J Cancer Res Clin Oncol 2017; 143: 2505–2509. [DOI] [PubMed] [Google Scholar]
- 30. Sun JY, Wu SG, Li FY, et al. Progesterone receptor loss identifies hormone receptor-positive and HER2-negative breast cancer subgroups at higher risk of relapse: a retrospective cohort study. Onco Targets Ther 2016; 9: 1707–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dunnwald L, Rossing M, Li C. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 2007; 9: R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Snell CE, Gough M, Liu C, et al. Improved relapse-free survival on aromatase inhibitors in breast cancer is associated with interaction between oestrogen receptor-α and progesterone receptor-b. Br J Cancer 2018; 119: 1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bae SY, Kim S, Lee JH, et al. Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer 2015; 15: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McNamara KM, Moore NL, Hickey TE, et al. Complexities of androgen receptor signalling in breast cancer. Endocr Relat Cancer 2014; 21: T161–181. [DOI] [PubMed] [Google Scholar]
- 35. Bronte G, Rocca A, Ravaioli S, et al. Androgen receptor in advanced breast cancer: is it useful to predict the efficacy of anti-estrogen therapy? BMC Cancer 2018; 18: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vera-Badillo FE, Templeton AJ, De Gouveia P, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2014, 106. [DOI] [PubMed] [Google Scholar]
- 37. Qu Q, Mao Y, Fei XC, et al. The impact of androgen receptor expression on breast cancer survival: a retrospective study and meta-analysis. PLoS One 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bozovic-Spasojevic I, Zardavas D, Brohee S, et al. The prognostic role of androgen receptor in patients with early-stage breast cancer: a metaanalysis of clinical and gene expression data. Clin Cancer Res 2017; 23: 2702–2712. [DOI] [PubMed] [Google Scholar]
- 39. Kensler KH, Regan MM, Heng YJ, et al. Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor-positive breast cancer: results from the Breast International Group Trial 1-98. Breast Cancer Res 2019; 21: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castellano I, Allia E, Accortanzo V, et al. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat 2010; 124: 607–617. [DOI] [PubMed] [Google Scholar]