Abstract
Background:
Frailty is a clinical phenotype of decreased physiologic reserve that is associated with increased morbidity and mortality. The most meaningful way to assess frailty in patients with end-stage kidney disease (ESKD) is unknown.
Objective:
To assess the prevalence of frailty in ESKD patients using the easy-to-administer FRAIL scale and, to determine its association with mortality, transplantation, and hospitalization.
Design:
A cohort study was used.
Setting:
The Ottawa Hospital, Ottawa, Ontario, Canada, was the setting of this study.
Patients:
All eligible adult ESKD patients treated with dialysis from August to November 2017 at The Ottawa Hospital were invited to participate.
Measurements:
The FRAIL scale.
Methods:
Eligible patients completed an exercise survey with FRAIL questions embedded within the instrument. Number of comorbid illnesses was determined from the electronic medical record and weight loss was calculated from target weight in the patients’ dialysis prescription. Mortality, transplant status, and hospitalizations were ascertained from the electronic medical record 18 months later; differences by frailty status were evaluated using descriptive statistics. Kaplan-Meier and Cox regression models were used to examine the association between frailty and transplant.
Results:
Of 476 ESKD patients screened, 261 participated; 101 receiving peritoneal dialysis, 135 intermittent hemodialysis, and 25 home hemodialysis. Thirty-nine, 145, and 77 were frail, pre-frail, and not frail, respectively. Employment status, ethnicity, and comorbid illnesses differed significantly by frailty status, but mortality did not. In univariate analysis, frail patients were less likely to be listed for (P = .05) and to receive a kidney transplant (P = .02). However, after adjusting for age and modality, frailty was not statistically associated with a decreased likelihood of transplant (Hazard Ratio: 0.15; confidence interval [CI], 0.02-1.15; P = .068). The results were similar when accounting for the competing risk of death (P = .060). Frail patients were more likely to be hospitalized (P = .01) and spend more time in the hospital (P = .04).
Limitations:
Single-center design with a relatively short follow-up and small sample size limiting the number of variables that could be assessed in analysis. We also excluded patients who were unable to communicate in English or French and those patients with physical limitations such as amputations, potentially affecting generalizability.
Conclusions:
Frail ESKD patients as identified by the FRAIL scale are less likely to receive a renal transplant; this association diminished statistically after adjusting for age and modality and when accounting for the competing risk of death. Frail patients were at increased risk of hospitalization. Further study with larger patient numbers and longer follow-up is needed to determine the usefulness of the FRAIL scale in predicting adverse outcomes.
Trial registration:
Not required as this was an observational study.
Keywords: frailty, dialysis, cohort, transplantation
Abrégé
Contexte:
La fragilité est un phénotype clinique d’une réduction de la réserve physiologique et est associée à une augmentation de la morbidité et de la mortalité. La meilleure façon d’évaluer la fragilité des patients atteints d’insuffisance rénale terminale (IRT) demeure toutefois inconnue.
Objectifs:
Mesurer la prévalence de la fragilité chez les patients atteints d’IRT à l’aide de l’échelle FRAIL et examiner les liens entre la fragilité et la mortalité, la transplantation et le nombre d’hospitalisations.
Type d’étude:
Étude de cohorte
Cadre:
L’Hôpital d’Ottawa (Ontario) au Canada
Sujets:
Tous les adultes admissibles atteints d’IRT et traités par dialyze entre août et novembre 2017 à l’Hôpital d’Ottawa ont été invités à participer à l’étude.
Mesures:
L’échelle FRAIL mesurant la fragilité.
Méthodologie:
Les patients admissibles ont répondu à un sondage sur l’activité physique où des questions issues de l’échelle FRAIL avaient été intégrées. Le nombre de maladies concomitantes a été obtenu à partir du dossier médical électronique et la perte de poids a été calculée à partir du poids cible figurant dans la prescription de dialyze du patient. La mortalité, le statut de la transplantation et le nombre d’hospitalisations ont été déterminés à partir du dossier médical électronique 18 mois plus tard. La statistique descriptive a servi à évaluer les différences selon l’état de fragilité. Des modèles de régression de Kaplan Meier et de Cox ont été utilisés pour examiner l’association entre la fragilité et la transplantation.
Résultats:
Des 476 patients atteints d’IRT dépistés, 261 ont participé à l’étude (101 traités par dialyze péritonéale, 135 par hémodialyse intermittente et 25 en hémodialyse à domicile). Ces patients ont été jugés fragiles (n=39), préfragiles (n=145) ou non fragiles (n=77). La situation d’emploi, l’ethnicité et les maladies concomitantes différaient considérablement en fonction de l’état de fragilité, mais la mortalité s’est avérée similaire. L’analyze univariée a révélé que les patients jugés fragiles étaient moins susceptibles d’être inscrits sur la liste d’attente (p=0,05) et de recevoir une greffe rénale (p=0,02). Cependant, après correction selon l’âge et la modalité de dialyze, la fragilité n’a montré aucune corrélation statistiquement significative avec une diminution de la probabilité de subir une transplantation (RR : 0,15; IC à 95 %: 0,02-1,15; p=0,068). Les résultats étaient similaires en tenant compte du risque concurrent de décès (p=0,060). Enfin, les patients jugés fragiles étaient plus susceptibles d’être hospitalisés (p=0,01), et ce, pour de plus longs séjours (p=0,04).
Limites:
Le nombre de variables pouvant être évaluées dans l’analyze est limité par le fait qu’il s’agit d’une étude monocentrique avec un suivi relativement court et portant sur un faible échantillon de patients. L’exclusion des patients incapables de communiquer en anglais ou en français et des patients présentant des limitations physiques, notamment des amputations, pourrait affecter la généralisabilité des résultats.
Conclusion:
Les patients atteints d’IRT jugés fragiles par l’échelle FRAIL sont moins susceptibles de recevoir une greffe rénale. Une réduction statistiquement significative de cette association a été observée après une correction selon l’âge et la modalité de dialyze, et en tenant compte du risque concurrent de décès. Les patients fragiles présentent également un risque accru d’être hospitalisés. Une étude plus approfondie sur une plus grande cohorte de patients et avec un suivi à plus long terme est nécessaire pour déterminer l’utilité de l’échelle FRAIL pour prédire les issues défavorables.
Enregistrement de l’essai:
N’est pas requis, étude observationnelle.
What was known before
Frailty is common in patients with end-stage kidney disease (ESKD) treated with dialysis and has been associated with increased morbidity and mortality. However, the most clinically useful way to assess frailty such that targeted interventions can be applied to the population is unknown.
What this adds
The FRAIL survey is simple to use in the ESKD population and is associated with a greater risk of hospitalization.
Introduction
Frailty is a clinical phenotype of decreased cognitive, functional, and health reserve that is associated with an increased vulnerability to stressors.1,2 Depending on the instrument used, 41% to 67% of end-stage kidney disease (ESKD) patients treated with hemodialysis are classified as frail; a prevalence that is 5-fold to 7-fold higher than in the general population.1,3,4 Although frailty has commonly been referred to as a geriatric syndrome, 35.4% of ESKD patients less than 65 years old were classified as frail using Fried criteria.3 Frailty in ESKD patients is more common in those individuals with more comorbid illnesses, female sex, increasing age, low serum albumin, higher body mass index, unemployment, lower levels of education, inpatient dialysis initiation and treatment with hemodialysis compared with peritoneal dialysis.1,4,5 Independent of age, sex, number of comorbid illnesses, and disability, frail ESKD patients have increased morbidity and mortality.1,3 For ESKD patients with moderate to severe frailty, there is also a lower likelihood of renal transplantation.1 In spite of the importance of frailty in ESKD patients, there is no consensus about the most clinically meaningful way to assess frailty in dialysis patients that may allow for risk stratification and/or intervention.
The commonly cited Fried Frailty Index assesses frailty by measuring 5 of its physical components directly making it less suitable for use in large population studies or clinical practice.2 Modifications to the scale have been used in ESKD patients with variable success and numerous other frailty scales have been developed for the general population.2,4,6 The International Academy of Nutrition and Aging (IANA) task force performed a comprehensive systematic review of existing scales with expert opinion from a European, Canadian, and American Geriatric Advisory Panel (GAP). The GAP considered disability a consequence of frailty and suggested that it not be included in frailty definitions and assessment tools.7 As a consequence of this work, the FRAIL questionnaire was constructed and validated (Figure 1).7-9 The FRAIL questionnaire assesses fatigue, resistance (defined as the ability to climb stairs), ambulation (walk a block), number of comorbid illnesses, and weight loss of more than 5% in the last year.10
Figure 1.
Frail questionnaire.
The primary outcome of our study was to determine the prevalence of frailty in ESKD patients treated with hemodialysis and peritoneal dialysis using the FRAIL questionnaire within the larger context of an exercise survey.11 The secondary outcomes were to determine the association between frailty and subsequent mortality, transplantation, and hospitalization 18 months after completion of the original survey.
Materials and Methods
This study was approved by the Ottawa Health Science Network Research Ethics Board (Ottawa, Ontario; 20180902-01H) and conducted in accordance with the Helsinki Declaration.
All adult (age >18 years) English- or French-speaking prevalent ESKD patients (>3 months on hemodialysis or peritoneal dialysis) from The Ottawa Hospital in Ottawa, Ontario, and selected satellite dialysis units were invited to complete an exercise survey during their clinic visits from August to November 2017.11 Patients with disabilities that would impair their ability to participate in a walking exercise program such as wheelchair dependency were excluded from the study. Patients who were unable to complete the survey secondary to impairments in cognition, vision, or severe illness were also excluded. Completion of the survey implied consent by study participants.
Embedded within the survey was the FRAIL Questionnaire (Figure 1). Basic demographic information including the participants’ age, sex, ethnic background, and marital status was collected in the survey. Length of time on dialysis and number of comorbid illness were obtained from the patient’s electronic medical records (EMRs) vOacis (Telus Health Inc, British Columbia, Canada) and Nephrocare (version 7.5b, Fresenius Inc, Bad Hombourg, Germany). The Charlson Comorbidity Index (CCI) was also calculated for each patient.12 Weight change during the last 12 months was calculated from the patient’s dialysis orders that include a target dry weight. If the patient had been treated with dialysis for less than 12 months, the longest possible interval for calculation was used. However, we excluded any patient who had been on dialysis for <3 months as weight loss during that time might be reflective of reductions in extracellular fluid volume as opposed to loss of lean body mass.
Eighteen months after completion of the survey, we collected information about mortality, transplantation status, number of hospitalizations (excluding transplant), average length of hospital stay (excluding transplant), and total length of hospital stay using patients’ EMRs.
Descriptive statistics were used to describe the patient population, with mean and standard deviation for normally distributed numerical variables, and median and interquartile range otherwise and proportions for nominal variables. Patients were categorized as frail, pre-frail, and not frail as per the FRAIL scale system (Figure 1) with a score of ≥3 being frail, 1 to 2 pre-frail, and 0 being robust (non-frail). Univariate comparisons between the 3 groups were made using Fisher’s exact test, Student’s t-tests, Wilcoxon rank sum tests, and chi-square tests as appropriate. We performed a Kaplan-Meier survival analysis to assess the impact of frailty (vs pre-frail and not frail) on mortality and likelihood of receiving a kidney transplant. We further explored the relationship using Cox regression after adjusting for age and modality (variables selected a priori based on previous literature) and censoring for death. A P value of <.05 was considered significant. Descriptive analysis was performed with JMP (version 8.0.3, SAS Inc, Cary, NC), while survival analysis models were performed with R (version 3.5.2, R Foundation for Statistical Computing).
Results
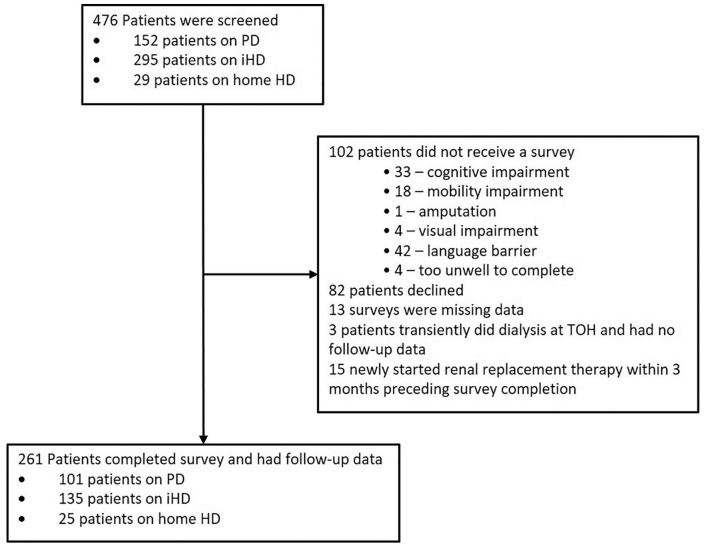
A total of 476 patients were screened for study, 215 patients were not included (102 were ineligible, 82 declined to participate, 15 had started dialysis within the last 3 months, 3 were at the dialysis center short term and 13 had missing data; Figure 2). Of the 261 patients who were included, 101 were treated with peritoneal dialysis, 135 with intermittent in-center hemodialysis, and 25 with home hemodialysis. Mean age of participants was 63.3 ± 15.6 years; the majority (n = 164) were men (Table 1). Thirty-nine (15%) were frail, 145 (55%) were pre-frail, and 77 (30%) were not frail. There were significant differences between the 3 frailty categories with respect to employment status, ethnicity, and comorbid illnesses (Table 1). Age, sex, dialysis modality, marital status, and income did not differ between the groups. Only 8% of frail patients compared with 25% of pre-frail and 19% of not-frail patients were listed for transplant at study start (P = .05).
Figure 2.
Study flow.
Note. PD = peritoneal dialysis; HD = hemodialysis.
Table 1.
Patient Demographics by Frail, Pre-frail, and Not-Frail Status.
| Demographics | Overall (N = 261) | Frail (n = 39) | Pre-frail (n = 145) | Not frail (n = 77) | P value |
|---|---|---|---|---|---|
| Age in years (mean ± standard deviation) | 63.3 ± 15.6 | 66.7 ± 12.6 | 63.6 ± 15.6 | 61.1 ± 16.0 | .17 |
| Sex | M: 164 (63%) F: 97 (37%) |
M: 21 (54%) F: 18 (46%) |
M: 92 (63%) F: 53 (37%) |
M: 51 (66%) F: 26 (34%) |
.42 |
| Diabetes mellitus | 120 | 31 (80%) | 65 (45%) | 24 (31%) | <.001 |
| Dialysis modality | .29 | ||||
| Peritoneal dialysis | 101 (39%) | 16 (41%) | 52 (36%) | 33 (43%) | |
| Hemodialysis | 135 (51%) | 20 (51%) | 82 (56%) | 33 (43%) | |
| Home hemodialysis | 25 (10%) | 3 (8%) | 11 (8%) | 11 (14%) | |
| Marital status | .30 | ||||
| No response/prefer not to answer | 17 | 2 (5%) | 8 (5%) | 7 (9%) | |
| Single/separated/divorced/widowed | 100 | 16 (41%) | 56 (39%) | 28 (36%) | |
| Married/Common Law | 144 | 21 (54%) | 81 (56%) | 42 (55%) | |
| Employment status | .05 | ||||
| No response/prefer not to answer | 8 | 2 (5%) | 2 (1%) | 4 (5%) | |
| Full-time | 31 | 1 (3%) | 17 (12%) | 13 (17%) | |
| Part-time | 15 | 0 (0%) | 6 (4%) | 9 (12%) | |
| Disability | 62 | 12 (31%) | 37 (26%) | 13 (17%) | |
| Not in paid workforce | 9 | 1 (3%) | 6 (4%) | 2 (3%) | |
| Student | 4 | 0 (0%) | 3 (2%) | 1 (1%) | |
| Retired | 132 | 23 (59%) | 74 (51%) | 35 (46%) | |
| Income | .34 | ||||
| No response/prefer not to answer | 72 | 9 (23%) | 39 (30%) | 24 (31%) | |
| Less than $10 000 | 18 | 6 (15%) | 8 (6%) | 4 (5%) | |
| $10 000-$24 999 | 54 | 9 (23%) | 31 (21%) | 14 (18%) | |
| $25 000-$79 999 | 78 | 12 (31%) | 42 (29%) | 24 (31%) | |
| $75 000+ | 39 | 3 (8%) | 25 (17%) | 11 (14%) | |
| Education | .65 | ||||
| No response/prefer not to answer | 25 | 5 (13%) | 10 (7%) | 10 (13%) | |
| Elementary or less | 34 | 5 (13%) | 23 (16%) | 6 (8%) | |
| High school | 66 | 10 (26%) | 38 (26%) | 18 (23%) | |
| Post-secondary education or higher | 136 | 19 (49%) | 74 (51%) | 43 (56%) | |
| Ethnicity | .01 | ||||
| White | 163 | 25 (64%) | 95 (66%) | 43 (56%) | |
| Black | 21 | 0 (0%) | 9 (6%) | 12 (16%) | |
| Arab | 10 | 1 (3%) | 7 (5%) | 2 (3%) | |
| Aboriginal | 9 | 3 (8%) | 4 (3%) | 2 (3%) | |
| Other | 31 | 7(18%) | 19 (13%) | 5 (7%) | |
| Multiple ethnicities | 13 | 1 (3%) | 3 (2%) | 9 (12%) | |
| Prefer not to answer/no response | 14 | 2 (5%) | 8 (6%) | 4 (5%) | |
| Charlson Comorbidity Index | 4.49 ± 2.19 | 6.4 ± 2.4 | 4.4 ± 1.9 | 3.8 ± 2.0 | <.001 |
| Wait-listed for transplant | 54 (21) | 3 (8%) | 36 (25%) | 15 (19%) | .05 |
Note. All data as n (proportion in percentage) unless stated otherwise. M = men; F = women.
During the 18-month follow-up period, 45 participants died, 1 participant transferred programs, and 1 participant recovered renal function (Table 2). Forty-three participants received a kidney transplant and 145 patients were hospitalized at least once (not including hospitalizations for kidney transplant or transplant-related complications).
Table 2.
Outcomes Based on Frailty.
| Outcome | Overall (N = 261) | Frail (n = 39) | Pre-frail (n = 145) | Not frail (n = 77) | P value |
|---|---|---|---|---|---|
| Death/withdrew care | 45 (17%) | 7 (18%) | 27 (19%) | 11 (15%) | .71 |
| Transferred | 1 (0.4%) | 0 (0%) | 1 (0.7%) | 0 (0%) | — |
| Recovered | 1 (0.4%) | 0 (0%) | 1 (0.7%) | 0 (0%) | — |
| Transplant | 43 (16%) | 1 (3%) | 27 (19%) | 15 (20%) | .02 |
| Any hospitalization? | 145 (56%) | 27 (69%) | 84 (58%) | 34 (44%) | .01 |
| Hospitalization specific data (n = 145) | |||||
| n of hospital staysa | 2 (1-3) | 2 (1-3) | 2 (1-3) | 1 (1-2) | .02 |
| Total LOS in daysa | 11 (5.5-31.5) | 17 (5-63) | 13 (13-32.8) | 9 (3-16) | .04 |
| Average LOS in daysa | 6 (3.1-12.6) | 8.8 (4-15.8) | 6 (3.6-13.4) | 5.5 (3-10.6) | .21 |
Note. All data as n (proportion in percentage) unless stated otherwise. LOS = length of stay.
Median (interquartile range).
The risk of death did not differ by frailty status (P = .78, Table 2) or time to death (frail vs pre-frail and not frail [log-rank test, P = .7]). Overall, frail patients were less likely to receive a kidney transplant, with only 3% of frail versus 19% of pre-frail and 20% of non-frail patients receiving a transplant during the 18 months of follow-up (P = .02, Table 2; log-rank test, P = .05, Figure 3). A Cox regression including only frailty found frailty to be associated with a longer time to transplant (Hazard Ratio [HR]: 0.13; confidence interval [CI], 0.02-0.95; P = .04). After adjusting for age (HR: 0.98; CI, 0.96-0.99; P = .03) and modality (not statistically significant), frailty was no longer statistically associated with a decreased likelihood of transplant (HR: 0.15; CI, 0.02-1.15; P = .068). This result was not materially changed with accounting for the competing risk of death (P = .060)
Figure 3.
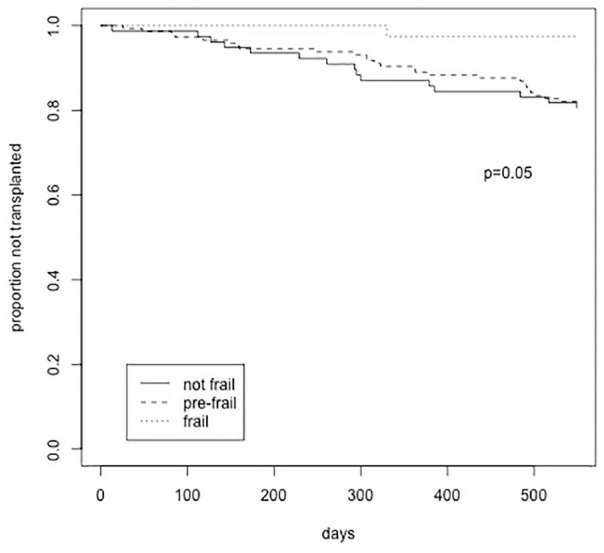
Kaplan-Meier survival analysis of impact of frailty on transplantation.
Frail patients were more likely to be hospitalized (P = .01), to be hospitalized more often (P = .02), and to spend more time in the hospital (P = .04) (Table 2). However, their average length of stay in hospital was not different (P = .21) compared with patients who were pre-frail or not frail.
Discussion
Patients with ESKD have a greater prevalence of frailty using a variety of scales compared with the general population that appears to start as renal function declines.13 Frailty in ESKD patients has been associated with an increased risk of morbidity and mortality.1,4 Moderate to severe frailty as assessed by the Clinical Frailty Scale (CFS) has also been associated with reduced kidney transplantation.1 In spite of the importance of frailty, the most clinically meaningful method to assess it remains unclear. A frailty measurement should be able to predict adverse clinical events and patient response to therapies but also be easy to use for research and/or clinical patient management.2 Therefore, we aimed to determine the usefulness of the FRAIL questionnaire by embedding it within an exercise survey and assessing outcomes of relevance, including mortality, kidney transplantation, and hospitalization in ESKD patients treated with dialysis.
The FRAIL questionnaire categorized a lower percentage of our participants as frail (15%) but similar percentages as pre-frail (55%) and not frail (30%) compared with other studies. In the study by Lee et al, 34.8% of Korean patients were frail and 45.7% were pre-frail using the Kidney Disease Quality of Life questionnaire to determine frailty phenotype.5 Two-thirds of incident dialysis patients in a US study were found to be frail using a frailty construct that included poor self-reported physical functioning, exhaustion/fatigue, low physical activity, and undernutrition.4 In another US study of prevalent hemodialysis patients, 41.8% of participants were frail using the Fried Frailty Index. A further 32.2% were classified as intermediately frail and 26% as non-frail.3 Twenty-six percent of incident Canadian dialysis patients had a score of 5 or greater (1, very fit; 2, well; 3, managing well; 4, vulnerable; 5, mildly frail; 6, moderately frail; 7, severely frail) and 27% were vulnerable using the Clinical Frailty Scale (CFS).1 The differences are likely due to the scales being used and the patient populations being studied. In one study, the percent agreement was poor between measured frailty (Fried criteria) and perceived frailty (reported by nephrologists, nurse practitioners, or patients).14 The average age of the populations were different, incident versus prevalent dialysis patients, and inclusion of different dialysis modalities in the reported studies. We also excluded participants from our study if they were deemed incapable of undertaking a walking exercise program likely skewing our population to the pre-frail and not-frail categories. However, a scale the identifies greater than 50% of the population in need of a targeted intervention is not likely to be useful clinically.
In other studies, older age and female sex have been associated with frailty.1,15 In univariate analysis, we were unable to show a statistically significant difference in these demographic factors but this may be related to the overall study numbers. On average, frail patients were 66.7 (±12.6) years compared with 61.1 (±16) years for non-frail patients. Similarly, 46% and 34% of frail and non-frail participants, respectively, were women. Employment status did differ, with frail patients being more likely to be on disability or retired compared with non-frail patients similar to other studies.5 Ethnicity was also different between frail and non-frail patients. This has been previously shown in the general population16 and may be related to intrinsic genetic factors but there may also be cultural differences in maintenance of activities of daily living or dietary preferences leading to nutritional deficiencies that could predispose certain populations to becoming frail. Frailty, defined by the FRAIL questionnaire and CFS, has been associated with hypoalbuminemia.
Unlike other studies of ESKD patients, we did not find an association between frailty and increased mortality.1,3-5 This is likely a reflection of our sample size; only 39 patients were frail limiting our ability to detect differences if they exist. We also excluded patients who had been on dialysis for less than 3 months when the risk of mortality is highest.17 Our study does support the increased risk of hospitalization with frailty, similar to other cohort studies.3-5 Frail patients were also more likely to have multiple hospital admissions such that total hospital length of stay was longer for frail patients.
Early identification of frail or pre-frail patients may allow for implementation of interventions to prevent or slow progression of frailty with the aim to reduce adverse outcomes and decrease the cost to the health care system. Such interventions may include exercise programs to increase physical strength and nutritional supplementations to mitigate weight loss.18-20 Use of the FRAIL questionnaire allows for identification of a focused area of intervention. Patients who are unable to climb a flight of stairs or walk a block may be more likely to benefit from a rehabilitation program whereas patients with weight loss maybe more likely to benefit from a dietary intervention. In a study reported by D. Moorman et al, patients are interested in exercise programs that will improve their strength and energy.11 The FITNESS trial from the United Kingdom is currently assessing if frailty can be lessened in hemodialysis patients through dietary changes and physical rehabilitation.21
Frail ESKD patients have been previously shown to be less likely to receive a kidney transplant.1 We found similar results in our univariate analysis. The apparent effect of frailty was unchanged when the survival analysis included modality and age, but the association was no longer statistically significant. Kidney transplantation is the optimal treatment for ESKD and dramatically alters the disease trajectory. Early identification of frailty and pre-frailty with frank discussions about the implications of the diagnosis on likelihood of receiving a kidney transplant may help patients to remain motivated to participate in exercise or nutrition intervention programs assuming the results of the FITNESS trial are positive.
Our study has several strengths including a high response rate (>70%) of eligible patients and almost complete follow-up of the cohort. We have also demonstrated that the FRAIL questionnaire is easy to administer and does identify frail patients who are at increased risk for hospitalization and may be less likely to receive a kidney transplant. Moreover, the survey allows for focused interventions in areas of difficulty. However, our study does have several limitations including the single-center design with a relatively small sample size and short follow-up limiting our ability to detect subgroup differences in outcomes and numbers of variables that could be assessed in subgroup analysis. We also excluded patients who were unable to communicate in English or French and those patients with physical limitations such as amputations, potentially affecting generalizability.
Conclusions
Using the easy-to-administer FRAIL Questionnaire, 15% of our dialysis cohort was frail. Ethnicity and employment status were different between our frail and non-frail patients, with the frail patients more likely to be on disability or retired. Although increased risk of mortality was not seen in the frail patients with ESKD, they were at an increased risk of hospitalization with more days spent in hospital. Similarly, frail ESKD patients were less likely to receive a transplant; however, this association was not significant when adjusting for age, modality, and competing risk of death. Further multi-center studies with larger patient numbers and longer follow-up is needed to determine the usefulness of the FRAIL scale in predicting adverse outcomes in patients with ESKD.
Acknowledgments
Special thanks to the participants who completed the survey and the research staff at the Kidney Research Center.
Footnotes
Ethics approval and consent to participate: This study was approved by the Ottawa Health Science Network Research Ethics Board (Ottawa, Ontario; 20180902-01H). Completion of the survey implied consent by study participants.
Consent to publication: All authors consented to the publication of this manuscript.
Availability of Data and Materials: The data underlying this article will be shared on reasonable request to the corresponding author.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Drs Zimmerman and Hiremath receive salary support from the Department of Medicine at the Ottawa Hospital. R.S.S. is supported by a Junior 2 Chercheur-Boursier Award from the Fonds de Recherche du Québec—Santé. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Swapnil Hiremath  https://orcid.org/0000-0003-0010-3416
https://orcid.org/0000-0003-0010-3416
Rita S. Suri  https://orcid.org/0000-0002-0519-3927
https://orcid.org/0000-0002-0519-3927
Deborah Zimmerman  https://orcid.org/0000-0003-0000-8806
https://orcid.org/0000-0003-0000-8806
References
- 1. Alfaadhel TA, Soroka SD, Kiberd BA, et al. Frailty and mortality in dialysis: evaluation of a clinical frailty scale. Clin J Am Soc Nephrol. 2015;10:832-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3-10. [DOI] [PubMed] [Google Scholar]
- 3. McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967. [DOI] [PubMed] [Google Scholar]
- 5. Lee SY, Yang DH, Hwang E, et al. The prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J Ren Nutr. 2017;27(2):106-112. [DOI] [PubMed] [Google Scholar]
- 6. Painter P, Kuskowski M. A closer look at frailty in ESRD: getting the measure right. Hemodial Int. 2013;17(1):41-49. [DOI] [PubMed] [Google Scholar]
- 7. Abellan van Kan G, Rolland Y, Bergman H, Morley JE, Kritchevsky SB, Vellas B. The I.A.N.A Task Force on frailty assessment of older people in clinical practice. J Nutr Health Aging. 2008;12(1):29-37. [DOI] [PubMed] [Google Scholar]
- 8. Lopez D, Flicker L, Dobson A. Validation of the frail scale in a cohort of older Australian women. J Am Geriatr Soc. 2012;60(1):171-173. [DOI] [PubMed] [Google Scholar]
- 9. Gleason LJ, Benton EA, Alvarez-Nebreda ML, et al. FRAIL questionnaire screening tool and short-term outcomes in geriatric fracture patients. J Am Med Direct Assoc. 2017;18:1082-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9(2):71-72. [DOI] [PubMed] [Google Scholar]
- 11. Moorman D, Suri R, Hiremath S, et al. Benefits and barriers to and desired outcomes with exercise in patients with ESKD. Clin J Am Soc Nephrol. 2019;14:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47:1245-1251. [DOI] [PubMed] [Google Scholar]
- 13. Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43(5):861-867. [DOI] [PubMed] [Google Scholar]
- 14. Salter ML, Gupta N, Massie AB, et al. Perceived frailty and measured frailty among adults undergoing hemodialysis: a cross-sectional analysis. BMC Geriatrics. 2015;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansen KL. The frail dialysis population: a growing burden for the dialysis community. Blood Purif. 2015;40(4):288-292. [DOI] [PubMed] [Google Scholar]
- 16. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med. 2011;27:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiner DE, Tighiouart H, Ladik V, Meyer KB, Zager PG, Johnson DS. Oral intradialytic nutritional supplement use and mortality in hemodialysis patients. Am J Kidney Dis. 2014;63(2):276-285. [DOI] [PubMed] [Google Scholar]
- 20. Manfredini F, Mallamaci F, D’Arrigo G, et al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol. 2017;28(4):1259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson BM, Dutton M, Day E, et al. Frailty Intervention Trial iN End-Stage patientS on haemodialysis (FITNESS): study protocol for a randomised controlled trial. Trials. 2018;19:457. [DOI] [PMC free article] [PubMed] [Google Scholar]