Abstract
In patients with ischemic stroke who receive systemic recombinant tissue plasminogen activator (rt-PA), the risk of secondary hemorrhage is 1-7%. Fibrinogen supplementation with cryoprecipitate is recommended in patients with rt-PA-associated symptomatic hemorrhage. We examined whether fibrinogen concentrate can be used safely in this setting. A single-center retrospective case series was performed in patients who received fibrinogen concentrate for post-rt-PA hemorrhage between January-2012 and December-2017. The primary outcome was the incidence of in-hospital thromboembolic events and infusion reactions. Secondary outcomes included incidence of clinically significant ICH expansion within 24-hours and patient serum fibrinogen response to fibrinogen concentrate therapy. Thromboembolic events occurred in 3 (12.5%) of 24 patients included in the analysis. No patients experienced infusion-related reactions. Five of 22 patients with ICH experienced clinically significant hemorrhage expansion. Hypofibrinogenemia was corrected in 87.5%(7/8) of patients with baseline hypofibrinogenemia, with a median increase in serum fibrinogen 166 mg/dL. Median fibrinogen increase in patients without baseline hypofibrinogenemia was 18 mg/dL. Fibrinogen concentrate is a safe potential therapeutic option to restore fibrinogen levels in acute ischemic stroke patients with thrombolysis-associated hemorrhage.
Keywords: intracerebral hemorrhage, thrombolysis, fibrinogen, cerebral infarction, stroke, hemostasis
Introduction
Intravenous recombinant tissue plasminogen activator (rt-PA) has become the mainstay of treatment in patients presenting within 4.5 hours of the onset of acute ischemic stroke.1,2 Early thrombolysis has been associated with improved functional outcomes but is not without significant bleeding risks. In patients who receive systemic rt-PA for the treatment of ischemic stroke, the risk of secondary hemorrhage is 3-7% for intracerebral hemorrhage (ICH) and 1-7% for extracranial hemorrhage.2–4 Acquired coagulopathies due to decreased fibrinogen levels, increased international normalized ratio (INR), prolonged prothrombin (PT) and activated partial thromboplastin (aPTT) times have all been observed.5 Despite the short plasma half-life of rt-PA (5 minutes) and rapid clearance of the drug after cessation of the infusion, coagulopathy may occur within 2 hours of thrombolysis, and hemorrhage risk can persist for over 24 hours.4 This may be in part due to a longer biologic half-life (15-20 minutes) of rt-PA compared to its plasma half-life. The longer antifibrinolytic effect of rt-PA after plasma clearance is attributable to persistent presence of rt-PA in the thrombus after administration and a slower beta clearance from the peripheral compartment.5 Hypofibrinogenemia occurs in approximately 5% of rt-PA treated patients and has been identified as an important risk factor for bleeding in rt-PA treated patients.4,6
Limited evidence guides the management of patients who experience hemorrhagic complications from rt-PA administration after acute ischemic stroke. Treatment strategies include empiric replacement of platelets or coagulation factors with fresh frozen plasma (FFP), cryoprecipitate, prothrombin complex concentrates (PCC), aminocaproic acid, tranexamic acid, and vitamin K.7 The 2018 American Heart Association/American Stroke Association (AHA/ASA) guideline for early management of patients with acute ischemic stroke and Neurocritical Care Society’s guideline for the reversal of anti-thrombotic agents in ICH suggests cryoprecipitate for fibrinogen supplementation in patients with thrombolysis-associated symptomatic ICH. If cryoprecipitate is not readily available or contraindicated, alternative antifibrinolytic agents are recommended.8,9
Purified human fibrinogen concentrate, RiaSTAP™, may be a reasonable alternative to cryoprecipitate for the repletion of serum fibrinogen in post-thrombolysis hemorrhage management. Compared to cryoprecipitate, there is negligible risk for immunological reactions or pathogen transmission with fibrinogen concentrate. The product is available as a lyophilized powder that can be stored at room temperature and rapidly reconstituted at the patient bedside, which may expedite administration times.10 Fibrinogen concentrate also has standardized contents, while cryoprecipitate and fresh frozen plasma do not. Per the American Association of Blood Banks (AABB), one unit of cryoprecipitate must contain a minimum of 150 mg fibrinogen and 80 IU Factor VIII per unit. Cryoprecipitate also contains von Willebrand factor and Factor XIII in appreciable amounts, though not standardized. FFP contains approximately 280 mg/dL fibrinogen, 81 IU/dL Factor VIII, along with appreciable amounts of Factor II, Factor V, Factor VII, Factor IX, Factor X, von Willebrand factor, antithrombin, Protein C, and Protein S.11
Despite limited use in the neurocritical care patient population, fibrinogen concentrate has been used in the treatment of congenital and acquired fibrinogen deficiency due to hemorrhage secondary to surgery, trauma, in obstetrics.12 The objective of this study was to evaluate the safety and effectiveness of fibrinogen concentration for the treatment of thrombolysis-associated hemorrhage at our institution.
Methods
We performed a single-center retrospective case series of patients who received fibrinogen concentrate for the treatment of major hemorrhage after systemic thrombolysis between January 2012 and December 2017 at Brigham and Women’s Hospital in Boston, Massachusetts. We included adult ischemic stroke patients treated with intravenous rt-PA who received at least one dose of fibrinogen concentrate for the management of secondary rt-PA related hemorrhage. We excluded patients who were less than 18 years old or who received fibrinogen concentrate for other indications. Our local institutional review board approved the study.
The primary safety outcomes assessed were the incidence of in-hospital thromboembolic events and infusion or anaphylactic reactions. Secondary outcomes evaluated included ICH expansion in the first 24 hours after fibrinogen concentrate administration and adequate patient response to fibrinogen concentrate administration in patients with post rt-PA serum fibrinogen levels collected. Response to fibrinogen concentrate was defined as serum fibrinogen normalization to > 200 mg/dL in patients with hypofibrinogenemia. Target serum fibrinogen of > 200 mg/dL, is consistent with the 2018 AHA/ASA Guidelines for the early management of patients with acute ischemic stroke.9 Hypofibrinogenemia was defined as a serum fibrinogen level ≤200 mg/dL. Other outcomes included change in fibrinogen levels from post rt-PA baseline levels, in-hospital mortality, intensive care unit (ICU) length of stay, and hospital length of stay.
Since 2013, our institutional guideline recommends a STAT fibrinogen level immediately after a CT scan confirmed thrombolysis-related symptomatic ICH, followed by administration of 2 vials of fibrinogen concentrate (approximately 900-1300 mg of fibrinogen per vial) without waiting for results of the baseline fibrinogen level. A repeat fibrinogen level is checked 1 hour after fibrinogen concentrate administration and every 2 hours thereafter until serum fibrinogen is > 200 mg/dL. Additional fibrinogen concentrate is administered until the serum fibrinogen level is greater than 200 mg/dL. Supplemental FFP, platelets, aminocaproic acid, vitamin K, or PCC may be administered based on individual patient need per treating provider’s clinical judgment. (Supplemental Figure E-1).
Hemorrhagic events were classified based on clinical and radiographic criteria. ICH were classified as either hemorrhagic infarction 1 (HI1), hemorrhagic infarction 2 (HI2), parenchymal hemorrhage 1 (PH1), or parenchymal hemorrhage 2 (PH2) in accordance with the ECASS II trial hemorrhage grading scale.13 ICH expansion was determined to be clinically significant if classification changed or new intraventricular extension was found on follow up imaging. Extracranial hemorrhages were identified based on clinical exam. Descriptive statistics were performed for this analysis.
Results
We identified 24 patients who received fibrinogen concentrate for the management of thrombolysis-associated hemorrhage post-acute ischemic stroke (Table 1). The median patient age was 74.1 years and 66.7% of patients were female. Median NIHSS score prior to rt-PA administration was 12 [interquartile range (IQR) 5-19]. The median time to administration of IV rt-PA was 2.2 hours [IQR 1.6-2.7] after ischemic stroke symptom onset. Most rt-PA associated bleeds were ICH (91.7%) with a median time from rt-PA administration to confirmation of hemorrhage of 4.6 hours [IQR 3.3-6.0]. The median time of administration of fibrinogen concentrate was 1.2 hours after hemorrhage confirmation. In addition to fibrinogen concentrate, 25% (6/20) of patients received concurrent cryoprecipitate. Other hemostatic products administered included FFP, platelets, tranexamic acid, aminocaproic acid, intravenous vitamin K, and additional clotting factors (Table 2). Three patients underwent urgent neurosurgical procedures including an external ventricular drain placement (n = 1), hemicraniectomy (n = 1), and dural arteriovenous fistula embolization (n = 1).
Table 1.
Patient Characteristics.
| Baseline characteristics—no. (%) | N = 24 |
|---|---|
| Age, yearsa | 74.1 [61.8-87.9] |
| Female gender | 16 (66.7) |
| Anti-thrombotic therapy | |
| Aspirin | 12 (50) |
| Clopidogrel | 2 (8.3) |
| Warfarin | 2 (8.3) |
| Ischemic Stroke Presentation and Management—no. (%) | |
| NIHSS Scorea | 12 [5 –19] |
| Stroke onset to rt-PA treatment time, hoursa | 2.2 [1.6–2.7] |
| Intravenous rt-PA—no. (%) | 24 (100) |
| rt-PA initiated at OSH—no. (%) | 20 (83.3) |
| Mechanical recanalization—no. (%) | 4 (16.7) |
| Intra-arterial rt-PA—no. (%) | 1 (4.2) |
| Laboratory Parameters Pre rt-PAa | |
| Platelets, k/mm3 | 203 [161–303] |
| INR | 1 [1–1.1] |
| PT, seconds | 12.6 [11.2–13.4] |
| aPTT, seconds | 28.4 [26.3–29.6] |
| Glucose | 159 [110–199] |
| Hemorrhage Presentation—no. (%) | |
| Symptom onset at OSH | 8 (33.3) |
| Time from rt-PA to hemorrhage confirmation, hoursa,b | 4.6 [3.3–6.0] |
| Hemorrhage type | |
| ICH | 22 (91.7) |
| GIc | 2 (8.3) |
| Groin puncture site | 1 (4.2) |
| Time from hemorrhage confirmationb to fibrinogen concentrate administration, hoursa | 1.2 [0.5–2.3] |
| Laboratory Parameters Post rt-PA Prior to Fibrinogen Concentratea | |
| Fibrinogen, mg/dL | 256 [130–347] |
| Platelets, k/mm3 | 197 [150–287] |
| INR | 1.2 [1.1–1.6] |
| PT, seconds | 15.1 [14.4–18.9] |
| aPTT, seconds | 33.4 [27.8–39.6] |
| Hematocrit, % | 37.4 [34.1–41.4] |
| Hemoglobin, mg/dL | 12.6 [11–13.9] |
OSH: Outside hospital; ICH: Intracerebral Hemorrhage; GI: Gastrointestinal.
a Data presented as median [interquartile range.
b On radiographic imaging (ICH) or clinical presentation (gastrointestinal, groin puncture hemorrhage).
c One patient had concurrent ICH and GI bleed.
Table 2.
Pharmacologic Management of Hemorrhage.
| No. (%) | Dosea | |
|---|---|---|
| Fibrinogen concentrate, mg | 24 (100) | 2215 [2188–4371] |
| Cryoprecipitate, units | 6 (25) | 20 [10–20] |
| Fresh frozen plasma, units | 7 (29.2) | 2 [1–4] |
| Platelets, units | 6 (25) | 1 [1–1] |
| Intravenous Vitamin K, mg | 4 (16.7) | 10 [10–10] |
| Aminocaproic acid, grams | 3 (12.5) | 5.5 [5.3–6.3] |
| Tranexamic acid, grams | 2 (8.3) | 1 [1–1] |
| Prothrombin Complex Concentrate, units | 2 (8.3) | 2417 [2060–2773] |
a Data presented as median [interquartile range].
Safety Outcomes
Documented thromboembolic events occurred in 3 patients (12.5%) who received fibrinogen concentrate. Two patients with thromboembolic complications had post rt-PA hypofibrinogenemia. Patient 11 was found to have a left lower extremity deep vein thrombosis (DVT) 10 days after fibrinogen concentrate administration. Pertinent coagulation products the patient received were 6,846 mg of fibrinogen concentrate along with 10 units of cryoprecipitate, 2 units of FFP, and 1 gram of tranexamic acid. Venous thromboembolism (VTE) prophylaxis with heparin 5,000 units subcutaneously 3 times daily was started on day 3 of hospital admission. Patient 14 was found to have an upper extremity superficial and deep vein thrombosis 9 days after presentation and treatment with 2,198 mg fibrinogen concentrate (no additional coagulation products). VTE prophylaxis was started on day 2 of hospital admission. Patient 20 was found to have a superficial upper extremity vein thrombosis on hospital day 3. Patient’s initial treatment course included 4,356 mg of fibrinogen concentrate and was not receiving VTE prophylaxis at the time of VTE diagnosis. Both upper extremity thromboembolic events (Patient 14 and Patient 20) were associated with access lines. No patients who received fibrinogen concentrate had a documented infusion or anaphylactic reaction.
Serum Fibrinogen Response to Fibrinogen Concentrate Administration
Of 20 patients with post rt-PA fibrinogen levels collected prior to fibrinogen concentrate administration, 8 (40%) patients had serum fibrinogen ≤200 mg/dL and 12 (60%) patients had serum fibrinogen > 200 mg/dL (Table 3).
Table 3.
Plasma Fibrinogen Response to Fibrinogen Concentrate Administration.
| All patients N = 24 | Patients with baseline hypofibrinogenemia N = 8 | Patients without hypofibrinogenemia at baseline N = 12 | |
|---|---|---|---|
| Baseline fibrinogen, mg/dL | 256 [130–347] | 119 [68–159] | 306 [263–388] |
| Initial fibrinogen concentrate dose administered, mg | 2188 [2181–2198] | 2198 [2188–2282] | 2193 [2178–2198] |
| Increase in fibrinogen after initial dose, mg/dL | 35 [16–65] | 40 [31–130] | 25 [-6–54] |
| Total fibrinogen concentrate dose administered, mg | 2215 [2188–4371] | 4376 [2232–5495] | 2193 [2116–2198] |
| Increase in fibrinogen levels after completion of therapy, mg/dL | 72 [13–117] | 133 [108–153] | 27 [-7–57] |
| Final fibrinogen level by the end of the 24 hour follow-up period, mg/dL | 316 [246–391] | 287 [238–331] | 344 [316–401] |
| Increase in fibrinogen levels by the end of the 24 hour follow-up period, mg/dL | 73 [2–131] | 166 [121–201] | 18 [-10–56] |
Data presented as median [interquartile range].
Note: Four patients did not have baseline fibrinogen levels prior to fibrinogen concentrate administration.
In the 8 patients with post rt-PA hypofibrinogenemia prior to fibrinogen concentrate administration, median fibrinogen level was 119 mg/dL. Six patients with post rt-PA hypofibrinogenemia presented with ICH post thrombolysis. Fibrinogen levels normalized to > 200 mg/dL in 7 (87.5%) hypofibrinogenemic patients. The median time required to increase plasma fibrinogen above 200 mg/dL in hypofibrinogenemic patients was 4.1 hours (range 1.5–14.8 hours). The median dose administered was 4366 mg (range 2174–6846 mg). Three of 8 patients with post rt-PA hypofibrinogenemia had fibrinogen levels > 200 mg/dL after receiving a median fibrinogen concentrate dose of 2,205 mg. One patient’s fibrinogen increased from 166 mg/dL to 200 mg/dL after 2178 mg fibrinogen concentrate, and subsequently received an additional 2178 mg dose to target fibrinogen > 200 mg/dL. Finally, one patient’s fibrinogen level increased from 60 to 188 mg/dL after administration of 4,396 mg but repeat labs were not available to confirm that serum fibrinogen returned to more than 200 mg/dL.
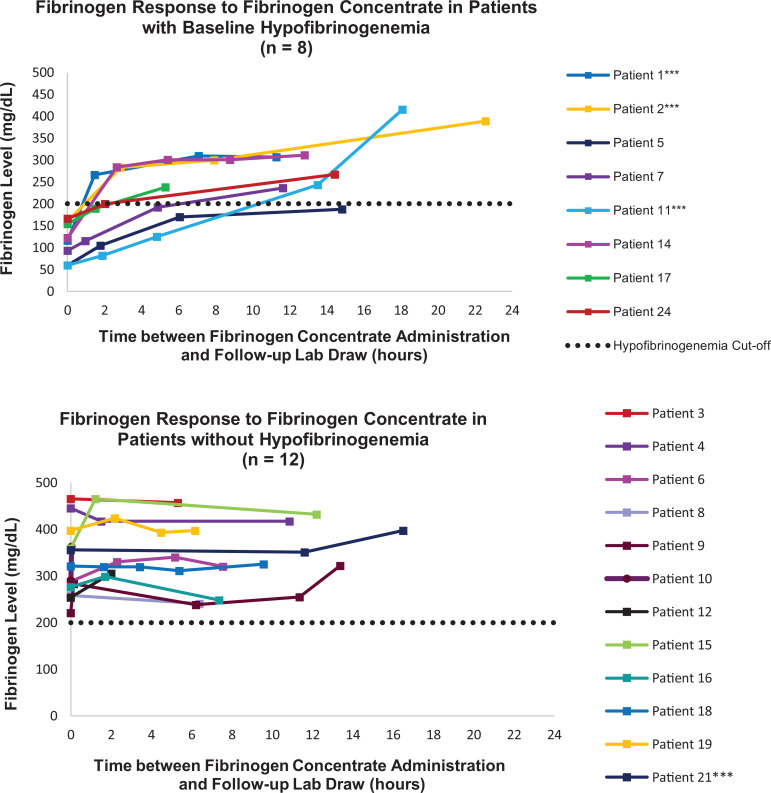
Twelve patients had normal fibrinogen levels post rt-PA, with a median baseline fibrinogen of 306 mg/dL. After a median total dose of 2,193 mg, fibrinogen levels increased by a median of 18 mg/dL. Further information on coagulation results pre- and post- fibrinogen concentrate administration is reported in Figure 1 and Supplemental Table E-1.
Figure 1.
Patient-specific fibrinogen response to fibrinogen concentrate administration. ***Patient received concurrent cryoprecipitate. Note: Four patients did not have baseline fibrinogen and were excluded from evaluation of serum fibrinogen response to fibrinogen concentrate administration.
Intracranial Hemorrhagic Expansion After Fibrinogen Concentrate Administration
Twenty-two patients with ICH were included in this analysis. Twenty patients were noted to have symptomatic ICH and 2 patients were found to have an ICH on routine imaging post-thrombectomy procedure or repeat imaging upon transfer to our institution’s emergency department. On neuroimaging of the 22 patients with ICH prior to fibrinogen concentrate administration, 45.5% (10/22) were originally classified as PH2 classified hemorrhage, followed by 31.8% (7/22) PH1, 13.6% (3/22) HI2, 4.5% (1/22) HI1, and 4.5% (1/22) isolated subarachnoid hemorrhage (Table 4). Additionally, 36% (8/22) had intraventricular extension, 40.9% (9/22) exhibited SAH, and 13.6% (3/22) subdural hematoma (SDH). Five patients suffered clinically significant hemorrhage expansion despite fibrinogen concentrate administration (Patient 7,10, 12, 21, 24). All 5 patients were treated within 3.5 hours of stroke onset with intravenous rt-PA and 3 patients were over 80 years of age. One patient had post rt-PA hypofibrinogenemia with concurrent coagulation laboratory elevations prior to fibrinogen concentrate therapy and 2 patients without baseline fibrinogen levels were noted to have hypofibrinogenemia on follow up laboratory testing post-treatment. While the other 2 patients did not exhibit coagulation abnormalities, they were on antiplatelet therapy at baseline (clopidogrel and aspirin). Four of the 5 patients received fibrinogen concentrate as the only source of fibrinogen replacement, 1 patient received concurrent aminocaproic acid and vitamin K, 1 patient received additional platelets, and 1 patient received additional cryoprecipitate and fresh frozen plasma (Table 2).
Table 4.
Intracerebral Progression After Fibrinogen Concentrate Administration.
| Patient number | ICH classification pre fibrinogen concentrate | Intraventricular extension pre-fibrinogen concentrate | ICH classification post fibrinogen concentrate 24 hour head-CT | Intraventricular extension post fibrinogen concentrate | Baseline fibrinogen level (mg/dL) | Final fibrinogen level after fibrinogen concentrate treatment (mg/dL) |
|---|---|---|---|---|---|---|
| 1 | PH1 | No | PH1 | No | 116 | 307 |
| 2 | PH1/IVH | Yes | PH1/HI1 | Yes | 160 | 389 |
| 3 | PH1/IVH | Yes | PH1 | Yes | 465 | 457 |
| 4 | PH1/IVH | Yes | PH1multiple | Yes | 445 | 417 |
| 6 | PH1/SAH | No | PH1multiple | No | 288 | 320 |
| 7a | SAH | No | PH2/SAH | Yes | 93 | 236 |
| 8 | PH2/SAH/SDH | Yes | PH2/SAH/SDH | Yes | 258 | 241 |
| 9 | PH2/SAH | Yes | PH2/SAH | Yes | 221 | 322 |
| 10a | HI2 | No | P H2 | No | 291 | 363 |
| 11 | HI2 | No | HI2 | No | 60 | 415 |
| 12a | PH2 | No | PH2 | Yes | 254 | 305 |
| 13a | PH1 | No | PH1/SDH | Yes | Not available | 226 |
| 14 | PH1 | No | PH1 | No | 122 | 311 |
| 15 | PH2 | No | PH2/SAH | No | 359 | 432 |
| 16 | HI2 | No | HI2 | No | 276 | 248 |
| 18 | PH2/SAH | No | PH2/SAH | No | 321 | 325 |
| 19 | PH2/SAH/SDH | No | PH2/SAH/SDH | No | 397 | 393 |
| 20 | PH2/SAH | Yes | PH2/SAH | Yes | Not available | Not available |
| 21 | HI1 | Yes | HI1 | Yes | 356 | 397 |
| 22 | PH2/SAH | Yes | PH2/SAH | Yes | Not available | 309 |
| 23a | PH2/SDH | No | PH2/SDH | Yes | Not available | 354 |
| 24 | PH2/SAH | No | PH2/SAH | No | 166 | 267 |
aPatient 7, 10, 12, 13, 23 were considered to have clinically significant progression after fibrinogen concentrate administration.
Note: Patient 5 and 17 was excluded due to extracerebral hemorrhage origin.
Extracranial Hemorrhage Management
Three patients included in our analysis developed extracranial hemorrhage post rt-Pa and were treated with fibrinogen concentrate. Two patients had a gastrointestinal hemorrhage (1 with concurrent ICH) while 1 patient had a groin puncture hemorrhage. Two of these patients were hypofibrinogenemic post rt-PA prior to fibrinogen concentrate administration. All patients who developed extracranial hemorrhages had elevated coagulation laboratory results (INR, PT, PTT) compared to baseline.
Clinical Outcomes
The median ICU length of stay was 4 days (range 1–17) and the median hospital length of stay was 5.5 days (range 1–22). In-hospital mortality occurred in 50% (12/24) of patients. All 12 patients with in-hospital mortality were transitioned to comfort measures only. Four of these 12 patients had documented post rt-PA hypofibrinogenemia.
Subgroup Analysis of Thrombolysis-Associated Hemorrhage Management With Fibrinogen Concentrate Monotherapy
Of the 15 patients who received fibrinogen concentrate as the sole source of fibrinogen supplementation, 5 had post rt-PA hypofibrinogenemia. Two patients experienced significant radiographic hematoma expansion (Patient 7 and 21). Patient 7 had a post rt-PA fibrinogen level of 93 mg/dL. The patient’s fibrinogen level rose to 115 mg/dL within 1 hour of treatment initiation, 192 mg/dL 4.9 hours after initiation and to a final level of 236 mg/dL after 11.6 hours and a total of 5,495 mg fibrinogen concentrate in conjunction with 5 grams of aminocaproic acid and 10 mg intravenous vitamin K administered. Patient 21 did not have post rt-PA baseline fibrinogen, but after receiving an initial dose of 2,174 mg fibrinogen concentration fibrinogen level was 136 mg/dL which rose to 225 mg/dL after an additional 2,175 mg were administered. No other hemostatic agents were administered.
In hospital mortality was 46.7% (7/15) for patients managed with fibrinogen concentrate monotherapy. No patients who received fibrinogen concentrate therapy as the sole fibrinogen repletion treatment modality experienced VTE events.
Discussion
In this descriptive report, we identified 24 patients who were treated with fibrinogen concentrate for the management of thrombolysis-associated hemorrhage after acute ischemic stroke. We found fibrinogen concentrate administration to be associated with a low rate of complications in our patient population. No patients experienced infusion reactions, secondary ischemic stroke, or myocardial infarction.
Approximately 12.5% of patients in our cohort experienced thromboembolic events. One patient in our study was diagnosed with a lower extremity DVT 10 days after fibrinogen concentrate administration while on VTE prophylaxis. This patient received higher than average doses of fibrinogen concentrate along with concurrent cryoprecipitate and tranexamic acid to manage their ICH and thus the VTE complication could not be attributed to the fibrinogen concentrate alone. Two patients experienced upper extremity thromboembolic events associated with access lines. The thromboembolic rate described in our case-series is higher than reported when fibrinogen concentrate was used in the non-ischemic stroke patient population, however patients with acute ischemic stroke are at a 20-fold increased risk of VTE events within the first month of diagnosis.14–16 This increased risk is attributed to prolonged hospitalization, prolonged immobilization in the setting of neurologic deficits, increased risk of secondary acute infection (e.g. aspiration pneumonia, urinary tract infection), high rate of co-morbid inflammatory conditions, and prolonged access line placement.15
Additionally, we found that fibrinogen concentrate corrected hypofibrinogenemia in all but one patient and maintained fibrinogen levels close to baseline in non-hypofibrinogenemic patients. There is a paucity of data to guide clinicians in the management of thrombolysis associated bleeding secondary to acquired coagulopathy. Administration of rt-PA has been associated with prolongation of the PT, reduction in fibrinogen and plasminogen levels, and increased fibrinogen degradation products in the immediate 24-hours post administration. A study by Huang et al. found that within 3–12 hours after rt-PA administration, the mean decrease in fibrinogen was 29% in patients treated with rt-PA and 3% in those treated with tenecteplase.17 In a cohort of 128 patients with thrombolysis-associated hemorrhage, a fibrinogen level of ≤150 mg/dL was the only risk factor on univariate analysis associated with an increased risk of hematoma expansion.18 A decrease in fibrinogen levels more than 200 mg/dL from baseline within 6 hours of thrombolysis has also been identified as a significant predictor for bleeding risk, with a 39.9% risk for major bleeding events.19 Baseline fibrinogen levels prior to rt-PA administration were not available in our patient population, and therefore we cannot determine if patients with normal fibrinogen levels had a decrease of ≥200 mg/dL from their baseline, thus increasing the risk of major hemorrhage. Hypofibrinogenemic patients who had a major hemorrhage as a complication from systemic thrombolysis for acute ischemic stroke were more likely to have a prolonged PT/INR prior to fibrinogen concentrate administration than non-hypofibrinogenemic patients; the PT/INR normalized after administration of fibrinogen concentrate.
Systemic fibrinogen decrease in response to rt-PA administration has been correlated with an increase in fibrinogen degradation products, signifying excessive fibrinogenolysis—an important predictor for parenchymal hematomas.3 The majority (70.8%) of patients with ICH included in our retrospective case series exhibited parenchymal hematomas with approximately 40% experiencing an intraventricular extension. While symptomatic ICH after rt-PA administration is infrequent, it is associated with significant morbidity and mortality.20 Early identification and empiric treatment of rt-PA induced fibrinogen deficiency may mitigate clinical hematoma progression. In our retrospective analysis, after administration of fibrinogen concentrate (and limited cryoprecipitate), 22.7% (5/22) of patients had clinically relevant hemorrhagic expansion on neuroimaging. Of those with clinically relevant hemorrhagic expansion, only 1 patient had baseline hypofibrinogenemia with elevated INR, PT, and PTT. Two patients with clinically relevant hemorrhagic expansion who did not have post rt-PA serum fibrinogen available were hypofibrinogenemic on follow up laboratory monitoring despite initial fibrinogen concentration administration. This suggests that fibrinogen deficiency may not be the only risk factor for hematoma progression. Whether antiplatelet therapy at baseline played a significant role in hematoma expansion in the 2 patients without hypofibrinogenemia is unknown.
Cryoprecipitate is often used to restore fibrinogen levels in the management of symptomatic intracranial hemorrhage after rt-PA administration, despite limited published evidence.21 Cryoprecipitate has several limitations, including the need for ABO matching, delay in administration due to product thawing, and concerns regarding potential transmission of viral pathogens.
Given the limited data in treating thrombolysis-associated hemorrhages, a task force at our institution was developed to create institutional guidelines for the management of symptomatic ICH after thrombolysis for acute ischemic stroke. In non-ischemic stroke patients, a median 4,000 mg dose has been associated with a 109 mg/dL increase in plasma fibrinogen.22 Fibrinogen concentrate was approved for use by our Pharmacy and Therapeutics Committee in patients for thrombolysis associated bleeding in place of the previous institutional standard—cryoprecipitate—in October 2013, prior to the release of the Neurocritical Care Society’s guideline for reversal of anti-thrombotic agents in intracranial hemorrhage in December 2015, AHA/ASA 2017 Consensus statement on the treatment of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke, or publication of the updated 2018 AHA/ASA Guidelines for the early management of patients with acute ischemic stroke.8,9,23 In our patient population, the median increase in fibrinogen at 24 hours was 73 mg/dL after a median dose of fibrinogen concentrate of 2,198 mg. However, upon post hoc subgroup analysis, increases in fibrinogen levels were more pronounced in the hypofibrinogenemic group compared to the non-hypofibrinogenemic group (median increase of 166 mg/dL versus 18 mg/dL, respectively). Fibrinogen concentrate did not result in a hyperfibrinogenemic state in patients with normal fibrinogen when used in the management of hemorrhages secondary to rt-PA administration, possibly due to an ongoing consumptive process in those patients with normal post rt-PA fibrinogen levels. Vandelli and colleagues evaluated 39 ischemic stroke patients with severe hypofibrinogenemia after rt-PA, 25.6% of which had symptomatic ICH, who received fibrinogen concentrate therapy to normalize serum fibrinogen.24 Similar to our findings, serum fibrinogen increased from a median of 133 mg/dL (range 18-231) pre-fibrinogen concentrate administration to a median of 160.5 mg/dL (range 56-337) post-fibrinogen concentrate administration. Two patients experienced thromboembolic events after administration, with unclear onset.
Additional agents have been explored in case reports for the management of rt-PA related hemorrhage, such as tranexamic acid and aminocaproic acid. These agents competitively inhibit activation of plasminogen to plasmin compared to direct fibrinogen replenishment. Tranexamic acid has been associated with decreased hematoma expansion in ICH not associated with rt-PA administration and may be a useful adjunct agent.7,25 Four patients in our study received adjunct tranexamic acid or aminocaproic acid, 2 of whom had hemorrhage expansion on repeat imaging. While the use of antifibrinolytic agents is recommended when cryoprecipitate cannot be administered within a reasonable timeframe, the role of concurrent antifibrinolytic agents or platelet transfusions with fibrinogen products remains unclear.8
In addition to rt-PA induced coagulopathy, reperfusion of ischemic vasculature and damage to the blood brain barrier secondary to activation of matrix metalloproteinases in the setting of ischemia may also play a role in the risk for ICH after rt-PA administration.26 While there is much to be discovered in the pathophysiology of hemorrhagic complications following rt-PA administration, utilization of products containing fibrinogen has become standard practice. Further investigations are warranted to determine optimal agents and the role of adjunct treatments. Fibrinogen concentrate appears to be a promising alternative to cryoprecipitate in this patient population.
This descriptive report has several limitations. This was a single-center retrospective analysis with a small sample size. Many patients received thrombolysis at an outside facility, and records were based on transfer documents, limiting our baseline demographic knowledge. In addition, baseline fibrinogen prior to rt-PA administration was often not collected at outside institutions. Because most patients received rt-PA at an outside institution and transferred, we were unable to determine the true incidence of post rt-PA hemorrhage or capture the quantity of patients who received rt-PA within the study period through medication dispense reports. Additionally, our institution established a standardized protocol for the management of symptomatic ICH after rt-PA administration utilizing fibrinogen concentrate as the primary treatment modality in 2013, not all patients were treated uniformly over the study period. Some patients received both cryoprecipitate and fibrinogen concentrate, limiting our ability to assess the effect of fibrinogen concentrate alone. Lastly, our population consists mostly of patients with thrombolysis-associated ICH with only 2 isolated extra-cranial hemorrhages, and therefore our interpretations are limited to this patient group. Due to the heterogeneous patient population and small sample size, the effectiveness of fibrinogen concentrate therapy could not be inferred. Furthermore, the institutional guideline (Supplemental Figure 1) was developed through expert opinion based on limited data available in this patient population. Despite these limitations, we believe our study provides further insight into the management of rt-PA induced hemorrhage, a rare but serious complication of thrombolytic treatment. There are limited data to guide clinicians in the optimal agents to manage these patients and this study highlights our experience using fibrinogen concentrate with or without adjunctive agents as empiric therapy.
Conclusion
Our study found fibrinogen concentrate to be a safe alternative therapeutic option for restoring fibrinogen levels in ischemic stroke patients with post rt-PA hypofibrinogenemia complicated by hemorrhage. Fibrinogen concentrate administration did not induce a hyperfibrinogenemic state in patients without initial hypofibrinogenemia. Most patients who were treated with fibrinogen concentrate had stable hemorrhages at 24 hours. Further research is required to determine the efficacy of fibrinogen concentrate in comparison to alternative therapies in the treatment of patients with post rt-PA hemorrhage.
Supplemental Material
Supplemental Material, MBarra_CATH_Fibrinogen_Concentrate_STROBE_checklist_ for Fibrinogen Concentrate for the Treatment of Thrombolysis-Associated Hemorrhage in Adult Ischemic Stroke Patients by Megan E. Barra, Steven K. Feske, Katelyn W. Sylvester, Charlene Ong, Sarah E. Culbreth, Patricia Krause, Galen V. Henderson and Eva Rybak in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, MBarra_CATH_Fibrinogen_Concentrate_Supplemental_Figure_1 for Fibrinogen Concentrate for the Treatment of Thrombolysis-Associated Hemorrhage in Adult Ischemic Stroke Patients by Megan E. Barra, Steven K. Feske, Katelyn W. Sylvester, Charlene Ong, Sarah E. Culbreth, Patricia Krause, Galen V. Henderson and Eva Rybak in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, MBarra_CATH_Fibrinogen_Concentrate_Supplemental_Table_E1 for Fibrinogen Concentrate for the Treatment of Thrombolysis-Associated Hemorrhage in Adult Ischemic Stroke Patients by Megan E. Barra, Steven K. Feske, Katelyn W. Sylvester, Charlene Ong, Sarah E. Culbreth, Patricia Krause, Galen V. Henderson and Eva Rybak in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: Ethical approval to report this case series was obtained from Brigham and Women’s Hospital (Protocol Number 2016P002079). Informed consent for patient information to be published in this article was not obtained because a wavier of consent was approved by the Brigham and Women’s Hospital Institutional Review Board.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Megan E. Barra  https://orcid.org/0000-0002-7696-1540
https://orcid.org/0000-0002-7696-1540
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Gross H, Guilliams KP, Sung G. Emergency neurological life support: acute ischemic stroke. Neurocritical Care. 2015;23(suppl 2):S94–102. [DOI] [PubMed] [Google Scholar]
- 2. Albers GW, Olivot JM. Intravenous alteplase for ischaemic stroke. Lancet. 2007;369(9558):249–250. [DOI] [PubMed] [Google Scholar]
- 3. Trouillas P, Derex L, Philippeau F, et al. Early fibrinogen degradation coagulopathy is predictive of parenchymal hematomas in cerebral rt-PA thrombolysis: a study of 157 cases. Stroke. 2004;35(6):1323–1328. [DOI] [PubMed] [Google Scholar]
- 4. Matrat A, De Mazancourt P, Derex L, et al. Characterization of a severe hypofibrinogenemia induced by alteplase in two patients thrombolysed for stroke. Thromb Res. 2013;131(1):e45–48. [DOI] [PubMed] [Google Scholar]
- 5. Sane DC, Califf RM, Topol EJ, Stump DC, Mark DB, Greenberg CS. Bleeding during thrombolytic therapy for acute myocardial infarction: mechanisms and management. Ann Intern Med. 1989;111(12):1010–1022. [DOI] [PubMed] [Google Scholar]
- 6. Alonso A, Dempfle CE, Szabo K, Zohsel K, Hennerici MG. Drop of PT Quick percent value is associated with both symptomatic and asymptomatic intracranial hemorrhage in patients treated with rt-PA for acute ischemic stroke. Thromb Res. 2011;127(1):65–66. [DOI] [PubMed] [Google Scholar]
- 7. Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA Neurol. 2014;71(9):1181–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frontera J. Guideline for reversal of antithrombotics in intracranial hemorrhage. Neurocritical care. 2016;24:6–46. [DOI] [PubMed] [Google Scholar]
- 9. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professions from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. [DOI] [PubMed] [Google Scholar]
- 10. Sorensen B, Bevan D. A critical evaluation of cryoprecipitate for replacement of fibrinogen. Br J Haematol. 2010;149(6):834–843. [DOI] [PubMed] [Google Scholar]
- 11. AABB ARC, America’s Blood Centers, Armed Services Blood Program. Circular of information for the use of human blood and blood components. AABB, 2017. [Google Scholar]
- 12. Soloman C, Groner A, Ye J, Pendrak I. Safety of fibrinogen concentrate: analysis of more than 27 years of pharmacovigilance data. Thromb Haemost. 2015;113(4):759–771. [DOI] [PubMed] [Google Scholar]
- 13. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. [DOI] [PubMed] [Google Scholar]
- 14. Bilecen S, de Groot JA, Kalkman CJ, et al. Effect of fibrinogen concentrate on intraoperative blood loss among patients with intraoperative bleeding during high-risk cardiac surgery: a randomized clinical trial. JAMA. 2017;317(7):738–747. [DOI] [PubMed] [Google Scholar]
- 15. Rinde LB, Småbrekke B, Mathiesen EB, et al. Ischemic stroke and risk of venous thromboembolism in the general population: the Tromsø Study. J Am Heart Assoc. 2016;5(11):e004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wikkelsø AJ, Edwards HM, Afshari A, et al. Pre-emptive treatment with fibrinogen concentrate for postpartum haemorrhage: randomized controlled trial. Br J Anaesth. 2015;114(4):623–633. [DOI] [PubMed] [Google Scholar]
- 17. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543–3546. [DOI] [PubMed] [Google Scholar]
- 18. Yaghi S, Boehme AK, Dibu J, et al. Treatment and outcome of thrombolysis-related hemorrhage: a multicenter retrospective study. JAMA Neurol. 2015;72(12):1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matosevic B, Knoflach M, Werner P, et al. Fibrinogen degradation coagulopathy and bleeding complications after stroke thrombolysis. Neurology. 2013;80(13):1216–1224. [DOI] [PubMed] [Google Scholar]
- 20. Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38(8):2279–2283. [DOI] [PubMed] [Google Scholar]
- 21. Franchini M, Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfu. 2012;10(1):23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danes AF, Cuenca LG, Bueno SR, Mendarte Barrenechea L, Ronsano JB. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sang. 2008;94(3):221–226. [DOI] [PubMed] [Google Scholar]
- 23. Yaghi S, Willey JZ, Cucchiara B, et al. Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke. Stroke. 2017;48(12):343–361. [DOI] [PubMed] [Google Scholar]
- 24. Vandelli L, Marietta M, Trenti T, et al. Fibrinogen concentrate replacement in ischemic stroke patients after recombinant tissue plasminogen activator treatment. Adv Clin Exp Med. 2019;28(2):219–222. [DOI] [PubMed] [Google Scholar]
- 25. French KF, White J, Hoesch RE. Treatment of intracerebral hemorrhage with tranexamic acid after thrombolysis with tissue plasminogen activator. Neurocrit Care. 2012;17(1):107–111. [DOI] [PubMed] [Google Scholar]
- 26. Leigh R, Jen SS, Hillis AE, Krakauer JW, Barker PB. Pretreatment blood-brain barrier damage and post-treatment intracranial hemorrhage in patients receiving intravenous tissue-type plasminogen activator. Stroke. 2014;45(7):2030–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, MBarra_CATH_Fibrinogen_Concentrate_STROBE_checklist_ for Fibrinogen Concentrate for the Treatment of Thrombolysis-Associated Hemorrhage in Adult Ischemic Stroke Patients by Megan E. Barra, Steven K. Feske, Katelyn W. Sylvester, Charlene Ong, Sarah E. Culbreth, Patricia Krause, Galen V. Henderson and Eva Rybak in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, MBarra_CATH_Fibrinogen_Concentrate_Supplemental_Figure_1 for Fibrinogen Concentrate for the Treatment of Thrombolysis-Associated Hemorrhage in Adult Ischemic Stroke Patients by Megan E. Barra, Steven K. Feske, Katelyn W. Sylvester, Charlene Ong, Sarah E. Culbreth, Patricia Krause, Galen V. Henderson and Eva Rybak in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, MBarra_CATH_Fibrinogen_Concentrate_Supplemental_Table_E1 for Fibrinogen Concentrate for the Treatment of Thrombolysis-Associated Hemorrhage in Adult Ischemic Stroke Patients by Megan E. Barra, Steven K. Feske, Katelyn W. Sylvester, Charlene Ong, Sarah E. Culbreth, Patricia Krause, Galen V. Henderson and Eva Rybak in Clinical and Applied Thrombosis/Hemostasis